Abstract
INTRODUCTION
In recent years, growing attention is rising to virtual reality (VR) tools and exergaming in rehabilitation management of patients with Parkinson disease (PD). However, no strong evidence supports the effectiveness of these cutting-edge technologies on cognitive function and the integration of these promising tool in the rehabilitation framework of PD patients is still challenging. Therefore, the present systematic review of randomized controlled trials (RCTs) aimed at assessing the effects of VR and exergames/telerehabilitation in the cognitive rehabilitation management of patients with PD.
EVIDENCE ACQUISITION
PubMed, Scopus and Web of Science databases were systematically searched up to February 14th, 2022, to identify RCTs assessing patients with PD undergoing cognitive rehabilitation including VR or exergames/telerehabilitation. The intervention was compared to conventional rehabilitation protocols. The primary outcome was cognitive function. The quality assessment was performed following the Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2). PROSPERO registration code: CRD42022319788.
EVIDENCE SYNTHESIS
Out of 1419 identified studies, 66 articles were assessed for eligibility, and, at the end of the screening process, 10 studies were included in the present systematic review. Five RCTs (50%) assessed the exergaming devices, reporting significant positive results on cognitive outcomes scales (Trail Making test scale, Digit Span backward, MoCA, and MyCQ score). The other 5 RTCs (50%) assessed VR approaches, reporting significant improvement in executive functions. The RoB 2 showed an overall high risk of bias for the 40% of studies included.
CONCLUSIONS
Exergaming and VR might be considered promising rehabilitation interventions in the cognitive rehabilitation framework of PD patients. Further high-quality studies are needed to define the role of exergames and VR in a comprehensive rehabilitation approach aiming at improving the multilevel cognitive impairment characterizing patients with PD.
Key words: Virtual reality, Exergaming, Parkinson disease, Cognitive dysfunction, Rehabilitation
Introduction
Parkinson disease (PD) is one of the most common progressive neurodegenerative disease worldwide and its prevalence is increasing with age and affects 1% of the population above 60 years.1 The patient with Parkinson’s disease is affected by motor disorders such as resting tremor, bradykinesia, rigidity and postural instability, which condition balance, gait, and quality of movement,2 but also non-motor disorders, such as dementia, dysautonomia and cognitive deficits.3, 4 Cognitive impairment is up to six times more expected in people with PD than in healthy populations.5 So, asunder from principal motor traits, PD is associated with a heterogeneous range of non-motor manifestations that contribute greatly to the general condition burden.6 Multiple cognitive domains are affected in those with cognitive impairment and PD, including, memory, attention, visuospatial abilities, and especially executive functions.7 Therefore, cognitive impairment seems to be typically associated with gait disorders, such as slower speed and shorter steps as well as poor postural control.8 Taken together, all these features lead to a relevant impairment in basic daily activities and quality of life with a high burden on caregivers and families, even in the early phases of PD.9 Nowadays, therapeutic management in the initial phase aims at an optimal compromise between symptom control and side effects deriving from the enhancement of the dopaminergic function.10 Levodopa administration might be delayed using other drugs, such as MAO-B inhibitors and dopamine agonists, to delay potential dyskinesia onset.11 In the second phase, the intent is to relieve symptoms, and in drug therapy, the surgical approach and deep brain stimulation might demonstrate usefully.12 On other hand, the treatment of cognitive impairment in PD lag far behind our knowledge. Most randomized controlled trials (RCTs) focusing on cognitive outcomes have been performed on PD with dementia. However, these patients together with dementia with Lewy bodies (DLB) are often considered as part of a broader and different clinic-pathological entity called Lewy body dementia,13-17 and to date, the only unambiguously positive RCT for PDD treatment was performed with the cholinesterase inhibitor (ChEI) rivastigmine.18 Continued efforts for a better comprehension of this complex feature of PD are required, considering that, at present, there is no pharmacologic treatment to prevent or delay cognitive decline in PD.5 Multidisciplinary is increasingly recognized as a crucial point in PD management, such as a complex rehabilitative approach.19-21 Conventional rehabilitation plays a pivotal role in PD and it is considered as an adjuvant to pharmacological and surgical treatments to improve many dysfunctions and self-care ability and even delay the progression of the disease.21, 22 In addition to conventional applications of physiotherapy and rehabilitation, the use of technological interventions are a promising feature of the complex PD rehabilitation framework, and in particular virtual reality approaches are suggested as potentially useful tools for these patients.23 The basic neuroscience pathways underpinning VR-based treatment are mirror neurons in the primary motor cortex, dorsal premotor cortex and supplementary motor area.24, 25 The evidence from human neuroimaging suggested that the neural effects of VR on neural plasticity and motor reorganization in humans might stimulate the internal sensorimotor system through the activation of mirror neurons in the cortical and subcortical motor control-related areas and cerebellum.26 Indeed, VR might enhance the interaction with surrounding artificial environment, created to appear similar to the original one, provided through a display that can be also head-mounted, with complementary motion tracking devices, visual and auditory cues and eventually end-effectors like joystick, or more advanced devices intercepting even muscle and brain signals.27 VR has been integrated in the rehabilitation of several neurological diseases with promising results.28-31 As a complementary tool of VR in rehabilitation programs,32 patients can also perform exergames, in association or not with tele-rehabilitation programs, defined as the activities of playing video games involving physical exertion.33 Active video game therapy could reduce the boredom of the rehabilitation process, increasing patient motivation, providing direct feedback, and enabling dual-task training. In this way, commercially available exergames (e.g. Nintendo Wii, Sony Move, Microsoft Kinect) have successfully transformed living rooms into playful training environments for about 10 years.34 Clinical and home trials have been conducted to investigate the effectiveness of Nintendo Wii Fit in PD, primarily for improving motor symptoms, but the results are significant but only preliminary.35-39 VR certainly has the potential to add value to conventional rehabilitation by providing scenarios and challenges that would be difficult to recreate safely in a real-world situation. It also facilitates a task being repeated multiple times using the same conditions and can track progress using different metrics within the environment providing immediate visual feedback.40 For example, in stroke patients, VR may be beneficial in improving activities of daily living function when used as an adjunct to usual care (to increase overall therapy time) but there was insufficient evidence to reach conclusions about their effects on gait speed, balance, participation, quality of life or cognitive function.41 Therefore, a recent survey on the use of immersive VR to improve cognitive function in dementia and mild cognitive impairment was unable to provide quantitative results regarding the use, acceptability, and effectiveness of this approach.42 To the best of our knowledge, few papers investigated the efficacy of VR to treat cognitive impairment occurring in PD patients. Therefore, by the present systematic review, we sought to evaluate the efficacy of virtual reality and exergames/telerehabilitation compared with conventional rehabilitation in terms of cognitive outcomes in PD.
Evidence acquisition
Search strategy
PubMed, Scopus, Web of Science and CENTRAL databases were systematically searched for English-language articles published from the inception until Feb 14th, 2022, according to each specific thesaurus, following the strategy depicted by Table I.
Table I. —Search strategy.
| PubMed (“Parkinson” OR “Parkinsonism” OR “Parkinson disease”) AND (“remote” OR “home” OR “tele” OR “video” OR “augmented reality” OR “visual augmentation” OR “VR” OR “virtual” OR “game” OR “exergaming” OR “wii” OR “kinect” OR “console” OR “consolle” OR “controller” OR “nirvana”) AND (“attention” OR “orientation” OR “mental” OR “memory” OR “memories” OR “cognitive” OR “cognition”) |
| Scopus TITLE-ABS-KEY(((“Parkinson” OR “Parkinsonism” OR “Parkinson disease”) AND (“remote” OR “home” OR “tele” OR “video” OR “augmented reality” OR “visual augmentation” OR “VR” OR “virtual” OR “game” OR “exergaming” OR “wii” OR “kinect” OR “console” OR “consolle” OR “controller” OR “nirvana”) AND (“attention” OR “orientation” OR “mental” OR “memory” OR “memories” OR “cognitive” OR “cognition”))) |
| Web of Science ((“Parkinson” OR “Parkinsonism” OR “Parkinson disease”) AND (“remote” OR “home” OR “tele” OR “video” OR “augmented reality” OR “visual augmentation” OR “VR” OR “virtual” OR “game” OR “exergaming” OR “wii” OR “kinect” OR “console” OR “consolle” OR “controller” OR “nirvana”) AND (“attention” OR “orientation” OR “mental” OR “memory” OR “memories” OR “cognitive” OR “cognition”)) |
| CENTRAL (“Parkinson” OR “Parkinsonism” OR “parkinson disease”) AND (“remote” OR “home” OR “tele” OR “video” OR “augmented reality” OR “visual augmentation” OR “VR” OR “virtual” OR “game” OR “exergaming” OR “wii” OR “kinect” OR “console” OR “consolle” OR “controller” OR “nirvana”) AND (“attention” OR “orientation” OR “mental” OR “memory” OR “memories” OR “cognitive” OR “cognition”) in Title Abstract Keyword - (Word variations have been searched) |
This systematic review followed the guidance of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines43 and the Cochrane Handbook for Systematic Reviews of Interventions.44 Systematic review protocol has been registered on the International Prospective Register of Systematic Reviews (PROSPERO) (number: CRD42022319788).
Selection criteria
After removing duplicates, two reviewers independently screened title and abstract of all articles for eligibility. In case of disagreement, a consensus was reached with the opinion of a third reviewer.
Full-text screening was subsequently performed by the two Authors. If a consensus was not achieved, any disagreement was resolved by consulting one of the other Authors.
RCTs were considered eligible if responding to the questions defined by the following PICOS model:
-
Participants: Patients with PD;
-
Intervention: virtual reality and exergames;
-
Comparator: conventional rehabilitation;
-
Outcome measure: cognitive impairments;
Study design: RCTs with two groups (study group and control group)
-
-
-
Moreover, we included only manuscripts providing data at the end of the intervention (after 1 week later as maximum). We excluded: 1) studies including patients aged <18 years; 2) cross-over study design; 3) studies written in a language different from English; 4) full-text unavailability (i.e., posters and conference abstracts); 5) studies involving animals.
Data extraction
Two reviewers independently extracted main data from the included RCTs, through a customized data extraction model on a Microsoft Excel sheet. We used for the independent evaluation of the studies the Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration, 2020. In case of disagreement, a consensus was obtained asking an opinion of another reviewer. We extracted the following data: 1) first author; 2) publication year; 3) nationality; 4) age of study participants; 5) type of virtual reality and/or exergames as intervention; 6) type of control (conventional rehabilitation); 7) population and number of patients included in the RCTs; 8) cognitive outcome scale values as outcome measure; 9) main findings.
Data synthesis and risk of bias
The RCTs were synthesized describing extracted data. To evaluate the quality of evidence included in this review, we adopted the Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2). As depicted in Figure 1, we assessed the five risk-of-bias domains,45 and discussed any disagreements until consensus was reached with a third reviewer.
Figure 1.
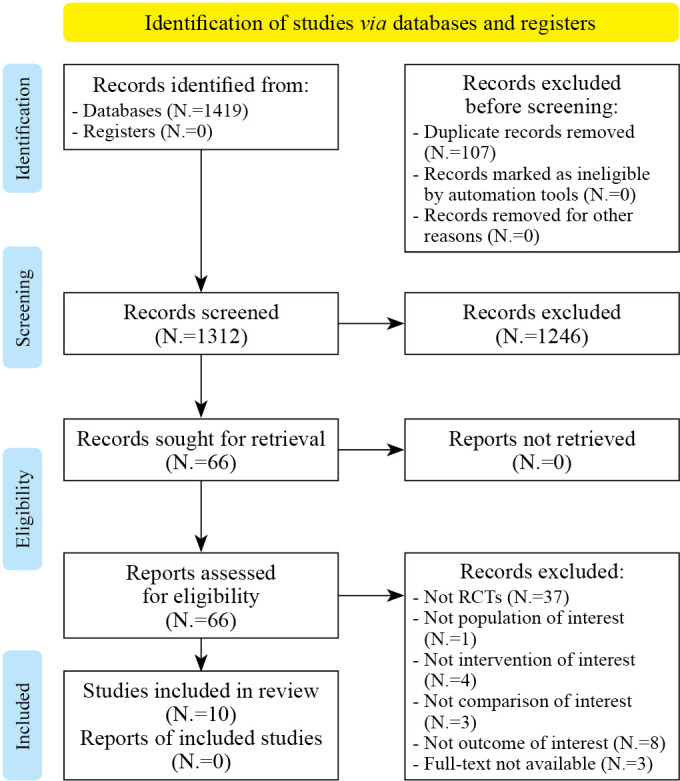
—PRISMA 2020 flow diagram for systematic review.
Evidence synthesis
Study characteristics
According to the guidance of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,43 at the end of the search, 1419 studies were identified. After the removal of 107 duplicates, 1312 records were considered suitable for title and abstract screening, after the removal of duplicates. Out of these, 1246 were excluded after the title and abstract screening, according to the PICO model. Thus, 66 articles were assessed for eligibility and 56 of them were excluded with reasons: not study design of interest N.=37), not population of interest N.=1), not intervention of interest (N.=4), not comparison of interest N.=3), not outcome of interest N.= 8), full text not available N.=3). Therefore, 10 RCTs36, 46-54 were included in this systematic review, as depicted by the PRISMA flowchart in Figure 1. The main characteristics of these studies are described in detail in Supplementary Digital Material 1 (Supplementary Table I). The included studies36, 46-54 have been published in the last 10 years (from 2012 to 2020). Seven36, 48-51, 53, 54 (70%) were conducted in Europe (336, 49, 50, from Italy, 148 from Belgium, 151 from Israel/Netherland, 153 from Netherland, 154 from Switzerland), 247, 52 (20%) from Brazil, and 146 (10%) from Australia. A total of 453 subjects were analyzed, of which 230 performed VR training and 223 were included in the control group (undergone conventional training). Study cohorts of the RCTs included ranged from 12148 to 2050 patients, with a mean age ranging from 58.89±11.16 years47 to 73.1±1.1 years.51 Concerning Hoehn & Yahr stage, three36, 46, 54 studies included patient in stage ≤V, three studies48, 49, 51 in stage II-III, two47, 53 stage ≤III, 150 in stage <III, and 152 in stage ≤II. Regarding the follow-up evaluations, 2 RCTs48, 53 performed a follow-up at 24 weeks from baseline, 147 at 9 weeks, and 152 at 15 weeks. Five RCTs46, 47, 52-54 investigated the effectiveness of exergaming, five36, 48-51 investigated the effectiveness of VR.
Exergaming
Five RCTs46, 47, 52-54 assessed exergames as an intervention to improve cognitive function. Allen et al.46 showed a significant improvement in Trail Making test part A in the experimental group after therapy (32.3±10.7 vs. 38.6±15.2: P<0.07). Alves et al.47 reported a significant improvement in Nintendo Wii group in Digit Span backward at follow-up when comparing with baseline and T1 (4.44±2.24 vs. 4.43±1.29 vs. 6.00±2.12; P=0.002) and in Back anxiety inventory at T1 (11.33±9.92 vs. 7.19±4.73; P=0.045), and T2 (6.24±3.97; P=0.031), compared to another exergame device (Microsoft Kinect). On other hand, Pompeu et al.52 showed a significant difference in MOCA score after treatment and at follow-up both in EG group (20.6±4.5 vs. 22.2±4.5 vs. 21.8±4.5; P<0.05) and in CG group (21.7±4.6 vs. 23.1±4.6 vs. 23.3±3.4; P<0.05). Van de Weijer et al.53 investigated the efficacy of an online cognitive game (AquaSnapTM) at home, showing a significant improvement in Global cognition MyCQ score in EG group compared with waiting list group (0.149±0.275 and -0.175±0.680; P=0.049). However, no significant difference between groups was observed at follow-up. At last, Zimmermann et al.54 compared Nintendo Wii and a specific cognitive exergame program (CogniPlus), showing a significantly greater enhancement of attention after Wii training compared with CogniPlus (0.5±0.8 vs. -0.3±1.2; P<0.024).
Virtual reality
Five36, 48-51 studies have investigated the effectiveness of VR on cognitive function in PD patients. Bekkers et al.48 in 2020 reported a significant effect of time for Trail Making test part B equally in treadmill+VR group (173.87 vs. 150.67 vs. 164.30; P<0.001) and in treadmill group (171.05 vs. 153.01 vs. 158.44; P<0.001) after training and at 6-month follow-up. A similar protocol was used by Maidan et al.51 They found a significant improvement in executive function in treadmill+VR and in treadmill group (P=0.032 both) after training, but no differences were found between groups. Fundarò et al.49 used a Robotic Assisted Gait Training+VR compared with a conventional training, but no differences were found between groups in changes for FIM cognitive subscale (0.5±1.0 vs. 0.3±0.7; P=0.65). Maggio et al.50 used a semi-immersive VR system (Nirvana, BTS) compared with a conventional training. Their results showed a significant difference between groups in favor of VR group in global cognitive score (77.5 [59.0-88.3] vs. 70.5 [57.5-72.5]; P<0.0001), in Attention and Orientation, (16.0 [15.3-18.0] vs. 14.5 [12.0-16.8]; P<0.001), in language fluency (9.0 [4.3-11.8] vs. 6.0 [4.3-7.0]; P<0.001), and visual spatial ability (14.0 [11.0-14.8] vs. 9.5 (6.0-10.0); P<0.0001). Moreover, significant improvement between groups in favor of VR were found in frontal ability at FAB scale (15.3 [11.8-15.9] vs. 13.9 [12.3-15.0]; P<0.001) and at Mini-Mental State Examination (24.1 [22.7-27.1] vs. 23.9 [21.4-25.1]; P=0.014). Similar results were found by Pazzaglia et al.36 The authors showed a significant improvement in Short-form 36 mental composite score only in semi-immersive VR group (37.7±11.4 vs. 43.5±9.2; P<0.05).
Risk of bias
To evaluate the quality of evidence included in this review, we adopted the Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2). As depicted in Figure 2, we assessed the five risk-of-bias domains.46-55
Figure 2.
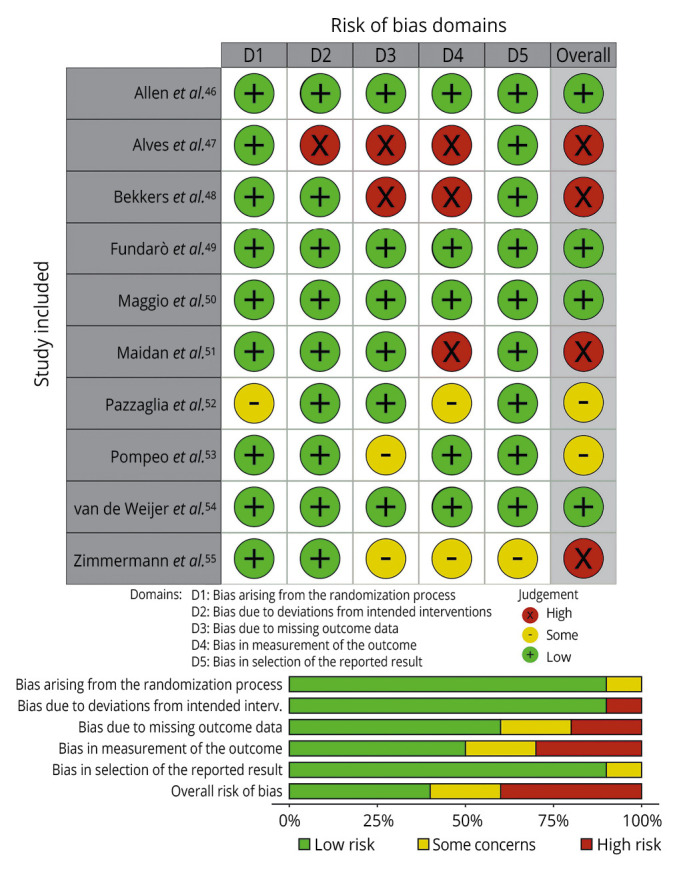
—Risk of bias of the included studies.46-55
All included items had full-text availability. Out of 10 studies, 4 RCTs were judged to have a low overall risk of bias, in 2 there are some concerns, and 4 had a high risk of bias. Despite this, we reported low risk with respect to randomization (90% of low risk) and deviations from intended intervention (90% of low risk). A low risk of bias was judged regarding the lack of outcome data; however, the included studies reported a lack of appropriate outcome measurement with only 50% of low-risk studies. Finally, we reported a low risk of bias for outcome selection reported in 90% low-risk studies.
Discussion
Cognitive impairment in neurodegenerative diseases is an often-neglected issue with relevant implications in terms of disability, HRQol and care-givers burden. In this context, digital innovation and new technologies might be crucial to implement the complex rehabilitative framework of these patients in both clinical and home setting. In this systematic review we tried to synthesize the existing evidence about VR and exergames/telerehabilitation to explore the impact on cognitive outcomes of these interventions in PD patients. Moreover, as far as we know, this is the first systematic review focusing on this topic.
The approaches identified in this review differed greatly across studies. Five trials46, 47, 52-54 used the exergaming devices, reporting significant results on the Trail Making test scale, Digit Span backward, MoCA, and MyCQ Score. The other five RTCs36, 48-51 explored VR approaches, showing significant improvement in executive function in combination with the treadmill but without significant quantitative differences in cognitive outcomes. However, the immersive and semi-immersive models, decoupled to the treadmill, showed significant cognitive improvements compared to conventional rehabilitation.55
More in detail, Exergaming is a novel exercise model that likewise integrates physical and cognitive exercise in an interactive digital, augmented, or virtual game-like environment.55To date, playing video games appears to be advantageous for cognitive functions, suggesting its possible use as a computerized cognitive training.56 The types of exergame can be approximately divided into three separate classes: 1) dance and step video games; 2) commercial video game consoles; and 3) interactive virtual ergometers (cyber cycle and virtual kayak ergometer).56 Although exergaming is a combination of gaming and exercise, due to the diversity of the platforms used to manage these interventions, it was difficult to determine which exergame was the best to improve cognitive functions in PD patients.57 Alves et al. reported that Nintendo Wii, compared to Microsoft Kinect, significantly improved anxiety levels, memory and attention these patients.47 In this scenario, Nintendo Wii is a commercial gaming device that employs a manual wireless controller and a force platform dubbed “Balance Board”. On the other hand, the Microsoft Kinect tracks body motions with an infrared camera that identifies real-time three-dimensional movements, permitting user interaction with the game environment without any controller.47 Consequently, Xbox KinectTM does not enclose visual references that delimit the sensor-capable space. As PD patients commonly show impairments in terms of divided attention, dual tasks, inhibition of response and sustained attention, Xbox Kinect games may be more complex and less compelling rehabilitative tools for these patients.30 Moreover, the Microsoft Kinect graphic has more details, providing more interference through distractors for PD patients whose cognitive alterations may comprise visuospatial disorders, slow decision strategies and selective attention problems.58 In light of these considerations, Alves et al. reported that PD patients appear to experience more advantages in the less detailed and augmented interface, with material references such as the force platform and controllers of the Nintendo Wii, compared to the Microsoft Kinect.47
Another important consideration should be done about distinct exergame approaches that varies in their physical and cognitive needs. However, as most studies have not systematically reported and checked physical and cognitive demands, we can only speculate on the distinguishing needs of the diverse exergame approaches.59 In this context, virtual ergometers, dance video game platforms or mats can provide limited physical-cognitive training outcomes based on lower play contexts, similar physical activity conditions and cognitive demand.60 On the other hand, exercise intensity may be easier to manage with virtual ergometers or dance systems by controlling game pace and heart rate measurements, which guarantees a relatively steady physical-cognitive and individually flexible intensity level.57 In contrast, commercial device consoles exert variable and relatively determined training requests relying on standard commercial games, standard breaks and standard success in each game level up.47 In conclusion, exergames could vary considerably in physical-cognitive demands, anyhow commercial devices are more accessible, with a simpler more user-friendly environment. Lastly, with a visual reference as the platform, the Wii device appears to provide, but above all satisfy appropriate PD cognitive needs.
VR models simulate the activities of the real world, in the context of an environment enriched by graphic interfaces and audio-visual feedback, allowing the patient to carry out cognitive and motor activities at the same time. These VR settings can virtually involve the outside world, in a safe and regulated clinical environment, avoiding risky activities during therapeutic sessions. Furthermore, they can engage patients in more intense and frequent rehabilitative programs as these approaches are more stimulating and entertaining than conventional rehabilitation programs.61 Through the replication of real-life scenarios, VR technology provides greater potential for transfer to functional activities of daily living. However, to date, it remains unclear how VR technology may be optimally used and adjusted to the specific cognitive demands of PD patients.62
In almost all the included studies on VR,36, 48, 49, 51 these novel rehabilitation approach showed to obtain to an improvement in the global mental domain of HRQoL, and not only in specific cognitive facets. This result may be due, in part, to the fact that during the VR program the patient perceives himself as an active part and center of the treatment, compared to a more passive role in conventional rehabilitative interventions.63 Nevertheless, this might also imply that PD patients have usually gone through a long period of conventional rehabilitation thus, a VR model may be perceived as new and more intriguing.36 Nor can it be underestimated that the coupling to a conventional rehabilitative program does not bring further benefits, but this could be justified by the complex of dual-task models in the complex management of this disease and this could be supported by the positive benefits of VR when decoupled from the treadmill.36, 48, 49, 51 Lastly, cognitive disorders can be different in both clinical manifestation and severity and patients with major deficits are probably less friendly and uncomfortable in interacting with technological devices and may be less inclined to use them.64
Limitations of the study
This systematic review is not free from limitations. The first is that we found a moderate-high methodological quality in all the included studies. However, the lack of standardized consensus on the cognitive assessment of these patients led to a large number of inconsistent outcome measures, which prevented a quantitative meta-analysis from being performed. Furthermore, we also found a high heterogeneity of the interventions performed with great differences across studies. There was only one study comparing commercial devices and considering the small number of trials it is not clear to define the role of coupling to VR gait training. Moreover, we should underline that in most of the included studies the cognitive assessment was a secondary outcome. Lastly, almost all studies had cognitive assessment as a secondary outcome. However, it should be noted that, to the best of our knowledge, this is the first systematic review that investigated the role of exergaming and virtual reality on cognition in patients with PD.
Conclusions
Taken together, findings of this systematic review showed a positive impact of exergames and VR on cognitive impairment in PD patients. Thus, these two innovative and technological interventions might be part of the complex rehabilitative treatment framework of PD patients. However, the clinical significance of the effects observed on the parameters of visuospatial disorders, slow decision strategies and selective attention disturbances remain still unclear, although a multidisciplinary approach involving exergames and VR can be regardless beneficial in these patients in terms of stimulation, participation, and engagement. In light of these considerations, more high-quality research is needed to better define the role of exergames and VR to implement cognitive rehabilitation strategies in PD patients. This could implement standardized, comprehensive, and appropriate strategies to implement the clinical management of these patients.
Supplementary Digital Material 1
Supplementary Table I
Main characteristics of the studies included in this systematic review.
References
- 1.Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 2017;124:901–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28150045&dopt=Abstract 10.1007/s00702-017-1686-y [DOI] [PubMed] [Google Scholar]
- 2.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9923759&dopt=Abstract 10.1001/archneur.56.1.33 [DOI] [PubMed] [Google Scholar]
- 3.Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson’s disease. Lancet Neurol 2006;5:75–86. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16361025&dopt=Abstract 10.1016/S1474-4422(05)70285-4 [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, Karlsen K. Neuropsychiatric aspects of Parkinson’s disease. Curr Psychiatry Rep 1999;1:61–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11122906&dopt=Abstract 10.1007/s11920-999-0011-3 [DOI] [PubMed] [Google Scholar]
- 5.Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers 2021;7:47. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34210995&dopt=Abstract 10.1038/s41572-021-00280-3 [DOI] [PubMed] [Google Scholar]
- 6.Chandler JM, Nair R, Biglan K, Ferries EA, Munsie LM, Changamire T, et al. Characteristics of Parkinson’s Disease in Patients with and without Cognitive Impairment. J Parkinsons Dis 2021;11:1381–92. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33720850&dopt=Abstract 10.3233/JPD-202190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey PD. Domains of cognition and their assessment . Dialogues Clin Neurosci 2019;21:227–37. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31749647&dopt=Abstract 10.31887/DCNS.2019.21.3/pharvey [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SM, Kim DH, Yang Y, Ha SW, Han JH. Gait Patterns in Parkinson’s Disease with or without Cognitive Impairment. Dement Neurocognitive Disord 2018;17:57–65. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30906393&dopt=Abstract 10.12779/dnd.2018.17.2.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leroi I, McDonald K, Pantula H, Harbishettar V. Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol 2012;25:208–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23172765&dopt=Abstract 10.1177/0891988712464823 [DOI] [PubMed] [Google Scholar]
- 10.Silva RC, Domingues HS, Salgado AJ, Teixeira FG. From regenerative strategies to pharmacological approaches: can we fine-tune treatment for Parkinson’s disease? Neural Regen Res 2022;17:933–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34558504&dopt=Abstract 10.4103/1673-5374.324827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Paula Vasconcelos A. Parkinson’s disease rehabilitation: effectiveness approaches and new perspectives. In: Bernardo-Filho M, Cunha de Sá-Caputo D, Taiar R. Physical Therapy Effectiveness. London: IntechOpen; 2019. [Google Scholar]
- 12.Gilbert R, Khemani P. Treatment of Advanced Parkinson’s Disease. J Geriatr Psychiatry Neurol 2022;35:12–23. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33511915&dopt=Abstract 10.1177/0891988720988904 [DOI] [PubMed] [Google Scholar]
- 13.Varela-López A, Giampieri F, Battino M, Quiles JL. Coenzyme Q and Its Role in the Dietary Therapy against Aging. Molecules 2016;21:373. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26999099&dopt=Abstract 10.3390/molecules21030373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emre M, Tsolaki M, Bonuccelli U, Destée A, Tolosa E, Kutzelnigg A, et al. ; 11018 Study Investigators. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:969–77. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20729148&dopt=Abstract 10.1016/S1474-4422(10)70194-0 [DOI] [PubMed] [Google Scholar]
- 15.Manenti R, Brambilla M, Benussi A, Rosini S, Cobelli C, Ferrari C, et al. Mild cognitive impairment in Parkinson’s disease is improved by transcranial direct current stimulation combined with physical therapy. Mov Disord 2016;31:715–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26880536&dopt=Abstract 10.1002/mds.26561 [DOI] [PubMed] [Google Scholar]
- 16.Cerasa A, Quattrone A. The effectiveness of cognitive treatment in patients with Parkinson’s disease: a new phase for the neuropsychological rehabilitation. Parkinsonism Relat Disord 2015;21:165. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25443555&dopt=Abstract 10.1016/j.parkreldis.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 17.Wang HF, Yu JT, Tang SW, Jiang T, Tan CC, Meng XF, et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J Neurol Neurosurg Psychiatry 2015;86:135–43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24828899&dopt=Abstract 10.1136/jnnp-2014-307659 [DOI] [PubMed] [Google Scholar]
- 18.Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, De Deyn PP, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med 2004;351:2509–18. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15590953&dopt=Abstract 10.1056/NEJMoa041470 [DOI] [PubMed] [Google Scholar]
- 19.van der Marck MA, Munneke M, Mulleners W, Hoogerwaard EM, Borm GF, Overeem S, et al. IMPACT study group . Integrated multidisciplinary care in Parkinson’s disease: a non-randomised, controlled trial (IMPACT). Lancet Neurol 2013;12:947–56. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23988337&dopt=Abstract 10.1016/S1474-4422(13)70196-0 [DOI] [PubMed] [Google Scholar]
- 20.Ferrazzoli D, Ortelli P, Zivi I, Cian V, Urso E, Ghilardi MF, et al. Efficacy of intensive multidisciplinary rehabilitation in Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry 2018;89:828–35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29321141&dopt=Abstract 10.1136/jnnp-2017-316437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seid AA, Demirdel E, Aychiluhm SB, Mohammed AA. Multidisciplinary Rehabilitation for People with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Parkinsons Dis 2022;2022:2355781. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35265314&dopt=Abstract 10.1155/2022/2355781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petraroli A, de Sire A, Pino I, Moggio L, Marinaro C, Demeco A, et al. Effects of rehabilitation on reducing dyskinesias in a Parkinson’s disease patient abusing therapy with levodopa-carbidopa intestinal gel: a paradigmatic case report and literature review. J Biol Regul Homeost Agents 2021;35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34337930&dopt=Abstract [DOI] [PubMed]
- 23.Lu Y, Ge Y, Chen W, Xing W, Wei L, Zhang C, et al. The effectiveness of virtual reality for rehabilitation of Parkinson disease: an overview of systematic reviews with meta-analyses. Syst Rev 2022;11:50. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35305686&dopt=Abstract 10.1186/s13643-022-01924-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizzolatti G, Sinigaglia C. The mirror mechanism: a basic principle of brain function. Nat Rev Neurosci 2016;17:757–65. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27761004&dopt=Abstract 10.1038/nrn.2016.135 [DOI] [PubMed] [Google Scholar]
- 25.Mekbib DB, Zhao Z, Wang J, Xu B, Zhang L, Cheng R, et al. Proactive Motor Functional Recovery Following Immersive Virtual Reality-Based Limb Mirroring Therapy in Patients with Subacute Stroke. Neurotherapeutics 2020;17:1919–30. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32671578&dopt=Abstract 10.1007/s13311-020-00882-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Y, Ma L, Lin C, Zhu S, Yao L, Fan H, et al. Effects of Virtual Reality-Based Intervention on Cognition, Motor Function, Mood, and Activities of Daily Living in Patients With Chronic Stroke: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Aging Neurosci 2021;13:766525. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34966267&dopt=Abstract 10.3389/fnagi.2021.766525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual Reality-Based Training to Improve Obstacle-Crossing Performance and Dynamic Balance in Patients With Parkinson’s Disease. Neurorehabil Neural Repair 2015;29:658–67. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25539782&dopt=Abstract 10.1177/1545968314562111 [DOI] [PubMed] [Google Scholar]
- 28.García-Bravo S, Cuesta-Gómez A, Campuzano-Ruiz R, López-Navas MJ, Domínguez-Paniagua J, Araújo-Narváez A, et al. Virtual reality and video games in cardiac rehabilitation programs. A systematic review. Disabil Rehabil 2021;43:448–57. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31258015&dopt=Abstract 10.1080/09638288.2019.1631892 [DOI] [PubMed] [Google Scholar]
- 29.Calafiore D, Invernizzi M, Ammendolia A, Marotta N, Fortunato F, Paolucci T, et al. Efficacy of Virtual Reality and Exergaming in Improving Balance in Patients With Multiple Sclerosis: A Systematic Review and Meta-Analysis. Front Neurol 2021;12:773459. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34956054&dopt=Abstract 10.3389/fneur.2021.773459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marotta N, Demeco A, Indino A, de Scorpio G, Moggio L, Ammendolia A. Nintendo WiiTM versus Xbox KinectTM for functional locomotion in people with Parkinson’s disease: a systematic review and network meta-analysis. Disabil Rehabil 2022;44:331–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32478581&dopt=Abstract 10.1080/09638288.2020.1768301 [DOI] [PubMed] [Google Scholar]
- 31.Kashif M, Ahmad A, Bandpei MA, Syed HA, Raza A, Sana V. A Randomized Controlled Trial of Motor Imagery Combined with Virtual Reality Techniques in Patients with Parkinson’s Disease. J Pers Med 2022;12:450. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35330450&dopt=Abstract 10.3390/jpm12030450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoury AR. Motion capture for telemedicine: a review of nintendo wii, microsoft kinect, and playstation move. J Int Soc Telemed eHealth 2018;6:e14. [Google Scholar]
- 33.Chesser BT, Blythe SA, Ridge LD, Roskone Tomaszewski RE, Kinne BL. Effectiveness of the Wii for pediatric rehabilitation in individuals with cerebral palsy: a systematic review. Phys Ther Rev 2020;106–17. 10.1080/10833196.2020.1740402 [DOI]
- 34.Feys P, Straudi S. Beyond therapists: technology-aided physical MS rehabilitation delivery. Mult Scler 2019;25:1387–93. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31469352&dopt=Abstract 10.1177/1352458519848968 [DOI] [PubMed] [Google Scholar]
- 35.Valipoor S, Ahrentzen S, Srinivasan R, Akiely F, Gopinadhan J, Okun MS, et al. The use of virtual reality to modify and personalize interior home features in Parkinson’s disease. Exp Gerontol 2022;159:111702. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35038568&dopt=Abstract 10.1016/j.exger.2022.111702 [DOI] [PubMed] [Google Scholar]
- 36.Pazzaglia C, Imbimbo I, Tranchita E, Minganti C, Ricciardi D, Lo Monaco R, et al. Comparison of virtual reality rehabilitation and conventional rehabilitation in Parkinson’s disease: a randomised controlled trial. Physiotherapy 2020;106:36–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32026844&dopt=Abstract 10.1016/j.physio.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 37.Santos P, Machado T, Santos L, Ribeiro N, Melo A. Efficacy of the Nintendo Wii combination with Conventional Exercises in the rehabilitation of individuals with Parkinson’s disease: A randomized clinical trial. NeuroRehabilitation 2019;45:255–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31498138&dopt=Abstract 10.3233/NRE-192771 [DOI] [PubMed] [Google Scholar]
- 38.Ribas CG, Alves da Silva L, Corrêa MR, Teive HG, Valderramas S. Effectiveness of exergaming in improving functional balance, fatigue and quality of life in Parkinson’s disease: A pilot randomized controlled trial. Parkinsonism Relat Disord 2017;38:13–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28190675&dopt=Abstract 10.1016/j.parkreldis.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 39.Gandolfi M, Geroin C, Dimitrova E, Boldrini P, Waldner A, Bonadiman S, et al. Virtual Reality Telerehabilitation for Postural Instability in Parkinson’s Disease: A Multicenter, Single-Blind, Randomized, Controlled Trial. BioMed Res Int 2017;2017:7962826. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29333454&dopt=Abstract 10.1155/2017/7962826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chatterjee K, Buchanan A, Cottrell K, Hughes S, Day TW, John NW. Immersive Virtual Reality for the Cognitive Rehabilitation of Stroke Survivors. IEEE Trans Neural Syst Rehabil Eng 2022;30:719–28. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35271448&dopt=Abstract 10.1109/TNSRE.2022.3158731 [DOI] [PubMed] [Google Scholar]
- 41.Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev 2015;(2):CD008349. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25927099&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sayma M, Tuijt R, Cooper C, Walters K. Are We There Yet? Immersive Virtual Reality to Improve Cognitive Function in Dementia and Mild Cognitive Impairment. Gerontologist 2020;60:e502–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31600389&dopt=Abstract 10.1093/geront/gnz132 [DOI] [PubMed] [Google Scholar]
- 43.Moher D, Altman DG, Liberati A, Tetzlaff J. PRISMA statement. Epidemiology 2011;22:128, author reply 128. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21150360&dopt=Abstract 10.1097/EDE.0b013e3181fe7825 [DOI] [PubMed] [Google Scholar]
- 44.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019;10:ED000142. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31643080&dopt=Abstract 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed]
- 45.Minozzi S, Cinquini M, Gianola S, Gonzalez-Lorenzo M, Banzi R. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J Clin Epidemiol 2020;126:37–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32562833&dopt=Abstract 10.1016/j.jclinepi.2020.06.015 [DOI] [PubMed] [Google Scholar]
- 46.Allen NE, Song J, Paul SS, Smith S, O’Duffy J, Schmidt M, et al. An interactive videogame for arm and hand exercise in people with Parkinson’s disease: A randomized controlled trial. Parkinsonism Relat Disord 2017;41:66–72. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28528804&dopt=Abstract 10.1016/j.parkreldis.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 47.Alves ML, Mesquita BS, Morais WS, Leal JC, Satler CE, Dos Santos Mendes FA. Nintendo Wii™ Versus Xbox Kinect™ for Assisting People With Parkinson’s Disease. Percept Mot Skills 2018;125:546–65. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29665760&dopt=Abstract 10.1177/0031512518769204 [DOI] [PubMed] [Google Scholar]
- 48.Bekkers EM, Mirelman A, Alcock L, Rochester L, Nieuwhof F, Bloem BR, et al. Do Patients With Parkinson’s Disease With Freezing of Gait Respond Differently Than Those Without to Treadmill Training Augmented by Virtual Reality? Neurorehabil Neural Repair 2020;34:440–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32202203&dopt=Abstract 10.1177/1545968320912756 [DOI] [PubMed] [Google Scholar]
- 49.Fundarò C, Maestri R, Ferriero G, Chimento P, Taveggia G, Casale R. Self-selected speed gait training in Parkinson’s disease: robot-assisted gait training with virtual reality versus gait training on the ground. Eur J Phys Rehabil Med 2019;55:456–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30370751&dopt=Abstract 10.23736/S1973-9087.18.05368-6 [DOI] [PubMed] [Google Scholar]
- 50.Maggio MG, De Cola MC, Latella D, Maresca G, Finocchiaro C, La Rosa G, et al. What About the Role of Virtual Reality in Parkinson Disease’s Cognitive Rehabilitation? Preliminary Findings From a Randomized Clinical Trial. J Geriatr Psychiatry Neurol 2018;31:312–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30360679&dopt=Abstract 10.1177/0891988718807973 [DOI] [PubMed] [Google Scholar]
- 51.Maidan I, Nieuwhof F, Bernad-Elazari H, Bloem BR, Giladi N, Hausdorff JM, et al. Evidence for Differential Effects of 2 Forms of Exercise on Prefrontal Plasticity During Walking in Parkinson’s Disease. Neurorehabil Neural Repair 2018;32:200–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29546797&dopt=Abstract 10.1177/1545968318763750 [DOI] [PubMed] [Google Scholar]
- 52.Pompeu JE, Mendes FA, Silva KG, Lobo AM, Oliveira TP, Zomignani AP, et al. Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: a randomised clinical trial. Physiotherapy 2012;98:196–204. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22898575&dopt=Abstract 10.1016/j.physio.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 53.van de Weijer SC, Duits AA, Bloem BR, de Vries NM, Kessels RP, Köhler S, et al. Feasibility of a Cognitive Training Game in Parkinson’s Disease: The Randomized Parkin’Play Study. Eur Neurol 2020;83:426–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32756067&dopt=Abstract 10.1159/000509685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimmermann R, Gschwandtner U, Benz N, Hatz F, Schindler C, Taub E, et al. Cognitive training in Parkinson disease: cognition-specific vs nonspecific computer training. Neurology 2014;82:1219–26. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24623840&dopt=Abstract 10.1212/WNL.0000000000000287 [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y, Feng H, Wu X, Du Y, Yang X, Hu M, et al. Effectiveness of Exergaming in Improving Cognitive and Physical Function in People With Mild Cognitive Impairment or Dementia: systematic Review. JMIR Serious Games 2020;8:e16841. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32602841&dopt=Abstract 10.2196/16841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stojan R, Voelcker-Rehage C. A Systematic Review on the Cognitive Benefits and Neurophysiological Correlates of Exergaming in Healthy Older Adults. J Clin Med 2019;8:734. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31126052&dopt=Abstract 10.3390/jcm8050734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gallou-Guyot M, Nuic D, Mandigout S, Compagnat M, Welter ML, Daviet JC, et al. Effectiveness of home-based rehabilitation using active video games on quality of life, cognitive and motor functions in people with Parkinson’s disease: a systematic review. Disabil Rehabil 2022;1–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34982599&dopt=Abstract 10.1080/09638288.2021.2022780 [DOI] [PubMed]
- 58.Mangone M, Agostini F, de Sire A, Cacchio A, Chiaramonte A, Butterini G, et al. Effect of virtual reality rehabilitation on functional outcomes for return-to-work patients with Parkinson’s disease: an umbrella review of systematic reviews. NeuroRehabilitation 2022. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35599505&dopt=Abstract 10.3233/NRE-220029 [DOI] [PubMed]
- 59.Torre MM, Temprado JJ. Effects of Exergames on Brain and Cognition in Older Adults: A Review Based on a New Categorization of Combined Training Intervention. Front Aging Neurosci 2022;14:859715. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35431905&dopt=Abstract 10.3389/fnagi.2022.859715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamnardsiri T, Phirom K, Boripuntakul S, Sungkarat S. An Interactive Physical-Cognitive Game-Based Training System Using Kinect for Older Adults: Development and Usability Study. JMIR Serious Games 2021;9:e27848. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34704953&dopt=Abstract 10.2196/27848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang W. Wong SS-l, Lai FH-y. The Effect of Virtual Reality Rehabilitation on Balance in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Electronics (Basel) 2021;10:1003. 10.3390/electronics10091003 [DOI] [Google Scholar]
- 62.Mirelman A, Maidan I, Deutsch JE. Virtual reality and motor imagery: promising tools for assessment and therapy in Parkinson’s disease. Mov Disord 2013;28:1597–608. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24132848&dopt=Abstract 10.1002/mds.25670 [DOI] [PubMed] [Google Scholar]
- 63.Wu J, Zhang H, Chen Z, Fu R, Yang H, Zeng H, et al. Benefits of Virtual Reality Balance Training for Patients With Parkinson Disease: Systematic Review, Meta-analysis, and Meta-Regression of a Randomized Controlled Trial. JMIR Serious Games 2022;10:e30882. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35230242&dopt=Abstract 10.2196/30882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imbimbo I, Coraci D, Santilli C, Loreti C, Piccinini G, Ricciardi D, et al. Parkinson’s disease and virtual reality rehabilitation: cognitive reserve influences the walking and balance outcome. Neurol Sci 2021;42:4615–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33661481&dopt=Abstract 10.1007/s10072-021-05123-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Main characteristics of the studies included in this systematic review.


