Abstract
BACKGROUND
Limited prospective outcome data exist regarding transgender and nonbinary youth receiving gender-affirming hormones (GAH; testosterone or estradiol).
METHODS
We characterized the longitudinal course of psychosocial functioning during the 2 years after GAH initiation in a prospective cohort of transgender and nonbinary youth in the United States. Participants were enrolled in a four-site prospective, observational study of physical and psychosocial outcomes. Participants completed the Transgender Congruence Scale, the Beck Depression Inventory–II, the Revised Children’s Manifest Anxiety Scale (Second Edition), and the Positive Affect and Life Satisfaction measures from the NIH (National Institutes of Health) Toolbox Emotion Battery at baseline and at 6, 12, 18, and 24 months after GAH initiation. We used latent growth curve modeling to examine individual trajectories of appearance congruence, depression, anxiety, positive affect, and life satisfaction over a period of 2 years. We also examined how initial levels of and rates of change in appearance congruence correlated with those of each psychosocial outcome.
RESULTS
A total of 315 transgender and nonbinary participants 12 to 20 years of age (mean [±SD], 16±1.9) were enrolled in the study. A total of 190 participants (60.3%) were transmasculine (i.e., persons designated female at birth who identify along the masculine spectrum), 185 (58.7%) were non-Latinx or non-Latine White, and 25 (7.9%) had received previous pubertal suppression treatment. During the study period, appearance congruence, positive affect, and life satisfaction increased, and depression and anxiety symptoms decreased. Increases in appearance congruence were associated with concurrent increases in positive affect and life satisfaction and decreases in depression and anxiety symptoms. The most common adverse event was suicidal ideation (in 11 participants [3.5%]); death by suicide occurred in 2 participants.
CONCLUSIONS
In this 2-year study involving transgender and nonbinary youth, GAH improved appearance congruence and psychosocial functioning. (Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development.)
Transgender and nonbinary youth comprise 2 to 9% of high-school–aged persons in the United States.1–3 Many transgender and nonbinary youth have gender dysphoria, the persistent distress arising from incongruence between gender identity and external phenotype. Increasingly, transgender and nonbinary youth receive medical care to alleviate gender dysphoria, including gonadotropin-releasing hormone (GnRH) agonists to suppress gender-incongruent puberty and gender-affirming hormones (GAH; testosterone or estradiol) to foster gender-congruent secondary sex characteristics. An important goal of such treatment is to attenuate gender dysphoria by increasing appearance congruence — that is, the degree to which youth experience alignment between their gender and their physical appearance.
The available prospective research indicates that gender-affirming medical care is associated with improvements in psychosocial functioning.4–9 Previously published studies with modest sample sizes5,6,9 have examined outcomes for relatively short follow-up periods (approximately 1 year on average),5,6,9 focused exclusively on outcomes of GnRH agonists,7,8 or examined outcomes for mixed samples of youth initiating GnRH agonists or GAH,4,6,9 despite evidence that such cohorts have distinct psychosocial profiles.10 Evidence has been lacking from longitudinal studies that explore potential mechanisms by which gender-affirming medical care affects gender dysphoria and subsequent well-being.
We characterized the longitudinal course of psychosocial functioning over a period of 2 years after GAH initiation in a prospective cohort of more than 300 transgender and nonbinary young people in the United States. We hypothesized that appearance congruence, positive affect, and life satisfaction would increase and that depression and anxiety symptoms would decrease. We also hypothesized that improvements would be secondary to treatment for gender dysphoria, such that increasing appearance congruence would be associated with concurrent improvements in psychosocial outcomes. We also explored the potential moderating effects of demographic and clinical characteristics, including age, designated sex at birth, racial and ethnic identity, and the initiation of GAH in early as compared with later stages of puberty.
METHODS
STUDY DESIGN AND PARTICIPANT RECRUITMENT
Participants were recruited from gender clinics at the Ann and Robert H. Lurie Children’s Hospital of Chicago, UCSF Benioff Children’s Hospitals, Boston Children’s Hospital, and Children’s Hospital Los Angeles from July 2016 through June 2019 for the Trans Youth Care–United States (TYCUS) Study,11 a prospective, observational study evaluating the physical and psychosocial outcomes of medical treatment for gender dysphoria in two distinct cohorts of transgender and nonbinary youth — those initiating GnRH agonists and those initiating GAH as part of their clinical care. All participating clinics employ a multidisciplinary team that includes medical and mental health providers and that collaboratively determines whether gender dysphoria is present and whether gender-affirming medical care is appropriate. For minors, parental consent is required to initiate medical treatment. Publications by individual study teams provide details on site-specific approaches to care.12–15
Study visits occurred at baseline and at 6, 12, 18, and 24 months after treatment initiation. Details on study procedures have been published previously,11 and the protocol is available with the full text of this article at NEJM.org. The present analyses focus on the GAH cohort; outcomes for the cohort initiating GnRH agonists are being analyzed separately, given differences in baseline functioning between the two cohorts10 and distinct outcomes of GnRH agonists8 as compared with GAH treatment.4 Participants provided written informed consent or assent; parents provided permission for minors to participate. Procedures were approved by the institutional review board at each study site.
The first and second authors analyzed the data and wrote the initial draft of the manuscript. All the authors critically reviewed the manuscript. The authors vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol. There were no agreements regarding confidentiality of the data among the sponsor (Eunice Kennedy Shriver National Institute of Child Health and Human Development), the authors, and the participating institutions. The sponsor had no role in the design of the study; the collection, analysis, or interpretation of data; the writing of the manuscipt; or the decision to submit the manuscript for publication.
MEASURES
Participants reported age, racial and ethnic identity, gender identity, and designated sex at birth (details are provided in the Supplementary Appendix, available at NEJM.org). A small subgroup had been treated with GnRH agonists in early puberty (Tanner stage 2 or 3) (20 participants) or had a relatively late age at onset of endogenous puberty, such that they began receiving GAH in Tanner stage 3 (at 13 to 15 years of age) even without previous treatment with GnRH agonists (4 participants). These 24 participants comprise a subcohort in that they did not undergo extensive gender-incongruent puberty. Participants with a history of GnRH agonist treatment that was initiated in Tanner stage 4 (5 participants) were not included in this subcohort, because their experience of substantial gender-incongruent puberty is more similar to that of youth initiating GAH in Tanner stage 4 or 5.
With respect to longitudinal outcomes, participants completed the Transgender Congruence Scale,16 the Beck Depression Inventory–II,17 the Revised Children’s Manifest Anxiety Scale (Second Edition),18 and the Positive Affect and Life Satisfaction measures from the NIH (National Institutes of Health) Toolbox Emotion Battery19 at each study visit. Scoring information and sample items from each scale are provided in the Supplementary Appendix. Higher scores on these measures reflect greater appearance congruence, depression, anxiety, positive affect, and life satisfaction, respectively.
STATISTICAL ANALYSIS
Trajectories of psychosocial functioning were examined with the use of repeated-measures multivariate analysis of variance and mixed-effects models. Multivariate analysis of variance provided a preliminary omnibus test for significant within-person change over time. Owing to listwise deletion, 150 participants were excluded from the multivariate analysis of variance (the analysis involved 141 participants). Mixed-effects modeling was therefore selected owing to greater flexibility in accommodating missing data and nonnormal distributions and examining parallel processes. Specifically, we used latent growth curve modeling, which uses a structural equation modeling framework to examine changes in mean scores over time.20 Repeated measures are treated as indicators of latent factors: an intercept factor (estimates of initial levels) and a slope factor (rate of change). Intercept and slope factors can be regressed on covariates in adjusted models to explore moderation effects. In addition, growth curves for two different outcomes can be combined to examine how intercepts and slopes of those constructs correlate with each other. Data were Winsorized at the 95th percentile to reduce the influence of outliers.
Analyses involving latent growth curve modeling proceeded in three steps. First, we modeled trajectories of appearance congruence and psychosocial outcomes (i.e., effects of time only). Second, we adjusted models to estimate the effects of covariates on baseline scores and rates of change over time. Third, because changes in appearance congruence and psychosocial outcomes occur as parallel, simultaneous processes during GAH treatment, we examined how initial levels and rates of change in appearance congruence correlated with those of each psychosocial outcome. Standardized β levels were used as indicators of effect sizes for longitudinal models using conventional ranges (small, 0.20; medium, 0.50; and large, 0.80). Our conceptual model is shown in Figure S1 in the Supplementary Appendix. All statistical analyses were conducted with the use of SPSS software, version 27, and Mplus software, version 8.8.
RESULTS
ANALYTIC SAMPLE
There were a total of 6114 observations from 315 participants, who were assessed up to five times over a period of 2 years (data were available for 81% of all possible observations). Most participants (238 [75.6%]) completed either four study visits (76 participants) or five visits (162 participants). Tables S1 and S2 show the number of completed visits by time point and data coverage for key variables. The analytic sample for longitudinal models included 291 participants with follow-up data on primary outcome variables (Fig. S2). The analytic sample did not differ substantially from the overall sample with respect to age, designated sex at birth, racial and ethnic identity, initiation of GAH in early puberty, or baseline scores on psychosocial measures (Table S3).
SAMPLE CHARACTERISTICS
We enrolled 315 eligible participants 12 to 20 years of age (mean [±SD], 16±1.9 years) (Table 1). Most were transmasculine (i.e., persons designated female at birth who identify along the masculine spectrum; 60.3%), designated female at birth (64.8%), and non-Latinx or non-Latine White (58.7%). Transmasculine, non-Latinx or non-Latine White, and multiracial participants were overrepresented and nonbinary and Black participants were underrepresented as compared with the study sample in the Williams Institute Executive Report21 (Table S4); however, the study sample was representative of transgender and nonbinary youth presenting to pediatric subspecialty gender programs22 and generalizable to this population. Two participants died by suicide during the study (one after 6 months of follow-up and the other after 12 months of follow-up), and 6 participants withdrew from the study. For these eight participants, data that had been collected before death or study withdrawal were included in the analyses. Data on adverse events are provided in Table 2.
Table 1.
Demographic and Clinical Characteristics of the Participants.*
| Characteristic | Participants (N = 315) |
|---|---|
| no. (%) | |
| Gender identit† | |
| Transmasculine | 190 (60.3) |
| Transfeminine | 106 (33.7) |
| Nonbinary | 19 (6.0) |
| Designated sex at birth | |
| Female | 204 (64.8) |
| Male | 111 (35.2) |
| Racial and ethnic identity | |
| Non-Latinx or non-Latine White | 185 (58.7) |
| Latinx or Latine non-White | 50 (15.9) |
| Latinx or Latine White | 25 (7.9) |
| Black | 11 (3.5) |
| Asian or Pacific Islander | 10 (3.2) |
| Multiracial | 32 (10.2) |
| Other | 1 (03) |
| Unknown | 1 (03) |
| Age at baseline | |
| 12 yr | 6 (1.9) |
| 13 yr | 23 (7.3) |
| 14 yr | 38 (12.1) |
| 15 yr | 67 (21.3) |
| 16 yr | 55 (17.5) |
| 17 yr | 51 (16.2) |
| 18 yr | 48 (15.2) |
| 19 yr | 15 (4.8) |
| 20 yr | 12 (3.8) |
| Tanner stage at GAH initiation‡ | |
| 1 | 2 (0.6) |
| 2 | 13 (4.1) |
| 3 | 9 (2.9) |
| 4 | 29 (9.2) |
| 5 | 262 (83.2) |
| Past use of GnRH agonist | |
| No | 290 (92.1) |
| Yes | 25 (7.9) |
| Tanner stage at initiation of GnRH agonist | |
| 2 | 12 (3.8) |
| 3 | 8 (2.5) |
| 4 | 5 (1.6) |
| Not applicable | 290 (92.1) |
| Initiation of GAH in early puberty subcohort§ | |
| No | 291 (92.4) |
| Yes | 24 (7.6) |
The table does not include demographic and clinical characteristics for one participant who was accidentally enrolled and did not meet criteria for study eligibility. Percentages may not total 100 because of rounding. GAH denotes gender-affirming hormones, and GnRH gonadotropin-releasing hormone.
Transmasculine refers to persons designated female at birth who identify along the masculine spectrum. Transfeminine refers to persons designated male at birth who identify along the feminine spectrum.
Three participants began receiving GnRH agonists in either Tanner stage 2 or 3 and subsequently had pubertal regression to Tanner stage 1 or 2 by the time of GAH initiation.
This subcohort includes 20 participants who began receiving GnRH agonists at Tanner stage 2 or 3 and 4 participants who had not previously received GnRH agonists but had begun receiving GAH in Tanner stage 3 owing to a relatively late onset of puberty (13 to 15 years of age) and thus did not have physical changes associated with later stages of endogenous puberty. This subcohort does not include 5 participants with a history of initiation of GnRH agonists in Tanner stage 4 and who thus did undergo substantial gender-incongruent puberty.
Table 2.
Adverse Events.
| Event | No. of Events in Sample |
|---|---|
| Any event | 15 |
| Death by suicide | 2 |
| Suicidal ideation reported during study visit | 11 |
| Severe anxiety triggered by study visit | 2 |
APPEARANCE CONGRUENCE AND PSYCHOSOCIAL OUTCOMES OVER TIME
Table S5 depicts mean scores for appearance congruence, depression, anxiety, positive affect, and life satisfaction at baseline and 24 months. Results for multivariate analysis of variance indicated that there were significant within-participant changes over time for all psychosocial outcomes in hypothesized directions (Wilk’s lambda, 0.32; F statistic with 20 and 122 degrees of freedom; 12.86; P<0.001). Specifically, scores for appearance congruence, positive affect, and life satisfaction increased significantly, and scores for depression and anxiety decreased significantly.
Means and variances of the variables for latent growth curve modeling, with estimated baseline levels and change over time for both time-only and adjusted models, are provided in Table 3. Scores for appearance congruence increased (annual increase on a 5-point scale, 0.48 points; 95% confidence interval [CI], 0.42 to 0.54; standardized β = 1.47), as did T scores for positive affect (annual increase on a 100-point scale, 0.80 points; 95% CI, 0.08 to 1.54; β = 0.19) and life satisfaction (annual increase on a 100-point scale, 2.32 points; 95% CI, 1.64 to 3.00; β = 0.52). We observed decreased scores for depression (annual change on a 63-point scale, −1.27 points; 95% CI, −1.98 to −0.57; standardized β = −0.29) and decreased T scores for anxiety (annual change on a 100-point scale, −1.46 points; 95% CI, −2.13 to −0.79; β = −0.35) over a period of 2 years of GAH treatment.
Table 3.
Variable Estimates for Individual Latent Growth Curve Models of 2-Year Outcomes.*
| Model | Appearance Congruence† | Depression‡ | Anxiety§ | Positive Affect¶ | Life Satisfaction‖ |
|---|---|---|---|---|---|
| value (95% confidence interval) | |||||
| Unconditional model: time | |||||
| Intercept mean | 2.99 (2.90 to 3.08) | 15.46 (14.27 to 16.70) | 59.58 (58.22 to 60.68) | 42.93 (41.82 to 44.03) | 40.12 (38.99 to 41.26) |
| Intercept variance | 0.35 (0.27 to 0.50) | 86.23 (68.13 to 106.85) | 17.84 (11.38 to 24.54) | 63.50 (46.23 to 81.79) | 75.21 (59.76 to 93.98) |
| Slope mean | 0.48 (0.42 to 0.54) | −1.27 (−1.98 to −0.57) | −1.46 (−2.13 to −0.79) | 0.80 (0.08 to 1.54) | 2.32 (1.64 to 3.00) |
| Slope variance | 0.11 (0.07 to 0.15) | 19.44 (12.23 to 27.14) | 17.84 (11.38 to 24.54) | 17.98 (9.25 to 27.57) | 20.33 (14.12 to 27.70) |
| Conditional model | |||||
| Time | |||||
| Intercept mean | 2.59 (1.91 to 3.27) | 20.01 (10.79 to 29.48) | 60.82 (53.56 to 67.95) | 47.27 (38.93 to 55.81) | 38.86 (29.90 to 47.75) |
| Intercept variance | 0.32 (0.25 to 0.42) | 80.92 (63.35 to 100.47) | 114.74 (91.96 to 138.23) | 56.96 (41.19 to 74.75) | 71.93 (57.15 to 90.22) |
| Slope mean | 0.51 (0.07 to 0.96) | −0.92 (−3.82 to −0.06) | −1.95 (−3.81 to −0.09) | 1.79 (0.14 to 3.43) | 4.54 (2.66 to 6.43) |
| Slope variance | 0.10 (0.06 to 0.14) | 18.81 (11.71 to 26.34) | 18.37 (11.78 to 25.63) | 17.97 (9.29 to 27.66) | 19.74 (13.61 to 27.06) |
| Time-invariant effects on intercept | |||||
| Baseline age | 0.02 (−002 to 0.06) | −0.23 (−0.08 to 0.36) | −0.20 (−0.78 to 0.38) | −0.32 (−0.84 to 0.21) | 0.06 (−0.49 to 0.62) |
| Designated sex at birth** | −0.12 (−0.31 to 0.06) | 1.74 (−0.69 to 4.09) | 0.05 (−2.37 to 2.49) | −1.26 (−3.53 to 0.91) | −2.36 (−4.89 to 0.18) |
| Racial and ethnic identity†† | 0.19 (0.03 to 0.36) | −2.60 (−4.82 to −0.32) | −2.22 (−4.48 to 0.06) | 2.30 (0.22 to 4.38) | 1.70 (−0.58 to 3.98) |
| Early gender-affirming care‡‡ | 0.70 (0.35 to 1.04) | −5.88 (−9.67 to −1.96) | −7.41 (−11.30 to −3.52) | 5.34 (1.70 to 8.98) | 7.55 (2.82 to 12.28) |
| Time-invariant effects on slope | |||||
| Baseline age | 0.00 (−0.03 to 0.03) | −0.04 (−0.18 to 0.10) | −0.02 (−0.15 to 0.12) | −0.03 (−0.15 to 0.10) | −0.09 (−0.22 to 0.05) |
| Designated sex at birth** | 0.03 (−0.09 to 0.15) | 1.91 (0.33 to 3.50) | 1.56 (0.01 to 3.10) | −0.43 (−2.10 to 1.31) | −1.86 (−3.49 to −0.24) |
| Racial and ethnic identity†† | −0.10 (−0.20 to 0.01) | 1.70 (0.23 to 3.15) | 0.62 (−0.77 to 1.98) | −1.42 (−2.98 to 0.13) | −1.08 (−2.52 to 0.36) |
| Early gender-affirming care‡‡ | −0.42 (−0.66 to −0.19) | −0.73 (−3.41 to 1.93) | 0.04 (−2.53 to 2.59) | −0.78 (−3.56 to 2.06) | −1.08 (−4.01 to 1.86) |
Shown are unstandardized variable estimates with 95% confidence intervals. Slope means indicate change over time, and slope variances indicate heterogeneity within the sample.
Scores on the Appearance Congruence subscale of the Transgender Congruence Scale range from 1 to 5, with higher scores indicating greater appearance congruence.
Scores on the Beck Depression Inventory–II range from 0 to 63, with scores of 20 to 28 indicating moderate depression and scores of 29 to 63 indicating severe depression.
T scores on the Revised Children’s Manifest Anxiety Scale (Second Edition) have a mean of 50 and a standard deviation of 10, with scores of 60 or more indicating clinical levels of anxiety.
T scores for the Positive Affect measure from the NIH (National Institutes of Health) Toolbox Emotion Battery have a mean of 50 and a standard deviation of 10, with higher scores indicating greater positive affect.
T scores for the Life Satisfaction measure from the NIH Toolbox Emotion Battery have a mean of 50 and a standard deviation of 10, with higher scores indicating greater life satisfaction.
Coding for designated sex at birth was as follows: 0 = assigned female at birth (reference) and 1 = assigned male at birth.
Coding for racial and ethnic identity was as follows: 0 = non-Latinx or non-Latine White (reference) and 1 = other racial and ethnic identities.
Coding for early gender-affirming care was as follows: 0 = initiated GAH in later puberty (Tanner stage 4 or 5) (reference) and 1 = initiated GAH in early puberty (Tanner stage 2 or 3).
Unadjusted models can be interpreted on their original scale. For instance, depression scores range from 0 to 63 (ranges of severity, minimal, 0 to 13; mild, 14 to 19; moderate, 20 to 28; and severe, 29 to 63). The model had an intercept (baseline mean) of 15.46 and estimated slope (change per year) of −1.27. Thus, on average, depression started in the mild range and decreased to the subclinical level by 24 months. Table S6 shows the percentages of youth scoring in the clinical range for depression and anxiety at each time point. Of 27 participants with depression scores in the severe range at baseline, 18 (67%) reported a depression score in the minimal or moderate ranges at 24 months. Similarly, 21 of 33 participants (64%) with depression scores in the moderate range at baseline reported a depression score in the minimal or moderate ranges at 24 months (chi-square statistic with 9 degrees of freedom, 49.85; P<0.001). With respect to anxiety, 47 of 122 participants (38.5%) with baseline scores in the clinical range (T scores, >60) were in the nonclinical range at 24 months (chi-square statistic with 1 degree of freedom, 22.05; P<0.001).
ASSOCIATIONS BETWEEN APPEARANCE CONGRUENCE AND PSYCHOSOCIAL OUTCOMES
Figure 1 depicts parallel processes between appearance congruence and each psychosocial outcome as analyzed by means of latent growth curve modeling. As described above, we used linear latent growth curve modeling to estimate baseline scores (intercepts) and linear rates of change (slopes) of each outcome (see Table 3 for details of each model). In parallel-process models, we examined how the components for latent growth curve modeling for appearance congruence related to those for scores for depression (Fig. 1A) and T scores for anxiety (Fig. 1B), positive affect (Fig. 1C), and life satisfaction (Fig. 1D). Higher appearance congruence at baseline was associated with lower baseline scores for depression (r = −0.60) and T scores for anxiety (r = −0.40), and increases in appearance congruence were associated with decreases in scores for depression (r = −0.68) and T scores for anxiety (r = −0.52) over time. In addition, higher appearance congruence at baseline was associated with higher baseline T scores for positive affect (r = 0.46) and life satisfaction (r = 0.72), and increases in appearance congruence were associated with increases in T scores for positive affect (r = 0.74) and life satisfaction (r = 0.84) over time.
Figure 1. Appearance Congruence and Depression, Anxiety, Positive Affect, and Life Satisfaction.
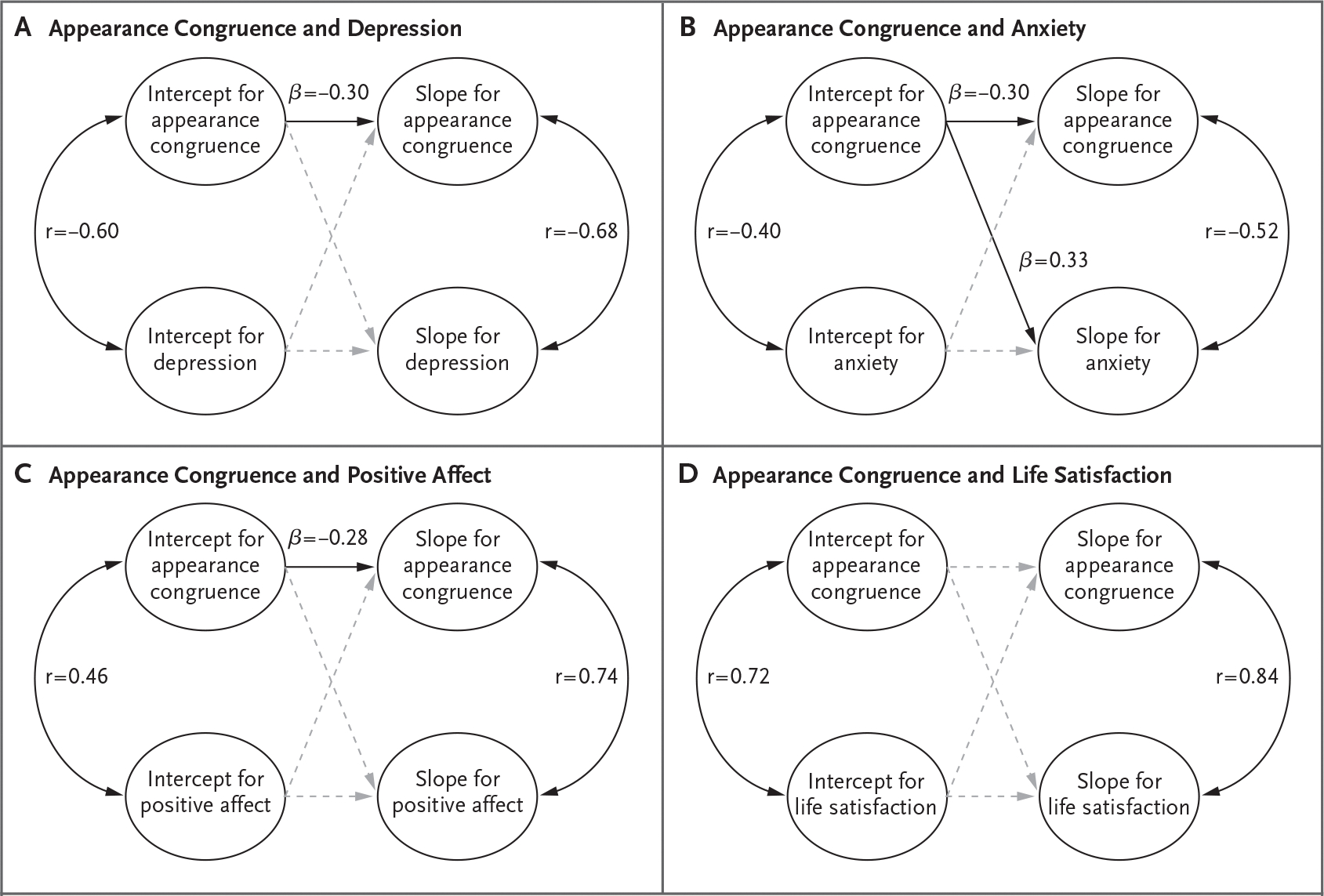
Parallel-process latent growth curve models are depicted. A linear latent growth curve model was fitted for each outcome, with model-based estimates of baseline scores (intercept) and rates of linear change over time (slope). Parallel-process models can provide tests of how aspects of trajectories relate to each other. Each panel provides estimates for correlations between baseline scores of appearance congruence and each outcome (intercept correlations, arcs displayed on the left side of each panel), correlations between rate of change of appearance congruence and rate of change of each outcome (slope correlations, arcs displayed on the right side of each panel), and effects of baseline scores on slopes (straight lines in the middle of each panel). Solid black lines and arcs indicate significant effects (confidence intervals for variable estimates do not contain 0); nonsignificant effects are shown with dashed gray lines. All models were controlled for age, designated sex at birth, racial and ethnic identity, and early gender-affirming care (not shown for ease of interpretation).
MODERATING EFFECTS OF DEMOGRAPHIC AND CLINICAL COVARIATES
Table 3 shows the effects of covariates on scores for appearance congruence and depression and T scores for anxiety, positive affect, and life satisfaction. Age was not associated with any outcomes at baseline or over time.
Designated Sex at Birth
Depression and anxiety scores decreased among youth designated female at birth but not among those designated male at birth. Similarly, T scores for life satisfaction increased among youth designated female at birth but not among those designated male at birth (Fig. S3). Designated sex at birth was not associated with any other outcomes at baseline or over time.
Effects of Racial and Ethnic Identity
At baseline, youth of color had higher scores for appearance congruence, lower scores for depression, and higher scores for positive affect than non-Latinx or non-Latine White youth. With respect to change over time, non-Latinx or non-Latine White youth had greater decreases in depression scores than youth of color (Fig. S4). Racial and ethnic identity were not associated with any other outcomes at baseline or over time.
Initiation of GAH in Early Puberty
Youth who had initiated GAH in early puberty had higher scores for appearance congruence, positive affect, and life satisfaction at baseline and lower scores for depression and anxiety at baseline than those who had initiated GAH in later puberty. Tables S7, S8, and S9 provide more information regarding differences between youth initiating GAH in early puberty and those initiating GAH in late puberty. With respect to change over time, youth initiating GAH in later puberty had greater improvements in appearance congruence than those initiating GAH in early puberty (Fig. 2).
Figure 2. Psychosocial Outcomes during 2 Years of GAH.
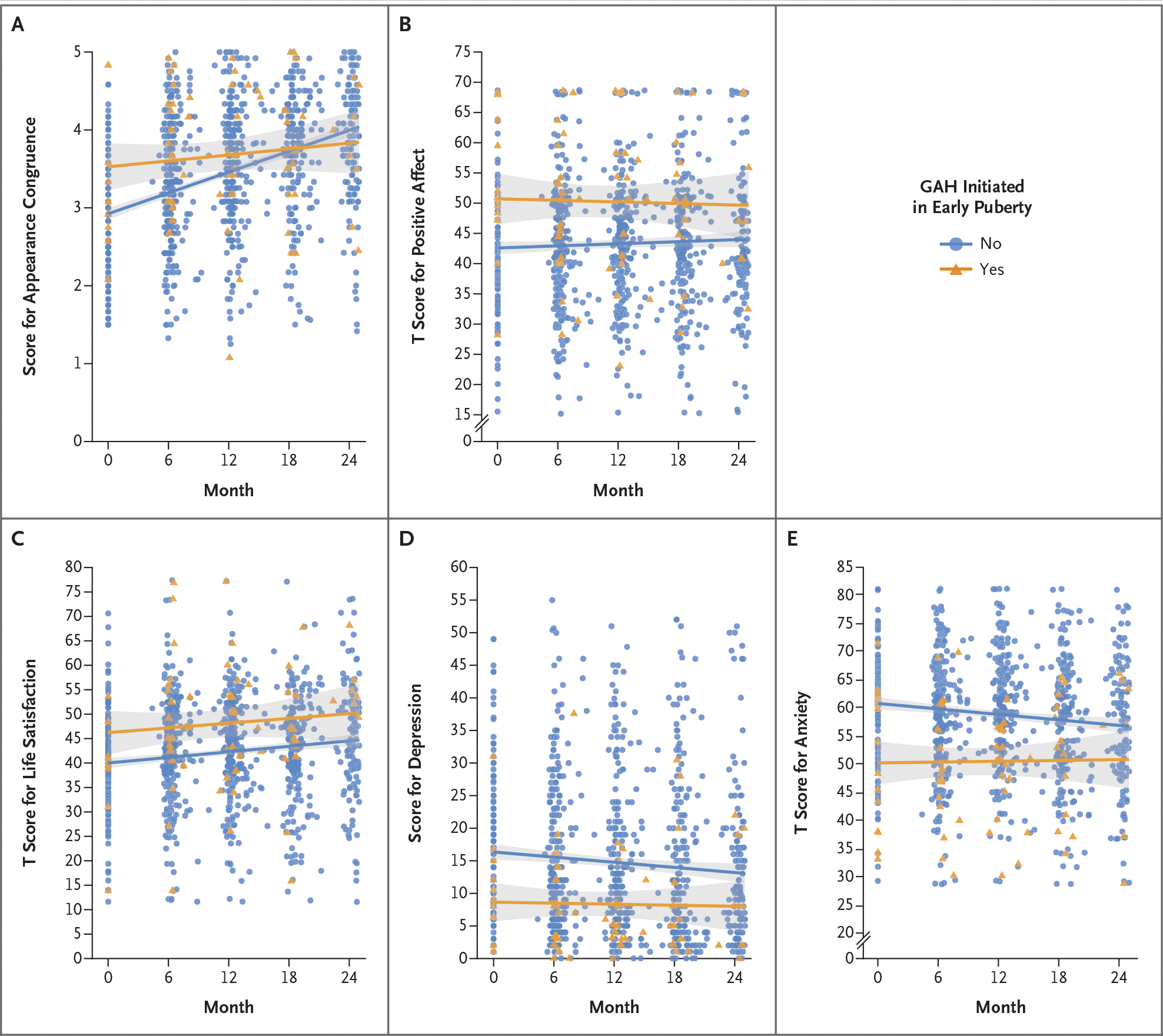
Shown are changes in participant-reported measures over a period of 2 years of treatment with gender-affirming hormones (GAH). Scores on the Appearance Congruence subscale of the Transgender Congruence Scale (Panel A) range from 1 to 5, with higher scores indicating greater appearance congruence. T scores for the Positive Affect measure from the NIH (National Institutes of Health) Toolbox Emotion Battery (Panel B) range from 0 to 100, with higher scores indicating greater positive affect. T scores for the Life Satisfaction measure from the NIH Toolbox Emotion Battery (Panel C) range from 0 to 100, with higher scores indicating greater life satisfaction. Scores on the Beck Depression Inventory–II (Panel D) range from 0 to 63, with higher scores indicating greater depression. T scores on the Revised Children’s Manifest Anxiety Scale (Second Edition) (Panel D), range from 0 to 100, with higher scores indicating greater anxiety. Individual scores are depicted with orange triangles for youth initiating GAH in early puberty (“Yes”) and with blue circles for youth who did not initiate GAH in early puberty (“No”). Lines indicate mean scores for each group, with gray shaded bands for 95% confidence intervals.
DISCUSSION
Understanding the effect of GAH on the psychosocial outcomes of transgender and nonbinary youth would appear crucial, given the documented mental health disparities observed in this population,10,15,23,24 particularly in the context of increasing politicization of gender-affirming medical care.25 In our U.S.-based cohort of transgender and nonbinary youth treated with GAH, we found decreases in depression and anxiety symptoms and increases in positive affect and life satisfaction as assessed through validated instruments. Our findings are consistent with those of other longitudinal studies involving transgender and nonbinary youth receiving GAH, which showed reductions in depression6,9 and anxiety6 and increases in overall well-being5 with small-to-moderate effects over a follow-up period of up to 1 year. We replicated these findings in a larger sample of racially and ethnically diverse transgender and nonbinary youth recruited from four geographically distinct regions in the United States and found sustained improvements over a period of 2 years.
Increasing appearance congruence is a primary goal of GAH, and we observed appearance congruence improve over 2 years of treatment. This was a moderate effect, and the strongest effect observed across our outcomes, consistent with the effect seen in research involving other samples, which has noted large effects of GAH on body image and small-to-moderate effects on mental health.6 Appearance congruence was also associated with each psychosocial outcome assessed at baseline and during the follow-up period, such that increases in appearance congruence were associated with decreases in depression and anxiety symptoms and increases in positive affect and life satisfaction. These findings suggest that appearance congruence is a candidate mechanism by which GAH influences psychosocial functioning.
The importance of appearance congruence for psychosocial well-being is further highlighted by the effect of avoiding gender-incongruent pubertal changes. Youth who had not undergone substantial gender-incongruent puberty had higher scores for appearance congruence, positive affect, and life satisfaction and lower scores for depression and anxiety at baseline than youth who had undergone substantial endogenous puberty. These observations align with other published reports that earlier access to gender-affirming medical care is associated with more positive psychosocial functioning.10,26 Alternatively, youth who first recognize their gender incongruence in adolescence may represent a distinct subgroup of transgender and nonbinary youth who have more psychosocial complexities than youth recognizing gender incongruence in childhood.27
The effects of GAH on some psychosocial outcomes varied on the basis of designated sex at birth. Depression and anxiety symptoms decreased significantly, and life satisfaction increased significantly, among youth designated female at birth but not among those designated male at birth. Given that some key estrogen-mediated phenotypic changes can take between 2 and 5 years to reach their maximum effect (e.g., breast growth),28 we speculate that a longer follow-up period may be necessary to see an effect on depression, anxiety, and life satisfaction. Furthermore, changes that are associated with an endogenous testosterone-mediated puberty (e.g., deeper voice) may be more pronounced and observable than those associated with an endogenous estrogen-mediated puberty. Thus, we hypothesize that observed differences in depression, anxiety, and life satisfaction among youth designated female at birth as compared with those designated male at birth may be related to differential experiences of gender minority stress, which could arise from differences in societal acceptance of transfeminine (i.e., persons designated male at birth who identity along the feminine spectrum) as compared with transmasculine persons. Indeed, gender minority stress is consistently associated with more negative mental health outcomes,29 and research suggests that transfeminine youth may experience more minority stress than transmasculine youth.30
Our study has certain limitations. Because participants were recruited from four urban pediatric gender centers, the findings may not be generalizable to youth without access to comprehensive interdisciplinary services or to transgender and nonbinary youth who are self-medicating with GAH. In addition, despite improvement across psychosocial outcomes on average, there was substantial variability around the mean trajectory of change. Some participants continued to report high levels of depression and anxiety and low positive affect and life satisfaction, despite the use of GAH. We plan to examine other factors that are known to contribute to psychosocial functioning among transgender and nonbinary youth and may not be affected by GAH, such as parental support,31,32 in this cohort. Finally, our study lacked a comparison group, which limits our ability to establish causality. However, the large effects in parallel-process models examining associations between improvements in appearance congruence and improvements in psychosocial outcomes provide support for the concept that GAH may affect psychosocial outcomes through increasing gender congruence.
Despite these limitations, our findings showed improvements in psychosocial functioning across 2 years of GAH treatment, which supports the use of GAH as effective treatment for transgender and nonbinary youth. We are now following this cohort to see whether gains in functioning are sustained over a longer follow-up period, and — given substantial variability in outcomes even after controlling for a number of factors — we hope to discover additional predictors of change to identify youth for whom GAH alone is not adequate to address mental health challenges. We intend to initiate further work with this cohort to focus on understanding reasons for discontinuing GAH among the small subgroup of youth who stopped medical treatment. Overall, our results provide evidence that GAH improved appearance congruence and psychosocial functioning in transgender and nonbinary youth.
Supplementary Material
Acknowledgments
Supported by a grant (R01 HD082554) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
We thank the participants, their families, their referring clinicians, and the many research staff for their contributions in conducting this study, and Norman Spack, one of the original principal investigators, for his contributions to the study.
Appendix
The authors’ affiliations are as follows: the Gender and Sex Development Program, Potocsnak Family Division of Adolescent and Young Adult Medicine (D.C., R.G.), and the Pritzker Department of Psychiatry and Behavioral Health (D.C.), Ann and Robert H. Lurie Children’s Hospital of Chicago, the Departments of Pediatrics (D.C., R.G.) and Psychiatry and Behavioral Sciences (D.C., J.B.), Northwestern University Feinberg School of Medicine, and the Institute for Sexual and Gender Minority Health and Wellbeing, Northwestern University (J.B.) — all in Chicago; the Division of Endocrinology, Department of Pediatrics, Boston Children’s Hospital (Y.-M.C.), and the Department of Pediatrics, Harvard Medical School (Y.-M.C.), Boston, and the Department of Psychology and Neuroscience, Boston College, Newton (A.C.T.) — all in Massachusetts; the Department of Pediatrics, Division of Pediatric Endocrinology (D.E., S.M.R.), and the Child and Adolescent Gender Center, Benioff Children’s Hospital (D.E., S.M.R.), University of California, San Francisco, San Francisco, and the Gender Health Program, UCLA Health (M.A.H.), and the Division of General Internal Medicine and Health Services Research, Medicine–Pediatrics Section, Department of Medicine, David Geffen School of Medicine (M.A.H.), University of California, Los Angeles, the Center for Transyouth Health and Development, Division of Adolescent and Young Adult Medicine, Children’s Hospital Los Angeles (J.O.-K.), and the Department of Pediatrics, Keck School of Medicine, University of Southern California (J.O.-K.), Los Angeles — all in California.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Diane Chen, Gender and Sex Development Program, Potocsnak Family Division of Adolescent and Young Adult Medicine, Chicago; Pritzker Department of Psychiatry and Behavioral Health, Chicago; Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago; Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago; Department of Psychiatry and Behavioral Sciences, Northwestern University Feinberg School of Medicine, Chicago
Johnny Berona, Department of Psychiatry and Behavioral Sciences, Northwestern University Feinberg School of Medicine, Chicago; Institute for Sexual and Gender Minority Health and Wellbeing, Northwestern University, Chicago
Yee-Ming Chan, Division of Endocrinology, Department of Pediatrics, Boston Children’s Hospital, Boston, Massachusetts; Department of Pediatrics, Harvard Medical School, Boston, Massachusetts
Diane Ehrensaft, Department of Pediatrics, Division of Pediatric Endocrinology, University of California, San Francisco, San Francisco, California; Child and Adolescent Gender Center, Benioff Children’s Hospital, University of California, San Francisco, San Francisco, California
Robert Garofalo, Gender and Sex Development Program, Potocsnak Family Division of Adolescent and Young Adult Medicine, Chicago; Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago; Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago
Marco A. Hidalgo, University of California, San Francisco, San Francisco, California; Gender Health Program, UCLA Health, California; Division of General Internal Medicine and Health Services Research, Medicine–Pediatrics Section, Department of Medicine, David Geffen School of Medicine, California
Stephen M. Rosenthal, Department of Pediatrics, Division of Pediatric Endocrinology, University of California, San Francisco, San Francisco, California; Child and Adolescent Gender Center, Benioff Children’s Hospital; University of California, San Francisco, San Francisco, California
Amy C. Tishelman, Department of Psychology and Neuroscience, Boston College, Newton, Massachusetts
Johanna Olson-Kennedy, Center for Transyouth Health and Development, Division of Adolescent and Young Adult Medicine, Children’s Hospital Los Angeles, California; Department of Pediatrics, Keck School of Medicine, University of Southern California, Los Angeles, California
REFERENCES
- 1.Johns MM, Lowry R, Andrzejewski J, et al. Transgender identity and experiences of violence victimization, substance use, suicide risk, and sexual risk behaivors among high school students — 19 states and large urban school districts, 2017. MMWR Morb Mortal Wkly Rep 2019;68:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rider GN, McMorris BJ, Gower AL, Coleman E, Eisenberg ME. Health and care utilization of transgender and gender nonconforming youth: a population-based study. Pediatrics 2018;141(3):e20171683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidd KM, Sequeira GM, Douglas C, et al. Prevalence of gender-diverse youth in an urban school district. Pediatrics 2021;147(6):e2020049823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Vries AL, McGuire JK, Steensma TD, Wagenaar ECF, Doreleijers TA, Cohen-Kettenis PT. Young adult psychological outcome after puberty suppression and gender reassignment. Pediatrics 2014;134:696–704. [DOI] [PubMed] [Google Scholar]
- 5.Allen LR, Watson LB, Egan AM, Moser CN. Well-being and suicidality among transgender youth after gender-affirming hormones. Clin Pract Pediatr Psychol 2019;7:302–11. [Google Scholar]
- 6.Kuper LE, Stewart S, Preston S, Lau M, Lopez X. Body dissatisfaction and mental health outcomes of youth on gender-affirming hormone therapy. Pediatrics 2020;145(4): e20193006. [DOI] [PubMed] [Google Scholar]
- 7.Costa R, Dunsford M, Skagerberg E, Holt V, Carmichael P, Colizzi M. Psychological support, puberty suppression, and psychosocial functioning in adolescents with gender dysphoria. J Sex Med 2015;12:2206–14. [DOI] [PubMed] [Google Scholar]
- 8.de Vries AL, Steensma TD, Doreleijers TA, Cohen-Kettenis PT. Puberty suppression in adolescents with gender identity disorder: a prospective follow-up study. J Sex Med 2011;8:2276–83. [DOI] [PubMed] [Google Scholar]
- 9.Achille C, Taggart T, Eaton NR, et al. Longitudinal impact of gender-affirming endocrine intervention on the mental health and well-being of transgender youths: preliminary results. Int J Pediatr Endocrinol 2020;2020:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen D, Abrams M, Clark L, et al. Psychosocial characteristics of transgender youth seeking gender-affirming medical treatment: baseline findings from the trans youth care study. J Adolesc Health 2021;68:1104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson-Kennedy J, Chan Y-M, Garofalo R, et al. Impact of early medical treatment for transgender youth: protocol for the longitudinal, observational trans youth care study. JMIR Res Protoc 2019;8(7):e14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen D, Hidalgo MA, Leibowitz S, et al. Multidisciplinary care for gender-diverse youth: a narrative review and unique model of gender-affirming care. Transgend Health 2016;1:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherer I, Rosenthal SM, Ehrensaft D, Baum J. Child and Adolescent Gender Center: a multidisciplinary collaboration to improve the lives of gender nonconforming children and teens. Pediatr Rev 2012;33:273–5. [DOI] [PubMed] [Google Scholar]
- 14.Spack NP, Edwards-Leeper L, Feldman HA, et al. Children and adolescents with gender identity disorder referred to a pediatric medical center. Pediatrics 2012;129:418–25. [DOI] [PubMed] [Google Scholar]
- 15.Olson J, Schrager SM, Belzer M, Simons LK, Clark LF. Baseline physiologic and psychosocial characteristics of transgender youth seeking care for gender dysphoria. J Adolesc Health 2015;57:374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozee HB, Tylka TL, Bauerband LA. Measuring transgender individuals’ comfort with gender identity and appearance: development and validation of the Transgender Congruence Scale. Psychol Women Q 2012;36:179–96. [Google Scholar]
- 17.Beck AT, Steer RA, Brown GK. BDI-II, Beck depression inventory. San Antonio, TX: Psychological Corporation, 1996. [Google Scholar]
- 18.Reynolds CR, Richmond BO. Revised children’s manifest anxiety scale (RCMAS-2), second edition. Torrance, CA: Western Psychological Services, 2008. [Google Scholar]
- 19.Slotkin J, Nowinski CJ, Hays RD, et al. NIH Toolbox Scoring and Interpretation Guide. Washington, DC: National Institutes of Health, September 18, 2012 (https://repository.niddk.nih.gov/media/studies/look-ahead/Forms/Look_AHEAD_Cognitive_Function/NIH%20Toolbox%20Scoring%20and%20Interpretation%20Manual%209-27-12.pdf). [Google Scholar]
- 20.Muthén LK, Muthén BO. Mplus user’s guide, version 8. 2017. (https://www.statmodel.com/download/usersguide/MplusUserGuideVer_8.pdf).
- 21.Herman JL, Flores AR, O’Neill KK. How many adults and youth identify as transgender in the United States? UCLA School of Law, June 2022. (https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/). [Google Scholar]
- 22.Chen D, Lash B, Kim E, et al. A comparison of demographic and psychosocial characteristics between transgender youth enrolling versus not enrolling in a multisite study. Transgend Health 2020;6:229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reisner SL, Vetters R, Leclerc M, et al. Mental health of transgender youth in care at an adolescent urban community health center: a matched retrospective cohort study. J Adolesc Health 2015;56:274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toomey RB, Syvertsen AK, Shramko M. Transgender adolescent suicide behavior. Pediatrics 2018;142(4):e20174218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Civil Liberties Union. Legislation affecting LGBT rights across the country. December 17, 2021 (https://www.aclu.org/legislation-affecting-lgbt-rights-across-country).
- 26.Sorbara JC, Chiniara LN, Thompson S, Palmert MR. Mental health and timing of gender-affirming care. Pediatrics 2020;146(4):e20193600. [DOI] [PubMed] [Google Scholar]
- 27.de Vries ALC. Challenges in timing puberty suppression for gender-nonconforming adolescents. Pediatrics 2020;146(4):e2020010611. [DOI] [PubMed] [Google Scholar]
- 28.Coleman E, Bockting W, Botzer M, et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgenderism 2012;13:165–232. [Google Scholar]
- 29.Delozier AM, Kamody RC, Rodgers S, Chen D. Health disparities in transgender and gender expansive adolescents: a topical review from a minority stress framework. J Pediatr Psychol 2020;45:842–7. [DOI] [PubMed] [Google Scholar]
- 30.Poquiz JL, Coyne CA, Garofalo R, Chen D. Comparison of gender minority stress and resilience among transmasculine, transfeminine, and nonbinary adolescents and young adults. J Adolesc Health 2021;68:615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simons L, Schrager SM, Clark LF, Belzer M, Olson J. Parental support and mental health among transgender adolescents. J Adolesc Health 2013;53:791–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pariseau EM, Chevalier L, Long KA, Clapham R, Edwards-Leeper L, Tishelman AC. The relationship between family acceptance-rejection and transgender youth psychosocial functioning. Clin Pract Pediatr Psychol 2019;7:267–77. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


