Abstract
Chronic inflammatory conditions including allergic, autoimmune, metabolic, and neuropsychiatric disorders are constantly increasing and leading to a high burden, especially in more industrialized countries. The prevalence is still on the rise in developing countries. The start of the steep increase in asthma, atopic dermatitis, and allergic rhinitis dates to the 1960s, whereas a second wave with an increase in eosinophilic gastrointestinal disease, food allergy, and drug hypersensitivity started after the 2000s. These diseases also started to appear more with neuropsychiatric and autoimmune conditions during the last few decades. Many theories have been proposed to explain this outbreak. The hygiene hypothesis was consolidated by “old friends” and biodiversity, although some gaps remained unresolved. The introduction of the epithelial barrier hypothesis gave us a new perspective to explain the effects of industrialization without environment control and health concerns creeping into our daily lives. The present review touches on the possible explanations of why epithelial barrier hypothesis covers all previous ones, which are not contradictory but mostly complementary.
Keywords: Allergy, autoimmune disorders, epithelial barrier hypothesis, hygiene hypothesis, industrialization
Introduction
Allergic diseases have been increasing for decades and represent an enormous psychosocial and economic burden. Development of novel medical techniques, in addition to therapeutic advances, has broadened our perspectives and diagnostic capabilities. There is still lack of clear knowledge about the exact pathogenesis of allergic, autoimmune, metabolic, and neuropsychiatric diseases, particularly the steep increases in their prevalence. Since the emergence of allergic diseases, many explanations have been proposed which are described below in comparison.
The Maturation of the Allergy Idea
At the end of the nineteenth century, the “immune system” was first described by scientists such as Louis Pasteur, Paul Erhlich, Elie Metchnikoff, Jules Bordet, and Emil Von Behring.1 The fundamental definitions had focused on the protection of the body, and no one could imagine that it could hurt itself. However, with the discovery of antitoxin treatments and vaccines, physicians documented some “reactions” due to these treatments. Clemens von Pirquet was one of the few who had suspected a connection between the immune system and these adverse reactions2 and came up with the idea that the immune system could damage the host itself.
In the same year, the French immunologist Nicolas Maurice Arthus published his experiment describing local reactions after the injection of horse serum, which became more and more severe when repeated.3 With the description of serum sickness and the precipitating antibodies, knowledge of these reactions was expanded. Meanwhile, the first terminology for “allergy” was proposed by von Pirquet in 1906; it was defined as altered reactions of the body to foreign substances that get severe upon subsequent exposures.4
The Allergy Epidemics
Besides the death of Pharaoh Menes due to bee sting, the first “realistic” description of an allergic disease was made by Charles Blackley in 1873 as hay fever.5 With the arrival of “hay fever,” the seasonal association with different pollens in different locations was defined, and this has been proposed to be linked to extensive lawn making. In addition, the increase in arable farming in the USA and many other countries led to a large-scale spread of ragweed.6
Furthermore, public hygiene gained momentum soon after sewage and intestinal diseases became known. At the end of the nineteenth century, drinking water was separated from sewage. By 1920, chlorination of water became widespread.6
The increase in hay fever in the 1940s led to the first attempts at immunotherapy against grass pollen. With regard to asthma, increased numbers of reports showing high numbers of cases point to the 1960s. Many of these children were found to be allergic to house dust mites.7 Increasingly, denser and warmer homes with more carpeting and indoor activities were blamed.8 During the 1960s, 2 studies from Sweden9 and Canada emphasized that living in urban sites was more frequently associated with allergic disorders than populations living in rural sites.10 In the early 1960s, it was noticed that asthma suddenly increased in almost every age group in England.7 In 1961, asthma increased in Finnish soldiers.11 From 1965 to 1980, there was a 10-fold increase in the number of hospitalized children with asthma in Australia, England, New Zealand, Canada, and the USA.12 Between 1971 and 1981, it was found that asthma increased by 3-fold in Swedish soldiers, especially in those who came from the cities.13 When East and West Germany were united, the prevalence of asthma and atopic dermatitis (AD) was very low in the East in 1990. They have caught up with the West within 10 years (5-10 times increase).14,15 An increase in AD was similarly reported after the 1960s. The frequency differed from 1.1% to 3.1% among the population born before 1960,16,17 whereas an increasing trend was suggested by tabulating individual year groups after the 1960s, reaching 12% in 1974.18
Hygiene Hypothesis
In 1989, David Strachan came up with the idea of “hygiene hypothesis” to explain the increase in allergic disorders. He suggested that the changes in the microbial environment would shape the development of the immune system. It was hypothesized that fewer infections would cause a shift toward allergic responses. Recurrent microbial exposure would initiate T-helper 1 (Th1) response rather than a Th2-mediated immune response associated with elevated interleukin-4 (IL-4) and IL-5 levels and eosinophilia.19 Rook’s “old friends hypothesis” almost supported this idea by arguing that infectious diseases evolve with human body, and adequate exposure is necessary for prompt development of the immune system.20 Further “Alpine farm studies” reflected the allergo-protective effects of traditional farming habits such as close contact with farm animals and unprocessed milk consumption.21,22 All in all, it was convincing that the more diverse the microbial environment, the better the immune system functions. The study “Prevention of Allergy Risk factors for Sensitization In children related to Farming and Anthroposophic Lifestyle (PARSIFAL)” then showed that this link is already established during pregnancy.23 Maternal exposure to a diverse microbial environment, such as is present in farming activities, was associated with lower atopic sensitization in the offspring. This kind of exposure modulated allergen-specific responses toward a Th1 pattern.24 The concomitant increase in autoimmune disorders in Westernized populations has been explained by the need for a microbial environment to fine-tune the Th1 and Th2 responses.25
The mechanism underlying the hygiene hypothesis consists of elements of the innate system such as Toll-like receptors (TLRs). After encountering bacterial products such as muramic acid and endotoxin, TLRs relay the microbial signals to the immune system and also to regulatory T cells. In short, it was clear that TLR2 and TLR4 ligands were protective in allergen-induced lung inflammation. The same protective functions were also attributed to TLR9 in mouse models.26 After recognition of bacterial endotoxins, Th1 cells can exert their protective function in several ways: inhibiting respiratory tract damage by antiviral defenses and reducing the abnormal repair mechanisms responsible for mucosal and smooth muscle hyperplasia. Excessive endotoxin exposure could also be harmful, as is the case with occupational asthma. In this context, it appears that the dose and timing of exposure are critical to the subsequent response.27 The biodiversity hypothesis endorsed the hygiene hypothesis. Briefly, the greater the diversity of microbial species in a given space, the less dominant their existence, and consequently the immune system balance is maintained. Similar to hygiene hypothesis, it supports the idea that more contact with natural environments would enrich the microbiota.28
In recent decades, several shortcomings of the hygiene hypothesis, the old friends hypothesis, and the biodiversity hypothesis have been discussed, suggesting that these hypotheses do not fully explain the rise in allergic and autoimmune diseases. These include the fact that water sanitation was introduced in many Western cities in the 1920s, but allergy and asthma epidemics did not begin until the 1960s. The protective role of parasite infections in increasing biodiversity has been questioned for the same reason. Moreover, allergic asthma is still increasing in some Asian and African cities with low hygiene standards.29 Another pitfall of the hygiene hypothesis is that it does not seem to protect against allergic diseases, despite the increase in respiratory diseases and measles.30 Moreover, allergic diseases are not uncommon in rural Africa, where children are exposed to a traditional, unhygienic lifestyle.30
Another limitation of the hygiene hypothesis and the biodiversity hypothesis is that probiotics are not viable alternatives for the prevention or treatment of allergies.31 Moreover, studies of migrants moving from developing countries to affluent regions show a rapid increase in asthma and allergic diseases, as well as autoimmune diseases such as type 1 diabetes and multiple sclerosis.32-34 It appears that home living conditions, the increase in births by cesarean section, antibiotic use, dietary habits, urbanization, and indoor air pollution are more important factors than general public hygiene.35-37
The protective role of growing up on a farm on the development of asthma and allergies has received the most attention in this context, and a substantial number of studies have supported the initial findings.38 For example, children in Amish communities in the USA where traditional dairy farming is practiced have been found to be highly protected from asthma and allergies.39 In contrast, Hutterite communities practice industrialized agriculture with extensive cleaning practices and have a significantly higher prevalence of asthma and allergies in children.39 The Amish community uses homemade detergents and cleaning products whose main ingredient is washing soda (Na2CO3) and does not use commercial cleaning products that may contain barrier surfactants and enzymes.
Epithelial Barrier Hypothesis
The first links between the epithelial barrier and inflammatory diseases were established in the early 1990s with the description of a disrupted intestinal barrier in celiac disease and inflammatory bowel disease.40 Later, this was also demonstrated for other diseases such as asthma, AD, chronic rhinosinusitis, and eosinophilic esophagitis. The mechanism underlying epithelial barrier hypothesis is that disrupted epithelia are prone to bacterial leakage and dysbiosis. Therefore, bacterial translocation leads to inflammation in the adjacent tissue.41-43 It has been suggested that this could have different consequences: either local pathologies as in AD or triggering chronic metabolic or autoimmune diseases such as diabetes or obesity and neurodegenerative disorders.41-43
In addition to known allergens and pathogens, various toxins we encounter daily can also cause epithelial damage. Air pollutants such as smoke and diesel exhaust are well described, but substances we use for hygiene measures can also hide in cleaning products or even in personal hygiene products (Table 1).
Table 1.
Experimental Models of Barrier Disruption
| Substance | Evidence |
|---|---|
| Anionic surfactants and commercial detergents | Cultures of human skin keratinocytes show that anionic surfactants and commercial detergents reduce the integrity of the tight junction barrier54,74 |
| Cigarette smoke | Mouse models show that cigarette smoke causes acute lung damage46 |
| Detergent residue | Cultures of human bronchial epithelial cells at the air–liquid interface show that detergent residues disrupt the integrity of the tight junction barrier in human bronchial epithelial cells even at low concentrations44 |
| Diesel exhaust particulates | Human and rat alveolar epithelial cells exposed to diesel exhaust particles exhibit low occludin expression and a leaky barrier53 |
| Emulsifiers in processed food | Emulsifiers increased damage to hamster small intestine structure in vivo and the translocation ofEscherichia coliacross M-cells in vitro.47,75 It has been shown in rat models that food emulsifier polysorbate 80 decreased the expression of proteins related to mucus barrier and mucosal barrier in the intestine, changed the integrity of intestinal epithelial cell, and increased the permeability of intestinal epithelial mucosa76 |
| Nanoparticles | Human cell cultures show that nanoparticles disrupt gut barrier homeostasis77 |
| Ozone | Mouse models show damage to the airway barrier by ozone78 |
| Particulate matter | Ex vivo experiments with human and rat alveolar epithelial cells show that particulate matter affects occludin distribution and the alveolar barrier. Particulate matter 2.5 causes defects in the nasal epithelial barrier in noninflamed nasal biopsies from patients with sinusitis. Particulate matter 10 stimulates myeloid dendritic cells to induce Th17 cells in vitro with the property of migrating to the brain.53-55 Mice exposed to particulate matter showed epithelial barrier dysfunction and an increase in eosinophilic inflammation in the sinonasal airways.79 |
| Polystyrene microplastic | Mouse models show the effect of polystyrene microplastics on the intestinal barrier80,81 |
The hygiene and epithelial barrier hypotheses overlap with the increase in cleaning products such as detergents and also air pollution as a result of industrialization. Even exposure to highly diluted laundry and dishwasher detergents has been shown to upregulate genes involved in oxidative stress and cell survival.44,45 On the contrary, genes involved in wound healing appear to have been downregulated in response to laundry detergents.44 It is now clear that some of the most harmful toxins, namely surfactants and emulsifiers, in detergents and processed foods, respectively, are part of our daily lives (Table 2). These chemicals have been overused in parallel with the increase in allergic and autoimmune diseases.46,47 Besides toxins, it is well known that proteolytic allergens such as house dust mites may cause epithelial barrier defects by cleaving the tight junctions.48
Table 2.
Comparison of Hygiene and Epithelial Barrier Hypotheses
| Hygiene Hypothesis | Epithelial Barrier Hypothesis |
|---|---|
| Water sanitation started in the 1920s but allergy epidemics only started in the 1960s | This correlates with the general use of everyday substances |
| Lack of rationale for increased Th1 response | Disruption of the barrier may contribute to both Th2 and Th1 responses |
| Increased allergy prevalence even in countries with low hygiene conditions | Allergic diseases are less pronounced in communities using less toxic substances |
| Probiotics does not prevent allergic disorders | Evidence is shown by in vitro models |
Th1, T-helper 1, Th2, T-helper 2.
The epithelial barrier, with its physical, chemical, and immunological properties, is the first line of defense of the innate immune system. It mainly lines the intestine, skin, urogenital system, and respiratory tract. The epithelial cells are tightly bound to each other with tight junctions and are well organized with the contribution of mucus and microbiota. Their immunological functions include the clearance of particles and the activation of the immune cells by the production of antimicrobial peptides and cytokines. In addition to its antimicrobial action, it is also essential for a prompt tissue repair. Once the epithelial barrier is impaired, in addition to tissue injury, an inflammatory state occurs and exacerbates epithelial damage.49 Healthy tight junctions prevent the entrance of foreign substances, while a disrupted barrier allows passage from both sides, either by the outflow of immune cells from the subepithelium to the surface or by the translocation of microbiota to deeper tissues. The latter can lead to inflammation due to colonization by opportunistic pathogens (Figure 1). Consequently, an inflammatory microenvironment disrupts epithelial barrier and regeneration from epithelial stem cells. These sequential events can lead to a local or systemic inflammatory state that may be causative for many immune-related disorders.50 Local epithelial damage in the skin and mucosa can lead to type 2 inflammation, which manifests as AD, asthma, allergic rhinitis, and eosinophilic esophagitis. The changes in the microbiome due to leakage of the epithelial barrier can trigger autoimmune processes in the gut.51
Figure 1.
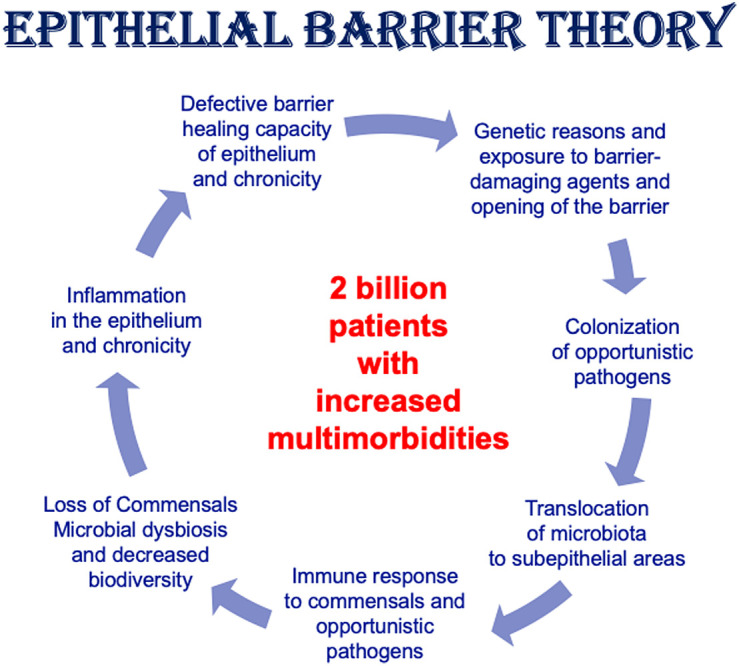
Exposure to barrier degraders or genetic deficiency of barrier molecules leads to colonization by opportunistic pathogens and epithelial inflammation.
In several autoimmune diseases, a link between disruption of the epithelial barrier in the intestine or lung and inflammation in distant organs has been demonstrated. For example, an association between intestinal barrier disruption and inflammation in distant organs was recently found in a mouse model of arthritis. In this study, Th1 and Th17 effector T cells accumulated in the lamina propria of leaky gut and migrated to affected joints, where they triggered pathology.52 Similarly, barrier disruption due to environmental exposures, such as particulate matter in the lungs, can trigger inflammation in distant organs in multiple sclerosis.53-55 In a mouse model of multiple sclerosis, the disease was induced by intra-tracheal administration of the autoantigen myelin basic protein in combination with a barrier-damaging adjuvant. Autoantigen-specific effector T cells were shown to be “licenced” in the airways to migrate to the brain, where they caused multiple sclerosis-like inflammation.56
Dendritic cells, macrophages, innate lymphoid cells (ILCs), T cells, and their cytokines interact with stem cells in the chronic inflammatory environment and are critical for both damage and regeneration of mucosal epithelial barriers.57-58 B cells, mast cells, eosinophils, type 2 ILCs, and Th2 cells are also usually involved in the response to the translocated microbiome.
Impaired epithelial barriers are in parallel to the development of extensive immune response against harmless environmental agents. Increased immunoglobulin G (IgG) and IgE responses to allergens, documented from the 1970s to the present, link disrupted epithelial barriers and demonstrate that antigens are reaching deeper tissues: In the 1970s and 1980s, healthy individuals occasionally showed IgG responses to environmental antigens.59,60 However, after the 1970s, there was an increase in allergen-specific IgE and IgG levels to environmental antigens.60-63 Comparing frozen serum samples from 1998 with those from 1990, the 1998 samples had more allergen-specific IgE, even when analyzed with the same assay.63 In 2015, 49.8% of Norwegian children aged 10 to 16 years were found to be IgE sensitized to at least 1 environmental allergenic protein.64 In 2017, most adults had IgG antibodies to grass pollen, olive/ash pollen, birch pollen, and house dust mites.65 A year later, almost every baby aged 1 year had IgG antibodies to cow’s milk and hen’s egg.66 In 2019, more than 90% of individuals with asthma, rhinitis, and conjunctivitis had elevated IgE levels to at least 1 allergen in a panel of 64 aeroallergen components.67 Similarly, IgE response toStaphylococcus aureusincreased in the 1980s. In 1985, serum-specific IgE toS. aureuswas not detected in individuals colonized only in the skin.68 In individuals with infected skin pustules withS. aureus, only 12% showedS. aureus-specific IgE.68 However, in 2019,S. aureus-specific IgE was found in 39% of healthy controls, 58% of patients with mild asthma, and 76% of patients with severe asthma.69 Today, nearly 90% of patients with AD and chronic rhinosinusitis haveS. aureuscolonization andS. aureus-specific IgE.70,71
Conclusion
Soon after the hygiene hypothesis was first put forward, many others followed with similar ideas such as the old friends and biodiversity. However, the main gap in hygiene hypothesis was that less exposure to pathogens in childhood would be protective for allergies. Today, we know that the type, dose, and nature of microorganisms are important for this type of protection. Additionally, recent studies have shown that our homes may not be as clean as we first thought. It is also almost impossible to reduce the microbial load only by daily cleaning routines.72 In a German birth cohort study, although personal cleanliness was associated with decreased endotoxin levels, the same could not be demonstrated for household cleanliness.73 So far, no direct connection in the sense of the hygiene hypothesis with an increase in allergic diseases could be confirmed. Hygiene measures are not sufficient to change the microbiota in the sense we understand it. Microbial interactions are undoubtedly necessary for adequate development of the immune system, but dietary habits and foreign exposure should not be excluded.73,82 Nevertheless, the parallel increase of westernization and allergic diseases seems quite convincing. From this point of view, epithelial barrier hypothesis seems more reasonable regarding the environmental exposures that we encounter every day which also have an impact on biodiversity.
Microbial dysbiosis and translocation of commensals and opportunistic pathogens across the epithelial barrier is usually followed by a type 2 immune response characterized by a predominance of Th2 cells, ILC2, and eosinophils. Mast cells, macrophages, and antibody-producing B cells may also be involved in this response. The epithelium cannot fully repair and close the barrier, setting in motion a vicious cycle of leaky barriers, microbial dysbiosis, and chronic inflammation.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Literature Review – A.K., I.O., D.Y., C.A., M.A., H.C.; Writing – A.K., I.O., D.Y., C.A.; Critical Review – M.A., C.A.
Declaration of Interests: The authors declare that they have no competing interest.
Funding: This study received no funding.
References
- 1. Igea JM. The history of the idea of allergy. Allergy. 2013;68(8):966 973. ( 10.1111/all.12174) [DOI] [PubMed] [Google Scholar]
- 2. Parish HJ. Clemens von pirquet, his life and work, by Richard Wagner, London, Oxford University Press, 1968, pp. xx, 214, illus., 66 s. 6 d. Med Hist. 1970;14(4):422. ( 10.1017/S0025727300015994) [DOI] [Google Scholar]
- 3. M. A. Injections répétées de sérum de cheval chez le lapin. Compt Rendu Soc Biol. 1903;50:20. [Google Scholar]
- 4. C. VP. Allergie. Munch Med Wochenschr. 1906;30:1457 1458. [Google Scholar]
- 5. Blackley CH. Experimental Researches on the Causes and Nature of Catarrhus Aestivus: Facsimile of the First Edition 1873. Balliere: Tindall & Cox;1873. [Google Scholar]
- 6. Platts-Mills TA. The allergy epidemics: 1870-2010. J Allergy Clin Immunol. 2015;136(1):3 13. ( 10.1016/j.jaci.2015.03.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Platts-Mills TAE Tovey ER Chapman MD Wilkins SR. Airborne allergen exposure, allergen avoidance and bronchial hyperreactivity. In: Kay AB Austen KF Lichtenstein LM, eds. Asthma: Physiology, Immunopharmacology and Treatment, Third International Symposium. London. Academic Press; Cambridge; 1984:297 314. [Google Scholar]
- 8. Tovey ER, Chapman MD, Wells CW, Platts-Mills TA. The distribution of dust mite allergen in the houses of patients with asthma. Am Rev Respir Dis. 1981;124(5):630 635. ( 10.1164/arrd.1981.124.5.630) [DOI] [PubMed] [Google Scholar]
- 9. Irnell L, Kiviloog J. Bronchial asthma and chronic bronchitis in a Swedish urban and rural population. With special reference to prevalence, respiratory function and socio-medical condition. Scand J Respir Dis Suppl. 1968;66:1 86. [PubMed] [Google Scholar]
- 10. Gerrard JW, Geddes CA, Reggin PL, Gerrard CD, Horne S. Serum IgE levels in white and metis communities in Saskatchewan. Ann Allergy. 1976;37(2):91 100. [PubMed] [Google Scholar]
- 11. Haahtela T, Lindholm H, Björkstén F, Koskenvuo K, Laitinen LA. Prevalence of asthma in Finnish young men. BMJ. 1990;301(6746):266 268. ( 10.1136/bmj.301.6746.266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitchell EA. International trends in hospital admission rates for asthma. Arch Dis Child. 1985;60(4):376 378. ( 10.1136/adc.60.4.376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aberg N. Asthma and allergic rhinitis in Swedish conscripts. Clin Exp Allergy. 1989;19(1):59 63. ( 10.1111/j.1365-2222.1989.tb02345.x) [DOI] [PubMed] [Google Scholar]
- 14. Krämer U, Schmitz R, Ring J, Behrendt H. What can reunification of East and West Germany tell us about the cause of the allergy epidemic? Clin Exp Allergy. 2015;45(1):94 107. ( 10.1111/cea.12458) [DOI] [PubMed] [Google Scholar]
- 15. von Mutius E, Martinez FD, Fritzsch C, Nicolai T, Roell G, Thiemann HH. Prevalence of asthma and atopy in two areas of West and East Germany. Am J Respir Crit Care Med. 1994;149(2 Pt 1):358 364. ( 10.1164/ajrccm.149.2.8306030) [DOI] [PubMed] [Google Scholar]
- 16. Walker RB, Warin RP. The incidence of eczema in early childhood. Br J Dermatol. 1956;68(5):182 183. ( 10.1111/j.1365-2133.1956.tb12805.x) [DOI] [PubMed] [Google Scholar]
- 17. Brereton EM, Carpenter RG. Rook AJ TP. The prevalence and prognosis of eczema and asthma in Cambridgeshire school-children. Br Med J. 1959:317 322. 13618630 [Google Scholar]
- 18. Schultz Larsen F, Hanifin JM. Secular change in the occurrence of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1992;176:7 12. [PubMed] [Google Scholar]
- 19. Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259 1260. ( 10.1136/bmj.299.6710.1259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rook GA, Brunet LR. Microbes, immunoregulation, and the gut. Gut. 2005;54(3):317 320. ( 10.1136/gut.2004.053785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riedler J, Braun-Fahrländer C, Eder W, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358(9288):1129 1133. ( 10.1016/S0140-6736(01)06252-3) [DOI] [PubMed] [Google Scholar]
- 22. Braun-Fahrländer C, Gassner M, Grize L, et al. Prevalence of hay fever and allergic sensitization in farmer’s children and their peers living in the same rural community. SCARPOL team. Swiss study on childhood allergy and respiratory symptoms with respect to air pollution. Clin Exp Allergy. 1999;29(1):28 34. ( 10.1046/j.1365-2222.1999.00479.x) [DOI] [PubMed] [Google Scholar]
- 23. Ege MJ, Bieli C, Frei R, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol. 2006;117(4):817 823. ( 10.1016/j.jaci.2005.12.1307) [DOI] [PubMed] [Google Scholar]
- 24. Pfefferle PI, Keber CU, Cohen RM, Garn H. The hygiene hypothesis - learning from but not living in the past. Front Immunol. 2021;12:635935. ( 10.3389/fimmu.2021.635935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001;1(1):69 75. ( 10.1038/35095579) [DOI] [PubMed] [Google Scholar]
- 26. Vercelli D. Mechanisms of the hygiene hypothesis--molecular and otherwise. Curr Opin Immunol. 2006;18(6):733 737. ( 10.1016/j.coi.2006.09.002) [DOI] [PubMed] [Google Scholar]
- 27. Liu AH. The hygiene hypothesis: promises and pitfalls. Prim Care Respir J. 2004;13(2):65 67. ( 10.1016/j.pcrj.2004.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haahtela T. A biodiversity hypothesis. Allergy. 2019;74(8):1445 1456. ( 10.1111/all.13763) [DOI] [PubMed] [Google Scholar]
- 29. Wong GW, Leung TF, Ko FW. Changing prevalence of allergic diseases in the Asia-pacific region. Allergy Asthma Immunol Res. 2013;5(5):251 257. ( 10.4168/aair.2013.5.5.251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benn CS, Melbye M, Wohlfahrt J, Björkstén B, Aaby P. Cohort study of sibling effect, infectious diseases, and risk of atopic dermatitis during first 18 months of life. BMJ. 2004;328(7450):1223. ( 10.1136/bmj.38069.512245.FE) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fiocchi A, Burks W, Bahna SL, et al. Clinical use of probiotics in pediatric allergy (CUPPA): A World Allergy Organization position paper. World Allergy Organ J. 2012;5(11):148 167. ( 10.1097/WOX.0b013e3182784ee0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grüber C, Illi S, Plieth A, Sommerfeld C, Wahn U. Cultural adaptation is associated with atopy and wheezing among children of Turkish origin living in Germany. Clin Exp Allergy. 2002;32(4):526 531. ( 10.1046/j.0954-7894.2002.01331.x) [DOI] [PubMed] [Google Scholar]
- 33. Bodansky HJ, Staines A, Stephenson C, Haigh D, Cartwright R. Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ. 1992;304(6833):1020 1022. ( 10.1136/bmj.304.6833.1020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hammond SR, English DR, McLeod JG. The age-range of risk of developing multiple sclerosis: evidence from a migrant population in Australia. Brain. 2000;123(5):968 974. ( 10.1093/brain/123.5.968) [DOI] [PubMed] [Google Scholar]
- 35. Renz H, Skevaki C. Early life microbial exposures and allergy risks: opportunities for prevention. Nat Rev Immunol. 2021;21(3):177 191. ( 10.1038/s41577-020-00420-y) [DOI] [PubMed] [Google Scholar]
- 36. Ernst SA, Schmitz R, Thamm M, Ellert U. Lower prevalence of atopic dermatitis and allergic sensitization among children and adolescents with a two-sided migrant background. Int J Environ Res Public Health. 2016;13(3).( 10.3390/ijerph13030265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu JE, Mallapaty A, Miller RL. It’s not just the food you eat: environmental factors in the development of food allergies. Environ Res. 2018;165:118 124. ( 10.1016/j.envres.2018.03.028) [DOI] [PubMed] [Google Scholar]
- 38. von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10(12):861 868. ( 10.1038/nri2871). [DOI] [PubMed] [Google Scholar]
- 39. Stein MM, Hrusch CL, Gozdz J, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med. 2016;375(5):411 421. ( 10.1056/NEJMoa1508749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schulzke JD, Riecken EO. [Principles of epithelial transport mechanisms: importance for pathophysiologic understanding, differential diagnosis and treatment of diarrheal diseases]. Z Gastroenterol. 1989;27(11):693 700. [PubMed] [Google Scholar]
- 41. Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21(11):739 751. ( 10.1038/s41577-021-00538-7). [DOI] [PubMed] [Google Scholar]
- 42. Celebi Sozener Z, Ozdel Ozturk B, Cerci P, et al. Epithelial barrier hypothesis: effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy. 2022;77(5):1418 1449. ( 10.1111/all.15240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pat Y, Ogulur I, Yazici D, et al. Effect of altered human exposome on the skin and mucosal epithelial barrier integrity. Tissue Barriers. 2022:2133877. ( 10.1080/21688370.2022.2133877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang M, Tan G, Eljaszewicz A, et al. Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J Allergy Clin Immunol. 2019;143(5):1892 1903. ( 10.1016/j.jaci.2018.11.016) [DOI] [PubMed] [Google Scholar]
- 45. Ogulur I, Pat Y, Aydin T, et al. Gut epithelial barrier damage caused by dishwasher detergents and rinse aids. J Allergy Clin Immunol. 2023;151(2):469 484. ( 10.1016/j.jaci.2022.10.020) [DOI] [PubMed] [Google Scholar]
- 46. Aghapour M, Raee P, Moghaddam SJ, Hiemstra PS, Heijink IH. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am J Respir Cell Mol Biol. 2018;58(2):157 169. ( 10.1165/rcmb.2017-0200TR) [DOI] [PubMed] [Google Scholar]
- 47. Roberts CL, Rushworth SL, Richman E, Rhodes JM. Hypothesis: increased consumption of emulsifiers as an explanation for the rising incidence of Crohn’s disease. J Crohns Colitis. 2013;7(4):338 341. ( 10.1016/j.crohns.2013.01.004) [DOI] [PubMed] [Google Scholar]
- 48. Akbarshahi H, Menzel M, Ramu S, Mahmutovic Persson I, Bjermer L, Uller L. House dust mite impairs antiviral response in asthma exacerbation models through its effects on TLR3. Allergy. 2018;73(5):1053 1063. ( 10.1111/all.13378) [DOI] [PubMed] [Google Scholar]
- 49. Sugita K, Steer CA, Martinez-Gonzalez I, et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL-13 in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):300-310.e11. ( 10.1016/j.jaci.2017.02.038) [DOI] [PubMed] [Google Scholar]
- 50. Yazici D, Ogulur I, Kucukkase O, et al. Epithelial barrier hypothesis and the development of allergic and autoimmune diseases. Allergo J Int. 2022;31(4):91 102. ( 10.1007/s40629-022-00211-y) [DOI] [Google Scholar]
- 51. Abdelhamid L, Luo XM. Retinoic acid, leaky gut, and autoimmune diseases. Nutrients. 2018;10(8).( 10.3390/nu10081016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tajik N, Frech M, Schulz O, et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun. 2020;11(1):1995. ( 10.1038/s41467-020-15831-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Caraballo JC, Yshii C, Westphal W, Moninger T, Comellas AP. Ambient particulate matter affects occludin distribution and increases alveolar transepithelial electrical conductance. Respirology. 2011;16(2):340 349. ( 10.1111/j.1440-1843.2010.01910.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xian M, Ma S, Wang K, et al. Particulate matter 2.5 causes deficiency in barrier integrity in human nasal epithelial cells. Allergy Asthma Immunol Res. 2020;12(1):56 71. ( 10.4168/aair.2020.12.1.56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cortese A, Lova L, Comoli P, et al. Air pollution as a contributor to the inflammatory activity of multiple sclerosis. J Neuroinflammation. 2020;17(1):334. ( 10.1186/s12974-020-01977-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Odoardi F, Sie C, Streyl K, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488(7413):675 679. ( 10.1038/nature11337) [DOI] [PubMed] [Google Scholar]
- 57. Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16(1):19 34. ( 10.1038/s41575-018-0081-y) [DOI] [PubMed] [Google Scholar]
- 58. Hou Q, Huang J, Ayansola H, Masatoshi H, Zhang B. Intestinal stem cells and immune cell relationships: potential therapeutic targets for inflammatory bowel diseases. Front Immunol. 2020;11:623691. ( 10.3389/fimmu.2020.623691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lichtenstein LM, Ishizaka K, Norman PS, Sobotka AK, Hill BM. IgE antibody measurements in ragweed hay fever. Relationship to clinical severity and the results of immunotherapy. J Clin Invest. 1973;52(2):472 482. ( 10.1172/JCI107204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Prahl P, Skov P, Minuva U, Weeke B, Nexø B. Estimation of affinity and quantity of human antigen-specific serum IgG (blocking antibodies). Allergy. 1981;36(8):555 560. ( 10.1111/j.1398-9995.1981.tb01873.x) [DOI] [PubMed] [Google Scholar]
- 61. Johansson SG. IgE in allergic diseases. Proc R Soc Med. 1969;62(9):975 976. [PMC free article] [PubMed] [Google Scholar]
- 62. Bjerg A, Ekerljung L, Eriksson J, et al. Increase in pollen sensitization in Swedish adults and protective effect of keeping animals in childhood. Clin Exp Allergy. 2016;46(10):1328 1336. ( 10.1111/cea.12757) [DOI] [PubMed] [Google Scholar]
- 63. Linneberg A, Nielsen NH, Madsen F, Frølund L, Dirksen A, Jørgensen T. Increasing prevalence of specific IgE to aeroallergens in an adult population: two cross-sectional surveys 8 years apart: the Copenhagen Allergy Study. J Allergy Clin Immunol. 2000;106(2):247 252. ( 10.1067/mai.2000.108312). [DOI] [PubMed] [Google Scholar]
- 64. Skrindo I, Lupinek C, Valenta R, et al. The use of the MeDALL-chip to assess IgE sensitization: a new diagnostic tool for allergic disease? Pediatr Allergy Immunol. 2015;26(3):239 246. ( 10.1111/pai.12366). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Siroux V, Lupinek C, Resch Y, et al. Specific IgE and IgG measured by the MeDALL allergen-chip depend on allergen and route of exposure: the EGEA study. J Allergy Clin Immunol. 2017;139(2):643-654.e6. ( 10.1016/j.jaci.2016.05.023) [DOI] [PubMed] [Google Scholar]
- 66. Huang X, Tsilochristou O, Perna S, et al. Evolution of the IgE and IgG repertoire to a comprehensive array of allergen molecules in the first decade of life. Allergy. 2018;73(2):421 430. ( 10.1111/all.13269) [DOI] [PubMed] [Google Scholar]
- 67. Siroux V, Boudier A, Nadif R, Lupinek C, Valenta R, Bousquet J. Association between asthma, rhinitis, and conjunctivitis multimorbidities with molecular IgE sensitization in adults. Allergy. 2019;74(4):824 827. ( 10.1111/all.13676) [DOI] [PubMed] [Google Scholar]
- 68. Friedman SJ, Schroeter AL, Homburger HA. IgE antibodies to Staphylococcus aureus. Prevalence in patients with atopic dermatitis. Arch Dermatol. 1985;121(7):869 872. ( 10.1001/archderm.1985.01660070059015) [DOI] [PubMed] [Google Scholar]
- 69. Sintobin I, Siroux V, Holtappels G, et al. Sensitisation to staphylococcal enterotoxins and asthma severity: a longitudinal study in the EGEA cohort. Eur Respir J. 2019;54(3).( 10.1183/13993003.00198-2019) [DOI] [PubMed] [Google Scholar]
- 70. Teufelberger AR, Bröker BM, Krysko DV, Bachert C, Krysko O. Staphylococcus aureusorchestrates Type 2 airway diseases. Trends Mol Med. 2019;25(8):696 707. ( 10.1016/j.molmed.2019.05.003) [DOI] [PubMed] [Google Scholar]
- 71. Altunbulakli C, Reiger M, Neumann AU, et al. Relations between epidermal barrier dysregulation and Staphylococcus species-dominated microbiome dysbiosis in patients with atopic dermatitis. J Allergy Clin Immunol. 2018;142(5):1643 1647.e12. ( 10.1016/j.jaci.2018.07.005) [DOI] [PubMed] [Google Scholar]
- 72. Bloomfield SF, Rook GA, Scott EA, Shanahan F, Stanwell-Smith R, Turner P. Time to abandon the hygiene hypothesis: new perspectives on allergic disease, the human microbiome, infectious disease prevention and the role of targeted hygiene. Perspect Public Health. 2016;136(4):213 224. ( 10.1177/1757913916650225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Weber J, Illi S, Nowak D, et al. Asthma and the hygiene hypothesis. Does cleanliness matter? Am J Respir Crit Care Med. 2015;191(5):522 529. ( 10.1164/rccm.201410-1899OC) [DOI] [PubMed] [Google Scholar]
- 74. Leoty-Okombi S, Gillaizeau F, Leuillet S, et al. Effect of sodium lauryl sulfate (SLS) applied as a patch on human skin physiology and its microbiota. Cosmetics. 2021;8(1). ( 10.3390/cosmetics8010006) [DOI] [Google Scholar]
- 75. Mitamura Y, Ogulur I, Pat Y, et al. Dysregulation of the epithelial barrier by environmental and other exogenous factors. Contact Dermatitis. 2021;85(6):615 626. ( 10.1111/cod.13959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhu YT, Yuan YZ, Feng QP, et al. Food emulsifier polysorbate 80 promotes the intestinal absorption of mono-2-ethylhexyl phthalate by disturbing intestinal barrier. Toxicol Appl Pharmacol. 2021;414:115411. ( 10.1016/j.taap.2021.115411) [DOI] [PubMed] [Google Scholar]
- 77. Vita AA, Royse EA, Pullen NA. Nanoparticles and danger signals: oral delivery vehicles as potential disruptors of intestinal barrier homeostasis. J Leukoc Biol. 2019;106(1):95 103. ( 10.1002/JLB.3MIR1118-414RR) [DOI] [PubMed] [Google Scholar]
- 78. Michaudel C, Mackowiak C, Maillet I, et al. Ozone exposure induces respiratory barrier biphasic injury and inflammation controlled by IL-33. J Allergy Clin Immunol. 2018;142(3):942 958. ( 10.1016/j.jaci.2017.11.044) [DOI] [PubMed] [Google Scholar]
- 79. Ramanathan M, London NR, Tharakan A, et al. Airborne particulate matter induces nonallergic eosinophilic sinonasal inflammation in mice. Am J Respir Cell Mol Biol. 2017;57(1):59 65. ( 10.1165/rcmb.2016-0351OC). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jin Y, Lu L, Tu W, Luo T, Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci Total Environ. 2019;649:308 317. ( 10.1016/j.scitotenv.2018.08.353) [DOI] [PubMed] [Google Scholar]
- 81. Yee MS, Hii LW, Looi CK, et al. Impact of microplastics and nanoplastics on human health. Nanomaterials (Basel). 2021;11(2).( 10.3390/nano11020496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kıykım A. Serving Healthy (?) Food on Toxic Plates. Turk Arch Pediatr. 2023;58(1):1 2. ( 10.5152/TurkArchPediatr.2022.151222) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a