Abstract
Significance:
Foreign body response (FBR), wherein a fibrotic capsule forms around an implanted structure, is a common surgical complication that often leads to pain, discomfort, and eventual revision surgeries. Although believed to have some mechanistic overlap with normal wound healing, much remains to be discovered about the specific mechanism by which this occurs.
Recent Advances:
Current understanding of FBR has focused on the roles of the immune system and the biomaterial, both major contributors to FBR. However, another key player, the fibroblast, is often overlooked. This review summarizes key contributors of FBR, focusing on the roles of fibroblasts. As much remains to be discovered about fibroblasts' specific roles in FBR, we draw on current knowledge of fibroblast subpopulations and functions during wound healing. We also provide an overview on candidate biomaterials and signaling pathways involved in FBR.
Critical Issues and Future Directions:
While the global implantable medical devices market is considerable and continues to appreciate in value, FBR remains one of the most common surgical implant complications. In parallel with the continued development of candidate biomaterials, further exploration of potential fibroblast subpopulations at a transcriptional level would provide key insights into further understanding the underlying mechanisms by which fibrous encapsulation occurs, and unveil novel directions for antifibrotic and regenerative therapies in the future.
Keywords: foreign body response, fibroblast, fibrosis, implant

Michael T. Longaker, MD, MBA, FACS
Scope and Significance
With uses in the fields of orthopedic, cardiac, and plastic and reconstructive surgery, to name a few, the global implantable medical devices market is considerable. Unfortunately, foreign body response (FBR), an immune reaction that leads to dense scar tissue formation around the implant, is a common surgical complication. Multiple articles have been published on FBR; however, fibroblasts, a key player in this process, has often been overlooked. This review will summarize key contributors and mechanisms of FBR, focusing on fibroblast morphology and function.
Translational Relevance
Current research aims to further understand the mechanisms that underlie FBR with the hope of developing treatment modalities that reduce fibrotic capsule formation. Some researchers have focused on elucidating how the various cell types and signals at different stages of FBR result in fibrotic capsule formation, while other groups are exploring biomaterials and implant coatings with antifibrotic properties. Research from both basic science and engineering may lead to the development of novel antifibrotic strategies.
Clinical Relevance
FBR is a common clinical complication that may lead to pain, discomfort, and device failure. In the context of breast implants, revision surgery is the standard treatment for FBR; however, patients frequently experience recurrence. That said, investigations into biomaterial candidates and molecular therapies derived from an increased understanding of the cell types and fundamental mechanisms that lead to FBR show promise as potential future directions in the management and treatment of this condition.
Background
With applications ranging from drug delivery, tissue replacement and reconstruction, biosensors, scaffolds for tissue engineering, and prostheses, implantable devices and materials have become a ubiquitous component of modern medicine.1 In 2021, the global implantable medical devices market reached a value of $120 billion USD and is expected to rise in value to $168.3 billion USD by 2027.2 Though offering significant benefits clinically, use of these materials often results in an inflammatory reaction known as FBR. This reaction is characterized by a series of stages: (1) provisional extracellular matrix (ECM) formation; (2) acute inflammatory stage; (3) chronic inflammatory stage; and (4) fibrotic capsule formation.3 The eventual fibrotic capsule that forms around the implant often leads to pain, discomfort, and eventual revision surgery.4
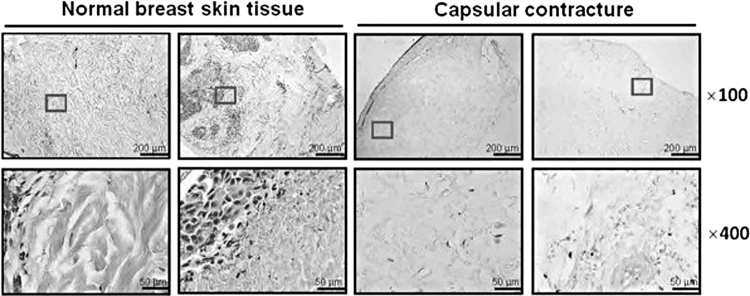
Capsular contracture, a form of FBR, remains one of the most common complications of breast implant surgery (Fig. 1). Although the percentage of patients experiencing capsular contracture varies based on procedure and implant type, an estimated 8–15% of patients receiving breast implants will experience capsular contracture.5 Reoperation is currently the standard treatment for patients experiencing capsular contracture; however, reported recurrence rates have reached up to 54%.4 Alongside breast augmentation and reconstruction, complications related to FBRs also result in device failure and extraction in orthopedic, cardiac, and dental implants, to name a few.6–8
Figure 1.
Capsular contracture as an example of foreign body response. Capsular contracture is the clinical term for fibrous encapsulation that occurs among patients who receive breast reconstruction or augmentation surgery. In this study, normal breast tissue (left) is compared with capsular contracture (right), using H&E staining. H&E, Hematoxylin and Eosin. Boxes indicate the areas from which magnified x400 images were captured. Taken with permission from “Extracellular matrix (ECM) structure in tissue from patients with capsular contracture” (panel A) by Kuo et al.62
Numerous reviews have been published on FBR; however, most discuss FBR through the lens of either the immune system or the biomaterial. Although both are major contributors to FBR, another key player, fibroblasts, is often overlooked. In this study, we begin our discussion with wound healing given its similarities with the acute stage of FBR. We then proceed with an overview of FBR, followed by a discussion of key cell types involved, focusing particularly on fibroblasts. As much remains to be explored regarding the morphology and function of fibroblasts in the context of FBR, we draw on current knowledge of fibroblast subpopulations and functions during wound healing. Although not the focus of this work, we will also touch briefly on biomaterials and signaling pathways involved in FBR.
Discussion
Wound healing
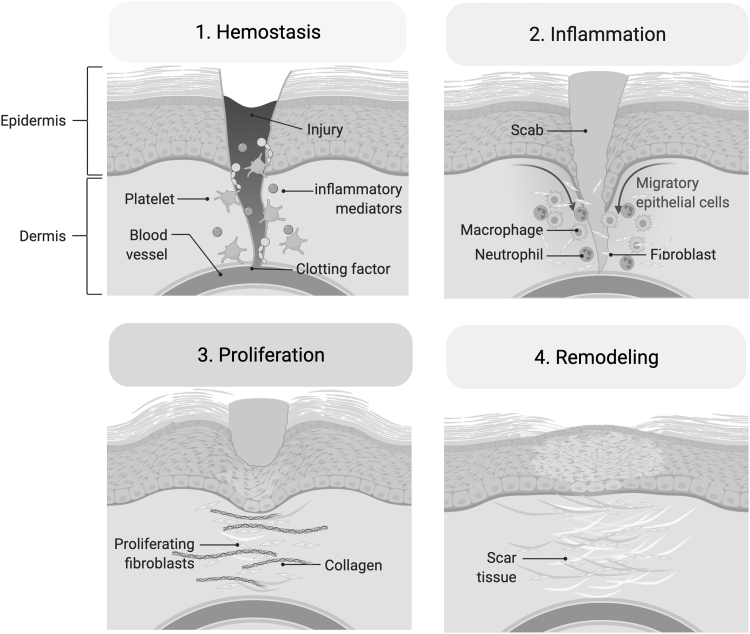
During normal wound healing, a series of overlapping stages is involved: hemostasis and inflammation, cell proliferation and matrix deposition, followed by maturation and tissue remodeling (Fig. 2).9 Immediately after injury, the coagulation cascade controls bleeding from damaged blood vessels. Inflammatory mediators, such as tumor necrosis factor-alpha (TNF-α) and transforming growth factor-beta (TGF-β) build in the wound bed, bringing in neutrophils and monocytes during the first week postinjury. Neutrophils remove bacteria and cellular debris from the wound, while monocytes activate into macrophages.10,11 In addition to their phagocytic role in the inflammatory phase, macrophages also stimulate numerous processes involved in the proliferative phase.
Figure 2.
Stages of wound healing. Wounds undergo a series of stages before scar formation. 1. Hemostasis: the coagulation cascade controls bleeding and platelets clot off the injury site. 2. Inflammation: macrophages and neutrophils remove bacteria and debris, while also stimulating processes during the proliferative phase. 3. Proliferation: endothelial cells reform capillaries and repair vasculature, while fibroblasts at the wound site secrete collagen and contribute to ECM formation. 4. Remodeling: the ECM matrix is gradually replaced by one that is more organized and structured, resulting in the observed scar. ECM, extracellular matrix. Adapted from “Wound Healing,” by BioRender.com (2022). Retrieved from http://app.biorender.com/biorender-templates
During proliferation and matrix deposition, endothelial cells reform capillaries to repair damaged vasculature, while fibroblasts begin depositing collagen and other components of the ECM that forms within the wound at this time.10 Activated fibroblasts known as myofibroblasts also initiate wound contracture through TGF-β1 signaling.9 Finally, during maturation and remodeling, the temporary matrix is gradually replaced by one that is more organized and structured. This stage is considered critical to ensure proper wound healing.9
FBR overview
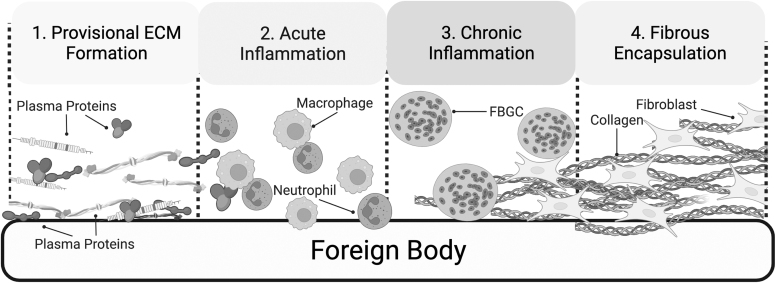
There are many parallels between normal wound healing and FBR. During FBR, the process similarly begins with an inflammatory response. The presence of the biomaterial, however, often results in a progression of the acute response into a chronic condition. In this study, we will provide an overview of FBR and will then proceed to discuss specific cellular components of the process in detail in sections to follow (Fig. 3).
Figure 3.
Stages of foreign body response. 1. Provisional ECM formation: Plasma proteins released due to damaged vasculature get adsorbed by the foreign body, forming a provisional matrix around the implant. 2. Acute inflammation: Acting as a source of bioactive agent, the provisional matrix attracts immune cells, including neutrophils and macrophages to the implant site. These attempt to phagocytose the foreign body. 3. Chronic inflammation: Due to the continued attempt by macrophages to phagocytose the implant, these cells undergo additional differentiation, forming FBGC, which continue to attempt to degrade the implant in a process known as frustrated phagocytosis. 4. Fibrous encapsulation: fibroblasts attracted to the implant site by immune cell signaling deposit a collagen-rich ECM that surrounds the implant. FBGC, foreign body giant cell.
When a device is implanted into the body, damage of vascularized connective tissue at the surgical site releases a variety of plasma proteins, including vitronectin, fibrinogen, complement, and fibronectin, which get adsorbed to the biomaterial surface.12,13 These proteins form what is known as a provisional matrix on the surface of the implant. The matrix acts as a source of bioactive agents comprising cytokines, growth factors, and chemoattractants, which leads to the recruitment and proliferation of a number of cell types involved in wound healing, such as immune cells and fibroblasts.3 While macrophages and neutrophils attempt to phagocytose the foreign body, fibroblasts attracted to the implant by immune cell signaling deposit a collagen-rich ECM that surrounds the implant, completing the final two phases of FBR: granulation tissue deposition and fibrotic capsule formation.
FBR is a complicated and dynamic response, incorporating different cell types, materials, and signaling pathways (Fig. 3). We begin our discussion of FBR at the acute phase, where the macrophage is the predominant cell type, before delving into fibroblast subpopulations and functions in more detail.
Macrophage involvement
During normal wound healing, M1 macrophages release inflammatory cytokines, such as TNF-α and interleukin (IL)-6, antimicrobial peptides, and reactive oxygen species.14 As this type of macrophage is phagocytic, it plays an important role in clearing debris and bacteria initially from the wound bed. In contrast to M1 macrophages that play a proinflammatory role in repair, M2 cells release anti-inflammatory mediators (TGF-β, IL-10, platelet-derived growth factor), which help stabilize the wound environment and promote angiogenesis, ECM deposition and remodeling, and cell proliferation.15,16 Although macrophages are often placed into these binary categories, evidence suggests that macrophage phenotypes fall on a continuum with M1- and M2-type macrophages on either end.14
An important distinction between normal wound healing and FBR is the formation of multinucleated cells from macrophages.17 Over the course of multiple days, as they continue attempting to phagocytose the foreign body, macrophages undergo additional differentiation and proceed to fuse into multinucleated foreign body giant cells (FBGCs). FBGCs surround the implant and are believed to secrete compounds such as reactive oxygen species and enzymes in an attempt to degrade the implant.17 This process is known as frustrated phagocytosis.
During the acute inflammatory phase of FBR, mast cells release IL-4 and IL-13, attracting monocytes to the surgical site.18 These monocytes differentiate into macrophages, which become the primary immune cell type in the implant bed during the chronic inflammatory phase.3 There is conflicting evidence available regarding the phenotype of macrophages involved in FBR.19 Some studies indicate that M1 macrophages are the primary macrophage subtype in all stages, while other in vitro assays indicate that formation of FBGCs relies on IL-4 and IL-13 signaling, both strong modulators of M2 type macrophages.17 Other studies also indicate that macrophages associated with FBR lie in between the phenotypes of M1 and M2 macrophages.19
Following the acute and chronic inflammatory phases of the FBR, granulation tissue forms around the implant, slowly transitioning to the formation of a chronic fibrotic capsule that will persist around the implant for as long as it is in the body.3 Diverse cell types, including fibroblasts, keratinocytes, immune cells, endothelial cells, thrombocytes, and adipocytes, are involved in fibrotic capsule formation; however, it is believed that fibroblasts play the central role.20
Fibroblast involvement
Historically, fibroblasts were considered a homogeneous group of spindle-shaped cells that deposit collagen and are present in fibrotic tissue. However, growing evidence suggests that fibroblasts are a heterogeneous cell population exhibiting diverse morphologies and functions.21,22 For one, fibroblasts demonstrate diversity at different sites of the body. Fibroblasts within the muscle, heart, gastrointestinal tract, and skin all exhibit specialized functions.
Murine dermal fibroblast heterogeneity
Notably, heterogeneity is also seen within a given tissue. In the dermis, for instance, fibroblasts exhibit different ECMs and distinct functional activities within the reticular and papillary dermis layers (Table 1).21 During wound healing, reticular fibroblasts contribute to formation of the fibrillar ECM and participate in the initial stages of repair. Meanwhile, papillary fibroblasts demonstrate key roles in hair growth regulation, piloerection control, and are only recruited during the late stage of wound healing when re-epithelialization occurs.21 Further multiomic analysis of neonatal murine skin cells revealed two additional specialized fibroblast subpopulations: (1) the dermal papilla defined by Lamc3, Alx4, Bmp3, Sobp, and Draxin; and (2) the preadipocyte defined by Fabp4, Adipoq, Adig, Fabp12, and CD36.23
Table 1.
Dermal fibroblast subpopulations
| Subtype | Role |
|---|---|
| Fibroblasts within murine dermis | |
| Reticular | Fibrillar ECM formation; initial stages of wound repair21 |
| Papillary | Hair growth regulation; piloerection control; late stage of wound repair21 |
| Dermal papilla (Lamc3, Alx4, Bmp3, Sobp, and Draxin) | Hair follicle development23 |
| Preadipocyte (Fabp4, Adipoq, Adig, Fabp12, and CD36) | Skin repair, regeneration, adipogenesis23,30 |
| En1-positive | Dorsal scar-forming, profibrotic fibroblast24 |
| En1-negative | Dorsal proregenerative fibroblast24 |
| Prrx1-positive | Ventral scar-forming, profibrotic fibroblast25 |
| Prrx1-negative | Ventral proregenerative fibroblast25 |
| Hic1-positive extrafollicular | Hair follicle regeneration27 |
| Crabp1-positive upper wound | Wound-induced hair follicle neogenesis26 |
| Fibroblasts within human dermis | |
| Hypothesized types and subtypes | |
| Reticular dermis; deep dermis; upper dermis31 Alternatively: 3 major subtypes and 10 subtypes33 |
1. Dermal cell, ECM homeostasis 2. Immune surveillance and inflammatory promotion 3. Specialized roles (dermal papilla, dermo-hypodermal junction |
Growing evidence suggests that fibroblasts are a heterogeneous population of cells that vary in morphology and function. In this study, we highlight the different fibroblast subpopulations identified within murine and human dermis as an example of fibroblast heterogeneity.
Adig, adipogenin; Adipoq, adiponectin; Alx4, ALX homeobox 4; BMP3, bone morphogenetic protein 3; Crabp1, cellular retinoic acid-binding protein 1; ECM, extracellular matrix; En1, engrailed-1; Fabp12, fatty acid-binding protein 12; Fabp4, fatty acid-binding protein 4; Hic1, HIC ZBTB transcriptional repressor 1; Lamc3, laminin subunit gamma 3; Prrx1, paired-related homeobox 1; Sobp, Sine oculis-binding protein homolog.
In the context of murine wound healing, our group has identified four additional fibroblast lineages. Engrailed-1 (En1)-positive and En1-negative fibroblasts are predominantly found on the mouse dorsum.24 Meanwhile, paired-related homeobox 1 (Prrx1)-positive and Prrx1-negative fibroblasts are analogously present in the ventral dermis.25 During wounding, En1-positive and Prrx1-positive fibroblasts are considered the dorsal and ventral scar-forming fibroblasts, respectively. When these fibroblast populations are ablated, cutaneous wounds heal with decreased scarring.
Murine skin has some capacity to regenerate and produce skin appendages such as hair follicles (HFs). Exactly how murine dermis regenerates and undergoes wound-induced hair follicle neogenesis (WIHN) is not well understood. It has been hypothesized that stem cells within HFs may be the primary contributors.26–28 One study explored the concept of hair follicular stem cells (HFSCs) through single-cell RNA sequencing (scRNAseq) analysis of young and aged murine skin.28 Aged HSFCs transplanted into young dermis were able to regenerate HFs, while young HFSCs transplanted into aged dermis failed to do so. These results suggested that the niche environment plays a crucial role in establishing cellular properties within the dermis.
Interestingly, complementary fate mapping within murine dermis performed by another group demonstrated that progenitor cells from HFs contributed very little to wound regeneration.27 Instead, their single-cell profiling of murine skin indicated that it was wound activation along with exposure to a permissive environment that activates a latent regenerative capacity of fibroblasts within the wound bed. They conclude that Hic1-lineage-comprising extrafollicular fibroblasts were the primary cells contributing to HF regeneration within the permissive microenvironment.
In parallel, another study compared recently published scRNAseq data of small scarring wounds with wounds that regenerated to identify a potential subpopulation of fibroblasts that contributed to WIHN. They identified a novel upper wound fibroblast within the regenerative condition that expresses retinoic acid-binding protein Crabp1.26
Heterogeneity also exists within the myofibroblast subpopulation. Characterized by a contractile phenotype, myofibroblasts within the wound bed participate in the organization of the new collagen-rich ECM into a mechanically resistant scar.9 The cell progenitors of myofibroblasts for many tissues fall under the umbrella term of “fibroblasts”; however, variation is seen depending on organ type.29
In the context of skin, a recent study analyzing mouse dermal wound healing and aging established that there were two classes of myofibroblasts: smooth muscle actin (SMA)+ and collagen 1+ cells.30 These cell groups varied in their cellular origins, with one expressing adipocyte precursor (AP) cell markers, and the other expressing high levels of CD29 (CD29High). Interestingly, AP cells were shown to be derived from En1-lineage fibroblasts, and during wound healing, a large proportion of APs expressed profibrotic markers, such as CD26 and CD9.30 Myofibroblasts also varied in spatial organization, with APs spread throughout the wound, whereas CD29High cells were localized mainly at the outer, superficial areas of the wound.
Human dermal fibroblast heterogeneity
Although there have been diverse murine fibroblast populations described to date, fibroblast subtypes present in human dermis are less well understood.31,32 Recent studies are emerging that indicate human fibroblasts exhibit similar heterogeneity to that present in mice, although conclusions currently vary between groups (Table 1). In one study, five distinct fibroblast subpopulations were found in human skin using spatial and single-cell transcriptional profiling.31 Their work demonstrated the following subpopulations of fibroblasts: three subtypes in the reticular dermis, a preadipocyte population within the deep dermis, and one population in the upper dermis.
More recently, another group reanalyzed transcriptomic data from ∼14,000 human adult dermal fibroblasts and assigned them to 3 major types and 10 subtypes.33 Major types functioned in (1) dermal cell and ECM homeostasis; (2) immune surveillance and inflammatory promotion; and (3) more specialized roles such as in the dermal papilla or dermo-hypodermal junction. A recent study conducted scRNAseq of human eyelid skin. These data confirmed the presence of reticular and papillary fibroblasts previously described.34
The group harvested eyelid skin from human patients across different ages, which revealed gradual increases in photoaging-related and chronic inflammatory changes with age. Interestingly, this team found that fibroblasts had the highest extent of age-related transcriptional variability of all the clusters they identified in their analyses. They determined transcription factor HES1 expression within fibroblasts declined during aging. Inhibition of HES1 decreased cell proliferation, and increased inflammation and cell senescence.
Although little consensus has been achieved about specific subpopulations that make up human fibroblasts, these studies illustrate the growing evidence of human fibroblast heterogeneity.
Fibroblast heterogeneity within FBR
As mentioned, much remains to be explored regarding the morphology and function of fibroblasts in the context of FBR. When macrophages begin releasing TGF-β into the implant bed during FBR acute phase, fibroblasts migrate to the area and surround the biomaterial's surface.3,35,36 The mechanism by which fibroblasts localize to the implant site is not yet well understood. Once there, similar to normal wound healing, the fibroblasts attempt to re-establish the tissue architecture and mechanical integrity of the implant site by replacing the temporary fibrin with collagen-rich ECM. In parallel, some fibroblasts transition into myofibroblasts.37 Altogether, fibroblasts and myofibroblasts become the primary cells surrounding the implant 4 weeks after implantation of the biomaterial.38 In cases where FBR is severe, the capsule surrounds the entirety of the implant, effectively walling off the foreign material from the rest of the body.
Biomaterials
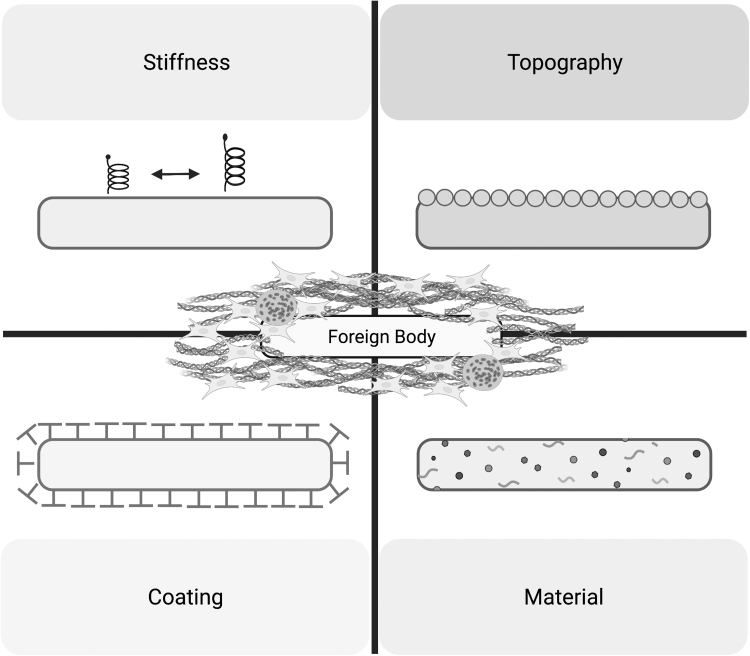
Many factors influence protein adsorption, a key component of provisional matrix formation during FBR.3 It has been established that different features of the biomaterial influence protein adsorption and alter the magnitude of the FBR. Furthermore, the degradation of the biomaterial itself has also been shown to be an important contributor of FBR. In the case of dental implants, for instance, ions and particles that get released from the material have been shown to further evoke an immune response, leading to bone resorption and peri-implant disease.39 Below, we will summarize investigations in the following features of a given biomaterial: chemical composition and coatings, implant topography, and implant stiffness (Fig. 4).
Figure 4.
Examples of biomaterial modification used to modulate foreign body response. Changes to stiffness, topography, coating, and material have all been shown to alter implant foreign body response.
Materials and coatings
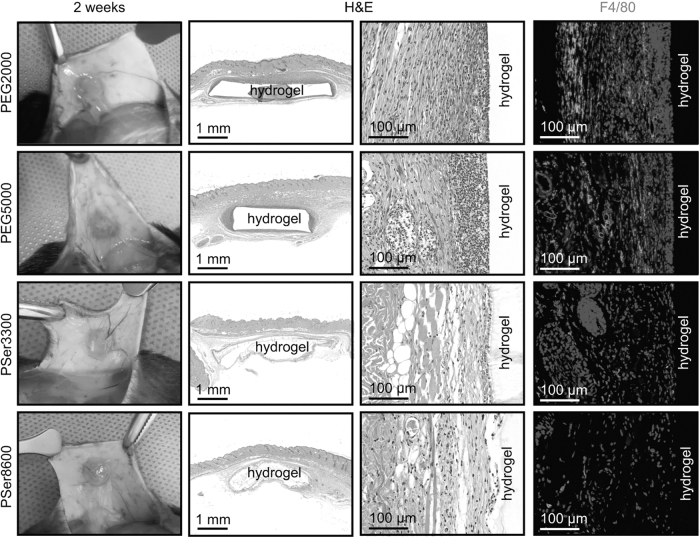
Materials used in implants are variable and dependent on their applications in the body. For instance, electrode arrays require coating conductivity, while joint replacements need to meet the demands of weight-bearing impact and locomotion. Meanwhile, extensive research is being conducted to develop materials that invoke a subdued FBR while still providing the desired function. Figure 5 illustrates the difference in FBR created by two different materials: poly(ethyleneglycol) (PEG) and poly-dl-serine (PSer) hydrogels.
Figure 5.
Comparison between foreign body response formation around PEG and PSer hydrogels as an example of the effect of biomaterial on fibrotic capsule formation. A visible reduction in the thickness and density of the fibrotic capsule is seen with the use of PSer hydrogels (bottom two rows) when compared with PEG hydrogels (top two rows). PEG, poly(ethyleneglycol); PSer, poly-dl-serine. Taken with permission from “Explantation and staining images of PEG and PSer hydrogels after subcutaneously implanted in mice for 1 week (a) and 2 weeks (b)” (panel B) by Zhang et al.63
The development of novel, low-fouling polymers has been a significant focus. Some polymers such as PEG, poly(ethylene oxide) (POE), and polyhydroxymethacrylate (PHEMA) result in a lower fibrotic response; however, these materials still demonstrate a degree of protein adsorption and cell adhesion.40 Zwitterionic polymers, particularly ones used to coat implants, have been shown to produce intrinsically low FBR responses, and are a promising option for hydrogel-based cell therapies. Novel materials are also in development. One set of compounds known as triazole-modified alginates, has been shown to incur lower inflammation and fibrosis markers.41 Another compound makes use of composite materials known as immobilized liquid interfaces and has a repellent liquid such as perfluorocarbon liquid at its surface.42 Preliminary results for both materials suggest they hold promise in decreasing FBR in future implant development.
In addition to the implant material itself, others are conducting research into coating implants with antifibrotic drugs. For instance, microstents coated with pirfenidone, an antifibrotic agent, implanted in rats demonstrated decreased fibrotic capsule formation relative to controls.43
Topography
Altering the surface architecture is another widely explored strategy to modulate immune response and fibrosis associated with FBR. In the context of breast implants, smooth, micro, and macrotextured implants are currently available.44 However, macrotextured implants have been associated with breast implant-associated anaplastic large cell lymphoma.45 A detailed investigation of the effect of surface topography on fibrotic capsule formation recently described that there appears to be a “goldilocks” surface roughness that reduces foreign body capsule formation.44 Any lower or higher, and the immune system responds more significantly.
Stiffness
Often, tissues in which implants are placed have a stiffness that can be substantially lower compared with the implant itself. Breast implants, for example, although they may appear soft as they are often filled with a liquid or gel, have an outer shell that is a 1,000 times stiffer compared with the dermis.46 Mismatch in the stiffness between the tissue and implant can lead to mechanical activation of inflammatory and fibroblastic cells seen in FBR. Indeed, a soft silicone coating with an elastic modulus similar to that of dermis around implants placed in mice led to a decreased fibrous encapsulation when compared with stiffer implants of the same size and shape.46
Signaling and FBR
Various signaling molecules have been associated with FBR, although further investigation is needed to grasp a more complete understanding of the specific pathways involved in the different stages of FBR. We will describe profibrotic and mechanotransduction pathways believed to direct fibroblast function and fibrotic capsule formation, specifically.
Fibrotic signaling
It is well known that TGF-β promotes fibrosis in many tissues, and numerous studies have demonstrated elevated TGF-β levels during fibrotic capsule formation.47 TGF-β signaling primarily functions through transmembrane receptor serine/threonine kinases to activate Smad proteins, signaling intermediates that in turn activate transcription of profibrotic target genes.48 However, TGF-β also acts through Smad-independent pathways, including extracellular signal-regulated kinase 1 and 2 (ERK1/2), c-Jun-N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3′-kinase (PI3K)–protein kinase B (AKT) pathways.49 Inhibition of TGF-β has been shown to decrease fibrotic capsule formation in mice, and its association with a variety of signaling pathways underscores the multiple avenues investigators could take in inhibiting TGF-β to subdue FBR.46
Although it does play a role in fibrosis, TGF-β is also crucial for multiple homeostatic processes, including embryogenesis, angiogenesis, immune modulation, and cell differentiation.50 In human studies, inhibition of TGF-β1 signaling at the levels of its isoforms and receptors has failed numerous times due to systemic complications, and has been shown to impede tissue healing.50,51 It is therefore believed that the development of a phased, lineage-targeted approach bodes more promise as a therapeutic option.
Mechanotransduction
Mechanotransduction allows cells to respond to mechanical cues through electrochemical signaling. There are a number of extracellular and cellular components that act as mechanosensors, which transmit signal to cells and lead to transcriptional changes when under mechanical stress.52 It has been previously shown that sustained mechanical load to an incision increased fibrotic response and hypertrophic scar formation in a mouse model, indicating that mechanical stress plays a key role in scarring and fibrosis.53 The importance of mechanical stress in FBR has been similarly demonstrated.54
Key contributors to detecting mechanical stress are transmembrane proteins known as integrins. Formed with different alpha and beta subunits, integrins bind to a variety of ECM proteins, act as bidirectional cell receptors transmitting signals across the plasma membrane, and control cytoskeletal organization.55 There are many integrins with various functions in FBR, ranging from immune cell recruitment and FBGC formation, to fibroblast recruitment and activation. Integrins can sense extracellular forces applied to the cell by the biomaterial in proximity to it, and in turn facilitate communication and coordination of these cell types during the acute and chronic stages of FBR.56
Importantly, additional mechanotransduction pathways alongside mechanical-sensing integrins may present essential areas of exploration in the field of FBR. YAP, Hippo, and Rho/ROCK signaling have all been shown to play key roles in mechanotransduction signaling in wound healing.24 Given parallels seen between wound healing and FBR, exploration of these pathways in the context of foreign materials may provide additional possibilities for potential drug targets in the future. A few studies exploring inhibitors of fibrotic and mechanotransduction pathways have been conducted, although further study will be necessary to identify effective antifibrotic therapies that could be used in patients. A list of these compounds along with their targeted pathways and FBR models used are summarized in Table 2.
Table 2.
Potential therapeutics to target foreign body response
| Therapeutic compound | FBR fibrotic pathway targeted | Model tested | Reference |
|---|---|---|---|
| Pirfenidone | TGF-β | Rat; daily injection; 8 weeks | Gancedo et al57 |
| Decorin | TGF-β | In vitro; decorin-modified titanium surface | He et al58 |
| CWHM-12 | TGF-β | Mouse; mini osmotic pump; 7 and 28 days | Noskovicova et al46 |
| Roxatidine | MAPK | Mouse; oral gavage with roxatidine for 14 days postimplant surgery; 90 days | Ji et al59 |
| Verteporfin | YAP; mechanotransduction | Rabbit; injection within implant pocket upon implantation; 60 days | Yi et al60 |
| AM-111 (brimapitide) | JNK | Guinea pig; drug administered 30 min before cochlear implantation; 90 days | Eshraghi et al61 |
Below we outline therapeutic strategies tested in implant models targeting antifibrotic pathways associated with FBR.
FBR, foreign body response; JNK, c-Jun-N-terminal kinase; MAPK, p38 mitogen-activated protein kinase; TGF-β, transforming growth factor-beta; YAP, yes-associated protein 1.
Future Directions and Conclusions
As alluded in this study, FBR continues to be a serious clinical complication for which the mechanisms are not completely understood. Although the field has elucidated cell types and signaling molecules responsible for the acute and chronic stages of FBR, gaps remain in appreciating the roles of fibroblasts. Although we are aware of their function in forming the fibrotic capsule around a foreign biomaterial, the exact mechanisms providing fibroblasts with the necessary cues remain elusive.
Furthermore, given the heterogeneity in both morphology and function of fibroblasts described within healthy and wounded dermis, one is left contemplating whether subpopulations of fibroblasts are also seen in the context of FBR. Indeed, many parallels are already appreciated between wound healing and FBR, and so it would not come as a surprise if a similar spectrum of fibroblast morphology and function is seen upon biomaterial implantation. Exploration of potential fibroblast subpopulations at a transcriptional level would provide key insights into answering this question, and these investigations may also provide deeper understanding of the nuances of different fibrotic and mechanical signaling pathways involved.
In addition, although a number of animal models exist to study FBR, the field would benefit from the use of transgenic animal studies along with genetic engineering approaches that could add necessary depth to decipher the mechanisms and subpopulations of fibroblasts associated with fibrotic encapsulation. Altogether, these future perspectives may help unveil novel directions for antifibrotic and regenerative therapies in the future.
Summary
Used in most surgical specialties, implants have become a ubiquitous component of modern medicine. Although the use of implants has benefited countless patients, an inflammatory reaction known as FBR remains a common and significant surgical complication. The field has gained an increased understanding of the cell types and signaling molecules that participate in the different stages of FBR; however, specific details regarding the role fibroblasts play remain elusive. In parallel to the work currently being conducted to develop antifibrotic biomaterials and implant coatings, a deeper understanding of the subpopulations of fibroblasts and mechanisms by which they are involved in FBR may provide the field with additional avenues for antifibrotic and regenerative therapies.
Take-Home Messagess
The global implantable devices market is a multi-billion-dollar industry.
A common surgical complication known as FBR results in the formation of a fibrotic capsule around the implanted medical device, leading to pain, discomfort, and device failure.
FBR is an immunologic reaction that involves multiple cell types and signals at the implant site.
During the acute phase of FBR, immune cells, including neutrophils and macrophages attempt to phagocytose the implant.
Meanwhile, signals released by immune cells attract fibroblasts to the implant site. They deposit a collagen-rich, fibrotic matrix around the implant, effectively walling it off from the rest of the body.
Although fibroblasts are believed to be key players in fibrotic capsule formation, the specific fibroblast subpopulations involved, and the roles that these play in FBR is not well understood.
Research into different biomaterials and implant coatings to reduce fibrotic capsule formation due to FBR is ongoing.
Increased understanding of the fibroblast subpopulations involved in FBR, and the mechanisms by which these fibroblasts form a fibrotic capsule around the implant may allow for the development of novel antifibrotic and regenerative therapies.
Abbreviations and Acronyms
- Adig
adipogenin
- Adipoq
adiponectin
- AKT
protein kinase B
- Alx4
ALX homeobox 4
- AP
adipocyte precursor
- Bmp3
bone morphogenetic protein 3
- Crabp1
cellular retinoic acid-binding protein 1
- ECM
extracellular matrix
- En1
engrailed-1
- ERK1/2
extracellular signal-regulated kinase 1 and 2
- Fabp12
fatty acid-binding protein 12
- Fabp4
fatty acid-binding protein 4
- FBGC
foreign body giant cell
- FBR
foreign body response
- H&E
Hematoxylin and Eosin
- HES1
Hes family BHLH Transcription Factor 1
- HF
hair follicle
- HFSC
hair follicular stem cell
- Hic1
HIC ZBTB transcriptional repressor 1
- IL
interleukin
- JNK
c-Jun-N-terminal kinase
- Lamc3
laminin subunit gamma 3
- MAPK
p38 mitogen-activated protein kinase
- PEG
poly(ethyleneglycol)
- PHEMA
polyhydroxymethacrylate
- PI3K
phosphatidylinositol 3′-kinase
- POE
poly(ethylene oxide)
- Prrx1
paired-related homeobox 1
- PSer
poly-dl-serine
- scRNAseq
single-cell RNA sequencing
- SMA
smooth muscle actin
- Sobp
Sine oculis-binding protein homolog
- TGF-β
transforming growth factor-beta
- TNF-α
tumor necrosis factor-alpha
- WIHN
wound-induced hair follicle neogenesis
- YAP
yes-associated protein 1
Authors' Contributions
J.B.P. wrote the article. M.F.G., A.F.S., D.C.W., and M.T.L. edited the article. Written consent was received from all authors to submit the article and all authors accept complete responsibility for the contents of the article.
Acknowledgments and Funding Sources
Figures 2, 3, and 4 were created with BioRender.com. This work was supported by the Hagey Laboratory for Pediatric Regenerative Medicine and the California Institute for Regenerative Medicine. M.T.L. was supported by NIH Grant R01 GM136659, NIH Grant U24 DE029463, and the Gunn-Olivier Fund. D.C.W. was supported by NIH Grant R01 DE027346.
Author Disclosure and Ghostwriting
The authors declare no competing or financial interests.
About the Authors
Jennifer B. Parker, BS, is a medical student at the University of Toronto and a PhD student at the Stanford School of Medicine in the Stem Cell Biology and Regenerative Medicine graduate program. Michelle Griffin, MBChB, MRes, MSc, PhD, MRCS, is a postdoctoral fellow in Dr. Longaker's laboratory. Amanda F. Spielman, BS is an MD/MPH student at the University of Miami. Derrick C. Wan, MD, is the Johnson and Johnson Distinguished Professor of Surgery (Plastic and Reconstructive Surgery) at Stanford. Michael T. Longaker, MD, MBA, is the Deane P. Louise Mitchell Professor of Surgery and Co-Director of the Institute for Stem Cell Biology and Regenerative Medicine at Stanford.
References
- 1. Kulinets I. 1—Biomaterials and Their Applications in Medicine. In: Regulatory Affairs for Biomaterials and Medical Devices. (Amato SF, Ezzell RM. eds.) Cambridge, UK: Woodhead Publishing; 2015; pp 1–10. [Google Scholar]
- 2. Implantable Medical Devices Market: Global Industry Trends, Share, Size, Growth Opportunity and Forecast 2022–2027. IMARC Serv. Priv. Ltd. 2021. Available from: https://www.imarcgroup.com/implantable-medical-devices-market/toc [Last accessed: January 31, 2022]. [Google Scholar]
- 3. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol 2008;20:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wan D, Rohrich RJ. Revisiting the management of capsular contracture in breast augmentation: A systematic review. Plast Reconstr Surg 2016;137:826–841. [DOI] [PubMed] [Google Scholar]
- 5. Malahias M, Jordan DJ, Hughes LC, Hindocha S, Juma A. A literature review and summary of capsular contracture: An ongoing challenge to breast surgeons and their patients. Int J Surg Open 2016;3:1–7. [Google Scholar]
- 6. Keiler J, Schulze M, Sombetzki M, et al. Neointimal fibrotic lead encapsulation—Clinical challenges and demands for implantable cardiac electronic devices. J Cardiol 2017;70:7–17. [DOI] [PubMed] [Google Scholar]
- 7. Albrektsson T, Chrcanovic B, Östman P-O, Sennerby L. Initial and long-term crestal bone responses to modern dental implants. Periodontol 2000 2017;73:41–50. [DOI] [PubMed] [Google Scholar]
- 8. Gibon E, Córdova LA, Lu L, et al. The biological response to orthopedic implants for joint replacement. II: Polyethylene, ceramics, PMMA, and the foreign body reaction. J Biomed Mater Res B Appl Biomater 2017;105:1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci Transl Med 2014;6:265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sindrilaru A, Scharffetter-Kochanek K. Disclosure of the culprits: Macrophages-versatile regulators of wound healing. Adv Wound Care 2013;2:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phillipson M, Kubes P. The healing power of neutrophils. Trends Immunol 2019;40:635–647. [DOI] [PubMed] [Google Scholar]
- 12. Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial–cell interactions by adsorbed proteins: A review. Tissue Eng 2005;11:1–18. [DOI] [PubMed] [Google Scholar]
- 13. Gorbet MB, Sefton MV. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004;25:5681–5703. [DOI] [PubMed] [Google Scholar]
- 14. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, Castegna A. The metabolic signature of macrophage responses. Front Immunol 2019;10:1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jetten N, Verbruggen S, Gijbels MJ, et al. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014;17:109–118. [DOI] [PubMed] [Google Scholar]
- 17. Sheikh Z, Brooks PJ, Barzilay O, Fine N, Glogauer M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Mater (Basel) 2015;8:5671–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: Roles in homeostasis and disease. Annu Rev Immunol 2013;31:317–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin KE, García AJ. Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies. Acta Biomater 2021;133:4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noskovicova N, Hinz B, Pakshir P. Implant fibrosis and the underappreciated role of myofibroblasts in the foreign body reaction. Cells 2021;10:1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Driskell RR, Lichtenberger BM, Hoste E, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013;504:277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Driskell RR, Watt FM. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol 2015;25:92–99. [DOI] [PubMed] [Google Scholar]
- 23. Thompson SM, Phan QM, Winuthayanon S, Driskell IM, Driskell RR. Parallel single-cell multiomics analysis of neonatal skin reveals the transitional fibroblast states that restrict differentiation into distinct fates. J Invest Dermatol 2021;142:1812–1823.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mascharak S, DesJardins-Park H, Davitt M, et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 2021;372:eaba2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leavitt T, Hu MS, Borrelli MR, et al. Prrx1 fibroblasts represent a pro-fibrotic lineage in the mouse ventral dermis. Cell Rep 2020;33:108356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Phan QM, Sinha S, Biernaskie J, Driskell RR. Single-cell transcriptomic analysis of small and large wounds reveals the distinct spatial organization of regenerative fibroblasts. Exp Dermatol 2021;30:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abbasi S, Sinha S, Labit E, et al. Distinct regulatory programs control the latent regenerative potential of dermal fibroblasts during wound healing. Cell Stem Cell 2020;27:396–412.e6. [DOI] [PubMed] [Google Scholar]
- 28. Yejing G, Yuxuan M, Shiri G-C, et al. The aging skin microenvironment dictates stem cell behavior. Proc Natl Acad Sci U S A 2020;117:5339–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pakshir P, Noskovicova N, Lodyga M, et al. The myofibroblast at a glance. J Cell Sci 2020;133:ics227900. [DOI] [PubMed] [Google Scholar]
- 30. Shook BA, Wasko RR, Rivera-Gonzalez GC, et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 2018;362:eaar2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Philippeos C, Telerman SB, Oulès B, et al. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J Invest Dermatol 2018;138:811–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Griffin MF, desJardins-Park HE, Mascharak S, Borrelli MR, Longaker MT. Understanding the impact of fibroblast heterogeneity on skin fibrosis. Dis Model Mech 2020;13:dmm044164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ascensión AM, Fuertes-Álvarez S, Ibañez-Solé O, Izeta A, Araúzo-Bravo MJ. Human dermal fibroblast subpopulations are conserved across single-cell RNA sequencing studies. J Invest Dermatol 2021;141:1735–1744.e35. [DOI] [PubMed] [Google Scholar]
- 34. Zou Z, Long X, Zhao Q, et al. A single-cell transcriptomic atlas of human skin aging. Dev Cell 2021;56:383–397.e8. [DOI] [PubMed] [Google Scholar]
- 35. Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993;122:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ratner BD. Reducing capsular thickness and enhancing angiogenesis around implant drug release systems. J Control Release 2002;78:211–218. [DOI] [PubMed] [Google Scholar]
- 37. Coleman DJ, Sharpe DT, Naylor IL, Chander CL, Cross SE. The role of the contractile fibroblast in the capsules around tissue expanders and implants. Br J Plast Surg 1993;46:547–556. [DOI] [PubMed] [Google Scholar]
- 38. Mooney JE, Rolfe BE, Osborne GW, et al. Cellular plasticity of inflammatory myeloid cells in the peritoneal foreign body response. Am J Pathol 2010;176:369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Noronha Oliveira M, Schunemann WVH, Mathew MT, et al. Can degradation products released from dental implants affect peri-implant tissues? J Periodontal Res 2018;53:1–11. [DOI] [PubMed] [Google Scholar]
- 40. Welch NG, Winkler DA, Thissen H. Antifibrotic strategies for medical devices. Adv Drug Deliv Rev 2020;167:109–120. [DOI] [PubMed] [Google Scholar]
- 41. Vegas AJ, Veiseh O, Doloff JC, et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol 2016;34:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mackie G, Gao L, Yau S, Leslie DC, Waterhouse A. Clinical potential of immobilized liquid interfaces: Perspectives on biological interactions. Trends Biotechnol 2019;37:268–280. [DOI] [PubMed] [Google Scholar]
- 43. Stahnke T, Siewert S, Reske T, et al. Development of a biodegradable antifibrotic local drug delivery system for glaucoma microstents. Biosci Rep 2018;38:BSR20180628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Doloff JC, Veiseh O, de Mezerville R, et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nat Biomed Eng 2021;5:1115–1130. [DOI] [PubMed] [Google Scholar]
- 45. Tevis SE, Hunt KK, Miranda RN, et al. Breast implant-associated anaplastic large cell lymphoma: A prospective series of 52 patients. Ann Surg 2022;275:e245–e249. [DOI] [PubMed] [Google Scholar]
- 46. Noskovicova N, Schuster R, van Putten S, et al. Suppression of the fibrotic encapsulation of silicone implants by inhibiting the mechanical activation of pro-fibrotic TGF-β. Nat Biomed Eng 2021;5:1437–1456. [DOI] [PubMed] [Google Scholar]
- 47. Li AG, Quinn MJ, Siddiqui Y, et al. Elevation of transforming growth factor beta (TGFβ) and its downstream mediators in subcutaneous foreign body capsule tissue. J Biomed Mater Res Part A 2007;82A:498–508. [DOI] [PubMed] [Google Scholar]
- 48. Chen S-J, Yuan W, Mori Y, et al. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-β: involvement of Smad 3. J Invest Dermatol 1999;112:49–57. [DOI] [PubMed] [Google Scholar]
- 49. Anahita H, Jie S, Noopur T, et al. TGF-β promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85α. Sci Signal 2017;10:eaal4186. [DOI] [PubMed] [Google Scholar]
- 50. Györfi AH, Matei A-E, Distler JHW. Targeting TGF-β signaling for the treatment of fibrosis. Matrix Biol 2018;68–69:8–27. [DOI] [PubMed] [Google Scholar]
- 51. Xu X, Zheng L, Yuan Q, et al. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res 2018;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape—The rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol 2014;15:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aarabi S, Bhatt KA, Shi Y, et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J 2007;21:3250–3261. [DOI] [PubMed] [Google Scholar]
- 54. Hilborn J, Bjursten LM. A new and evolving paradigm for biocompatibility. J Tissue Eng Regen Med 2007;1:110–119. [DOI] [PubMed] [Google Scholar]
- 55. Sun Z, Guo SS, Fässler R. Integrin-mediated mechanotransduction. J Cell Biol 2016;215:445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jo J, Abdi Nansa S, Kim D-H. Molecular regulators of cellular mechanoadaptation at cell-material interfaces. Front Bioeng Biotechnol 2020;8:608569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gancedo M, Ruiz-Corro L, Salazar-Montes A, et al. Pirfenidone prevents capsular contracture after mammary implantation. Aesthetic Plast Surg 2008;32:32–40. [DOI] [PubMed] [Google Scholar]
- 58. He R, Lu Y, Ren J, et al. Decreased fibrous encapsulation and enhanced osseointegration in vitro by decorin-modified titanium surface. Colloids Surf B Biointerfaces 2017;155:17–24. [DOI] [PubMed] [Google Scholar]
- 59. Ji L, Wang T, Tian L, Song H, Gao M. Roxatidine inhibits fibrosis by inhibiting NF‑κB and MAPK signaling in macrophages sensing breast implant surface materials. Mol Med Rep 2020;21:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yi Z, Zeng J, Chen Z, et al. The role of verteporfin in prevention of periprosthetic capsular fibrosis: An experimental study. Aesthetic Surg J 2022;:sjac083. [DOI] [PubMed] [Google Scholar]
- 61. Eshraghi AA, Gupta C, Van De Water TR, et al. Molecular mechanisms involved in cochlear implantation trauma and the protection of hearing and auditory sensory cells by inhibition of c-jun-N-terminal kinase signaling. Laryngoscope 2013;123:S1–S14. [DOI] [PubMed] [Google Scholar]
- 62. Kuo Y-L, Jou I-M, Jeng S-F, et al. Hypoxia-induced epithelial-mesenchymal transition and fibrosis for the development of breast capsular contracture. Sci Rep 2019;9:10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang D, Chen Q, Bi Y, et al. Bio-inspired poly-DL-serine materials resist the foreign-body response. Nat Commun 2021;12:5327. [DOI] [PMC free article] [PubMed] [Google Scholar]