Abstract
Objectives:
Complementary and alternative medicine (CAM) has become increasingly popular among cancer patients and is often used concomitantly with standard cancer therapies. Nonetheless, disclosure of CAM utilization by cancer patients to physicians, along with the provision of information on CAM therapies by physicians, is poor. This review explores the literature to synthesize existing information on communication about CAM usage, reasons for nondisclosure, and the clinical implications thereof.
Methods:
A search of medical literature published between December 1, 2009, and October 1, 2021 (last searched on April 18, 2022), on communications between physicians and cancer patients about CAM treatments was conducted through MEDLINE and EMBASE. Results were screened for inclusion, dually reviewed, and assessed using the QualSyst quality appraisal instrument. Findings were categorized and synthesized for review.
Results:
A total of 30 articles were located (n = 8721 total participants), which discussed elements related to patient disclosure of CAM use (n = 16), provider experiences or perceptions related to communication about CAM (n = 3), patterns of this communication (n = 6), and recommendations for effectively discussing CAM with cancer patients (n = 5). Reports indicate that nondisclosure is common throughout the cancer care spectrum. Factors influencing nondisclosure range from patient beliefs and attitudes about their provider, demographic characteristics, disease progression, physician–patient relationship, physician noninquiry, and type of CAM used; ultimately creating a gap in care that may have serious medical implications.
Discussion:
Many of the studies identified are small and confined to a single-center, hospital-network, or geographic setting, thereby limiting the applicability of findings and recommendations. Nonetheless, improving patient–physician communication is essential in delivering evidence-informed, patient-centered care and crucial for achieving patient satisfaction and positive health outcomes. The lack of adequate CAM dialogue about CAM use increases the risk of adverse interactions with conventional cancer treatments and results in missed opportunities for providers and patients to engage in vital information exchange. Future research and education are necessary to further identify barriers surrounding patient–provider communication about CAM treatments.
Keywords: cancer, alternative medicine, complementary medicine, communication, physician–patient relations
Introduction
Effective patient–doctor communication is the cornerstone of exceptional medical care and an important factor for achieving patient satisfaction and positive health outcomes.1 Good communication is vital in establishing trust between physicians and patients, enabling adequate gathering of important information, increasing compliance to medical treatment, as well as allowing for patients to be active participants in decision-making.2 These factors are especially important in cancer care.
The term complementary and alternative medicine (CAM) refers to medical products and practices that are not part of, and may be used with or without, standard medical care.3 Integrative medicine is a physician-coordinated approach to medical care that combines standard medicine with CAM practices that, by some definitions, emphasizes a holistic, patient-focused approach to health care and wellness.3
Many cancer patients believe CAM is beneficial for alleviating cancer symptoms and treatment side effects, increasing quality of life, and addressing other unmet needs.4,5 As such, CAM use is increasing worldwide,6,7 with cancer patients7 and survivors4,6 in the United States reporting greater use than the general population without cancer. An average of 51% of cancer patients engage in CAM use7; although despite this high prevalence, literature suggests that communication between patients and physicians regarding CAM remains poor. In fact, it is reported that up to 70% of cancer patients do not disclose their use of CAM therapies with oncologists.8 The impact of this nondisclosure on patient outcomes and the physician–patient relationship have been only minimally explored.
Most patients initiate CAM use based on recommendations from family and friends, the media or the internet.9–11 This is of concern as the information provided may be unreliable and inaccurate. Evidence in the literature suggests that cancer patients would like to receive information about CAM from their physicians.2,12 Might this situation provide doctors with an opportunity to build a stronger therapeutic relationship with patients through engaging in dialogue about CAM?
The relatively high rates of use and nondisclosure of CAM use among cancer patients highlight the importance of understanding the barriers to disclosure and the need for solutions to reduce these barriers. Few studies have explored and discussed the importance of enhancing the communication between physicians and patients on this topic. The aim of this review is to identify, summarize, and synthesize the existing literature on communication about cancer patient usage and disclosure of CAM use to physicians, barriers to communication, and the clinical implications associated with this communication, or lack thereof.
Methods
Search strategy
A comprehensive search was conducted by a medical librarian of published medical literature on communications between physicians and cancer patients about CAM treatments. The MEDLINE database was searched (via PubMed) using the following terms: (medical oncology[mh] OR “medical oncology”[tiab] OR oncology[tiab] OR neoplasms/therapy[mh]) AND (physician-patient relations[mh] OR physician patient[tiab]) AND (complementary therapies[mh] OR CAM[tiab] OR “complementary medicine therapy”[tiab] OR “complementary therapy”[tiab]) AND (communication[mh] OR communicate[tiab] OR communication[tiab]). To examine a sufficient body of literature while also gathering relatively current information, all results published between December 1, 2009, and October 1, 2021, were included for review. The search results were independently examined by at least two authors, and articles were initially screened for inclusion based on information supplied by the titles and abstracts.
The findings from all relevant, English-language articles were dually reviewed and selected based on further analysis of the full-text. Discrepancies, although infrequent, were resolved through discussion between two authors. Selected articles were then categorized into groups of common themes in this review.
The EMBASE database was also searched using the following terms: (“medical oncology”/exp OR “medical oncology” OR “oncology” OR “neoplams/therapy”) AND (“doctor patient relationship”/exp OR “doctor patient relationship” OR “physician patient relationship”/exp OR “physician patient relationship”) AND (“complementary therapies”/exp OR “complementary therapies” OR “cam”/exp OR “cam” OR “alternative medicine”/exp OR “alternative medicine” OR “alternative medicine therapy” OR “complementary medicine therapy” OR “complementary therapy” OR “alternative therapy”/exp OR “alternative therapy”) AND (“communication”/exp OR “communication” OR “communicate” OR “communicating” OR “interpersonal communication”/exp OR “interpersonal communication”); Filters: published between December 1, 2009 to October 1, 2021.
Duplicate results from the original MEDLINE search were eliminated, while the remaining articles were independently assessed by two authors for inclusion based on information supplied in the titles and abstracts. Findings from pertinent, English-language articles were dually reviewed and selected based on further analysis of the full-text. Selected articles were grouped into themes previously defined during the initial MEDLINE search (no novel themes were identified among the selected EMBASE literature). Data collected by both MEDLINE and EMBASE were then synthesized for discussion in this review. This review was not registered.
Eligibility criteria
To be included in this review, all studies must have fulfilled each of the following content criteria:
-
(1)
Investigates a cancer patient or cancer provider population involving the use of CAM, subscribing to the definition of CAM provided above.3
-
(2)
Investigates the discussion of CAM use (and not, e.g., the sole presence of CAM use or lack thereof) between patients, or their parent/guardian, and providers.
-
(3)
Investigates the discussion of CAM use between cancer patients and conventional health care providers, rather than integrative physicians or other CAM-specific practitioners.
Quality assessment criteria
The QualSyst13 assessment tool was used to appraise the internal validity of included studies, or the extent to which their design and conduct minimize errors and biases.13 This validated instrument uses a 14-item checklist with a quantitative scoring approach to assess the quality of a broad range of study designs and research topics, including qualitative studies. Each of the 14 items were scored depending on the degree to which the specific criteria were met (“yes” = 2, “partial” = 1, “no” = 0). Items not applicable to a particular study design were marked “N/A” and therefore excluded from the calculation of the summary score. The total assessment score ranges from 0 to 1, with a higher score indicating better quality. A score of 0.75 represents a relatively conservative threshold to indicate a good quality study.13
Individual study assessments were performed independently and later reviewed by two authors. These assessments are made available upon request to the authors.
Results
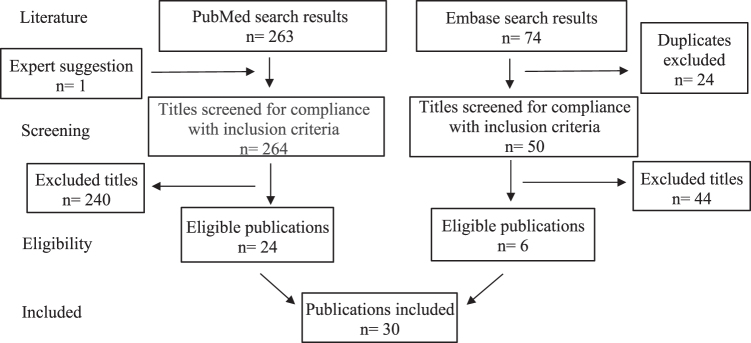
A total of 337 publications were generated in the initial MEDLINE (n = 263) and ensuing EMBASE (n = 74) search process. All EMBASE articles identical to those found in MEDLINE were resolved before screening (n = 24). One other MEDLINE article, not identified in this search, was recommended by an expert on the topic (n = 1). While 314 titles (264 and 50 articles from MEDLINE and EMBASE, respectively) were screened for compliance with the inclusion criteria, only 30 articles (n = 8721 total participants; 8017 cancer patients and 704 cancer providers [588 of whom were specifically characterized as physicians]) were deemed eligible for discussion in this review (Fig. 1). All 30 articles were included and classified into the 4 main subcategories discussed below based on the outcome measures of each study.
FIG. 1.
Study selection summary.
The overall quality of the articles selected for this review was high, with a mean assessment score of 0.89 (range: 0.63–1.0). Two studies2,14 scored below the “good” quality threshold due to inadequacies in methodology reporting. Study characteristics are summarized in Table 1.
Table 1.
Eligible Studies with Information on the Discussion of Complementary and Alternative Medicine Use Between Cancer Patients and Providers
| Study | Date published | Objective | Design | Setting | Participants | Relevant results | QualSyst score |
|---|---|---|---|---|---|---|---|
| Arslan and Guler17 | 2017 | (1) To investigate CAM usage and knowledge levels of chemotherapy-receiving cancer patients. | Questionnaire | Tepecik Research and Training Hospital in Izmir, Turkey | Cancer patients (n = 289) | Sixty-two of 289 patients (22%) received CAM. Nearly half of these patients did not inform their clinicians about their use of CAM. | 0.89 |
| Black et al18 | 2016 | (1) To understand the prevalence and patterns of CIH use among Hispanics. | In-person and/or telephone-based interview | Cases were identified from the California Cancer Registry and/or from the LAC+USC County Hospital and USC Norris Comprehensive Cancer Center in Los Angeles, CA | Colorectal cancer patients (n = 631) | Among 631 Hispanic patients, 40.1% reported ever using CIH. About 60% of participants reported CIH use to address specific health conditions; however, most patients did not discuss CIH use with their physicians (76.3%). | 0.86 |
| Butler et al19 | 2011 | (1) To examine the prevalence and predictors of CAM use among rural patients with localized prostate cancer and (2) the disclosure of CAM use to their physicians. | Three in-person semistructured interview surveys (conducted within 1, 6, and 9 months of diagnosis) | Undescribed center(s) in rural, southwest Georgia | Prostate cancer patients (n = 321) | At baseline, 26.4% reported ever using CAM. Fifty-six percent of these patients did not disclose their CAM use to their physicians. While 44% of the study sample disclosed using CAM to their doctors before treatment, 61% disclosed after treatment began (p = 0.05). | 0.86 |
| Choi et al20 | 2012 | (1) To investigate in depth the use of CAMs by cancer patients at the EOL and (2) how they communicate with physicians about them. | Questionnaire | Seventeen hospitals in Korea | Cancer patients (n = 1662) | The prevalence of CAM use among cancer patients at the EOL was 37.0%. 93.1% of CAM-users were using pharmacologic agents. Only 42.5% discussed CAM use with their physicians. | 1.0 |
| Chow et al24 | 2010 | (1) To evaluate determinants, expectations, association with QOL, and doctor's awareness of CAM use in Singapore cancer patients. | Cross-sectional survey | Cancer Centre of the National University Hospital in Singapore | Cancer patients (n = 316) | Fifty-one percent of CAM users informed their doctors about their use and 15% of doctors reported to be aware of CAM use in these patients. Twenty-five percent of patients reported concurrent use of oral CAM and chemotherapy, of which oncologists were unaware in 86% of cases. | 0.89 |
| Corina et al31 | 2016 | (1) To learn about the values, norms and defining features that characterize oncologist–patient discussions on CAM. | Semistandardized telephone interviews | Inpatient and outpatient settings in Germany | Oncologists (n = 17) | Discussions on CAM tend to reflect the idea that CAM belongs “to another world.” Many interviewees mentioned an apparent lack of scientific proof, especially when their aim was to warn patients against the use of CAM. Advice on CAM is seen by oncologists as an important service they provide to their patients, even though their knowledge of the subject is often limited. | 0.78 |
| Davis et al6 | 2012 | (1) To explore the nondisclosure of CAM use among cancer patients, including reasons for and outcomes from nondisclosure of CAM use, within the context of patient–doctor communication. | Systematic review | Various | Twenty-one articles | Studies reported a prevalence of CAM use among patients with cancer ranging between 11% and 95%. Patient–doctor communication about the use of CAM was associated with an enhanced patient–doctor relationship and higher patient satisfaction. | 1.0 |
| Frenkel and Cohen2 | 2014 | (1) To realize the components of effective communication about the use of CIM in cancer care. | Literature review | Various | Not reported | The communication process requires a very sensitive approach that depends on effective communication skills, experience in listening, encouraging hope, and the ability to convey empathy and compassion. This process is coupled with the use of reliable information sources that can be shared with the patient and his or her family in making decisions about this use. | 0.63 |
| Frenkel et al14 | 2010 | (1) To present an overview of the literature regarding communication in cancer care related to CAM use and (2) discuss a possible model of effective patient–physician communication about CAM use in cancer care. | Literature review | Various | Not reported | A communication approach that fosters a collaborative relationship that includes adequate information exchange, responds to emotional needs, and manages uncertainty can lead to informed decisions about CAM use. | 0.63 |
| Ge et al25 | 2013 | (1) To quantify the extent of patient–physician communication about CAM and (2) identify factors associated with its discussion in RT settings. | Cross-sectional survey | Department of Radiation Oncology at the Hospital of the University of Pennsylvania in Philadelphia, PA | RT patients (n = 305) | In multivariate analyses, female patients (AOR 0.45, 95% CI 0.21–0.98) were less likely to discuss CAM with their radiation oncologists. CAM users were more likely to discuss CAM with their radiation oncologists than were non-CAM users (AOR 4.28, 95% CI 1.93–9.53). | 0.91 |
| Hunter et al15 | 2014 | (1) To investigate the type and prevalence of CAM among an Australian regional radiotherapy patient cohort and the disclosure of information to the consultant radiation oncologist. | Questionnaire | Peter MacCallum Cancer Centre, University of Melbourne in Melbourne, Victoria, Australia | Cancer patients (n = 152) | Sixty-nine of the 152 patients (45.4%) reported active CAM use. | 1.0 |
| Juraskova et al33 | 2010 | (1) To describe communication patterns between oncologists and breast cancer patients regarding CAM use and (2) assess the relationship between CAM discussions and anxiety levels. | Interaction analysis of audiotaped initial consultations | Seven public hospitals in Australia and New Zealand | Breast cancer patients (n = 102); Oncologists (n = 24) | At least 1 instance of CAM discussion in 24 of the 102 consultations (24%). CAM discussions were mainly patient initiated (73%). | 1.0 |
| King et al30 | 2015 | (1) To understand why communication about CAM is not occurring between patients and HCPs and (2) how CAM communication could be improved during HCP–patient interactions. | Questionnaire | Two outpatient clinics in Calgary, Alberta | Cancer outpatients (n = 481); HCPs (n = 100) | HCPs reported limited training about CTs but most (90%) expressed interested in receiving more training. The majority of HCPs (>80%) reported limited knowledge about the role of CTs in cancer care or evidence to support CT use. Questions about communication and interactions revealed that 80% of patients reported not having had an HCP speak to them about CTs. However, 63% of HCPs reported addressing CT use. | 0.91 |
| Koenig et al35 | 2015 | (1) To characterize how providers respond to patient mentions of CIM during routine oncology visits. | Longitudinal ethnographic study | Four oncology clinics in the Western United States | Advanced cancer patients (n = 82) | CIM was mentioned in 78/229 (34%) of the total observed visits. Patients initiated talk about CIM (76%) more than providers (24%). Providers' responses inhibited further talk in 44% of observations and promoted talk in 56% of observations. | 0.89 |
| Lee et al32 | 2014 | (1) To explore oncologists' knowledge, attitudes, and practice patterns regarding HS use by their patients. | Questionnaire (e-mail and snail mail) | United States | Oncologists (n = 392) | Fifty-nine percent of respondents had not received any education on the topic of CAM. | 1.0 |
| Luo and Asher37 | 2017 | (1) To define CAM use by cancer patients and (2) investigate factors that might influence changes in CAM use in relation to cancer diagnoses. | Questionnaire (snail mail) | NCCH | Cancer patients (n = 603) | Initiation of CAM use after cancer diagnosis was positively associated with a patient having a conversation about CAM use with his or her oncology provider. Patients practicing mind–body medicine before cancer diagnosis who engaged in a CAM-related conversation with their oncologist were less likely to cease practice. | 0.89 |
| Lüthi et al29 | 2021 | (1) To explore CAM use by pediatric oncology patients in relation to specific time intervals and (2) communication about CAM use between parents and oncologists. | Retrospective cross-sectional study | Pediatric hematology-oncology center at Lausanne University Hospital in Switzerland | Parents of cancer patients (n = 140) | CAM was used by 54.3% of patients before diagnosis and 69.3% of patients after diagnosis. Forty percent of respondents did not discuss CAM with their oncologist. | 0.86 |
| Oh et al23 | 2010 | (1) To examine patient–doctor communication about the use of CAM by adult patients with cancer and (2) compare patients' satisfaction with the consultation between patients who had and those who had not discussed the use of CAM with their doctors. | Questionnaire | Three major university teaching hospitals in Sydney, Australia | Cancer patients (n = 381) | Sixty-five percent of cancer patients used at least one form of CAM. Use of CAM was not discussed with the oncologist by 55% of respondents using biologically based CAM and by 80% of those using non–biologically based CAM since the diagnosis of cancer. | 0.83 |
| Rausch et al16 | 2011 | (1) To evaluate the frequency of CAM use among radiation oncology patients, the coping strategies that influenced this use, and the rates of disclosure of CAM use to their health care providers. | Questionnaire | Two rural radiation oncology clinics in south central Minnesota | RT patients (n = 153) | CAM use was reported in 95% of the participants. One hundred and twelve participants reported taking vitamins, minerals, or supplements, and 47% of those 112 did not disclose this use to their providers. | 0.89 |
| Rogge et al8 | 2021 | (1) To evaluated whether using blended learning (e-learning plus a workshop) to train oncology physicians in providing advice on CIM therapies to their patients with cancer, in addition to distributing an information leaflet on reputable CIM websites, had different effects on patient-reported outcomes for the consultation than only distributing the leaflet. | Multicenter, cluster-randomized trial | Private practices and hospital departments in Germany | Cancer patients (n = 291; 128 in the intervention group and 169 in the control group); Oncologists (n = 41) | Patients in the intervention group rated physician–patient communication higher on all EORTC QLQ-COMU26 scales (mean total score, 84.3 [95% CI, 79.5–89.2] vs. 73.6 [95% CI, 69.3–78.0]; p = 0.002), were more satisfied with the advice (mean, 4.2 [95% CI, 4.0–4.4] vs. 3.7 [95% CI, 3.5–3.8]; p < 0.001), and were readier to make a decision (mean, 63.5 [95% CI, 57.4–69.6] vs. 53.2 [95% CI, 47.8–58.7]; p = 0.016) than the control group. | 0.92 |
| Roter et al34 | 2016 | (1) To describe CAM discussions in oncology visits, (2) the communication patterns that facilitate these discussions, and (3) their association with visit satisfaction. | Prospective, observational study and questionnaire | Academic medical oncology practices in the Upper Midwest of the United States | Cancer patients (n = 327); oncology physicians and NPs (n = 37) | CAM was discussed in 36 of 327 visits; discussions were brief (<1 min), the majority patient initiated (65%) and more common for patients in early stages of cancer care. | 0.95 |
| Salamonsen36 | 2013 | (1) To explore possible connections between cancer patients' communication experiences with doctors and the decision to use CAM as either supplement or alternative to CT. | Face-to-face interviews | The NAFKAM in Tromsø, Norway | Cancer patients (n = 13); Doctors (n = 46) | The analysis revealed three connections between doctor–patient communication and patients' treatment decisions: (1) negative communication experiences because of the use of CAM; (2) negative communication experiences resulted in the decision to use CAM, and in some cases to decline CT; and (3) positive communication experiences led to the decision to use CAM as supplement, not alternative to CT. | 0.80 |
| Schofield et al39 | 2010 | (1) To develop evidence-based guidelines to assist oncology HP to have respectful, balanced and useful discussions with patients about CAM. | Systematic review | Various | Thirty-six articles | Evidence-based guidelines are presented as a sequence of recommended steps: (1)eElicit the person's understanding of the situation; (2) respect cultural and linguistic diversity and different epistemologic frameworks; (3) ask questions about CAM use at critical points in the illness trajectory; (4) explore details and actively listen; (5) respond to the person's emotional state; (6) discuss relevant concerns while respecting the person's beliefs; (7) provide balanced, evidence-based advice; (8) summarize discussions; (9) document the discussion; (10) monitor and follow-up. | 1.0 |
| Sewitch et al28 | 2011 | (1) To gather data about CAM use by site of cancer, (2) reasons for use and rates of use disclosure to treating physicians, and (3) to better understand CAM use and patient perspectives of CAM discussions that occur or not between cancer patients and their physicians. | Questionnaire; focus group discussion | St. Mary's Hospital Center in Montreal, Canada | Cancer patients (n = 100) | In the past 1 and 12 months, natural health products were used by 70% and 80% of respondents, respectively; mind–body therapies by 61% and 64%, respectively, and CAM practitioners by 11% and 29%, respectively. | 0.73 |
| Sohl et al26 | 2015 | (1) To examine CHA disclosure to follow-up care physicians in a diagnostically diverse sample of cancer survivors by describing rates, reasons, and predictors of CHA disclosure with a focus on patient-centered communication and responses from physicians. | Cross-sectional survey | CPIC SEER registry | Cancer survivors (n = 623); CHA-users (n = 196) | Disclosure was significantly associated with patient-centered communication even when adjusting for hypothesized covariates (OR = 1.37; 95% CI, 1.09–1.71]). Perceived physician knowledge of the patient-as-person (OR = 1.28; CI, 1.10–1.48) and information exchange (OR = 1.27; CI, 1.02–1.60) were the aspects of patient-centered communication that contributed to this association. | 1.0 |
| Stub et al38 | 2016 | (1) To examine the qualitative research literature on the perception of and communication about the risk of complementary therapies between different health care providers and cancer patients. | Literature review | Various | Twenty-seven articles | The main risk situations identified were (1) differences in treatment concepts and philosophical values among complementary and conventional health care providers, (2) adverse effects from complementary products and herbs due to their contamination/toxicity and interactions with conventional cancer treatment, (3) health care physicians and oncologists find it difficult to recommend many complementary modalities due to the lack of scientific evidence, and (4) lack of knowledge and information about complementary and conventional cancer treatments among different HCPs. | 1.0 |
| Sullivan et al21 | 2015 | (1) To investigate trends and regional variations in CAM use by cancer patients in regional and remote populations. | Questionnaire | Regional cancer care center in Toowoomba, South East Queensland, Australia | Cancer patients (n = 142) | Sixty-eight percent of patients were currently or had previously used at least one form of CAM. Disclosure of CAM use to either the general practitioner or specialist was reported by 46% and 33% of patients, respectively. | 0.86 |
| Ustundag and Demir Zencirci10 | 2015 | (1) To determine the use and effects of CAM on cancer patients receiving chemotherapy. | Cross-sectional survey | Daytime Chemotherapy Unit of the College District Outpatients in the Ankara Numune Education and Research Hospital in Ankara, Turkey | Cancer outpatients (n = 397) | Most of patients resorting to CAM were women (52.6%), housewives (51.5%), and patients with a family history of cancer (37.7%). | 0.94 |
| Witt et al40 | 2020 | (1) To develop and pretest a CIM-specific physician-consultation framework for potential implementation. | Implementation study | Germany | Oncologists (n = 47) | The manual-guided consultation was considered suitable. The structure and time frame (20 min or less) of the consultation as well as the training were feasible and well accepted. | 0.95 |
| Wortmann et al22 | 2016 | (1) To evaluate the prevalence and predictors for the use of CAM by cancer patients under active treatment with chemo- or radiotherapy or in aftercare. | Questionnaire | Department of Radiotherapy–Radiooncology of the University of Munster/Westfalia, the ambulance for oncology of the St. Franziskus Hospital at Munster, and offices of 13 GPs from a network in Munster, Germany | Cancer patients (n = 506) | Fifty-one percent admitted using CAM. Thirty-five percent informed the oncologist about using CAM, 56% informed the GP, and 26% did not inform any physician. | 0.80 |
AOR, adjusted odds ratio; CAM, complementary and alternative medicine; CHA, complementary health approach; CI, confidence interval; CIH, complementary and integrative health; CIM, complementary and integrative medicine; CPIC, Cancer Prevention Institute of California; CT, conventional treatment; EORTC QLQ-COMU26, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire for the assessment of communication between patients and professionals; EOL, end-of-life; GP, General Practitioner; HCP, health care provider; HP, health professionals; HS, herbal supplement; NAFKAM, National Research Center in Complementary and Alternative Medicine; NCCH, North Carolina Cancer Hospital; NPs, Nurse Practitioners; OR, odds ratio; QOL, quality of life; RT, radiation therapy; SEER, Surveillance Epidemiology and End Results.
Disclosure of CAM use and implications thereof (n = 16)
Nondisclosure of CAM use is common throughout the cancer care spectrum. Several surveys10,15–23 have assessed the frequency at which patients of varying cancer populations revealed their use of CAM to a doctor, with results primarily aggregated between 40% and 50%.6
Discrepancies have been found between patients' and providers' recollection of disclosure. A study24 among Singapore cancer patients (n = 316) and physicians (n = 18) revealed that while 51% of CAM users verified that they communicated CAM use to their physician, only 15% of their doctors reported being aware of such use in these patients. Among those being treated with chemotherapy (n = 200), 25% of patients were also using some form of dietary supplement. The oncologists of 86% of these patients were unaware of this CAM use. Similarly, within the group of patients under radiotherapy (n = 219), 12% were using a dietary supplement; however, 77% of these oncologists were unaware of concurrent use. Alarmingly, up to 27% of patients taking dietary supplements during oncotherapy are estimated to use potentially hazardous combinations.
Three studies found an association between CAM-use disclosure and patient or physician characteristics. One study22 found that highly educated females, patients under active therapy, and those whose cancer had metastasized were more likely to disclose CAM use, while the other studies found that male25,26 and minority race/ethnicity patients26 were more likely to disclose use. The latter two studies25,26 also found that patients were more likely to disclose use to primary care or oncology/hematology physicians compared with other specialties. Another study did not find patient demographics to be significantly related to disclosure.23
Physician–patient relationship was also revealed as a significant factor related to CAM disclosure. Disclosure of CAM use was found to increase the overall “patient centeredness” of patient–provider communication. Patients were more likely to discuss CAM use when they believed that their doctor viewed them “as a person,” was respectful, open-minded, and engaged in information exchange.26
Similar information was revealed in a qualitative study (not included within the time frame of this search) comparing cancer patients' experiences between consultations with CAM practitioners and conventional physicians.27 Patients preferred communication styles that prioritize recognition of the “whole person,” rather than sole interest in their “disease or tumor.” Furthermore, patient-centered communication styles were found to influence patients to initiate CAM-related questions that were not raised by their doctor.
Two studies reported a notable difference in disclosure depending on the type of CAM used. Both studies found that patients using natural health products/biological CAMs (i.e., herbs, dietary supplements, vitamins) had lower rates of nondisclosure than those using nonbiological CAMs (i.e., chiropractic, yoga, mind–body therapy).23,28
Six studies investigated the reasons for patient nondisclosure of CAM use. Common reasons for nondisclosure of CAM use included physician noninquiry,19,23,29 perceptions that the physician will not be receptive to CAM use,23,29 presumed physician lack of knowledge about CAM,23 usage perceived as not important to tell their physician,23,25,26,29 or the ideology that CAM does not interfere with conventional treatments.23 Despite this, when the topic was discussed, both patients and doctors believed it enhanced their relationship and this was associated with higher levels of doctor satisfaction.6,23
Health care providers' experiences or perceptions related to communication about CAM (n = 3)
Various perspectives surrounding communication about CAM have been uncovered through assessments of patient–provider encounters. Among providers surveyed in King et al (n = 100),30 70% indicated that at least one patient had discussed CAM use during an encounter within the past month, and roughly 63% indicated that this interaction was prompted by specifically asking patients about their use of CAM (Interestingly, 80% of patients participating in this same survey [n = 418] denied having spoken to a health care provider [HCP] about CAM therapies.). HCPs reported that approximately one in every five of their cancer patients was disclosing use of a CAM therapy. Nearly half (45%) of these providers were providing recommendations of CAM use with their patients.
Nevertheless, <20% of surveyed providers felt knowledgeable of the risks and benefits of CAM, where to find evidence-based information on this topic, or its role in cancer care. Nearly all providers though (90%) reported being “interested” or “very interested” in receiving education or training on the use of CAM in cancer care.30
Corina et al31 conducted structured audiotaped interviews with 17 oncologists and documented the physicians' ideology of CAM based on their experiences, discussions, and interactions with patients. Three major patterns of the patient–physician discussion were found. Some physicians believed CAM to be “other-worldly”; meaning, for example, that complementary medicine has “no place in standard therapies.” Many of these interviewees communicated that when “cancer patients feel they are well cared for, they tend to show less interest in CAM.” Others discredited the role of CAM in conventional medicine due to a perceived lack of scientific “knowledge and evidence,” and therefore feel obligated to warn interested patients against its use.
Lastly, some oncologists described discussions of CAM as a service provided to patients to foster a degree of openness that can be utilized to glean information about CAM usage, patient symptom/side effect burden, and quality-of-life alterations, as well as to protect patients from health and financial risks associated with CAM usage.31
In a national survey32 investigating U.S. oncologists' perceptions and knowledge regarding herbal supplement (HS) use, 93% of oncologists indicated concern about potential herb/drug interactions in their patients; however, only 64% of these oncologists believed that they were knowledgeable enough to answer their patients' questions about HS. Furthermore, less than half of responders reported having received education on the topic of HS. When asked about specific HS-chemotherapy interactions, only 14% of U.S. oncologists who participated in this survey were able to correctly answer four questions regarding which herbs/supplements their patients should avoid during cancer treatment.
Patterns of communication related to CAM and its use (n = 6)
Studies investigating CAM-related communication patterns between physicians and patients during oncology visits noted that most of these conversations were infrequent and patient initiated. Juraskova et al33 reported that only about one-quarter (24/102) of the consultations observed at a public hospital included discussions of CAM. CAM was discussed only once in 12 consultations and twice in 10 consultations; 2 consultations had 3 or more mentions of CAM, yielding a total of 40 occasions of CAM discussion within the 24 consultations. While 72.5% of these CAM discussions were initiated by the patient, ∼25% were clinician initiated. Similarly, Roter et al34 noted CAM discussions in 11% (36/327) of recorded patient visits in an academic medical oncology practice. CAM was discussed more than once in two-thirds of these visits, resulting in a total of 65 conversations with an average of 1.8 distinctive CAM discussions per visit.
While 61% of CAM discussions (n = 22) were patient initiated, 33% (n = 12) were clinician initiated, and two discussions were initiated by a companion. Within these studies, it was frequently observed that when patients mentioned CAM it was either ignored or strongly discouraged by the physician.
Koenig et al35 observed routine oncology encounters between patients and providers and characterized how providers responded to patient-initiated mentions of complementary and integrative medicine (CIM). CIM was mentioned in 78/229 (34%) of the total observed encounters. Of the interactions about CIM, researchers found that the provider's responses inhibited further talk in 44% of observations and promoted talk in 56% of observations.
A small (n = 13) Nordic study36 identified two main categories of communication behaviors by physicians, traditional or supportive, which were shown to influence a patient's decision to use or not use CAM. Traditional communication behavior is described as more rigid and technical with a focus on medical terminology and is often accompanied by a negative response to CAM use. Several patients who encountered physicians with this style of communication felt as though their communication expectations had not been fulfilled, thus reporting a negative communication experience with their physician. Many of these patients asked for a change of doctor and a few others (n = 5) elected to forgo conventional therapies all together for alternative treatments.
Supportive communication behaviors often involved open dialogue about the use of CAM and its associated risk. These physicians did not endorse CAM use but respected the patient's autonomy. Patients who encountered physicians with this communication behavior opted for more complementary treatments than alternative ones and adhered to recommended conventional treatment regimens. These patients also expressed satisfaction with their communication with their physician.
Luo and Asher37 investigated factors that might influence initiation or cessation of CAM use among cancer patients. Among never-users of CAM, conversations with oncology providers about CAM therapies after cancer diagnosis were positively correlated with initiation of use. Similarly, newly diagnosed cancer patients who had conversations about CAM with their oncologist were less likely to cease mind–body medicine therapies.
Stub et al38 conducted a systematic review that examined the literature on the perception of risk and communication about the risk of CAM therapies between different health care providers and cancer patients. This study identified central themes related to risk perception, direct risk association, indirect risk association, risk communication, and information regarding complementary therapies and conventional medicine. The researchers revealed that lack of communication poses a serious risk to patients who aim to integrate CAM and conventional therapies into their care. Studies within this review reported that negative experiences from doctor–patient interactions and negative outcomes, or the adverse effects of conventional treatment, also influence the cancer patient's decision to use CAM or to potentially decline or delay conventional standard treatment, which poses an additional risk. Consuming biological CAMs containing various chemicals with potentially direct toxicity, or that might interfere with established cancer therapies, may also be a threat to patient safety.
Finally, the lack of scientific evidence of the effects of many CAM therapies and differences in treatment ideologies between doctors and patients further hinder effective communication.
Recommendations for effectively discussing CAM (n = 5)
Schofield et al39 and Frenkel et al2,14 have provided recommendations for how health professionals could communicate more effectively with their patients regarding CAM.
Frenkel and Cohen2 suggest it is best to approach effective communication by dividing this process into two parts: the “how” and the “what.” The “how” addresses what clinicians can do to facilitate safe integration of CAM into patient care and includes awareness of affective components that may influence effective communication such as clinician attitude, patient emotions about uncertainty and unmet needs, as well as the process of gathering information and learning the skill of effective communication. The “what” encompasses the process of information exchange and informed decision-making. It details bilateral information exchange between the patient and physician, thus fostering a collaborative approach to care to address patients' information needs.
Another article published by Frenkel et al14 identified six core functions needed for effective communication: fostering a healing relationship, exchanging information, responding to emotions, managing uncertainty, making decisions, and enabling self-management. These guidelines emphasize the importance of developing trust and rapport, information gathering and sharing, responding to and managing patients' emotions, and making decisions based on a mutual understanding between the patient and physician.
Schofield et al's recommendations expand upon those previously mentioned by Frenkel et al14 and are comprehensively centered around 10 essential items.39 These recommendations assert that physicians need to elicit an understanding of the patient's situation, respect their values and beliefs, ask questions related to previous and/or current CAM use, explore details and actively listen, respond to their emotions, discuss the risks and benefits of both conventional cancer treatment and CAM, provide evidence-based advice, summarize treatment decisions, document the discussion, and finally, monitor and follow-up with the patient. Ultimately, these guidelines encourage and emphasize the importance of informed and collaborative decision-making related to CAM use.39
While these recommendations are helpful in theory, physician-led consultations based on these guidelines are often difficult to implement into clinical practice for reasons such as insufficient visit time or poor CAM knowledge.40 Witt et al40 have not only developed but also evaluated a framework to resolve these complications, among others, for oncology physicians. The KKOKON-KTO (a German acronym for Competence Network for Complementary Medicine-Consultation Training for Oncology Physicians) framework40 was designed to equip physicians with knowledge of the safety and effectiveness of CAM, as well as the tools to communicate this advice in an empathetic, nonjudgmental, and timely manner. Development of this framework first involved the construction of a consultation manual, which was systematically developed through literature review and analysis by a panel of experts on the topics of integrative oncology, psychology, psycho-oncology, medical ethics, epidemiology, communication, and public health, and later refined through pilot testing and evaluation by participating physicians.
An associated training procedure was then developed, involving both an “e-learning” component and a “workshop” component, where physicians applied their newfound knowledge and skills in consultations with standardized patients. Feedback was acquired and training was refined accordingly.8,40
Following this implementation study, Rogge et al8 conducted a multicenter, cluster-randomized trial involving KKOKON-KTO-trained oncologists (n = 41) and their patients (n = 291) who expressed interest in receiving CAM-related information. Cancer patients were blinded to group assignments and randomized to receive either a CAM consultation and leaflet of reputable CAM websites (n = 128) or an information leaflet only (n = 169) from their oncologist. Patients in the intervention group rated physician–patient communication higher on all the assessment scales, were more satisfied with the advice, and felt better prepared to make CAM-related decisions than those in the control group (p ≤ 0.016).8
Discussion and Conclusion
Discussion
This review provides a brief examination of literature on the prevalence of CAM-use disclosure, or the lack thereof, among cancer patients, with an emphasis on communication barriers and patterns between patients and physicians. While this information is limited to articles supplied in the English language, these findings—revealed by a variety of countries—substantiate worldwide concerns over the lack of communication regarding CAM use between providers and their patients.38 Among the limited body of research dedicated to this topic, several of these studies are small and confined to a single-center, hospital-network, or geographic setting—all of which limit patient and physician-selection demographics. Guidelines for the scope of this review, and its associated abstract, can be found within Supplementary Data S1 and S2, respectively.
Future research on the communication of CAM use should involve a more representative sample of patients, physicians, and treatment settings, to better extrapolate these findings and henceforth develop or evaluate mitigation strategies accordingly. Nonetheless, given the well-documented large variance in rates of patient nondisclosure within the reviewed studies,6 an understanding of the barriers to communication, the development of effective interventions to improve communication, and their implementation is necessary.
Reported factors that influenced nondisclosure ranged from patient and physician demographic characteristics,22 disease progression,22 physician–patient relationship,26 and type of CAM used.23,28 The reviewed literature also finds that patients' beliefs and attitudes about their provider to be the most pervasive influence on communication,36,38 thereby elucidating the impression that perhaps these perceptions pose the greatest barrier to an effective patient–physician communication.
The problem of patient nondisclosure is contributed to by the fact that providers are often reluctant to initiate the discussion of CAM use. This reluctance may be due, in part, to clinicians not having relevant knowledge about CAMs, lack of a complete overview of the CAMs available and in use, or an underestimation of the prevalence of usage.41 A 2004 U.S. study42 found that few physicians (39%) felt knowledgeable enough to discuss CAM with patients and 81% thought they needed to learn more to adequately address patient concerns. Unfortunately, more current information of this sort is not available; thus, this topic warrants greater research attention.
Physicians should be aware of CAM use in their patients because some of these therapies may be harmful with continuous use or may have potential adverse interactions with conventional cancer treatments.24,32,38 It is also important for doctors to be interested in talking about CAM to help identify and address significant unmet information needs, and other needs, of cancer patients.30,43
Communication style was also identified as a factor affecting the quality of patient–provider CAM communication. The aforementioned studies36,38 reveal that patients often report negative communication experiences with their physicians. Unfortunately, consequences of some of these experiences may lead to a decision to delay or even decline important conventional treatment.38 Discussion of CAM use was encouraged by patient impressions of acceptance and nonjudgment from providers, while communication was inhibited by dispiriting responses from previous discussions with providers about CAM.36
Lack of communication about CAM use could potentially be addressed by medical providers through communication with patients about CAM in a direct, supportive, nonjudgmental manner to build trust and rapport. Research indicates that positive communication styles drive “patient-centered” care approaches to clinical medicine.26,27 This type of approach involves a combination of patient sharing in decision-making, provider empathy, and recognition of a patient's values, thus encouraging and promoting further conversation and subsequent discussion of CAM use.35 This style of communication may also ease patient anxiety, increase patient satisfaction, and expand treatment compliance. Further research is warranted to determine whether these hypothetical benefits can be realized.
At least two studies (excluded for review as per eligibility criteria) have explored approaches to educating providers about how to discuss CAM with cancer patients. Researchers from Germany44 developed a consultation training program to aid physician communication related to CAM usage in breast cancer patients. Eight breast cancer centers, two physicians per center, were randomized to either a complementary communication training program or to a control group without training. Each physician consulted 10 patients who were not in his or her care. The results from this randomized-controlled study found that the training program was beneficial for physicians with less consultation experience related to CAM usage. Patients reported very positive interactions and appreciated talking about CAM in both groups.
A similar study,45 conducted in community clinics within the United States, examined the efficacy of a brief educational intervention to increase communication about CAM between oncology nurses and patients. Nurses were divided into an intervention and control group. The intervention included a 20-min video emphasizing the importance of discussing CAM, resources for obtaining CAM information, and a laminated card reminding nurses to ask about CAM use. Results indicated that nurses in the intervention group were significantly more likely to report communication about CAM than those in the control group. Nurses in the intervention group also reported being more comfortable with having CAM-focused communication with patients.
Analyses from the authors of these reports align with suggestions from Frenkel et al14 to emphasize the potential benefits of educational interventions targeted toward physicians. These benefits include an increased ability to deliver high-quality care, allowing informed decision-making, enhancing the patient–physician relationship, enhancing patient satisfaction, and reducing patient exposure to misleading and harmful information.
Practice implications
Consideration should be given to developing educational interventions that target the patients and their caregiver/families to increase their knowledge base relating to CAM while also helping the patient and/or caregiver/families to develop their own skills of communication with their health care team. This may increase and normalize conversations about CAM and cancer while also encouraging open dialogue between physicians and patients. Furthermore, developing and encouraging standardized CAM-specific question prompts and/or guidelines for doctors may motivate comfort in discussions surrounding CAM use, and thus improve both patient and physician satisfaction with these encounters.
Lastly, improving the CAM information capture and assessment components of electronic health record systems may aid physicians in integrating CAM into patient care, flagging potential interactions, and identifying appropriate CAM interventions and practitioners for referral. This then would allow for more evidence-based care, provide a more accurate measurement of outcomes, and facilitate large-scale, real-world assessments of which complementary medical practices are most effective or harmful when coupled with standard treatments. With increased documentation of the risk and benefits, physicians may be more comfortable discussing and/or recommending specific evidence-based, or at least evidence-informed, CAM interventions to their cancer patients.
Conclusion
Despite increased and widespread use of CAM among cancer patients, many obstacles persist to doctors and patients comfortably engaging in dialogue related to its use. The relative lack of adequate CAM dialogue increases the risk of adverse interactions with conventional cancer treatments and results in missed opportunities for providers and patients to engage in vital information exchange and relationship building. More research and education are needed to further identify barriers surrounding patient–provider communication about CAM treatments. A few models for improving this dialogue have been studied, however, more research on physician-specific, as well as patient-specific, training about CAM may be useful to address and promote open communication on this topic.
Supplementary Material
Acknowledgment
The authors thank Diane Cooper, MS, biomedical librarian and informationist, National Institutes of Health Library, for her assistance in conducting the literature search.
Authors' Contributions
A.A.A.: Conceptualization, methodology, formal analysis, data curation, writing—original draft preparation, investigation, and visualization. S.M.K.: Formal analysis, data curation, writing—review and editing, investigation, and visualization. O.O.: Conceptualization, methodology, and supervision. J.D.W.: Conceptualization, methodology, validation, resources, formal analysis, writing—review and editing, supervision, and project administration.
Author Disclosure Statement
No conflicts of interest.
Funding Information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. No specific funding was required for this review.
Supplementary Material
References
- 1. Arora NK, Street RL Jr., Epstein RM, et al. Facilitating patient-centered cancer communication: A road map. Patient Educ Couns 2009;77(3):319–321; doi: 10.1016/j.pec.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 2. Frenkel M, Cohen L. Effective communication about the use of complementary and integrative medicine in cancer care. J Altern Complement Med 2014;20(1):12–18; doi: 10.1089/acm.2012.0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Center for Complementary and Integrative Health. Complementary, Alternative, or Integrative Health: What's in a Name? 2018. Available from: https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name [Last accessed: May 14, 2022].
- 4. Mao JJ, Palmer CS, Healy KE, et al. Complementary and alternative medicine use among cancer survivors: A population-based study. J Cancer Surviv 2011;5(1):8–17; doi: 10.1007/s11764-010-0153-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saghatchian M, Bihan C, Chenailler C, et al. Exploring frontiers: Use of complementary and alternative medicine among patients with early-stage breast cancer. Breast 2014;23(3):279–285; doi: 10.1016/j.breast.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 6. Davis EL, Oh B, Butow PN, et al. Cancer patient disclosure and patient-doctor communication of complementary and alternative medicine use: A systematic review. Oncologist 2012;17(11):1475–1481; doi: 10.1634/theoncologist.2012-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keene MR, Heslop IM, Sabesan SS, et al. Complementary and alternative medicine use in cancer: A systematic review. Complement Ther Clin Pract 2019;35:33–47; doi: 10.1016/j.ctcp.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 8. Rogge AA, Helmer SM, King R, et al. Effects of training oncology physicians advising patients on complementary and integrative therapies on patient-reported outcomes: A multicenter, cluster-randomized trial. Cancer 2021;127(15):2683–2692; doi: 10.1002/cncr.33562 [DOI] [PubMed] [Google Scholar]
- 9. Scarton LA, Del Fiol G, Oakley-Girvan I, et al. Understanding cancer survivors' information needs and information-seeking behaviors for complementary and alternative medicine from short- to long-term survival: A mixed-methods study. J Med Libr Assoc 2018;106(1):87–97; doi: 10.5195/jmla.2018.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ustundag S, Demir Zencirci A. Complementary and alternative medicine use among cancer patients and determination of affecting factors: A questionnaire study. Holist Nurs Pract 2015;29(6):357–369; doi: 10.1097/hnp.0000000000000113 [DOI] [PubMed] [Google Scholar]
- 11. Bauer F, Schmidt T, Eisfeld H, et al. Information needs and usage of complementary and alternative medicine in members of a German self-help group for gastrointestinal stroma tumours, sarcoma, and renal cancer. Complement Ther Med 2018;41:105–110; doi: 10.1016/j.ctim.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 12. Pihlak R, Liivand R, Trelin O, et al. Complementary medicine use among cancer patients receiving radiotherapy and chemotherapy: Methods, sources of information and the need for counselling. Eur J Cancer Care 2014;23(2):249–254; doi: 10.1111/ecc.12132 [DOI] [PubMed] [Google Scholar]
- 13. Kmet LM, Lee RC, Cook LS. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields. Institute of Health Economics: Alberta, Canada; 2004. [Google Scholar]
- 14. Frenkel M, Ben-Arye E, Cohen L. Communication in cancer care: Discussing complementary and alternative medicine. Integr Cancer Ther 2010;9(2):177–185; doi: 10.1177/1534735410363706 [DOI] [PubMed] [Google Scholar]
- 15. Hunter D, Oates R, Gawthrop J, et al. Complementary and alternative medicine use and disclosure amongst Australian radiotherapy patients. Support Care Cancer 2014;22(6):1571–1578; doi: 10.1007/s00520-014-2120-8 [DOI] [PubMed] [Google Scholar]
- 16. Rausch SM, Winegardner F, Kruk KM, et al. Complementary and alternative medicine: Use and disclosure in radiation oncology community practice. Support Care Cancer 2011;19(4):521–529; doi: 10.1007/s00520-010-0846-5 [DOI] [PubMed] [Google Scholar]
- 17. Arslan C, Guler M. Alternative medicine usage among solid tumour patients receiving chemotherapy. Eur J Cancer Care 2017;26(5):e12530; doi: 10.1111/ecc.12530 [DOI] [PubMed] [Google Scholar]
- 18. Black DS, Lam CN, Nguyen NT, et al. Complementary and integrative health practices among Hispanics diagnosed with colorectal cancer: Utilization and communication with physicians. J Altern Complement Med 2016;22(6):473–479; doi: 10.1089/acm.2015.0332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Butler S, Owen-Smith A, DiIorio C, et al. Use of complementary and alternative medicine among men with prostate cancer in a rural setting. J Community Health 2011;36(6):1004–1010; doi: 10.1007/s10900-011-9402-6 [DOI] [PubMed] [Google Scholar]
- 20. Choi JY, Chang YJ, Hong YS, et al. Complementary and alternative medicine use among cancer patients at the end of life: Korean national study. Asian Pac J Cancer Prev 2012;13(4):1419–1424; doi: 10.7314/apjcp.2012.13.4.1419 [DOI] [PubMed] [Google Scholar]
- 21. Sullivan A, Gilbar P, Curtain C. Complementary and alternative medicine use in cancer patients in rural Australia. Integr Cancer Ther 2015;14(4):350–358; doi: 10.1177/1534735415580679 [DOI] [PubMed] [Google Scholar]
- 22. Wortmann JK, Bremer A, Eich HT, et al. Use of complementary and alternative medicine by patients with cancer: A cross-sectional study at different points of cancer care. Med Oncol 2016;33(7):78; doi: 10.1007/s12032-016-0790-4 [DOI] [PubMed] [Google Scholar]
- 23. Oh B, Butow P, Mullan B, et al. Patient-doctor communication: Use of complementary and alternative medicine by adult patients with cancer. J Soc Integr Oncol 2010;8(2):56–64. [PubMed] [Google Scholar]
- 24. Chow WH, Chang P, Lee SC, et al. Complementary and alternative medicine among Singapore cancer patients. Ann Acad Med Singap 2010;39(2):129–135 [PubMed] [Google Scholar]
- 25. Ge J, Fishman J, Vapiwala N, et al. Patient-physician communication about complementary and alternative medicine in a radiation oncology setting. Int J Radiat Oncol Biol Phys 2013;85(1):e1–e6; doi: 10.1016/j.ijrobp.2012.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sohl SJ, Borowski LA, Kent EE, et al. Cancer survivors' disclosure of complementary health approaches to physicians: The role of patient-centered communication. Cancer 2015;121(6):900–907; doi: 10.1002/cncr.29138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eliott JA, Mignone J. ‘It's a very difficult balance’: A qualitative study of cancer specialists' perceptions of discussing complementary and alternative medicine with their patients. Asia Pac J Clin Oncol 2012;8:228; doi: 10.1111/ajco.12030 [DOI] [Google Scholar]
- 28. Sewitch MJ, Yaffe M, Maisonneuve J, et al. Use of complementary and alternative medicine by cancer patients at a Montreal hospital. Integr Cancer Ther 2011;10(4):305–311; doi: 10.1177/1534735410395136 [DOI] [PubMed] [Google Scholar]
- 29. Lüthi E, Diezi M, Danon N, et al. Complementary and alternative medicine use by pediatric oncology patients before, during, and after treatment. BMC Complement Med Ther 2021;21(1):96; doi: 10.1186/s12906-021-03271-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. King N, Balneaves LG, Levin GT, et al. Surveys of cancer patients and cancer health care providers regarding complementary therapy use, communication, and information needs. Integr Cancer Ther 2015;14(6):515–524; doi: 10.1177/1534735415589984 [DOI] [PubMed] [Google Scholar]
- 31. Corina G, Christine H, Klein G. Oncologists' experiences of discussing complementary and alternative treatment options with their cancer patients. A qualitative analysis. Support Care Cancer 2016;24(9):3857–3862; doi: 10.1007/s00520-016-3205-3 [DOI] [PubMed] [Google Scholar]
- 32. Lee RT, Barbo A, Lopez G, et al. National survey of US oncologists' knowledge, attitudes, and practice patterns regarding herb and supplement use by patients with cancer. J Clin Oncol 2014;32(36):4095–4101; doi: 10.1200/JCO.2014.55.8676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Juraskova I, Hegedus L, Butow P, et al. Discussing complementary therapy use with early-stage breast cancer patients: Exploring the communication gap. Integr Cancer Ther 2010;9(2):168–176; doi: 10.1177/1534735410365712 [DOI] [PubMed] [Google Scholar]
- 34. Roter DL, Yost KJ, O'Byrne T, et al. Communication predictors and consequences of Complementary and Alternative Medicine (CAM) discussions in oncology visits. Patient Educ Couns 2016;99(9):1519–1525; doi: 10.1016/j.pec.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koenig CJ, Ho EY, Trupin L, et al. An exploratory typology of provider responses that encourage and discourage conversation about complementary and integrative medicine during routine oncology visits. Patient Educ Couns 2015;98(7):857–863; doi: 10.1016/j.pec.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salamonsen A. Doctor-patient communication and cancer patients' choice of alternative therapies as supplement or alternative to conventional care. Scand J Caring Sci 2013;27(1):70–76; doi: 10.1111/j.1471-6712.2012.01002.x [DOI] [PubMed] [Google Scholar]
- 37. Luo Q, Asher GN. Complementary and alternative medicine use at a comprehensive cancer center. Integr Cancer Ther 2017;16(1):104–109; doi: 10.1177/1534735416643384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stub T, Quandt SA, Arcury TA, et al. Perception of risk and communication among conventional and complementary health care providers involving cancer patients' use of complementary therapies: A literature review. BMC Complement Altern Med 2016;16(1):353; doi: 10.1186/s12906-016-1326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schofield P, Diggens J, Charleson C, et al. Effectively discussing complementary and alternative medicine in a conventional oncology setting: Communication recommendations for clinicians. Patient Educ Couns 2010;79(2):143–151; doi: 10.1016/j.pec.2009.07.038 [DOI] [PubMed] [Google Scholar]
- 40. Witt CM, Helmer SM, Schofield P, et al. Training oncology physicians to advise their patients on complementary and integrative medicine: An implementation study for a manual-guided consultation. Cancer 2020;126(13):3031–3041; doi: 10.1002/cncr.32823 [DOI] [PubMed] [Google Scholar]
- 41. Shelley BM, Sussman AL, Williams RL, et al. ‘They don't ask me so I don't tell them’: Patient-clinician communication about traditional, complementary, and alternative medicine. Ann Fam Med 2009;7(2):139–147; doi: 10.1370/afm.947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Milden SP, Stokols D. Physicians' attitudes and practices regarding complementary and alternative medicine. Behav Med 2004;30(2):73–82; doi: 10.3200/bmed.30.2.73-84 [DOI] [PubMed] [Google Scholar]
- 43. Choi KH, Park JH, Park SM. Cancer patients' informational needs on health promotion and related factors: A multi-institutional, cross-sectional study in Korea. Support Care Cancer 2011;19(10):1495–1504; doi: 10.1007/s00520-010-0973-z [DOI] [PubMed] [Google Scholar]
- 44. Blodt S, Mittring N, Schutzler L, et al. A consultation training program for physicians for communication about complementary medicine with breast cancer patients: A prospective, multi-center, cluster-randomized, mixed-method pilot study. BMC Cancer 2016;16(1):843; doi: 10.1186/s12885-016-2884-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parker PA, Urbauer D, Fisch MJ, et al. A multisite, community oncology-based randomized trial of a brief educational intervention to increase communication regarding complementary and alternative medicine. Cancer 2013;119(19):3514–3522; doi: 10.1002/cncr.28240 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.