Abstract
Salivary gland epithelial cells (SGECs) play an active role in primary Sjogren’s syndrome (pSS) pathogenesis. Quantitative and qualitative abnormalities of saliva might expose SGECs to chronic hyperosmolarity. We aimed to decipher the links between hyperosmolar stimulation of SGECs and lymphocytic infiltration of the salivary glands (SG) observed in pSS. RNAseq was performed on NS-SV-AC cells stimulated with hyperosmolar media containing NaCl (100 mM) or sucrose (200 mM), or with iso-osmolar (Iso) medium. RNAseq was performed on primary cultured SGECs from pSS and controls, in the presence or not of B cells. Hyperosmolar stimulation of NS-SV-AC-cells identified an upregulation of interferon-induced (MX1, IFIT2) and MMPs genes. Enrichment analysis revealed an over-representation of fibrosis pathway. In parallel, RNAseq of SGECs comparing pSS to controls identified an over-representation of a pathway involving MMPs. Given the unexpected upregulation of collagen (COL3A1, COL1A2) and ADAMTS genes in pSS SGECs, we hypothesized that SGECs might undergo epithelial–mesenchymal transition. ZEB2 was upregulated and SLUG was down regulated in SGECs from pSS versus controls. MMP24 and ZEB2 were higher in SGECs from pSS with a focus score ≥1 versus <1. Lastly, SGECs cocultured with B cells expressed higher levels of COL1A2. These results suggest the existence of a vicious circle. Alteration of SGECs in pSS participates in the establishment of a hyperosmolar microenvironment, which in turn promotes SGECs transcriptomic modifications. These modifications include extracellular matrix remodeling and promote SG lymphocytic infiltration.
Keywords: Sjögren’s syndrome, hyperosmolarity, epithelial–mesenchymal transition, extracellular matrix, RNAseq
Graphical Abstract
Graphical Abstract.
Introduction
Primary Sjögren syndrome (pSS) is a systemic autoimmune disease characterized by a lymphocytic infiltration of exocrine glands leading to sicca symptoms including xerostomia and kerato-conjonctivitis sicca. In addition to quantitative abnormalities of saliva and tears (decreased production), qualitative abnormalities have also been described [1, 2]. Salivary gland epithelial cells (SGECs) play an active role in pSS pathophysiology, by participating in the over-activation of the immune system. The origin of this specific capacity of SGECs to support immune system stimulation remains to be understood. SGECs may play an early role in the initiation of the disease and their intrinsic characteristics may explain their capacity to promote activation of the immune system in pSS patients. At the same time, defective exocrine function may alter both quantitatively [3] and qualitatively salivary secretion [4]. In saliva formation triggered by nerve stimulation, the initial secretion of NaCl into the acini lumen leads to the formation of an osmotic gradient and aquaporin-5 (AQP5, a transmembrane water channel) trafficking to the apical membrane of acinar cells. The latter allows the passage of water to the gland lumen and the formation of a primary isotonic saliva that is modified by the ductal cells when flowing through the ductal lumen to produce a final hypotonic saliva when reaching the mouth [5, 6]. Both acinar cell apoptosis [7], AQP4, and AQP5 altered localization [8–11] may contribute to the formation of an osmotic gradient subjecting SGECs to chronic hyperosmolar stimulation. To our knowledge, saliva osmolarity and in situ osmolarity measures are unknown. However, the increase of sodium concentration in saliva from pSS compared to controls has been described in several studies [12–14]. Cells subjected to hyperosmolar stress are known to rapidly encounter cell shrinkage followed by a phase of cell volume recovery involving a mechanism named regulatory volume increase [15, 16]. In addition, hyperosmolar stress can also induce DNA damage, cell cycle arrest, apoptosis, mitochondrial depolarization, alteration of transcription and translation, oxidative stress, cytoskeleton modification, modification of stress proteins [15], modulation of innate, and adaptative immune response [17–19], differentiation of CD4+ lymphocytes into Th17 cells [20, 21], induction of autoimmune diseases [20, 22]. Therefore, our hypothesis is that quantitative and qualitative modifications of saliva may affect SGECs osmotic microenvironment and perpetuate immune activation within salivary glands (SG).
In this study, we aimed to decipher the links between hyperosmolar stimulation of SGECs and SG lymphocytic infiltration observed in pSS. First, we assessed the transcriptomic modifications induced by hyperosmolar stimulation of an acinar SG cell line. Then, we compared the transcriptome of cultured SGECs from pSS primary and controls and evaluated the correlation between the expression of several genes involved in modulated pathways and SG lymphocytic infiltration. Lastly, we assessed the effects of coculture of SGECs with B lymphocytes on gene expression.
Methods
NS-SV-AC culture and stimulation
NS-SV-AC (Normal SG-SV40 transformed-squamous cells resembling acinar cells) was kindly provided by Prof. M. Azuma (Second Department of Oral and Maxillofacial Surgery, Tokushima University School of Dentistry) [23]. Cells were grown and passaged twice a week as previously described [24]. Cells were confirmed to be free of mycoplasma contamination using the LookOut® Mycoplasma PCR Detection Kit (Sigma-Aldrich, St Louis, MO, USA) and to present unique cell identity using short tandem repeat (STR) DNA profile (European Collection for Authenticated Cell cultures, Public Health England, England). Cells were stimulated for 8 h with standard iso-osmolar culture medium (Iso) or standard culture medium containing an additional 100 mM of NaCl (Na100) or 200 mM of Sucrose (Su200).
RNA extraction of NS-SV-AC
Total RNA was extracted from stimulated NS-SV-AC cells using an Ezna Total RNA kit II (Omega Bio-tek, Norcross, GA, USA). RNA purity and integrity were verified using a Bioanalyzer (Agilent technologies, Palo Alto, CA, USA).
RNA-Seq of NS-SV-AC
Total NS-SV-AC RNA was used to prepare indexed cDNA libraries using the TruSeq Stranded mRNA Sample Prep kit (Illumina, San Diego, CA, USA) following manufacturer recommendations. The multiplexed libraries were loaded on a NovaSeq 6000 (Illumina, San Diego, CA, USA) using an S2 flow cell, and sequences were produced using a 200 cycles reagent Kit. Paired-end reads were mapped against the human reference genome GRCh38 using STAR_2.5.3a software to generate read alignments for each sample. Annotations Homo_sapiens.GRCh38.90.gtf were obtained from sftp.Ensembl.org. After transcripts assembling, gene-level counts were obtained using HTSeq-0.9.1 and normalized to 20 million aligned reads. Data were computed on these values between the experimental conditions using the https://degust.erc.monash.edu/ software suite.
Minor salivary gland biopsies and B lymphocytes preparation
Minor salivary gland biopsies (MSGB) were obtained from consecutive patients referred for suspicion of pSS to the Rheumatology Department of Bicêtre Hospital, AP-HP, Paris-Saclay university, a tertiary reference center for systemic auto-immune diseases. pSS was defined according to the 2016 ACR/EULAR criteria [25]. To compose a homogenous group, all included pSS patients in the RNAseq analysis presented a focus score ≥ 1 for their MSGB and positive anti-SSA antibodies in their sera. Controls subjects presented with sicca symptoms but without anti-SSA/SSB antibodies and with normal or sub-normal MSGB (i.e. focus score <1).
Peripheral mononuclear cells were isolated from residual blood of apheresis from healthy subjects (French blood donors) by Ficoll gradient separation. B lymphocytes were isolated using CD19 magnetic bead according to the manufacturer’s instructions (CD19 Microbeads human and Fc-Block, Miltenyi Biotec, Bergisch Gladbach, Germany) to achieve a purity of greater than 80% as assessed by FACS analysis (percentage of CD20+ cells).
Primary cultures of SGECs and co-culture with B lymphocytes
Primary cultures of SGECs from pSS and controls were established from MSGBs as previously described [26]. After 2–3 weeks of culture, cells at 70–80% confluence were dissociated with 0.125% trypsin-EDTA. Then, cells were suspended in basal epithelial medium, seeded at 80 000 cells/cm2 to a 6-well plate coated with collagen type I (Institut de Biotechnologies, Reims, France), and incubated at 37°C and 5% CO2 in a humidified atmosphere. The basal epithelial medium was changed at day 1 to remove non-adherent SGECs. When SGECs reached 70% confluency, we added the B cells (isolated as described above). The coculture lasted 5 days in 2 mL RPMI-1640 (Lonza, Verviers, Belgium) supplemented with 10% heat-inactivated fetal bovine serum (PAN biotech, Aidenbach, Germany) and 100 Units/mL penicillin and 100 Units/mL streptomycin (Lonza, Verviers, Belgium). After 5 days, B lymphocytes were harvested, SGECs were washed twice with PBS, and then harvested and frozen (−80°C).
RNA extraction of primary cultured SGECs
Total RNA from SGECs was extracted using the RNeasy Minikit (Qiagen, Hidden, Germany) according to the manufacturer’s specifications. Contaminating DNA was removed using an RNase-free Dnase set (Qiagen, Hidden, Germany) according to the manufacturer’s instructions. After RNA extraction, RNA concentrations were obtained using a fluorometric Qubit RNA HS assay (Life Technologies, Grand Island, New York, USA). The quality of the RNA (RNA integrity number 8.2) was determined on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). An amount of 1 µg RNA was reverse transcribed in cDNA using the First strand synthesis kit (Sigma Aldrich, Palo Alto, CA, USA).
RNA-seq of primary cultured SGECs
To construct cDNA libraries, 1 μg of high-quality total RNA sample (RIN >8) was processed using Truseq stranded total RNA kit (Illumina, San Diego, CA, USA) according to manufacturer instructions. Briefly, after the removal of human ribosomal RNA (using Ribo-zero rRNA) confirmed by quality control on pico chip on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA), total RNA molecules were fragmented and reverse-transcribed using random primers. Replacement of dTTP by dUTP during the second strand synthesis allowed to achieve the strand specificity. The addition of a single A base to the cDNA was followed by the ligation of adapters.
Libraries were quantified by qPCR using the KAPA Library Quantification Kit (KapaBiosystems, Wilmington, MA, USA), and library profiles were assessed using the DNA High Sensitivity LabChip kit on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Libraries were sequenced on a Nextseq 500 instrument (Illumina, San Diego, CA, USA) using 75 base-lengths read V2 chemistry in a paired-end mode. After sequencing, a primary analysis based on AOZAN software (ENS, Paris) was applied to demultiplex and control the quality of the raw data (based on FastQC modules/version 0.11.5). Obtained fastq files were then aligned using Star algorithm (version 2.5.2b) and quality control of the alignment realized with Picard tools (version 2.8.1). Reads were then counted using Feature count (version Rsubread 1.24.1).
RNA-seq data analyses: the Interferome v2.01 database was used to identify and characterize interferon (IFN)-induced genes. Functional enrichment analysis of differentially expressed genes was performed on differentially expressed up and down-regulated genes using Ingenuity Pathway Analysis software (Qiagen, Hidden, Germany). STRING DB software v 11.5 was used to analyze the protein–protein interaction networks [27]. Volcano plot representations were performed using Prism software.
Real-time quantitative PCR (RT-qPCR)
To confirm the results of RNA-seq on SGECs, new samples of primary cultured SGECs of pSS and controls were added. Total RNA was extracted with the GeneJET kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer specifications and cDNA was obtained using the STR1 1KT kit (Sigma Aldrich, Saint Louis, MO). The quantification of MMP24, COL3A1, COL1A2, ADAM12, SNAIL, SLUG, and ZEB2 mRNA expression was determined by TaqMan real time PCR according to the manufacturer’s instructions.
Statistical analysis of NS-SV-AC RNA-seq
Differentially expressed genes were identified with EdgeR quasi-likelihood method (false discovery rate (FDR) <0.05) and log2fold change (log2FC)>1 or <−1.
Statistical analysis of SGECs RNA-seq
Statistical analyses on the read counts were performed with the DESeq2 package version 1.14.1. Comparisons were performed between pSS and control samples based on a non-parametric t-test. Differentially expressed genes were identified based on P-value < 0.05 and log2FC >0.58 or <−0.58.
Statistical analysis of RT-qPCR results
Relative mRNA expression was determined from normalized Ct values by using GAPDH as housekeeping gene and the 2−ΔCt method. To compare means the Mann–Whitney test was applied.
Results
Hyperosmolar stimulation induces NS-SV-AC transcriptomic modifications
Transcriptomic analysis was performed on NS-SV-AC exposed to a hyperosmolar stimulation (n = 4). Volcano plots show the comparison of gene expression under stimulation with Na100 or Su200 compared to ISO control with respectively 1981 (1347 upregulated and 634 downregulated) and 3339 (2262 upregulated and 1077 downregulated) differentially expressed genes (Fig. 1A). To avoid bias linked to intracellular action of Na and focus on the hyperosmolarity effect, subsequent analyzes were performed using genes differentially regulated by both Na100 and Su200 stimulation: a total of 1376 genes, with 1035 upregulated and 341 downregulated (Fig. 1B).
Figure 1:
A: Volcano plot of differentially expressed genes in NS-SV-AC stimulated with hyperosmolar media versus iso-osmolar medium. The horizontal line indicates the cut-off for significance at log10 FDR >1.30 (P < 0.05) and the vertical lines indicate the cut-off for expression at log2FC > 1 and <−1. B: Venn diagram representation of differentially expressed genes in NS-SV-AC stimulated with Na100 or Su200 versus iso-osmolar medium. C: Representation using StringDB software of the existing links between 150 most upregulated genes between NS-SV-AC stimulated with hyperosmolar media versus iso-osmolar medium. D: Normalized counts of MX1, IFIT2, MMP1, MMP3, MMP13, MMP25, CCL2, IL15, PECAM1 and CCL26 in NS-SV-AC cells stimulated with Na100 or Su200 versus ISO. P values were determined using EdgeR quasi-likelihood method; false discovery rate (FDR) <0.05 or −log10 FDR >1.30 were considered statistically significant. -Log10FDR values are given above bars to indicate the level of statistical significance. E: Venn diagram representation of the repartition of the differentially expressed genes among the 3 subtypes of IFN (Interferome v2.01 database)
Upregulation of IFN-induced genes
Among the top 150-upregulated genes by hyperosmolarity, we identified using String DB software one hub corresponding to interferon (IFN)-induced genes (Fig. 1C). In this hub, the expression of MX1 (MX dynamin-like GTPase 1) (Na100: log2FC = 3.26; Su200: log2FC = 2.74) and IFIT2 (interferon-induced protein with tetratricopeptide repeats 2) (Na100: log2FC = 5.26; Su200: log2FC = 6.59) were upregulated (Fig. 1D). Among the 634 most upregulated genes (with a log2FC> 1.5), 332 were recognized by the Interferome database as IFN-induced genes. Among them, 153 were induced by types I and II; 5 were induced by types I, II, and III; and 139 were induced by type II only (Fig. 1E). Among the 91 down-regulated genes (with log2FC <−1.5), 42 were IFN-induced genes according to the Interferome database; 17 were induced by types I and II; 1 was induced by types I, II and III; and 17 were induced by type II only (Fig. 1E). These results on cell line with hyperosmolar stimulation are in line with previous results we obtained in SGECs sorted from SG from pSS patients and controls, which showed an over-representation of the IFN signaling pathway [28].
Over-representation of fibrosis and extracellular matrix remodeling pathways
Functional enrichment pathway analysis of the differentially expressed genes in response to hyperosmolarity was performed with IPA software. The analysis highlighted an over-representation of pathways involving fibrosis signaling, as well as tumor microenvironment pathways (Table 1). These pathways included several MMP genes. The upregulation of MMP genes, such as MMP1, MMP3, MMP13, and MMP25 (Fig. 1D) suggested an implication of epithelial cells in extracellular matrix remodeling. Moreover, the IL-17 signaling pathway was also over-represented, including several genes such as CCL2, IL15, MMP3, and MMP13 (Table 1; Fig. 1D).
Table 1:
Enrichment pathway analysis of NS-SV-AC stimulated with hyperosmolar media versus iso-osmolar medium
| Ingenuity canonical oathways | –Log(p-value) | Molecules |
|---|---|---|
| Pulmonary healing signaling pathway | 7·13 | ACKR3,BMP4,CXCR4,ETV5,FGFR2,FZD7,MMP1,MMP11,MMP13,MMP25,MMP3,NFKBIA,NGFR,PECAM1,PGF,PRKCH,PRKCQ,RASD1,TLR4,VEGFA,VEGFC,WNT16,WNT2B |
| Osteoarthritis pathway | 5·81 | ALPG,COL2A1,DDIT4,DKK1,FGF2,FZD7,HTRA1,ITGA10,ITGA8,ITGB8,MMP1,MMP13,MMP3,NOS2,PGF,PTHLH,S1PR3,SOX9,TIMP3,TLR4,VEGFA,VEGFC,WNT16 |
| Role of macrophages, fibroblasts and endothelial cells in rheumatoid arthritis | 5·76 | APC2,CCL2,DKK1,F2RL1,FCGR1A,FGF2,FZD7,IL15,MMP1,MMP13,MMP3,NFAT5,NFKBIA,NGFR,NOS2,NOTUM,PDGFA,PGF,PIK3C2B,PRKCH,PRKCQ,RASD1,TLR4,TLR9,VEGFA,VEGFC,WNT16,WNT2B |
| Axonal guidance signaling | 5·72 | ADAMTS9,BDNF,BMP4,CXCR4,EPHA2,FZD7,GNA14,GNAZ,ITGA10,ITGA8,ITGB8,MMP1,MMP11,MMP13,MMP25,MMP3,MYL4,NFAT5,NGFR,NOTUM,PAK6,PDGFA,PGF,PIK3C2B,PRKCH,PRKCQ,RASD1,SDCBP,SEMA6D,SEMA7A,SLIT1,STK36,UNC5B,VEGFA,VEGFC,WNT16,WNT2B |
| HIF1α signaling | 5·6 | ADRA1B,CDKN1A,FGF2,FLT1,IGF2,MMP1,MMP11,MMP13,MMP25,MMP3,NOS2,PGF,PIK3C2B,PRKCH,PRKCQ,RASD1,SAT1,SLC2A14,SLC2A3,VEGFA,VEGFC |
| Tumor microenvironment pathway | 5·44 | CCL2,CXCR4,FGF13,FGF2,FOXO6,IGF2,MMP1,MMP11,MMP13,MMP25,MMP3,NOS2,PDGFA,PGF,PIK3C2B,RASD1,SLC2A3,VEGFA,VEGFC |
| Hepatic fibrosis signaling pathway | 5·41 | APC2,BAMBI,CCL2,COL2A1,FGF2,FLT1,FZD7,IRS2,ITGA10,ITGA8,ITGB8,MMP1,MMP13,MYL4,NFKBIA,NGFR,PDGFA,PGF,PIK3C2B,PRKCH,PRKCQ,RASD1,RHOF,RHOU,SIRT3,SNAI1,TGFBR3,TLR4,VEGFA,VEGFC,WNT16,WNT2B |
| Hepatic fibrosis/hepatic stellate cell activation | 4·94 | BAMBI,CCL2,CD14,COL2A1,EDNRB,FGF2,FGFR2,FLT1,IGF2,IGFBP5,MMP1,MMP13,MYL4,NGFR,PDGFA,PGF,TLR4,VEGFA,VEGFC |
| Leukocyte extravasation signaling | 4·42 | ARHGAP5,CLDN5,CLDN6,CLDN9,CXCR4,MMP1,MMP11,MMP13,MMP25,MMP3,PECAM1,PIK3C2B,PRKCH,PRKCQ,PTK2B,SELPLG,TIMP3,VAV3 |
| Colorectal cancer metastasis signaling | 4·33 | ADCY1,BCL2L1,FZD7,GNA14,GNAZ,MMP1,MMP11,MMP13,MMP25,MMP3,NOS2,PGF,PIK3C2B,RASD1,RHOF,RHOU,TLR4,TLR9,VEGFA,VEGFC,WNT16,WNT2B |
| Bladder cancer signaling | 4·16 | CDKN1A,DAPK1,FGF13,FGF2,MMP1,MMP11,MMP13,MMP25,MMP3,PGF,RASD1,VEGFA,VEGFC |
| CREB signaling in neurons | 4·04 | ACKR3,ADCY1,ADORA2A,ADRA1B,ADRA2A,ADRB2,CXCR6,EDNRB,F2RL1,FGF2,FGFR2,FLT1,FZD7,GNA14,GNAZ,GPER1,GPR135,GPR3,GPR35,GPR45,GPRC5D,GRIK5,GRIN2C,GRM2,HRH1,HTR1B,HTR7,NGFR,NOTUM,PIK3C2B,PRKCH,PRKCQ,RASD1,S1PR3,SSTR2,TGFBR3,VEGFA |
| Granulocyte adhesion and diapedesis | 4·02 | ACKR3,CCL2,CCL26,CLDN5,CLDN6,CLDN9,CXCR4,CXCR6,HRH1,MMP1,MMP11,MMP13,MMP25,MMP3,NGFR,PECAM1,SELPLG |
| Agranulocyte adhesion and diapedesis | 3·84 | ACKR3,AOC3,CCL2,CCL26,CLDN5,CLDN6,CLDN9,CXCR4,CXCR6,HRH1,MMP1,MMP11,MMP13,MMP25,MMP3,MYL4,PECAM1,SELPLG |
| VDR/RXR activation | 3·81 | CALB1,CD14,CDKN1A,IGFBP5,KLF4,MXD1,NCOA3,PDGFA,PRKCH,PRKCQ |
| G-protein coupled receptor signaling | 3·67 | ACKR3,ADCY1,ADORA2A,ADRA1B,ADRA2A,ADRB2,CXCR6,DUSP1,DUSP4,DUSP6,EDNRB,F2RL1,FOXO6,FZD7,GNA14,GNAZ,GPER1,GPR135,GPR3,GPR35,GPR45,GPRC5D,GRM2,HCN3,HRH1,HTR1B,HTR7,KCNN4,MYL4,NFAT5,NFKBIA,PAK6,PDE6G,PIK3C2B,PTK2B,RASD1,RGS16,RGS4,S1PR3,SSTR2 |
| Human embryonic stem cell pluripotency | 3·64 | APC2,BDNF,BMP4,FGF2,FGFR2,FZD7,GNA14,GNAZ,INHBA,NOG,PDGFA,PIK3C2B,S1PR3,WNT16,WNT2B |
| IL-17 signaling | 3·57 | CCL2,CNTF,IL11,IL15,MMP13,MMP3,NOS2,PGF,PIK3C2B,RASD1,RGS16,TAB2,TNFSF15,TNFSF9,VEGFA,VEGFC |
| Phagosome formation | 3·52 | ACKR3,ADORA2A,ADRA1B,ADRA2A,ADRB2,C3,CD14,CXCR6,EDNRB,F2RL1,FCGR1A,FZD7,GPER1,GPR135,GPR3,GPR35,GPR45,GPRC5D,GRM2,HRH1,HTR1B,HTR7,ITGA10,ITGA8,ITGB8,MALT1,MYL4,PAK6,PIK3C2B,PLA2G4B,PRKCH,PRKCQ,PTK2B,RASD1,S1PR3,SSTR2,TLR4,TLR9,VAV3 |
| Wound healing signaling pathway | 3·41 | CNTF,COL2A1,FGF2,FGFR2,IL11,IL15,LAMB3,MMP1,NFKBIA,NGFR,PDGFA,PGF,RASD1,SNAI2,TGFBR3,TNFSF15,TNFSF9,VEGFA,VEGFC |
| Cardiac hypertrophy signaling (enhanced) | 3·32 | ADCY1,ADRA1B,ADRA2A,ADRB2,CNTF,EDNRB,FGF13,FGF2,FGFR2,FZD7,GNA14,IL11,IL15,IL3RA,ITGA10,ITGA8,ITGB8,MYOCD,NFAT5,NGFR,NOTUM,PDE6G,PIK3C2B,PRKCH,PRKCQ,RASD1,RCAN1,TGFBR3,TNFSF15,TNFSF9,WNT16,WNT2B |
| HOTAIR regulatory pathway | 3·21 | AR,CDKN1A,MMP1,MMP11,MMP13,MMP25,MMP3,NFKBIA,PCDH10,PIK3C2B,SNAI2,TLR4,WNT16,WNT2B |
| Role of osteoblasts, osteoclasts and chondrocytes in rheumatoid arthritis | 3·15 | APC2,BMP4,CSF1R,DKK1,FZD7,IL11,MMP1,MMP13,MMP3,NFAT5,NFKBIA,NGFR,PIK3C2B,PTK2B,TAB2,WNT16,WNT2B |
Upregulation of homing chemokines genes
In the functional enrichment pathway analysis, another over-represented pathway was the leukocyte extravasation signaling pathway (Table 1). This pathway included, for example, PECAM-1 which was also one of the 150 most upregulated genes (Na100: log2FC = 5.46; Su200: log2FC = 6.63; Fig. 1D). PECAM-1 is a cell adhesion molecule required for leukocyte trans-endothelial migration under most inflammatory conditions [29].
CCL2 was upregulated by hyperosmolar stimulation (Na100: log2FC = 4.42; Su200: log2FC = 4.99; Fig. 1D). CCL2, chemokine (C–C) motif ligand 2, is a pro-inflammatory cytokine playing a role in the recruitment of monocytes. Interestingly, pSS patients have higher levels of CCL2 in saliva [30] but lower CCL2 levels in tears [31]. In addition, greater serum levels of CCL2 have also been found in pSS patients with germinal centers [32].
CCL26, also known as eotaxin-3, was also upregulated (Na100: log2FC = 4.66; Su200: log2FC = 4.10; Fig. 1D). CCL26 is a functional ligand for CCR3 and acts as a chemoattractant for eosinophils and basophils.
Given these results showing the multiple effects of hyperosmolar stimulation on the NS-SV-AC cells, we wondered whether modifications of extracellular matrix were also observed in SGECs from pSS patients.
Primary cultured SGECs from patients express genes involved in extracellular matrix remodeling
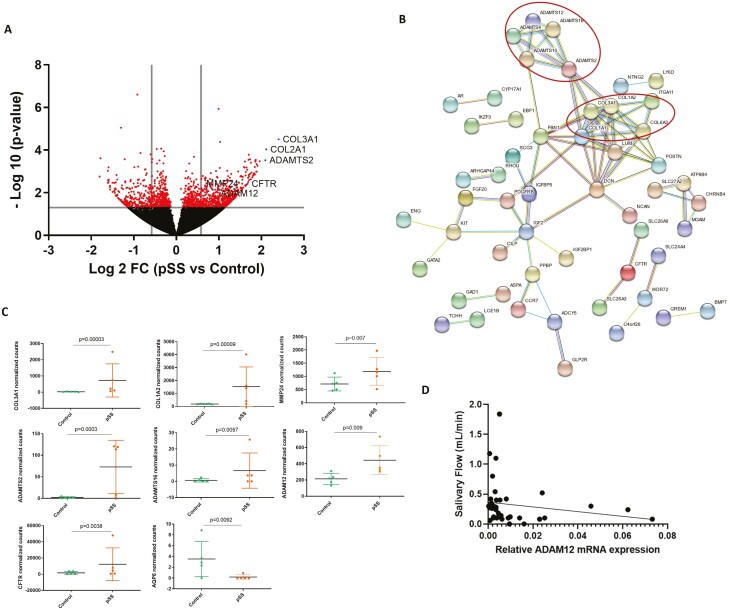
RNAseq transcriptomic analysis was performed on SGECs from five pSS and five controls. The characteristics of the patients and controls included in the study are presented in Table 2. The comparison of gene expression in primary cultured unstimulated SGECs of pSS compared to controls showed 511 differentially expressed genes: 251 upregulated and 260 downregulated (Fig. 2A). Functional enrichment pathway analysis highlighted an over-representation of a signaling pathway involving matrix metalloproteases, including the upregulated MMP12, MMP21, MMP24 genes (log2FC = 1.62, log2FC = 1.62, and log2FC = 0.70, respectively) (Table 3). Among the 150 most upregulated genes, using string DB software, we identified hubs corresponding to genes involved in extracellular matrix formation (Fig. 2B). These hubs included several collagen genes: COL3A1 and COL1A2 with the higher fold-change (log2FC = 2.40, log2FC = 2.12, respectively) and MMP24 (log2FC = 0.70). There were also several ADAM and ADAMTS genes (A disintegrin and metalloproteinases with a thrombospondin motif) related genes such as ADAMTS2, ADAMTS16, and ADAM12 (log2FC = 2.09, log2FC = 1.57, log2FC = 0.95, respectively) (Fig. 2C). Other genes that might have a role in saliva secretion such as CFTR and AQP6 were differentially expressed; CFTR, which code for a HCO3-transporter [33], was upregulated (log2FC = 1.67), whereas AQP6, which code for a water channel reported to be expressed in rat parotid acinar cells [34], was downregulated (log2FC = −1.44) (Fig. 2C). RT-qPCR performed using a larger number of pSS and controls SGECs samples for MMP24, COL3A1, COL1A2, and ADAM12 did not confirm these results, likely because of the low basal gene expression levels (data not shown).
Table 2:
Characteristics of the patients included in the study
| RNA-seq experiments on primary cultured SGECs | ||
|---|---|---|
| pSS (n = 5) | Control (n = 5) | |
| Median age (min–max) | 31 (23-53) | 52 (34-56) |
| Female sex, n (%) | 5 (100) | 5 (100) |
| Pathologic Schirmer, n (%)* | 2 (50) | 2 (50) |
| Pathologic salivary flow, n (%)** | 0 (0) | 0 (0) |
| Focus score ≥ 1, n (%) | 5 (100) | 0 (0) |
| Anti- SSA+, n (%) | 5 (100) | 0 (0) |
| RT-qPCR experiments on primary cultured SGECs | ||
| pSS (n = 19) | Control (n = 20) | |
| Median age (min–max) | 50 (25–74) | 55 (34–74) |
| Female sex, n (%) | 18 (95) | 17 (85) |
| Pathologic Schirmer, n (%)* | 6 (32) | 10/19 (53) |
| Pathologic salivary flow, n (%)** | 8/18 (44) | 5 (25) |
| Focus score ≥ 1, n (%) | 12 (63) | 0 (0) |
| Anti-SSA+, n (%) | 14 (74) | 0 (0) |
Pathologic Schirmer: result <5mm in 5 min; pathologic salivary flow: salivary flow <0.10 ml/min.
Figure 2:
A: Volcano plot of differentially expressed genes in SGECs from patients with pSS (n = 5) versus controls (n = 5). The horizontal line indicates the cut-off for significance at P < 0.05 and the vertical lines indicate fold changes at ≥1.5 and ≤−1.5. B: Representation using StringDB software of the existing links between differentially expressed genes between pSS and controls in primary cultured SGECs. Minimum required interaction score = 0.7 (high confidence). C: Normalized counts of COL3A1, COL1A2, MMP24, ADAMTS2, ADAMTS16, ADAMT12, CFTR, and AQP6 in SGECs from controls and pSS. P values were determined by using DESeq2. D: Correlation between ADAM12 relative to GAPDH expression in primary cultured SGECs from pSS (n = 18) and controls (n = 20) and salivary flow (ml/min)
Table 3:
Enrichment pathway analysis of primary cultured SGECs from pSS compared to controls. Selection of the 10 most significant pathways
| Ingenuity canonical pathways | –log(P-value) | Molecules |
|---|---|---|
| Inhibition of matrix metalloproteases | 3·85 | HSPG2,MMP21,TIMP4,ADAM12,THBS2,MMP12,TFPI2,MMP24 |
| Stearate biosynthesis I (animals) | 3·46 | BDH2,SLC27A2,PPT1,SLC27A6,ELOVL2,ACOT7,ACSL1,ACOT8 |
| Glycine biosynthesis I | 2·76 | SHMT1,SHMT2 |
| Superpathway of serine and glycine biosynthesis I | 2·65 | PHGDH,SHMT1,SHMT2 |
| γ-Linolenate biosynthesis II (animals) | 2·34 | SLC27A2,SLC27A6,FADS2,ACSL1 |
| Mitochondrial L-carnitine shuttle pathway | 2·34 | SLC27A2,CPT1B,SLC27A6,ACSL1 |
| Folate transformations I | 2·3 | MTHFD2,SHMT1,SHMT2 |
| Glycine betaine degradation | 2·16 | SHMT1,BHMT2,SHMT2 |
| GP6 signaling pathway | 2·06 | COL1A2,LAMC1,COL1A1,CALM1 (includes others),COL5A2,COL6A3,COL4A3,PRKCD,PIK3C2G,RAC1,KLF12,COL3A1 |
| Acyl-CoA hydrolysis | 2·03 | PPT1,ACOT7,ACOT8 |
Relation between SGECs modifications in pSS and saliva secretion
To assess if these modifications could be associated with saliva production in patients, we determined the possible correlation between COL3A1, COL1A2, MMP24, and ADAM12 gene expression and saliva flow in 19 pSS and 20 controls. Only ADAM12 mRNA expression was significantly correlated with the salivary flow with a negative correlation (Spearman coefficient r = −0.3438, P = 0.0346) (Fig. 2D).
SGECs might undergo epithelial–mesenchymal transition during pSS
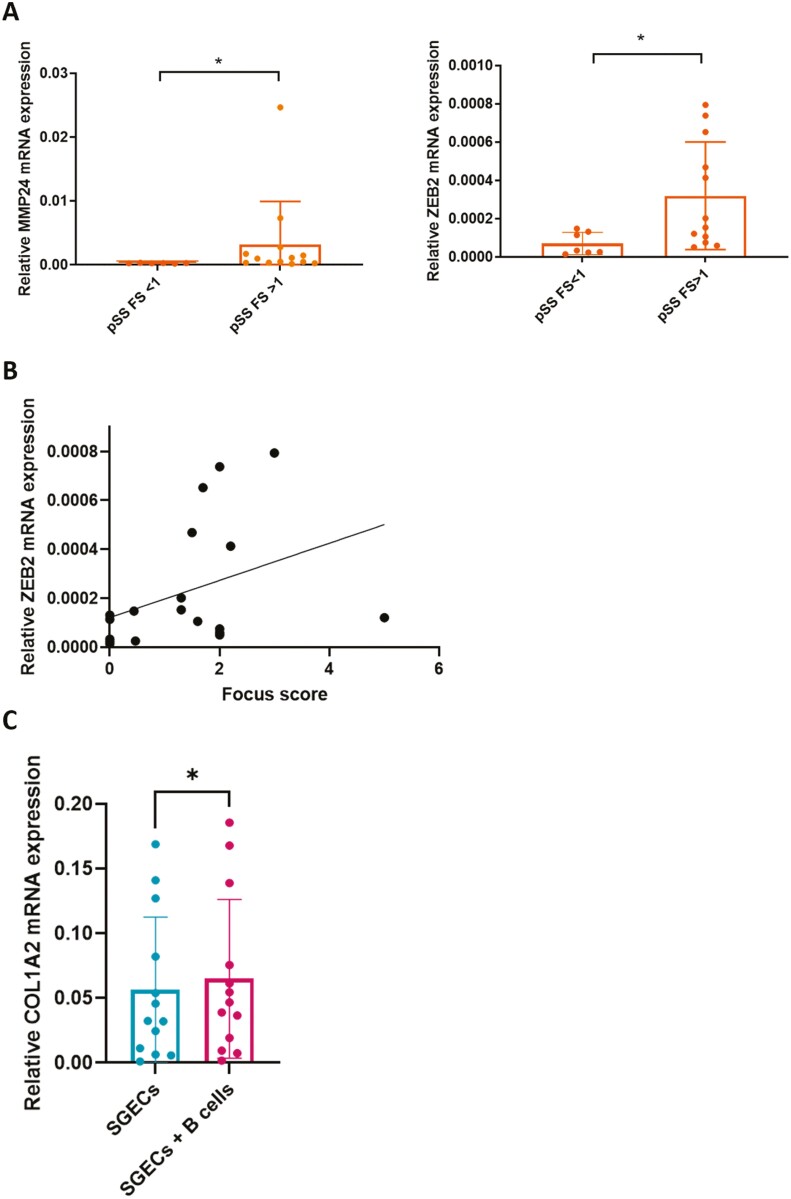
The production of collagen is usually due to fibroblastic cells. Given the unexpected COL3A1 and COL1A2 expression found in pSS SGECs, we hypothesized that SGECs from pSS may acquire fibroblastic properties due to epithelial–mesenchymal transition (EMT). Several transcription factors have been involved in EMT, such as Snail (encoded by SNAI1), Slug (encoded by SLUG or SNAI2), and Zeb2 (encoded by ZEB2) [35]. The RNAseq analysis of SGECs showed a trend for an upregulation of SNAIL and a down-regulation of SLUG in SGECs from pSS compared to controls (Fig. 3A). SLUG down-regulation was confirmed by RT-qPCR in SGECs from pSS compared to controls (Fig. 3B). Lastly, there was an upregulation of ZEB2 in pSS compared to controls (log2FC = 1.21, P = 0.019) (Fig. 3A). Interestingly, SNAI1, SNAI2, and ZEB were all increased by Na100 and Su200 in NS-SV-AC cells (data not shown).
Figure 3:
A: Normalized counts of SNAIL, SLUG, and ZEB2 in SGECs from controls and pSS. Data from RNAseq analysis. P values were determined by using DESeq2; ns: not significant. B: SLUG RNA expression in SGECs form pSS and controls (n = 19 pSS and 20 controls) determined by RT-qPCR. *P-value < 0.05
To sum up, these results support a potential switch of SGECs from the epithelial to mesenchymal fibroblast-like cell shape in pSS.
Genes involved in matrix remodeling and EMT are associated with SG lymphocytic infiltration
We then hypothesized that the changes in extracellular remodeling and EMT in SGECs may participate in sialadenitis observed in pSS. Interestingly, the changes were more pronounced in patients with a focus score (FS) ≥1 for genes associated with matrix remodeling (MMP24) and EMT (ZEB2) (P = 0.0125, and P = 0.0171, respectively) (Fig. 4A). Furthermore, there was a correlation between ZEB2 expression and the FS in SG (Fig. 4B).
Figure 4:
A: Relative mRNA expression of MMP24 and ZEB2 in SGECs from pSS with a focus score (FS) <1 and FS ≥1 were normalized to the expression of GPADH mRNA. B: Correlation between ZEB2 expression and the salivary gland focus score in pSS patients (Spearman: r = −0.47, P = 0.042). C: COL1A2 expression in SGECs from pSS and controls cultured alone or cocultured with B lymphocytes or cultured alone for 5 days. *P-value < .05. FS: focus score.
Given the association between the expression of genes involved in extracellular remodeling and the FS, we decided to look at the potential impact of B lymphocytes on the modification observed in primary cultured SGECs from pSS or controls. We showed an increase of COL1A2 expression in SGECs when co-cultured with B lymphocytes compared to SGECs cultured alone (Fig. 4C). There was no difference between SGECs from pSS and controls. This result supports the hypothesis that B lymphocytes infiltrate might sustain the phenotypic switch of SGECs.
Discussion
In this study, we showed that hyperosmolar stress impacts transcriptomic profile of NS-SV-AC leading to the up-regulation of IFN-induced genes, several chemokine genes, and genes promoting the expression of extracellular matrix remodeling. Interestingly, the induction of genes involved in extracellular matrix remodeling was also observed in SGECs from pSS patients. In addition, we observed a transcriptomic profile suggestive of EMT in SGECs from pSS patients. These modifications correlated with higher lymphocytic infiltration. In turn, it was likely that co-culture with B lymphocytes could sustain the phenotypic switch of SGECs. Considering these results, we made the hypothesis of the existence of a vicious circle. Indeed, abnormal saliva secretion, a key symptom of pSS, may be responsible for chronic hyperosmolar stimulation of SGECs leading to the modifications of SGECs transcriptome notably by upregulating genes involved in extracellular matrix remodeling and fibrosis. This switch seems to be accentuated by co-culture with B lymphocytes. Furthermore, hyperosmolar stress could amplify immune activation and aggravate sialadenitis by induction of pro-inflammatory cytokines, IFNs, and chemokines. A graphical summary of this hypothesis is presented in Fig. 5.
Figure 5:
Graphical abstract of the main results and the hypothesis of a vicious circle.
One of the main symptoms of pSS is the xerostomia. The factors responsible for SG hypofunction are not fully deciphered and might result not only from SG destruction but also involve other mechanisms, such as the presence of anti-muscarinic autoantibodies [36], altered mucin expression, nitric oxide-mediated SG dysfunction, and modified aquaporin-5 distribution [37]. In addition to quantitative abnormality, saliva in pSS patients presents qualitative abnormalities [38] including hyperosmolarity. For the first time, we showed that hyperosmolarity stress has an impact on SG acinar epithelial cells transcriptome. The effect of hyperosmolar stimulation has been studied in primary cultured human corneal epithelial cells, showing an induction of several MMP genes expression, including MMP9, MMP1, MMP13, and MMP3 [39]. Otherwise, Grauso et al. studied the effect of hyperosmolar media on intestinal epithelial cells using the human colonocyte Caco-2 cell line model. They showed that hyperosmolar stimulation slowed down cell proliferation and induced IL-8 secretion, a pro-inflammatory cytokine [40]. The strength of this work is the observation of similar characteristics in hyperosmolar stimulated NS-SV-AC and in SGECs from pSS patients including, for example, MMP genes upregulation. The comparison between SGECs from pSS and controls showed an upregulation of collagen genes as well as ADAMTS family genes. These results suggested a potential involvement of SGECs in fibrosis formation in SG, which does not constitute a diagnostic criterion for pSS, but can be observed in SG from numbers of pSS patients. Leehan et al. showed that pSS subjects had significantly more fibrotic tissue in their SG than controls. In their study, SG fibrosis was related to focus score and was not solely attributable to age, suggesting an intimate relationship between lymphocytic infiltration and fibrotic tissue replacement [41]. In addition, structural alterations of ductal and acini microarchitecture, as well as changes in the expression and activation of MMP, which are involved in basal membrane remodeling, have been described in pSS [42]. The alteration of basal membrane and modifications of cell polarization might precede periductal lymphocyte infiltration [43]. Several ADAMTS genes were also upregulated in pSS SGECS compared to controls, these genes code for complex extracellular proteases that can be secreted by cancer and stromal cells and contribute to tumor microenvironment modification. ADAMTS enzymes can cleave or interact with a wide range of extracellular matrix components or regulatory factors, and therefore affect cell adhesion, migration, proliferation, and angiogenesis [44]. Delaleu et al. analyzed the salivary proteome of pSS patients compared to controls and identified proteins related to collagen synthesis [45]. Moreover, in another study, they showed that proteins relative to tissue remodeling, including MMP3, were associated with hyposalivation in pSS patient’s saliva [46]. All together, these observations support the hypothesis of a role for SGECs in SG fibrosis development and extracellular matrix remodeling. One hypothesis suggested by Sisto et al. is that these cells could undergo epithelial–mesenchymal transition (EMT), with a potential role of IL-17 and IL-22 in the EMT program [47]. Of note, in our study, we found that the IL-17 pathway was over-represented in NS-SV-AC in hyperosmolar stimulated conditions. Recently, Fernández-Torres et al., described polymorphisms of genes involved in the Wnt/B-catenin signaling pathway, which is associated with fibrogenesis, increasing the risk of pSS [48]. The relation between SG fibrosis and SG hypofunction is not well understood. In this study, we observed an inverse correlation between ADAM12 expression and salivary flow. Yin et al. observed a correlation between the SG interstitial fibrosis and the SG hypofunction (evaluated by the unstimulated saliva flow rate); however, in this study, there was no correlation between the interstitial fibrosis and the focus score [49]. Further functional experiments are needed to investigate more precisely the impact of SGECs on the extracellular matrix and to assess whether they could, for example, allow the formation of niches for B cells and plasma cells, as described by Szyszko et al. [50].
SG modifications observed in pSS may represent an intermediate state between a purely fibrotic impairment, as seen in radiation-induced fibrosis [51], and an immune-driven SG impairment as seen in sicca syndrome induced by immune checkpoint inhibitor therapy [52]. Besides these two opposing models, some diseases associate fibrosis and immune impairment of SG. Xerostomia is often present during systemic sclerosis (SSc) and can involve immune-mediated destruction of the acinar tissues or fibrosis. Of note, in a population of 133 SSc patients, Avouac et al. found a prevalence of pSS of 14% and pSS was markedly associated with limited cutaneous SSc [53]. A potential involvement of mTOR signaling pathway in minor SG from pSS and SSc patients has been suggested [54]. In IgG4-related disease, salivary secretion is usually normal or slightly reduced and xerostomia is present in 30% of the patients [55], SG histology shows marked lymphoplasmacytic infiltration and fibrosis with IgG4+ plasma cells [56]. In pSS, salivary secretion impairment might result from both fibrotic and immune mechanisms. This study has some limitations; the hypothesis of the hyperosmolar environment effect on epithelial cells has been studied by an in vitro experimental protocol using possibly high hyperosmolar solutions which do not entirely reflect the tissue microenvironment, especially the impact of immune cells. The co-culture experiment was performed using B cells sorted from blood, which might have different characteristics than salivary gland B cells. However, due to technical reasons, using B cells sorted from biopsies would have not been feasible. SGECs play a central and active role in pSS pathophysiology. Several lines of evidence support this hypothesis: they can interact with B and T lymphocytes by secreting cytokines and chemokines and expressing adhesion and co-stimulation molecules. We previously showed that SGECs increase B cells survival and activation [28]; here, we showed that, in turn, B lymphocytes might induce modifications of SGECs transcriptome, especially COL1A2 expression. Thus, the crosstalk between SGECs and lymphoid cells could be bi-directional: from SGECs to immune cells, but also from immune cells to SGECs. As suggested by these results showing the upregulation of collagen genes or genes involved in extracellular matrix remodeling, SGECs might undergo EMT and therefore acquire specific skills. While no data are currently available concerning the measurement of osmolarity in situ in salivary glands or in the saliva, increased concentrations of sodium and/or chloride have been found in unstimulated and/or stimulated pSS saliva [1, 12, 13, 57–59], and may render saliva hyperosmolar. The saliva hyperosmolarity, whch remains to be determined by further studies, could be one key to explaining these transcriptomic modifications, being at the same time a cause and a consequence of these modifications. Thus, multiple factors may be involved in SGECs modification, including hyperosmolarity but also the crosstalk with B lymphocytes.
In conclusion, local hyperosmolarity could be one of the factors promoting SGECs changes in pSS, responsible for both fibrosis and T and B lymphocytes attraction, activation, and survival. In return, B cells could worsen the fibrosis process of these SGECs.
Glossary
Abbreviations
- CCL
C–C motif chemokine ligand
- EMT
epithelia-mesenchymatous transition
- FS
focus score
- IFN
interferon
- IL
interleukin
- NS-SV-AC
normal SG-SV40 transformed-squamous cells resembling acinar cells
- pSS
primary Sjogren’s syndrome
- RNA
ribonucleic acid
- RNA-seq
RNA sequencing
- SSA
Sicca syndrome A
- SG
salivary gland
- SGECs
salivary gland epithelial cells
Contributor Information
Elodie Rivière, Université Paris-Saclay, INSERM UMR 1184, Autoimmune disease laboratory, Center for immunology of viral infections and autoimmune diseases, Le Kremlin Bicêtre, France; Rheumatology Department, Assistance Publique-Hôpitaux de Paris (AP-HP), Hôpital Bicêtre, Le Kremlin Bicêtre, France.
Clara Chivasso, Laboratory of Pathophysiological and Nutritional Biochemistry, Faculty of Medicine, Université Libre de Bruxelles, Brussels, Belgium.
Juliette Pascaud, Université Paris-Saclay, INSERM UMR 1184, Autoimmune disease laboratory, Center for immunology of viral infections and autoimmune diseases, Le Kremlin Bicêtre, France.
Rami Bechara, Université Paris-Saclay, INSERM UMR 1184, Autoimmune disease laboratory, Center for immunology of viral infections and autoimmune diseases, Le Kremlin Bicêtre, France.
Bineta Ly, Université Paris-Saclay, INSERM UMR 1184, Autoimmune disease laboratory, Center for immunology of viral infections and autoimmune diseases, Le Kremlin Bicêtre, France.
Christine Delporte, Laboratory of Pathophysiological and Nutritional Biochemistry, Faculty of Medicine, Université Libre de Bruxelles, Brussels, Belgium.
Xavier Mariette, Université Paris-Saclay, INSERM UMR 1184, Autoimmune disease laboratory, Center for immunology of viral infections and autoimmune diseases, Le Kremlin Bicêtre, France; Rheumatology Department, Assistance Publique-Hôpitaux de Paris (AP-HP), Hôpital Bicêtre, Le Kremlin Bicêtre, France.
Gaetane Nocturne, Université Paris-Saclay, INSERM UMR 1184, Autoimmune disease laboratory, Center for immunology of viral infections and autoimmune diseases, Le Kremlin Bicêtre, France; Rheumatology Department, Assistance Publique-Hôpitaux de Paris (AP-HP), Hôpital Bicêtre, Le Kremlin Bicêtre, France.
Acknowledgements
We thank C. Le Pajolec, Hôpital Bicêtre, Assistance Publique-Hôpitaux de Paris, Paris-Saclay University, Le Kremlin Bicêtre, France; E. Berge, Hôpital Bicêtre, Rheumatology department, Assistance Publique-Hôpitaux de Paris, Paris-Saclay University, Le Kremlin Bicêtre, France who participated in the recruitment of patients. We thank F. Letourneur, for his help concerning RNAseq of SGECs performed at Université de Paris, Institut Cochin, Paris, France. We thank Prof. M. Azuma (Second Department of Oral and Maxillofacial Surgery, Tokushima University School of Dentistry) for kindly providing us with the NS-SV-AC cell line, Dr A. Lefort and Dr F. Libert for their help concerning RNAseq performed at the Brussels Interuniversity Genomics High Throughput core (www.brightcore.be), and F. Gregoire and N.Bolaky for their technical assistance.
Ethical approval
The study received approval from the local ethics committee, and informed consent was obtained from all patients and control subjects.
Conflict of interests
None declared.
Funding
Support was obtained from: the Laboratory of Excellence in Research on Medication and Therapeutic Innovation (LERMIT) (grant ANR10), the Fondation pour la Recherche Médicale (grant DEQ20150934719), an unrestricted grant from Biogen to Université Paris-Sud (grant UPSud/SAIC N 97731), the Innovative Medicines Initiative 2 Joint Undertaking (IMI 2 JU) (NECESSITY grant 806975) and the EU H2020 contract HarmonicSS (H2020-SC1-2016-RTD/731944). The Joint Undertaking received support from the European Union’s Horizon 2020 Research and Innovation Program and from the European Federation of Pharmaceutical Industries and Associations.
Author contributors
E.R. participated in designing research studies, conducting experiments, acquiring data, analyzing data, and writing the manuscript. J.P. and C.C. participated in designing research studies, conducting experiments, acquiring data, and analyzing data. R.B. and B.L.: participated in designing research studies, conducting experiments, and acquiring and analyzing data. C.D, G.N., and X.M. participated in designing research studies, analyzing data, and writing the manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Kalk WW, Vissink A, Spijkervet FK, Bootsma H, Kallenberg CG, Nieuw Amerongen AV.. Sialometry and sialochemistry: diagnostic tools for Sjögren’s syndrome. Ann Rheum Dis 2001, 60, 1110–6. doi: 10.1136/ard.60.12.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baldini C, Giusti L, Ciregia F, Da Valle Y, Giacomelli C, Donadio E, et al. Proteomic analysis of saliva: a unique tool to distinguish primary Sjögren’s syndrome from secondary Sjögren’s syndrome and other sicca syndromes. Arthritis Res Ther 2011, 13, R194. doi: 10.1186/ar3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Billings M, Dye BA, Iafolla T, Baer AN, Grisius M, Alevizos I.. Significance and implications of patient-reported xerostomia in Sjögren’s Syndrome: findings From the National Institutes of Health Cohort. EBioMedicine 2016, 12, 270–9. doi: 10.1016/j.ebiom.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parisis D, Chivasso C, Perret J, Soyfoo MS, Delporte C.. Current state of knowledge on primary Sjögren’s syndrome, an autoimmune exocrinopathy. J Clin Med 2020, 9, E2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Delporte C. Aquaporins in salivary glands and pancreas. Biochim Biophys Acta 2014, 1840, 1524–32. doi: 10.1016/j.bbagen.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 6. Delporte C. Aquaporins in secretory glands and their role in Sjögren’s syndrome. Handb Exp Pharmacol 2009, 190, 185–201. doi: 10.1007/978-3-540-79885-9_9 [DOI] [PubMed] [Google Scholar]
- 7. Manganelli P, Fietta P.. Apoptosis and Sjögren syndrome. Semin Arthritis Rheum 2003, 33, 49–65. doi: 10.1053/sarh.2003.50019. [DOI] [PubMed] [Google Scholar]
- 8. Sisto M, Lorusso L, Ingravallo G, Nico B, Ribatti D, Ruggieri S, et al. Abnormal distribution of AQP4 in minor salivary glands of primary Sjögren’s syndrome patients. Autoimmunity 2017, 50, 202–10. doi: 10.1080/08916934.2017.1341495. [DOI] [PubMed] [Google Scholar]
- 9. Steinfeld S, Cogan E, King LS, Agre P, Kiss R, Delporte C.. Abnormal distribution of aquaporin-5 water channel protein in salivary glands from Sjögren’s syndrome patients. Lab Investig J Tech Methods Pathol 2001, 81, 143–8. [DOI] [PubMed] [Google Scholar]
- 10. Soyfoo MS, De Vriese C, Debaix H, Martin-Martinez MD, Mathieu C, Devuyst O, et al. Modified aquaporin 5 expression and distribution in submandibular glands from NOD mice displaying autoimmune exocrinopathy. Arthritis Rheum 2007, 56, 2566–74. doi: 10.1002/art.22826. [DOI] [PubMed] [Google Scholar]
- 11. Beroukas D, Hiscock J, Jonsson R, Waterman SA, Gordon TP.. Subcellular distribution of aquaporin 5 in salivary glands in primary Sjögren’s syndrome. Lancet 2001, 358, 1875–6. [DOI] [PubMed] [Google Scholar]
- 12. Pedersen AML, Bardow A, Nauntofte B.. Salivary changes and dental caries as potential oral markers of autoimmune salivary gland dysfunction in primary Sjogren’s syndrome. BMC Clin Pathol 2005, 5, 4. doi: 10.1186/1472-6890-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pringle SA, Berkhof B, van Ginkel M, Liefers S, van der Vegt B, Spijkervet FKL, et al. Parotid salivary sodium levels of Sjögren’s syndrome patients suggest B-cell mediated epithelial sodium channel disruption. Clin Exp Rheumatol 2021, 39, 30–8. [DOI] [PubMed] [Google Scholar]
- 14. Asashima H, Inokuma S, Onoda M, Oritsu M.. Cut-off levels of salivary beta2-microglobulin and sodium differentiating patients with Sjögren’s syndrome from those without it and healthy controls. Clin Exp Rheumatol 2013, 31, 699–703. [PubMed] [Google Scholar]
- 15. Burg MB, Ferraris JD, Dmitrieva NI.. Cellular response to hyperosmotic stresses. Physiol Rev 2007, 87, 1441–74. doi: 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- 16. Pedersen SF, Kapus A, Hoffmann EK.. Osmosensory mechanisms in cellular and systemic volume regulation. J Am Soc Nephrol 2011, 22, 1587–97. [DOI] [PubMed] [Google Scholar]
- 17. Shapiro L, Dinarello CA.. Osmotic regulation of cytokine synthesis in vitro. Proc Natl Acad Sci U S A 1995, 92, 12230–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang D, Wang C, Cao S, Ye Z, Deng B, Kijlstra A, et al. High-salt enhances the inflammatory response by retina pigment epithelium cells following lipopolysaccharide stimulation. Mediators Inflamm 2015, 2015, 197521. doi: 10.1155/2015/197521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yi B, Titze J, Rykova M, Feuerecker M, Vassilieva G, Nichiporuk I, et al. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res J Lab Clin Med 2015, 166, 103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013, 496, 518–22. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013, 496, 513–7. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manzel A, Muller DN, Hafler DA, Erdman SE, Linker RA, Kleinewietfeld M.. Role of « Western diet » in inflammatory autoimmune diseases. Curr Allergy Asthma Rep 2014, 14, 404. doi: 10.1007/s11882-013-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Azuma M, Tamatani T, Kasai Y, Sato M.. Immortalization of normal human salivary gland cells with duct-, myoepithelial-, acinar-, or squamous phenotype by transfection with SV40 ori- mutant deoxyribonucleic acid. Lab Investig J Tech Methods Pathol 1993, 69, 24–42. [PubMed] [Google Scholar]
- 24. Chivasso C, Nesverova V, Järvå M, Blanchard A, Rose KL, Öberg FK, et al. Unraveling human AQP5-PIP molecular interaction and effect on AQP5 salivary glands localization in SS patients. Cells 2021, 10, 2108. doi: 10.3390/cells10082108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three International Patient Cohorts. Arthritis Rheumatol 2017, 69, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dimitriou ID, Kapsogeorgou EK, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN.. Establishment of a convenient system for the long-term culture and study of non-neoplastic human salivary gland epithelial cells. Eur J Oral Sci 2002, 110, 21–30. doi: 10.1034/j.1600-0722.2002.00152.x. [DOI] [PubMed] [Google Scholar]
- 27. Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 2021, 49, D605–12. doi: 10.1093/nar/gkaa1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rivière E, Pascaud J, Tchitchek N, Boudaoud S, Paoletti A, Ly B, et al. Salivary gland epithelial cells from patients with Sjögren’s syndrome induce B-lymphocyte survival and activation. Ann Rheum Dis 2020, 79, 1468–77. doi: 10.1136/annrheumdis-2019-216588. [DOI] [PubMed] [Google Scholar]
- 29. Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol 2015, 15, 692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 30. Hernández-Molina G, Michel-Peregrina M, Hernández-Ramírez DF, Sánchez-Guerrero J, Llorente L.. Chemokine saliva levels in patients with primary Sjögren’s syndrome, associated Sjögren’s syndrome, pre-clinical Sjögren’s syndrome and systemic autoimmune diseases. Rheumatology 2011, 50, 1288–92. [DOI] [PubMed] [Google Scholar]
- 31. Hernández-Molina G, Ruiz-Quintero N, Lima G, Hernández-Ramírez D, Llorente-Chávez A, Saavedra-González V, et al. Chemokine tear levels in primary Sjögren’s syndrome and their relationship with symptoms. Int Ophthalmol 2022, 42, 2355–61. doi: 10.1007/s10792-022-02233-5. [DOI] [PubMed] [Google Scholar]
- 32. Szodoray P, Alex P, Jonsson MV, Knowlton N, Dozmorov I, Nakken B, et al. Distinct profiles of Sjögren’s syndrome patients with ectopic salivary gland germinal centers revealed by serum cytokines and BAFF. Clin Immunol 2005, 117, 168–76. [DOI] [PubMed] [Google Scholar]
- 33. Lee MG, Ohana E, Park HW, Yang D, Muallem S.. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev 2012, 92, 39–74. doi: 10.1152/physrev.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsuki-Fukushima M, Hashimoto S, Shimono M, Satoh K, Fujita-Yoshigaki J, Sugiya H.. Presence and localization of aquaporin-6 in rat parotid acinar cells. Cell Tissue Res 2008, 332, 73–80. doi: 10.1007/s00441-007-0558-4. [DOI] [PubMed] [Google Scholar]
- 35. Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, et al.; EMT International Association (TEMTIA). Guidelines and definitions for research on epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 2020, 21, 341–52. doi: 10.1038/s41580-020-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beroukas D, Goodfellow R, Hiscock J, Jonsson R, Gordon TP, Waterman SA.. Up-regulation of M3-muscarinic receptors in labial salivary gland acini in primary Sjögren’s syndrome. Lab Investig J Tech Methods Pathol 2002, 82, 203–10. [DOI] [PubMed] [Google Scholar]
- 37. Soyfoo MS, Chivasso C, Perret J, Delporte C.. Involvement of aquaporins in the pathogenesis, diagnosis and treatment of Sjögren’s syndrome. Int J Mol Sci 2018, 19, E3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aqrawi LA, Galtung HK, Guerreiro EM, Øvstebø R, Thiede B, Utheim TP, et al. Proteomic and histopathological characterisation of sicca subjects and primary Sjögren’s syndrome patients reveals promising tear, saliva and extracellular vesicle disease biomarkers. Arthritis Res Ther 2019, 21, 181. doi: 10.1186/s13075-019-1961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li DQ, Chen Z, Song XJ, Luo L, Pflugfelder SC.. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci 2004, 45, 4302–11. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- 40. Grauso M, Lan A, Andriamihaja M, Bouillaud F, Blachier F.. Hyperosmolar environment and intestinal epithelial cells: impact on mitochondrial oxygen consumption, proliferation, and barrier function in vitro. Sci Rep 2019, 9, 11360. doi: 10.1038/s41598-019-47851-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leehan KM, Pezant NP, Rasmussen A, Grundahl K, Moore JS, Radfar L, et al. Minor salivary gland fibrosis in Sjögren’s syndrome is elevated, associated with focus score and not solely a consequence of aging. Clin Exp Rheumatol 2018, 36, 80–8. [PMC free article] [PubMed] [Google Scholar]
- 42. Pérez P, Goicovich E, Alliende C, Aguilera S, Leyton C, Molina C, et al. Differential expression of matrix metalloproteinases in labial salivary glands of patients with primary Sjögren’s syndrome. Arthritis Rheum 2000, 43, 2807–17. doi:. [DOI] [PubMed] [Google Scholar]
- 43. Páez MC, González MJ, Serrano NC, Shoenfeld Y, Anaya JM.. Physiological and pathological implications of laminins: from the gene to the protein. Autoimmunity 2007, 40, 83–94. doi: 10.1080/08916930600911519. [DOI] [PubMed] [Google Scholar]
- 44. Bonnans C, Chou J, Werb Z.. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014, 15, 786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Delaleu N, Mydel P, Kwee I, Brun JG, Jonsson MV, Jonsson R.. High fidelity between saliva proteomics and the biologic state of salivary glands defines biomarker signatures for primary Sjögren’s syndrome. Arthritis Rheumatol 2015, 67, 1084–95. doi: 10.1002/art.39015. [DOI] [PubMed] [Google Scholar]
- 46. Delaleu N, Mydel P, Brun JG, Jonsson MV, Alimonti A, Jonsson R.. Sjögren’s syndrome patients with ectopic germinal centers present with a distinct salivary proteome. Rheumatology 2016, 55, 1127–37. doi: 10.1093/rheumatology/kew013. [DOI] [PubMed] [Google Scholar]
- 47. Sisto M, Lorusso L, Ingravallo G, Ribatti D, Lisi S.. TGFβ1-Smad canonical and -Erk noncanonical pathways participate in interleukin-17-induced epithelial-mesenchymal transition in Sjögren’s syndrome. Lab Investig J Tech Methods Pathol 2020, 100, 824–36. [DOI] [PubMed] [Google Scholar]
- 48. Fernández-Torres J, Pérez-Hernández N, Hernández-Molina G, Martínez-Nava GA, Garrido-Rodríguez D, López-Reyes A, et al. Risk of Wnt/β-catenin signalling pathway gene polymorphisms in primary Sjögren’s syndrome. Rheumatology 2020, 59, 418–25. [DOI] [PubMed] [Google Scholar]
- 49. Yin H, Pranzatelli TJF, French BN, Zhang N, Warner BM, Chiorini JA, et al.; NIDCD/NIDCR Genomics and Computational Biology CoreNIDCD/NIDCR Genomics and Computational Biology Core. Sclerosing sialadenitis is associated with salivary gland hypofunction and a unique gene expression profile in Sjögren’s syndrome. Front Immunol 2021, 12, 699722. doi: 10.3389/fimmu.2021.699722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Szyszko EA, Brokstad KA, Oijordsbakken G, Jonsson MV, Jonsson R, Skarstein K.. Salivary glands of primary Sjögren’s syndrome patients express factors vital for plasma cell survival. Arthritis Res Ther 2011, 13, R2. doi: 10.1186/ar3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Acauan MD, Figueiredo MAZ, Cherubini K, Gomes APN, Salum FG.. Radiotherapy-induced salivary dysfunction: structural changes, pathogenetic mechanisms and therapies. Arch Oral Biol 2015, 60, 1802–10. doi: 10.1016/j.archoralbio.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 52. Warner BM, Baer AN, Lipson EJ, Allen C, Hinrichs C, Rajan A, et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist 2019, 24, 1259–69. doi: 10.1634/theoncologist.2018-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Avouac J, Sordet C, Depinay C, Ardizonne M, Vacher-Lavenu MC, Sibilia J, et al. Systemic sclerosis-associated Sjögren’s syndrome and relationship to the limited cutaneous subtype: results of a prospective study of sicca syndrome in 133 consecutive patients. Arthritis Rheum 2006, 54, 2243–9. doi: 10.1002/art.21922. [DOI] [PubMed] [Google Scholar]
- 54. Soypaçacı Z, Gümüş ZZ, Çakaloğlu F, Özmen M, Solmaz D, Gücenmez S, et al. Role of the mTOR pathway in minor salivary gland changes in Sjogren’s syndrome and systemic sclerosis. Arthritis Res Ther 2018, 20, 170. doi: 10.1186/s13075-018-1662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li W, Chen Y, Sun ZP, Cai ZG, Li TT, Zhang L, et al. Clinicopathological characteristics of immunoglobulin G4-related sialadenitis. Arthritis Res Ther 2015, 17, 186. doi: 10.1186/s13075-015-0698-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Puxeddu I, Capecchi R, Carta F, Tavoni AG, Migliorini P, Puxeddu R.. Salivary gland pathology in IgG4-related disease: a comprehensive review. J Immunol Res 2018, 2018, 6936727. doi: 10.1155/2018/6936727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vissink A, Kalk WWI, Mansour K, Spijkervet FKL, Bootsma H, Roodenburg JLN, et al. Comparison of lacrimal and salivary gland involvement in Sjögren’s syndrome. Arch Otolaryngol Head Neck Surg 2003, 129, 966–71. doi: 10.1001/archotol.129.9.966. [DOI] [PubMed] [Google Scholar]
- 58. Pijpe J, Kalk WWI, Bootsma H, Spijkervet FKL, Kallenberg CGM, Vissink A.. Progression of salivary gland dysfunction in patients with Sjogren’s syndrome. Ann Rheum Dis 2007, 66, 107–12. doi: 10.1136/ard.2006.052647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mandel ID, Baurmash H.. Sialochemistry in Sjögren’s syndrome. Oral Surg Oral Med Oral Pathol 1976, 41, 182–7. doi: 10.1016/0030-4220(76)90229-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.