Abstract
Objective:
To evaluate safety and effectiveness of MiniMed™ 670G hybrid closed loop (HCL) in comparison with continuous subcutaneous insulin infusion (CSII) therapy for 6 months in persons with type 1 diabetes (T1D).
Methods:
Adults (aged 18–80 years), adolescents, and children (aged 2–17 years) with T1D who were using CSII therapy were enrolled and randomized (1:1) to 6 months of HCL intervention (n = 151, mean age of 39.9 ± 19.8 years) or CSII without continuous glucose monitoring (n = 151, 35.7 ± 18.4 years). Primary effectiveness endpoints included change in A1C for Group 1 (baseline A1C >8.0%), from baseline to the end of study, and difference in the end of study percentage of time spent below 70 mg/dL (%TBR <70 mg/dL) for Group 2 (baseline A1C ≤8.0%), to show superiority of HCL intervention versus control. Secondary effectiveness endpoints were change in A1C and %TBR <70 mg/dL for Group 2 and Group 1, respectively, to show noninferiority of HCL intervention versus control. Primary safety endpoints were rates of severe hypoglycemia and diabetic ketoacidosis (DKA).
Results:
Change in A1C and difference in %TBR <70 mg/dL for the overall group were significantly improved, in favor of HCL intervention. In addition, a significant mean (95% confidence interval) change in A1C was observed for both Group 1 (−0.8% [−1.1% to −0.4%], P < 0.0001) and Group 2 (−0.3% [−0.5% to −0.1%], P < 0.0001), in favor of HCL intervention. The same was observed for difference in %TBR <70 mg/dL for Group 1 (−2.2% [−3.6% to −0.9%]) and Group 2 (−4.9% [−6.3% to −3.6%]) (P < 0.0001 for both). There was one DKA event during run-in and six severe hypoglycemic events: two during run-in and four during study (HCL: n = 0 and CSII: n = 4 [6.08 per 100 patient-years]).
Conclusions:
This RCT demonstrates that the MiniMed 670G HCL safely and significantly improved A1C and %TBR <70 mg/dL compared with CSII control in persons with T1D, irrespective of baseline A1C level.
Keywords: Type 1 diabetes, Hybrid closed loop, A1C, Time below range, Time in range, Diabetes treatment satisfaction, Adult, Pediatric
Introduction
Findings from the Diabetes Control and Complications Trial (DCCT)1 and the follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) studies2–4 established that intensive insulin therapy for type 1 diabetes (T1D) significantly reduced development of chronic diabetes complications and mortality.5,6 Although the increased risk of severe hypoglycemia observed in the DCCT intensive insulin therapy group was shown to decline to a rate similar to that observed in the conventional therapy group over time,7 hypoglycemia exposure has remained a challenge for many living with T1D.
Thus, a first step in automated insulin delivery technology was reducing time spent in hypoglycemia by suspending insulin delivery at a given threshold of low sensor glucose (SG). The Medtronic MiniMed 530G and MiniMed Veo systems (Medtronic, Northridge, CA)8,9 provided this with “threshold suspend” and “low-glucose suspend,” and were available as early as 2013. The next step was suspending insulin delivery before a low SG threshold was reached, which was possible with the MiniMed 640G system10,11 and t:slim X2™ with Basal-IQ™ system (Tandem Diabetes Care, San Diego, CA).12 The randomized controlled trials of these predictive systems in children, adolescents, or adults demonstrated significantly reduced percentages of time spent below 70 mg/dL (%TBR <70 mg/dL) and below 54 mg/dL (%TBR <54 mg/dL), compared with sensor-augmented pump (SAP) therapy and without increasing time in hyperglycemia.
Toward a combined reduction in hyperglycemic excursion and hypoglycemia, system algorithms automatically delivering basal insulin, in addition to suspending insulin delivery when predicting hypoglycemia, were developed. The MiniMed 670G system was the first available hybrid closed-loop (HCL) therapy13 and uses a proportional integrated derivative-insulin feedback module algorithm that provides adaptive basal automated insulin delivery every 5 min based on current and predicted SG readings to achieve a glucose target of 120 mg/dL (or 150 mg/dL, if using the temporary target).
The single-arm, 3-month, pivotal trials of the MiniMed 670G system showed significantly reduced A1C and a significantly increased percentage of time in target range (%TIR, 70–180 mg/dL) in children,14,15 adolescents,16 and adults16 with T1D, compared with 2-week open-loop baseline. In addition, clinical MiniMed 670G system use was shown to significantly reduce or not change (i.e., in children) the percentage of time spent at <70 mg/dL (%TBR <70 mg/dL). Since then, real-world MiniMed 670G system use analyses of thousands living in the United States17,18 and throughout Europe19 have confirmed the findings from the pivotal trials and begun to highlight performance of the system in people with different levels of baseline glycemia.
In this study, we report on changes in A1C and %TBR <70 mg/dL in pediatric and adult persons with T1D randomized to MiniMed 670G HCL intervention versus continuous subcutaneous insulin infusion (CSII) control (without CGM) for 6 months, stratified on baseline A1C levels of >8% and ≤8.0%.
Methods
Study design
The Multicenter Trial in Adult and Pediatric Patients with T1D Using a HCL System and Control at Home trial (ClinicalTrials.gov NCT02748018) involves three separate randomized, parallel, adaptive study evaluations enrolling persons aged 2–80 years with T1D, to assess safety and effectiveness of MiniMed 670G HCL intervention when used for 6 months compared with control. Each evaluation includes a multiple daily injections (MDI), SAP, or CSII comparator. The present article reports on the HCL intervention versus CSII control evaluation of those who were enrolled from May 25, 2017 to October 6, 2020.
Review and Ethics Board approvals
Study enrollment occurred at 23 sites across the United States (n = 22) and Canada (n = 1) and complied with the Declaration of Helsinki, the United States Food and Drug Administration Code of Federal Regulations Title 21, the Medical Devices Regulations SOR/98-282, and applicable laws and requirements (federal and local). The protocol was approved by the central Internal Review Board (IRB) Advarra (formerly Quorum IRB) and local site IRBs, as applicable. Informed consent/assent was obtained in accordance with the CFR Title 21, Part 50 (U.S. only) or Tri-Council Policy Statement, Article 3.2 (Canada only) before study start.
A clinical events committee consisting of external physicians with expertise in endocrinology, diabetes technology, and diabetes management reviewed adverse events, diabetic ketoacidosis (DKA), severe hypoglycemic, and severe hyperglycemic events. A data monitoring committee reviewed study progress and safety.
Inclusion and exclusion criteria
In brief, study inclusion required participants (2–80 years of age) with a clinical diagnosis of T1D for ≥3 months before screening and who had requisite support, if 2–21 years of age, to participate successfully in the trial; a minimum daily insulin requirement of ≥8 U of insulin; ≥3 months of CSII therapy use before screening; and the ability to perform or reliably undergo ≥4 blood glucose meter (BGM) measurements daily. Exclusion criteria included participation in any previous automated insulin delivery therapy study; inability to tolerate tape adhesive around the glucose sensor; and any unresolved adverse skin condition around the glucose sensor or transcutaneous infusion set. There was no exclusion criterion for A1C and the complete list of study eligibility criteria is provided in Supplementary Table S1.
Study schedule and randomization
During run-in, informed consent/assent and screening were completed at Visit 1 (Supplementary Table S2). At Visit 2, all participants underwent study and CareLink™ Clinical training that included initiation of masked CGM (Guardian™ Sensor 3 glucose sensor with Guardian Link 3 transmitter [Medtronic]) for 2 weeks, while using their existing CSII diabetes management therapy. Bloodwork for a central laboratory A1C test was collected at either Visit 1 or Visit 2.
During the 2 weeks of masked CGM, participants were expected to demonstrate appropriate glucose sensor wear and perform requested daily BGM (CONTOUR®NEXT LINK 2.4 meter [Ascensia Diabetes Care, Parsippany, NJ]) measurements that were used for sensor calibration. The SG data captured during masked CGM provided baseline metrics for both arms. At the end of the run-in period (Visit 3), participants underwent computer-generated 1:1 randomization into HCL intervention or CSII control based on their baseline A1C such that Group 1 comprised participants with baseline A1C >8.0% and Group 2 included only those with baseline A1C ≤8.0%.
For the study period, the HCL intervention arm started integrated CGM (Visit 4), enabled Auto Mode at least 7 days later (Visit 5), and continued MiniMed 670G system use for 6 months. Follow-up office and phone visits occurred until the end of the study period (Visit 9), when bloodwork for a central laboratory A1C test was collected and study questionnaires were completed. The CSII control arm underwent pump settings review (Visit 4) and continued MiniMed 670G pump use with follow-up office or phone visits for 6 months without CGM. The control arm started 2 weeks of masked CGM that ended at Visit 9. Similar to the HCL intervention arm, bloodwork for a central laboratory A1C test was collected and study questionnaires were completed at Visit 9.
System settings
For the HCL intervention arm, the automated basal glucose target was 120 mg/dL with an allowed temporary target of 150 mg/dL. It was recommended to set the high SG alert at 300 mg/dL and the low SG alert at 70 mg/dL. For participants 2–6 years of age, the recommended low SG alert was 80 mg/dL and advised to be no lower than 70 mg/dL. The insulin-to-carbohydrate ratios and active insulin time were adjusted as needed at the investigator's discretion. For the CSII control arm, insulin-to-carbohydrate ratios, active insulin time, and basal rates were adjusted as needed at the investigator's discretion.
Statistical and descriptive analyses
Primary effectiveness endpoints underwent hierarchical analyses that included determining superiority of the HCL intervention compared with CSII control in reducing A1C from baseline to the end of the study period in Group 1 (baseline A1C >8.0%) and determining superiority of the HCL intervention compared with CSII control in reducing %TBR <70 mg/dL at the end of the study period in Group 2 (baseline A1C ≤8.0%).
Secondary effectiveness endpoints included determining noninferiority of the HCL intervention compared with the CSII control in reducing %TBR <70 mg/dL at the end of the study for Group 1 and determining noninferiority of the HCL intervention compared with the CSII control in reducing A1C from baseline to the end of the study period in Group 2. Additional secondary effectiveness endpoints were explored for the overall group (Group 1 and Group 2 combined) and included the difference between HCL intervention and CSII control end-of-study %TBR <70 mg/dL and %TIR (70–180 mg/dL) during the overall (24 h day) and nighttime periods (12:00 AM–5:59 AM) and change in A1C.
For primary and secondary endpoints involving A1C, multiple imputation (MI) was applied for missing A1C data using an imputation regression method (ŷ + z) where ŷ was the predicted value, z was a standard normal random variable, and was the estimated standard deviation (SD) from the regression model. The independent variables in the model were age, gender, baseline A1C, diabetes duration, BMI, and the treatment group indicator. Imputations were conducted five times using the MI procedure and results were combined to form one inference using the MIANALYZE procedure in SAS™ 9.4 (SAS Institute, Cary, NC).
Detailed sample size estimations for the statistical analyses of the primary effectiveness endpoints are shown in Supplementary Table S3. Comparisons of mean (95% confidence interval [CI]) difference in change in A1C, difference in end of study %TBR, and %TIR between the HCL intervention arm and the CSII control arm within Group 1 and Group 2 were analyzed with one-way analysis of variance (ANOVA) (SAS 9.4) and P < 0.05 was considered statistically significant.
The mean SG, SD of SG, coefficient of variation (CV) of SG, and percentage of time spent at additional SG ranges (i.e., <54 mg/dL, <70 mg/dL, 70–180 mg/dL, >180 mg/dL, >250 mg/dL, and >300 mg/dL) were assessed for each group to determine the mean difference (95% CI) between HCL intervention and CSII control. Scores for the diabetes treatment satisfaction questionnaire status (DTSQs) and change (DTSQc) that were completed by adults (≥18 years of age), teens (baseline age of 13–17 years), and parents of younger children were assessed for each group to determine the mean difference (95% CI) between HCL intervention and CSII control. Exploratory descriptive analysis of glycemic outcomes by age group (2–17 years and 18–90 years) was also determined for each group.
Safety endpoints
Safety endpoints were analyzed based on the rates (100 patient-years) of events and included DKA, defined as blood glucose >250 mg/dL, arterial pH <7.3, bicarbonate <15 mEq/L, and moderate ketonuria or ketonemia, requiring treatment within a health care facility; and severe hypoglycemia, defined as an event requiring the active assistance of another person to administer carbohydrate, glucagon, or other resuscitative actions due to altered participant consciousness. In addition, all serious adverse events, serious adverse device effects (SADEs), unanticipated adverse device effects (UADEs), and deaths were reported.
Results
Study disposition and participant baseline demographics and characteristics
Of the 321 enrolled, there were 13 screen failures and two early withdrawals (Supplementary Fig. S1). A total of 306 entered the run-in period and 302 were randomized to either the HCL intervention arm (n = 151; n = 78 in Group 1 and n = 73 in Group 2) or CSII control arm (n = 151; n = 77 in Group 1 and n = 74 in Group 2). The Group 1 baseline A1C ranged from 8.1% to 15.3% and that for Group 2 ranged from 5.3% to 8.0%.
Ninety-two percent of randomized participants (140 within the HCL intervention arm [n = 74 in Group 1 and n = 66 in Group 2] and 138 within the CSII control arm [n = 73 in Group 1 and n = 65 in Group 2]) completed the study. The baseline demographics and characteristics of randomized participants are shown in Table 1. The baseline demographics and characteristics of participants by age group (2–17 years and 18–80 years) are listed in Supplementary Tables S4 and S5, respectively.
Table 1.
Demographics and Characteristics at Randomization, by Baseline A1C Group
| Group 1 (baseline A1C >8%) |
Group 2 (baseline A1C ≤8%) |
|||
|---|---|---|---|---|
| HCL (n = 78) | CSII (n = 77) | HCL (n = 73) | CSII (n = 74) | |
| Age, years | 38.0 ± 20.7 | 31.2 ± 17.5 | 41.9 ± 18.7 | 40.4 ± 18.2 |
| Sex | ||||
| Female | 33 (42.3%) | 42 (54.5%) | 38 (52.1%) | 51 (68.9%) |
| Male | 45 (57.7%) | 35 (45.5%) | 35 (47.9%) | 23 (31.1%) |
| Diabetes duration, years | 18.7 ± 11.8 | 16.4 ± 11.1 | 24.5 ± 14.8 | 22.9 ± 14.2 |
| A1C, % | 9.2 ± 1.2 | 9.0 ± 0.9 | 7.3 ± 0.6 | 7.2 ± 0.5 |
| BMI, kg/m2 | 26.0 ± 6.4 | 27.0 ± 7.2 | 27.7 ± 5.0 | 26.9 ± 6.7 |
| Height, cm | 168.5 ± 12.6 | 167.5 ± 12.1 | 171.9 ± 11.3 | 166.0 ± 12.2 |
| Weight, kg | 75.1 ± 22.4 | 76.9 ± 25.1 | 82.1 ± 17.8 | 75.2 ± 22.7 |
| Race | ||||
| African American/Black | 2 (2.6%) | 1 (1.3%) | 2 (2.7%) | 2 (2.7%) |
| American Indian/Alaska Native | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.4%) |
| American Indian/Alaska Native; White | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.4%) |
| Asian | 1 (1.3%) | 0 (0.0%) | 1 (1.4%) | 3 (4.1%) |
| Other | 1 (1.3%) | 0 (0.0%) | 1 (1.4%) | 1 (1.4%) |
| White | 74 (94.9%) | 76 (98.7%) | 69 (94.5%) | 66 (89.2%) |
Data are presented as mean ± SD or n (%).
BMI, body mass index; CSII, continuous subcutaneous insulin infusion; HCL, hybrid closed loop; SD, standard deviation.
Glycemic endpoints
Table 2 shows that Group 1 demonstrated a significant mean (95% CI) difference in A1C change between HCL intervention and CSII control that favored the intervention arm (−0.8% [−1.1% to −0.4%], P < 0.0001). For Group 2, there was a significant difference (−4.9% [−6.3% to −3.6%], P < 0.0001) in the end-of-study %TBR <70 mg/dL between arms, where the duration of time in hypoglycemia was 0.6 ± 0.5 h/day for HCL intervention, but 1.8 ± 1.3 h/day for CSII control. A significant difference in %TBR <70 mg/dL was also observed with HCL intervention for Group 1 (P < 0.0001) (Table 2), where the duration of time spent in hypoglycemia was 0.6 ± 0.6 h/day, but 1.1 ± 1.2 h/day for CSII control. The significant difference in A1C observed for Group 2 (−0.3% [−0.5% to −0.1%]) also favored the HCL intervention arm (P < 0.0001).
Table 2.
Difference in Primary and Secondary Endpoints Between the Hybrid Closed-Loop Intervention and Continuous Subcutaneous Insulin Infusion Control
| HCL (age 39.9 ± 19.8 years) |
CSII (age 35.7 ± 18.4 years) |
Difference (HCL − CSII) | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Study end | Change | n | Baseline | Study end | Change | |||
| Group 1 (baseline A1C >8.0%) | ||||||||||
| Primary endpoint | ||||||||||
| A1C, %a | 78 | 9.2 ± 1.2 | 7.7 ± 0.9 | −1.6 ± 1.3 | 77 | 9.0 ± 0.9 | 8.2 ± 0.9 | −0.8 ± 0.9 | −0.8 (−1.1 to −0.4) | <0.0001b |
| Secondary endpoint | ||||||||||
| TBR <70 mg/dL, %c | 78 | 4.4 ± 4.4 | 2.3 ± 2.3 | NA | 77 | 4.5 ± 4.9 | 4.6 ± 5.2 | NA | −2.2 (−3.6 to −0.9) | <0.0001d |
| Group 2 (baseline A1C ≤8.0%) | ||||||||||
| Primary endpoint | ||||||||||
| TBR <70 mg/dL, %a | 73 | 8.2 ± 6.0 | 2.4 ± 1.9 | NA | 74 | 8.7 ± 6.0 | 7.3 ± 5.3 | NA | −4.9 (−6.3 to −3.6) | <0.0001d |
| Secondary endpoint | ||||||||||
| A1C, %e | 73 | 7.3 ± 0.6 | 7.0 ± 0.6 | −0.4 ± 0.5 | 74 | 7.2 ± 0.5 | 7.2 ± 0.7 | 0.0 ± 0.6 | −0.3 (−0.5 to −0.1) | <0.0001b |
Primary and secondary glycemic endpoints that included change in A1C and %TBR <70 mg/dL (based on a baseline A1C of >8.0% [Group 1] or a baseline A1C of ≤8.0% [Group 2]) are shown. All analyses for A1C compared the change in A1C difference between the HCL intervention and CSII control (i.e., difference [HCL − CSII]) and all analyses for %TBR <70 mg/dL compared the end-of-study difference in %TBR <70 mg/dL between the HCL intervention and CSII control (one-way ANOVA).
Data are shown as mean ± SD or mean (95% CI) to one decimal place.
The n (number of randomized participants) for study end and change may differ due to study period withdrawals.
Time in HCL was 75.7% ± 17.9% and 83.9% ± 12.9% for the Group 1 HCL arm and Group 2 HCL arm, respectively.
Superiority test.
Comparison of change in A1C between HCL intervention and CSII control.
Noninferiority test with 2% margin.
Comparison of end-of-study %TBR <70 mg/dL between HCL intervention and CSII control.
Noninferiority test with 0.4% margin.
ANOVA, analysis of variance; CI, confidence interval; NA, not applicable; TBR, time below range.
The mean (95% CI) difference in additional secondary glycemic endpoints between the HCL intervention and CSII control arms for the combined Group 1 and Group 2 were determined for the nighttime and 24 h day periods (Table 3). Nighttime %TBR <70 mg/dL was significantly reduced and nighttime %TIR was significantly increased with HCL intervention compared with CSII control. The 24 h day %TBR <70 mg/dL was also significantly reduced with HCL intervention, such that time in hypoglycemia was 0.6 ± 0.5 h/day compared with 1.4 ± 1.3 h/day for the CSII control arm. Improvement in 24 h day %TIR was also observed with HCL intervention compared with CSII control, such that 16.2 ± 2.6 h/day versus 13.3 ± 3.6 h/day were spent in target range, respectively (Table 3).
Table 3.
Difference in Additional Secondary Endpoints Between the Hybrid Closed-Loop Intervention and Continuous Subcutaneous Insulin Infusion Control
| HCL (age 39.9 ± 19.8 years) |
CSII (age 35.7 ± 18.4 years) |
Difference (HCL − CSII) | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Study end | Change | n | Baseline | Study end | Change | |||
| Group 1 + Group 2 | ||||||||||
| TBR <70 mg/dL, %a (nighttime) | 151 | 8.7 ± 10.3 | 2.3 ± 3.3 | NA | 151 | 8.0 ± 9.7 | 7.7 ± 9.4 | NA | −5.4 (−7.1 to −3.7) | <0.0001b |
| TBR <70 mg/dL, %a | 151 | 6.2 ± 5.5 | 2.4 ± 2.1 | NA | 151 | 6.5 ± 5.8 | 6.0 ± 5.4 | NA | −3.6 (−4.6 to −2.6) | <0.0001b |
| TIR, %a (nighttime) | 151 | 53.6 ± 20.7 | 73.8 ± 14.3 | NA | 151 | 51.9 ± 17.5 | 55.0 ± 20.8 | NA | 18.8 (14.5, 23.1) | <0.0001b |
| TIR, %a | 151 | 52.5 ± 16.1 | 67.4 ± 10.8 | NA | 151 | 51.8 ± 13.1 | 55.4 ± 15.0 | NA | 12.0 (8.8, 15.1) | <0.0001b |
| A1C, %a | 151 | 8.3 ± 1.4 | 7.3 ± 0.8 | −1.0 ± 1.2 | 151 | 8.1 ± 1.1 | 7.7 ± 1.0 | −0.4 ± 0.9 | −0.6 (−0.8 to −0.3) | <0.0001c |
Additional secondary glycemic endpoints that included the %TBR <70 mg/dL, %TIR, and change in A1C (Groups 1 + 2 combined) are shown. All analyses for %TBR <70 mg/dL and %TIR compared the end-of-study difference in %TBR <70 mg/dL and %TIR between the HCL intervention and CSII control (one-way ANOVA) and the analysis for A1C compared the change in A1C difference between the HCL intervention and CSII control (i.e., difference [HCL − CSII]).
Data are shown as mean ± SD or mean (95% CI) to one decimal place.
The n (number of randomized participants) for study end and change may differ due to study period withdrawals.
Time in HCL was 79.7% ± 16.2%.
Nighttime was 12:00 AM–5:59 AM.
Superiority test.
Comparison of end-of-study %TBR <70 mg/dL or %TIR between HCL intervention and CSII control.
Comparison of change in A1C between HCL intervention and CSII control.
TIR, time in target range.
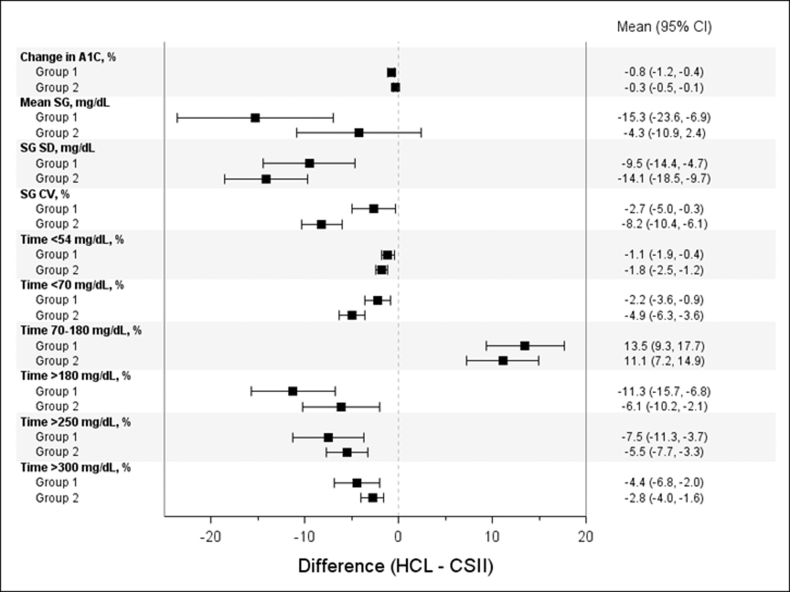
The reduction in overall A1C was significantly greater for those randomized to HCL intervention compared with CSII control (Δ = −1.0% ± 1.2% vs. Δ = −0.4% ± 0.9%, P < 0.0001). The mean (95% CI) difference in mean SG, SD of SG, CV of SG, and the percentage of time spent at additional SG ranges between the HCL intervention and CSII control arms are shown by baseline A1C group (Fig. 1).
FIG. 1.
Difference in glycemic endpoints between the HCL intervention and CSII control arms, by baseline A1C group. The mean (95% CI) difference in change in A1C, mean SG, SG SD, SG CV, and the percentage of time spent in SG ranges between the HCL intervention arm and CSII control arm are shown for Group 1 (baseline A1C >8.0%) and Group 2 (baseline A1C ≤8.0%). CSII, continuous subcutaneous insulin infusion; CV, coefficient of variation; HCL, hybrid closed-loop; SD, standard deviation; SG, sensor glucose.
Exploratory analysis of glycemic endpoints of participants 2–17 years of age (Supplementary Table S6) and 18–80 years of age (Supplementary Table S7) are listed by group. For the HCL intervention arm, the Group 1 (n = 21) and Group 2 (n = 7) younger cohorts spent 67.8% ± 21.9% and 68.8% ± 13.8% of time in HCL, respectively, and showed a trend in increased %TIR and reduced %TBR <70 mg/dL. For the older cohort, similar trends in %TIR and %TBR <70 mg/dL with HCL intervention were observed for Group 1 (n = 57, 78.7% ± 15.4% of time in HCL) and Group 2 (n = 66, 85.3% ± 12.0% of time in HCL).
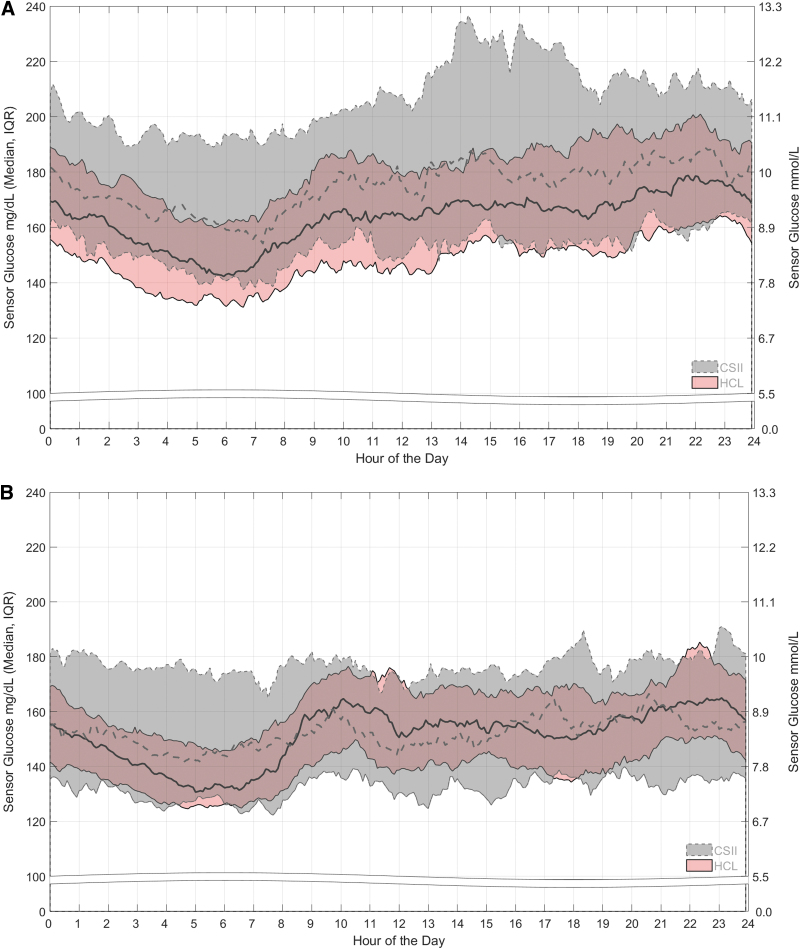
The 24 h day SG profiles during HCL intervention and CSII control are shown for each group (Figs. 2A, B). For Group 1, the median of SG during CSII use remained above the median of SG for the HCL intervention arm, with an upper interquartile range (IQR) of SG that reached 200 mg/dL for most of the 24 h day. For Group 2, the median and IQR of SG during CSII use were lower than those for Group 1 and were relatively steady throughout the 24 h day. While the IQR of SG (124.5–185.3 mg/dL) for the HCL intervention arm fell primarily within that of the CSII control arm, it was reduced (124.5–157.0 mg/dL) during the early morning (2:00 AM–8:00 AM).
FIG. 2.
SG profiles for the 24 h day. The medians and IQRs of SG after randomization to the HCL intervention arm (pink band with solid lines) or the CSII control arm (gray band with dashed lines) are shown across the 24 h day for (A) Group 1 with baseline A1C >8.0% and (B) Group 2 with baseline A1C ≤8.0%. For both arms, the SG data are based on the 2 weeks before the 6-month follow-up visit (Visit 9). IQR, interquartile range.
Participant-reported outcome measures
A summary of the total diabetes treatment satisfaction (DTSQs and DTSQc) scores, in addition to the perceived diabetes control, frequency of hyperglycemia, and frequency of hypoglycemia scores, reported by adult and teen participants after randomization are provided in Supplementary Table S8 (Group 1) and Supplementary Table S9 (Group 2), respectively. For both summaries, the mean ± SD change and mean difference (95% CI) between the HCL intervention and CSII control arms are also shown. A summary of the same scores, in addition to the scores for the effects of treatment on the parent's life, is listed in Supplementary Table S10.
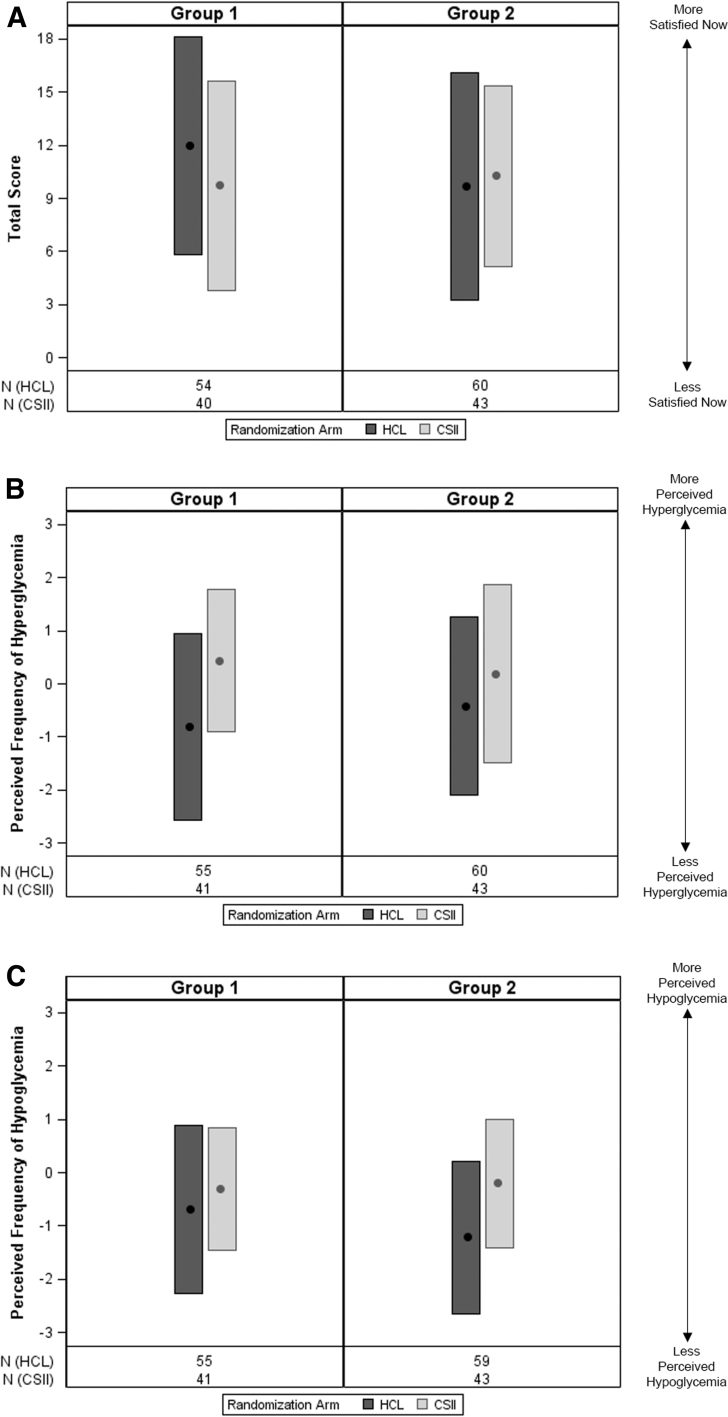
Figure 3 shows the total score and perceived frequency of hyperglycemia and hypoglycemia results of the DTSQc completed at study end by participants ≥18 years of age. A higher total DTSQc score for the Group 1 HCL intervention arm was observed, compared with the CSII control arm (Supplementary Table S8), while a lower total score was observed for the Group 2 HCL intervention arm (Supplementary Table S9). For both Group 1 and Group 2, the end-of-study perceived frequency of hyperglycemia score was lower during HCL intervention. The reduced perceived frequency of hypoglycemia score in the HCL intervention arm versus CSII control arm was more apparent for Group 2.
FIG. 3.
Diabetes treatment satisfaction and perceived frequency of hyperglycemia and hypoglycemia scores at the end of study, in adult participants. The mean and SD of the DTSQc (A) total score, (B) perceived frequency of hyperglycemia score and (C) perceived frequency of hypoglycemia score at the end of the study are shown for participants ≥18 years of age randomized to the HCL intervention arm or the CSII control arm for Group 1 (baseline A1C >8.0%) and Group 2 (baseline A1C ≤8.0%). The total DTSQc score ranged from −18 to +18, where a higher value indicated “more satisfied.” The perceived frequency of hypoglycemia/hyperglycemia scores ranged from −3 to +3, where a higher value indicated “more perceived hyperglycemia/hypoglycemia.” DTSQc, Diabetes Treatment Satisfaction Questionnaire-change.
Safety endpoints
There was one DKA event (run-in period) and a total of six severe hypoglycemic events. Two severe hypoglycemic events were during the run-in period and four were during the study period (HCL: 0 and CSII: 4 [6.08 per 100 patient-years]). There were no SADEs, UADEs, or deaths.
Discussion
This multicenter randomized controlled trial showed that 6-month MiniMed 670G HCL use, compared with CSII control, provided clinically significant improvement in A1C in the overall group of participants with T1D, in addition to significantly increased %TIR and significantly reduced %TBR <70 mg/dL. Study findings also indicate that HCL intervention in comparison to CSII provided not only significant glycemic benefits but was safe, as demonstrated by no DKA event and no severe hypoglycemic event during HCL use. Relatively similar improvements in overall group A1C, %TIR, and %TBR <70 mg/dL were reported in one other RCT of 6-month MiniMed 670G system use in adults randomized to HCL intervention (89% of time in HCL) versus control (either CSII or MDI).20
Study of the recently approved Omnipod™ 5 system21 (Insulet Corporation, Acton, MA) that provides automatic basal insulin delivery every 5 min but with a personalized model predictive control algorithm22 also demonstrated glycemic benefits that included increased %TIR and reductions in A1C in children, adolescents, and adults with T1D. As most of the children in both system pivotal trials had a low %TBR <70 mg/dL at baseline, the time at level 1 hypoglycemia was either reduced or unchanged. Nevertheless, these automated basal insulin delivery investigations of young and adult T1D participants in different studies have confirmed HCL safety and effectiveness.
The present RCT evaluated MiniMed 670G system use outcomes based on participant baseline A1C, which allowed determination of HCL impact on the varied glycemic range often observed in many persons living with T1D. In participants with an A1C >8.0% before randomization, clinically significant improvements in A1C and %TBR <70 mg/dL were observed. For those with the lower baseline A1C ≤8.0%, reductions in A1C and %TBR <70 mg/dL were statistically and clinically significant, respectively. The latter finding is especially important as this group had a mean %TBR <70 mg/dL of 8.2% (2.0 ± 1.4 h/day) at baseline that reduced to 2.4% (0.6 ± 0.5 h/day) by study end.
The 24 h day SG profiles of Group 1 and Group 2 participants provided a distinct visual of the effects of HCL intervention compared with CSII control. The SG profile of Group 1 participants using CSII displayed high SG excursions that were reduced with HCL intervention, without increasing hypoglycemia. Such reductions in hyperglycemic excursion have also been shown in other investigations of MiniMed 670G HCL versus different insulin delivery therapies.14–16,20 For the present study's Group 2 participants, who had lower SG IQRs compared to those of Group 1 participants, HCL intervention substantially reduced overnight hyperglycemia and minimized hypoglycemia throughout most of the 24 h day.
Participant-reported outcomes
With the expansion of automated insulin delivery therapy use and the reported improvement in A1C, %TIR, %TBR, and %TAR with different systems,23 there have been prospective20,24–28 and retrospective29,30 studies that assessed psychosocial participant-reported outcomes (PROs) compared with control (either MDI, CSII, or SAP with and without low-glucose management). Although PROs were based on persons from a wide age range, not every study has determined significant between-arm changes or differences. Regarding diabetes treatment satisfaction and the MiniMed 670G HCL system, McAuley et al. assessed participant total DTSQs score and determined an end-of-study mean (95% CI) difference of only 1.0 (−0.8 to 2.7) between HCL intervention and control that was not statistically significant, although participants reported significantly greater positive well-being with HCL use (P < 0.0048).20
Diabetes treatment satisfaction outcomes in the present study underwent descriptive analysis, and the end-of-study mean (95% CI) difference between the HCL intervention and CSII control adult DTSQs scores were low for Group 1 and even lower for Group 2. The difference in the DTSQc score for Group 1 was also low and that for Group 2, lower. These observations between groups may be, in part, due to a relatively high degree of satisfaction in the control arm or specific rigors of a clinical trial involving a new diabetes management technology, as suggested by McAuley et al. authors. However, others have reported greater expectations of HCL therapy in persons with more ideal glycemic management at baseline,31,32 which aligns with what was observed in Group 2 participants of the present study.
There was only one DKA event (prerandomization) and six severe hypoglycemic events, none of which occurred during 6-month HCL use. In contrast, the McAuley et al. 6-month RCT reported three DKA events (HCL = 1 and Control = 2) and 15 severe hypoglycemic events (HCL = 8 and Control = 7).20 Although the authors mentioned that the proportion of participants using MDI (51%) versus CSII, in the control group, required more days (median of 28 vs. 14 days) before HCL initiation and that run-in comprised detailed diabetes self-management training, it is not clear as to what factors may have contributed to the higher event rates. Nevertheless, DKA and severe hypoglycemic events observed in the present study aligned with the safety profile demonstrated for HCL systems as reported in systematic metanalysis reviews of closed-loop therapy.33,34
A limitation of the present study was that enrollment did not reflect estimates of diagnosed T1D prevalence in Hispanic (0.5% [0.3%–0.7%]), Asian (0.2% [0.1%–0.4%]), and non-Hispanic black (0.4% [0.2%–0.6%]) adults in the United States,35 which are likely underestimates today. This highlights an apparent need to address shortfalls of underrepresented groups in diabetes technology trials. It is also important to note that while there have been increased recommendations encouraging CGM use with or without CSII,36,37 the current study focused on a CSII control, as many with T1D may be using insulin pump therapy alone.38,39
However, the current study evaluation is one of three that are investigating glycemic and safety outcomes after randomization to 6-month HCL intervention versus 6-month control. The evaluations of the MDI and SAP control cohorts will provide further evidence on the impact of HCL versus a wider spectrum of insulin delivery therapy.
Strengths of the current study include the randomized controlled design and the large number of participants representing 23 sites that included children, adolescents, and adults who were relatively age- and diabetes duration-matched within each study arm. An important strength, not previously analyzed, was the powered assessment of 6-month HCL therapy versus CSII control on suboptimal baseline glycemia. While the 1:1 randomization was based solely on baseline A1C level, the between-arm assessment was not restricted by an A1C exclusion criterion, which allowed determination of HCL therapy benefits for a range of glycemic outcomes.
Conclusion
The present study further supports the advantages of HCL therapy in children, adolescents, and adults with T1D and that the MiniMed 670G system safely provides significantly improved A1C and TIR, while significantly reducing time in hypoglycemia. With HCL system advancements in automated dose correction and meal detection, closed-loop therapies will continue to improve glycemia, reduce diabetes management burden, and improve quality of life.
Supplementary Material
Acknowledgments
The authors thank the participants and their families for devoting time and effort to the study. The authors also thank the investigational coordinators and staff for their support and contributions to the study and Zheng Dai, PhD (Medtronic) for help with statistical analyses.
Contributor Information
Adult and Pediatric MiniMed™ HCL Outcomes 6-month RCT: HCL versus CSII Control Study Group:
Ashleigh Downs, Samantha Lange, Emily Jost, Angela Karami, John “Chip” H. Reed, Donna Hamill, Vinaya Simha, Donna Desjardins, Shelly McCrady Spitzer, Corey Reid, Ravinderjeet Kaur, Joann Malone, Mark Sulik, Mary Halvorson, Laya Ekhlaspour, Christine Weir, Luis Casaubon, Lynn Ang, Kara Mizokami-Stout, Cindy Plunkett, Brittany Williams, Carol L. Recklein, and Mary Jane Clifton
Collaborators: for the Adult and Pediatric MiniMed™ HCL Outcomes 6-month RCT: HCL versus CSII Control Study Group and Adult and Pediatric MiniMed™ HCL Outcomes 6-month RCT: HCL versus CSII Control Study Group
Authors' Contributions
The principal investigator authors provided substantial contributions to the acquisition and interpretation of data, and critical review of the article to its finalized and submitted version. The Medtronic employee authors (A.K., C.L., B.M., T.L.C., J.S., S.W.L., A.S.R., and R.A.V.) contributed substantially to the study design development, data analyses and validation, and/or interpretation of data; and drafting or critical review of the article to its finalized and submitted version.
Declarations
Copyrights in the DTSQ for adults, teenagers (DTSQ-Teen), and parents (DTSQ-Parent) in both status (s) and change (c) versions are owned by Professor Clare Bradley and licensed by Health Psychology Research Ltd. Medtronic obtained license for use of these questionnaires in the present study. Portions of data within this article were presented at the 15th Advanced Technologies & Treatments for Diabetes Congress (Barcelona, Spain, 2022).
Adult and Pediatric MiniMed™ HCL Outcomes 6-month Randomized Controlled Trial: HCL versus CSII Control Study Group: Atlanta Diabetes Associates (Atlanta, Georgia, USA): Bruce W. Bode, MD, FACE. Barbara Davis Center for Diabetes (Aurora, Colorado, USA): Satish K. Garg, MD; Christie Beatson, MS, RD, CDCES and Ashleigh Downs, BS. Barbara Davis Center for Childhood Diabetes (Aurora, Colorado, USA): Robert H. Slover, MD; Greg P. Forlenza, MD; Cari Berget, RN, CDCES; Emily Boranian, RN; Samantha Lange, MSN, FNP-C, CDCES; Emily Jost, RD, CDCES and Angela Karami, BS. Children's Hospital of Eastern Ontario (Ottawa, Ontario, CA): Margaret L. Lawson, MD, FRCPC. Diabetes and Glandular Disease Clinic (San Antonio, Texas, USA): Mark Kipnes, MD. Diablo Clinical Research (Walnut Creek, California, USA): Mark P. Christiansen, MD. Endocrine Research Solutions, Inc. (Roswell, Georgia, USA): John “Chip” H. Reed, MD. Grunberger Diabetes Institute (Bloomfield Hills, Michigan, USA): George Grunberger, MD, FACP, MACE and Donna Hamill, DNP. Indiana University—Riley Hospital for Children (Indianapolis, Indiana, USA): Linda A. DiMeglio, MD. Mayo Clinic (Rochester, Minnesota, USA): Yogish C. Kudva, MBBS; Vinaya Simha, MD; Donna Desjardins APRN, CNP, MS; Shelly McCrady Spitzer, MS; Corey Reid, BS and Ravinderjeet Kaur, MBBS. Medtronic (Northridge, California, USA): Ashleigh Keiter, MS; Chenxiao Ling, PhD; Briggitte Marinos, MS; Toni L. Cordero, PhD; John Shin, PhD, MBA; Scott W. Lee, MD; Andrew S. Rhinehart, MD and Robert A. Vigersky, MD. Park Nicollet International Diabetes Center (Minneapolis, Minnesota, USA): Anders L. Carlson, MD and Amy B. Criego, MD, MS. Rainier Clinical Research Center (Renton, Washington, USA): Ronald L. Brazg, MD, FACE. Rocky Mountain Diabetes and Osteoporosis Center (Idaho Falls, Idaho, USA): David R. Liljenquist, MD; Joann Malone, CCRC and Mark Sulik, PharmD, CCRP. Scripps Whittier Diabetes Institute (La Jolla, California, USA): Athena Philis-Tsimikas, MD. SoCal Diabetes (Torrance, California, USA): Kevin B. Kaiserman, MD and Mary Halvorson, RN, MSN, CDE. Stanford University School of Medicine (Stanford, California, USA): Bruce A. Buckingham, MD; Laya Ekhlaspour, MD; Marissa Town, RN, CDE and Christine Weir, BS. Texas Diabetes and Endocrinology (Austin, Texas, USA): Luis Casaubon, MD. SUNY Upstate Medical University (Syracuse, New York, USA): Ruth Weinstock, MD, PhD. University of Michigan Health System—University Hospital (Ann Arbor, Michigan, USA): Rodica Pop-Busui, MD, PhD; Lynn Ang, MD; Kara Mizokami-Stout, MD; Cindy Plunkett, RN and Brittany Williams, BS. University of South Dakota—Sanford Research (Sioux Falls, South Dakota, USA): Kurt J. Griffin, PhD, MD. University of Washington (Seattle, Washington, USA): Irl B. Hirsch, MD, MACP. Washington University School of Medicine (St. Louis, Missouri, USA): Janet B. McGill, MD, MA, FACE, FACP; Carol L. Recklein, BSN, CDCES, MHS and Mary Jane Clifton, BA, CCRC.
Author Disclosure Statement
All principal investigator authors received research support from Medtronic to conduct the clinical trial at their respective investigational site. A.K., C.L., B.M., T.L.C., J.S., S.W.L., A.S.R., and R.A.V. are or were employees of Medtronic.
Funding Information
Medtronic supported and funded this study.
Supplementary Material
References
- 1. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. : The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 2. Epidemiology of Diabetes Interventions and Complications (EDIC): Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nathan DM, Cleary PA, Backlund JY, et al. : Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. White NH, Cleary PA, Dahms W, et al. : Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Pediatr 2001;139:804–812. [DOI] [PubMed] [Google Scholar]
- 5. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group: Mortality in type 1 diabetes in the DCCT/EDIC versus the general population. Diabetes Care 2016;39:1378–1383.27411699 [Google Scholar]
- 6. Writing Group for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group, Orchard TJ, Nathan DM, et al. : Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gubitosi-Klug RA, Braffett BH, White NH, et al. : Risk of severe hypoglycemia in type 1 diabetes over 30 years of follow-up in the DCCT/EDIC study. Diabetes Care 2017;40:1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weiss R, Garg SK, Bode BW, et al. : Hypoglycemia reduction and changes in hemoglobin A1c in the ASPIRE in-home study. Diabetes Technol Ther 2015;17:542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riemsma R, Corro Ramos I, Birnie R, et al. : Integrated sensor-augmented pump therapy systems [the MiniMed(R) Paradigm Veo system and the Vibe and G4(R) PLATINUM CGM (continuous glucose monitoring) system] for managing blood glucose levels in type 1 diabetes: a systematic review and economic evaluation. Health Technol Assess 2016;20:v–xxxi, 1–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhong A, Choudhary P, McMahon C, et al. : Effectiveness of automated insulin management features of the MiniMed(R) 640G sensor-augmented insulin pump. Diabetes Technol Ther 2016;18:657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abraham MB, Nicholas JA, Smith GJ, et al. : Reduction in hypoglycemia with the predictive low-glucose management system: a long-term randomized controlled trial in adolescents with type 1 diabetes. Diabetes Care 2018;41:303–310. [DOI] [PubMed] [Google Scholar]
- 12. Forlenza GP, Li Z, Buckingham BA, et al. : Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care 2018;41:2155–2161. [DOI] [PubMed] [Google Scholar]
- 13. Food and Drug Administration—Center for Devices and Radiological Health. MiniMed 670G system approval letter (P160017). September 28, 2016. www.accessdata.fda.gov/cdrh_docs/pdf16/P160017a.pdf Accessed December 13, 2022.
- 14. Forlenza GP, Ekhlaspour L, DiMeglio LA, et al. : Glycemic outcomes of children 2–6 years of age with type 1 diabetes during the pediatric MiniMed 670G system trial. Pediatr Diabetes 2022;23:324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, et al. : Safety evaluation of the MiniMed 670G system in children 7–13 years of age with type 1 diabetes. Diabetes Technol Ther 2019;21:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garg SK, Weinzimer SA, Tamborlane WV, et al. : Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arunachalum S, Velado K, Vigersky R, Cordero TL: Glycemic outcomes during real-world hybrid closed-loop system use by individuals with type 1 diabetes in the United States. J Diabetes Sci Technol 2022. [Online ahead of print]; DOI: 10.1177/19322968221088608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stone MP, Agrawal P, Chen X, et al. : Retrospective analysis of 3-month real-world glucose data after the MiniMed 670G system commercial launch. Diabetes Technol Ther 2018;20:689–692. [DOI] [PubMed] [Google Scholar]
- 19. Da Silva J, Bosi E, Jendle J, et al. : Real-world performance of the MiniMed 670G system in Europe. Diabetes Obes Metab 2021;23:1942–1949. [DOI] [PubMed] [Google Scholar]
- 20. McAuley SA, Lee MH, Paldus B, et al. : Six months of hybrid closed-loop versus manual insulin delivery with fingerprick blood glucose monitoring in adults with type 1 diabetes: a randomized, controlled trial. Diabetes Care 2020;43:3024–3033. [DOI] [PubMed] [Google Scholar]
- 21. Food and Drug Administration—Center for Devices and Radiological Health: SmartAdjust technology—Omnipod 5 iAGC approvlal letter (K203774). January 27, 2022. https://www.accessdata.fda.gov/cdrh_docs/pdf20/K203774.pdf Accessed December 13, 2022.
- 22. Forlenza GP, Buckingham BA, Brown SA, et al. : First outpatient evaluation of a tubeless automated insulin delivery system with customizable glucose targets in children and adults with type 1 diabetes. Diabetes Technol Ther 2021;23:410–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Noor N, Kamboj MK, Triolo T, et al. : Hybrid closed-loop systems and glycemic outcomes in children and adults with type 1 diabetes: real-world evidence from a U.S.-based multicenter collaborative. Diabetes Care 2022;45:e118–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beato-Vibora PI, Gallego-Gamero F, Lazaro-Martin L, et al. : Prospective analysis of the impact of commercialized hybrid closed-loop system on glycemic control, glycemic variability, and patient-related outcomes in children and adults: a focus on superiority over predictive low-glucose suspend technology. Diabetes Technol Ther 2020;22:912–919. [DOI] [PubMed] [Google Scholar]
- 25. Barnard KD, Wysocki T, Ully V, et al. : Closing the loop in adults, children and adolescents with suboptimally controlled type 1 diabetes under free living conditions: a psychosocial substudy. J Diabetes Sci Technol 2017;11:1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bisio A, Gonder-Frederick L, McFadden R, et al. : The impact of a recently approved automated insulin delivery system on glycemic, sleep, and psychosocial outcomes in older adults with type 1 diabetes: a pilot study. J Diabetes Sci Technol 2022;16:663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharifi A, De Bock MI, Jayawardene D, et al. : Glycemia, treatment satisfaction, cognition, and sleep quality in adults and adolescents with type 1 diabetes when using a closed-loop system overnight versus sensor-augmented pump with low-glucose suspend function: a Randomized crossover study. Diabetes Technol Ther 2016;18:772–783. [DOI] [PubMed] [Google Scholar]
- 28. Adams RN, Tanenbaum ML, Hanes SJ, et al. : Psychosocial and human factors during a trial of a hybrid closed loop system for type 1 diabetes management. Diabetes Technol Ther 2018;20:648–653. [DOI] [PubMed] [Google Scholar]
- 29. Cobry EC, Hamburger E, Jaser SS: Impact of the hybrid closed-loop system on sleep and quality of life in youth with type 1 diabetes and their parents. Diabetes Technol Ther 2020;22:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boscari F, Ferretto S, Cavallin F, Bruttomesso D: Switching from predictive low glucose suspend to advanced hybrid closed loop control: effects on glucose control and patient reported outcomes. Diabetes Res Clin Pract 2022;185:109784. [DOI] [PubMed] [Google Scholar]
- 31. DuBose SN, Bauza C, Verdejo A, et al. : Real-world, patient-reported and clinic data from individuals with type 1 diabetes using the MiniMed 670G hybrid closed-loop system. Diabetes Technol Ther 2021;23:791–798. [DOI] [PubMed] [Google Scholar]
- 32. Wang LR, Malcolm J, Arnaout A, et al. : Real-world patient experience of long-term hybrid closed-loop insulin pump use. Can J Diabetes 2021;45:750.e3–756.e3. [DOI] [PubMed] [Google Scholar]
- 33. Fang Z, Liu M, Tao J, et al. : Efficacy and safety of closed-loop insulin delivery versus sensor-augmented pump in the treatment of adults with type 1 diabetes: a systematic review and meta-analysis of randomized-controlled trials. J Endocrinol Invest 2022;45:471–481. [DOI] [PubMed] [Google Scholar]
- 34. Weisman A, Bai JW, Cardinez M, et al. : Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–512. [DOI] [PubMed] [Google Scholar]
- 35. Xu G, Liu B, Sun Y, et al. : Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ 2018;362:k1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martin CT, Criego AB, Carlson AL, Bergenstal RM: Advanced technology in the management of diabetes: which comes first-Continuous glucose monitor or insulin pump? Curr Diab Rep 2019;19:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mulinacci G, Alonso GT, Snell-Bergeon JK, Shah VN: Glycemic outcomes with early initiation of continuous glucose monitoring system in recently diagnosed patients with type 1 diabetes. Diabetes Technol Ther 2019;21:6–10. [DOI] [PubMed] [Google Scholar]
- 38. DeSalvo DJ, Miller KM, Hermann JM, et al. : Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: international comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes 2018;19:1271–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DeSalvo DJ, Noor N, Xie C, et al. : Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D Exchange real-world observational study. J Diabetes Sci Technol 2021:19322968211049783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.