Abstract
Objective:
Prior to initial distribution of Glucose-6-phosphate dehydrogenase (G6PD) proficiency testing (PT) materials, we evaluated G6PD enzyme stability in dried blood spots (DBS) under various temperature and humidity environments to develop storage and usage guidelines for our new materials. Design&methods: We prepared fresh G6PD-normal DBS materials and conducted stability evaluations of daily use and short and long-term storage under various temperature and humidity environments.
Results:
G6PD DBS PT materials retained 92% of initial activity after 30 days of use at 4 °C. Materials stored at −20 °C and 4 °C with desiccant for 30 days retained 95% and 90% of initial activity, respectively. When stored for one year at −20 °C or six months at 4 °C specimens retained > 90% of initial activity. Specimens stored at 37 °C with desiccant lost 10% activity in three days. At the end of 30 days, specimens stored under ‘Extreme’—humidity> 50% without desiccant— conditions at 37 °C assayed below the NSQAP cut off for G6PD. Humidity exacerbated loss of enzyme activity with increasing temperature and time duration.
Conclusion:
Data suggest that G6PD PT materials can be stored at 4 °C and used for up to one month and can be stored at −20 °C for one year and yield > 90% enzyme activity. Exposure to warm temperatures, especially with elevated humidity, should be avoided. Desiccant should always be used to mitigate humidity effects.
Keywords: Glucose-6-phosphate dehydrogenase, Newborn screening, Dried blood spot, Proficiency testing, Stability
1. Introduction
1.1. Background
Glucose-6-phosphate dehydrogenase (G6PD) (EC 1.1.1.49) deficiency is an X-linked disease of the red blood cells that affects> 400 million people globally [1], and is most common in those of African, Asian and Middle-Eastern descent [2]. During the newborn period, G6PD defi ciency can cause jaundice. Severe forms can lead to kernicterus, an irreversible form of brain damage caused by bilirubin accumulation [3]. The disorder can also cause episodes of hemolytic anemia in patients after consumption of certain drugs or foods or following infection. It is estimated that G6PD deficiency occurs in 4–7% of the U.S. population [4], although in the African-American male population it may be as high as 10% [5]. In the United States (US), newborn screening (NBS) programs are administered at the state level without federal oversight. The US Department of Health and Human Services does, however, encourages all states to cover the disorders included its US Recommended Uniform Screening Panel (RUSP) [6]. At present, the RUSP consists of 34 conditions and, although it does not currently include G6PD, it is continually updated to include new disorders. G6PD is the most common enzyme defect in the world [1] and was recommended for routine screening by the World Health Organization (WHO) working group, in countries where incidence is> 3% in males [7].
The Newborn Screening Quality Assurance Program (NSQAP) at the Centers for Disease Control and Prevention (CDC) provides dried blood spot (DBS) proficiency testing (PT) materials to 534 domestic and international newborn screening (NBS) laboratories [8] three times each year. Our program assists laboratories in maintaining technical proficiency in the assays they perform and meeting certain accreditation requirements. In 2015 we prepared G6PD PT materials and began offering them to NBS laboratories for the first time. Prior to the initial distribution, we evaluated mean G6PD activity in a set of de-identified newborn DBS specimens and used it as the target value for our laboratory-prepared G6PD-normal specimens. We also conducted analyses on our laboratory-prepared DBS to determine G6PD enzyme stability and to evaluate optimal storage conditions to ensure high- quality PT specimens [9].
2. Materials and methods
2.1. Analysis of newborn specimens
To ascertain a typical range of G6PD activity in infants we obtained a random sampling of 144 de-identified newborn DBS samples from the Wisconsin State Laboratory of Hygiene. Newborn specimens were shipped from the birth hospital to the screening laboratory at ambient temperature. After initial newborn screening, they were stored at room temperature for one week before being stored at 4 °C for less than one year. Specimens were shipped to Centers for Disease Control and Prevention in Atlanta at ambient temperature and stored at −20 °C upon arrival. All specimens in this study were evaluated using a PerkinElmer Life and Analytical Sciences Neonatal G6PD kit.
2.2. Preparation of dried blood spots
Cord blood was selected for use throughout this study after preliminary experiments showed it to have consistently higher endogenous G6PD enzyme activity compared to adult blood. Twenty-one cord blood units were purchased from a cord blood bank (Cord: Use Cord Blood Bank, Orlando, FL) and screened for G6PD activity. Three cord blood units with highest normal G6PD activity were combined to prepare a single, G6PD-normal blood pool which was adjusted to a hematocrit of 50 ± 1%. An automated handheld pipette (Eppendorf) was used to dispense 75 |iL aliquots onto Grade 903 filter paper (GE Healthcare Biosciences). Blood spot cards were dried overnight, separated by weigh paper (www.fishersci.com) and packaged in low gas permeable zip closure bags (www.fishersci.com). Desiccant (www.polylam.com) and humidity indicator cards (www.desicare.com) were added before bags were sealed and stored at −20°C. A one-way random effects analysis of variance was used to determine between and among card variability to establish homogeneity. Twelve punches were analyzed from six different cards as described previously [10], to test G6PD activity across the entire pool.
2.3. Stability study design
2.3.1. Daily use study
We cycled G6PD-normal DBS specimens between 4 °C and room temperature (RT) to observe effects on enzyme activity. Thirty specimens were packaged into separate Mylar zip-seal bags (ULine, Pleasant Prairie, WI) with desiccant. For 30 days all bags were removed from storage at 4 °C and placed at RT for 45 min. Each day, one bag was then placed in storage at −70 °C while remaining bags were returned to 4 °C. At the end of the study, all samples were analyzed in triplicate.
2.3.2. Analysis of short-term temperature and humidity e ffects
We placed G6PD-normal DBS specimens in 14 storage environments and evaluated the change in enzyme activity over 30 days. Methods similar to previous studies were used [11]; briefly, eight groups containing 10 G6PD-normal DBS each were individually labeled and packaged in Mylar zip-seal bags to evaluate enzyme activity at low humidity (< 30%). Each group of 10 was stored for one month at −20°C, 4 °C, RT or 37 °C; with desiccant (Controlled) or without desiccant (Open). Six identical groups of 10dB were prepared with desiccant (Zipped) or without desiccant (Extreme) and were stored at the same temperatures (except −20 °C), but in plastic containers with damp paper towel to create a high humidity (> 50%) environment. Humidity was monitored using an indicator card (www.desicare.com). On the first day of the study, a reference specimen for each storage environment was stored at −70 °C. One specimen was transferred to −70 °C from each of the 14 storage environments at bi-weekly intervals. Fresh desiccant was added to bags that had none prior to storage at −70 °C. At the end of the study period each set of samples was analyzed in triplicate.
2.3.3. Analysis of long-term temperature effects
To evaluate long-term stability, we stored G6PD-normal DBS specimens under Controlled humidity conditions at four different temperatures for extended time periods. Four groups containing 14 DBS each were individually labeled and packaged with desiccant and humidity indicator cards, and stored at 37 °C, RT, 4°C and −20 °C for three month, six month and twelve month time periods. On the first day of the study and at regular intervals, one specimen from each storage environment was removed and stored at −70 °C. Each set was analyzed in duplicate at the end of the study period.
3. Results
3.1. Newborn specimen results
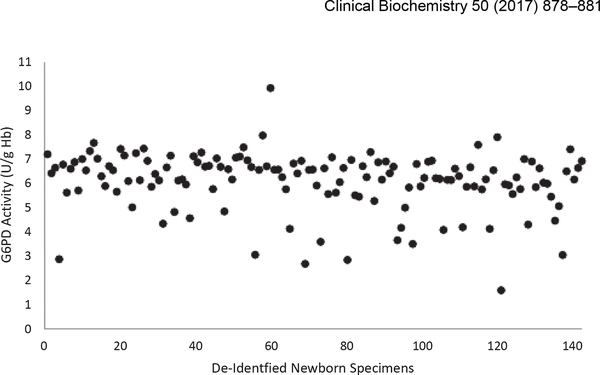
The mean G6PD enzyme activity of de-identified newborn DBS samples was 6.1 U/g Hb with a range of 2.7–9.9 U/g Hb; standard deviation was 1.2 U/g Hb. None of the de-identified newborn samples assayed below the NSQAP cut off of 2.6 U/g Hb (Fig. 1).
Fig. 1.
G6PD activity in sampling of de-identified newborn specimens mean G6PD activity was 6.1 U/g Hb.
3.2. DBS homogeneity results
Analysis of the pooled cord blood units yielded a homogenous pool with mean G6PD activity of 7.2 U/g Hb (95% confidence limits 7.0–7.5 U/g Hb). Within-card variance was 0.011 and among-card variance was 0.047.
3.3. Stability study design
3.3.1. Daily use study
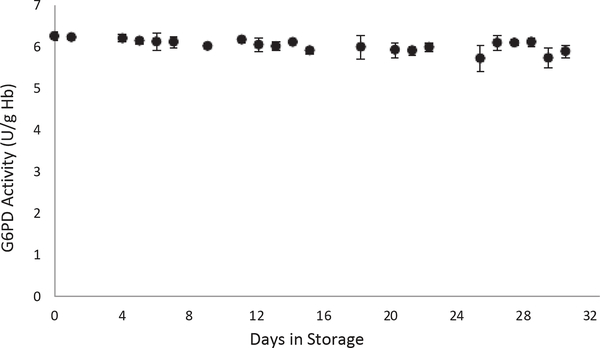
DBS specimens that were cycled between 4 °C storage and RT for 30 days lost an average of 2% activity after two weeks and 8% by day 30. Assayed values ranged between 5.7 and 6.2 U/g Hb (Fig. 2). Regression analysis yielded a slope of-0.0103.
Fig. 2.
G6PD Daily Use G6PD activity decreased by 8% after cycling DBS between 4 °C and RT for 30 days.
3.3.2. Analysis of short-term temperature and humidity effects
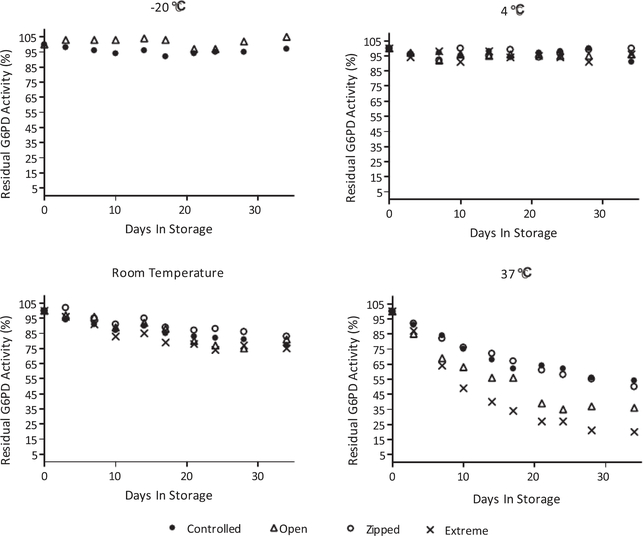
At the end of 30 days, specimens stored at −20 °C retained > 95% average G6PD activity. Those stored at 4 °C retained > 90% of initial G6PD activity at the end of 30 days. Regression analysis of specimens stored under Controlled conditions at −20 °C and 4 °C yielded slopes of −0.0058 and −0.0055, respectively. Specimens stored under Controlled conditions lost 16% G6PD activity after one week at 37 °C. Specimens stored under Controlled conditions at RT and 37 °C lost 23% (slope −0.0436) and 46% (slope −0.0903), respectively after 30 days. Two weeks into the study, specimens stored under Extreme conditions at 37 °C assayed at 2.4 U/g Hb; below the NSQAP cut off of 2.6 U/g Hb (Fig. 3).
Fig. 3.
Short-term stability analysis all specimens maintained > 95% initial G6PD activity under Controlled humidity conditions at −20 °C. Specimens stored at 4 °C under all humidity conditions retained > 90% G6PD activity. Specimens stored at RT under Extreme humidity conditions lost 25% activity. Specimens stored at 37 °C under Extreme humidity conditions lost 80% activity.
3.3.3. Analysis of long-term temperature effects
Specimens evaluated for long-term temperature effects were stored under Controlled conditions only. Specimens stored at −20 °C and 4 °C for three months retained>90% G6PD activity with slopes of −0.0022 and slope −0.0032, respectively; G6PD activity decreased by 30% (slope −0.0178) in specimens stored at RT for the same time interval. G6PD activity declined by 70% by the end of three months (slope −0.0472) when specimens were stored at 37 °C.
Specimens stored at −20°C for six months retained> 90% G6PD activity (slope 0.0006). Those stored at 4°C, RT and 37 °C lost 17% (slope −0.0005), 37% (slope −0.0107) and 80% (slope −0.0238) activity, respectively, during the same time interval.
G6PD activity remained > 90% (slope 0.0000) after 12 months of storage at −20 °C. G6PD activity decreased by 20% (slope −0.0033), 50% (slope −0.0072) and 87% (slope −0.0118) in specimens stored at 4 °C, RT and 37 °C, respectively, at the end of one year.
4. Discussion
The NSQAP continuously develops quality assurance materials for laboratories that perform newborn screening tests. DBS stability analysis is an integral step in this process to ensure the integrity of the materials we provide. Prior to the inaugural distribution of G6PD PT materials, we began analysis by evaluating a random sampling of newborn specimens for G6PD activity to ascertain a typical enzyme activity range in newborn DBS specimens. We used mean G6PD activity in these specimens as a reference value and selected cord blood units with similar G6PD-normal activity to prepare the blood pool used in this stability analysis.
The NSQAP routinely characterizes newly produced materials to ensure analyte homogeneity post-production. A homogenous blood pool ensures that study outcomes are not compromised due to preexisting variations in enzyme activity. We evaluated the effect of cycling G6PD PT materials between 4 °C and RT to mimic what occurs during in-house production, analysis and after distribution of the materials. Although the slope was negative, it was close to zero, reflecting the slight decline in G6PD activity over the study period, with a total loss of 8% activity. Since values remained within normal range, G6PD DBS materials can be stored at 4 °C and be used for 30 days.
DBS are routinely stored in zip-seal bags with desiccant during inhouse storage and transit. To evaluate the effects of temperature and humidity on G6PD activity we placed G6PD-normal specimens in 14 different storage environments and evaluated enzyme activity after 30 days. G6PD PT materials can be stored at −20 °C and 4 °C for 30 days under Controlled humidity conditions and still retain >95% and > 90% activity, respectively. They can also be stored at −20 °C for one S.R. Flores et al. year or 4 °C for three months under Controlled humidity conditions and retain>90% initial activity. Due to pronounced decline in enzyme activity by 30 days, specimens should not be stored at RT and exposure to warm environments should be avoided since as much as 10% activity was lost in the first three days at 37 °C even under Controlled humidity conditions. The largest losses in G6PD activity were observed with increasing temperature and this was exacerbated by humidity> 50%. Desiccant should always be used to optimize storage conditions.
In the U.S., most laboratories that report patient data are required to participate in an external PT program on a regular basis [12]; for many newborn screening laboratories, the NSQAP is the source of the materials to meet this requirement. The addition of G6PD PT materials continues NSQAP’s mission of assisting domestic and international newborn screening laboratories in maintaining technical proficiency and high-quality performance while meeting these accreditation guidelines. Because of the potentially devastating effects and high penetrance, newborn screening for G6PD deficiency routinely occurs in many countries where the disease is endemic; these include Israel [14], Saudi Arabia [2], Greece and many parts of Asia [13]. While G6PD deficiency has origins in the Middle East, Africa, Asia, and the Mediterranean [4], human migration has created the possibility for it to now exist in most areas of the world. Although two laboratories in the U.S. routinely screen for G6PD deficiency, prevalence in various ethnic groups in this country can range from 10 to 22% [13]. International NBS laboratories constitute 97% of recipients enrolled in the NSQAP G6PD PT program. While other G6PD PT programs are available [15], the NSQAP offers all of its DBS PT materials— including those for G6PD —to any screening laboratory that requests them, free of charge. This serves a major public health need by supporting laboratories who might otherwise be challenged in finding a source of PT materials on a regular basis. Determining the stability and appropriate storage conditions of these materials is imperative to assuring we continue to provide high-quality materials to laboratories that rely on them to support the vital public health work of newborn screening.
Acknowledgements
We thank the Wisconsin State Laboratory of Hygiene for providing de-identified newborn DBS specimens.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention, or the authors’ affiliated institutions. Use of trade names is for identification only and does not imply endorsement.
Abbreviations:
- G6PD
glucose-6-phosphate dehydrogenase
- DBS
dried blood spot
- CDC
Centers for Disease Control and Prevention
- NSQAP
Newborn Screening Quality Assurance Program
- PT
proficiency testing
- RUSP
United States Recommended Uniform Screening Panel
References
- [1].Cappellini MD, Fiorelli G, Glucose-6-phosphate dehydrogenase de ficiency, Lancet 371 (9606)(2008) 64–74. [DOI] [PubMed] [Google Scholar]
- [2].Mohamed S, Newborn screening for glucose-6-phosphate dehydrogenase deficiency in Eastern Province, SaudiArabia, Curr. Pediatr. Res 16 (2) (2012) 125–128. [Google Scholar]
- [3].Leong A, Is there a need for neonatal screening of glucose-6-phosphate dehydrogenase deficiency in Canada? McGill J. Med 10 (1) (2007) 31–34. [PMC free article] [PubMed] [Google Scholar]
- [4].Watchko JF, et al. , Should we screen newborns for glucose-6-phosphate dehydrogenase deficiency in the United States? J. Perinatol 33 (7) (2013) 499–504. [DOI] [PubMed] [Google Scholar]
- [5].FRANK JE, Diagnosis and management of G6PD deficiency, Am. Fam. Physician 72 (7) (2005) 1277–1282. [PubMed] [Google Scholar]
- [6].Sebelius K, Letter from the Secretary of Health and Human Services. http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendations/correspondence/uniformpanelsecre052110.pdf, (2010) (accessed September 06, 2016).
- [7].Working WG, Glucose-6-phosphate dehydrogenase deficiency, Bull. World Health Organ 67 (6) (1989) 601–611. [PMC free article] [PubMed] [Google Scholar]
- [8].Zobel S, Newborn Screening Quality Assurance Program Annual Summary Report, 33b (2016), p. 5.
- [9].De Jesus VR , et al., The newborn screening quality assurance program at the Centers for Disease Control and Prevention: thirty-five year experience assuring newborn screening laboratory quality, Int. J. Neonatal Screen 1 (1) (2015) 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Adam BW, et al. , Galactose-1-phosphate uridyltransferase dried blood spot quality control materials for newborn screening tests, Clin. Biochem 48 (6) (2015) 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Adam BW, Chafin DL, De Jesus VR, Stabilities of hemoglobins A and S in dried blood spots stored under controlled conditions, Clin. Biochem 46 (12) (2013) 1089–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Clinical Laboratory Improvement Amendments, https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/index.html, (2016) (accessed September 6, 2016).
- [13].Kaplan M, Hammerman C, The need for neonatal glucose-6-phosphate dehydro genase screening: a global perspective, J. Perinatol 29 (Suppl. 1) (2009) S46–S52. [DOI] [PubMed] [Google Scholar]
- [14].Algur N, et al. , Quantitative neonatal glucose-6-phosphate dehydrogenase screen ing: distribution, reference values, and classification by phenotype, J. Pediatr 161 (2)(2012) 197–200. [DOI] [PubMed] [Google Scholar]
- [15].Chiang SH, F.M., Hsiao KJ, External quality assurance programme for newborn screening of glucose-6-phosphate dehydrogenase deficiency, Ann. Acad. Med 37 (12)(2008) 84–87. [PubMed] [Google Scholar]