SUMMARY
Genomic DNA is a crowded track where motor proteins frequently collide. It remains underexplored whether these collisions carry physiological function. In this work, we developed a single-molecule assay to visualize the trafficking of individual E. coli RNA polymerases (RNAPs) on DNA. Based on transcriptomic data, we hypothesized that RNAP collisions drive bidirectional transcription termination of convergent gene pairs. Single-molecule results showed that the head-on collision between two converging RNAPs is necessary to prevent transcriptional readthrough, but insufficient to release the RNAPs from the DNA. Remarkably, co-directional collision of a trailing RNAP into the head-on collided complex dramatically increases the termination efficiency. Furthermore, stem-loop structures formed in the nascent RNA are required for collisions to occur at well-defined positions between convergent genes. These findings suggest that physical collisions between RNAPs furnish a mechanism for transcription termination and that programmed genomic conflicts can be exploited to co-regulate the expression of multiple genes.
Keywords: Transcription termination, RNA polymerase, GreB, Convergent transcription, Collision, Bidirectional terminator, SEnd-seq, Single-molecule fluorescence, Optical tweezers
Graphical Abstract
eTOC Blurb
The genomic DNA is a crowded molecular highway where motor protein collisions are inevitable. Using a single-molecule visualization platform, Wang et al. investigated the outcome of collisions between Escherichia coli RNA polymerases and found that, paradoxically, frequent collisions solve traffic jams, leading to efficient transcription termination of convergent gene pairs.
INTRODUCTION
Efficient and precise transcription termination ensures the generation of accurate gene products and the recycling of RNA polymerases (RNAPs) for next rounds of transcription1,2. By restricting the RNAP movement within specific genomic regions, transcription termination also minimizes conflicts between RNAPs and other DNA-based molecular machines such as the DNA replication machinery3. In bacteria, two mechanisms for transcription termination have been documented, one mediated by a stem-loop structure formed in the nascent RNA transcript (intrinsic termination) and the other mediated by an ATP-dependent RNA translocase Rho (Rho-dependent termination)1,4,5.
Recently, we developed a transcriptomic method termed SEnd-seq (simultaneous 5’ and 3’ end RNA sequencing) to map full-length bacterial RNAs, which led us to identify a class of bidirectional transcription terminators located between convergent gene pairs that prevalently exist in the Escherichia coli genome6. These bidirectional terminators—controlling hundreds of E. coli genes—do not resemble intrinsic terminators in terms of the nucleotide sequence, nor do they depend on the activity of Rho6. We thus proposed a model for bacterial transcription termination in which the physical collisions between convergent transcription elongation complexes (ECs) drive their own dissociation. This model, if proven, could represent a conserved mechanism to terminate gene transcription given that convergent gene arrangement is universally found in different organisms7–13. However, direct evidence for such collision-driven termination mechanism is still lacking.
Single-molecule techniques are powerful tools to study dynamic biomolecular processes and have greatly aided our understanding of the mechanism of transcription and its regulation14–18. Of the two major types of single-molecule approaches, fluorescence-based detection reveals the composition of transcription complexes [e.g.,19], while force-based manipulation reports the position of RNAP on the DNA template [e.g.,20]. In this work, we sought to combine these two complementary approaches to visualize RNAP trafficking and collisions on DNA.
RESULTS
A single-molecule platform for studying RNAP trafficking
We first constructed a DNA template (Template1) composed of a transcription unit flanked by biotinylated DNA handles for bead conjugation (Figure 1A). The transcription unit contains a σ70-dependent T7A1 promoter, a C-less segment, a cassette harboring seven tandem repeats for RNA detection, a 1,831-basepair (bp) transcribed region, and a λ tR’ intrinsic terminator (Figure 1A and Table S1). Transcription was initiated and stalled in a test tube by mixing Cy3-labeled E. coli RNAP/σ70 holoenzyme, Template1, and ribonucleoside triphosphates (NTPs) except CTP. Stalled ECs were injected into the microfluidic flow cell of a dual optical trap instrument combined with multicolor confocal fluorescence microscopy21,22. A single DNA molecule loaded with the stalled EC was tethered between a pair of streptavidin-coated beads held by optical traps and verified by 2D scanning of the Cy3 fluorescence (Figure 1B). The tethered complex was then moved to a separate channel containing the full set of NTPs where unidirectional movement of Cy3-RNAP along the DNA template towards the terminator was observed, indicating successful restart of the EC (Figure 1C). Time-resolved positions of the Cy3-RNAP on DNA were converted to distances traveled in bp (Figure 1D). The average elongation speed of RNAP derived from these trajectories is 16 ± 6 bp/s (Figure 1E, Inset), consistent with previous single-molecule measurements under similar conditions23,24.
Figure 1. A single-molecule assay for visualizing transcription elongation and termination.

(A) Schematic of Template1 that contains a λ tR’ intrinsic terminator. See main text and Table S1 for a detailed description and sequence of the transcription unit.
(B) Experimental setup. Streptavidin-coated beads, stalled elongation complexes (ECs) and imaging buffer were flown into channels 1–3, respectively. A single DNA tether loaded with a stalled EC (visualized by the Cy3-RNAP fluorescence) was moved to channel 4 containing all NTPs to restart transcription.
(C) A representative kymograph showing directional translocation of a restarted EC along Template1.
(D) Position of the Cy3-RNAP on DNA as a function of time extracted from the kymograph in (C). Raw data and smoothed values (± 10-s moving average of tracked points) are shown in green and black dots, respectively. Gray region indicates the intrinsic terminator position (2150 ± 200 bp).
(E) Ten individual RNAP trajectories on Template1 showing RNAP release within the termination zone. (Inset) Distribution of the average elongation speed for 57 individual RNAPs. Bars represent mean ± SD.
(F) A representative two-color kymograph showing the concomitant translocation and release (white arrows) of RNAP (green) and nascent RNA (red).
(G) Pie chart showing the fraction of EC release, retention, and readthrough events observed on Template1. N denotes the total number of ECs.
See also Figures S1 and S2.
We found that the RNAP trajectories often lost the Cy3 fluorescence signal within the termination zone (Figure 1E). The center of the zone corresponds to the terminator position on Template1 and its width corresponds to the spatial resolution of the fluorescence microscopy. The signal loss was unlikely due to dye photobleaching because the fluorescence lifetime of Cy3-RNAP was much longer under our imaging conditions (>400 s). Thus, the signal disappearance most likely signified the release of RNAP at the intrinsic terminator. To confirm that these translocating ECs were active in synthesizing RNA, we included a Cy5-labeled DNA probe complementary to the RNA sequence transcribed from the repeat cassette (Figure 1A). Indeed, we observed the onset of Cy5 signal shortly after the stalled EC resumed translocation (Figure 1F). The lag period (22 ± 9 s) accounts for the time needed for the RNAP to transcribe from the stall site to the repeat cassette. The Cy3 and Cy5 signals co-migrated and concomitantly disappeared at the terminator (Figure 1F and Figure S1A), indicating bona fide transcription termination events at which both RNAP and nascent RNA within the same transcription complex dissociated from the DNA template. Immediate release of RNAP and RNA was observed in the vast majority (83%) of ECs that reached the intrinsic terminator (“Release” in Figure 1G), consistent with the high termination efficiency previously reported for the λ tR’ terminator25. We also observed minor subpopulations of ECs that remained associated with DNA at the terminator position, as well as those that translocated across the terminator (“Retention” and “Readthrough” in Figure 1G and Figure S1B–C). These observations highlight the stochastic nature of the termination process and exemplify the power of single-molecule analysis in revealing heterogeneous molecular behaviors within population. We note that there also existed a fraction of stalled ECs that failed to restart, or restarted but arrested before reaching the terminator site (Figure S2). In the following we focused on the processive ECs that arrived at the terminator within our observation window (~300 s).
Visualizing collision between convergent RNAPs
Having demonstrated the utility of our assay in visualizing transcription elongation and termination, we sought to test our hypothesis that the collision between a pair of convergent ECs can result in bidirectional transcription termination. To this end, we constructed a convergent transcription template (Template2) (Figure 2A and Table S1), which contains two opposite T7A1 promoters, each followed by a transcribed region with a stall site and a distinct repeat cassette, and a bidirectional terminator from the E. coli genome located between the ynaJ-uspE gene pair (Figure 2B). We also constructed two unidirectional transcription templates (Template3 and Template4), which contain only one promoter but otherwise identical elements including the ynaJ-uspE terminator as in Template2 (Figure 2A).
Figure 2. Unidirectional transcription on templates containing a bidirectional terminator.
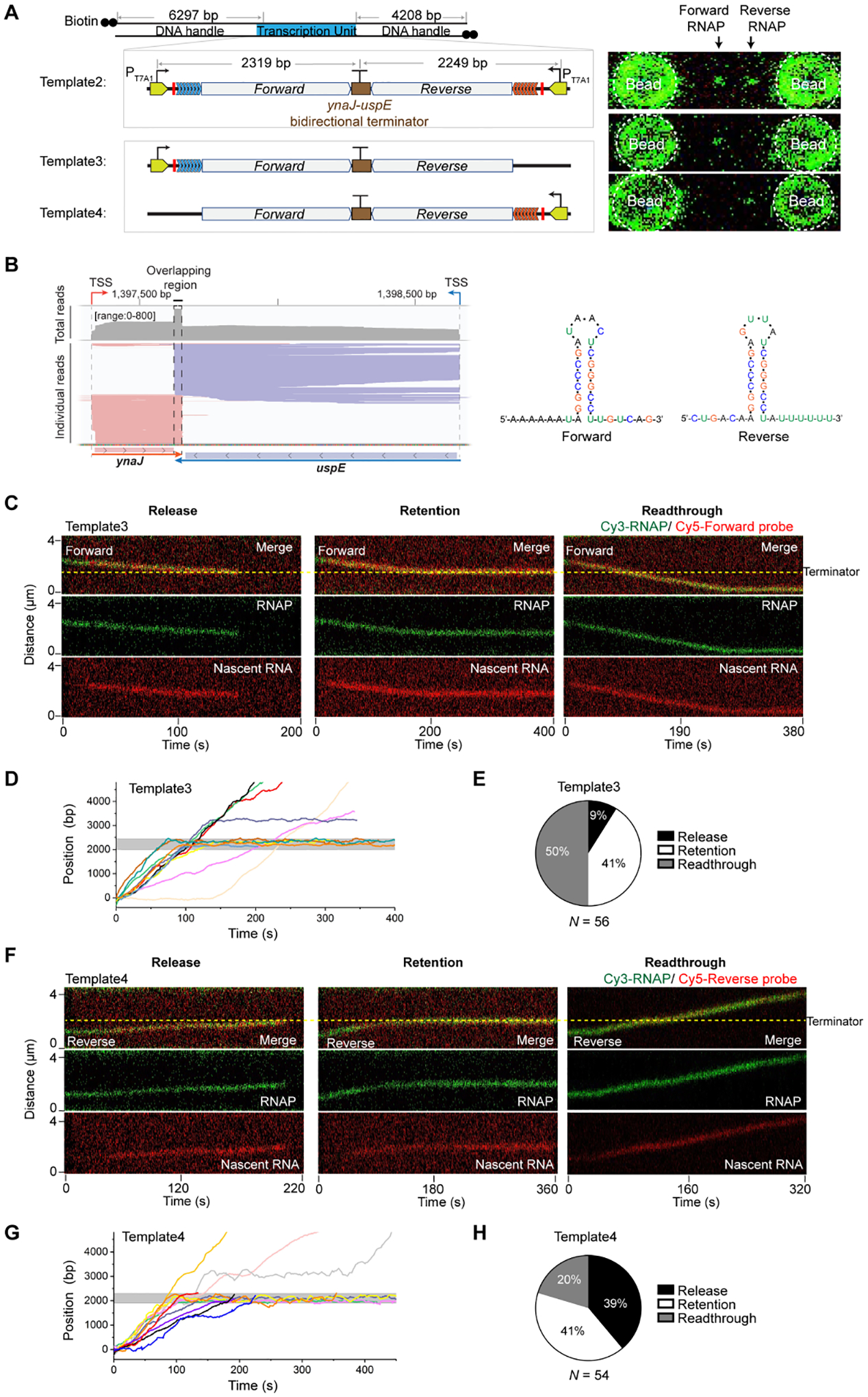
(A) (Left) Schematic of Template2 containing two opposing promoters and the ynaJ-uspE bidirectional terminator, Template3 containing the same bidirectional terminator but only one promoter in the forward direction, and Template4 containing the bidirectional terminator and one promoter in the reverse direction. (Right) 2D scan images showing Cy3-RNAPs at their respective stall sites on each template.
(B) (Left) SEnd-seq data track for the convergent ynaJ-uspE gene pair exhibiting strong bidirectional termination. (Right) Predicted secondary structures for the RNA transcribed from the overlapping region of the ynaJ-uspE bidirectional terminator sequence in both forward and reverse directions. This bidirectional terminator element was incorporated into Templates 2–4.
(C) Representative kymographs showing a forward EC (RNAP in green, nascent RNA in red) displaying the “Release” (Left), “Retention” (Middle), and “Readthrough” (Right) behavior at the bidirectional terminator on Template3.
(D) Ten individual RNAP trajectories on Template3 displaying distinct behaviors at the terminator (gray zone).
(E) Pie chart showing the fraction of forward EC release, retention, and readthrough events observed on Template3. N denotes the total number of ECs.
(F) Representative kymographs showing a reverse EC (RNAP in green, nascent RNA in red) displaying the “Release” (Left), “Retention” (Middle), and “Readthrough” (Right) behavior at the bidirectional terminator on Template4.
(G) Ten individual RNAP trajectories on Template4 displaying distinct behaviors at the terminator (gray zone).
(H) Pie chart showing the fraction of reverse EC release, retention, and readthrough events observed on Template4. N denotes the total number of ECs.
We observed that only 9% of restarted ECs on Template3 released their RNAP and RNA at the terminator, while the majority fell within the Retention (41%) or Readthrough (50%) category (Figure 2C–E). For the latter two categories, we can rule out the possibility that the nascent RNA was released at the terminator and rapidly reassociated to the RNAP in a nonspecific manner26,27, because the addition of heparin—which prevents released RNA from re-binding—did not increase the frequency of observing a Retention or Readthrough RNAP with no RNA bound. On Template4, the Release events were observed at a higher frequency (Figure 2F–H), an expected result given the longer 3’ U tract of the terminator hairpin in the reverse direction than in the forward direction (Figure 2B). Nevertheless, the Release fraction for Template4 was still much lower than that observed on Template1 (Figure 1G), indicating that the ynaJ-uspE terminator does not cause strong intrinsic termination in either direction.
Next, we used Template2 to examine the fate of ECs during convergent transcription. Satisfyingly, we observed that stalled ECs in both forward and reverse directions can restart, translocate towards each other, and collide within the termination zone (Figure 3A–B). The collided RNAPs never bypassed each other, thereby efficiently preventing transcriptional readthrough. This observation suggests that the opposing RNAP presents a strong physical barrier against transcription elongation, consistent with previous work28. The readthrough frequency in either direction on Template2 was significantly reduced compared to that observed on the corresponding unidirectional transcription template (Template3 and Template4) (Figure 3C). The readthrough RNAP across the terminator on Template2 can still be blocked by an opposite RNAP stalled within the convergent transcription unit (Figure S3).
Figure 3. Visualization of convergent transcription.
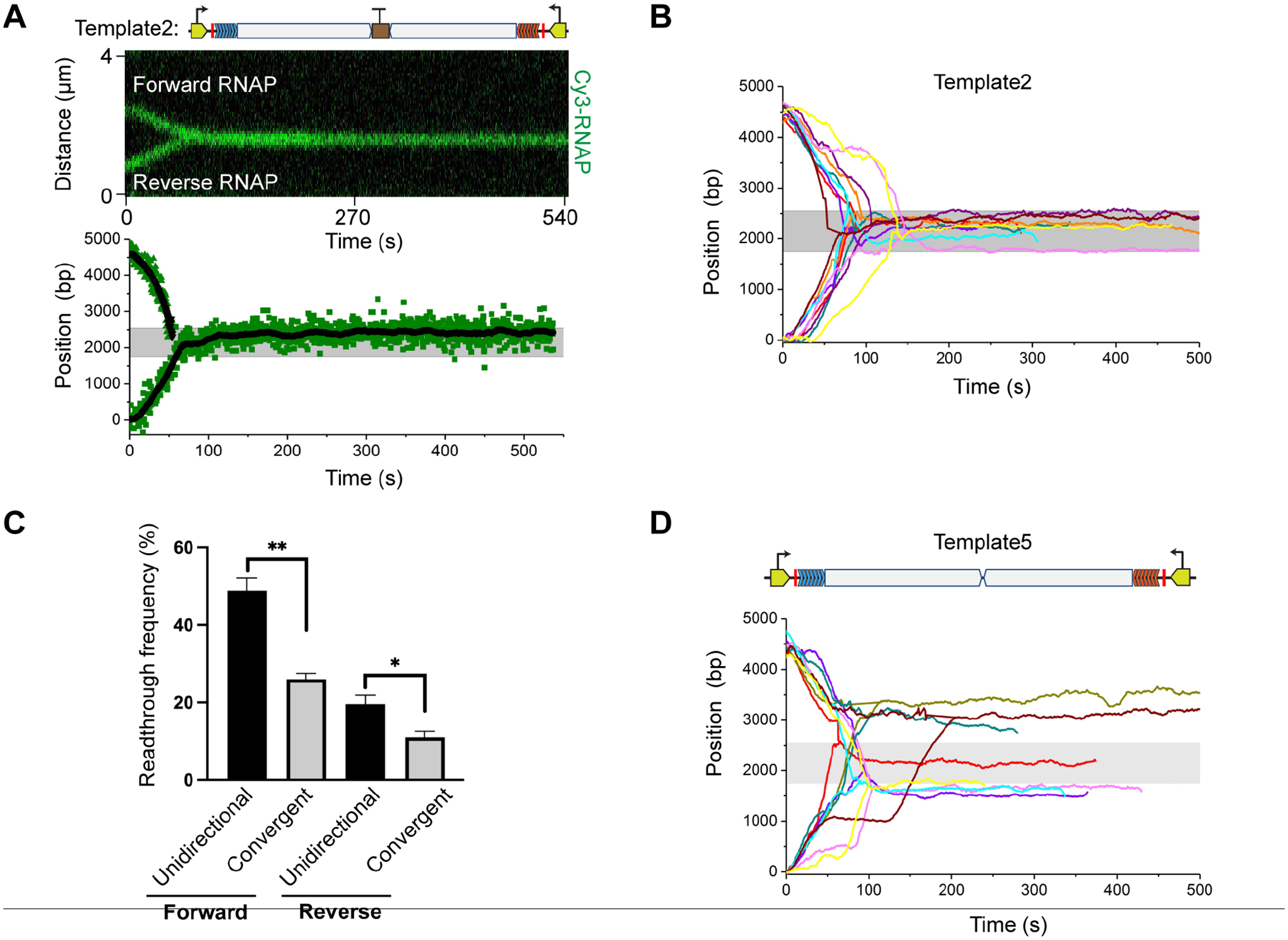
(A) (Top) A representative kymograph showing the convergence and head-on collision of two RNAPs on Template2. (Bottom) Extracted positions of the two RNAPs as a function of time for the same kymograph. Raw data and smoothed values (± 10-s moving average of tracked points) are shown in green and black dots, respectively. Gray region indicates the bidirectional terminator position (2250 ± 400 bp).
(B) Multiple trajectories of convergent RNAP pairs on Template2 overlaid showing that collisions occur uniformly within the termination zone.
(C) Frequencies of RNAP reading through the ynaJ-uspE terminator in the forward and reverse directions observed on DNA templates that only allow unidirectional transcription (Template3 and Template4; see Figure 2) versus on Template2 that allows convergent transcription. Data are represented as mean ± SEM from three independent datasets. Significance was determined using two-sided unpaired Student’s t-tests (*P < 0.05; **P < 0.01).
(D) Multiple trajectories of convergent RNAP pairs on Template5 that lacks the bidirectional terminator overlaid showing heterogeneous collision sites.
See also Figures S3 and S4.
Bidirectional terminator element is necessary for controlled collisions
How could the two converging RNAPs initially located thousands of base pairs apart always collide at the same position (i.e., the bidirectional terminator site)? We postulated that the DNA sequence at the collision site—even though it does not cause strong termination per se—may serve as a pausing signal for the converging ECs due to the RNA hairpin structure that it encodes. This would ensure that head-on collision events uniformly take place at the terminator site. The high Retention frequency observed on Template3 and Template4 supports this speculation (Figure 2E and 2H). To directly test this notion, we removed the bidirectional terminator element from Template2, creating Template5. Interestingly, single-molecule transcription experiments using Template5 yielded heterogeneous collision sites among different tethers (Figure 3D), in contrast to the homogeneous collision sites observed on Template2 (Figure 3B). This result is corroborated by bulk transcription assays, which showed RNA products of largely uniform sizes when the terminator element was present in the template but of varied lengths when the element was absent (Figure S4).
Fate of transcription complexes after the head-on collision
By monitoring the Cy3 fluorescence intensity, we noticed that in more than half of the head-on collision events, both RNAPs remained bound to the DNA after collision (i.e., the fluorescence intensity of the collided complex equaled the sum of the fluorescence intensities of both pre-collision RNAPs) (Figure 4A Left and Figure 4B). Only a minor fraction of events showed one RNAP dissociated from the DNA (post-collision fluorescence intensity matched the pre-collision value) (Figure 4A Middle) or both RNAPs dissociated after collision (fluorescence intensity dropped to zero) (Figure 4A Right). We then performed three-color imaging experiments to simultaneously track the fates of convergent RNAPs (by Cy3 fluorescence) and their respective nascent RNAs (by AlexaFluor488 and Cy5 fluorescence). We again observed that in the majority of the head-on collision events, the two converging ECs—including both RNAP and nascent RNA—were retained on the DNA (Figure 4C–D). Therefore, even though collision halts the converging ECs, it is often insufficient to dismantle the collided complex. Other factors must be involved to complete the collision-driven termination process.
Figure 4. Outcomes of head-on collisions at the bidirectional terminator.
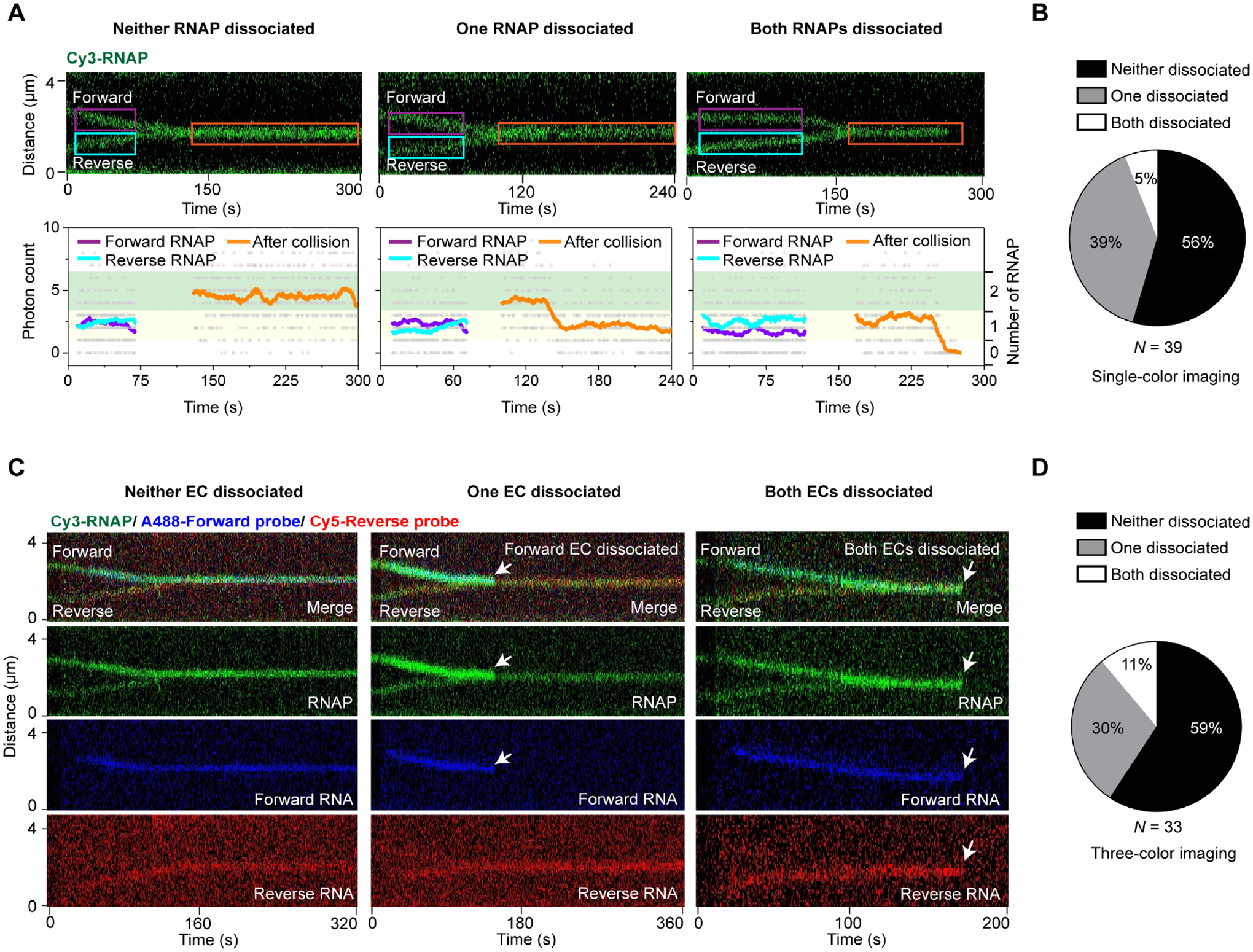
(A) Three possible outcomes of the head-on collision between two convergent RNAPs at the bidirectional terminator on Template2 (depicted in Figure 2A). (Top) Representative kymographs showing both RNAPs remaining on DNA (Left), one of the RNAPs dissociated (Middle), and both RNAPs dissociated (Right) after collision. (Bottom) Cy3 intensity profiles for the selected regions from the top kymographs (colored boxes). The summed pixel intensities of individual frames were displayed as gray dots. The filtered values (± 10-s average of frames) were shown as colored lines matching the colored boxes. The number of RNAPs within the selected regions were assigned based on the photon count: under 1 as zero RNAP; between 1 and 3.5 as one RNAP (yellow shade); between 3.5 and 6.5 as two RNAPs (green shade).
(B) Pie chart showing the fraction of each outcome illustrated in (A). N denotes the total number of collision events.
(C) Three-color imaging experiments using Cy3-RNAP (green) and AlexaFluor488- and Cy5-labeled oligo probes (blue and red) that hybridize to forward and reverse RNA, respectively. Representative kymographs showing both ECs remaining on DNA (Left), one of the ECs dissociated (Middle; forward EC dissociated in this example), and both ECs dissociated (Right) after collision. Some fluorescence crosstalk from the blue channel to the green channel was visible.
(D) Pie chart showing the fraction of each outcome illustrated in (C). N denotes the total number of collision events.
Screening for factors required for collision-driven termination
To identify the missing factor, we developed a bulk assay to evaluate the effect of several E. coli elongation and termination factors—namely Rho, NusA, NusG, Mfd, GreA and GreB29,30—on the collision outcome. In this assay, transcription took place on biotinylated DNA templates immobilized on magnetic streptavidin beads (Figure 5A). Dissociated transcription complexes upon termination were washed away, and the amount of complexes retained on the DNA was assessed by the intensity of fluorescent oligo probes on a gel. We observed a higher level of retained nascent RNA on Template2 that allows convergent transcription than on Template1 that contains an intrinsic terminator (Figure 5B), consistent with the single-molecule results described in Figure 1 and Figure 4. Of all the factors tested, only GreB induced a substantially reduced level of retained RNA on Template2 (Figure 5C). Notably, GreA did not confer the same effect, and GreB’s effect was only observed under the multi-round transcription condition (Figure 5D). When we used rifampicin to inhibit RNAP re-initiation, GreB no longer affected the level of retained RNA (Figure 5D). We then included GreB in the single-molecule experiments, which by design only allow single-round transcription. We found that most collided complexes still remained stable (i.e., neither RNAP dissociated) even in the presence of GreB (Figure 5E). However, we did notice that GreB significantly decreased the frequency of occurrence for arrested ECs in both forward and reverse directions (Figure 5F).
Figure 5. Screening for factors that facilitate the dissociation of head-on collided complexes.
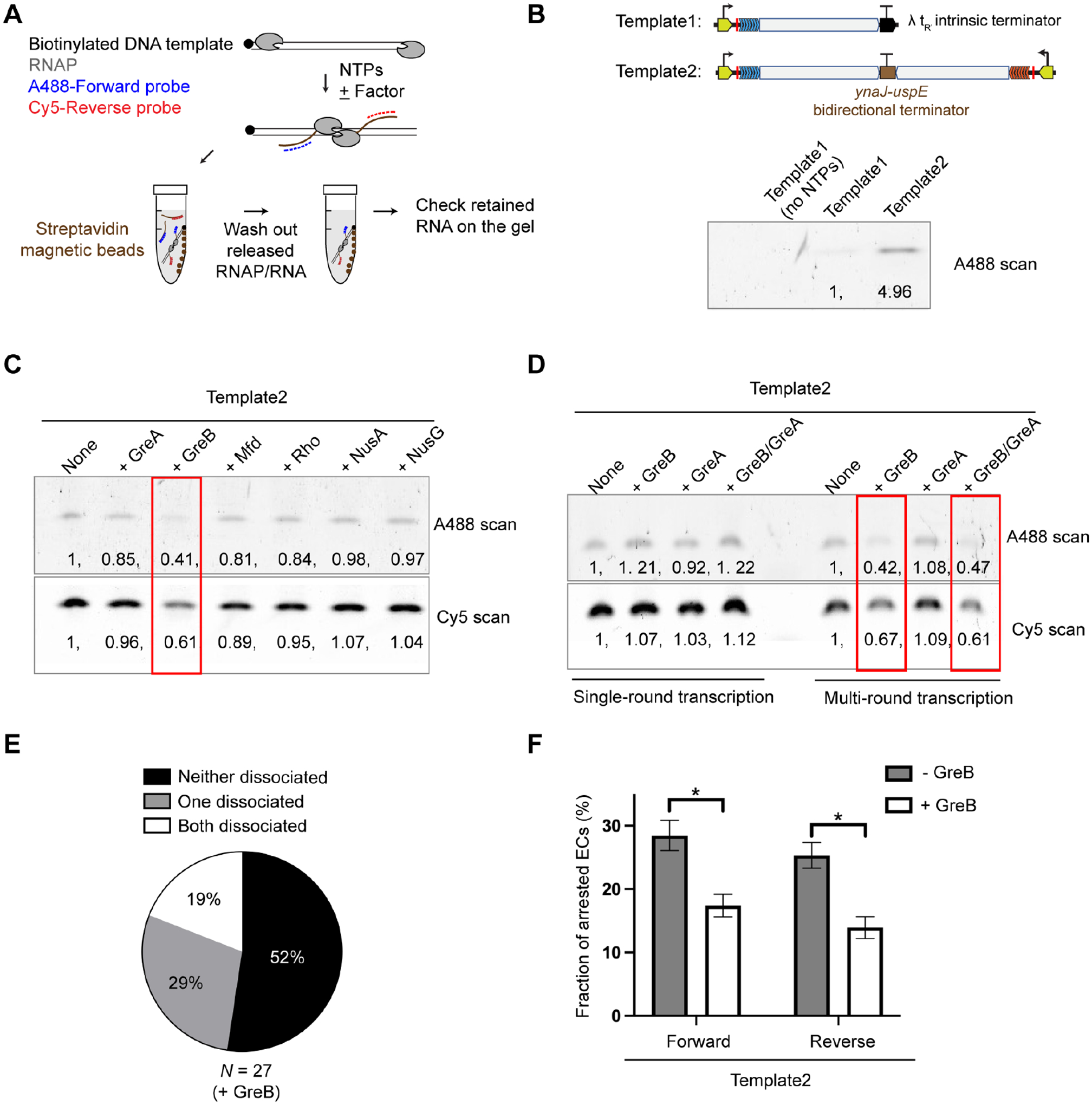
(A) Workflow of the bulk assay. In vitro transcription reactions were performed on biotinylated DNA templates in the presence of fluorescently labeled probes to detect nascent RNA. After reaction, DNA substrates were pulled down by streptavidin magnetic beads. After thoroughly washing out the released RNAP and RNA products, the amount of RNA retained on the DNA template was assessed by the probe intensity on a gel.
(B) Gel results for the assay described in (A) using DNA substrates derived from Template1 (with an intrinsic terminator) and Template2 (with a bidirectional terminator). AlexaFluor488-labeled probe intensities were normalized to the Template1 value.
(C) Gel results showing the effects of a panel of E. coli transcription factors on the amount of RNA products retained on Template2. AlexaFluor488-labeled forward probe and Cy5-labeled reverse probe intensities were normalized to the no-factor condition. The +GreB condition (outlined in red) exhibited reduced probe intensities.
(D) Gel results showing the effects of GreB and GreA on the amount of RNA retained on Template2 under single-round and multi-round transcription conditions. Probe intensities were normalized to the no-factor condition. The factors were added at a final concentration of 600 nM. Using higher concentrations did not qualitatively change the results.
(E) Pie chart showing the fraction of different outcomes for head-on collisions observed on Template2 in the presence of 600 nM GreB in the single-molecule assay. N denotes the total number of collision events.
(F) Fraction of arrested ECs (forward and reverse) observed on Template2 in the absence and presence of GreB. Data are represented as mean ± SEM from three independent datasets. Significance was determined using two-sided unpaired Student’s t-tests (*P < 0.05).
See also Figures S5 and S6.
GreB is a transcript cleavage factor that can rescue long backtracked RNAPs, as opposed to GreA that only rescues 1–2 bp short backtracked RNAPs31,32. We confirmed these activities of our GreA/B samples by assembling mismatched DNA:RNA scaffolds to mimic backtracked ECs (Figure S5). Based on these bulk and single-molecule results, we proposed that GreB increases the RNAP “trafficking density” on DNA by rescuing arrested ECs that have likely undergone backtracking, thereby elevating the likelihood of additional RNAPs running into a head-on collided complex (Figure S6). The combination of head-on and co-directional RNAP collisions then lead to efficient bidirectional transcription termination.
Multi-RNAP collision is key to bidirectional termination
If our interpretation for the GreB effect were true, we reasoned that creating a condition where multiple RNAPs can collide should yield a similar stimulatory effect on termination even without GreB. To test this idea, we constructed a three-promoter template (Template6) by placing an additional T7A1 forward promoter upstream of the original forward promoter in Template2 (Figure 6A). This template enabled us to stall three Cy3-RNAPs (two forward, one reverse) and then restart all of them to observe both head-on and co-directional collisions in succession. Remarkably, we found that most collisions that involved three RNAPs at the bidirectional terminator ended with the release of two RNAPs from the DNA (i.e., the Cy3 intensity at the collision site dropped to one-RNAP level) (Figure 6B). Next, we performed three-color experiments to distinguish between forward and reverse ECs. We observed that the head-on collided complex—formed by two leading ECs—was dislodged by a trailing EC (Figure 6C). Overall, after head-on and co-directional collisions had sequentially occurred at the bidirectional terminator, the final number of RNAPs remaining on the DNA was predominantly one, occasionally two, and rarely three (Figure 6D). These results lend support to our model that rear-ending by a trailing RNAP induces dissociation of the head-on collided complex. In other words, heavy RNAP trafficking, counterintuitively, alleviates the traffic jam rather than exacerbating it.
Figure 6. Outcomes of sequential head-on and co-directional collisions at the bidirectional terminator.
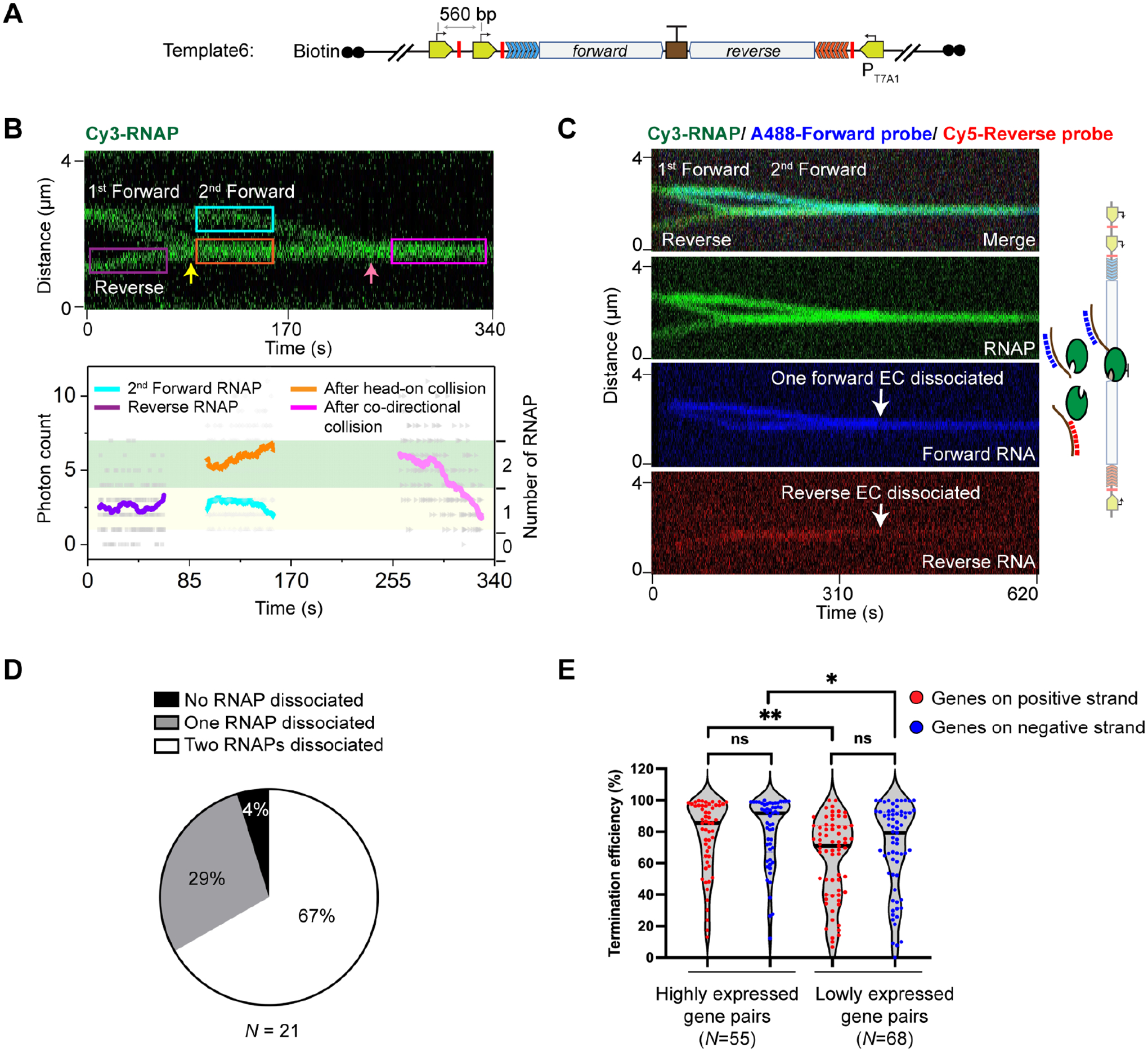
(A) Schematic of Template6, which contains an additional forward promoter and stall site compared to Template2.
(B) A representative kymograph (Top) and the corresponding fluorescence intensity profile (Bottom) showing a head-on collision (yellow arrow) followed by a co-directional collision (pink arrow) that involved two forward RNAPs and one reverse RNAP on Template6. The number of RNAPs for the selected regions (colored boxes) were analyzed and displayed in the same fashion as in Figure 4A. Two of the three RNAPs eventually dissociated in this example.
(C) A representative three-color kymograph (green for RNAP, blue for forward RNA, and red for reverse RNA) showing that co-directional collision by the second forward EC dislodged both forward and reverse ECs in the head-on collided complex from Template6 (white arrows).
(D) Pie chart showing the fraction of different outcomes (none, one, or two of the three RNAPs dissociated) after sequential head-on and co-directional collisions at the bidirectional terminator on Template6. N denotes the total number of sequential collision events.
(E) Violin plot showing the distribution of termination efficiencies for convergent E. coli gene pairs (positive-strand genes in red, negative-strand genes in blue) that were highly expressed (read counts > 100 for both genes) and lowly expressed (10 < read counts < 100 for both genes) in the SEnd-seq dataset. N denotes the number of gene pairs in each group. Significance was determined using two-sided unpaired Student’s t-tests (ns, not significant; *P < 0.05; **P < 0.01).
See also Figure S7.
This model then makes the prediction that a high bidirectional termination efficiency would correlate with strong convergent promoters from which RNAPs initiate frequently, creating more chances for collision. We thus analyzed our SEnd-seq dataset and indeed found that highly expressed gene pairs on average exhibited higher termination efficiencies than lowly expressed pairs (Figure 6E and S7).
DISCUSSION
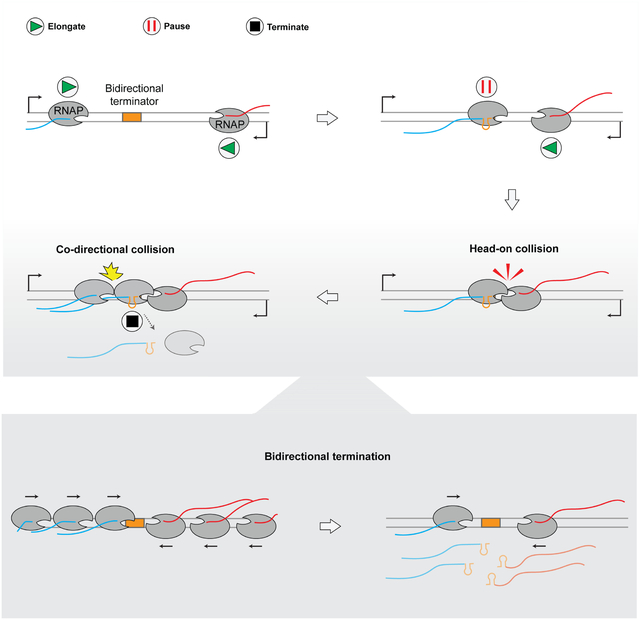
Based on results from this work, we propose a mechanism for transcription termination in addition to the well-documented intrinsic termination and Rho-dependent termination (Figure 7): (1) an RNA hairpin structure transcribed from the bidirectional terminator element causes pausing of the EC from either direction, which serves as a barrier to prevent the opposite EC from transcribing across the terminator, forming a stable head-on collided complex; (2) a trailing RNAP subsequently runs into the head-on collided complex, releasing one or both RNAPs within the complex and their associated RNA; (3) the remaining RNAP continues to block any incoming RNAP and the whole process repeats. Collisions occur more frequently for highly expressed convergent gene pairs and are assisted by transcription factors like GreB. This mechanism entails a direct link between the initiation phase and the termination phase of the transcription cycle, adding to the functional roles of cooperative RNAP trafficking in transcriptional regulation33,34.
Figure 7. A working model for RNAP-collision-driven bidirectional transcription termination.
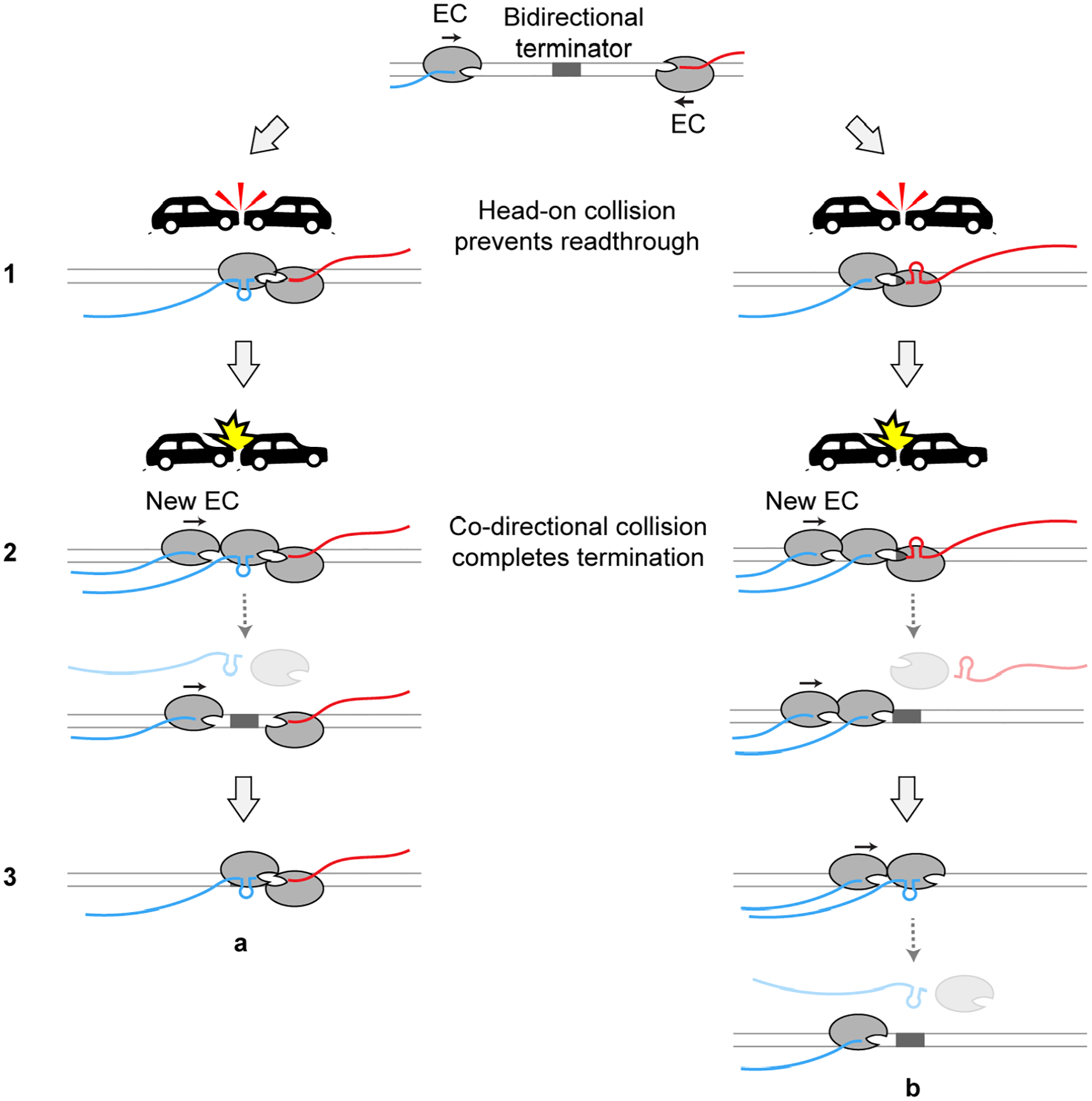
The RNA hairpin formed at the overlapping region of the bidirectional terminator induces pausing of the EC from either direction. An opposite RNAP collides into the paused RNAP, forming a stable head-on collided complex that tends not to dissociate by itself (Step 1). When a trailing RNAP newly initiated from the promoter runs into the head-on collided complex, the RNAP that has transcribed past the overlapping region is dislodged from the DNA along with its nascent RNA (Step 2). The trailing EC either transcribes the overlapping region and forms a new head-on collided complex with the remaining EC (Step 3, a), or pushes its leading EC across the overlapping region and dislodges it (Step 3, b). Note that the new EC in this illustration travels in the forward direction. A reciprocal scenario where the new EC travels in the reverse direction is not shown for simplicity.
The SEnd-seq data show that, during bidirectional termination, both forward and reverse RNAPs have moved past the hairpin-encoding region when they eventually terminate transcription (Figure 2B and S7). We speculate that the RNAP harboring the RNA hairpin is prone to dissociation upon successive head-on and co-directional collisions, while the other RNAP in the collided complex may undergo backtracking. In accordance with this view, we occasionally observed that when one RNAP dissociated after collision, the remaining RNAP resumed elongation and the frequency of such occurrences became higher if GreB was present, likely because GreB facilitates the restart of backtracked RNAPs. Elucidation of the structural details of the collided complexes awaits future studies.
Given the ubiquitous existence of convergent gene pairs and antisense transcripts7–13, programmed RNAP collisions may represent an evolutionarily conserved mechanism for transcription termination. In E. coli, this mechanism is coupled with the strategic placement of bidirectional terminator elements between convergent genes, a feature critical to shaping the 3’ boundaries of transcripts. It will be interesting to assess the prevalence of similar elements and their influence on the transcriptome in other species.
Collisions between DNA-based motors are pervasive and generally thought to be deleterious to genome stability35. Our work suggests that motor collisions can in some contexts be harnessed to control the output of gene expression and potentially be beneficial to cell fitness. By enabling real-time observation of motor trafficking on DNA, our single-molecule platform can be used to investigate a wide range of genomic conflicts and their physiological functions.
Limitation of the Study
Because of the limited spatial resolution of the confocal fluorescence microscopy, the current study did not answer the question of whether the RNAPs in the collided complex are in direct contact. Due to the relatively low throughput of the single-molecule assay, we did not survey other terminator sequences or experimental conditions where certain RNAP behaviors such as post-termination diffusion on DNA were reported36,37. Finally, it warrants further investigation whether other mechanical processes such as DNA supercoiling38 and transcription-translation coupling39 affect the outcome of collision-driven transcription termination.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Requests for reagents and further information should be directed to the lead contact, Shixin Liu (shixinliu@rockefeller.edu).
Material availability
Plasmids generated in this study are available upon request.
Data and code availability
All generated data have been deposited at Mendeley Data and are publicly available as of the date of publication. DOIs are listed in the key resources table.
All original code has been deposited at Zenodo and is publicly available as of the date of publication. DOIs are listed in the key resources table.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| Escherichia coli DH5α | Thermo Fisher | Cat# 18265017 |
| Escherichia coli BL21 (DE3) | Thermo Fisher | Cat# EC0114 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| AlexaFluor488 (A488) NHS ester | Thermo Fisher | Cat# A20000 |
| Cy3 maleimide mono-reactive dye | GE Healthcare | Cat# PA23031 |
| Cy5 NHS ester mono-reactive dye | GE Healthcare | Cat# PA15101 |
| RNaseOUT Recombinant ribonuclease inhibitor | Thermo Fisher | Cat# 10777019 |
| Gibson Assembly Master mix | New England Biolabs | Cat# E2611S |
| Klenow Fragment | New England Biolabs | Cat# M0212 |
| Biotin-11-dUTP | Thermo Fisher | Cat# R0081 |
| Biotin-14-dCTP | Thermo Fisher | Cat# 19518018 |
| Biotin-14-dATP | Thermo Fisher | Cat# 19524016 |
| Trolox | Sigma-Aldrich | Cat# 238813 |
| 4-Nitrobenzyl alcohol (NBA) | Sigma-Aldrich | Cat# N12821 |
| Cyclooctatetraene (COT) | Sigma-Aldrich | Cat# 138924 |
| Protocatechuate 3,4-Dioxygenase from Pseudomonas sp. (PCD) | Sigma-Aldrich | Cat# P8279 |
| 3,4-Dihydroxybenzoic acid (PCA) | Sigma-Aldrich | Cat# 37580 |
| Streptavidin-coated polystyrene particles (2.0–2.9 μm) | Spherotech | Cat# SVP-20-5 |
| Rifampicin | Sigma-Aldrich | Cat# R3501 |
| S6-RNAP | This paper | N/A |
| σ70 | 40 | N/A |
| Sfp synthase | 41 | N/A |
| Recombinant DNA | ||
| pVS11-Sfp-rpoC (expressing S6-RNAP) | This paper | N/A |
| pLW83 (Template1) | This paper | N/A |
| pLW86 (Template2) | This paper | N/A |
| pLW87 (Template3) | This paper | N/A |
| pLW88 (Template4) | This paper | N/A |
| pLW89 (Template5) | This paper | N/A |
| pLW103 (Template6) | This paper | N/A |
| Software and Algorithms | ||
| Bluelake | LUMICKS | https://lumicks.github.io/bluelake-api/ |
| Origin | OriginLab | https://www.originlab.com |
| Prism | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| Python scripts for single-molecule data analysis | This paper | doi:10.5281/zenodo.7618698 |
| Deposited data | ||
| Unprocessed single-molecule images | This paper | doi:10.17632/djgmvpxjj9.1 |
| Unprocessed gels | This paper | doi:10.17632/yp3x9k53gf.1 |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Bacterial strains and growth conditions
The Escherichia coli DH5α was used for all cloning and plasmid extraction. The E. coli BL21 (DE3) was used for all protein expression. Cells were grown in LB medium containing 100 μg/ml ampicillin or 50 μg/ml kanamycin as necessary.
METHOD DETAILS
DNA substrate preparation
DNA templates for single-molecule experiments
The 2.2-kbp transcription unit in Template1 (Table S1) is adapted from pCDW114 (a gift from Jeff Gelles, addgene plasmid #70061), which was shown to allow processive transcription and robust detection of nascent RNA by complementary probes42. It contains a λ PR’ promoter, a cassette harboring seven tandem repeats of a 21-bp sequence (5’-AGACACCACAGACCACACACA), a portion of the E. coli rpoB gene, and a λ tR’ terminator. To make Template1, pCDW114 was digested with EcoRI/BamHI to remove its original λ PR’ promoter. A 171-bp DNA fragment that contains a stall site located at the +39 position and flanking EcoRI/BamHI recognition sites (synthesized by GENEWIZ, Azenta Life Sciences) was inserted between those restriction sites. Additionally, a 4-kbp DNA fragment (PCR from the Myo7a gene) and a 5-kbp DNA fragment (PCR from the λ genome) were inserted upstream and downstream, respectively, of the transcription unit by Gibson Assembly (New England BioLabs). The assembled 13.8-kbp plasmid (pLW83) contains a unique XbaI cutting site that generates a linear DNA upon XbaI digestion where the transcription unit is flanked between two asymmetric DNA handles. The 5’ overhangs of the linearized plasmid were then filled in with biotinylated nucleotides by the exonuclease-deficient DNA polymerase I Klenow fragment (New England BioLabs) to create terminally biotinylated DNA for bead conjugation. The fill-in reaction was conducted by incubating 10 nM linearized plasmid DNA, 33 μM each of dGTP/biotin-14-dATP/biotin-11-dUTP/biotin-14-dCTP (Thermo Fisher), and 5 U of Klenow in 1x NEB2 buffer at room temperature for 15 min. To stop the reaction, EDTA was added at a final concentration of 10 mM and the reaction mixture was heat inactivated at 75 °C for 20 min. The DNA was then ethanol precipitated overnight at −20 °C by 3x volumes of cold ethanol and 300 mM NaOAc pH 5.2. Precipitated DNA was recovered by centrifugation at 15,000 rpm at 4 °C for 30 min. The DNA pellet was washed by 75% ethanol and then air-dried, resuspended in TE buffer (10 mM Tris-HCl pH 8.0, 1 mM EDTA), and stored at −20 °C.
To generate Template2, a 2.6-kbp partial convergent transcription unit was synthesized by GENEWIZ into a pUC19 vector. The synthesized DNA contains the ynaJ gene, ynaJ-uspE bidirectional terminator, uspE gene and a nearby gene from the E. coli genome, followed by the reverse repeat cassette (seven tandem repeats of a 19-bp sequence: 5’-CCCTATCCCTTATCTTAAC) and a reverse T7A1 promoter with the same stall site as Template1. The whole fragment was amplified and inserted into pLW83 to replace the λ tR’ intrinsic terminator by Gibson Assembly, yielding a full convergent transcription unit (Table S1). To unambiguously determine the orientation of the DNA tether, the downstream DNA handle was truncated by replacing the 5-kbp DNA fragment with a 3.8-kbp fragment (PCR from the λ genome), resulting in a 15-kbp plasmid (pLW86). Following XbaI digestion, a linear DNA containing the convergent transcription unit flanked by a 6.3-kbp DNA handle and a 4.2-kbp DNA handle on each side was produced. Terminally biotinylated DNA was generated in the same way as described above.
To make Template3 and Template4, either the reverse or the forward T7A1 promoter and repeat cassette were deleted from pLW86, yielding forward-only construct (plasmid pLW87) and reverse-only construct (plasmid pLW88). For Template5, the ynaJ-uspE bidirectional terminator was deleted from pLW86, yielding plasmid pLW89. For Template6, an additional T7A1 promoter and a stall site were inserted into a location 560 bp upstream of the forward T7A1 promoter of pLW86 by Gibson Assembly, yielding a three-promoter construct (plasmid pLW103). Linearized and terminally biotinylated DNA substrates were generated in the same way as described above.
DNA templates for bulk transcription assays
The DNA templates used in Figure S4 were prepared from each corresponding single-molecule construct by PCR with a universal pair of primers (LW8F: 5’-AACGCCAGCAACGCGGCCTTTTTACGGTTC; LW10R: 5’-CCTTATCTAAATTATAATGACGCACGATATG) that recognize the DNA handle regions of the plasmid and amplify the transcription unit region. The biotinylated DNA templates used in Figure 5B–D were prepared by PCR with the same primer sequences (LW8F/LW10R) containing a 5’ biotin modification (IDT).
Protein and oligonucleotide probe labeling
To site-specifically label the E. coli RNAP core enzyme, we engineered a 12-residue S6 peptide tag (GDSLSWLLRLLN)41 to the C-terminus of β’ subunit. Expression and purification of S6-RNAP (provided by Brandon Malone, Darst/Campbell Laboratory, Rockefeller University) followed the same procedure as previously described for wild-type RNAP40. For fluorescent labeling, the S6-RNAP protein, Sfp synthase and Cy3-CoA dye were incubated at a 1:2:5 molar ratio for 2 h at room temperature in the presence of 10 mM MgCl2. Excess dye and Sfp synthase were removed by a 100-kDa Amicon spin filter (Millipore). Final protein samples were aliquoted, flash frozen, and stored at −80 °C.
The oligonucleotide probes (Forward: 5’-GTGTGTGGTCTGTGGTGTCT; Reverse: 5’-GTTAAGATAAGGGATAGGG) were synthesized by IDT with a 5’ amino modification. The oligos were incubated with AlexaFluor488 NHS ester (Thermo Fisher) or Cy5 NHS ester (Cytiva) at room temperature for 3 h. Excess free dye was subsequently removed by a Sephadex G-25 column (Cytiva).
Single-molecule experiments
Formation of stalled elongation complexes
Cy3-labeled E. coli RNAP core enzyme was incubated with σ70 (provided by James Chen, Darst/Campbell Laboratory, Rockefeller University) at a 1:3 molar ratio for 2 h on ice. A 20-μl reaction mixture (4 μl of 100 ng/μl biotinylated DNA template, 5 μl of 3 μM Cy3-RNAP-σ70 holoenzyme, 4 μl of 5x transcription buffer (see below), 0.5 μl of RNaseOUT, and nuclease-free water) was incubated at 37 °C for 10 min. Transcription was initiated by the addition of 50 μM of ApU dinucleotide (TriLink), 150 μM each of ATP, UTP, and GTP (New England Biolabs) and incubation for 10 min at 37 °C. Heparin (Sigma-Aldrich) was then added to a final concentration of 100 μg/ml for another 5-min incubation at 37 °C. The final reaction mixture was diluted with 500 μl of 1x transcription buffer (25 mM Tris-HCl pH 7.5, 150 mM KCl, 10 mM MgCl2, 1mM DTT) before loading onto the single-molecule instrument.
Single-molecule data collection
Single-molecule experiments were performed at room temperature on a LUMICKS C-Trap instrument. Channels of the microfluidic chip were first passivated by flowing BSA (0.1% w/v in PBS, Sigma-Aldrich) and then Pluronic F127 (0.5% w/v in PBS, Sigma-Aldrich) for 10 min each. Streptavidin-coated polystyrene beads (2.07-μm diameter, Spherotech), stalled ECs, imaging buffer, imaging buffer with all four ribonucleotides (1 mM each) were injected into channels 1–4, respectively. Imaging buffer included an oxygen scavenging system [10 nM protocatechuate-3,4-dioxygenase (Sigma-Aldrich) and 2.5 mM protocatechuic acid (Sigma-Aldrich)] and a triplet-state quenching cocktail [1 mM cyclooctatetraene (Sigma-Aldrich), 1 mM 4-nitrobenzyl alcohol (Sigma-Aldrich) and 1 mM Trolox (Sigma-Aldrich)] in transcription buffer. A single DNA tether was caught between a pair of streptavidin beads in optical traps, and the tether was held under 5 pN of tension. The tether was moved to channel 3 for confocal scanning using a 532-nm laser to verify the existence of stalled EC. Empty DNA and protein aggregates were discarded during this step. A DNA tether containing the stalled EC was then moved to channel 4 to resume transcription. When needed, AlexaFluor488- and/or Cy5-labeled oligo probes (5 nM each) were included in channel 4 to detect nascent RNA. Kymographs were generated via confocal line scanning through the center of the two beads at 0.32 s/line (pixel time: 0.1 ms). Data acquisition was performed using the Bluelake software (LUMICKS).
Single-molecule data analysis
Single-molecule force and fluorescence data from the .h5 files generated from Bluelake were analyzed using tools in the lumicks.pylake Python library supplemented with other Python modules in a custom GUI Python script titled “CTrapVis.py” (http://harbor.lumicks.com/single-script/c5b103a4-0804-4b06-95d3-20a08d65768f), which was used to export confocal scans and kymographs in TIFF format, as well as to extract real-time force, distance, and photon count data.
Extracting the position of a translocating RNAP
Positions of a Cy3-RNAP along the DNA template as a function of time were extracted from kymographs by a custom Python script titled “kymotracker_calling_script.py” (https://harbor.lumicks.com/single-script/4db9d63e-1f93-0c0-9b90-e99066469578), which applies the lumicks.pylake Python package’s greedy line tracking algorithms to define line traces. In general, a pixel threshold of 1 photon count, line width of 5 pixels, window of 20 frames, minimum line length of 50 frames provided the best tracking of moving RNAPs. Extracted trajectories were further smoothed by averaging adjacent ± 10-s time points of each position. Individual trajectories were then aligned based on the starting position of stalled RNAPs. Traveling distance in nm was converted to bp using a conversion factor of 0.327 nm/bp for B-form DNA under 5 pN tension. When multiple RNAPs came in close proximity, discontinued lines resulted from overlapping fluorescence signals.
Extracting the fluorescence intensity of colliding RNAPs
A custom Python script titled “area_photon_count_extractor.py” (https://harbor.lumicks.com/single-script/23f33367-7b02-4762-9457-37348da59194) was used to extract the intensity of RNAP lines at user-defined regions to compare the intensity before and after collision. This method of intensity analysis provided more reliable results than summing the photon counts around the tracked lines because the line tracking analysis biases selection of frames by setting a pixel threshold. The summed photon counts across each line scan within the selected regions were smoothed by averaging adjacent ± 10-s time points of the individual scan values.
Bulk transcription assays
The general reaction mixture contained 0.5 μg of DNA template, 0.5 μl of E. coli RNAP holoenzyme (New England Biolabs), 4 μl of 5x reaction buffer (New England Biolabs), 0.5 μl of RNaseOUT (Thermo Fisher), and nuclease-free water to a final volume of 20 μl. The mixture was incubated at 37 °C for 10 min and then 1 μl of 20 mM ribonucleotide mix (New England Biolabs) was added to initiate transcription. After 10 min of incubation at 37 °C, the DNA template and proteins were digested with 0.3 μl of TURBO DNase (Thermo Fisher) for 10 min and then 0.5 μl of Proteinase K (Thermo Fisher) for 10 min. Reaction products were mixed with 2x RNA loading buffer (95% formamide, 0.02% SDS, 1 mM EDTA) and separated by 1% agarose gel in 0.5x TBE buffer. The gel was stained by SYBR Gold (Thermo Fisher) and scanned by Axygen Gel System (Corning).
Probing the effect of accessory factors on releasing head-on collided ECs
The expression plasmids for Rho, NusA, NusG, GreA and GreB were provided by Rachel Anne Mooney (Landick Laboratory, University of Wisconsin-Madison), and the proteins were purified by Rui Gong (Alushin Laboratory, Rockefeller University) following the protocols described previously43,44. The Mfd protein was provided by Joshua Brewer (Darst/Campbell Laboratory, Rockefeller University). All proteins were verified to be active by their respective functional assays. After incubating the above reaction mixture at 37 °C for 10 min, indicated factors were added at a final concentration of 600 nM, a value close to the working concentrations used in previous studies on these factors32,45–48, and incubated for another 5 min at 37 °C. Then 1 μl of 20 mM ribonucleotide mix, 0.5 μl of 2 μM AlexaFluor488-forward probe and 0.5 μl of 2 μM Cy5-reverse probe were added to the reaction and incubated for 10 min at 37 °C. After convergent transcription, 20 μl of streptavidin magnetic beads (New England Biolabs) were added to the reaction and mixed on a rotator for 20 min at room temperature. Released RNA and RNAP were washed out by 300 μl of 1x transcription buffer three times. The remaining products were mixed with 2x RNA loading buffer and loaded on a 8% Urea-PAGE gel after incubating at 90 °C for 5 min. The gel was scanned by Typhoon FLA 7000 (GE Healthcare) and quantified by ImageJ.
Single-round transcription
The same reaction mixture was incubated at 37 °C for 10 min and stalled ECs were formed by adding 50 μM of ApU dinucleotide, 150 μM each of ATP, UTP, and GTP for another 10 min at 37 °C. Transcription was restarted by supplying 1 mM ribonucleotide mix, as well as 50 μg/ml rifampicin (Sigma Aldrich) which blocks re-initiation. The reaction was incubated at 37 °C for 10 min in the presence of fluorescently labeled probes and the following procedures were the same as above.
GreA/B activity assays
The template DNA strand (5’-CTCTGAATCTCTTCCCCTCTAGCTTAGGACGTACTGACC), non-template DNA strand (5’-GGTCAGTACGTCCATTCGATCTCCCGAAGAGATTCAGAG), 15-nt Cy3-RNA (5’-AGCUAGA*G*G*UUUUUU; * denotes the phosphorothioate linkage designed to minimize cleavage by the intrinsic endonuclease activity of RNAP), and 11-nt Cy3-RNA (5’-AGCUAGA*G*G*UU) were synthesized by IDT. The two DNA strands were mixed and annealed in a buffer (10 mM Tris-HCl pH 8.0, 40 mM KCl, 5 mM MgCl2) by raising the temperature to 95 °C for 5 min and then slowly cooling down to 50 °C. Then an equal volume of the RNA strand was added and annealed to the DNA scaffold by incubating at 50 °C for 5 min and then slowly cooling down to 25 °C. The annealed DNA:RNA scaffolds were mixed with E. coli RNAP core enzyme (New England Biolabs) at 1:1 (vol/vol) and incubated at 37 °C for 10 min to form long-backtracked (using 15-nt Cy3-RNA) and short-backtracked (using 11-nt Cy3-RNA) ECs. The reconstituted backtracked ECs were stored at 4 °C and used within 2 h. The activity assays were performed at 37 °C for 10 min by adding GreB or GreA at a final concentration of 600 nM in the absence or presence of NTPs. The reactions were stopped by adding an equal volume of 2x RNA loading buffer and incubating at 90 °C for 2 min. Reaction products were separated on a 25% Urea-PAGE gel and visualized by Typhoon FLA 7000.
SEnd-seq data analysis
Bidirectional terminators were identified from our published SEnd-seq dataset6. Highly expressed convergent gene pairs were defined as those with >100 read counts for each gene in the pair; lowly expressed gene pairs were those with 10 < read counts < 100 for each gene in the pair.
QUANTIFICATION AND STATISTICAL ANALYSIS
The number of molecules or events analyzed is indicated in the figure legends. P values were determined using two-sided unpaired Student’s t-tests with Prism (GraphPad). The difference between two groups was considered statistically significant when P < 0.05 (*P < 0.05; **P < 0.01; ns, not significant).
Supplementary Material
Highlights.
Head-on collision between convergent RNAPs prevents transcriptional readthrough
Co-directional collision from a trailing RNAP helps disassemble the collided complex
Hairpin formed in the nascent RNA synchronizes the RNAPs for timed collisions
Higher RNAP trafficking density leads to more efficient transcription termination
ACKNOWLEDGMENTS
We thank J. Chen, B. Malone, J. Brewer (Darst/Campbell Laboratory, Rockefeller University), R. Gong (Alushin Laboratory, Rockefeller University), and R. Mooney (Landick Laboratory, University of Wisconsin-Madison) for sharing reagents and providing technical assistance. S.L. acknowledges support from the Robertson Foundation, the Alfred P. Sloan Foundation Matter-to-Life Program, the Pershing Square Sohn Cancer Research Alliance, and the National Institutes of Health (DP2HG010510).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
INCLUSION AND DIVERSITY
We support inclusive, diverse, and equitable conduct of research.
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures and one table.
REFERENCES
- 1.Porrua O, Boudvillain M, and Libri D (2016). Transcription Termination: Variations on Common Themes. Trends Genet 32, 508–522. 10.1016/j.tig.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Santangelo TJ, and Artsimovitch I (2011). Termination and antitermination: RNA polymerase runs a stop sign. Nat Rev Microbiol 9, 319–329. 10.1038/nrmicro2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Muse T, and Aguilera A (2016). Transcription-replication conflicts: how they occur and how they are resolved. Nat Rev Mol Cell Biol 17, 553–563. 10.1038/nrm.2016.88. [DOI] [PubMed] [Google Scholar]
- 4.Ray-Soni A, Bellecourt MJ, and Landick R (2016). Mechanisms of Bacterial Transcription Termination: All Good Things Must End. Annu Rev Biochem 85, 319–347. 10.1146/annurev-biochem-060815-014844. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JW (2019). Mechanisms of Bacterial Transcription Termination. J Mol Biol 431, 4030–4039. 10.1016/j.jmb.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Ju X, Li D, and Liu S (2019). Full-length RNA profiling reveals pervasive bidirectional transcription terminators in bacteria. Nat Microbiol 4, 1907–1918. 10.1038/s41564-019-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callen BP, Shearwin KE, and Egan JB (2004). Transcriptional interference between convergent promoters caused by elongation over the promoter. Mol Cell 14, 647–656. DOI 10.1016/j.molcel.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Gullerova M, and Proudfoot NJ (2012). Convergent transcription induces transcriptional gene silencing in fission yeast and mammalian cells. Nat Struct Mol Biol 19, 1193–1201. 10.1038/nsmb.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobson DJ, Wei W, Steinmetz LM, and Svejstrup JQ (2012). RNA polymerase II collision interrupts convergent transcription. Mol Cell 48, 365–374. 10.1016/j.molcel.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prescott EM, and Proudfoot NJ (2002). Transcriptional collision between convergent genes in budding yeast. P Natl Acad Sci USA 99, 8796–8801. 10.1073/pnas.132270899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yelin R, Dahary D, Sorek R, Levanon EY, Goldstein O, Shoshan A, Diber A, Biton S, Tamir Y, Khosravi R, et al. (2003). Widespread occurrence of antisense transcription in the human genome. Nat Biotechnol 21, 379–386. 10.1038/nbt808. [DOI] [PubMed] [Google Scholar]
- 12.Shearwin KE, Callen BP, and Egan JB (2005). Transcriptional interference - a crash course. Trends Genet 21, 339–345. 10.1016/j.tig.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georg J, and Hess WR (2018). Widespread Antisense Transcription in Prokaryotes. Microbiol Spectr 6. 10.1128/microbiolspec.RWR-0029-2018. [DOI] [PubMed] [Google Scholar]
- 14.Bai L, Santangelo TJ, and Wang MD (2006). Single-molecule analysis of RNA polymerase transcription. Annu Rev Biophys Biomol Struct 35, 343–360. 10.1146/annurev.biophys.35.010406.150153. [DOI] [PubMed] [Google Scholar]
- 15.Larson MH, Landick R, and Block SM (2011). Single-molecule studies of RNA polymerase: one singular sensation, every little step it takes. Mol Cell 41, 249–262. 10.1016/j.molcel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, and Greene EC (2011). Single-Molecule Studies of Transcription: From One RNA Polymerase at a Time to the Gene Expression Profile of a Cell. Journal of Molecular Biology 412, 814–831. 10.1016/j.jmb.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dangkulwanich M, Ishibashi T, Bintu L, and Bustamante C (2014). Molecular Mechanisms of Transcription through Single-Molecule Experiments. Chem Rev 114, 3203–3223. 10.1021/cr400730x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CY, and Myong S (2021). Probing steps in DNA transcription using single-molecule methods. J Biol Chem 297(3). 10.1016/j.jbc.2021.101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman LJ, and Gelles J (2012). Mechanism of Transcription Initiation at an Activator-Dependent Promoter Defined by Single-Molecule Observation. Cell 148, 679–689. 10.1016/j.cell.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbondanzieri EA, Greenleaf WJ, Shaevitz JW, Landick R, and Block SM (2005). Direct observation of base-pair stepping by RNA polymerase. Nature 438, 460–465. 10.1038/nature04268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Candelli A, Wuite GJ, and Peterman EJ (2011). Combining optical trapping, fluorescence microscopy and micro-fluidics for single molecule studies of DNA-protein interactions. Phys Chem Chem Phys 13, 7263–7272. 10.1039/c0cp02844d. [DOI] [PubMed] [Google Scholar]
- 22.Wasserman MR, Schauer GD, O’Donnell ME, and Liu S (2019). Replication Fork Activation Is Enabled by a Single-Stranded DNA Gate in CMG Helicase. Cell 178, 600–611 e616. 10.1016/j.cell.2019.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schafer DA, Gelles J, Sheetz MP, and Landick R (1991). Transcription by single molecules of RNA polymerase observed by light microscopy. Nature 352, 444–448. 10.1038/352444a0. [DOI] [PubMed] [Google Scholar]
- 24.Wang MD, Schnitzer MJ, Yin H, Landick R, Gelles J, and Block SM (1998). Force and velocity measured for single molecules of RNA polymerase. Science 282, 902–907. 10.1126/science.282.5390.902. [DOI] [PubMed] [Google Scholar]
- 25.Rees WA, Weitzel SE, Das A, and vonHippel PH (1997). Regulation of the elongation-termination decision at intrinsic terminators by antitermination protein N of phage lambda. Journal of Molecular Biology 273, 797–813. 10.1006/jmbi.1997.1327. [DOI] [PubMed] [Google Scholar]
- 26.Kashlev M, and Komissarova N (2002). Transcription termination: primary intermediates and secondary adducts. J Biol Chem 277, 14501–14508. 10.1074/jbc.M200215200. [DOI] [PubMed] [Google Scholar]
- 27.Berlin V, and Yanofsky C (1983). Release of transcript and template during transcription termination at the trp operon attenuator. J Biol Chem 258, 1714–1719. [PubMed] [Google Scholar]
- 28.Crampton N, Bonass WA, Kirkham J, Rivetti C, and Thomson NH (2006). Collision events between RNA polymerases in convergent transcription studied by atomic force microscopy. Nucleic Acids Research 34, 5416–5425. 10.1093/nar/gkl668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Washburn RS, and Gottesman ME (2015). Regulation of transcription elongation and termination. Biomolecules 5, 1063–1078. 10.3390/biom5021063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zenkin N, and Yuzenkova Y (2015). New Insights into the Functions of Transcription Factors that Bind the RNA Polymerase Secondary Channel. Biomolecules 5, 1195–1209. 10.3390/biom5031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borukhov S, Sagitov V, and Goldfarb A (1993). Transcript cleavage factors from E. coli. Cell 72, 459–466. 10.1016/0092-8674(93)90121-6. [DOI] [PubMed] [Google Scholar]
- 32.Marr MT, and Roberts JW (2000). Function of transcription cleavage factors GreA and GreB at a regulatory pause site. Mol Cell 6, 1275–1285. 10.1016/s1097-2765(00)00126-x. [DOI] [PubMed] [Google Scholar]
- 33.Epshtein V, and Nudler E (2003). Cooperation between RNA polymerase molecules in transcription elongation. Science 300, 801–805. 10.1126/science.1083219. [DOI] [PubMed] [Google Scholar]
- 34.Jin J, Bai L, Johnson DS, Fulbright RM, Kireeva ML, Kashlev M, and Wang MD (2010). Synergistic action of RNA polymerases in overcoming the nucleosomal barrier. Nat Struct Mol Biol 17, 745–U122. 10.1038/nsmb.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le TT, and Wang MD (2018). Molecular Highways-Navigating Collisions of DNA Motor Proteins. Journal of Molecular Biology 430, 4513–4524. 10.1016/j.jmb.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Harden TT, Herlambang KS, Chamberlain M, Lalanne JB, Wells CD, Li GW, Landick R, Hochschild A, Kondev J, and Gelles J (2020). Alternative transcription cycle for bacterial RNA polymerase. Nat Commun 11, 448. 10.1038/s41467-019-14208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang W, Ha KS, Uhm H, Park K, Lee JY, Hohng S, and Kang C (2020). Transcription reinitiation by recycling RNA polymerase that diffuses on DNA after releasing terminated RNA. Nat Commun 11, 450. 10.1038/s41467-019-14200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S, Beltran B, Irnov I, and Jacobs-Wagner C (2019). Long-Distance Cooperative and Antagonistic RNA Polymerase Dynamics via DNA Supercoiling. Cell 179, 106–119 e116. 10.1016/j.cell.2019.08.033. [DOI] [PubMed] [Google Scholar]
- 39.Proshkin S, Rahmouni AR, Mironov A, and Nudler E (2010). Cooperation Between Translating Ribosomes and RNA Polymerase in Transcription Elongation. Science 328, 504–508. 10.1126/science.1184939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J, Gopalkrishnan S, Chiu C, Chen AY, Campbell EA, Gourse RL, Ross W, and Darst SA (2019). E. coli TraR allosterically regulates transcription initiation by altering RNA polymerase conformation. Elife 8. 10.7554/eLife.49375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Z, Cironi P, Lin AJ, Xu Y, Hrvatin S, Golan DE, Silver PA, Walsh CT, and Yin J (2007). Genetically encoded short peptide tags for orthogonal protein labeling by Sfp and AcpS phosphopantetheinyl transferases. ACS Chem Biol 2, 337–346. 10.1021/cb700054k. [DOI] [PubMed] [Google Scholar]
- 42.Harden TT, Wells CD, Friedman LJ, Landick R, Hochschild A, Kondev J, and Gelles J (2016). Bacterial RNA polymerase can retain sigma70 throughout transcription. Proc Natl Acad Sci U S A 113, 602–607. 10.1073/pnas.1513899113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hao ZT, Epshtein V, Kim KH, Proshkin S, Svetlov V, Kamarthapu V, Bharati B, Mironov A, Walz T, and Nudler E (2021). Pre-termination Transcription Complex: Structure and Function. Mol Cell 81, 281–292. 10.1016/j.molcel.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perederina AA, Vassylyeva MN, Berezin IA, Svetlov V, Artsimovitch I, and Vassylyev DG (2006). Cloning, expression, purification, crystallization and initial crystallographic analysis of transcription elongation factors GreB from Escherichia coli and Gfh1 from Thermus thermophilus. Acta Crystallogr Sect F Struct Biol Cryst Commun 62, 44–46. 10.1107/S1744309105040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tetone LE, Friedman LJ, Osborne ML, Ravi H, Kyzer S, Stumper SK, Mooney RA, Landick R, and Gelles J (2017). Dynamics of GreB-RNA polymerase interaction allow a proofreading accessory protein to patrol for transcription complexes needing rescue. Proc Natl Acad Sci U S A 114, E1081–E1090. 10.1073/pnas.1616525114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deaconescu AM, Chambers AL, Smith AJ, Nickels BE, Hochschild A, Savery NJ, and Darst SA (2006). Structural basis for bacterial transcription-coupled DNA repair. Cell 124, 507–520. 10.1016/j.cell.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 47.Mooney RA, Schweimer K, Rosch P, Gottesman M, and Landick R (2009). Two structurally independent domains of E. coli NusG create regulatory plasticity via distinct interactions with RNA polymerase and regulators. J Mol Biol 391, 341–358. 10.1016/j.jmb.2009.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gusarov I, and Nudler E (2001). Control of intrinsic transcription termination by N and NusA: the basic mechanisms. Cell 107, 437–449. 10.1016/s0092-8674(01)00582-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All generated data have been deposited at Mendeley Data and are publicly available as of the date of publication. DOIs are listed in the key resources table.
All original code has been deposited at Zenodo and is publicly available as of the date of publication. DOIs are listed in the key resources table.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| Escherichia coli DH5α | Thermo Fisher | Cat# 18265017 |
| Escherichia coli BL21 (DE3) | Thermo Fisher | Cat# EC0114 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| AlexaFluor488 (A488) NHS ester | Thermo Fisher | Cat# A20000 |
| Cy3 maleimide mono-reactive dye | GE Healthcare | Cat# PA23031 |
| Cy5 NHS ester mono-reactive dye | GE Healthcare | Cat# PA15101 |
| RNaseOUT Recombinant ribonuclease inhibitor | Thermo Fisher | Cat# 10777019 |
| Gibson Assembly Master mix | New England Biolabs | Cat# E2611S |
| Klenow Fragment | New England Biolabs | Cat# M0212 |
| Biotin-11-dUTP | Thermo Fisher | Cat# R0081 |
| Biotin-14-dCTP | Thermo Fisher | Cat# 19518018 |
| Biotin-14-dATP | Thermo Fisher | Cat# 19524016 |
| Trolox | Sigma-Aldrich | Cat# 238813 |
| 4-Nitrobenzyl alcohol (NBA) | Sigma-Aldrich | Cat# N12821 |
| Cyclooctatetraene (COT) | Sigma-Aldrich | Cat# 138924 |
| Protocatechuate 3,4-Dioxygenase from Pseudomonas sp. (PCD) | Sigma-Aldrich | Cat# P8279 |
| 3,4-Dihydroxybenzoic acid (PCA) | Sigma-Aldrich | Cat# 37580 |
| Streptavidin-coated polystyrene particles (2.0–2.9 μm) | Spherotech | Cat# SVP-20-5 |
| Rifampicin | Sigma-Aldrich | Cat# R3501 |
| S6-RNAP | This paper | N/A |
| σ70 | 40 | N/A |
| Sfp synthase | 41 | N/A |
| Recombinant DNA | ||
| pVS11-Sfp-rpoC (expressing S6-RNAP) | This paper | N/A |
| pLW83 (Template1) | This paper | N/A |
| pLW86 (Template2) | This paper | N/A |
| pLW87 (Template3) | This paper | N/A |
| pLW88 (Template4) | This paper | N/A |
| pLW89 (Template5) | This paper | N/A |
| pLW103 (Template6) | This paper | N/A |
| Software and Algorithms | ||
| Bluelake | LUMICKS | https://lumicks.github.io/bluelake-api/ |
| Origin | OriginLab | https://www.originlab.com |
| Prism | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| Python scripts for single-molecule data analysis | This paper | doi:10.5281/zenodo.7618698 |
| Deposited data | ||
| Unprocessed single-molecule images | This paper | doi:10.17632/djgmvpxjj9.1 |
| Unprocessed gels | This paper | doi:10.17632/yp3x9k53gf.1 |


