Abstract
Objectives: Acute kidney injury (AKI) is associated with increased mortality among coronavirus disease 2019 (COVID-19) patients. This meta-analysis aimed to identify risk factors for the development of AKI in patients with COVID-19.
Methods: A systematic literature search was conducted in PubMed and EMBASE from 1 December 2019 to 1 January 2023. Due to significant study heterogeneity, meta-analyses were conducted using random-effects models. Meta-regression and sensitivity analysis were also performed.
Results: A total of 153,600 COVID-19 patients from 39 studies were included, and 28,003 patients developed AKI. By meta-analysis, we discovered that age, male sex, obesity, black race, invasive ventilation, and the use of diuretics, steroids and vasopressors, in addition to comorbidities such as hypertension, congestive heart failure, chronic kidney disease, acute respiratory distress syndrome, and diabetes, were significant risk factors for COVID-19-associated AKI.
Conclusions: Early detection of these risk factors is essential to reduce the incidence of AKI and improve the prognosis of COVID-19 patients.
Keywords: Acute kidney injury, COVID-19, systematic review, meta-analysis
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a worldwide pandemic. According to the World Health Organization Coronavirus Dashboard, 651,918,402 confirmed cases and 6,656,601 deaths have occurred globally (https://covid19.who.int/) as of 23 December 2022. The virus primarily affects the respiratory system, causing fever, cough, headache, shortness of breath, sore throat, and chest pain.
Acute kidney injury (AKI) is defined as an absolute increase in serum creatinine (SCr) that occurs within 48 h of a SCr increase of as little as 0.3 mg/dL (26.5 µmol/L) and a time constraint of 48 h [1]. According to a recent meta-analysis, AKI is a severe complication of COVID-19 with a prevalence of 8.9% [2]. AKI can increase the severity of illness, duration of hospitalization, and mortality of COVID-19 patients [3]. Common risk factors for AKI include age, chronic kidney disease (CKD), sepsis, and nephrotoxic drugs. However, the precise relationship between these indicators and COVID-19-related AKI remains unclear, and relevant research is relatively hysteretic [4,5].
This study aimed to evaluate risk factors for AKI in patients with COVID-19 to improve patient prognosis.
Methods
The systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and have been registered prospectively in PROSPERO (CRD42022310561).
Search strategy
Articles for this review were identified through a combined PubMed and Embase search from 1 December 2019 to 1 January 2023. Studies assessing risk factors for AKI in patients with COVID-19 satisfied the inclusion criteria for the present meta-analysis. The following search inquiry was used in PubMed: ((((SARS-CoV-2) OR (COVID- 19)) OR (coronavirus disease 2019)) AND (acute kidney injury)) AND (risk factor). References to all relevant original articles were manually reviewed to identify additional eligible studies.
Selection criteria
JLZ and QP screened the abstracts independently, and any disagreements were resolved by consensus or by a third reviewer (AHZ). Inclusion criteria were as follows: (1) adults diagnosed with COVID-19 according to the guidelines for the diagnosis and treatment of novel coronavirus disease; (2) studies mentioning at least one of the risk factors; (3) studies evaluating AKI using the 2012 Kidney Disease Improving Global Outcomes (KDIGO) guidelines; and (4) studies reporting statistical data, including odds ratios (ORs) and 95% confidence intervals (CIs). Non-English studies and studies failing to include diagnostic criteria for COVID-19, diagnostic criteria for AKI, or OR value adjustment for specific potential confounders were excluded.
Statistical analysis
All statistical analyses were conducted using Stata software. OR adjustment was performed for confounding variables, and 95% CIs were extracted from the included studies. Statistical heterogeneity among studies was evaluated using the I2 index [6]. A random-effects model was adopted if the I2 index was >50%, representing substantial heterogeneity; otherwise, a fixed-effects model was employed. A p value <.05 was considered statistically significant. Egger’s test and funnel plots were utilized to assess potential publication bias, and a sensitivity analysis was conducted to identify studies that contributed to heterogeneity.
Results
Study selection and study characteristics
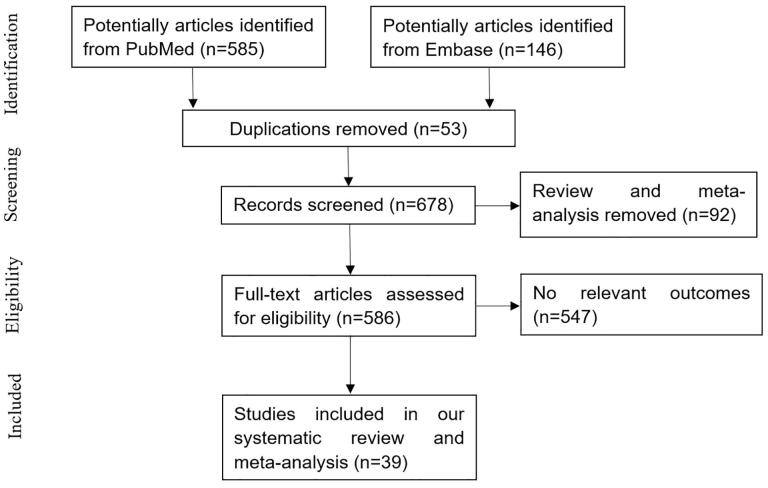
Based on the search terms, we identified a total of 731 articles. Among them, 39 studies met our inclusion criteria [7–45]. The identification procedure for these eligible articles is described in Figure 1. The meta-analysis exhibited good inter-reviewer agreement (κ = 0.895). Basic information and important characteristics of each study are listed in Table 1. A total of 153,600 COVID-19 patients and 28,003 confirmed cases of AKI were reported in this study. The average age of the individual study included in our meta-analysis ranged from 38 to 71.7 years. According to the KDIGO classification, stage 1 AKI was most common among AKI patients followed by stage 3 AKI. Six studies were performed in China, eight in the USA, one in Spain, four in Mexico, two in Korea, one in Italy, two in France, one in Brazil, seven in the UK, one in Portugal, one in Switzerland, one in the Netherlands, one in Norway, one in Singapore, one in Bahrain, and one in Iran.
Figure 1.
Flow diagram of the selection of studies.
Table 1.
Baseline of characteristics of studies of systematic review and meta-analysis.
| Study | Country | Study design | Sample size (no-AKI vs. AKI) | Age | Male (%) | CKD (%) | DM (%) | COPD (%) | HTN (%) | KDIGO classification (%) | ICU admission (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Erben et al. [45] | USA | Retrospective | 876 (671 vs. 205) | 64.4 ± 16.2 | 521 (59.5) | 220 (25.1) | 552 (63) | ||||
| Aukland et al. [43] | Norway | Observational cohort | 361 (256 vs. 105) | 63.6 (53.5–72.5) | 261 (72.3) | 74 (20.5) | 37 (10.2) | ||||
| Dehesa-Lópe et al. [44] | Mexico | Retrospective | 307 (204 vs. 103) | 56 ± 15 | 198 (64.5) | 15 (4.9) | 95 (30.9) | 6 (2) | 127 (41.4) | Stage 1 15.5; Stage 2 34; Stage 3 50.5 | |
| Sullivan et al. [13] | UK | Prospective multicenter cohort | 41294 (28294 vs. 13000) | 24407 (59.1) | 6810 (16.5) | 9345 (22.6) | 20845 (50.5) | Stage 1 65.9; Stage 2 20.1; Stage 3 14.1 | |||
| Marques et al. [7] | Portugal | Retrospective | 339 (237 vs. 87) | 71.7 ± 17.0 | 191 (56.3) | 86 (25.4) | 103 (30.4) | 43 (12.7) | 238 (70.2) | Stage 1 32.2; Stage 2 13.6; Stage 3 54.3 | 87 (25.7) |
| Procaccini et al. [9] | Spain | Retrospective, case control | 1096 (548 vs. 548) | 191 (17.4) | 270 (24.6) | 328 (29.9) | 171 (15.6) | 426 (78.02) | Stage 1 70.07; Stage 2 19.34; Stage 3 10.58 | ||
| Bowe et al. [10] | USA | Observational cohort | 5216 (3561 vs. 1655) | 70 (61–76) | 4908 (94) | 2537 (49) | 3985 (76) | Stage 1 58; Stage 2 13; Stage 3 16 | 1693 (33) | ||
| Wan et al. [11] | UK | Prospective | 1855 (1400 vs. 455) | 65 (51–79) | 1122 (60.5) | 248 (15.8) | 585 (37.2) | 365 (23.2) | 1120 (71.2) | Stage 1 44; Stage 2 19.8; Stage 3 36.3 | 338 (18.2) |
| Diebold et al. [15] | Switzerland | Retrospective | 188 (147 vs. 41) | 62 (48–73) | 115 (61) | 28 (15) | 35 (19) | 23 (12) | 86 (46) | Stage 1 39; Stage 2 24; Stage 3 37 | |
| Casas-Aparicio et al. [16] | Mexico | Retrospective | 99 (41 vs. 58) | 52.9 ± 13.2 | 74 (74.7) | 27 (26.7) | 30 (29.7) | Stage 1 20.7; Stage 2 27.6; Stage 3 50 | |||
| Geri et al. [17] | France | Retrospective multicenter | 379 (184 vs. 195) | 291 (76.8) | 65 (17.2) | 114 (30.1) | 188 (49.6) | Stage 1 29.7; Stage 2 22.6; Stage 3 47.7 | |||
| Genovesi et al. [21] | Italy | Retrospective | 2816 (2334 vs. 482) | 65 (54, 77) | 1856 (65.9) | 477 (17.0) | |||||
| Parker et al. [23] | UK | Retrospective | 1032 (822 vs. 210) | 71 (56–83) | 569 (55.1) | 144 (15.4) | 259 (27.7) | 457 (44.3) | Stage 1 58.6; Stage 2 17.6; Stage 3 23.8 | 165 (16) | |
| Bell et al. [25] | UK | Retrospective | 448 (330 vs. 118) | 69.4 ± 16.2 | 246 (54.8) | 45 (10) | 117 (26.1) | 195 (43.4) | Stage 1 55.1; Stage 2 18.6; Stage 3 26.3 | 62 (13.8) | |
| Jewell et al. [26] | UK | Retrospective | 1248 (761 vs. 487) | 69 ± 17.1 | 734 (58.8%) | 207 (26.6%) | 406 (32.7%) | 122 (9.8%) | 681 (54.6%) | Stage 1 50.9; Stage 2 13.1; Stage 3 35.9 | 192 (15.4%) |
| Li et al. [27] | China | Retrospective multicenter | 223 (188 vs. 35) | 55 ± 30.0 | 130 (58.3) | 5 (2.7) | 31 (16.8) | 63 (34.1) | Stage 1 51.4; Stage 2 20; Stage 3 28.6 | ||
| Charytan et al. [28] | USA | Retrospective | 4732 (3346 vs. 1386) | 65 (51–76) | 2702 (57.10) | 761 (16.08) | 1646 (34.78) | 815 (17.22) | 2738 (57.86) | Stage 1 51.7; Stage 2 9.5; Stage 3 38.7 | 1056 (40.9) |
| Martín-Del-Campo et al. [31] | Mexico | Retrospective | 773 (613 vs. 160) | 485 (62.7) | 293 (37.9) | 369 (47.7) | 182 (23.5) | ||||
| Chávez-Íñiguez et al. [32] | Mexico | Prospective | 877 (540 vs. 337) | 55.9 ± 15.7 | 548 (62.5) | 97 (11) | 311 (35.4) | 27 (3.0) | 332 (37.8) | Stage 1 31.2; Stage 2 24.3; Stage 3 44.5 | |
| Reese et al. [33] | USA | Retrospective multicenter | 66494 (62328 vs. 4166) | 31238 (47.0) | 11712 (17.6) | 20615 (31.0) | |||||
| van Son et al. [34] | Netherlands | Retrospective | 1634 (1456 vs. 178) | 1040 (63.6) | 161 (9.9) | 297 (18.2) | 748 (45.8) | 467 (28.6%) | |||
| See et al. [35] | Singapore | Retrospective | 707 (650 vs. 57) | 46 (29–57) | 405 (57.3) | 5 (1) | 82 (12) | 24 (3) | 137 (19) | Stage 1 68; Stage 2 16; Stage 3 16 | 46 (7) |
| Hansrivijit et al. [36] | USA | Retrospective | 283 (168 vs. 115) | 64.1 ± 15.9 | 159 (56.2) | 66 (23.3) | 108 (38.2) | 73 (25.8) | 189 (66.8) | Stage 1 41.8; Stage 2 29.6; Stage 3 28.6 | 89 (31.4) |
| Naser et al. [37] | Bahrain | Retrospective | 353 (185 vs. 168) | 55.8 ± 15.7 | 42 (11.9) | 167 (47.3) | 25 (7.2) | 160 (45.3) | |||
| Teoh et al. [38] | China | Retrospective | 1040 (974 vs. 66) | 38 ± 18 | 560 (53.8) | 81 (7.8) | 144 (13.8) | Stage 1 81.8; Stage 2 4.5; Stage 3 13.6 | 53 (5.1) | ||
| Rahimzadeh et al. [41] | Iran | Retrospective | 516 (322 vs. 194) | 57.6 ± 16.1 | 324 (62.8) | 20 (3.9) | 166 (32.2) | 38 (7.4) | 213 (41.3) | Stage 1 61.9; Stage 2 18; Stage 3 20.1 | 79 (15.3) |
| Doher et al. [8] | Brazil | Retrospective | 201 (100 vs. 101) | 64.0 (52.0–80.0) | 123 (61.2) | 64 (31.8) | 98 (48.8) | Stage 1 42.6; Stage 2 18.8; Stage 3 38.6 | |||
| Kolhe et al. [42] | UK | Retrospective | 1161 (857 vs. 304) | 657(56.6) | 224 (19.3) | 255 (22.0) | 96 (8.3) | ||||
| Dai et al. [12] | China | Retrospective | 492 (456 vs. 36) | 226 (45.93) | 6 (1.22) | 65 (13.21) | 141 (28.66) | Stage 1 72.2; Stage 2 5.6; Stage 3 22.2 | |||
| Hirsch et al. [14] | USA | Retrospective | 5449 (3456 vs. 1993) | 64.0 (52.0- 75.0) | 3317 (60.9) | 1797 (33.0) | 296 (5.4) | 3037 (55.7) | Stage 1 46.5; Stage 2 22.4; Stage 3 31.1 | 1395 (25.6) | |
| Louis et al. [18] | France | Observational cohort | 181 (101 vs. 80) | 127 (70%) | 13 (7.2%) | 54 (30%) | 22 (12%) | 132 (73%) | Stage 1 33.8; Stage 2 13.8; Stage 3 52.5 | ||
| Lim, et al. [19] | Korea | Retrospective | 130 (105 vs. 25) | 67.0 (57.0–78.0) | 70 (53.8) | 12 (9.2) | 33 (25.4) | 52 (40.0) | 38 (29.2) | ||
| Kang et al. [20] | Korea | Retrospective | 7341 (7314 vs. 27) | 47.1 ± 19.0 | 4,371 (59.5) | 1230 (16.8) | 1676 (22.8) | 1572 (21.4) | |||
| Hamilton et al. [22] | UK | Retrospective | 1032 (822 vs. 210) | 71 (56–83) | 569 (55.1) | 259 (25.1) | Stage 1 58; Stage 2 18; Stage 3 24 | ||||
| Pelayo et al. [24] | USA | Retrospective | 223 (113 vs. 110) | 115 (51.6) | 39 (17.5) | 104 (46.6) | 27 (12.1) | 180 (80.7) | |||
| Wang et al. [29] | China | Retrospective multicenter | 275 (139 vs. 136) | 69 (62–77) | 161 (58.4) | 16 (5.8) | 62 (22.5) | 37 (13.5) | 150 (54.5) | Stage 1 33.8; Stage 2 22.1; Stage 3 44.1 | |
| Nimkar et al. [30] | USA | Retrospective | 327 (148 vs. 179) | 71 (59-82) | 182 (55.7) | 40 (12.2) | 139 (42.5) | 44 (13.5) | 209 (63.9) | Stage 1 21.1; Stage 2 12.8; Stage 3 20.8 | |
| Sang et al. [39] | China | Retrospective | 210 (118 vs. 92) | 64 (56–71) | 131 (62.4) | 10 (4.8) | 44 (21.0) | 5 (2.4) | 98 (46.7) | Stage 1 14.1; Stage 2 16.3; Stage 3 69.6 | |
| Cheng et al. [40] | China | Retrospective | 1392 (1293 vs. 99) | 63 (50–71) | 711 (51) | 21 (2) | 241 (17) | 77 (6) | 499 (36) | Stage 1 42.4; Stage 2 22.2; Stage 3 35.4 | 140 (10) |
Note: DM: diabetes mellitus; CKD: chronic kidney disease; HTN: hypertension; COPD: chronic obstructive pulmonary disease; KDIGO: kidney disease: improving global outcomes; ICU: intensive care unit; AKI: acute kidney injury.
Meta-analysis results
The prevalence of AKI and associated mortality
Thirty-nine preliminary studies reported the prevalence of AKI among COVID-19 patients. Based on the meta-analysis, the overall prevalence of AKI was 29%. In addition, the mortality rate of COVID-19 patients who developed AKI in the included studies was 47% (shown in Supplementary Material).
General risk factors
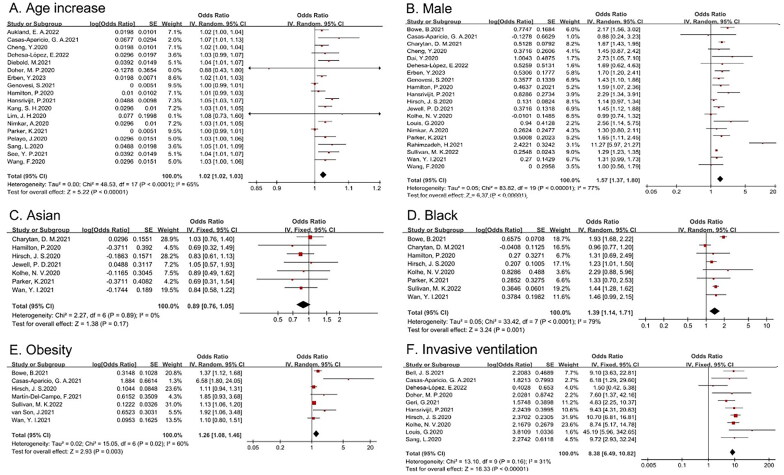
Nineteen studies explored the relationship between age and the development of AKI in COVID-19 patients, and twenty studies examined the relationship between sex and the development of AKI. The random-effects model was used to pool the data, yielding an OR of 1.02 (95% CI 1.02–1.03) for every additional year of age, and an OR of 1.57 (95% CI 1.37–1.8) for male sex. With respect to ethnicity, eight studies from Western societies compared the incidence of AKI in Asian or black COVID-19 patients with the incidence in white patients. After meta-analysis, only black race was identified as a risk factor for AKI in patients with COVID-19 in Western societies (OR 1.39, 95% CI 1.14–1.71). We also analyzed the association between the development of AKI and invasive ventilation or obesity. Given nonsignificant heterogeneity, a fixed-effects model was performed, yielding an OR of 8.38 (95% CI 6.49–10.82) for invasive ventilation and 1.26 (95% CI 1.08–1.46) for obesity (shown in Figure 2).
Figure 2.
Forest plot demonstrating the relationship between general indices and COVID-19-related AKI (A: age (per one-year increment); B: male sex; C: Asian; D: black; E: obesity (BMI ≥ 30 kg/m2); F: invasive ventilation).
Laboratory indices
The relationship between baseline creatinine and AKI was examined (OR 1.22, 95% CI 1.01–1.47), and significant heterogeneity was identified. However, the random-effects model did not determine the predictive value of C-reaction protein (CRP) for AKI.
Comorbidities
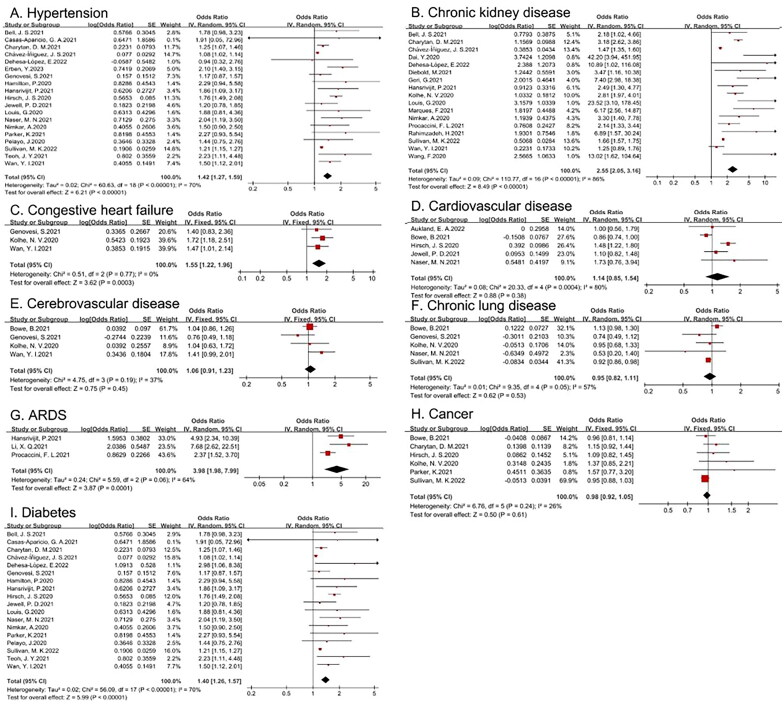
Various comorbidities were included in this meta-analysis to determine their relevance to the development of AKI in patients with COVID-19, including hypertension, congestive heart failure, cardiovascular disease, CKD, chronic lung disease, cerebrovascular disease, acute respiratory distress syndrome (ARDS), cancer, and diabetes mellitus. As shown in Table 2, only hypertension, congestive heart failure, CKD, ARDS, and diabetes mellitus were risk factors for AKI. The I2 test indicated that no statistically significant heterogeneity existed among the studies with respect to congestive heart failure (shown in Figure 3).
Table 2.
Risk factors of COVID-19-related AKI.
| Baseline characteristic | OR | 95% confidence interval | I2 | Egger test |
|---|---|---|---|---|
| Age (per one-year increment) | 1.02 | 1.02–1.03 | 65 | 0.768 |
| Male (reference: female) | 1.57 | 1.37–1.80 | 77 | 0.039 |
| Race | ||||
| White | Reference | |||
| Asian | 0.89 | 0.76–1.05 | 0 | |
| Black | 1.39 | 1.14–1.71 | 79 | 0.75 |
| Invasive ventilation | 8.38 | 6.49–10.82 | 31 | 0.52 |
| Obesity (BMI ≥ 30 kg/m2) | 1.26 | 1.08–1.46 | 60 | 0.029 |
| Laboratory index | ||||
| Creatinine (per 1-mg/dl increment) | 1.22 | 1.01–1.47 | 77 | 0.017 |
| C-reaction protein (per 1-mg/dl increment) | 1 | 0.93–1.07 | 85 | |
| Comorbidity | ||||
| Hypertension (yes vs no) | 1.42 | 1.27–1.59 | 70 | 0.01 |
| Congestive heart failure (yes vs no) | 1.55 | 1.22–1.96 | 0 | 0.612 |
| Cardiovascular disease (yes vs no) | 1.14 | 0.85–1.54 | 80 | |
| Chronic kidney disease (yes vs no) | 2.55 | 2.05–3.16 | 86 | 0.002 |
| Cerebrovascular disease (yes vs no) | 1.06 | 0.91–1.23 | 37 | |
| Chronic lung disease (yes vs no) | 0.95 | 0.82–1.11 | 57 | |
| ARDS (yes vs no) | 3.98 | 1.98–7.99 | 64 | 0.102 |
| Cancer (yes vs no) | 0.98 | 0.92–1.05 | 26 | |
| DM (yes vs no) | 1.40 | 1.26–1.57 | 70 | 0.002 |
| Drug | ||||
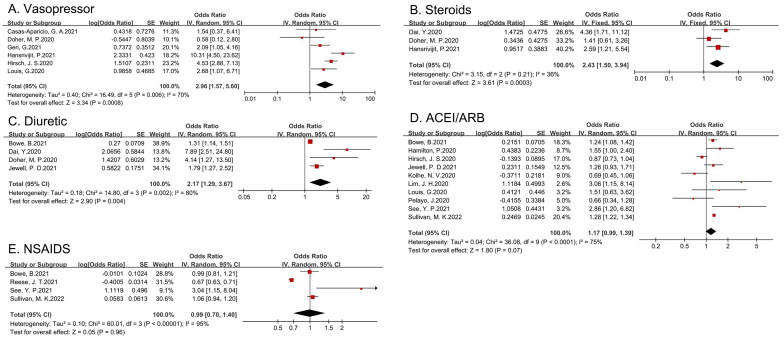
| Vasopressor | 2.96 | 1.57–5.6 | 70 | 0.268 |
| Steroids | 2.43 | 1.5–3.94 | 36 | 0.716 |
| Diuretic | 2.17 | 1.29–3.67 | 80 | 0.024 |
| ACEI or ARB use | 1.17 | 0.99–1.39 | 75 | |
| NSAIDS | 0.99 | 0.7–1.4 | 95 |
Note: OR: odds ratio; CI: confidence interval; ARDS: acute respiratory distress syndrome; DM: diabetes mellitus; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; NSAIDS: non-steroidal anti-inflammatory drugs.
Figure 3.
Forest plot demonstrating the relationship between comorbidities and COVID-19-related AKI (A: hypertension; B: chronic kidney disease; C: congestive heart failure; D: cardiovascular disease; E: cerebrovascular disease; F: chronic lung disease; G: ARDS; H: cancer; I: diabetes).
Drug exposure
The fixed-effects model was used to calculate an OR of 2.17 (95% CI 1.29–3.67) for diuretic treatment and the development of AKI in COVID-19 patients, suggesting that diuretic use is a risk factor for AKI in this patient population. Furthermore, the pooled estimates of the effect of vasopressors and steroids on the incidence of AKI, as shown in Table 2, were also significant. However, the pooled estimates of the effect of angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs) and nonsteroidal anti-inflammatory drug (NSAID) exposure on AKI was not statistically significant (shown in Figure 4).
Figure 4.
Forest plot demonstrating the relationship between medication use and COVID-19-related AKI (A: vasopressor; B: steroids; C: diuretic; D: ACEI or ARB; E: NSAID).
Meta-regression and sensitivity analysis
As mentioned previously, we identified significant heterogeneity in age, male sex, black race, and multiple comorbidities, including hypertension, CKD, and diabetes, as risk factors for AKI in COVID-19 patients. Given an I2>50%, we applied both a random effects model as well as meta-regression and sensitivity analyses. For age, male sex and black race, meta-regression based on sample size and country revealed no significant correlation. However, meta-regression identified a significant effect of country on the correlation between AKI and hypertension (p = .038) or CKD (p = .026). Sensitivity analysis was also performed by repeatedly omitting one individual study to explore the robustness of the results. As shown in the Supplementary Material, we determined that the Chávez-Íñiguez study may have led to heterogeneity with respect to diabetes and CKD.
Publication bias
The Egger test was applied to this meta-analysis and suggested no significant bias in the studies with respect to age, black race, invasive ventilation, congestive heart failure, ARDS, vasopressor use, or steroid exposure (shown in Table 2). The funnel plots were shown in Supplementary Material.
Discussion
This meta-analysis, including 39 studies, investigated risk factors for AKI in COVID-19 patients. Our results demonstrate that age, male sex, black race, drug exposure, and multiple comorbidities, including CKD, congestive heart failure, diabetes and hypertension, may significantly increase the risk of COVID-19-related AKI.
COVID-19, primarily manifesting as an acute respiratory illness, has spread rapidly worldwide. In addition to respiratory issues, severe COVID-19 can be accompanied by AKI as well as cerebrovascular and cardiovascular complications, such as myocardial infarction and acute stroke [46,47]. A recent meta-analysis indicated that the incidence of AKI in COVID-19 patients was approximately 8% [48]. A previous meta-analysis reported that approximately 11% of COVID-19 patients develop AKI between 2 and 28 days of follow-up [49]. Another recent meta-analysis of 60 articles reported a pooled incidence of AKI among COVID-19 patients of 19.45% and mortality in patients with AKI of 54.24% [50]. In our meta-analysis, we found that the incidence of AKI was 29%, whereas the mortality in patients with AKI was 47%. The development of AKI may increase the risk of mortality in patients with COVID-19 [51]. Renal replacement therapy (e.g., hemodialysis, peritoneal dialysis, sustained low-efficiency dialysis), fluid management, maintenance of oxygenation saturation, and avoidance of nephrotoxins, depending on patient characteristics, should be considered in the management of COVID-19 patients with AKI. Given the increased mortality, it is essential to define predictors of AKI in COVID-19 patients.
Cai, X. proposed that older age, male sex and obesity are significant risk factors for AKI in COVID-19 patients [5]. In addition, we observed that black race is another risk factor. Several mechanisms might explain the higher AKI risk with increasing age. Elderly patients may suffer from more comorbidities, greater organ injury, or severe infection. For instance, lymphocytopenia, neutrophilia and increased inflammation seem to be more common in older patients [52]. The increased AKI risk in male COVID-19 patients may be due to different innate and adaptive immune responses to infection. Sex-specific patterns of gene expression, sex-specific features of epigenomic organization, and sex steroids could contribute to the pathophysiology of sex bias [53]. Black race may be an independent risk factor for mortality, hospitalization, critical care admission, and AKI in COVID-19 patients, potentially interacting with age, sex, socioeconomic factors, and medical techniques [54]. In addition, this racial disparity in COVID-19 outcomes may be due to implicit bias among healthcare providers and inequalities in the healthcare system, which likely influence diagnosis and treatment decisions as well as the level of care [55].
With respect to comorbidities, the incidence of hypertension, ARDS, CKD, and diabetes may increase the risk of COVID-19-related AKI. However, the effect of CRP on the risk of AKI was not statistically significant in our study, which conflicts with previous reports [4]. The pathophysiology of COVID-19-induced AKI is complex and may include the volume response and subsequent renal tissue injury. Critically ill patients with pneumonia frequently develop ARDS. The development of ARDS may contribute to AKI due to positive-pressure ventilation and hypoxemia. Invasive ventilation can lead to systemic hemodynamic changes and reductions in renal blood flow and the glomerular filtration rate. Higher tidal volumes can increase various inflammatory mediators [56]. Consistent with this relationship, our study also confirmed the adverse effects of invasive ventilation on AKI. In addition, researchers have demonstrated that the receptor of SARS-CoV-2, ACE2, is upregulated and highly expressed in podocytes and proximal tubules [57]. Thus, the kidney may be more vulnerable to COVID-19 invasion. Chronic hypertension can damage renal function through contraction of renal vessels and the side effects of anti-hypertensive therapies. Renin-angiotensin-aldosterone system (RAAS) blockade and diuretics are essential treatments for patients with CKD, congestive heart failure, or diabetes. Activation of sympathetic nerves and RAAS could aggravate the decrease in glomerular perfusion and induce renal fibrosis. Systemic inflammation, hypercoagulability, and administration of ACEIs may lead to AKI, in addition to the direct virulence effects of the virus [37]. Several studies reported no significant correlation between all-cause mortality or AKI risk in COVID-19 patients with hypertension and ACEIs or ARB use [58–60], which is consistent with our findings and likely contributed to the recommendations of medical societies. However, a recent meta-analysis (including randomized controlled trials) identified an increased risk of AKI but not all-cause mortality in patients with COVID‐19 on ACEIs/ARBs [61]. Given that the association between ACEI use and AKI was inconclusive, more randomized clinical trials are warranted to determine causality. In our study, the use of inpatient diuretics and vasopressors was associated with a higher risk of AKI, likely indicating an adverse effect of these medications on renal perfusion. Our findings suggest that such medicines should be used with caution, especially in the presence of background renal impairment. Our data demonstrated that the incidence of AKI was not significantly elevated in patients taking NSAIDs. However, given their side effect profile, the use of such medicines may be disputed in older patients, who are more likely to experience negative effects from COVID-19. Additionally, the duration of medication use was not available, which may contribute to uncertainty.
Through our meta-analysis, we determined that hypertension, CKD, and diabetes were all independent risk factors for AKI in patients with COVID-19, consistent with the study of Cai X [5]. Considering the significant heterogeneity among studies, we not only applied a random-effect model but also subsequently performed meta-regression and sensitivity analysis. Through our meta-regression analysis, we observed an interaction among country, hypertension, and CKD. By further sensitivity analysis, we identified significant heterogeneity between the study of Chávez-Íñiguez et al. [32] and other studies. Chávez-Íñiguez et al. conducted a prospective, observational cohort study with a relatively young population from Mexico. Clinical heterogeneity is probably high for the country since the country is a heterogeneous group of diverse ethnicities. Disparities in socioeconomic conditions across racial lines were exacerbated during the COVID-19 pandemic [62]. In addition, study heterogeneity may be related to the proportion of subjects with comorbidities, study sample size, COVID severity, and differences among national and regional healthcare systems, which may affect the conclusions of the meta-analysis.
In this meta-analysis, we included recent studies exploring independent risk factors for COVID-19-related AKI and adjusted for other confounding factors. Although several previous meta-analyses have addressed risk factors for AKI in COVID-19 patients [63–65], our studies were larger and more extensive in number and risk factors. However, there are still some limitations. Despite conducting a detailed literature search, limitations of the review process include restriction of the search to published English language articles. In addition, the baseline characteristics of the populations, sample sizes, research methodologies, variation in AKI staging, and definitions of disease severity among countries varied in different studies, which may contribute to bias. The majority of studies included in our analysis were from China, America and Brazil and may not be representative of the general population in other countries. Despite the use of meta-regression and sensitivity analysis, country did not fully explain the heterogeneity. Further prospective studies and unpublished data are needed to explore additional potential risk factors for AKI in COVID-19 patients.
Conclusion
Our meta-analysis indicates that older age, male sex, black race, obesity, hypertension, diabetes mellitus, congestive heart failure, CKD, ARDS, invasive ventilation, and the use of diuretics and vasopressors are independent risk factors for the development of AKI in COVID-19 patients. Clinical attention should be paid to these risk factors, and early identification and intervention may reduce the incidence of AKI and improve the prognosis of patients with COVID-19.
Ethics statement
An ethics statement is not applicable because this study is based exclusively on published literature.
Supplementary Material
Funding Statement
This study was supported by the Xuanwu Hospital Huizhi talent leader training program to Aihua Zhang.
Author contribution
AHZ contributed to the study concept and design; JLZ, QP, TZ, JLM, XTD, and ZW contributed to data collection; JLZ, ZW, and AHZ contributed to the statistical analysis; JLZ, and AHZ contributed to the original draft. All authors approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
References
- 1.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen YT, Shao SC, Hsu CK, et al. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24(1):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabaghian T, Kharazmi AB, Ansari A, et al. COVID-19 and acute kidney injury: a systematic review. Front Med. 2022;9:705908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan KW, Yu KY, Lee PW, et al. Global REnal involvement of CORonavirus disease 2019 (RECORD): a systematic review and meta-analysis of incidence, risk factors, and clinical outcomes. Front Med. 2021;8:678200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai X, Wu G, Zhang J, et al. Risk factors for acute kidney injury in adult patients with COVID-19: a systematic review and meta-analysis. Front Med. 2021;8:719472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marques F, Gameiro J, Oliveira J, et al. Acute kidney disease and mortality in acute kidney injury patients with COVID-19. J Clin Med. 2021;10(19):4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doher MP, Torres de Carvalho FR, Scherer PF, et al. Acute kidney injury and renal replacement therapy in critically ill COVID-19 patients: risk factors and outcomes: a single-center experience in Brazil. Blood Purif. 2021;50(4-5):520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Procaccini FL, Alcázar Arroyo R, Albalate Ramón M, et al. Acute kidney injury in 3182 patients admitted with COVID-19: a single-center, retrospective, case-control study. Clin Kidney J. 2021;14(6):1557–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowe B, Cai M, Xie Y, et al. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2020;16(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan YI, Bien Z, Apea VJ, et al. Acute kidney injury in COVID-19: multicentre prospective analysis of registry data. Clin Kidney J. 2021;14(11):2356–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai Y, Liu Z, Du X, et al. Acute Kidney injury in hospitalized patients infected with COVID-19 from Wuhan, China: a retrospective study. Biomed Res Int. 2021;2021:6655185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan MK, Lees JS, Drake TM, et al. Acute kidney injury in patients hospitalized with COVID-19 from the ISARIC WHO CCP-UK study: a prospective, multicentre cohort study. Nephrol Dial Transplant. 2022;37(2):271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diebold M, Schaub S, Landmann E, et al. Acute kidney injury in patients with COVID-19: a retrospective cohort study from Switzerland. Swiss Med Wkly. 2021;151:w20482. [DOI] [PubMed] [Google Scholar]
- 16.Casas-Aparicio GA, León-Rodríguez I, Alvarado-de la Barrera C, et al. Acute kidney injury in patients with severe COVID-19 in Mexico. PLoS One. 2021;16(2):e0246595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geri G, Darmon M, Zafrani L, et al. Acute kidney injury in SARS-CoV2-related pneumonia ICU patients: a retrospective multicenter study. Ann Intensive Care. 2021;11(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louis G, Belveyre T, Goetz C, et al. Acute kidney injury in severe SARS-CoV-2 infection: an experience report in Eastern France. Anaesth Crit Care Pain Med. 2021;40(1):100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim JH, Cho JH, Jeon Y, et al. Adverse impact of renin-angiotensin system blockade on the clinical course in hospitalized patients with severe COVID-19: a retrospective cohort study. Sci Rep. 2020;10(1):20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang SH, Kim SW, Kim AY, et al. Association between chronic kidney disease or acute kidney injury and clinical outcomes in COVID-19 patients. J Korean Med Sci. 2020;35(50):e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genovesi S, Rebora P, Occhino G, et al. Atrial fibrillation and clinical outcomes in a cohort of hospitalized patients with Sars-Cov-2 infection and chronic kidney disease. J Clin Med. 2021;10(18):4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton P, Hanumapura P, Castelino L, et al. Characteristics and outcomes of hospitalised patients with acute kidney injury and COVID-19. PLoS One. 2020;15(11):e0241544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker K, Hamilton P, Hanumapura P, et al. Chronic anticoagulation is not associated with a reduced risk of acute kidney injury in hospitalised covid-19 patients. BMC Nephrol. 2023;24(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelayo J, Lo KB, Bhargav R, et al. Clinical characteristics and outcomes of community- and hospital-acquired acute kidney injury with COVID-19 in a US inner city hospital system. Cardiorenal Med. 2020;10(4):223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell JS, James BD, Al-Chalabi S, et al. Community- versus hospital-acquired acute kidney injury in hospitalised COVID-19 patients. BMC Nephrol. 2021;22(1):269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jewell PD, Bramham K, Galloway J, et al. COVID-19-related acute kidney injury; incidence, risk factors and outcomes in a large UK cohort. BMC Nephrol. 2021;22(1):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XQ, Liu H, Meng Y, et al. Critical roles of cytokine storm and secondary bacterial infection in acute kidney injury development in COVID-19: a multi-center retrospective cohort study. J Med Virol. 2021;93(12):6641–6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charytan DM, Parnia S, Khatri M, et al. Decreasing incidence of acute kidney injury in patients with COVID-19 critical illness in New York city. Kidney Int Rep. 2021;6(4):916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F, Ran L, Qian C, et al. Epidemiology and outcomes of acute kidney injury in COVID-19 patients with acute respiratory distress syndrome: a multicenter retrospective study. Blood Purif. 2021;50(4-5):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nimkar A, Naaraayan A, Hasan A, et al. Incidence and risk factors for acute kidney injury and its effect on mortality in patients hospitalized from COVID-19. Mayo Clin Proc Innov Qual Outcomes. 2020;4(6):687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martín-Del-Campo F, Ruvalcaba-Contreras N, Velázquez-Vidaurri AL, et al. Morbid obesity is associated with mortality and acute kidney injury in hospitalized patients with COVID-19. Clin Nutr ESPEN. 2021;45:200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chávez-Íñiguez JS, Cano-Cervantes JH, Maggiani-Aguilera P, et al. Mortality and evolution between community and hospital-acquired COVID-AKI. PLoS One. 2021;16(11):e0257619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reese JT, Coleman B, Chan L, et al. NSAID use and clinical outcomes in COVID-19 patients: a 38-center retrospective cohort study. medRxiv. 2022;19(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Son J, Oussaada SM, Şekercan A, et al. Overweight and obesity are associated with acute kidney injury and acute respiratory distress syndrome, but not with increased mortality in hospitalized COVID-19 patients: a retrospective cohort study. Front Endocrinol. 2021;12:747732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.See YP, Young BE, Ang LW, et al. Risk Factors for development of acute kidney injury in COVID-19 patients: a retrospective observational cohort study. Nephron. 2021;145(3):256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansrivijit P, Gadhiya KP, Gangireddy M, et al. Risk factors, clinical characteristics, and prognosis of acute kidney injury in hospitalized COVID-19 patients: a retrospective cohort study. Medicines. 2021;8(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naser MN, Al-Ghatam R, Darwish AH, et al. Risk factors, predictions, and progression of acute kidney injury in hospitalized COVID-19 patients: an observational retrospective cohort study. PLoS One. 2021;16(9):e0257253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teoh JY, Yip TC, Lui GC, et al. Risks of AKI and major adverse clinical outcomes in patients with severe acute respiratory syndrome or coronavirus disease 2019. J Am Soc Nephrol. 2021;32(4):961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sang L, Chen S, Zheng X, et al. The incidence, risk factors and prognosis of acute kidney injury in severe and critically ill patients with COVID-19 in mainland China: a retrospective study. BMC Pulm Med. 2020;20(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng Y, Luo R, Wang X, et al. The Incidence, risk factors, and prognosis of acute kidney injury in adult patients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15(10):1394–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahimzadeh H, Kazemian S, Rahbar M, et al. The Risk factors and clinical outcomes associated with acute kidney injury in patients with COVID-19: data from a large cohort in Iran. Kidney Blood Press Res. 2021;46(5):620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolhe NV, Fluck RJ, Selby NM, et al. Acute kidney injury associated with COVID-19: a retrospective cohort study. PLoS Med. 2020;17(10):e1003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aukland EA, Klepstad P, Aukland SM, et al. Acute kidney injury in patients with COVID-19 in the intensive care unit: evaluation of risk factors and mortality in a national cohort. BMJ Open. 2022;12(6):e059046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dehesa-López E, Galindo AE, Santos IMV, et al. Clinical characteristics and factors associated with acute kidney injury among patients hospitalized with coronavirus disease: an observational retrospective study. Sao Paulo Med J. 2022;140(4):566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erben Y, Marquez CP, Prudencio M, et al. Race affects adverse outcomes of deep vein thrombosis, pulmonary embolism, and acute kidney injury in coronavirus disease 2019 hospitalized patients. J Vasc Surg Venous Lymphat Disord. 2023;11(1):19.e3–24.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katz JM, Libman RB, Wang JJ, et al. Cerebrovascular complications of COVID-19. Stroke. 2020;51(9):e227–e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jafari-Oori M, Fiorentino M, Castellano G, et al. Acute kidney injury and covid-19: a scoping review and meta-analysis. Adv Exp Med Biol. 2021;1321:309–324. [DOI] [PubMed] [Google Scholar]
- 49.Kunutsor SK, Laukkanen JA.. Renal complications in COVID-19: a systematic review and meta-analysis. Ann Med. 2020;52(7):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raina R, Mahajan ZA, Vasistha P, et al. Incidence and outcomes of acute kidney injury in COVID-19: a systematic review. Blood Purif. 2022;51(3):199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu EL, Janse RJ, de Jong Y, et al. Acute kidney injury and kidney replacement therapy in COVID-19: a systematic review and meta-analysis. Clin Kidney J. 2020;13(4):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15(7):e0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scully EP, Haverfield J, Ursin RL, et al. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raharja A, Tamara A, Kok LT.. Association between ethnicity and severe COVID-19 disease: a systematic review and meta-analysis. J Racial Ethn Health Disparities. 2021;8(6):1563–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.FitzGerald C, Hurst S.. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Darmon M, Clec’h C, Adrie C, et al. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin J Am Soc Nephrol. 2014;9(8):1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morales DR, Conover MM, You SC, et al. Renin-angiotensin system blockers and susceptibility to COVID-19: an international, open science, cohort analysis. Lancet Digit Health. 2021;3(2):e98–e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126(12):1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baral R, Tsampasian V, Debski M, et al. Association between renin-angiotensin-aldosterone system inhibitors and clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(3):e213594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gnanenthiran SR, Borghi C, Burger D, et al. Renin-angiotensin system inhibitors in patients with COVID-19: a meta-analysis of randomized controlled trials led by the International Society of Hypertension. J Am Heart Assoc. 2022;11(17):e026143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopez L 3rd, Hart LH 3rd, Katz MH.. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325(8):719–720. [DOI] [PubMed] [Google Scholar]
- 63.Lin L, Wang X, Ren J, et al. Risk factors and prognosis for COVID-19-induced acute kidney injury: a meta-analysis. BMJ Open. 2020;10(11):e042573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Z, Zhang L, Zha D, et al. Clinical characteristics and risks of Chinàs 2019 novel coronavirus patients with AKI: a systematic review and meta-analysis. Ren Fail. 2020;42(1):926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hansrivijit P, Qian C, Boonpheng B, et al. Incidence of acute kidney injury and its association with mortality in patients with COVID-19: a meta-analysis. J Investig Med. 2020;68(7):1261–1270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.