Abstract
Objective:
Accumulating evidence demonstrates that gender-affirming hormone therapy improves mental health outcomes in transgender persons. Data specific to the risks associated with gender-affirming hormone therapy for transgender persons continue to emerge, allowing for improvements in understanding, predicting, and mitigating adverse outcomes while informing discussion about desired effects. Of particular concern is the risk of venous thromboembolism in the context of both longitudinal gender-affirming hormone therapy and the perioperative setting. Combining what is known about the risk of VTE in cisgender individuals on hormone therapy with the evidence for transgender persons receiving hormone therapy allows for an informed approach to assess underlying risk and improve care in the transgender community.
Observations:
Hormone formulation, dosing, route, and duration of therapy can impact thromboembolic risk, with transdermal estrogen formulations having the lowest risk. There are no existing risk scores for venous thromboembolism that consider hormone therapy as a possible risk factor. Risk assessment for recurrent venous thromboembolism and bleeding tendencies using current scores may be helpful when assessing individual risk. Gender-affirming surgeries present unique perioperative concerns, and certain procedures include a high likelihood that patients will be on exogenous estrogens at the time of surgery, potentially increasing thromboembolic risk.
Conclusions and Relevance:
Withholding gender-affirming hormone therapy due to potential adverse events may cause negative impacts for individual patients. Providers should be knowledgeable about the management of hormone therapy in transgender individuals of all ages, as well as in the perioperative setting, to avoid periods in which transgender individuals are off gender-affirming hormone therapy. Treatment decisions for both anticoagulation and hormone therapy should be individualized and tailored to patients’ overall goals and desired outcomes, given that the physical and mental health benefits of gender-affirming care may outweigh the risk of venous thromboembolism.
INTRODUCTION
As data continue to emerge regarding transgender persons receiving gender-affirming hormone therapy (GAHT), the ability to predict potential outcomes, both adverse and desired, is becoming more feasible. Evolving evidence allows for more informed discussion between patients and providers, ensuring that transgender persons of all ages can access GAHT and optimal health. More research is needed to understand the outcomes associated with longitudinal GAHT and better define the risks and benefits of treatment; however, accumulating evidence demonstrates that GAHT greatly improves mental health outcomes in transgender persons1–3. Withholding GAHT due to potential adverse events may cause substantially negative impacts for transgender patients.
What follows is a brief overview of evidence pertaining to the risk of venous thromboembolism (VTE) among cisgender and transgender persons receiving hormone therapy (HT), and a proposed approach to assessing VTE risk in transgender patients. As is often the case in transgender health, limited data require that lessons be extracted from experience with the cisgender population utilizing HT. For this reason, data are included pertaining to oral contraceptives (OCs), HT used for replacement in postmenopausal cisgender women, and GAHT in transgender populations (where available), and we make note of important differences between these three types of exogenous hormone administration (e.g., duration of treatment, endogenous hormone production, co-administration or absence of other hormones, and prior exposure to hormones).
Throughout the manuscript we use the term “transgender” to reflect the diversity of non-cisgender individuals to whom our discussions and recommendations should apply. There are some inconsistencies when describing existing evidence due to the limitations of gender identity collection or categorization in the studies reviewed, which may not have included participants with other non-cisgender identities (e.g., non-binary). Additionally, this discussion focuses on transgender patients who receive feminizing HT, given the known association of exogenous estrogen and VTE. There are limited data on VTE risk associated with masculinizing hormones.
VTE RISK STRATIFICATION AND PREDICTION IN TRANSGENDER PATIENTS
VTE and Exogenous Hormones Among Cisgender Adults
Among premenopausal cisgender women, meta-analyses of exogenous combined estrogen-progestin OCs found that compared to levonorgestrel with 30–40μg ethinyl estradiol, all OCs have a significantly increased VTE risk4. Additional meta-analyses have also confirmed that the use of OCs containing cyproterone acetate, desogestrel, drospirenone, or gestodene was associated with a significantly increased risk of VTE compared with the use of levonorgestrel-containing OCs (pooled RR ratios of 1.5–2.0)5. Evidence from the Food and Drug Administration highlights that while the incidence of VTE is increased among premenopausal women using OCs (3–9/10,000 person-years) compared to premenopausal women not using OCs (1–5/10,000 person-years), VTE incidence with exogenous hormone use is lower compared to pregnancy (5–20/10,000 person-years) and the postpartum period (40–65/10,000 person-years) (Figure 1)6.
Figure 1.
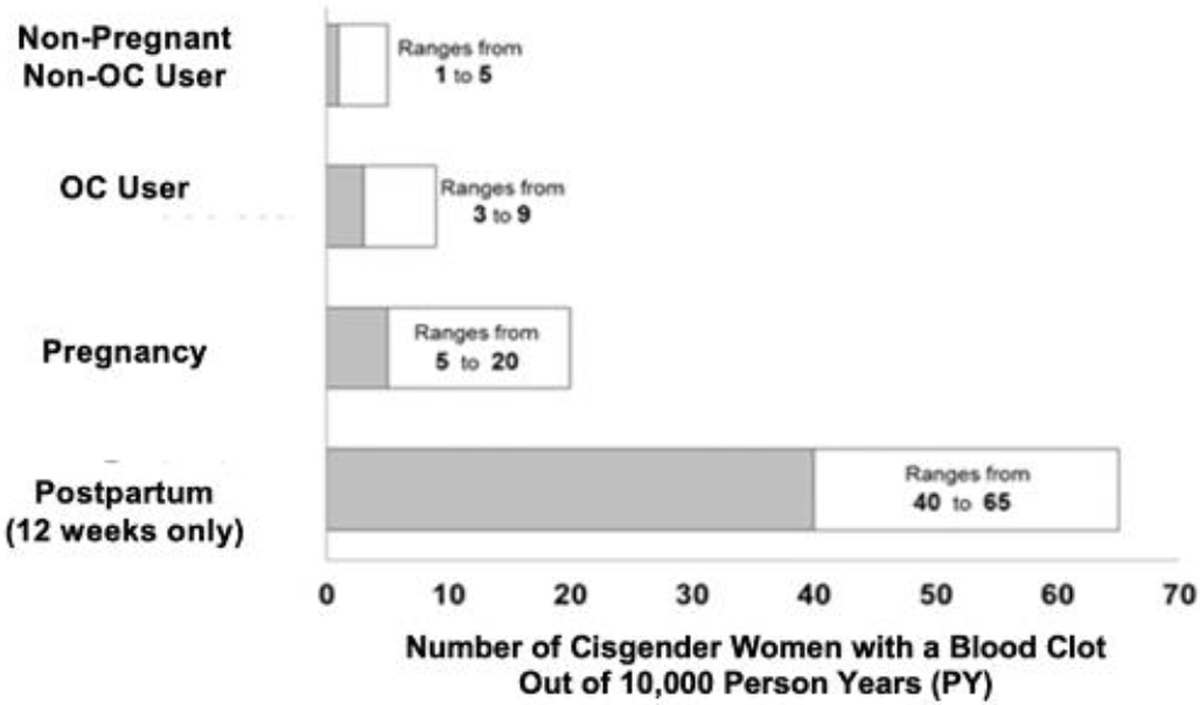
Estimated Risk of VTE in Cisgender Women6
*Pregnancy data based on actual duration of pregnancy in the reference studies. Based on a model assumption that pregnancy duration is nine months, the rate is 7 to 27 per 10,000 PY.
Among peri- and postmenopausal cisgender women, the most definitive data on VTE risk in the setting of HT come from the Women’s Health Initiative, which found an elevated risk of VTE with exogenous hormone use to treat peri- and postmenopausal symptoms (hazard ratio [HR] 2.06; 95% CI, 1.57–2.70)7. Increasing age, elevated body mass index (BMI), and factor V Leiden further increased the association between VTE and HT7. While the HRs were elevated, these findings translate into an absolute risk of 3.5 VTEs per 1,000 person-years among cisgender women taking estrogen plus progestin to treat peri- and postmenopausal symptoms, compared to 1.7 VTEs per 1,000 person-years among cisgender women taking placebo.
The route of HT administration is an additional consideration in VTE risk. A multicenter, hospital-based, case-control study of postmenopausal cisgender women found differences in risks of developing idiopathic VTE based on route of estrogen therapy. In this study, the odds ratio (OR) for VTE in users of oral and transdermal estrogen HT compared with nonusers was 3.5 (95% CI, 1.8–6.8) and 0.9 (95% CI, 0.5–1.6), respectively. The estimated risk for VTE in users of oral vs. transdermal estrogen HT was 4.0 (1.9–8.3). These data suggest a differential risk for VTE based on the route of HT8. The serum estradiol levels or estrogen doses used were not examined as potential VTE risk factors. Existing data have included nonequivalent doses of oral and transdermal estradiol when making these comparisons. The highest dose of transdermal estradiol studied was 0.1 mg/day; clinicians often use doses up to 0.2–0.3 mg/day of transdermal estradiol9.
VTE and Exogenous Hormones Among Transgender Adults
VTE research among transgender persons receiving GAHT has focused primarily on estrogen-based therapy. As in cisgender adults, estrogen formulation, dosing, route, and duration of therapy can impact VTE risk. The transdermal route is reported to be the least thrombogenic, though there are no head-to-head comparisons with other products8. A retrospective study including 816 transgender women found an association between VTE and high doses of oral ethinyl estradiol (standard regimens included 100μg oral ethinyl estradiol daily)10. In a follow-up study, no increased risk was seen among transgender women receiving preparations other than ethinyl estradiol, albeit also in a setting where standard doses of ethinyl estradiol were 100μg daily11. These studies are focused on older transgender women and have influenced the field of transgender care, leading to guidelines that discourage use of ethinyl estradiol for GAHT12. Subsequent studies on lower doses of oral ethinyl estradiol for GAHT have not been performed.
Other estrogen formulations have also been associated with VTE in transgender women. Several studies have found evidence that the risk of VTE in transgender individuals (particularly among transgender women taking estrogen) may be higher than the general population; however, these results are inconclusive9. In a retrospective study of 214 transgender women receiving oral estradiol, transdermal estradiol, or estradiol gel for 7.4 years on average, 5.1% experienced VTE, with almost half experiencing VTE within the first 12 months of GAHT initiation and three of 11 transgender women experiencing VTE in the setting of gender-affirming surgery (GAS); no events were observed among cisgender control groups13.
Recent retrospective data from 853 transgender women starting HT matched 10-to-1 to cisgender men and women found a higher incidence of VTE in transgender women (HR 3.2; 95% CI, 1.5–6.5 and HR 2.5; 95% CI, 1.2–5.0, respectively)14. However, these studies did not examine risk for VTE by HT formulation or duration of use.
A cohort study of 4,960 transgender individuals matched with cisgender controls found that transgender individuals taking estrogen had a higher incidence of VTE, with 2- and 8-year risk differences of 3.4 and 13.7 per 1000 persons relative to cisgender women15. This study controlled for BMI, blood pressure, smoking status, cholesterol level, and history of acute cardiovascular events, but not for other potential confounders (e.g., HIV status, concurrent antidepressant use, or psychosocial stressors). Similar risk differences were seen in a Dutch cohort of nearly 4,000 transgender women receiving GAHT16. In a representative sample of cisgender and transgender adults aged ≥40 years, there was no statistically significant association between hormone use and VTE (adjusted odds ratio [AOR] 1.49; 95% CI, 0.21–10.78)14.
Recommendations to Assess Risk Factors for VTE Among Transgender Adults
Initiating GAHT requires a thorough patient history relating to VTE and associated conditions and should include inquiries about smoking behavior, family history of VTE—particularly among first-degree relatives—and personal history of VTE17–19. For persons with a personal or family history of VTE in a first-degree relative, additional consultation with hematology may be considered; there are no data available to support specific thrombophilia testing in transgender persons with a family history of VTE. As the incidence of thrombophilias does not appear to be elevated in transgender populations, routine screening prior to initiating GAHT is not recommended for persons without a personal or family history of VTE20.
There continues to be a need for prospective data on the value of pretreatment screening for all persons with a personal history of VTE, as well as population-level data on the incidence of major gene thrombophilia among transgender persons.
VTE AND GAHT IN THE ADOLESCENT, ADULT, AND OLDER TRANSGENDER POPULATIONS
There is limited evidence on the correlation between duration of GAHT and risk and incidence of VTE among transgender persons10, 21. Adolescent data (ages 10 to 20) are limited. Risk for VTE following estrogen use is based on cisgender adolescent data, which suggest that events are rare (0.07–0.21 per 10,000 person-years) and with most occurring due to a secondary risk factor (e.g., a central venous catheter, inherited thrombophilia, malignancy, obesity, or immobility)22–24. No cohort data exclusively focus on adolescents; instead, adolescents are included in larger studies of adults.
The risk for VTE increases as people age. In a recent cohort study, an increase in procoagulant profiles and a decrease in protein C levels were associated with age, with the older age groups experiencing marked decreases in protein C levels25. The older adult population is unique because of age-related physiological changes in hemostatic, inflammatory, and vascular pathways26,27. Moreover, older adults are often faced with chronic medical conditions that may render them frail; these conditions also require management with numerous medications that augment risk for drug-drug interactions and adverse effects28,29. Additionally, renal function diminishes with age and directly impacts the dosing and clearance of anticoagulants30.
The presentation of VTE among older adults (especially those >80 years) may not follow the typical signs or symptoms experienced by adolescents and younger adult populations 26,27;31,32. There is no single sign or symptom that can rule in or rule out VTE in older adults. The diagnosis relies on existing clinical prediction tools adjusted for age, as well as age-adjusted D-dimer levels and imaging studies adjusted for renal function. Of note, current prediction tools such as the Wells score may be less specific for the older population26,27;30. At present, there are no clinical VTE prediction tools specifically for transgender patients.
It is recommended that treatment decisions for older adults be guided by the overall goals of care, risk of bleeding and thromboembolic complications, duration of treatment, and other considerations such as renal function, polypharmacy, fall risk, and medication adherence among others10,30 (Table 1). It is also important to consider that in older adults, there may be a higher risk of anticoagulation-related bleeding due to fall risk, a higher prevalence of ischemic stroke from comorbid conditions such as hypertension and diabetes, and a higher mortality risk from VTE33. In the palliative care setting, the decision to continue or discontinue GAHT in the presence of VTE has not been studied. Given the decreased thrombogenic risk with transdermal estrogen, older adults (particularly those >70 years) may have less risk of VTE continuing the transdermal formulation.
Table 1.
Suggested Recommendations for VTE Risk Assessment and Management for Older Transgender Adults
| VTE Risk Assessment | • Recognize VTE risk factors, including presence of a central venous catheter, inherited thrombophilia, malignancy, obesity, trauma, immobility, etc. • Use existing VTE risk assessment tools to guide shared decision-making; there are no special VTE risk assessment tools specific for transgender adults. • Routinely assess VTE risk factors in the geriatric population (age >65 years old), including comorbidities, recent surgery and/or hospitalization, malignancy, infection, immobility, chronic heart or lung disease, prior VTE, frailty, stroke, residence in a nursing home, HIV, or diabetes mellitus. • Use prediction scores such as Wells, PERC, DASH, HAS-BLED, or HEMORR2HAGES to assess one’s individual risk for recurrent VTE or bleeding33,a. • Monitor for subtle signs of VTE, such as subjective weakness or acute change in mental status. • Use the standard VTE workup for both transgender and cisgender older adults. These could include but are not limited to use of clinical prediction tools, D-dimer levels, and imaging that are all adjusted for age and renal function per clinician judgment. |
| Medication Reconciliation | • Review estrogen formulation, dosing, route, and duration of therapy with patients. The transdermal route of GAHT is preferred for older adults (age ≥70 years) and for those younger than 70 with active comorbidities. • Review all medications (prescription and nonprescription) and drug-drug interactions. ■ The brown bag method (i.e., home medications collected in a brown bag for review by provider) is an evidence-based strategy used by geriatricians34. |
| Treatment Decisions | • Implement a shared decision-making approach between provider and patient/family regarding anticoagulation in the setting of VTE and GAHT. • Integrate goals of care into primary and palliative care. • Individualize treatment decisions for both anticoagulation and GAHT and tailor to patients’ overall goals and desired outcomes, given that the physical and mental health benefits of GAHT may outweigh the risk of VTE in some individuals. |
VTE = venous thromboembolism; GAHT = gender-affirming hormone therapy; PERC = Pulmonary Embolism Rule-Out Criteria; DASH = D-dimer Age Sex Hormones; HAS-BLED = Hypertension Abnormal Liver/Renal Function Stroke History Bleeding History or Predisposition Labile INR Elderly Drug/Alcohol Use; HEMORR2HAGES = Hepatic/renal disease Ethanol abuse Malignancy Older age Reduced platelet function/count Rebleeding risk Hypertension Anemia Genetic factors Elevated fall risk Stroke history
Though only validated in the cisgender population, these risk scores may be useful in the transgender population as well. The PERC and DASH tools include hormonal use in both men and women. The first three scores pertain to the risk for developing VTE while the latter two refer to the bleeding risk based on specific variables such as age, hypertension, hepatic and renal disease, and stroke among others. These scoring tools are easily accessible online. In general, the higher the scores, the greater likelihood or pretest probability of having a VTE or bleed.
When weighing the risk of GAHT-associated VTE, it is also important to weigh the risk of not providing GAHT. GAHT is important for the primary health and quality of life for many transgender individuals. Withholding GAHT could have negative mental health consequences that may outweigh the risk of VTE. Some individuals may also obtain HT outside of the clinical care setting if not provided by a healthcare provider (HCP). Implementing a shared decision-making approach between provider(s) and the patient-family/caregiver unit is key. Such an approach fosters close collaboration with all stakeholders, including providers, patients, and families/caregivers, and can mitigate power imbalances by allowing equity in voices and input. Components may include, but are not limited to, a discussion of the risks and benefits of GAHT in the setting of VTE (or potential VTE); quality-of-life metrics and goal setting; offering of various treatment options and ranking of options to align with the patient’s goal(s); pursuit of prioritized treatment option(s); and periodic reevaluation of goals and treatment options.
TREATMENT AFTER THROMBOSIS
Transgender people who develop VTE on GAHT should be treated per current therapeutic anticoagulation recommendations for cisgender people35. The American Society of Hematology guidelines preferentially recommend direct oral anticoagulants (DOACs) over vitamin K antagonists or low-molecular-weight heparin (LMWH) for VTE treatment due to a lower risk for bleeding with these agents35. Although data are limited in transgender people, recent case reports demonstrate efficiency of DOACs in those who have developed VTE while on GAHT36,37. Among transgender people with severe VTE that threatens limbs, thrombolysis should be considered (as it is for cisgender people)19,35. Most clinicians will recommend discontinuation of GAHT during an acute episode of VTE, especially if levels are supraphysiologic; however, this practice is based on limited data. The clinician and patient should discuss the potential risks and benefits of continuing GAHT during an acute VTE episode.
Following acute treatment of VTE, the decision to resume GAHT can be challenging. As noted, shared decision-making is critically important. Given the importance of GAHT to the health of transgender patients, many clinicians continue its use—even in patients with prior VTE—so long as full-intensity anticoagulation is also continued. This strategy has been shown to prevent recurrent VTE in cisgender women on HT; data are lacking in transgender patients8. Many cisgender patients on extended-duration anticoagulation with DOACs can reduce the dose after initial therapy38,39. This strategy has not been systematically evaluated in patients on GAHT, so shared decision-making that emphasizes the risks and benefits of this approach remains critical.
In cisgender patients who have completed therapeutic anticoagulation after a first episode of unprovoked VTE and do not wish to remain on extended-duration therapy, there is evidence that aspirin confers some benefit in secondary VTE prophylaxis40. It is unclear if this benefit persists in the presence of GAHT, particularly because these studies were conducted in cisgender women who were randomized to conjugated equine estrogen and medroxyprogesterone acetate7,41. Ethinyl estradiol and conjugated equine estrogens are generally avoided in GAHT and the role of progestogens in GAHT remains questionable in clinical guidelines12,42. We recommend continued use of anticoagulation over antiplatelet therapy in a patient who has had VTE while on GAHT.
SURGERY: PRE-, PERI-, AND POSTOPERATIVE MANAGEMENT
Key Barriers and Challenges
GAS presents unique perioperative concerns related to VTE. For certain procedures (especially breast augmentation, facial feminization, and vaginoplasty) there is a high likelihood that patients will be on exogenous estrogens at the time of surgery, which can increase VTE risk43. However, VTE risk during surgery is less than 2% in the general population, and the incidence rate has fallen over the past several decades44.
There are several considerations with GAHT, though sparse data to guide practice. No trials exist demonstrating a reduction in postsurgical VTE with discontinuation of any HT (post-menopausal HT, GAHT, or OC use)45. A single retrospective study examining the effects of postmenopausal HT in presumed cisgender adults found an OR of 1.2 for VTE with estrogen-only HT, an OR of 2.7 in regimens that contained progesterone, and no increased risk with transdermal estrogens46. Among transgender people, a single study of 1858 GAS procedures looking at VTE incidence in those who did and did not discontinue GAHT preoperatively found one VTE event in a patient who had stopped estrogen perioperatively47. However, this study included both transgender men and women; only 407 of the surgeries were higher risk inpatient cases in estrogen-treated patients (e.g., vaginoplasty), so the power of the study may be limited due to low cumulative expected event rates. A recent meta-analysis pooled overall events to suggest that transfeminine patients had VTE rates that were higher than transmasculine patients and comparable to cisgender women on HRT, but included only retrospective studies with high heterogeneity and widely variable GAHT regimens21.
The most widely used approach to assess VTE risk at the time of surgery is the modified Caprini score (latest iteration 2013)48. However, different fields may consider surgery-specific issues of postoperative bleeding risk, mobilization approaches postoperatively, and field-specific data to determine cutoffs and risks for the use of chemoprophylaxis. Additionally, Caprini scoring only has options for whether a patient is taking estrogen at all and does not account for differences in estrogen formulations or route of administration48–50.
Current guidance is as follows:
For a modified Caprini score ≤3: utilize sequential compression devices (SCDs), ensure early mobilization, do not discontinue HT.
For a modified Caprini score >3 and ≤7: utilize SCDs, ensure early mobilization, provide chemoprophylaxis for 1 week, consider discontinuing HT 4 weeks prior to surgery and resuming 1 week after surgery (use a shared decision-making approach).
For a modified Caprini score >7: utilize SCDs, ensure early mobilization, provide chemoprophylaxis for 4 weeks, discontinue HT 4 weeks prior to surgery and resume 4–6 weeks after surgery.
As noted, stopping GAHT may have negative impacts on a patient’s mental health and cause distressing body changes. There are no data available on mental health outcomes when patients are off hormones perioperatively; however, copious data exist showing the benefits of GAHT51.
CONTROVERSIES AND FUTURE DIRECTIONS
Risk Assessment and Management
All clinical care decisions must carefully consider the balance between the risks and benefits of an intervention. One of the difficulties in providing appropriate counseling to transgender patients is understanding individual risk, as much of the data applied to transgender patients are indirectly derived from cisgender populations. In addition, many studies have not captured data on intersex traits; data are further lacking in transgender and cisgender intersex individuals.
Quantifying risk is even more difficult because of the heterogeneity of the patient population. There are many separate populations of interest, and therapies are applied in different ways. For example, tobacco/nicotine use is a known independent risk factor for VTE; however, the contribution of tobacco/nicotine use to VTE risk is modified by other risk factors such as age and which HT formulation is being used. Further research will need to examine whether risk varies by type and duration of HT, and how risk is modified by risk factors to help patients make an informed decision about use.
In addition, patients come to the decision-making process with known and unknown risk factors. Family history of VTE may be difficult to assess in individuals who have limited contact with family due to social circumstances. Furthermore, standard thrombophilia testing does not exclude an inherited thrombophilia.
Exogenous estrogen may require additional evaluation and treatment in patients with a history of VTE or for those with a known inherited thrombophilia, at times requiring discontinuation of the medication. However, it is important to understand that withholding GAHT due to the potential risk of thrombosis is not without inherent risks and can have significant consequences for transgender patients. Transgender patients with a history of VTE should not be discouraged from receiving GAHT without full consideration of potential risk mitigation strategies, such as prophylactic anticoagulation.
Another area of uncertainty is how to manage VTE risk in transgender persons who take estrogen perioperatively. Available data are based on formulations or doses of estrogen no longer used in clinical practice, and the correlation between a measured estradiol blood level and risk of VTE is unknown. There is a dose-dependent relationship between estrogen use and VTE risk, so using the lowest dose of estrogen therapy possible to achieve therapeutic effect is an important consideration, while recognizing that this dose will be different for each patient.
In patients with multiple VTE risk factors, such as obesity, tobacco/nicotine use, inherited thrombophilias, or a strong family history of VTE, prophylactic anticoagulation should be considered in conjunction with HT. Antifibrinolytic therapy is beneficial in many situations, including in the perioperative setting. Tranexamic acid carries a boxed warning cautioning against use in patients who are receiving estrogen therapy as hormonal contraception. This risk primarily applies to patients who were receiving higher doses of estrogen and had additional risk factors for VTE; however, the risks of using antifibrinolytic therapy in the transgender population are not known.
It remains unclear how to best manage VTE risk in the setting of malignancy and GAHT. Malignancy is an independent risk factor for VTE and there are additional risk contributions from cancer-directed therapies.
Lack of Inclusion in Health Professions Training
Traditionally, transgender care has not been included in HCP training (e.g., undergraduate medical education, nursing, and advance practice provider curricula)52. Unless trainees opt for a specific rotation focused on transgender care, postgraduate training does not provide standardized education. As transgender care is introduced into the undergraduate medical curriculum, developing a specific subset of standards related to VTE risk assessment and thrombosis mitigation strategies will be important.
To develop a specific curriculum around VTE risk and transgender care, there first needs to be an assessment of both existing gaps in HCP education and barriers to provider education; these knowledge gaps and barriers must then be addressed in any efforts to improve medical education and training.
Additional Considerations
It is also critical to recognize the barriers to patient education and communication that exist when providing VTE care to transgender patients. Beyond a lack of HCP training, conflicting guidelines with inconsistent messaging and recommendations—combined with negative experiences with the healthcare community—make it difficult for many patients to trust HCPs. These limitations and concerns may be improved through gender-affirming care, broader education efforts, and an investment in specific research focused on VTE risk within the transgender community.
Finally, while the conclusions and proposed recommendations in this report are directed towards all individuals with non-cisgender identities, future research on VTE should include a diversity of identities (e.g., nonbinary, genderfluid, agender, etc.) to ensure their representation in data and to explore VTE issues that may be specific to these communities. For example, future studies should seek to determine the relative risks and benefits regarding VTE in nonbinary individuals who may be using lower dose or intermittent GAHT.
Acknowledgements:
We thank the North American Thrombosis Forum, in Brookline, Massachusetts, as well as Troy Keyser and Diva Martinez for their invaluable comments and support during this work.
Grant support:
This work was supported by an educational grant from Bristol-Myers Squibb to the North American Thrombosis Forum. The funder of this work had no role in the design, preparation, or writing of the report.
Grants or contracts from any entity not listed grant support section:
For CS: grants from NHLBI, AHA, and NIAAA; For AS: grants from United Therapeutics, Bayer, Acceleron, Altavant, RareGen LLC, Alexion, Janssen, Cardinal Health, AstraZeneca, PeerView, PriMed, Boston Scientific, Abbott Medical, Temple Health, Bristol Myers Squibb, BMS Foundation, Bristol Myers Squibb-Pfizer Alliance; For VT: grants from Cystic Fibrosis Foundation, NIH; For MT: Harvard University Center for AIDS, NIH/NHLBI, Robert Wood Johnson Foundation and American Heart Association, MGH Department of Medicine
Footnotes
Other Disclosures of Interest
All authors had full access to all study data, take full responsibility for the accuracy of the analysis, and have authority over manuscript preparation and decisions to submit the manuscript for publication.
Consulting Fees: For CS: EverlyWell, LLC.; For FG: Next Gen Jane; For TP: Merck, ViiV Healthcare; For ZG: Thunermist Health Center, WPATH; For RG: Best Doctors, LLC
Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: For MM: Medpage Today, Integrity CME; For VT: American Assoc of Clinical Endocrinology; For AG: Cooper Hospital, NJ
Payment for expert testimony: For AT: Kirkland Ellis; For RG: Kellog & Van Aiken, LLP
Support for attending meetings and/or travel: For AT: American Assoc of Clinical Endocrinology
Patents Planned/Issued/Pending: None
Participation on a Data Safety Monitoring Board or Advisory Board: For NC: on advisory board of Takeda; For MT: Data Safety Monitoring Board Member for the Lite Plus Study
Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: For NC: on board of Michael H. Flanagan Foundation; For CS: on board of USPATH; For AS: on board of NATF; For AT: WPATH, Southern Society of Clinical Investigation; For MT: American Heart Association Bioethics Subcommittee Member and Diversity and Inclusion Working Group Member; Women in Endocrinology Membership Committee Chair and Executive Board Member; Nutrition and Obesity Research Center NIH/NIDDK Diversity and Inclusion Working Group; Nutrition and Obesity Research Center at Harvard Associate Member Council Co-Director and Executive Board Member; MGH Internal Medicine Residency Interview and Selection Committee Member; MGH Endocrinology Fellowship Interview and Selection Committee Member and URIM Subcommittee Member; For ZG: USPATH; For RG: HIVMA, MassEquality
Stock or stock options: For NC: stock in Doximity
Receipt of equipment, materials, drugs, medical writing, gifts or other services: No
Other financial or non-financial interests: None
References
- 1.Nguyen HB, Chavez AM, Lipner E, et al. Gender-affirming hormone use in transgender individuals: impact on behavioral health and cognition. Curr Psychiatry Rep. 2018;20(12):110. doi: 10.1007/s11920-018-0973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bränström R, Pachankis JE. Reduction in mental health treatment utilization among transgender individuals after gender-affirming surgeries: a total population study. Am J Psychiatry. 2020;177(8):727–734. doi: 10.1176/appi.ajp.2019.19010080. [DOI] [PubMed] [Google Scholar]
- 3.Coleman E, Bockting W, Botzer M, et al. Standards of Care for the Health of Transsexual, Transgender, and Gender Non-Conforming People. 7th version. World Professional Association for Transgender Health (WPATH); 2012. Accessed August 16, 2022. https://www.wpath.org/publications/soc. [Google Scholar]
- 4.Oedingen C, Scholz S, Razum O. Systematic review and meta-analysis of the association of combined oral contraceptives on the risk of venous thromboembolism: the role of the progestogen type and estrogen dose. Thromb Res. 2018;165:68–78. doi: 10.1016/j.thromres.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Dragoman MV, Tepper NK, Fu R, Curtis KM, Chou R, Gaffield ME. A systematic review and meta-analysis of venous thrombosis risk among users of combined oral contraception. Int J Gynaecol Obstet. 2018;141:287–294. doi: 10.1002/ijgo.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. FDA Drug Safety Communication: Updated Information About the Risk of Blood Clots in Women Taking Birth Control Pills Containing Drospirenone. Food and Drug Administration; 2018. Accessed August 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-updated-information-about-risk-blood-clots-women-taking-birth-control [Google Scholar]
- 7.Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA. 2004;292(13):1573–8150. doi: 10.1001/jama.292.13.1573. [DOI] [PubMed] [Google Scholar]
- 8.Scarabin PY, Oger E, Plu-Bureau G; EStrogen and THromboEmbolism Risk Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362(9382):428–432. doi: 10.1016/S0140-6736(03)14066-4. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein Z, Khan M, Reisman T, Safer JD. Managing the risk of venous thromboembolism in transgender adults undergoing hormone therapy. J Blood Med. 2019;10:209–216. doi: 10.2147/JBM.S166780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol. 1997;47(3):337–342. doi: 10.1046/j.1365-2265.1997.2601068.x. [DOI] [PubMed] [Google Scholar]
- 11.Asscheman H, Giltay EJ, Megens JA, Pim de Ronde W, van Trotsenburg MAA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164(4):635–642. doi: 10.1530/EJE-10-1038. [DOI] [PubMed] [Google Scholar]
- 12.Hembree WC, Cohen-Kettenis PT, Gooren LJ, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2017;102(11):3869–3903. doi: 10.1210/jc.2017-01658. [DOI] [PubMed] [Google Scholar]
- 13.Wierckx K, Elaut E, Declercq E, et al. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case-control study. Eur J Endocrinol. 2013;169(4):471–478. doi: 10.1530/EJE-13-0493. [DOI] [PubMed] [Google Scholar]
- 14.Poteat TC, Divsalar S, Streed CG, Feldman JL, Bockting WO, Meyer IH. Cardiovascular disease in a population-based sample of transgender and cisgender adults. Am J Prev Med. 2021;61(6):804–811. doi: 10.1016/j.amepre.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Getahun D, Nash R, Flanders WD, et al. Cross-sex hormones and acute cardiovascular events in transgender persons: a cohort study. Ann Int Med. 2018;169(4):205–213. doi: 10.7326/m17-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nota NM, Wiepjes CM, de Blok CJM, Gooren LJG, Kreukels BPC, den Heijer M. Occurrence of Acute Cardiovascular Events in Transgender Individuals Receiving Hormone Therapy. Circulation. 2019;139(11):1461–1462. doi: 10.1161/CIRCULATIONAHA.118.038584. [DOI] [PubMed] [Google Scholar]
- 17.Anand SS. Smoking: a dual pathogen for arterial and venous thrombosis. Circulation. 2017;135(1):17–20. doi: 10.1161/CIRCULATIONAHA.116.025024. [DOI] [PubMed] [Google Scholar]
- 18.Connors J Thrombophilia testing and venous thrombosis. New Engl J Med. 2017;377:1177–1187. doi: 10.1056/NEJMra1700365. [DOI] [PubMed] [Google Scholar]
- 19.Connors J, Middeldorp S. Transgender patients and the role of the coagulation clinician. J Thromb Haemost. 2019;17(11):1790–1797. doi: 10.1111/jth.14626. [DOI] [PubMed] [Google Scholar]
- 20.Ott J, Kaufmann U, Bentz EK, Huber JC, Tempfer CB. Incidence of thrombophilia and venous thrombosis in transsexuals under cross-sex hormone therapy. Fertil Steril. 2010;93(4):1267–1272. doi: 10.1016/j.fertnstert.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Kotamarti VS, Greige N, Heiman AJ, Patel A, Ricci JA. Risk for venous thromboembolism in transgender patients undergoing cross-sex hormone treatment: a systematic review. J Sex Med. 2021;18(7):1280–1291. doi: 10.1016/j.jsxm.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Andrew M, David M, Adams M, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood. 1994;83(5):1251–1257. [PubMed] [Google Scholar]
- 23.van Ommen CH, Heijboer H, Büller HR, Hirasing RA, Hiejmans HS, Peters M. Venous thromboembolism in childhood: a prospective two-year registry in The Netherlands. J Pediatr. 2001;139(5):676–681. doi: 10.1067/mpd.2001.118192. [DOI] [PubMed] [Google Scholar]
- 24.Tuckuviene R, Christensen AL, Helgestad J, Johnsen SP, Kristensen SR. Pediatric venous and arterial noncerebral thromboembolism in Denmark: a nationwide population-based study. J Pediatr. 2011;159(4):663–669. doi: 10.1016/j.jpeds.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 25.Scheres LJ, Selier NL, Nota NM, van Diemen JJK, Cannegieter SC, den Heijer M. Effect of gender-affirming hormone use on coagulation profiles in transmen and transwomen. J Thromb Haemost. 2021;19(4):1029–1037. doi: 10.1111/jth.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahan R, Bailey TA, Bibb TJ, Fenney M, Williams T. Drug therapy for gender transitions and health screenings in transgender older adults. J Am Geriatr Soc. 2016;64(12):2354–2359. doi: 10.1111/jgs.14350. [DOI] [PubMed] [Google Scholar]
- 27.Johnson SA, Eleazer GP, Rondina MT. Pathogenesis, diagnosis, and treatment of venous thromboembolism in older adults. J Am Geriatr Soc. 2016;64(9):1869–1878. doi: 10.1111/jgs.14279. [DOI] [PubMed] [Google Scholar]
- 28.Tritschler T, Aujesky D. Venous thromboembolism in the elderly: a narrative review. Thromb Res. 2017;155:140–147. doi: 10.1016/j.thromres.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Folsom AR, Boland LL, Cushman M, Heckbert SR, Rosamond WD,Walston JD. Frailty and risk of venous thromboembolism in older adults. J Gerontol. 2007;62A(1):79–82. doi: 10.1093/gerona/62.1.79. [DOI] [PubMed] [Google Scholar]
- 30.Bauersachs RM, Herold J. Oral anticoagulation in the elderly and frail. Hamostaseologie. 2020;40(1):74–83. doi: 10.1055/s-0040-1701476. [DOI] [PubMed] [Google Scholar]
- 31.Robert-Ebadi H, Righini M. Diagnosis and management of pulmonary embolism in the elderly. Eur J Intern Med. 2014;25(4):343–449. doi: 10.1016/j.ejim.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Le Gal G, Righini M, Roy PM, et al. Differential value of risk factors and clinical signs for diagnosing pulmonary embolism according to age. J Thromb Haemost. 2005;3(11):2457–2464. doi: 10.1111/j.1538-7836.2005.01598.x. [DOI] [PubMed] [Google Scholar]
- 33.Ko D, Hylek EM. Anticoagulation in the older adult: optimizing benefit and reducing risk. Semin Thromb Hemost. 2014;40(6):688–694. doi: 10.1055/s-0034-1389083. [DOI] [PubMed] [Google Scholar]
- 34.Murtha E, Elder B, Faragher M. Brown bag medication review: using AHRQ’s brown bag medication tool. J Nurs Care Qual. 2020;35(1):58–62. doi: 10.1097/NCQ.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 35.Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693–4638. doi: 10.1182/bloodadvances.2020001830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan W, Drummond A, Kelly M. Deep vein thrombosis in a transgender woman. CMAJ. 2017;189(13):e502–504. doi: 10.1503/cmaj.160408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanley K, Cooper J. Hormone therapy and venous thromboembolism in a transgender adolescent. J Pediatr Hematol Oncol. 2018;40(1):e38–e40. doi: 10.1097/MPH.0000000000000984. [DOI] [PubMed] [Google Scholar]
- 38.Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376(13):1211–1222. doi: 10.1056/NEJMoa1700518. [DOI] [PubMed] [Google Scholar]
- 39.Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368(8):699–708. doi: 10.1056/NEJMoa1207541. [DOI] [PubMed] [Google Scholar]
- 40.Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367(21):1979–1987. doi: 10.1056/NEJMoa121038. [DOI] [PubMed] [Google Scholar]
- 41.Hulley S, Furberg C, Barrett-Connor E, et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288(1):58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- 42.Tomlins L Prescribing for transgender patients. Aust Prescr. 2019;42(1):10–13. doi: 10.18773/austprescr.2019.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tollinche LE, Walters CB, Radix A, et al. The perioperative care of the transgender patient. Anesth Analg. 2018;127(2):359–366. doi: 10.1213/ANE.0000000000003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JYS, Khavanin N, Rambachan A, et al. Surgical duration and risk of venous thromboembolism. JAMA Surg. 2015;150(2):110–117. doi: 10.1001/jamasurg.2014.1841. [DOI] [PubMed] [Google Scholar]
- 45.The American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion: perioperative pathways: enhanced recovery after surgery. Obstet Gynecol. 2018;132(3):e120–e130. [DOI] [PubMed] [Google Scholar]
- 46.Douketis J. Hormone replacement therapy and risk for venous thromboembolism: what’s new and how do these findings influence clinical practice? Curr Opin Hematol. 2005;12(5):395–400. doi: 10.1097/01.moh.0000161047.53353.a8. [DOI] [PubMed] [Google Scholar]
- 47.Kozato A, Fox GWC, Yong PC, et al. No venous thromboembolism increase among transgender female patients remaining on estrogen for gender-affirming surgery. J Clin Endocrinol Metab. 2021;106(4):e1586–e1590. doi: 10.1210/clinem/dgaa966. [DOI] [PubMed] [Google Scholar]
- 48.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S–277S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810. Published erratum. BMJ. 2019;364:162. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanh BM, Cuong LQ, Son NT, et al. Determination of risk factors for venous thromboembolism by an adapted Caprini scoring system in surgical patients. J Pers Med. 2019;9(3):36. doi: 10.3390/jpm9030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White Hughto JM, Reisner SL. A systematic review of the effects of hormone therapy on psychological functioning and quality of life in transgender individuals. Transgender Health. 2016;1(1):21–31. doi: 10.1089/trgh.2015.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obedin-Maliver J, Goldsmith ES, Stewart L, et al. Lesbian, gay, bisexual, and transgender-related content in undergraduate medical education. JAMA. 2011;306(9):971–977. doi: 10.1001/jama.2011.1255. [DOI] [PubMed] [Google Scholar]


