Abstract
Spine fusion surgery is one of the most common orthopedic procedures, with over 400,000 performed annually to correct deformities and pain. However, complications occur in approximately one third of cases. While many of these complications may be related to poor bone quality, it is difficult to detect bone abnormalities prior to surgery. Areal BMD (aBMD) assessed by DXA may be artifactually high in patients with spine pathology, leading to missed diagnosis of deficits. In this study, we related preoperative imaging characteristics of both central and peripheral sites to direct measurements of bone quality in vertebral biopsies. We hypothesized that pre-operative imaging outcomes would relate to vertebral bone mineralization and collagen properties. Pre-operative assessments included DXA measurements of aBMD of the spine, hip, and forearm, central quantitative computed tomography (QCT) of volumetric BMD (vBMD) at the lumbar spine, and high resolution peripheral quantitative computed tomography (HRpQCT; Xtreme CT2) measurements of vBMD and microarchitecture at the distal radius and tibia. Bone samples were collected intraoperatively from the lumbar vertebrae and analyzed using Fourier-transform Infrared (FTIR) spectroscopy. Bone samples were obtained from 23 postmenopausal women (mean age 67 ± 7 years, BMI 28 ± 8 kg/m2). We found that patients with more mature bone by FTIR, measured as lower acid phosphate content and carbonate to phosphate ratio, and greater collagen maturity and mineral maturity/crystallinity (MMC), had greater cortical vBMD at the tibia and greater aBMD at the lumbar spine and one-third radius. Our data suggests that bone quality at peripheral sites may predict bone quality at the spine. As bone quality at the spine is challenging to assess prior to surgery, there is a great need for additional screening tools. Pre-operative peripheral bone imaging may provide important insight into vertebral bone quality and may foster identification of patients with bone quality deficits.
Introduction
Spine fusion surgery is a complex orthopedic procedure that is the gold standard for the treatment of myriad spinal conditions including deformity, spondylolistheses, and degenerative disc disease1,2. Its prevalence is increasing, with over 400,000 of these procedures currently performed annually in the United States2–5. However, complications have been reported to occur in approximately 30 percent of patients6–8 and often necessitate revision surgery, which is associated with substantial morbidity and healthcare costs9,10. The resultant morbidity and healthcare costs for patients with failed fusions provides impetus for prospective studies to better assess patient risk pre-operatively. Risk of post-operative complications has been linked to many demographics such as age11–15, sex16, menopausal status17–19 and low BMD20. The impact of low BMD is an important consideration as 10 percent – 40 percent of patients presenting for these procedures have osteoporosis21 and numbers may be even higher when modalities other than dual energy x-ray absorptiometry (DXA) are utilized22,23. While skeletal health is critical to hardware stability, de novo bone formation and therefore post-operative outcomes, few studies have directly investigated bone material properties, and how they relate to complications in patients undergoing spinal fusion surgery.
Previous work has linked pre-operative skeletal assessments and post-operative outcomes in patients who undergo fusion surgery. A few studies have found relationships between low areal bone mineral density (aBMD) measurements from DXA and complications after fusion, but this finding is not uniform. Although DXA is the gold standard for the diagnosis of osteoporosis, it may underestimate the prevalence of osteoporosis at the spine in patients with spinal deformities24–29. Low spine volumetric BMD (vBMD) by QCT has also been linked to risk of post-operative complications20,30,31. Further, in a recent prospective study, our group found that patients with low peripheral volumetric bone density (vBMD) and abnormal microarchitecture by high resolution peripheral QCT (HRpQCT) had higher rates of skeletal complications post-operatively, while DXA did not predict complications17. Few studies have examined the relationship between pre-operative imaging and intrinsic properties of bone obtained during surgery.
The goal of this study was to relate direct measurements of the collagen and mineral properties of the bone by Fourier-transform infrared (FTIR) spectroscopy to pre-operative imaging by DXA, QCT, and HRpQCT, as well as to the incidence of post-operative complications and revision surgery. Fourier-transform infrared spectroscopy provides a means of analyzing material properties of bone (including crystallinity, mineral-to-matrix ratio, and collagen maturity) and for assessment of bone quality in different disease states32,33. Properties assessed by this technique are associated with risk of fragility fractures and atypical femur fractures34–37. For this investigation, we focused on postmenopausal women, as this group is at an increased risk for developing skeletal complications17–19. We hypothesized that patients with lower aBMD, vBMD, and worse microarchitecture would have features characteristic of older bone by FTIR. We further performed an exploratory investigation of the relationships between FTIR indices and post-operative complications and revision surgeries.
Methods
Study procedures
This cohort included postmenopausal women undergoing spinal fusion surgery who were recruited from the Spine Service at the Hospital for Special Surgery (HSS) between February 2019 and October 2021. Patients were enrolled as part of a larger prospective study that included adult men and women. Patients were included who presented for single or multilevel spine fusion surgeries that involved the lumbar region, whether it was an initial surgery or a revision of a prior procedure. Patients in whom an intra-operative bone biopsy could not be obtained were excluded. A total of 23 women met the criteria for this analysis. This study was approved by the Institutional Review Board of HSS (IRB# 2016-0662), and all participants signed written informed consent.
At baseline pre-operative visits, patients provided information regarding their medical history and medications and a blood sample was obtained. DXA, QCT, and HRpQCT scans were performed. Upon enrollment, eight patients presented for a primary surgery, and fifteen for a revision of a prior procedure. The primary indication for initial surgery in the majority of patients (61%) was adult spinal deformity (ASD; scoliosis, kyphosis, flatback syndrome and/or kyphosis), though most patients (87%) had multiple indications for surgery. The most common indication for revision was pseudoarthrosis. The mean number of surgical levels was 6 ± 4. Graft material, including a combination of bone morphogenic protein (BMP), allograft, autograft, and bone marrow, was used in all patients. Radiographs were performed on patients at six weeks and six months post-operatively to identify any skeletal complications related to hardware failure or adjacent vertebral fractures. Re-operation and other complication rates were assessed for up to two years following the procedure.
Bone turnover markers
Participants had blood drawn pre-operatively. All labs were drawn fasting before 10 am. Samples were processed within 1 hour of collection and stored at −80°C for batch analysis. Bone-specific alkaline phosphatase (BSAP; BioVender, Asheville, NC) was used as a marker of bone formation, while C-telopeptide (CTX; Immunodiagnostics Systems, Gaithersburg, MD) was used to measure bone resorption. 25-hydroxy vitamin D (25OHD; Abbott Diagnostics, Abbott Park, IL) was also measured.
Areal BMD
Areal BMD was measured by DXA (Horizon A (S/N 201056) densitometer, Hologic Inc., Waltham, MA) of the lumbar spine L1-L4 (LS), right total hip (RTH), left total hip (LTH), right femoral neck (RFN), left femoral neck (LFN), and left 1/3 radius (1/3R). Short term in vivo precision is 0.70% for the spine, 1.36% for the total hip and 0.70% for the radius17,38.
Lumbar vertebrae with significant deformity, osteosclerosis, osteophytes, or degenerative disease were excluded from the analysis. One patient’s hips could not be scanned because of bilateral hardware and a second patient did not have any DXA measurements available for analysis. Osteoporosis was defined as a T-score of equal to or less than −2.5, and osteopenia was defined as a T-score less than −1.0, but greater than −2.5 at any site.
HRpQCT of the distal radius and tibia
HRpQCT (XtremeCT II, Scanco Medical, Brüttisellen, Switzerland) scans were collected and analyzed as previously described17,20,38. Images were acquired by using a microfocus X-ray source (68 kVp voltage, 900 μA current, 43 s integration time), scanning a region 10.2 mm long yielding an image volume of 60.7 μm isotropic voxel size, with a 140.010 field of view. The non-dominant forearm and ipsilateral tibia (or non-fractured arm or leg in subjects with prior wrist or ankle fractures) were immobilized in a radiolucent carbon fiber shell. A region of interest (ROI) was defined on a 2-D scout view by placing a reference line at the distal subchondral endplate and scans were acquired using a standard fixed offset of 9 mm and 22 mm from the reference line for radius and tibia, respectively. A single, highly trained operator acquired and analyzed all scans. Scans were scored for motion on a scale of 1–5 and scans with motion score > 3 were excluded from analysis. The manufacturer’s standard method was used to filter and binarize the HRpQCT images. Automated segmentation was used to segment the cortical and trabecular regions39. Morphologic microstructural HRpQCT bone features were assessed, including area; density - total, trabecular (Tb) and cortical (Ct) volumetric BMD (vBMD); microstructure - trabecular number (Tb.N), thickness (Tb.Th), and separation (Tb.Sp), cortical thickness (Ct.Th), and cortical porosity (Ct.Po). In vivo short-term reproducibility (CV) for HRpQCT measures at our center is between 0 and 5% for all measures except Ct.Po40. This has been previously described and validated in other studies41. HRpQCT scans at both the radius and tibia were available on all participants.
QCT measurement of lumbar spine
Central QCT was performed using asynchronous calibration and an accompanying commercially available software package (Mindways QCTpro). The ROIs were placed automatically, reviewed by a radiologist, and repositioned if necessary. An average of the two levels analyzed were collected for all patients. Volumetric BMD was measured at L1 and L2 for most patients. In one case in which L2 was not evaluable due to hardware, measurements were performed at T12 and L1. Vertebral bodies with evidence of sclerosis, prior fracture, or other anatomic abnormality were excluded from analysis. There were two subjects whose scans were not available for analysis.
Radiographic analysis
Full length scoliosis films or EOS films were obtained on patients at baseline, six weeks, six and 12 months post-operatively. These radiographs were analyzed for each of the following: rod breakage, screw loosening, pseudoarthrosis, adjacent segment fracture and proximal junctional kyphosis (PJK, a post-operative spinal deformity directly above the site of instrumentation42–45). PJK was defined using the criteria detailed in Glattes et al. (1976)46. PJK was measured from the upper instrumented vertebra (UIV) to the vertebra directly above (UIV + 1) and from the UIV to the UIV + 2 on both pre- and post-operative imaging.
Fourier transform-infrared spectroscopy
Intraoperative cortical bone biopsies were taken from the lumbar spine, most commonly cortical bone from the spinous process (21). In two cases where the spinous process was not available, samples were taken from the lamina (1) or facet joint (1). Twelve biopsies were taken from L1, the remainder from L2 (3), L3 (3), L4 (3), and L5 (2). Biopsies were available on all study participants. FTIR spectroscopy was used to investigate underlying changes in cortical bone tissue that might affect surgical outcomes in spine fusion patients by further analyzing the spatial distributions and composition of mineral and collagen within the vertebral bone. Following our previously published protocol47, samples were cleaned of soft tissue and marrow, defatted, lyophilized, and cryogenically ground to a powder (677; SPEX SamplePrep, Metuchen, NJ, USA). Pellets were created by combining 200 mg of dried KBr with 2 mg of bone powder and pressing the mixture in 13-mm-diameter die. Using an FTIR spectrometer (Spotlight 400; Perkin-Elmer Instruments, Waltham, MA), FTIR spectra were collected at a spectral resolution of 4 cm−1 over a range of 800 to 2000 cm−1.
Using a custom Matlab code (201a; The MathWorks Inc., Natick, MA, USA), the spectra were baseline corrected (as detailed in48) and analyzed to determine the following metrics: (1) mineral-to-matrix ratio (area ratio of the phosphate ν1-ν3 peak [916-1180 cm−1] to amide I peak [1596-1712 cm−1]), which characterizes tissue mineral content49; (2) collagen maturity (intensity ratio of 1660 cm−1 to 1690 cm−1(intensity ratio of 1660 cm−1 to 1690 cm−1), which is related to the ratio of mature trivalent to immature divalent enzymatic crosslinks50; (3) mineral maturity/crystallinity (MMC) (intensity ratio of 1030 cm−1 to 1020 cm−1), which is related to hydroxyapatite crystal size and stoichiometric perfection51; (4) carbonate to phosphate ratio (area ratio of the carbonate ν2 peak [852-890 cm−1] to phosphate ν1-ν3 peak [916-1180 cm1]), which characterizes carbonate substitution in the crystal structure52; and (5), the acid phosphate content (intensity ratio of 1127 cm−1 to 1096 cm−1), which characterizes acid phosphate substitution into stoichiometric hydroxyapatite and is an indicator of new mineral formation53 (Figure 1). These measurements provide insight into bone strength and fragility. To assess repeatability, multiple measurements were performed on cortical bone samples from three randomly chosen study specimens and two synthetic carbonate-substituted hydroxyapatite standards (10% CO3 and 25% CO3)54 (Supplemental Figure 1). When repeatability of each FTIR outcome measure was quantified as SD/sqrt(n), where n=3 repeated scans per specimen, the FTIR outcome measures were highly repeatable, with values for MMC ranging from 0.00021 to 0.000076 (Supplemental Table 1).
Figure 1.
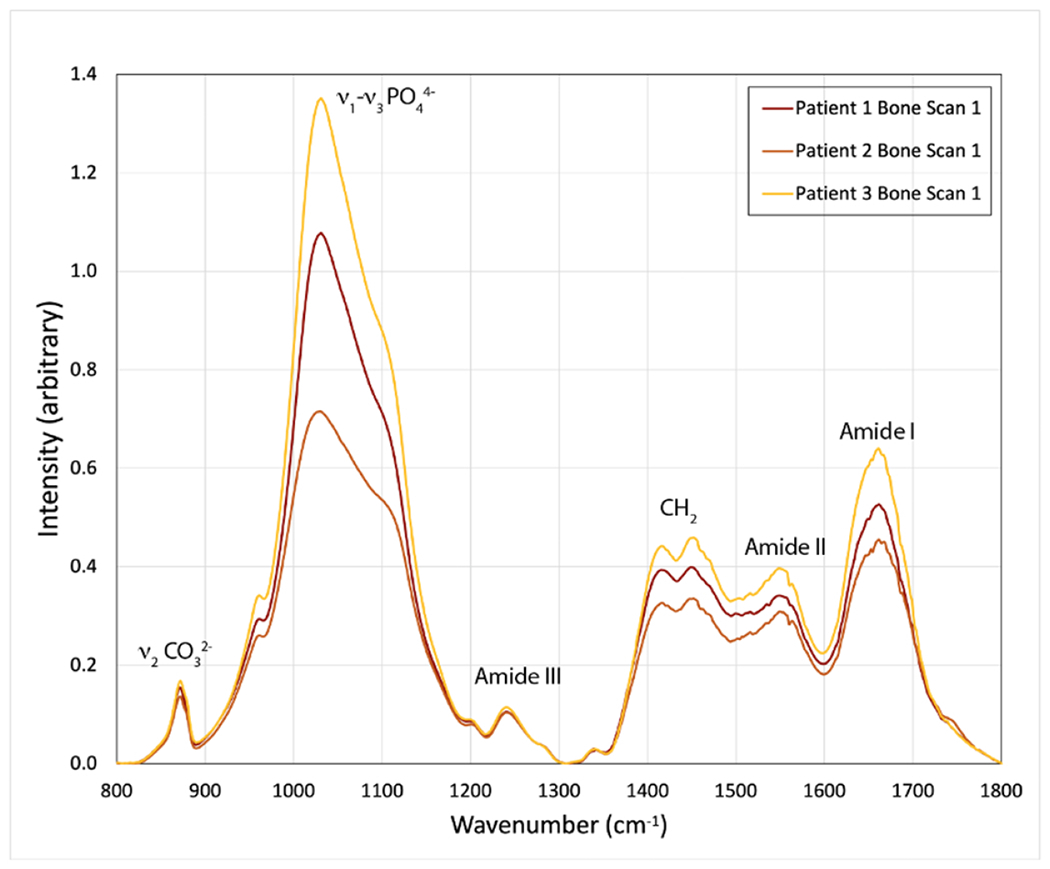
Representative FTIR spectra of cortical bone showing characteristic mineral and organic matrix peaks.
Statistical analysis
Data were merged into a single SAS 9.0 dataset (SAS Institute, Cary, NC) and checked for consistency and completeness. Distributions were evaluated for normality and variables were log-transformed as appropriate. Spearman correlations were used for all analyses. No data were imputed and no adjustment for multiple comparisons was made. T-tests were performed to assess the difference between FTIR properties and complication incidence (composite of hardware failure, complications in adjacent vertebrae and re-operation for the above indications).
Results
Study subjects
Characteristics of the 23 postmenopausal women, including co-morbidities, medications, and supplement use are outlined in Table 1. The cohort was predominantly Caucasian (91%). The mean age was 67 years. Mean BMI was 28 kg/m2; 35% of the cohort was obese (BMI > 30 kg/m2). Half of the cohort had a history of tobacco use, 17% were current smokers, and 35% were former smokers. Other relevant co-morbidities included hypertension (10), hyperlipidemia (8), and diabetes (3). Four patients (17%) reported a history of an atraumatic non-vertebral fracture. Of these, two patients reported multiple fractures. The skeletal sites of fractures included the forearm (1), humerus (1), hip (3), rib (2), and metatarsal (2). Additionally, 17% (4) reported current use of medication for osteoporosis, including teriparatide (1) and bisphosphonates (3).
Table 1.
Characteristics of the study population
| Total | |
|---|---|
| Age (years) (mean ± SD) | 67 ± 7 |
| Race | |
| White (N, %) | 21, 91% |
| Black/African American (N, %) | 1, 4.5% |
| Asian | 1, 4.5% |
| Ethnicity | |
| Hispanic (N, %) | 3, 13% |
| Non-Hispanic (N, %) | 20, 87% |
| BMI (kg/m2) (mean ± SD) | 28 ± 8 |
| Underweight (BMI < 18.5) | 0, 0% |
| Normal (18.5 ≤ BMI < 25) | 11, 48% |
| Overweight (25 ≤ BMI < 30) | 4, 17% |
| Obese (BMI ≥ 30) | 8, 35% |
| Revision | 8, 35% |
| Smoking history | |
| Current (N, %) | 4, 17% |
| Former (N, %) | 8, 35% |
| Never (N, %) | 11, 48% |
| Supplement history | |
| Vitamin D (N, %) | 17, 74% |
| Calcium (N, %) | 12, 52% |
| Current use of medication for Osteoporosis | |
| Bisphosphonate (N, %) | 3, 13% |
| Teriparatide (N, %) | 1, 4.5% |
| Serum biochemistries (mean ± SD) | |
| 25 OH vitamin D (20-50 ng/mL) | 42 ± 16 |
| C-telopeptide (postmenopausal ref: 104-1008 pg/mL) | 544 ± 238 |
| Bone-specific alkaline phosphatase (postmenopausal ref: 7-22.4 ug/L) | 12 ± 6 |
Mean 25OHD was in the sufficient range, and baseline bone turnover markers (BSAP and CTX) were within the normal postmenopausal reference range. Results from pre-operative imaging exams are detailed in Table 2. Mean Z-scores for the cohort at the LS, TH and 1/3 R were within the normal range. Measurements at the FN were within the osteopenic range. Among the study cohort, 30% had osteoporosis and 43% had osteopenia by T-score at any site. The average vBMD at the lumbar spine by QCT was 107 mg/cm3, falling within the osteopenic range as defined by the American College of Radiology55. According to those criteria, vBMD less than 80 mg/cm3 represents osteoporosis, between 80 and 120 mg/cm3 osteopenia, and above 120 mg/cm3 normal. Mean Z-scores for HRpQCT measures, comparing subjects to a reference population of similar age and sex are also presented56. Mean values for FTIR measures are presented in Table 3.
Table 2.
Baseline imaging values
| Average Value ± SD | Z-Score ± SD | |
|---|---|---|
| DXA | ||
| LS (g/cm2) | 1.05 ± 0.15 | 1.97 ± 1.56 |
| RFN (g/cm2) | 0.70 ± 0.12 | 0.21 ± 1.13 |
| LFN (g/cm2) | 0.69 ± 0.14 | 0.17 ± 1.25 |
| RTH (g/cm2) | 0.82 ± 0.14 | 0.23 ± 1.24 |
| LTH (g/cm2) | 0.82 ± 0.14 | 0.23 ± 1.21 |
| 1/3R (g/cm2) | 0.63 ± 0.07 | 0.82 ± 0.96 |
| Radius HRpQCT | ||
| Trabecular vBMD (mg HA/cm3) | 120 ± 38 | −0.66 ± 1.21 |
| Trabecular number (1/mm) | 1.26 ± 0.22 | −0.52 ± 0.99 |
| Trabecular bone volume fraction (unitless) | 0.17 ± 0.05 | −0.61 ± 1.23 |
| Trabecular separation (mm) | 0.79 ± 0.15 | −0.48 ± 1.02 |
| Cortical thickness (mm) | 0.89 ± 0.23 | 0.70 ± 1.40 |
| Cortical vBMD (mg HA/cm3) | 861 ± 87 | 0.82 ± 1.12 |
| Total area (mm2) | 261 ± 49 | −0.78 ± 1.29 |
| Tibia HRpQCT | ||
| Trabecular vBMD (mg HA/cm3) | 129 ± 39 | −0.85 ± 1.17 |
| Trabecular number (1/mm) | 1.22 ± 0.24 | −0.47 ± 1.05 |
| Trabecular bone volume fraction (unitless) | 0.20 ± 0.05 | −0.77 ± 1.13 |
| Trabecular separation (mm) | 0.83 ± 0.19 | −0.48 ± 1.14 |
| Cortical thickness (mm) | 1.30 ± 0.30 | 0.01 ± 1.22 |
| Cortical vBMD (mg HA/cm3) | 824 ± 68 | 0.07 ± 0.87 |
| Total area (mm2) | 689 ± 135 | 0.01 ± 1.31 |
| Spine QCT | ||
| vBMD (mg HA/cm3) | 107 ± 44 | Not available |
LS: lumbar spine; RFN: right femoral neck; LFN: left femoral neck; RTH: right total hip; LTH: left total hip; 1/3R: 1/3 radius
Table 3.
Mean absolute values of cortical bone FTIR metrics of tissue maturity
| FTIR Metric | Mean ± SD |
|---|---|
| MMC | 1.051 ± 0.019 |
| Acid:phosphate ratio | 0.648 ± 0.045 |
| Collagen maturity | 1.509 ± 0.110 |
| Mineral:matrix ratio | 4.211 ± 1.145 |
| Carbonate:phosphate ratio | 0.021 ± 0.003 |
Association between FTIR metrics and pre-operative assessments
We found that cortical bone measurements by HRpQCT were associated with material properties by FTIR. Patients with higher cortical vBMD at the tibia exhibited characteristics of more mature bone, as shown by a positive correlation between cortical vBMD with mineral maturity/crystallinity (r = 0.45, p = 0.034) and collagen maturity (r = 0.45, p = 0.032), for which higher values indicate mature bone. Further, there was an inverse relationship between cortical vBMD with carbonate to phosphate ratio (r = −0.42, p = 0.049) and acid phosphate content (r = −0.55, p = 0.0073), for which higher values indicate new bone (Figure 2). These relationships were not significant at the radius. We did not find significant relationships between FTIR compositional metrics and trabecular bone indices assessed by HRpQCT at either site.
Figure 2.
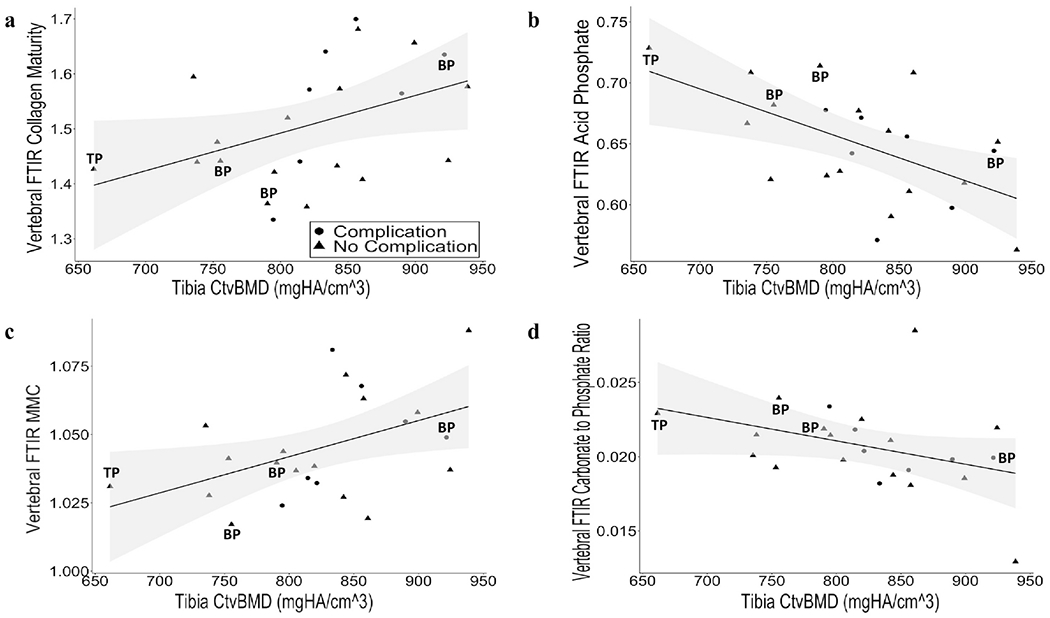
Association between FTIR metrics and cortical vBMD at the tibia by HRpQCT. Correlation between Ct vBMD at the tibia, measured by HRpQCT with (a) collagen maturity (r = 0.45, p = 0.032), (b) acid phosphate content (r = −0.55, p = 0.0073), (c) mineral maturity/crystallinity (MMC) (r = 0.45, p = 0.034), and (d) carbonate to phosphate ratio (r = −0.42, p = 0.049). Shaded area represents the 95% confidence interval. Patients currently using bisphosphonates (BP) and teriparatide (TP) are indicated. Patients who developed post-operative complications are denoted by circles, and patients who did not develop complications are denoted by triangles.
Greater aBMD at the LS (r = −0.55, p = 0.013) and 1/3R (r = −0.54, p = 0.010) assessed by DXA were both significantly associated with lower acid phosphate content assessed by FTIR (Figure 3). Analyses were repeated after excluding one patient whose LS aBMD was substantially lower than the others, and the relationships remained the same. There was not any other significant correlation with aBMD at any of the other sites with the other FTIR metrics. Relationships between DXA and HRpQCT parameters and FTIR were further assessed by correcting for treatment with osteoporosis medications, age, BMI, and smoking. Adjusting for treatment, age, BMI, and smoking minimally influenced the unadjusted correlations. The majority of the correlations remained significant and all retained p-values less than or equal to 0.1, except for one. Analyses adjusted for treatment for osteoporosis, age, BMI, and smoking are shown in Supplemental Table 2.
Figure 3.
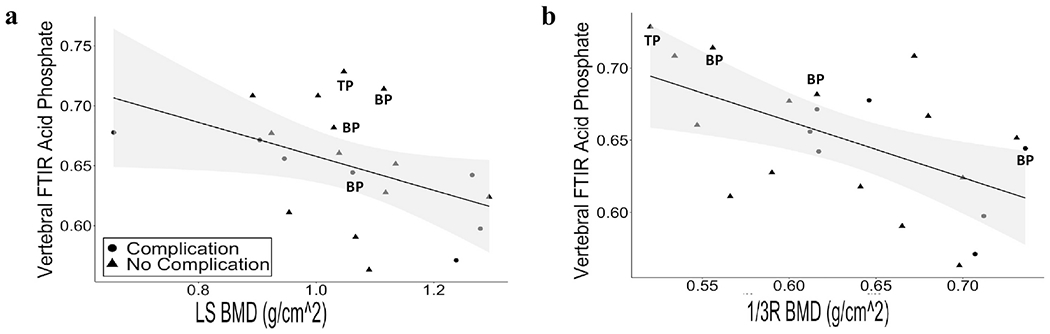
Association between FTIR metrics and aBMD by DXA. Correlation between acid phosphate content with (a) LS (r = −0.55, p = 0.013) and (b) 1/3R (r = −0.54, p = 0.010). Shaded area represents the 95% confidence interval. Patients currently on bisphosphonates (BP) and teriparatide (TP) are indicated. Patients who developed post-operative complications are denoted by circles, and patients who did not develop complications are denoted by triangles.
There was no association between any FTIR metrics and average spine vBMD by QCT. Further, there was no association between any of the FTIR metrics with 25OHD, nor with bone turnover measured by CTX and BSAP. Detailed correlations between FTIR and HRpQCT at the radius and tibia, DXA and lumbar QCT are included in Supplemental Table 3.
Association between FTIR metrics and complications
During our prospective study follow-up, skeletal complications occurred in seven patients during the first year after surgery. Three of these patients experienced multiple complications. The most common complications were PJK (7 patients), fracture (2 patients), and screw loosening (2 patients). Two of the patients who developed skeletal complications required revision surgeries.
We did not find a significant difference between development of skeletal complications, as a composite measure, and any of the FTIR metrics. Patients who developed complications were of similar age and BMI to those who did not. As only two patients had revision surgery, formal statistical testing comparing their FTIR metrics to those who did not undergo revision is not presented. However, the data suggests that mineral maturity/crystallinity was lower in patients who required revision for skeletal complications (mean = 1.033±0.02) by FTIR than patients who did not require a revision (mean = 1.047±0.001). This suggests that patients who required subsequent revision had smaller, less perfect mineral crystals, indicative of newer bone at the vertebrae, than patients who did not require revision.
Discussion
This study investigated the relationships between direct measurements of bone material properties with pre-operative imaging and post-operative outcomes. Contrary to our hypothesis, we found that patients with more mature bone by FTIR, measured as lower acid phosphate content and carbonate to phosphate ratio, and greater collagen maturity and mineral maturity/crystallinity, had greater cortical vBMD at the tibia and greater aBMD at the LS and 1/3R. Our results extend findings from previous studies17,20,57 by highlighting material properties as another feature of bone quality that directly relates to microarchitecture and may be important for successful fusion. Several studies have investigated the relationship between aBMD measured by DXA and the risk of complications among patients undergoing spine fusion surgery. Some of these studies suggest that low BMD is such a risk factor for skeletal complications—including PJK, fractures of adjacent vertebrae45,58, and screw loosening30,31,45—but others have not found a relationship between aBMD by DXA and post-operative complications15,17,59,60. Our group has previously demonstrated that patients who have low vBMD and microarchitectural abnormalities by pre-operative high resolution peripheral QCT had higher rates of complications than those with higher vBMD and more intact microarchitecture17.
FTIR spectroscopy is a commonly used method to investigate bone quality. During formation of mineralized tissues, crystal growth (maturation) occurs through the addition of ions to the crystal nucleus. Bone crystals generally grow larger and more perfect with greater tissue age (time since tissue formation). As primary and secondary mineralization progress, these physiologic processes are reflected in spectroscopic metrics of increasing tissue maturity, including increasing mineral matrix ratio and mineral maturity/crystallinity (MMC) and decreasing acid phosphate content61. In this study, we found that patients with greater cortical vBMD had higher MMC and collagen maturity and lower acid phosphate content and carbonate to phosphate ratio, indicators of greater tissue maturity, whereas individuals with lower vBMD had indicators of lower tissue maturity. Lower vBMD and lower aBMD may result in an adaptive response to promote formation of new tissue, whereas greater vBMD and greater aBMD may result in less formation of new bone, and secondary mineralization may proceed, with the growth of larger and more perfect crystals and formation of more mature crosslinks. Although, to our knowledge, our study is the first to report mineral properties assessed by FTIR in vertebral bone tissue, the mean absolute values of cortical bone FTIR metrics of tissue maturity reported here (Table 3) are similar to or modestly below those reported for cortical iliac bone of healthy postmenopausal women (mineral matrix 4.2 vs. 4.2; MMC 1.0 vs. 1.2; collagen maturity 1.5 vs. 2)62, suggesting that maturity of the tissue assessed here reflects physiologic mineralization processes. Further studies on vertebral bone are required to confirm these results.
Healthy bone tissue typically exhibits similar trends in mineral:matrix ratio and MMC63. However, only the relationship between MMC and vBMD reached statistical significance likely because MMC is one of the least variable FTIR parameters (COV=1.87%), while mineral:matrix is more variable (COV=27.18%) (Supplemental Table 3). Although HRpQCT measurements are obtained at peripheral sites, previous studies have suggested that these peripheral measures correlate with lower vBMD and fragility at central sites64–66. We found that vertebral FTIR measurements were associated with cortical measures at the tibia but not at the radius. Prior work by our group and others64,65 has demonstrated that tibial measurements are closely related to vertebral bone fragility.
Other studies have specifically investigated the mineral and collagen properties of patients undergoing spine fusion surgery, but none have looked at how these properties are associated with microarchitecture. One study demonstrated that dermal layer thickness and echogenicity by ultrasound were respectively negatively and positively correlated with collagen maturity assessed by FTIR spectroscopy in women67, suggesting that markers of collagen age may correlate between skin and bone. In one study that evaluated tibial plateau specimen in patients with osteoarthritis at the knee, samples with bone marrow edema-like lesions (BMEL) had lower mineral-to-matrix ratio and also exhibited greater vBMD, bone volume fraction, and trabecular thickness than non-BMEL regions68. In contrast, our study did not detect any relationship between mineral-to-matrix ratio and trabecular microarchitecture. This difference might be because we analyzed samples largely comprised of cortical bone or because our measurements of trabecular microarchitecture were obtained at sites different from that of the bone biopsy. To our knowledge, ours is the first study to relate skeletal assessments of aBMD, spine and peripheral vBMD, and microarchitecture, at both the tibia and radius, to FTIR metrics of vertebral bone in patients undergoing spinal fusion surgery.
In our study, we found that lower aBMD at the 1/3R and LS were significantly associated with greater acid phosphate content, which is indicative of newer bone formation53. This observation may relate to the fact that newer bone has less mineral content than older bone, which could explain the resulting lower aBMD. No other FTIR metrics exhibited statistically significant correlations with aBMD at any of the other anatomic sites.
We did not find that the FTIR metrics correlated with bone turnover markers. It is conceivable that we would have detected relationships with a larger sample size. Few studies have investigated the relationships between bone turnover and FTIR indices. In one other study, resorption measured by urine N-terminal telopeptide (uNTX) was positively correlated with mineral-to-matrix ratio in women, but conversely uNTX was positively correlated with acid phosphate content and negatively with collagen maturity in men69. As greater turnover is expected to be associated with indices of new bone formation (low mineral-to-matrix ratio, low collagen maturity, low mineral maturity/crystallinity, and high acid phosphate), the divergent correlations observed in that study among men and women between uNTX and indices of new bone formation previously observed are unexpected. Additional research investigating these relationships is needed.
In addition to the many studies investigating aBMD in patients presenting for spine fusion, many other studies have focused on vBMD by CT20,58. While we did not find any correlation here between vertebral FTIR measurements and QCT, it is conceivable that this is a consequence of the biopsy consisting mostly of cortical bone rather than trabecular bone, while the vBMD calculated by QCT is restricted to the trabecular bone.
We found that patients who required a revision surgery appeared to have lower MMC at the vertebrae than patients who did not require this intervention. These results suggest that patients who required a revision had smaller or less perfect mineral crystals, compared to patients who did not require revision. Since MMC reflects both stoichiometric perfection and crystal size, the lower MMC observed in the revision group may arise from less stoichiometric perfection, smaller crystals, or a combination of both factors. Our findings of lower MMC in the revision group are consistent with the numerically greater carbonate:phosphate ratio and acid phosphate observed in the revision group, suggesting that substitution of carbonate and acid phosphate into the hydroxyapatite structure may be one mechanism that contributes to lower MMC in the revision group. However, it is important to note that only two patients required a revision during the first year after surgery. It is likely that other factors may have contributed to this result. These results, suggestive of an initial relationship warrant investigation in future studies.
There are several important limitations to this work. The sample size of this cohort was relatively small, which may have limited our ability to detect more subtle differences between FTIR metrics and underlying microarchitectural properties. Further, the number of patients who experienced complications and required re-operation was very few. The majority of subjects were only followed for six months post-operatively. Future studies with larger sample sizes and a longer duration of follow-up are necessary to further investigate the sensitivity of the different pre-operative imaging exams to different FTIR features as well as between FTIR and surgical outcomes. Although the inclusion of women who were using osteoporosis medications may have influenced our findings, when we excluded the four patients who were taking osteoporosis medications at the time of surgery from the analysis, most comparisons remained significant. Further, since these are so commonly used among postmenopausal women, exclusion of those using these medications would have been a barrier to recruitment and limited the generalizability of our results. Similarly, although we did have some heterogeneity in our study, including age, BMI, and smoking status, adjusting for these factors did not substantially change the results. Our samples were almost exclusively cortical bone because they were collected from the spinous process and not the vertebral bodies. We did this because obtaining vertebral body samples would have been much more complicated and might disrupt the surgical construct.
In conclusion, we found direct relationships between pre-operative imaging and intra-operative measurements of bone quality in postmenopausal women having spine fusion. Greater tibial vBMD was associated with higher MMC and collagen maturity and lower carbonate to phosphate ratio and acid phosphate content. Greater aBMD at the LS and 1/3R was associated with lower acid phosphate content. Our results are important because they suggest that morphologic parameters assessed by pre-operative imaging directly relate to material properties of bone in patients have spine fusion surgery. They further provide the first suggestion that these material properties may predict those patients who will require revision surgery. Additional work is needed to further understand of the relationships between preoperative and intra-operative bone quality and surgical complications in order to optimize outcomes for the burgeoning population of older patients having spine fusion surgery.
Supplementary Material
Highlights.
BMD was related to material properties of bone in spine surgery patients
More mature bone by FTIR was associated with greater BMD at cortical sites
Imaging of peripheral skeletal sites may predict material properties at the spine
Acknowledgements:
This research was supported by UL1TR002384 NIH/NCATS awarded by the Weill Cornell CTSC NSF CMMI 1452852, and the Marina Kellen French Foundation.
Declaration of Interest:
AKH, AD, BES, LS, SG, KBA, AJ, SC, and EB have nothing to disclose. HJK reports personal fees from Alphatec, personal fees from K2M, personal fees from Zimmerbiomet, outside the submitted work. MC reports grants and personal fees from K2M, personal fees from Zimmerbiomet, grants from Radius Health, grants from RTI, outside the submitted work. DL reports. consultant role for Depuy Synthes, Stryker, Viseon, Inc., ownership interest in HS2,LLC, ISPH II,LLC, Remedy Logic, Vestia Ventures MiRus Investment LLC, Woven Orthopedic Technologies, Viseon, Inc; royalty fees from Nuvasive, Inc., Stryker; Advisory Board membership for Remedy Logic (all activities outside the submitted work). ED has nothing to disclose. EMS reports grants from Novartis, grants from Radius Health, all outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit Author Statement:
Alison K. Heilbronner: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing- Original Draft, Writing- Review & Editing
Alexander Dash: Data curation, Investigation, Methodology, Writing- Original Draft
Beth E. Straight: Data curation, Investigation
Leah J. Snyder: Data curation, Investigation
Sandhya Ganesan: Data curation, Investigation
Kobby Adu: Data curation, Investigation, Validation
Andy Jae: Data curation, Investigation
Shannon Clare: Data curation, Investigation, Methodology, Writing- Original Draft
Emma Billings: Data curation, Investigation, Writing- Original Draft, Writing- Review & Editing
Han Jo Kim: Data curation, Investigation, Methodology, Writing- Original Draft
Matthew Cunningham: Data curation, Investigation, Methodology, Writing- Original Draft
Darren R. Lebl: Data curation, Investigation, Methodology, Writing- Original Draft
Eve Donnelly: Conceptualization, Data curation, Validation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Visualization, Writing- Original Draft, Writing- Review & Editing
Emily M. Stein: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing- Original Draft, Writing- Review & Editing
References
- 1.Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI. United States Trends in Lumbar Fusion Surgery for Degenerative Conditions. Spine 2005;30:1441–5. 10.1097/01.brs.0000166503.37969.8a. [DOI] [PubMed] [Google Scholar]
- 2.Rajaee SS, Bae HW, Kanim LEA, Delamarter RB. Spinal Fusion in the United States: Analysis of Trends From 1998 to 2008. Spine 2012;37:67–76. 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- 3.Characteristics of Operating Room Procedures in U.S. Hospitals, 2011 - Statistical Brief #170. n.d. URL: https://hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.jsp (Accessed 16 October 2021).
- 4.Lee DD, Kim JY. A comparison of radiographic and clinical outcomes of anterior lumbar interbody fusion performed with either a cellular bone allograft containing multipotent adult progenitor cells or recombinant human bone morphogenetic protein-2. J Orthop Surg Res 2017;12:126. 10.1186/s13018-017-0618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report 2010:1–20, 24. [PubMed] [Google Scholar]
- 6.Dede O, Thuillier D, Pekmezci M, Ames CP, Hu SS, Berven SH, et al. Revision surgery for lumbar pseudarthrosis. Spine J 2015;15:977–82. 10.1016/j.spinee.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 7.Yoon ST, Boden SD. Spine fusion by gene therapy. Gene Ther 2004;11:360–7. 10.1038/sj.gt.3302203. [DOI] [PubMed] [Google Scholar]
- 8.Röllinghoff M, Schlüter-Brust K, Groos D, Sobottke R, Michael JW-P, Eysel P, et al. Mid-range outcomes in 64 consecutive cases of multilevel fusion for degenerative diseases of the lumbar spine. Orthop Rev (Pavia) 2010;2:e3. 10.4081/or.2010.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bess S, Boachie-Adjei O, Burton D, Cunningham M, Shaffrey C, Shelokov A, et al. Pain and disability determine treatment modality for older patients with adult scoliosis, while deformity guides treatment for younger patients. Spine (Phila Pa 1976) 2009;34:2186–90. 10.1097/BRS.0b013e3181b05146. [DOI] [PubMed] [Google Scholar]
- 10.Pichelmann MA, Lenke LG, Bridwell KH, Good CR, O’Leary PT, Sides BA. Revision rates following primary adult spinal deformity surgery: six hundred forty-three consecutive patients followed-up to twenty-two years postoperative. Spine (Phila Pa 1976) 2010;35:219–26. 10.1097/BRS.0b013e3181c91180. [DOI] [PubMed] [Google Scholar]
- 11.Worley N, Marascalchi B, Jalai CM, Yang S, Diebo B, Vira S, et al. Predictors of inpatient morbidity and mortality in adult spinal deformity surgery. Eur Spine J. 2016;25:819–27. 10.1007/s00586-015-4104-x. [DOI] [PubMed] [Google Scholar]
- 12.Andersen T, Christensen FB, Langdahl BL, Ernst C, Fruensgaard S, Ostergaard J, et al. Fusion mass bone quality after uninstrumented spinal fusion in older patients. Eur Spine J 2010;19:2200–8. 10.1007/s00586-010-1373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JS, Shaffrey E, Klineberg E, Shaffrey CI, Lafage V, Schwab FJ, et al. Prospective multicenter assessment of risk factors for rod fracture following surgery for adult spinal deformity. J Neurosurg Spine 2014;21:994–1003. 10.3171/2014.9.SPINE131176. [DOI] [PubMed] [Google Scholar]
- 14.Cloyd JM, Acosta FL, Cloyd C, Ames CP. Effects of age on perioperative complications of extensive multilevel thoracolumbar spinal fusion surgery. J Neurosurg Spine 2010;12:402–8. 10.3171/2009.10.SPINE08741. [DOI] [PubMed] [Google Scholar]
- 15.Sheinberg DL, Perez-Roman RJ, Lugo-Pico JG, Cajigas I, Madhavan KH, Green BA, et al. Effects of menopausal state on lumbar decompression and fusion surgery. J Clin Neurosci 2020;77:157–62. 10.1016/j.jocn.2020.04.117. [DOI] [PubMed] [Google Scholar]
- 16.Faciszewski T, Winter RB, Lonstein JE, Denis F, Johnson L. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults. A review of 1223 procedures. Spine (Phila Pa 1976) 1995;20:1592–9. 10.1097/00007632-199507150-00007. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Dash A, Cunningham M, Schwab F, Dowdell J, Harrison J, et al. Patients with abnormal microarchitecture have an increased risk of early complications after spinal fusion surgery. Bone 2021;143:115731. 10.1016/j.bone.2020.115731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg 1999;90:163–9. 10.3171/spi.1999.90.2.0163. [DOI] [PubMed] [Google Scholar]
- 19.Toyone T, Ozawa T, Kamikawa K, Watanabe A, Matsuki K, Yamashita T, et al. Subsequent vertebral fractures following spinal fusion surgery for degenerative lumbar disease: a mean ten-year follow-up. Spine (Phila Pa 1976) 2010;35:1915–8. 10.1097/BRS.0b013e3181dc846c. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Dash A, Krez A, Kim HJ, Cunningham M, Schwab F, et al. Low volumetric bone density is a risk factor for early complications after spine fusion surgery. Osteoporos Int 2020;31:647–54. 10.1007/s00198-019-05245-7. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer C, Mafi A, Beil FT, Schroeder M, Rolvien T. Skeletal Status in Patients Scheduled for Elective Lumbar Spine Surgery: Comparison of Discectomy, Decompression, Fusion, and Revision. Global Spine J 2022:21925682221105004. 10.1177/21925682221105005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burch S, Feldstein M, Hoffmann PF, Keaveny TM. Prevalence of Poor Bone Quality in Women Undergoing Spinal Fusion Using Biomechanical-CT Analysis. Spine (Phila Pa 1976) 2016;41:246–52. 10.1097/BRS.0000000000001175. [DOI] [PubMed] [Google Scholar]
- 23.Kadri A, Binkley N, Hare KJ, Anderson PA. Bone Health Optimization in Orthopaedic Surgery. J Bone Joint Surg Am 2020;102:574–81. 10.2106/JBJS.19.00999. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, Upadhyaya S, Patel A, Fogel HA, Cha T, Schwab J, et al. DEXA sensitivity analysis in patients with adult spinal deformity. Spine J 2020(20:174–80. 10.1016/j.spinee.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Pappou IP, Girardi FP, Sandhu HS, Parvataneni HK, Cammisa FP, Schneider R, et al. Discordantly high spinal bone mineral density values in patients with adult lumbar scoliosis. Spine (Phila Pa 1976) 2006;31:1614–20. 10.1097/01.brs.0000222030.32171.5f. [DOI] [PubMed] [Google Scholar]
- 26.Jaovisidha S, Sartoris DJ, Martin EM, De Maeseneer M, Szollar SM, Deftos LJ. Influence of spondylopathy on bone densitometry using dual energy X-ray absorptiometry. Calcif Tissue Int 1997;60:424–9. 10.1007/s002239900257. [DOI] [PubMed] [Google Scholar]
- 27.Blake GM, Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J 2007;83:509–17. 10.1136/pgmj.2007.057505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stone KL, Seeley DG, Lui L-Y, Cauley JA, Ensrud K, Browner WS, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 2003;18:1947–54. 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 29.Schuit SCE, van der Klift M, Weel AE a. M, de Laet CEDH, Burger H, Seeman E, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 2004;34:195–202. 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Schwaiger BJ, Gersing AS, Baum T, Nöel PB, Zimmer C, Bauer JS. Bone mineral density values derived from routine lumbar spine multidetector row CT predict osteoporotic vertebral fractures and screw loosening. AJNR Am J Neuroradiol 2014;35:1628–33. 10.3174/ajnr.A3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bredow J, Boese CK, Werner CML, Siewe J, Löhrer L, Zarghooni K, et al. Predictive validity of preoperative CT scans and the risk of pedicle screw loosening in spinal surgery. Arch Orthop Trauma Surg 2016;136:1063–7. 10.1007/s00402-016-2487-8. [DOI] [PubMed] [Google Scholar]
- 32.Mathavan N, Turunen MJ, Guizar-Sicairos M, Bech M, Schaff F, Tägil M, et al. The compositional and nano-structural basis of fracture healing in healthy and osteoporotic bone. Sci Rep 2018;8:1591. 10.1038/s41598-018-19296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt HB, Miller NA, Hemmerling KJ, Koga M, Lopez KA, Taylor EA, et al. Bone Tissue Composition in Postmenopausal Women Varies With Glycemic Control From Normal Glucose Tolerance to Type 2 Diabetes Mellitus. J Bone Miner Res 2021;36:334–46. 10.1002/jbmr.4186. [DOI] [PubMed] [Google Scholar]
- 34.Boskey A, Pleshko Camacho N. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials 2007;28:2465–78. 10.1016/j.biomaterials.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gourion-Arsiquaud S, Faibish D, Myers E, Spevak L, Compston J, Hodsman A, et al. Use of FTIR spectroscopic imaging to identify parameters associated with fragility fracture. J Bone Miner Res 2009;24:1565–71. 10.1359/jbmr.090414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lloyd AA, Gludovatz B, Riedel C, Luengo EA, Saiyed R, Marty E, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. PNAS 2017;114:8722–7. 10.1073/pnas.1704460114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt FN, Zimmermann EA, Campbell GM, Sroga GE, Püschel K, Amling M, et al. Assessment of collagen quality associated with non-enzymatic cross-links in human bone using Fourier-transform infrared imaging. Bone 2017;97:243–51. 10.1016/j.bone.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dash AS, Agarwal S, McMahon DJ, Cosman F, Nieves J, Bucovsky M, et al. Abnormal microarchitecture and stiffness in postmenopausal women with isolated osteoporosis at the 1/3 radius. Bone 2020;132:115211. 10.1016/j.bone.2019.115211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone 2007;41:505–15. 10.1016/j.bone.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal S, Rosete F, Zhang C, McMahon DJ, Guo XE, Shane E, et al. In vivo assessment of bone structure and estimated bone strength by first- and second-generation HR-pQCT. Osteoporos Int 2016;27:2955–66. 10.1007/s00198-016-3621-8. [DOI] [PubMed] [Google Scholar]
- 41.Stein EM, Kepley A, Walker M, Nickolas TL, Nishiyama K, Zhou B, et al. Skeletal structure in postmenopausal women with osteopenia and fractures is characterized by abnormal trabecular plates and cortical thinning. J Bone Miner Res 2014;29:1101–9. 10.1002/jbmr.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu F-Y, Wang T, Yang S-D, Wang H, Yang D-L, Ding W-Y. Incidence and risk factors for proximal junctional kyphosis: a meta-analysis. Eur Spine J 2016;25:2376–83. 10.1007/s00586-016-4534-0. [DOI] [PubMed] [Google Scholar]
- 43.Balci A, Kalemci O, Kaya FG, Akyoldas G, Yucesoy K, Ozaksoy D. Early and long-term changes in adjacent vertebral body bone mineral density determined by quantitative computed tomography after posterolateral fusion with transpedicular screw fixation. Clin Neurol Neurosurg 2016;145:84–8. 10.1016/j.clineuro.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Ma L, Yang D, Wang T, Yang S, Wang Y, et al. Incidence and risk factors for the progression of proximal junctional kyphosis in degenerative lumbar scoliosis following long instrumented posterior spinal fusion. Medicine (Baltimore) 2016;95:e4443. 10.1097/MD.0000000000004443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjerke BT, Zarrabian M, Aleem IS, Fogelson JL, Currier BL, Freedman BA, et al. Incidence of Osteoporosis-Related Complications Following Posterior Lumbar Fusion. Global Spine J 2018;8:563–9. 10.1177/2192568217743727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glattes RC, Bridwell KH, Lenke LG, Kim YJ, Rinella A, Edwards C. Proximal junctional kyphosis in adult spinal deformity following long instrumented posterior spinal fusion: incidence, outcomes, and risk factor analysis. Spine (Phila Pa 1976) 2005;30:1643–9. 10.1097/01.brs.0000169451.76359.49. [DOI] [PubMed] [Google Scholar]
- 47.Hunt HB, Torres AM, Palomino PM, Marty E, Saiyed R, Cohn M, et al. Altered tissue composition, microarchitecture, and mechanical performance in cancellous bone from men with type 2 diabetes mellitus. J Bone Miner Res 2019;34:1191–206. 10.1002/jbmr.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dehring KA, Crane NJ, Smukler AR, McHugh JB, Roessler BJ, Morris MD. Identifying chemical changes in subchondral bone taken from murine knee joints using Raman spectroscopy. Appl Spectrosc 2006;60:1134–41. 10.1366/000370206778664743. [DOI] [PubMed] [Google Scholar]
- 49.Boskey A, Mendelsohn R. Infrared analysis of bone in health and disease. Journal of Biomedical Optics 2005;10:31102. 10.1117/L1922927. [DOI] [PubMed] [Google Scholar]
- 50.Paschalis EP, Verdelis K, Doty SB, Boskey AL, Mendelsohn R, Yamauchi M. Spectroscopic Characterization of Collagen Cross-Links in Bone. Journal of Bone and Mineral Research 2001;16:1821–8. 10.1359/jbmr.2001.16.10.1821. [DOI] [PubMed] [Google Scholar]
- 51.Pleshko N, Boskey A, Mendelsohn R. Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophysical Journal 1991;60:786–93. 10.1016/S0006-3495(91)82113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor EA, Donnelly E, Yao X, Johnson ML, Amugongo SK, Kimmel DB, et al. Sequential Treatment of Estrogen Deficient, Osteopenic Rats with Alendronate, Parathyroid Hormone (1-34), or Raloxifene Alters Cortical Bone Mineral and Matrix Composition. Calcif Tissue Int 2020;106:303–14. 10.1007/s00223-019-00634-w. [DOI] [PubMed] [Google Scholar]
- 53.Spevak L, Flach CR, Hunter T, Mendelsohn R, Boskey A. FTIRI Parameters describing Acid Phosphate Substitution in Biologic Hydroxyapatite. Calcif Tissue Int 2013;92:418–28. 10.1007/s00223-013-9695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor EA, Mileti CJ, Ganesan S, Kim JH, Donnelly E. Measures of Bone Mineral Carbonate Content and Mineral Maturity/Crystallinity for FT-IR and Raman Spectroscopic Imaging Differentially Relate to Physical–Chemical Properties of Carbonate-Substituted Hydroxyapatite. Calcif Tissue Int 2021;109:77–91. 10.1007/s00223-021-00825-4. [DOI] [PubMed] [Google Scholar]
- 55.Brett AD, Brown JK. Quantitative computed tomography and opportunistic bone density screening by dual use of computed tomography scans. J Orthop Translat 2015;3:178–84. 10.1016/j.jot.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warden SJ, Liu Z, Fuchs RK, van Rietbergen B, Moe SM. Reference data and calculators for second-generation HR-pQCT measures of the radius and tibia at anatomically standardized regions in White adults. Osteoporos Int 2022;33:791–806. 10.1007/s00198-021-06164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Loughlin PF, Cunningham ME, Bukata SV, Tomin E, Poynton AR, Doty SB, et al. Parathyroid hormone (1-34) augments spinal fusion, fusion mass volume, and fusion mass quality in a rabbit spinal fusion model. Spine (Phila Pa 1976) 2009;34:121–30. 10.1097/BRS.0b013e318191e687. [DOI] [PubMed] [Google Scholar]
- 58.Formby PM, Kang DG, Helgeson MD, Wagner SC. Clinical and Radiographic Outcomes of Transforaminal Lumbar Interbody Fusion in Patients with Osteoporosis. Global Spine J 2016:6:660–4. 10.1055/s-0036-1578804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ehresman J, Ahmed AK, Lubelski D, Schilling A, Pennington Z, Cottrill E, et al. Vertebral Bone Quality Score and Postoperative Lumbar Lordosis Associated with Need for Reoperation After Lumbar Fusion. World Neurosurg 2020;140:e247–52. 10.1016/j.wneu.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 60.Uei H, Tokuhashi Y, Maseda M, Nakahashi M, Sawada H, Matsumoto K, et al. Exploratory analysis of predictors of revision surgery for proximal junctional kyphosis or additional postoperative vertebral fracture following adult spinal deformity surgery in elderly patients: a retrospective cohort study. J Orthop Surg Res 2018;13:252. 10.1186/s13018-018-0960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muretta JE, Prieto-Centurion D, LaDouceur R, Kirtley JD. Unique Chemistry and Structure of Pyrolyzed Bovine Bone for Enhanced Aqueous Metals Adsorption. Waste Biomass Valor 2022. 10.1007/s12649-022-01895-7. [DOI] [Google Scholar]
- 62.Boskey AL, Spevak L, Weinstein RS. Spectroscopic markers of bone quality in alendronate-treated postmenopausal women. Osteoporos Int 2009;20:793–800. 10.1007/s00198-008-0725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paschalis EP, DiCarlo E, Betts F, Sherman P, Mendelsohn R, Boskey AL. FTIR microspectroscopic analysis of human osteonal bone. Calcif Tissue Int 1996;59:480–7. 10.1007/BF00369214. [DOI] [PubMed] [Google Scholar]
- 64.Sornay-Rendu E, Cabrera-Bravo J-L, Boutroy S, Munoz F, Delmas PD. Severity of vertebral fractures is associated with alterations of cortical architecture in postmenopausal women. J Bone Miner Res 2009;24:737–43. 10.1359/jbmr.081223. [DOI] [PubMed] [Google Scholar]
- 65.Stein EM, Liu XS, Nickolas TL, Cohen A, McMahon DJ, Zhou B, et al. Microarchitectural abnormalities are more severe in postmenopausal women with vertebral compared to nonvertebral fractures. J Clin Endocrinol Metab 2012;97:E1918–1926. 10.1210/jc.2012-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Stein EM, Zhou B, Nishiyama KK, Yu YE, Shane E, et al. Deterioration of Trabecular Plate-Rod and Cortical Microarchitecture and Reduced Bone Stiffness at Distal Radius and Tibia in Postmenopausal Women with Vertebral Fractures. Bone 2016;88:39–46. 10.1016/j.bone.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salzmann SN, Okano I, Rentenberger C, Winter F, Miller CO, Schadler P, et al. Skin Ultrasound Measurement as a Potential Marker of Bone Quality: A Prospective Pilot Study of Patients undergoing Lumbar Spinal Fusion. J Orthop Res 2019;37:2508–15. 10.1002/jor.24438. [DOI] [PubMed] [Google Scholar]
- 68.Kazakia GJ, Kuo D, Schooler J, Siddiqui S, Shanbhag S, Bernstein G, et al. Bone and Cartilage Demonstrate Changes Localized to Bone Marrow Edema-like Lesions within Osteoarthritic Knees. Osteoarthritis Cartilage 2013;21:94–101. 10.1016/j.joca.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okano I, Salzmann SN, Ortiz Miller C, Rentenberger C, Schadler P, Sax OC, et al. Correlation between Urine N-Terminal Telopeptide and Fourier Transform Infrared Spectroscopy Parameters: A Preliminary Study. J Osteoporos 2020;2020:5725086. 10.1155/2020/5725086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


