Abstract
Organisms determine the transcription rates of thousands of genes through a few modes of regulation that recur across the genome1. These modes interact with a changing cellular environment to yield highly dynamic expression patterns2. In bacteria, the relationship between a gene’s regulatory architecture and its expression is well understood for individual model gene circuits3,4. However, a broader perspective of these dynamics at the genome-scale is lacking, in part because bacterial transcriptomics have hitherto captured only a static snapshot of expression averaged across millions of cells5. As a result, the full diversity of gene expression dynamics and their relation to regulatory architecture remains unknown. Here we present a novel genome-wide classification of regulatory modes based on each gene’s transcriptional response to its own replication, which we term the Transcription-Replication Interaction Profile (TRIP). We found that the response to the universal perturbation of chromosomal replication integrates biological regulatory factors with biophysical molecular events on the chromosome to reveal a gene’s local regulatory context. While the TRIPs of many genes conform to a gene dosage-dependent pattern, others diverge in distinct ways, including altered timing or amplitude of expression, and this is shaped by factors such as intra-operon position, repression state, or presence on mobile genetic elements. Our transcriptome analysis also simultaneously captures global properties, such as the rates of replication and transcription, as well as the nestedness of replication patterns. This work challenges previous notions of the drivers of expression heterogeneity within a population of cells, and unearths a previously unseen world of gene transcription dynamics.
Our ability to understand and manipulate bacteria, from design of synthetic regulatory circuits6 to determining how bacterial pathogens establish and maintain infection in their hosts, demands a sophisticated understanding of gene regulatory processes. Bacterial gene regulation occurs primarily at the level of transcription7, but while decades of research has produced a wealth of knowledge about RNA polymerase and its interactions with promoters, repressors, and activators of transcription, this work is primarily based on measurements averaged across a population of millions of cells. Therefore, much is still unclear about how transcription takes place in individual cells in the context of a constantly changing cellular environment2. In rapidly proliferating cells, transcription occurs on a chromosome that is under continuous replication8,9. However, although there has been some exploration of the effects of replication on individual genes10,11, the transcriptomes-wide consequences of this perturbation are unknown12,13. Measuring global gene expression during the replication cycle has traditionally been hampered by the requirement for analysis of synchronized populations at a bulk level, limiting this analysis to organisms such as Caulobacter crescentus14–16 where natural biological features facilitate synchronization, or to populations synchronized by batch synchronization methods such as starvation17 or temperature shift18 that may be both of questionable efficacy and liable to introduce artefacts19.
Here we combined state-of-the-art bacterial single cell RNA sequencing (scRNA-seq)20–23 with a new cell cycle analysis framework to reveal extensive transcriptional variation during the cell cycle in two unrelated species – the model organism and Gram-negative rod Eschericha coli (E. coli), and the Gram-positive coccus Staphylococcus aureus (S. aureus), both major bacterial pathogens. We identified first a global replication-dependent pattern that depends on a gene’s chromosomal location, then developed a predictive computational analysis framework to reveal diverse types of divergence from this pattern. In E. coli, we found an effect of a gene’s position within its operon on expression dynamics that is largely absent in S. aureus. Other genes diverged from the expected pattern in both amplitude and timing of their expression in ways that are sensitive to gene-specific factors such as repression state. Therefore, while DNA replication introduces a universal perturbation, how individual genes respond to this perturbation depends on their local regulatory context, providing a new lens through which to understand the behavior of genes at their native loci.
Global gene expression in proliferating bacterial populations is shaped by chromosomal organization.
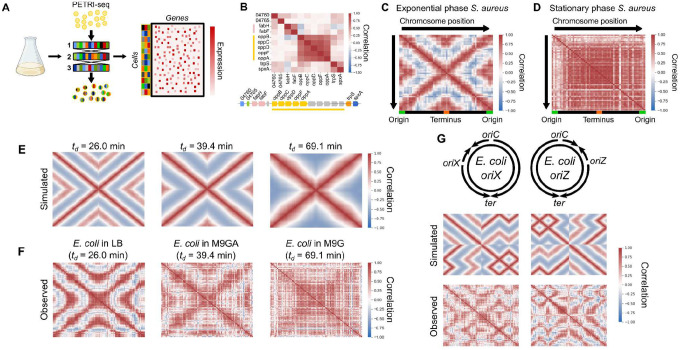
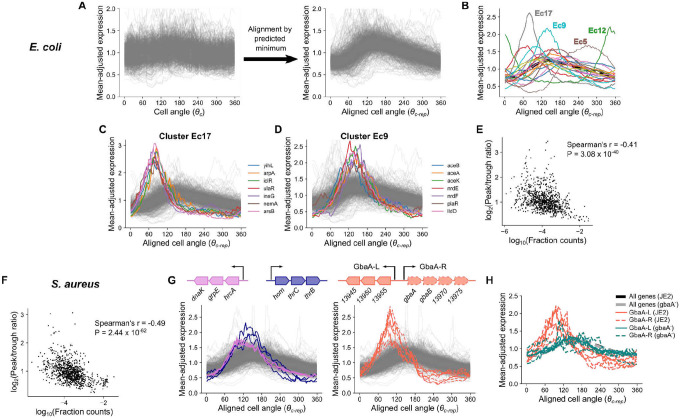
To investigate transcriptional heterogeneity in proliferating bacterial populations, we applied a recently-described scRNA-seq method, PETRI-seq20, to 73,053 individual S. aureus cells in exponential phase (Fig. 1A). S. aureus is an important human pathogen, yet little is known about heterogeneous gene expression dynamics within its populations. We detected on average 135 transcripts per cell (Fig. S1A), an increase on the 43 transcripts per cell previously published for this species with this method20. As the data are very sparse, we denoised them using the single-cell variational inference (scVI) method, an unsupervised deep learning approach24. Studying gene-gene correlations, we recovered the expected covariance of genes within operons (Fig. 1B). However, when we investigated gene-gene correlations on a genomic scale, we discovered a striking ‘X-shaped’ pattern of gene expression covariance (Fig. 1C, Fig. S2A). The central ‘X’ of this pattern reflects symmetry around the origin of replication, meaning that genes equidistant from the origin on each side of the chromosome correlate with each other. Beyond the ‘X’ itself, however, we observed an additional correlation directly between genes at the origin and terminus (Fig. 1C). This pattern was strengthened by averaging expression into 50 kb bins by chromosome position (Fig. 1C), and was reproducible in a second independent dataset under the same conditions of 21,257 cells (Fig. S2C). It was detectable even without the use of scVI, although the signal was noisier (Fig. S2B). The pattern was abolished when we studied 55,894 cells in stationary phase, suggesting that it is a property of proliferating cells (Fig. 1D).
Figure 1: scRNA-seq reveals a global pattern of replication-associated gene covariance.
A) PETRI-seq workflow20. Bacterial cells were fixed and permeabilized, then subjected to three rounds of cDNA barcoding to give transcripts of each cell a unique barcode combination. This method is highly scalable to multiple samples and tens of thousands of cells. B) Local operon structure is captured by gene-gene correlations (Spearman’s r). Operons are indicated by shared colors of genes. Gray genes indicate those removed by low-count filtering. Names of SAUSA300_RS04760 and SAUSA300_RS04765 are truncated. C & D) Global gene-gene correlations reflect chromosomal position in (C) exponential phase and (D) stationary phase S. aureus. Spearman correlations were calculated based on scVI-smoothed expression averaged in 50 kb bins by chromosome position. E) Simulated correlation patterns in unsynchronized E. coli populations at three different growth rates. F) Spearman correlations between scaled data averaged into 50 kb bins, as for (C) but for E. coli grown at three growth rates. G) Introducing ectopic origins of replication in E. coli leads to predictable perturbations in gene expression heterogeneity. Top: schematic of predicted replication patterns based on previous studies25–27. Middle: Predicted correlation patterns based on the copy number simulation. Bottom: Real correlation patterns in oriX and oriZ mutant strains, as in (C). Heatmaps of correlations without chromosome position-dependent binning are shown in Fig. S2D.
As we observed correlations among genes that are equidistant from the origin of replication and cells in stationary phase did not show such correlations, we hypothesized that the ‘X-shaped’ pattern reflects the effect of DNA replication on gene expression. In the model organism E. coli, replication patterns are growth rate-dependent: at high rates of proliferation, overlapping cycles of replication occur simultaneously, whereas at slower proliferation rates one round of replication is completed before the next one begins8,28. This arises because the ‘C-period’, the time for one complete round of replication from the origin to the terminus, remains approximately constant and can be greater than the doubling time8,28. The effect of replication on gene expression covariance should reflect this. To test this, we therefore measured the doubling times (td) of E. coli grown at 37 °C in three medium conditions (Fig. S3A): LB (26.0 ± 1.3 min), M9 minimal medium with glucose and amino acids (M9GA, 39.4 ± 2.3 min), and M9 medium with glucose only (M9G, 69.1 ± 9.8 min). We next developed a simulation to predict correlation patterns arising from gene dosage in cells proliferating with these doubling times (Fig. 1E & Fig. S4). At an intermediate growth rate (td = 39.4 min), we predicted a correlation pattern similar to that observed for S. aureus (Fig. 1C). However, simulating faster growth produced a nested “multi-X” pattern resulting from overlapping cycles of replication, and slower growth greatly reduced origin-terminus correlations (Fig. 1E).
When we compared these predictions to the observed data for E. coli grown under the three conditions, we observed a close correspondence between simulated and observed expression patterns (Fig. 1F). Correlations became less defined at slower growth rates, although this may reflect technical noise due to lower transcript counts (Fig. S1B), resulting from lower RNA content at slower growth rates29. The correlation pattern of E. coli grown in M9G, the slow-growth condition, further resembled bulk RNA-seq of synchronized C. crescentus (Fig. S4C)15, a species that undergoes a single round of replication prior to asymmetric division14, which is a similar situation to that of slower-growing E. coli. Next, we reasoned that if this pattern is driven by the effect of gene copy number on expression levels (as assumed in our simulation), we also expect to find a relationship between origin distance and expression levels. Indeed, despite high variation in intrinsic promoter activity, we found that on average gene expression decreased with distance from the origin, and this effect was stronger at faster growth rates30 (Fig. S5). Finally, while these patterns could theoretically arise due to reads from contaminating genomic DNA, multiple lines of evidence from the data (Fig. S6), as well as our observation of the X-shaped pattern in a previously published dataset of bulk RNA from synchronized C. crescentus15 (Fig. S4C), demonstrate that this is very unlikely to be the case and support our interpretation that the observed patterns are driven by the effect of DNA replication on mRNA abundance.
To further test our ability to predict global correlations from expected replication patterns, we examined strains in which normal replication is perturbed. We compared wild-type E. coli grown in LB to two strains with ectopic origins of replication at either 9 o’clock (oriX) or 3 o’clock (oriZ) positions in addition to oriC25–27. In these strains, replication initiates simultaneously at both native and inserted origins, while ending at the same terminus, ter25. Our simulation predicted perturbed correlation patterns that were almost mirror images of each other, given that the ectopic origins of the mutants we chose were nearly equidistant from oriC on each side of the chromosome (Fig. 2G). Again, we found that the observed patterns matched closely with our predictions (Fig. 2G). These results support the notion that DNA replication kinetics produce a predictable effect on transcriptional heterogeneity within a population of proliferating bacteria, and that this effect is sensitive to growth rate and genetic perturbations.
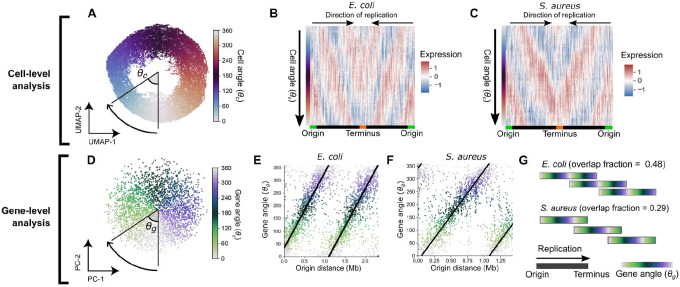
Figure 2: Ordering expression by cell angle and gene angle provides a quantitative description of cell cycle gene expression.
A) UMAP of LB-grown E. coli with expression averaged in 100 kb bins by chromosome position. Cell angle θc is the angle between UMAP dimensions relative to the center. For UMAP without averaging, see Fig. S7A. B & C) Heatmap of scaled gene expression in E. coli (B) or S. aureus (C) averaged in 100 bins by θc. D) Derivation of gene angle θg in LB-grown E. coli. Principal component analysis was performed on the transpose of the matrix in (B), and θg was defined as the angle between principal components (PCs) 1 and 2. Genes form a wheel in UMAP (Fig. S7C). E & F) The relationship between θg and origin distance for E. coli grown in LB (E) and S. aureus grown in TSB (F). G) Predicted replication patterns in LB-grown E. coli (td = 26.0 ± 1.3 min) and S. aureus (td = 24.9 ± 0.6 min). Overlapping rounds of replication lead to shared θg in simultaneously-replicated chromosomal regions. Note that greater overlap in replication rounds is observed for E. coli than for S. aureus.
The effect of chromosomal replication on transcription facilitates resolution of bacterial gene expression by cellular replication state.
Since DNA replication exerts a strong influence over gene expression, we reasoned that this effect can be used to resolve a cell’s position within the replication cycle given only its transcriptome. To examine the distribution of cellular states in a population of cells, we projected gene expression measurements of LB-grown E. coli cells in two dimensions by uniform manifold approximation and projection (UMAP31). Cells arranged into a “wheel” shape (Fig. 2A) when we performed UMAP on expression averaged by chromosomal position (which was found to strengthen global correlation patterns, Fig. 1C). To determine the order of cells along this wheel, we calculated cells’ angle θc between UMAP coordinates (Fig. 2A). Examining gene expression as a function of θc, we observed waves of gene expression progressing from the origin to the terminus (Fig. 2B), suggesting that cells’ positions on this wheel reveal their replication state. Performing equivalent analysis to resolve replication states in S. aureus, we observed a similar pattern (Fig. 2C, Fig. S7B). These data suggest that we can infer a cell’s replication state from the transcriptome alone, and that this holds across different bacterial species.
As we observed that the expression of most genes is strongly influenced by a cell’s replication state, we reasoned that we should also be able to order genes by their timing of expression within the cell cycle and that this would generally reflect their order of replication. To do this, we projected the genes themselves into two dimensions to derive a gene angle, θg (Fig. 2D). We observed a close relationship between the order of genes by θg and the distance from the origin of replication in both E. coli and S. aureus (Fig. 2E & F), suggesting that θg does indeed capture the order of replication. However, we also observed that the period of θg (i.e. the chromosomal distance associated with a 360° rotation) was less than the full origin-terminus distance, meaning that genes at multiple positions on the origin-terminus axis had the same θg value. We can interpret this to mean that at high growth rates, overlapping rounds of replication lead to simultaneous replication of genes at multiple distances from the origin. Furthermore, we observed that in E. coli, the gradient of change of θg with respect to origin distance decreased with slowing growth rate (Fig. S7D & F). We can use this gradient to infer two parameters about the replication pattern. Firstly, this gradient provides an estimate of the average DNA polymerase speed. For E. coli in LB, this estimate was 780 bp/s (Fig. S7F), very close to previously reported values of ~800 bp/s32,33. Secondly, the gradient can also be used to estimate an “overlap fraction” (Fig. 2G), the fraction of one round of replication happening before the previous one has finished. When we compared E. coli at different growth rates, we observed that, in line with expectations8,28, decreasing proliferation speed in E. coli is associated with reduced overlap in rounds of replication (Fig. S7E), while the average DNA polymerase speed (and hence the C-period) remains roughly consistent (Fig. 7F). In S. aureus, the reduced size of its genome (2.9 Mb vs 4.6 Mb in E. coli) explains why, despite similar proliferation rates and DNA polymerase speeds (Fig. S7F), less overlap in rounds of replication is observed than E. coli (Fig. 2G). Therefore, the gene angle θg and its relationship to distance from the replication origin provide a quantitative and interpretable description of the relationship between gene expression and global replication patterns.
Finally, the two parameters we introduce here – the cell angle θc and the gene angle θg (Fig. S7 G & H) – led us to construct an inference model to predict the expression of a given gene (by θg) at a given point in the cell cycle (by θc), based on global replication-dependent trends (Fig. S8). Thus based on a given pattern of gene expression, the model infers the state of the cell along the cell cycle; conversely, for a particular cell cycle state, the model infers an expected gene expression pattern based solely on a gene’s distance from the origin (and hence replication timing). Overall, we found a moderate correlation of this prediction with the observed data (Pearson’s r = 0.59, Fig. S9A), and subtraction of this prediction from the observed data eliminated the global correlation pattern (Fig. S9B), confirming that our model effectively captured position-dependent gene expression trends.
The global consensus pattern of gene expression reflects a replication-dependent gene dosage effect.
We next sought to confirm that the transcriptional dynamics we inferred from the scRNA-seq data represent cell cycle-dependent gene expression. To do this, we first identified three operons whose genes’ expression closely fits the model-predicted pattern (Fig. 3A), then compared our measurements for genes within the selected operons to cell cycle-dependent gene expression measurements obtained using single molecule fluorescence in situ hybridization (smFISH)10,34. Overall, population-averaged expression measurements from the two methods were in close quantitative agreement (Fig. S10D). The smFISH approach resolves cell cycle by using cell length to infer cell age, thus defining the cell cycle relative to division timing10. By contrast, we defined cell angle θc = 0 to be the assumed time of replication initiation (see Materials & Methods). As expected given these differing “start” points, we observed a phase shift in expression profiles between the two methods that was consistent across genes (Fig. S10E). Modeling of total DNA content as a function of cell length supported that this phase shift was roughly consistent with our choice of θc = 0 as the point of replication initiation (Fig. S10F), albeit with some discrepancy (see Materials & Methods).
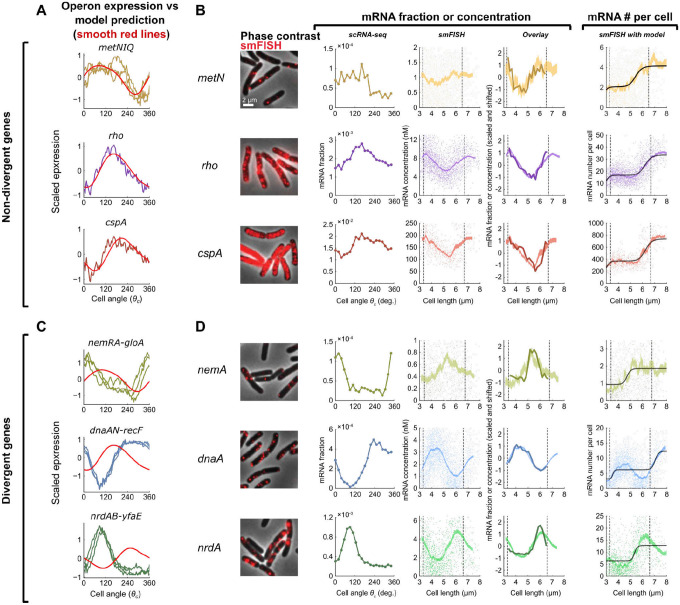
Figure 3: Genes show a spectrum of divergence from a dosage-driven consensus pattern.
A) Expression of genes in operons that conform to the consensus pattern across 100 bins averaged by θc. Expression is z-scores derived from scVI (jagged lines) or predicted as a replication effect (smooth, red lines). B) Comparison of scRNA-seq and smFISH data for genes within non-divergent operons. From left to right: 1) Microscopy images of E. coli cells labeled using smFISH against the indicated gene (cspA is visualized with alternative contrast; for negative control see Fig. S10A); 2) scRNA-seq expression shown as fraction of total cellular mRNA (expression is averaged in 100 bins by θc); 3) mRNA concentration, measured using smFISH, as a function of cell length. Single-cell data (scatter plot) was binned by cell length (shaded curve, moving average ± SEM, 10% sample size per bin). Dashed lines indicate the twofold length range where most cells reside, used to infer the mean values at birth and division; 4) Alignment of scaled data from smFISH and scRNA-seq measurements; 5) Absolute mRNA copy number, measured using smFISH, as a function of cell length. Single-cell data was processed as in column 3 (5% sample size per bin). Black line, fit to a sum of two Hill functions, corresponding to two gene replication rounds. C) Expression of divergent genes compared to model predictions (as in (A)). D) Comparison of scRNA-seq and smFISH as in (B) but for divergent genes. See Material and Methods for further details.
By correcting for this phase shift between methods, we aligned the scRNA-seq profile to that of the smFISH data (Fig. 3B). In doing so, we observed that expression dynamics inferred by the two methods were highly correlated, confirming that our scRNA-seq approach captures cell cycle-dependent expression. Moreover, while our scRNA-seq measurements capture only relative expression of a gene among total cellular mRNA, our smFISH experiments additionally provide us absolute abundance. This revealed a discrete twofold stepwise increase in expression (Fig. 3B), consistent with genes that are sensitive to gene dosage but otherwise exhibit constant expression10. These observations support an interpretation that the model-predicted pattern corresponds to cell cycle expression variation driven by gene dosage.
Genes that diverge from the global consensus pattern exhibit gene dosage-independent features.
While many genes conform to this gene dosage-driven expression pattern, others differ from it in a variety of ways. To identify genes that diverged from the expected pattern, we used the predictive model developed above to derive a score for divergence, which we found to be correlated between replicates for genes that showed high variance across the cell cycle (Pearson’s r = 0.80, Fig. S9D). We then focused on three operons whose genes strongly diverged from the expected pattern, two of which were involved in replication initiation and elongation (dnaAN-recF and nrdAB-yfaE, respectively) and one involved in the response to reactive electrophilic species (nemRA-gloA)35–37. Divergent genes within the same operon showed highly similar expression profiles (Fig. 3A & C), but showed reproducible patterns that differed markedly from predictions (Fig. 3C), while also closely aligning with smFISH measurements (Fig. 3D, Fig. S11). Moreover, both scRNA-seq and smFISH showed that the amplitude of cell cycle expression (i.e. the relative change between cell cycle minimum and maximum expression) was higher for these divergent genes than the non-divergent ones (Fig. S10G). Finally, absolute mRNA copy number measurement demonstrated that unlike the non-divergent genes, dnaA and nrdA do not conform to a dosage-related step function (Fig. 3D). Taken together, therefore, we observe that genes diverging from the predicted global pattern do so in both shape and timing of expression profile, as well as amplitude, suggesting that additional factors beyond gene dosage drive their expression dynamics. This motivated us to investigate further the factors shaping the divergences in each species.
The location of genes within operons influences cell cycle expression dynamics in E. coli.
We first sought to determine what contributes to differential timing of expression profiles among divergent genes. In E. coli, we observed the systematic bias that the majority of divergent genes showed delayed expression dynamics relative to predictions (θg is more “clockwise” than θg-pred, Fig. 4A). Many of these genes were encoded in large operons, such as those involved in energy biogenesis (e.g. nuo and atp operons) and cell surface synthesis (e.g. the mraZ-ftsZ operon). We found that genes with a more distal position within these operons exhibited a greater delay (Fig. 4B, Fig. S13A). Moreover, this delay was relative to the timing of replication: in genes whose replication-predicted pattern changed in the oriZ mutant, expression shifted in this strain so that the delay was relative to this new replication time (Fig. 4B). Across all genes, we observed a modest but highly significant correlation between this “angle difference” and distance from the transcriptional start site (TSS) (Fig. 4C). We hypothesized that this delayed phenotype arises due to the time for RNA polymerase (RNAP) to reach genes after replication by DNA polymerase (DNAP) has occurred. The speed of RNAP has previously been estimated as 40 nt/s10,38, much slower than the ~800 nt/s speed for DNAP (32,33 and Fig. S7F). By performing linear regression to measure the angle difference/transcriptional distance relationship (Fig. 4C) and converting θg into time by assuming that 360° is equivalent to one doubling time of 26 min, we infer that distance from the TSS is associated with a delay that is consistent an with average RNAP speed of 32 nt/s (38 nt/s in a second replicate, Fig. 13C). Therefore, our data support the hypothesis that when a gene is replicated, the time for expression to increase to the higher-expressed state (due to higher gene dosage) correlates with the time for RNAP to reach that same gene after transcription from the replicated locus restarts.
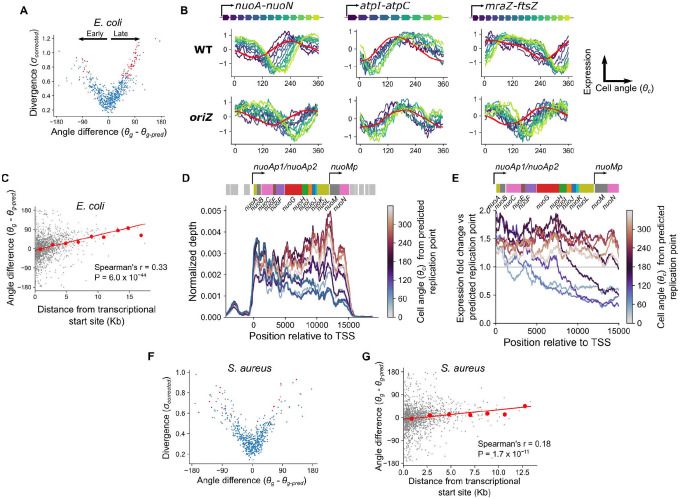
Figure 4: A gene’s position within its operon produces a characteristic delay in expression dynamics in E. coli but not S. aureus.
A) Plot of divergence from predictions against the difference between predicted and observed angles in E. coli, with divergent genes in red. Angle difference therefore represents whether a gene is expressed earlier or later than expected, as indicated by the black arrows. B) Cell cycle expression plots for operons showing “delayed” genes as in Fig. 3A & C but colored by position within the operon. Model-predicted expression is represented in red. Shown for WT and the oriZ mutant. C) Plot of maximum distance from a transcriptional start site against difference between predicted and observed angles in E. coli. Red line indicates the linear model fit and red points indicate averages of 2 kb bins. D) Normalized per-base read depth at the nuo operon locus for cells averaged in 10 bins by cell angle, θc. Traces are smoothed by a 1 kb centered rolling mean and colored by mean cell angle relative to the predicted timing of gene replication (see Materials & Methods). The nuo operon structure is indicated by the schematic above, with the surrounding genes in grey. E) Per-base read depth as shown in (D) for the nuo operon, but with expression shown as fold-change relative to expression at the predicted time of gene replication. F) Plot of divergence from predictions against the difference between predicted and observed angles, as in (A) but for S. aureus. G) Plot of maximum distance from a transcriptional start site against difference between predicted and observed angles, as in (B) but for S. aureus.
To further understand the nature of this transcriptional distance effect, we focused on a single operon encoding the NADH dehydrogenase I complex (nuo). We observed a delayed effect that increased with distance from the major TSS for this operon, similar to the delay recently observed for this operon in response to transcription initiation inhibition by rifampicin7 (Fig. 4B). Additionally, however, where coverage of genes close to the TSS increase in expression immediately after the predicted time of gene replication, coverage at the distal end of the operon dropped sharply before recovering to a higher level (Fig. 4D & E). A similar drop was observable for genes far from the TSS in the mraZ-ftsZ operon (Fig. S13B). A potential mechanistic explanation for this is as follows: since passage of the replication fork leads to local disruption of ongoing transcription39, genes at the distal end of a transcript are more likely to experience disruption before their transcription can be completed, and there will be a longer delay before new transcription of these genes resumes after replication. This in turn would lead to a post-replication drop in expression of genes far from the TSS, compared to an immediate rise in genes close to it. In turn, this would lead to higher amplitude of expression (maximum vs minimum expression) within the cell cycle for genes far from their TSS. Consistent with this, we observed a weak but significant correlation in E. coli between genes’ distance from their TSS and their amplitude of expression (Spearman’s r = 0.16, P = 2.3 × 10−10) (Fig. S13D). We note that many long operons in E. coli (e.g. the nuo and mraZ-ftsZ operons described here, Fig. 4D, Fig. S13B, and40) contain internal promoters, and we suggest that these may contribute to expression by buffering the effects of replication-associated abortive transcription in long operons.
Finally, we asked whether similar trends could be observed in S. aureus. In contrast to E. coli, we did not observe an excess of “delayed” genes among the divergent genes (Fig. 4F). Moreover, the relationship between operon position and the difference between observed and predicted gene angles was weaker in this species (Fig. 4G), with no observable effect of distance from the TSS on expression amplitude (Spearman’s r = 0.01, P = 0.73) (Fig. S13D). From the gradient of this relationship, we predicted that distance from the TSS introduces a delay of 64 nt/s (92 nt/s and 59 nt/s in additional replicates, Fig. S13C). These differences between species persisted even when operons were redefined according to simpler criteria (tandemly arrayed genes with intergenic distance less than 40 bp41, Fig. S13E). One potential explanation for this is that if the RNAP processivity rate were faster in S. aureus than in E. coli, the delay before it reached genes at the distal end of operons would be far less pronounced. In keeping with this, experimental measurement of RNAP by a reporter system in Bacillus subtilis, like S. aureus a firmicute of the order Bacillales, suggested that it was substantially faster (75–80 nt/s) than its counterpart in E. coli measured by the same method (~48 nt/s)42,43. Therefore, the interplay between DNAP and RNAP processivity may lead to species-specific effects of operon position on cell cycle expression dynamics.
Repressed genes exhibit higher amplitude pulses in cell cycle gene expression.
Although the position of genes within operons explains the delayed expression pattern observed in E. coli, it can not explain divergent patterns for many other genes in both E. coli and S. aureus. Therefore, we investigated more closely the shape of cell cycle expression curves for those genes that had reproducible dynamics across replicates (Fig. S14B). To compare genes at different chromosomal loci, we introduced an alignment procedure whereby time is represented as progression by cell angle relative to a gene’s predicted replication time, θc-rep (Fig. 5A). Most genes rise rapidly (presumably due to a doubling of gene dosage) before declining as a relative fraction of the transcriptome. Many genes, however, exhibited patterns that could not be explained by gene dosage effects alone.
Figure 5: Repression is associated with higher amplitude in cell cycle gene expression.
A) Procedure to align expression profiles of different genes. Smoothed expression for each gene normalized by division by its mean (left) is standardized by rotating cell angle so the predicted replication time expression is at zero. We term this aligned cell angle progression metric θc-rep. See Materials & Methods. B) Average aligned expression profiles for 20 k-means clusters in E. coli. The dotted black line represents average expression across all reproducible genes. C & D) Plots of individual genes from clusters in (B). E & F) Comparison of average expression to the log-ratio of peak to trough expression in E. coli (E) and S. aureus (F). G) Aligned expression profiles for select operons in clusters Sa11 and Sa18, with operon structure shown. H) Aligned expression profiles for GbaA regulon genes in JE2 and a gbaA− transposon mutant. Thick black and gray lines represent average expression across all reproducible genes.
To identify the range of behaviors, we partitioned E. coli genes into 20 clusters based on the aligned dynamics (Fig. 5B). Of these, several exhibited particularly divergent expression, differing from the expected pattern in both the timing of expression dynamics and the amplitude (i.e. the relative difference between maximal and minimal cell cycle expression). Cluster E. coli (Ec) 12 comprised the nrdAB-yfaE operon and cluster Ec5 contained the dnaAN-recF operon and other delayed expression genes, including some nuo genes. Cluster Ec17 showed an early-peaking pulse in expression with greater amplitude than most genes (Fig. 5C). Many genes in these clusters were in operons that encode repressors, at least some of which have autorepressive activity (including nemA, which is co-transcribed with the autorepressor nemR) (Table S4). Cluster Ec9, whose members peak at the expected time but show increased amplitude (Fig. 5D), also included several repressed genes (Table S4), such as the glyoxylate shunt operon, aceBAK, which is IclR-repressed. While these clusters showed the most dramatic patterns, other clusters composed of low-expressed genes showed similar trends (Fig. S14A). Globally, we observed that lower average expression was associated with expression amplitude when amplitude was measured either as peak-to-trough fold change or standard deviation after mean-adjustment (Fig. 5E, Fig. S14C), and this trend was stronger when we focused on only the most-reproducible genes (Fig. S14D & E). Previously, Wang and colleagues10 observed that for the lacZ gene in E. coli, gene replication is associated with a pulse in transcription, but that this effect is reduced as its repression by LacI is relieved. Our data suggest that similar repression-driven effects, while varying greatly between genes, may be present across the E. coli transcriptome.
Extending this analysis to S. aureus, we also observed a negative relationship between average expression and amplitude of cell cycle expression, suggesting similar principles (Fig. 5F, Fig. S14F & G). After clustering genes based on their aligned dynamics, we noted extreme divergence in several clusters, in which we identified genes belonging to genome-integrated mobile genetic elements (MGEs) (Fig. S15). Genes within these clusters were localized within the core of the MGE, suggesting a role in MGE mobilization as opposed to host-related functions (such as virulence factors)44–46. After excluding all MGE genes, however, a range of behaviors were still evident (Fig. S14H). For example, as in E. coli, we observed high amplitude and delayed dynamics in a cluster, S. aureus (Sa) 9, comprised of dnaAN. Analogous to clusters Ec17 and Ec9 in E. coli, we observed high-amplitude clusters with (Sa18) and without (Sa11) a “left” shift, indicating that expression peaked earlier than expected (Fig. S14I & J). Sa11 contained a range of genes including the heat shock response operon, hrcA-grpE-dnaK, and an amino acid biosynthesis operon, hom-thrCB, which showed a particularly large expression amplitude (Fig. 5G). Sa18 was almost exclusively composed of genes in the GbaA regulon (Fig. 5G). In contrast, another cluster (Sa12) showed delayed dynamics (Fig. S14K). Notably, this included several genes involved in stress and virulence.
Since high amplitude in gene expression is typically associated with low average expression levels, and based on previous observations10,47,48, we reasoned that transcriptional repression could be driving the high amplitude pulses observed for genes in certain clusters (Ec9, Ec17, Sa11, Sa18). Therefore, we focused on genes of the S. aureus GbaA regulon (Fig. 5G), which showed a particularly strong early pulse in expression. This regulon consists of two divergent operons (referred to here as “GbaA-L” and “GbaA-R”) that are repressed by GbaA. GbaA is a transcriptional repressor encoded by gbaA within the GbaA-R operon whose repression is relieved by reactive electrophilic species such as quinones or aldehydes49,50. To test whether GbaA repression was responsible for the divergent dynamics of its regulon, we compared wild-type expression dynamics to those of a gbaA transposon mutant, where GbaA-mediated repression should be relieved. Since transposon insertion happens within the GbaA-R operon, transcription of this locus was disrupted, whereas in the GbaA-L operon we observed a >100-fold increase in expression (Fig. S16A) due to loss of repression. As predicted, this loss of repression was accompanied by a clear reversion of GbaA-L expression to the expected pattern in the transposon mutant, as well as reduced expression amplitude (Fig. 5H). To further verify that this change resulted directly from loss of the regulator rather than disruption of the locus, we measured transcription from the GbaA-L promoter upon integration at an alternative chromosomal locus. While repression by GbaA was less efficient at this locus than for native GbaA-L (Fig. S16B), we nonetheless observed a spike in reporter expression on a wild-type JE2 background that was absent when the reporter was integrated on a gbaA− transposon mutant background (Fig. S16C), further supporting that the GbaA regulon dynamics arise due to repressor-promoter interactions. These observations suggest that repression drives the high-amplitude pulses in expression seen for low-expressed genes.
Discussion
Our analysis reveals, for the first time, the cell cycle transcriptomes of rapidly proliferating bacteria. Although the expression of most genes fluctuates, crucially, these fluctuations do not appear to be a response to cell cycle-dependent changes in the cellular environment (with a few exceptions: DnaA is not only the major regulator of replication initiation51, but also regulates its own transcription in a cell cycle-dependent fashion52,53, explaining its highly divergent expression in both species). Instead, gene expression fluctuations during the cell cycle appear to be responses to the local perturbation that each gene experiences upon passage of the replication fork. This appears to be the case even for major cell cycle regulators and explains why despite the known cell cycle-dependent fluctuations of ftsZ54,55, which encodes the major regulator of cell division in E. coli, division timing appears to be relatively insensitive to the expression patterns of this protein56–58. A direct link between ftsZ replication and transcriptional inhibition was previously postulated but the authors at the time could not provide a satisfactory mechanistic explanation55. Here, we explain these augmented fluctuations in ftsZ abundance as a consequence of transcription from a distant promoter40 (Fig. 4B, Fig. S13B). Our observations therefore support the view that the cytoplasm may be relatively invariant during cell cycle progression of bacteria in a state of balanced growth59, at least as it pertains to the activity of specific transcriptional modulators. Thus a gene is likely to experience few environmentally-induced changes to its transcription during the cell cycle besides its own replication. While it is important to consider the potential influence of global factors on gene expression (such as competition for RNA polymerase between genes60,61), it is not clear which of these could lead to the dynamics we describe here. By redefining cell cycle expression of a gene relative to its replication time, as measured by θc-rep (Fig. 5), we explicitly focus instead on the response of each gene after perturbation by its replication. This provides an expression trace specific to each gene, which we here term the Transcription-Replication Interaction Profile (TRIP).
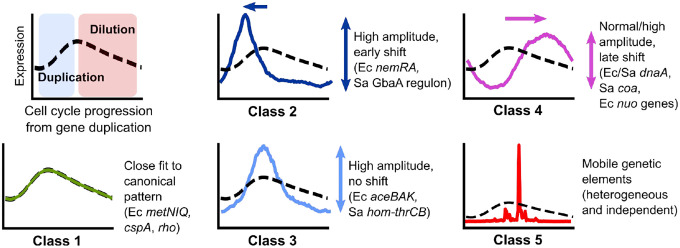
Analysis of each species reveals a diversity of TRIPs that may reflect gene-specific variation in local regulatory motifs. This variation may arise from each gene’s distance from the promoter, local repression state, and possibly other factors such as chromatin structure, together generating a high degree of complexity that we are only beginning to untangle. Nevertheless, we can distinguish several archetypal behaviors of TRIPs (Fig. 6). First, we delineate the non-divergent or “canonical” pattern (Class 1). For genes that fall into this category, expression increases in response to gene dosage at a rate that is likely to be proportional to mRNA half-life13, before being gradually diluted as a fraction of total mRNA as gene dosage increases the expression of subsequently-replicated genes. For genes outside this category, we observe divergence of TRIPs along two main axes: heterochrony, or differential expression timing, and heterometry, or differential amplitude (or “peak/trough ratio”). Many operons under repression exhibit heterometry (Class 2 & 3), while a subset of these peak earlier than expected (heterochrony) (Class 2). Genes can also exhibit heterochrony as a “delayed” expression profile (Class 4). Finally, we note that in S. aureus, many genes located in MGEs, particularly those involved in mobilization, exhibit heterogeneity patterns that are entirely distinct from those of the host genome (Class 5). Future work will be required to fully describe the heterogeneous expression of these elements.
Figure 6: Classes of Transcription-Replication Interaction Profiles of non-divergent and divergent genes.
Top left: Canonical TRIP driven by gene dosage. Other panels: Archetypal patterns of TRIPs that do not (Class 1) or do (Classes 2–5) diverge from this pattern. Genes in E. coli and S. aureus are represented as Ec and Sa, respectively.
Mechanistically, much remains to be explored. For genes with Class 2 or 3 TRIPs, many genes are under repression (or even autorepression). This suggests a possible mechanism in which the passage of the replication fork through the promoter transiently displaces the repressor, leading to a temporary increase in transcription shortly after replication10,62. Other modes of replication-induced transcription have also been suggested47,48. However, it is unclear what drives the precise timing of these transient increases. In E. coli, iclR, which encodes a transcriptional repressor that represses itself as well as the neighboring aceBAK operon, has a Class 2 TRIP, whereas its target, aceBAK, belongs to Class 3. This demonstrates that the presence of binding sites for a particular repressor may not alone be sufficient to determine the expression timing. For Class 4, the delayed pattern, the effect of gene position within operons in E. coli clearly points to the greater disruption experienced by genes far from their promoters, but in other cases, particularly in S. aureus, there must be other drivers. Overall, while certain themes emerge, many questions remain about how these myriad influences on gene expression interact to produce the observed patterns.
As our interpretation of these signatures continues to improve, we may be able to distinguish additional modes of regulation. For example, does low expression of a specific gene reflect weak intrinsic promoter strength (subject to positive regulation) or strong repression (subject to negative regulation)? A Class 2 or 3 TRIP would indicate the latter. Alternatively, what does the delay in expression of genes associated with stress responses or virulence in S. aureus tell us about their regulation, and how might this relate to the phenotypic heterogeneity in stress sensitivity and virulence observed in bacterial pathogens63? Our work demonstrates that this approach can be extended beyond standard model organisms to allow comparison across genes, genetic backgrounds, or even distantly-related species, helping to characterize control of virulence or resistance genes in an emergent pathogen, or regulation of a gene cassette with potential biotechnology applications64. Finally, our ability to infer global parameters directly from the data, including replication patterns and both RNA and DNA polymerase speeds, facilitates comparison across very different growth conditions and will allow us to connect gene-specific dynamics to the overall state of the cell.
This work represents only an initial effort in this direction, but provides a foundational framework for genome-wide exploration of novel bacterial regulatory phenomena. As bacterial scRNA-seq methods evolve in scale, capture efficiency, and cost5,65–67, we predict that these methods, in combination with microscopy and molecular genetics approaches that allow mechanistic dissection of these phenomena, will illuminate a diverse ecosystem of dynamic transcriptional processes.
Materials and Methods
Bacterial strains and growth conditions
Strains used are listed in Table S1. All E. coli strains (a gift from Dr. Christian Rudolph) were routinely grown in modified Luria Broth (LB) (1% tryptone (Sigma-Aldrich), 0.5% yeast extract (Sigma-Aldrich), 0.05% NaCl, pH adjusted to 7.426). For growth in minimal media, an M9 base (1X M9 minimal salts (Gibco), 2 mM MgSO4, 0.2 mM CaCl2) was supplemented with 0.4% glucose (M9G) or with both 0.4% glucose and 0.2% acid casein peptone (Acros Organics) (M9GA). All S. aureus strains were routinely grown in Bacto tryptic soy broth (TSB) (BD Biosciences). The gbaA transposon mutant was provided by the Network on Antimicrobial Resistance in Staphylococcus aureus (cat. # NR-46898).
Growth curves
Strains were grown overnight in LB (E. coli) or TSB (S. aureus) at 37°C, shaking at 225 rpm. For initial experiments with S. aureus (Datasets D3 & D4), strains were diluted to an A600 value of 0.05 in prewarmed TSB, after which A600 was measured at the times specified. A600 was measured on a BioMate 3S spectrophotometer (Thermo Scientific). For experiments with S. aureus in balanced growth (Datasets D5-D8), overnight cultures were diluted in TSB first to 0.005, then after 3 hr diluted again to 0.005 before measuring A600 at the time intervals specified. For E. coli growth curves, strains were diluted to an A600 value of 0.05 and incubated for 2 hr in the desired medium then diluted again in the same prewarmed medium to an A600 value of 0.005, after which A600 was measured at the time intervals specified. Where E. coli cells were diluted into a different medium, cells were washed once with PBS prior to dilution. To measure growth rate, a linear model log2(A600) ~ mT + c was calculated for the linear portion of this relationship (where T is the time in minutes) using the LINEST function in Microsoft Excel and the doubling time in minutes td was calculated as 1/m.
PETRI-seq analysis
Cells were grown as described for the growth curves except that after specific time intervals (for S. aureus, 2 hr 20 min in initial experiments, 1 hr 30 min in balanced growth experiments; for E. coli, 2 hr, 3 hr, and 7 hr in LB, M9GA, and M9G, respectively, when growth rates appeared constant (Fig. S3)) cells were harvested by centrifugation and resuspension in 4% formaldehyde in PBS. For S. aureus initial experiments, centrifugation was at 10,000 × g, 1 min at room temperature and for E. coli and balanced growth S. aureus experiments, centrifugation was at 3,220 × g, 5 min, 4°C. PETRI-seq was carried out as described previously20 with the following modifications. Initial fixing, permeabilization, and DNase treatment were carried out as described but with cell wall permeabilization using 100 μg/ml lysostaphin (Sigma-Aldrich) for S. aureus and 100 μg/ml lysozyme (Thermo Scientific) for E. coli. For Dataset D4, samples were split into processing with or without DNase treatment and subsequent wash steps, to test whether this would affect correlation patterns (suggesting contaminating genomic DNA could play a role). However, no difference was observed in the presence or absence of DNase treatment, although UMI/barcode was slightly higher after DNase treatment (Table S1). For barcoding, the number of cells included was reduced from 3 × 107 to a maximum of 1 × 107, since preliminary experiments indicated lower input at this stage was associated with a higher UMI/barcode for S. aureus. Tagmentation was performed using the EZ-Tn5 transposase (Lucigen) as described in the latest version of the PETRI-seq protocol (available at https://tavazoielab.c2b2.columbia.edu/PETRI-seq/updates_April2021/PETRI_Seq_Protocol.pdf). Briefly, the transposase was loaded by incubating EZ-Tn5 with pre-annealed oligonucleotides (/5Phos/CTGTCTCTTATACACATCT and GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG) at 4 μM and 40% glycerol at room temperature for 30 min. Tagmentation was then performed incubating samples with loaded EZ-Tn5 (at a final further dilution of 400x) and 2x Tagment DNA buffer; either using the Nextera 2x Tagment DNA (TD) buffer or 20 mM Tris(hydroxymethyl)aminomethane; 10 mM MgCl2; 20% (vol/vol) dimethylformamide, pH adjusted to 7.6 with acetic acid68. After incubating for 5 min at 55°C and decreasing the temperature to 10°C, either Nextera NT buffer (Illumina) or 0.2% sodium dodecyl sulfate was added, allowing neutralization to proceed for 5 min at room temperature. Final amplification was performed with Q5 polymerase (New England Biolabs) using the NEBNext Universal i5 primer (New England Biolabs) and the N7 indices from the Nextera XT Index Kit v2 Set A (Illumina) as also described in the updated PETRI-seq protocol. Sequencing was performed on an Illumina NextSeq 500 to obtain 58 × 26 base paired-end reads. For each barcoding experiment, multiple libraries of ~20,000 cells were prepared and sequenced, and no batch effects were noted across libraries.
Pre-processing and scVI analysis
Initial demultiplexing of barcodes, alignment, and feature quantification was performed using the analysis pipeline described in 20 except that feature quantification was performed at the gene level rather than operon level. Reference sequences and annotations were obtained from Genbank (https://www.ncbi.nlm.nih.gov/genbank/). E. coli reads were aligned to the K-12 MG1655 reference assembly (GCA_000005845.2) and S. aureus to the USA300_FPR3757 reference assembly (GCF_000013465.1). After initial processing, counts by cell barcode were pooled across different libraries and initial filtering was performed using Scanpy v1.7.169. Barcodes with UMI below a threshold (15 for Dataset D1, D2, D4; 20 for Dataset D3, D5–7, 40 for Dataset D8) were removed, as well as any genes with fewer than 50 UMI across all included barcodes (100 for Dataset D3). To generate the denoised representation of the data, scVI v0.9.024 was applied with the following hyperparameters, chosen through grid search to distinguish between closely related S. aureus strains in a pilot dataset: two hidden layers, 64 nodes per layer, five latent variables, a dropout rate of 0.1, and with a zero-inflated negative binomial gene likelihood (other hyperparameters maintained as defaults). Denoised expression values based on the scVI model were obtained using the scVI function “get_normalized_expression”.
Cell cycle analysis
Cells were assigned to cell cycle phases by calculating the angle θc relative to the origin between x and y coordinates in a two-dimensional UMAP embedding of the data as tan1(x / y), similar to the ZAVIT method our lab has described previously70,71. scVI-denoised expression values were first log2-transformed then converted to z-scores. Embeddings were computed by averaging these z-scores within bins according to chromosomal location (50–400 kb bins, depending on the dataset), and then performing two-dimensional UMAP analysis using the umap-learn v0.5.1 library in Python (https://umap-learn.readthedocs.io/en/latest/) with the ‘correlation’ distance metric. These embeddings were then mean-centered (Fig. 2A & Fig. S7B). To get the expression by cell angle matrix used in Fig. 2B, gene expression z-scores were then averaged within 100 equally spaced bins of θc to produce a cell angle-binned expression matrix. To order genes based on their cell cycle expression, gene angle, θg, was calculated as follows. PCA was performed on the transpose of the cell angle-binned expression matrix and θg was calculated as the angle between PCs 1 and 2 relative to the origin. Together, θc and θg are metrics for ordering of cells and genes, respectively, within the model of cell cycle gene expression described here.
Modeling the gene angle-origin distance relationship
While there was a strong relationship between origin distance D and gene angle θg, modeling this relationship is challenged by the fact that the relationship is “wrapped” with an unknown periodicity with respect to D (Fig. 2E & F, Fig. S7D) (i.e. after a period of increased θg with D, θg starts again at zero). To fit this relationship, a custom Bayesian regression analysis was developed according to the following model partially adapted from72, with both θg and D standardized to the range −π to π:
Where:
The von Mises probability distribution is a circular probability distribution here parameterized by A, the predicted mean angle, and κ, the concentration parameter (higher κ implies greater concentration of the distribution around A). The parameter ɣ can be interpreted as the gradient of D with respect to θg after standardizing both variables to to the range −π to π. The inverse of ɣ, 1/ɣ, is the gradient of θg with respect to D (after range standardization) and therefore is the fraction of the origin-terminus distance covered within a single span of θg. Therefore, 1 – 1/ɣ is the fraction of D during which the next round of replication has already initiated, referred to as the “overlap fraction” in Fig. 2G & Fig. S7E. Here, ɣ is constrained to be positive by the lognormal prior distribution (Fig. S17), which is appropriate since the ordering of angles θg are reversed (i.e. 360 - θg when θg is in degrees) if during analysis this relationship shows a negative trend. This can occur because the directionality of PCs used to calculate θg is arbitrary. Posterior distributions for the parameters were obtained by Hamiltonian Monte-Carlo sampling using Rstan v2.21.373. Fitted values for θg based on D (θg-pred) were calculated by determining θg-pred for all sampled parameter values and then calculating the mean value of θg-pred as tan−1(mean(sin(θg-pred)) / mean(cos(θg-pred))).
Calculating replication pattern statistics.
We can use the gradient parameter, ɣ, of the gene angle-origin distance model to calculate statistics of the replication pattern. The parameter ɣ can be interpreted as the gradient of D with respect to θg after standardizing both variables to to the range −π to π. To convert the gradient to °/Mb (as in Fig. S7F), this value is multiplied by 360 divided by origin-terminus distance in Mb. The average DNA polymerase speed can be estimated from this as follows:
Here, vDNAP is the DNAP speed in bp/s, td is the doubling time in min, ɣ°/Mb is the gradient of the gene angle-origin distance relationship in °/Mb.
Modeling the cell angle-gene angle relationship
To predict expression based on cell angle θc and gene angle θg, a linear regression model was constructed using scikit-learn v0.24.174 with features generated from θc and θg. Specifically, both angles were converted to radians and then transformed into cos(θc), sin(θc), cos(θg), and sin(θg). All interactions and combinations of these terms up to a fourth degree polynomial were constructed using the scikit-learn PolynomialFeatures function. The untransformed θc and θg values in radians were also included as features. These features were then used to fit a Ridge regression model (ɑ = 10). The model was trained on scVI expression z scores averaged first in 100 bins by θc then in 100 bins by θg (i.e. the expression matrix used for Fig. 3F). An alternative approach considered was a nonlinear approach using the scikit-learn implementation of kernel ridge regression with kernel “rbf”. However, the fourth degree polynomial model performed similarly and was computationally far more efficient so was chosen (increasing the polynomial degree further made little difference to performance).
Predicting expression dynamics based on DNA replication alone
To derive a prediction of cell cycle gene expression dynamics based on the expected effect of replication alone, the two regression models above were combined to yield the pipeline in Fig. S8. Firstly, the gene angle-origin distance model (see Section “Modeling the gene angle-origin distance relationship”) was used to predict the expected value θg-pred from origin distance D. Next, cell cycle expression was predicted using the cell angle-gene angle regression model (see Section “Modeling the cell angle-gene angle relationship”) using θg-pred values. For cell angle θc, values used were the average θc values of cells binned into 100 equally spaced bins by θc. This gives a replication-predicted gene expression matrix of 100 bins × number of genes. The success of this model fit was evaluated based on the correlation with the θc-binned expression z-scores derived from scVI (Fig. S9A & F), as well as the loss of global chromosome position-dependent gene-gene correlations upon correction of scVI expression with replication-predicted expression (Fig. S9B & G). Additionally, we used this modeling approach to set the zero angle for gene expression plots.
Setting the position of θc = 0.
Initially, the cell angle θc orders cells by their cell cycle position within a circle but the start point, when θc = 0, is arbitrary. This is not only challenging to interpret but impedes comparing across replicates. Therefore, we standardized θc so that θc = 0 was the predicted point of replication initiation. Using the inference approach described above, we predicted the gene expression profile by θc for an imaginary gene at D = 0 (i.e. at the origin of replication). We then determined the value of θc giving the minimum predicted expression, reasoning that if increased expression in this model is responsive to a doubling of copy number, the doubling event should occur at the expression minimum. Therefore, we determined this angle, θ0 to be the most likely value of θc at which replication initiation occurs, rotating the angles by the operation (θc - θ0) mod 360 to set this point as 0°. This interpretation is roughly in accordance with the estimated timing of replication initiation as determined directly from smFISH data (Fig. S10F and see Section “Inferring cell-cycle phase from the DAPI signal”). Crucially, however, it also provides a point of standardization that allows in-phase comparison of cell cycle expression profiles across independent replicates.
Identifying replication-divergent genes
We identified replication-divergent genes based on two criteria: absolute variability by cell angle θc and divergence from the replication model.
Identifying genes with high cell cycle variance.
First, we identified highly variable genes as follows (based on the method implemented in Seurat v375). We normalized raw counts for library size (so that the total sum of UMI for each barcode was the median UMI/barcode), then to reduce sparsity while retaining cycle information, we averaged counts across 20 bins by θc. Next, we log2-transformed the data (removing any genes with zero values after binning to allow log-transformation). We observed a negative overall relationship between the mean and variance of genes in log-transformed data (Fig. S9C), to which we fitted a regression line with locally weighted scatterplot smoothing (LOWESS) using the Python package statsmodels v0.12.276. We used this fit to develop a mean-dependent variance threshold. In all cases, genes were considered highly variable if they had a ratio of observed to LOWESS-predicted variance > 1.3 as well as a log2 mean normalized expression > −10. These thresholds typically classified ~25% of genes as highly variable.
Identifying genes with high divergence from predicted expression.
Next, to quantify divergence from the replication model, we subtracted the replication-predicted expression from the scVI-derived expression z-scores (both averaged in 100 bins by θc) to “correct” for the effect of replication, and then calculated the standard deviation of this replication-corrected value, σcorrected. A high σcorrected indicates that the dynamics behave differently from that expected based on replication alone. Thresholds for σcorrected (0.6 for E. coli, 0.5 for S. aureus) were determined manually based on inspection of the relationship between σcorrected across two datasets and choosing a value above which the correlation between datasets was stronger (Fig. S9E & I) (below the threshold, lack of reproducibility of σcorrected suggests divergences are small and dominated by noise). To calculate peak/trough fold changes in expression, normalized gene expression derived from scVI was averaged into 100 bins by θc and then the ratio between the fourth highest and fourth lowest values were calculated (this was chosen instead of maximum/minimum values to increase robustness to noise).
Analyzing the effect of operon gene position on expression dynamics
We identified the excess of genes with a “delayed” expression profile by calculating the angle difference as tan−1(sin(θg − θg-pred) / cos(θg − θg-pred)) where θg and θg-pred are the observed and predicted gene angles in radians, respectively. For operon annotations, E. coli transcription units from Biocyc77,78 (https://biocyc.org/) were used. To investigate the relationship between gene distance from transcriptional start sites and angle difference in E. coli, all genes in polycistrons (transcription units with more than one gene) were included. The distance was measured from the annotated transcription unit start site to the midpoint of each gene. Where genes were in multiple transcription units, the longest distance from a start site was taken. Angle difference was converted into time by dividing the angle by 360° then multiplying by the doubling time in seconds. For S. aureus, operon annotation was obtained from AureoWiki79 (aureowiki.med.uni-greifswald.de). Since this provided only the genes within an operon and not its start, the first base of the first gene was taken as the transcriptional start site.
Per-base analysis of the nuo and mraZ-ftsZ operons.
To analyze per-nucleotide coverage of the nuo operon (Fig. 4D & E), we obtained “.bam” alignment files from the analysis pipeline (see “Pre-processing and scVI analysis) and removed PCR duplicates with UMI-tools v0.5.580. Next, for a genomic interval encompassing the nuo operon and neighboring genes, we quantified per-base per-barcode read depth using the mpileup function in Samtools v1.3.181. This coverage was then normalized by total per-cell library depth (division by per-cell total mRNA count then multiplication by median mRNA count across all cells) and averaged in 10 bins by θc. For the plots in Fig. 4D & E, we recenter θc so that 0° is the predicted minimum expression of nuoA, the first gene in the operon, so that θc corresponds to the approximate time elapsed since the locus was replicated. Analysis of the mraZ-ftsZ locus was carried out as for the nuo operon except that θc was recentered so that 0° is the predicted minimum expression of mraZ.
Aligning gene expression profiles of based on their predicted minimum expression
To align cell cycle gene expression profiles as displayed in Fig. 5A & C, we use the replication-predicted expression profiles derived above to determine the minimum cell angle, θc-min, predicted for each gene. Profiles of gene expression by cell angle (averaged in 100 bins by θc as used elsewhere) are then rotated so that θc = 0 corresponds to this new minimum by the transformation (θc - θc-min) mod 360 to give the cell angle relative to the predicted timing of a gene (θc-rep). Gene expression profiles are then divided by their mean to center them, but they are not scaled (so that amplitude differences are preserved). These profiles are used to generate the k-means clusters described.
Simulating the effect of DNA replication on gene expression
We predicted the gene-gene correlation patterns arising from DNA replication using a simulation written in Python (see Fig. S4) as follows. Cells were represented by genomes with 200 genes, each represented as a single integer and divided into individual replication units. In the simplest case, genomes were divided into two units of 100 genes (i.e. the two “arms” of the chromosome). In each cell, replication initiation events were simulated at intervals determined by a Poisson distribution with expected value μ. After an initiation event, replication proceeds in stepwise fashion along the length of each replication unit, doubling the copy number at each point until the end of that replication unit has been reached. We also simulate “cell division” events in which all copy numbers are halved. These are timed independently from replication initiation but in the same way (at Poisson-distributed intervals with rate μ), with an additional offset from the first replication initiation event. In practice, we found that this offset did not affect correlations, since all genes are scaled equally. We used an initial offset of 150 steps (i.e. 1.5x the time to replicate a 100 gene replication unit, equivalent to the 40 min C-period + 20 min D-period originally proposed for E. coli B/r8). For each simulation, we generated 1,000 cells. Cells were initiated one at a time to yield an unsynchronized population, then the simulation was run for a further 1,000 steps with the whole population. We then normalized expression by total counts and calculated Spearman correlations across all genes. In order to simulate specific doubling times, the rate μ was calculated as μ = (n × td)/tc. where n is the number of genes in the longest replication unit (here, 100 genes), td is the doubling time, and tc is the C-period (here a value of 42 min was chosen for E. coli MG1655 based on82). The td/tc ratio represents the fraction of one round of chromosomal replication that can take place in one cell cycle. Finally, for simulation of cells with additional origins of replication, genes were split into replication units according to the following assumptions: a) all origins initiate replication simultaneously; b) replication stops at the termination site ter, which is halfway along the chromosome; c) genes are replicated by the nearest origin (unless the replication fork must pass through ter to reach that gene).
Bulk RNA-seq analysis
For the analysis of bulk RNA-seq from15 (Fig. S4C), we accessed data from the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) under accession ID GSE46915. Counts were size factor-normalized with DESeq2 v1.32.083, then data were standardized to z-scores and averaged into 100 kb bins by chromosomal position. Spearman correlations of binned values across all time points and replicates are shown.
Single-molecule fluorescence in situ hybridization (smFISH)
Our smFISH protocol was described previously34,84. Briefly, we first designed seven sets of antisense DNA oligonucleotide probes. Six probe sets were against E. coli mRNAs dnaA, nrdA, nemA, metN, rho, and cspA, and another against bacteriophage lambda cI mRNA (which serves as a negative control, since the probes have no target in the bacterial cell). All oligos were synthesized with a 3’ amine modification (LGC Biosearch Technologies). The oligos against a given gene (oligo set) were pooled and covalently linked to 5-Carboxytetramethylrhodamine succinimidyl ester (5’-TAMRA SE, Cayman Chemical) and purified using ethanol precipitation. Probe sequences are listed in Table S2.
Microscopy
An inverted microscope (Eclipse Ti2E, Nikon), equipped with motorized stage control (TI2-S-SE-E, Nikon), a universal specimen holder, an LED lamp (X-Cite XYLIS), a CMOS camera (Prime 95B, Photometrics), and a ×100, NA 1.45, oil-immersion phase-contrast objective (CFI60 Plan Apo, Nikon) was used for imaging. The following fluorescent filter sets were used: DAPI (Nikon, 96370) and Cy3 (Nikon, 96374).
E. coli cells were grown as described in Section “Bacterial strains and growth conditions”. After overnight culture, dilution, and re-dilution at 37°C, 220 rpm, cells were grown to a density of ≈ 0.2, then for each gene, 36 ml of culture was collected, immediately fixed and permeabilized, then incubated with the fluorescent probe set, washed. Next, we loaded 2 μl of the cell suspension on a circular coverslip, then covered it by a 1 × 1 cm agarose pad made of 1.5% agarose (Sigma) in 1× PBS, as described in 34. The coverslip was then lodged in an Attofluor Cell Chamber (Invitrogen), which was then placed onto the microscope’s slide holder and the cells were visually located using the phase-contrast channel. Images were taken in the following order: phase-contrast (100 ms; to detect the cell outline), Cy3 (400 ms; smFISH-labeled mRNA), and DAPI (4′,6-diamidino-2-phenylindole) (100 ms; bacterial DNA). Snapshots were taken at seven z-positions (focal planes) with steps of 300 nm. Images were acquired at multiple positions on the slide, to image a total of 500–2000 cells per sample (typically 9–16 positions).
Cell segmentation
Cells were identified in the phase-contrast channel, as described previously10,84. Briefly, we first defined the “in-focus” z-slice in every image stack by finding the one with the highest variance among pixels. We then used U-Net, a convolutional network for image segmentation85, previously trained on our E. coli images, to recognize all pixels that are within any given cell. Finally, the segmentation results were manually inspected, with poorly segmented cells manually corrected or removed.
To estimate the dimensions of each cell, the cell area A was first measured by counting the number of pixels within the cell, and the cell length L by calculating the length of its long axis. Approximating the bacterial cell as a spherocylinder86, we estimated the cell width d and cell volume V using the equations below:
The estimated cell volume V is used when measuring mRNA concentrations in each cell (Section “mRNA quantification”), and the cell length L serves as an indicator for cell cycle progression (Section “Cell-cycle analysis of smFISH data”).
mRNA quantification
Following cell segmentation (Section “Cell segmentation”), we estimated the mRNA copy number in individual cells using two methods: (i) based on the recognition of fluorescent foci (“spots”), and (ii) based on the measurement of whole-cell fluorescence. The two methods yielded consistent results (Fig. S12) and were used interchangeably in subsequent analysis.
Spot based quantification.
Spot recognition and the subsequent mRNA quantification were done as described previously34,84. Briefly, we used the Spätzcells software34 to identify the spots in the fluorescent images. The software fits the fluorescence intensity profile near each spot to a two-dimensional elliptical Gaussian. The fitting results yielded the properties of each spot, including the position, spot area, peak height (amplitude of the fitted Gaussian), and spot intensity (integrated volume under the fitted Gaussian), used in the subsequent analysis.
To discard false positive spots, such as the ones resulting from nonspecific binding of smFISH probes, we performed a gating procedure as described in 34,84. Briefly, we compared the 2D scatter plots of peak height versus spot area for all detected spots in the experimental samples to that from the negative control (the sample incubated with probes against lambda cI, see Section “smFISH”). We then defined a polygon in the 2D plane, such that most spots from the negative sample were located outside of it. All spots outside of this polygon were discarded, and the gating results were confirmed by manual inspection of a subset of images.
Following spot recognition, we estimated the fluorescence intensity of a single mRNA molecule as described in 34. We fitted the histogram of spot intensities in each experimental sample to a sum of three Gaussians corresponding to one, two, and three mRNA molecules per spot. The center of the first Gaussian was then used to estimate the fluorescence intensity of a single mRNA molecule. Using this procedure, we found that the Gaussian fitting results for genes dnaA, nrdA, nemA, metN, and rho were very close to each other, consistent to the fact that the probe sets against them have the same number of probes (see Table S2). Therefore, we used the mean of their first-Gaussian center as our estimated single-mRNA intensity. The high expression level of the cspA samples (Fig. S10B) was likely to hinder the identification of individual mRNA molecules34. Since the number of probes in the cspA set is 1/3 of that against other genes (Table S2), we assumed its single-mRNA intensity to be a third of that for the other genes. Finally, the mRNA copy number for a given gene in each cell was calculated by summing the mRNA spot intensities within the cell and dividing by the single-mRNA intensity34, and the mRNA concentration for a given gene in each cell was calculated by dividing the mRNA copy number by the estimated cell volume (Section “cell segmentation”).
Whole-cell based mRNA quantification.
An alternative approach to relying on spot recognition is the use of total cell fluorescence as a proxy for the total number of bound probes, in turn indicating the number of target mRNA molecules. We first chose the z-slice with the largest coefficient of variation among intracellular pixels, indicating maximum contrast. Next, we determined the background fluorescence intensity by calculating the average fluorescence per intracellular pixel in the negative control (the sample incubated with probes against lambda cI, see Section “smFISH”). After subtracting this background intensity from cells in each positive sample, we calculated the total and average (per pixel) fluorescence of each cell. These values exhibited a linear relation with the spot-based measurements of mRNA number and concentration, respectively (Fig. S12). The fitted slopes were used as calibration factors to convert the whole-cell fluorescent signals to mRNA numbers and concentrations.
Modeling the distribution of cell length
Within a population of exponentially growing cells, under the assumption that the instantaneous growth rate a cell is proportional its length, the cell length distribution is predicted to follow87:
with L0 the cell length at birth. To account for the stochasticity of cell-cycle processes88, as well as the experimental error, we described the measured cell length data using a Gaussian-smoothed version of the original function:
where σ represents the noise magnitude. Fitting this equation to the experimental data (Fig. S10C) yielded L0 = 3.43 ± 0.05 μm, σ = 0.56 ± 0.10 μm (N = 12 samples, each with > 500 cells. See Table S3 for detailed sample sizes).
Cell-cycle analysis of mRNA concentration
Comparing the mean expression levels of the six genes (dnaA, nrdA, nemA, metN, rho, and cspA) as measured by smFISH with the estimated abundance obtained by scRNA-seq showed that the two methods were highly correlated(Fig. S10D). We next aimed to test whether the cell-cycle dependence of transcription, revealed by scRNA-seq (Fig. 3 B & D, 2nd column) is too found in the smFISH data.
We first examined the cell cycle dependence of mRNA concentration, since we reasoned that those values would correspond closely to the mRNA fraction measured in scRNA-seq. For this purpose, we followed the approach of10 and used cell length as an indicator for cell cycle progression. In each sample, we first found the two-fold range of cell length containing most cells. The lower bound of this range provides an estimate for the cell length at birth (L0), and the value found (L0 = 3.34 ± 0.07 μm, N = 12) was consistent with the estimate in Section “Modeling the distribution of cell length”. The measured single-cell mRNA concentration was binned based on cell length (with each bin containing 10% of the cells in the sample, and a shift of 1 cell between adjacent bins), and the average mRNA concentration within each bin was calculated (Fig. 3 B & D, 3rd column). For all genes, we observed that the mRNA concentration fluctuates along the cell cycle, returning at cell division (length of 2L0) to a level similar to that at cell birth (length of L0), as expected.
To directly compare cell cycle patterns between smFISH and scRNA-seq, we needed to correct for differences in both amplitude and phase of the two signals. In particular, whereas the smFISH pattern is aligned by cell length, hence the bacterial birth-to-division cycle, the scRNA-seq data is aligned, through the cell angle, to the timing of genome replication (oriC replication to next oriC replication). Aligning the two signals was done as follows. We first linearly converted the cell length to a parameter β within the range 0 to 2π:
Next, we fitted the relationship between smFISH-measured mRNA concentration and β to a sinusoid:
In this function, A and B indicate the median level and fluctuation of the mRNA concentration, and C indicates the phase. Specifically, the maximal mRNA concentration is reached when or (Fig. S10E).
Similarly, for the scRNA-seq data, we fit the relationship between the mRNA fraction and cell angle θc to a sinusoid:
We then estimated the cell angle at cell birth using the phase difference φ = C − c between the fits for scRNA-seq and smFISH data (Fig. S10E). This estimated value (~155°) was consistent across the 6 genes examined (Fig. S10E).
To overlay the scRNA-seq and the smFISH data (Fig. 3B & D, 4th column and Fig. S11), we scaled and shifted the measured values using the fitting parameters above. The experimentally measured mRNA concentration (smFISH) and fraction (scRNA-seq) were converted using the equations below:
The cell angle θc was first shifted by the estimated phase difference, then linearly converted to the corresponding cell length using the equations below:
Specifically, the cell length at which oriC replicates is estimated to be .
Comparison to a replication-transcription model
In the simplest model of cell cycle dependent transcription, mRNA levels follow gene dosage, and will thus double following gene replication. To test whether the non-divergent patterns (revealed by scRNA-seq) correspond to this simple scenario, we first binned the smFISH-measured mRNA numbers based on cell length (each bin contains 5% cells in the sample, with a shift of 1 cell between adjacent bins) (Fig. 3B & D, 5th column). Following10, we then fitted the data to the sum of two Hill functions, corresponding to two gene replication rounds:
In this expression, the parameter Lr indicates the cell length at which gene replication occurred, and n2 indicates the fold change in cell length between successive replication events. As seen in Fig. 3B & D, 5th column, the data for the three genes defined as non-divergent (metN, rho, cspA) is well described by this expression, with the fitted n2 close to 2 as expected (n2 = 1.89, 2.04, and 2.04 respectively for metN, rho, and cspA). In contrast, two of the three divergent genes (dnaA and nrdA) exhibit a noticeable deviation from the expected form. In particular, mRNA levels appear to overshoot, consistent with our previous observation10.
Inferring cell-cycle phase from the DAPI signal
When comparing the cell cycle expression patterns obtained by scRNA-seq and smFISH (Section “Cell-cycle analysis of mRNA concentration”), we aligned the two datasets by horizontally shifting by a constant cell-length interval of ~1.4 μm, equivalent to cell angle of ~155° (Fig. S10E). This shift is interpreted as corresponding to the cell cycle interval between cell birth and oriC replication (which was estimated to take place at cell length of ~5.2 μm). Whereas in Section “Cell-cycle analysis of mRNA concentration” this value was inferred directly from the mRNA data, we also attempted to estimate the same parameter from single-cell measurements of DNA contents in the smFISH samples, obtained using DAPI labeling (Section “Microscopy”).
We assume that the replication speed is constant along the genome, and designate by T, TC, TD the cell doubling time, duration of genome replication, and the time between replication termination to cell division82. We specifically consider the case max(TD, T/2) < T < (TC + TD)/2 where genome replication initiates at cell age 3T − TC − TD89. Under these assumptions, the cellular DNA contents (in equivalent number of chromosomes) as a function of cell length (assuming cell length grows exponentially with time87, will be given by89:
T − TD is the cell age when one round of genome replication ends, and 3T − TC − TD is the cell age when another round of genome replication begins. When t < T − TD, there are three pairs of replication forks present. When T − TD ≤ t < 3T − TC − TD, there are only two pairs of replication forks. When t ≥ 3T − TC − TD, there are six pairs of replication forks. Therefore, the ratios of DNA production rates during these three phases are 3:2:6 (Fig. 10F). In particular, a 3-fold jump in slope takes place at the cell cycle age (length) when oriC replicates. We use this constraint to fit our experimental data. We first plotted the single-cell DAPI fluorescence against cell length. We then determined the two-fold range of cell length containing most cells (see Section “cell-cycle analysis of mRNA concentration”), and fitted the data within this length range to the equation above. Discarding those fits where the fitted parameters fell on the boundary of the allowable range and whose r-square value was less than 0.4, the average fitted cell length when the replication of oriC occurs is 4.0 ± 0.3 μm (N = 6, with 6 samples discarded). The imperfect agreement between this estimate and the one obtained from scRNA-seq/smFISH alignment (5.2 μm) reflects multiple sources of error. Most notably, the analyses above assumed a simple linear mapping from both cell angle (scRNA-seq) and cell length (smFISH) to cell age, but the relation between observables is in fact nonlinear and subject to stochastic effects. These conceptual errors are likely compounded by experimental ones, for example, the distortion of cell length during fixation, and heterogeneity in DAPI staining.
Generation of chromosome-integrated reporter constructs in S. aureus
For generation of the reporter construct, we modified the pJC1111 vector90, which integrates at the SaPI1 chromosomal attachment (attC) site. The vector was linearized with restriction enzymes SphI and XbaI (New England Biolabs) and insertion fragments were amplified using Q5 polymerase (New England Biolabs). For the GbaA-L promoter, the intergenic region of the GbaA regulon (130 bp upstream of the SAUSA300_RS13955 start codon) amplified from USA300 LAC genomic DNA using primers-5’CCGTATTACCGCCTTTGAGTGAGCTGGCGGCCGCTGCATGGATTACACCTACTTAAAATTCTCTAAAATTGACAAACGG-3’ and 5’-AGTTCTTCTCCTTTGCTCATTATCAACACTCTTTTCTTTTATGATATTTAATAGTTATTGCAAATTCA-3’. S. aureus codon-optimized sGFP was amplified from the genomic DNA of S. aureus USA300 LAC previously transformed with the pOS1 plasmid (VJT67.6391) using primers 5’-AAAAGAAAAGAGTGTTGATAATGAGCAAAGGAGAAGAACTTTTCACTG-3’ and 5’-ATAGGCGCGCCTGAATTCGAGCTCGGTACCCGGGGATCCTTTAGTGGTGGTGGTGGTGGTGGG-3’. Fragments were assembled using the NEBuilder HiFi assembly kit (New England Biolabs) and transformed into competent E. coli DH5ɑ (New England Biolabs). The plasmid was purified and then electroporated into RN9011 (RN4220 with pRN7023, a CmR shuttle vector containing SaPI1 integrase), and positive chromosomal integrants were selected with 0.1 mM CdCl2. Finally, this strain was lysed using bacteriophage 80ɑ and the lysate was used to transduce JE2 and JE2 gbaA− strains, selecting for transduction on 0.3 mM CdCl2.
Supplementary Material
Acknowledgements
We thank Yitzhak Pilpel, Timothée Lionnet, and Fanny Matheis for critical discussions on the project and the manuscript, and Saeed Tavazoie, Sydney Blattman, and Wenyan Jiang for initial advice on implementing PETRI-seq. We thank Christian Rudolph and his lab for providing the E. coli strains. We also further thank Menyu Wang and members of the Yanai and Golding labs for advice and suggestions. The following funding was provided by the National Institutes of Health: R21AI169350 (IY), R01AI143290 (IY), R01AI137336 (BS, VJT, IY), R35 GM140709 (IG).
Footnotes
Competing interests: The authors declare no competing interests.
Data and materials availability
All counts matrices and raw sequencing reads used to perform the scRNA-seq analysis are available in the Gene Expression Omnibus (GEO) under the accession number GSE217715.
References
- 1.Bervoets I. & Charlier D. Diversity, versatility and complexity of bacterial gene regulation mechanisms: opportunities and drawbacks for applications in synthetic biology. FEMS Microbiol. Rev. 43, 304–339 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez A. & Golding I. Genetic determinants and cellular constraints in noisy gene expression. Science 342, 1188–1193 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilar J. M. G., Guet C. C. & Leibler S. Modeling network dynamics: the lac operon, a case study. J. Cell Biol. 161, 471–476 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narula J., Devi S. N., Fujita M. & Igoshin O. A. Ultrasensitivity of the Bacillus subtilis sporulation decision. Proc. Natl. Acad. Sci. U. S. A. 109, E3513–22 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Homberger C., Hayward R. J., Barquist L. & Vogel J. Improved bacterial single-cell RNA-seq through automated MATQ-seq and Cas9-based removal of rRNA reads. Preprint at 10.1101/2022.11.28.518171. [DOI] [PMC free article] [PubMed]
- 6.Xie M. & Fussenegger M. Designing cell function: assembly of synthetic gene circuits for cell biology applications. Nat. Rev. Mol. Cell Biol. 19, 507–525 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Balakrishnan R. et al. Principles of gene regulation quantitatively connect DNA to RNA and proteins in bacteria. Science 378, eabk2066 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper S. & Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J. Mol. Biol. 31, 519–540 (1968). [DOI] [PubMed] [Google Scholar]
- 9.Schaechter M., Bentzon M. W. & Maaloe O. Synthesis of deoxyribonucleic acid during the division cycle of bacteria. Nature 183, 1207–1208 (1959). [DOI] [PubMed] [Google Scholar]
- 10.Wang M., Zhang J., Xu H. & Golding I. Measuring transcription at a single gene copy reveals hidden drivers of bacterial individuality. Nat Microbiol 4, 2118–2127 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narula J. et al. Chromosomal Arrangement of Phosphorelay Genes Couples Sporulation and DNA Replication. Cell 162, 328–337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slager J. & Veening J.-W. Hard-Wired Control of Bacterial Processes by Chromosomal Gene Location. Trends Microbiol. 24, 788–800 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson J. R., Cole J. A., Fei J., Ha T. & Luthey-Schulten Z. A. Effects of DNA replication on mRNA noise. Proc. Natl. Acad. Sci. U. S. A. 112, 15886–15891 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laub M. T., McAdams H. H., Feldblyum T., Fraser C. M. & Shapiro L. Global analysis of the genetic network controlling a bacterial cell cycle. Science 290, 2144–2148 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Fang G. et al. Transcriptomic and phylogenetic analysis of a bacterial cell cycle reveals strong associations between gene co-expression and evolution. BMC Genomics 14, 450 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou B. et al. The global regulatory architecture of transcription during the Caulobacter cell cycle. PLoS Genet. 11, e1004831 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Nisco N. J., Abo R. P., Wu C. M., Penterman J. & Walker G. C. Global analysis of cell cycle gene expression of the legume symbiont Sinorhizobium meliloti. Proc. Natl. Acad. Sci. U. S. A. 111, 3217–3224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandekar A. C., Subedi S., Ioerger T. R. & Sassetti C. M. Cell-Cycle-Associated Expression Patterns Predict Gene Function in Mycobacteria. Curr. Biol. 30, 3961–3971.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper S. The synchronization manifesto: a critique of whole-culture synchronization. FEBS J. 286, 4650–4656 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Blattman S. B., Jiang W., Oikonomou P. & Tavazoie S. Prokaryotic single-cell RNA sequencing by in situ combinatorial indexing. Nat Microbiol 5, 1192–1201 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuchina A. et al. Microbial single-cell RNA sequencing by split-pool barcoding. Science 371, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imdahl F., Vafadarnejad E., Homberger C., Saliba A.-E. & Vogel J. Single-cell RNA-sequencing reports growth-condition-specific global transcriptomes of individual bacteria. Nat Microbiol 5, 1202–1206 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Homberger C., Barquist L. & Vogel J. Ushering in a new era of single-cell transcriptomics in bacteria. microLife 3, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez R., Regier J., Cole M. B., Jordan M. I. & Yosef N. Deep generative modeling for single-cell transcriptomics. Nat. Methods 15, 1053–1058 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X., Lesterlin C., Reyes-Lamothe R., Ball G. & Sherratt D. J. Replication and segregation of an Escherichia coli chromosome with two replication origins. Proc. Natl. Acad. Sci. U. S. A. 108, E243–50 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimude J. U. et al. Origins Left, Right, and Centre: Increasing the Number of Initiation Sites in the Chromosome. Genes 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanova D. et al. Shaping the landscape of the Escherichia coli chromosome: replication-transcription encounters in cells with an ectopic replication origin. Nucleic Acids Res. 43, 7865–7877 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bremer H. & Dennis P. P. Modulation of Chemical Composition and Other Parameters of the Cell at Different Exponential Growth Rates. EcoSal Plus 3, (2008). [DOI] [PubMed] [Google Scholar]
- 29.Schaechter M., Maaloe O. & Kjeldgaard N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J. Gen. Microbiol. 19, 592–606 (1958). [DOI] [PubMed] [Google Scholar]
- 30.Korem T. et al. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science 349, 1101–1106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McInnes L., Healy J. & Melville J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv [stat.ML] (2018). [Google Scholar]
- 32.Khodursky A. B. et al. Analysis of topoisomerase function in bacterial replication fork movement: use of DNA microarrays. Proc. Natl. Acad. Sci. U. S. A. 97, 9419–9424 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pham T. M. et al. A single-molecule approach to DNA replication in Escherichia coli cells demonstrated that DNA polymerase III is a major determinant of fork speed. Mol. Microbiol. 90, 584–596 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Skinner S. O., Sepúlveda L. A., Xu H. & Golding I. Measuring mRNA copy number in individual Escherichia coli cells using single-molecule fluorescent in situ hybridization. Nat. Protoc. 8, 1100–1113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray M. J., Wholey W.-Y., Parker B. W., Kim M. & Jakob U. NemR is a bleach-sensing transcription factor. J. Biol. Chem. 288, 13789–13798 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozyamak E., de Almeida C., de Moura A. P. S., Miller S. & Booth I. R. Integrated stress response of Escherichia coli to methylglyoxal: transcriptional readthrough from the nemRA operon enhances protection through increased expression of glyoxalase I. Mol. Microbiol. 88, 936–950 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee C., Shin J. & Park C. Novel regulatory system nemRA-gloA for electrophile reduction in Escherichia coli K-12. Mol. Microbiol. 88, 395–412 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Proshkin S., Rahmouni A. R., Mironov A. & Nudler E. Cooperation between translating ribosomes and RNA polymerase in transcription elongation. Science 328, 504–508 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pomerantz R. T. & O’Donnell M. The replisome uses mRNA as a primer after colliding with RNA polymerase. Nature 456, 762–766 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de la Fuente A., Palacios P. & Vicente M. Transcription of the Escherichia coli dcw cluster: evidence for distal upstream transcripts being involved in the expression of the downstream ftsZ gene. Biochimie 83, 109–115 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Zaslaver A., Mayo A., Ronen M. & Alon U. Optimal gene partition into operons correlates with gene functional order. Phys. Biol. 3, 183–189 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Zhu M., Mu H., Han F., Wang Q. & Dai X. Quantitative analysis of asynchronous transcription-translation and transcription processivity in under various growth conditions. iScience 24, 103333 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu M., Mori M., Hwa T. & Dai X. Disruption of transcription-translation coordination in Escherichia coli leads to premature transcriptional termination. Nat Microbiol 4, 2347–2356 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novick R. P., Christie G. E. & Penadés J. R. The phage-related chromosomal islands of Gram-positive bacteria. Nat. Rev. Microbiol. 8, 541–551 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jamrozy D. M. et al. Pan-genomic perspective on the evolution of the USA300 epidemic. Microb Genom 2, e000058 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cervera-Alamar M. et al. Mobilisation Mechanism of Pathogenicity Islands by Endogenous Phages in Staphylococcus aureus clinical strains. Sci. Rep. 8, 16742 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golding I. Revisiting Replication-Induced Transcription in Escherichia coli. Bioessays 42, e1900193 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guptasarma P. Does replication-induced transcription regulate synthesis of the myriad low copy number proteins of Escherichia coli? Bioessays 17, 987–997 (1995). [DOI] [PubMed] [Google Scholar]
- 49.Ray A., Edmonds K. A., Palmer L. D., Skaar E. P. & Giedroc D. P. Glucose-Induced Biofilm Accessory Protein A (GbaA) Is a Monothiol-Dependent Electrophile Sensor. Biochemistry 59, 2882–2895 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Loi V. et al. The two-Cys-type TetR repressor GbaA confers resistance under disulfide and electrophile stress in Staphylococcus aureus. Free Radic. Biol. Med. 177, 120–131 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirota Y., Ryter A. & Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb. Symp. Quant. Biol. 33, 677–693 (1968). [DOI] [PubMed] [Google Scholar]
- 52.Atlung T., Clausen E. S. & Hansen F. G. Autoregulation of the dnaA gene of Escherichia coli K12. Mol. Gen. Genet. 200, 442–450 (1985). [DOI] [PubMed] [Google Scholar]
- 53.Braun R. E., O’Day K. & Wright A. Autoregulation of the DNA replication gene dnaA in E. coli K-12. Cell 40, 159–169 (1985). [DOI] [PubMed] [Google Scholar]
- 54.Garrido T., Sánchez M., Palacios P., Aldea M. & Vicente M. Transcription of ftsZ oscillates during the cell cycle of Escherichia coli. EMBO J. 12, 3957–3965 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou P. & Helmstetter C. E. Relationship between ftsZ gene expression and chromosome replication in Escherichia coli. J. Bacteriol. 176, 6100–6106 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willis L. & Huang K. C. Sizing up the bacterial cell cycle. Nat. Rev. Microbiol. 15, 606–620 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Palacios P., Vicente M. & Sánchez M. Dependency of Escherichia coli cell-division size, and independency of nucleoid segregation on the mode and level of ftsZ expression. Mol. Microbiol. 20, 1093–1098 (1996). [DOI] [PubMed] [Google Scholar]
- 58.Tétart F. & Bouché J. P. Regulation of the expression of the cell-cycle gene ftsZ by DicF antisense RNA. Division does not require a fixed number of FtsZ molecules. Mol. Microbiol. 6, 615–620 (1992). [DOI] [PubMed] [Google Scholar]
- 59.Cooper S. The Escherichia coli cell cycle. Res. Microbiol. 141, 17–29 (1990). [DOI] [PubMed] [Google Scholar]
- 60.Lin J. & Amir A. Homeostasis of protein and mRNA concentrations in growing cells. Nat. Commun. 9, 4496 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chandler M. G. & Pritchard R. H. The effect of gene concentration and relative gene dosage on gene output in Escherichia coli. Mol. Gen. Genet. 138, 127–141 (1975). [DOI] [PubMed] [Google Scholar]
- 62.Hammar P. et al. Direct measurement of transcription factor dissociation excludes a simple operator occupancy model for gene regulation. Nat. Genet. 46, 405–408 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weigel W. A. & Dersch P. Phenotypic heterogeneity: a bacterial virulence strategy. Microbes Infect. 20, 570–577 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Baral B., Akhgari A. & Metsä-Ketelä M. Activation of microbial secondary metabolic pathways: Avenues and challenges. Synth Syst Biotechnol 3, 163–178 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma P. et al. Bacterial droplet-based single-cell RNA-seq reveals antibiotic-associated heterogeneous cellular states. Cell (2023) doi: 10.1016/j.cell.2023.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McNulty R., Sritharan D., Liu S., Hormoz S. & Rosenthal A. Z. Droplet-based single cell RNA sequencing of bacteria identifies known and previously unseen cellular states. Preprint at 10.1101/2021.03.10.434868. [DOI]
- 67.Wang B. et al. Massively-parallel Microbial mRNA Sequencing (M3-Seq) reveals heterogeneous behaviors in bacteria at single-cell resolution. Preprint at 10.1101/2022.09.21.508688. [DOI]
- 68.Wang Q. et al. Tagmentation-based whole-genome bisulfite sequencing. Nat. Protoc. 8, 2022–2032 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Wolf F. A., Angerer P. & Theis F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levin M. et al. The mid-developmental transition and the evolution of animal body plans. Nature 531, 637–641 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zalts H. & Yanai I. Developmental constraints shape the evolution of the nematode mid-developmental transition. Nat Ecol Evol 1, 113 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Jammalamadaka S. R., Rao Jammalamadaka S. & SenGupta A. Topics in Circular Statistics. Series on Multivariate Analysis Preprint at 10.1142/4031 (2001). [DOI] [Google Scholar]
- 73.Stan Development Team. RStan: the R interface to Stan. (2021).
- 74.Buitinck L. et al. API design for machine learning software: experiences from the scikit-learn project. arXiv [cs.LG] (2013) doi: 10.48550/ARXIV.1309.0238. [DOI] [Google Scholar]
- 75.Stuart T. et al. Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902.e21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seabold S. & Perktold J. Statsmodels: Econometric and statistical modeling with python. in Proceedings of the 9th Python in Science Conference (SciPy, 2010). doi: 10.25080/majora-92bf1922-011. [DOI] [Google Scholar]
- 77.Keseler I. M. et al. The EcoCyc Database in 2021. Front. Microbiol. 12, 711077 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karp P. D. et al. The BioCyc collection of microbial genomes and metabolic pathways. Brief. Bioinform. 20, 1085–1093 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fuchs S. et al. AureoWiki-The repository of the Staphylococcus aureus research and annotation community. Int. J. Med. Microbiol. 308, 558–568 (2018). [DOI] [PubMed] [Google Scholar]
- 80.Smith T., Heger A. & Sudbery I. UMI-tools: modeling sequencing errors in Unique Molecular Identifiers to improve quantification accuracy. Genome Res. 27, 491–499 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Michelsen O., Teixeira de Mattos M. J., Jensen P. R. & Hansen F. G. Precise determinations of C and D periods by flow cytometry in Escherichia coli K-12 and B/r. Microbiology 149, 1001–1010 (2003). [DOI] [PubMed] [Google Scholar]
- 83.Love M. I., Huber W. & Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yao T., Coleman S., Nguyen T. V. P., Golding I. & Igoshin O. A. Bacteriophage self-counting in the presence of viral replication. Proc. Natl. Acad. Sci. U. S. A. 118, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ronneberger O., Fischer P. & Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. arXiv [cs.CV] (2015). [Google Scholar]
- 86.Acemel R. D., Govantes F. & Cuetos A. Computer simulation study of early bacterial biofilm development. Sci. Rep. 8, 5340 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cullum J. & Vicente M. Cell growth and length distribution in Escherichia coli. J. Bacteriol. 134, 330–337 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Heerden J. H. et al. Statistics and simulation of growth of single bacterial cells: illustrations with B. subtilis and E. coli. Sci. Rep. 7, 16094 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wallden M., Fange D., Lundius E. G., Baltekin Ö. & Elf J. The Synchronization of Replication and Division Cycles in Individual E. coli Cells. Cell 166, 729–739 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Chen J., Yoong P., Ram G., Torres V. J. & Novick R. P. Single-copy vectors for integration at the SaPI1 attachment site for Staphylococcus aureus. Plasmid 76, 1–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benson M. A. et al. Staphylococcus aureus regulates the expression and production of the staphylococcal superantigen-like secreted proteins in a Rot-dependent manner. Mol. Microbiol. 81, 659–675 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arndt D. et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 44, W16–21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bae T., Glass E. M., Schneewind O. & Missiakas D. Generating a collection of insertion mutations in the Staphylococcus aureus genome using bursa aurealis. Methods Mol. Biol. 416, 103–116 (2008). [DOI] [PubMed] [Google Scholar]
- 94.Fey P. D. et al. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. MBio 4, e00537–12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.So L.-H. et al. General properties of transcriptional time series in Escherichia coli. Nat. Genet. 43, 554–560 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karp P. D. et al. The EcoCyc Database. EcoSal Plus 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aquino P. et al. Coordinated regulation of acid resistance in Escherichia coli. BMC Syst. Biol. 11, 1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hayashi S. et al. Analysis of organic solvent tolerance in Escherichia coli using gene expression profiles from DNA microarrays. J. Biosci. Bioeng. 95, 379–383 (2003). [DOI] [PubMed] [Google Scholar]
- 99.Gui L., Sunnarborg A., Pan B. & LaPorte D. C. Autoregulation of iclR, the gene encoding the repressor of the glyoxylate bypass operon. J. Bacteriol. 178, 321–324 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Campos E., Baldoma L., Aguilar J. & Badia J. Regulation of expression of the divergent ulaG and ulaABCDEF operons involved in LaAscorbate dissimilation in Escherichia coli. J. Bacteriol. 186, 1720–1728 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kalivoda K. A., Steenbergen S. M. & Vimr E. R. Control of the Escherichia coli sialoregulon by transcriptional repressor NanR. J. Bacteriol. 195, 4689–4701 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cai J. & DuBow M. S. Expression of the Escherichia coli chromosomal ars operon. Can. J. Microbiol. 42, 662–671 (1996). [DOI] [PubMed] [Google Scholar]
- 103.Maloy S. R. & Nunn W. D. Genetic regulation of the glyoxylate shunt in Escherichia coli K-12. J. Bacteriol. 149, 173–180 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Torrents E. et al. NrdR controls differential expression of the Escherichia coli ribonucleotide reductase genes. J. Bacteriol. 189, 5012–5021 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vassinova N. & Kozyrev D. A method for direct cloning of fur-regulated genes: identification of seven new fur-regulated loci in Escherichia coli. Microbiology 146 Pt 12, 3171–3182 (2000). [DOI] [PubMed] [Google Scholar]
- 106.Ibañez E., Campos E., Baldoma L., Aguilar J. & Badia J. Regulation of expression of the yiaKLMNOPQRS operon for carbohydrate utilization in Escherichia coli: involvement of the main transcriptional factors. J. Bacteriol. 182, 4617–4624 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aguilera L. et al. Dual role of LldR in regulation of the lldPRD operon, involved in L-lactate metabolism in Escherichia coli. J. Bacteriol. 190, 2997–3005 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All counts matrices and raw sequencing reads used to perform the scRNA-seq analysis are available in the Gene Expression Omnibus (GEO) under the accession number GSE217715.