Abstract
This retrospective pooled analysis aims to identify factors predicting relapse despite a pathologic complete response (pCR) in patients with breast cancer (BC). 2066 patients with a pCR from five neoadjuvant GBG/AGO-B trials fulfill the inclusion criteria of this analysis. Primary endpoint is disease-free survival (DFS); secondary endpoints is distant DFS (DDFS) and overall survival (OS). After a median follow-up of 57.6 months, DFS is significantly worse for patients with positive lymph nodes (cN+ vs cN0 hazard ratio [HR] 1.94, 95%CI 1.48–2.54; p < 0.001). In patients with triple-negative tumors, lobular histology (lobular vs other HR 3.55, 95%CI 1.53–8.23; p = 0.003), and clinical nodal involvement (cN+ vs cN0 HR 2.45, 95%CI 1.59–3.79; p < 0.001) predict a higher risk of DFS events. Patients with HER2-positive cT3/4 tumors have a significantly higher risk of relapse (cT3/4 vs cT1 HR 2.07, 95%CI 1.06–4.03; p = 0.033). Initial tumor load and histological type predict relapse in patients with a pCR.
Subject terms: Breast cancer, Outcomes research
Introduction
Neoadjuvant chemotherapy as part of a multimodal approach has been widely used for decades in patients with inflammatory, locally advanced, or inoperable breast cancer to achieve or facilitate operability and increase both local and systemic control1. In operable breast cancer, a recently published meta-analysis by the EBCTCG demonstrated that neoadjuvant chemotherapy was as effective as adjuvant chemotherapy in reducing the risk of distant recurrence and death from breast cancer. The rates of breast-conserving surgery were increased with the neoadjuvant approach and clinical response was highest in patients with HER2-positive and triple-negative breast cancer (TNBC)2.
Several individual trials and two patient-level meta-analyses showed that a pathological complete response (pCR) after neoadjuvant therapy is associated with improved event-free and overall survival3–5. The magnitude of this effect varied by molecular subtype and was most likely seen in patients with HER2-positive or triple-negative disease but also some luminal B-like tumors. In contrast, the long-term outcome of patients without a pCR is generally poor. Two recent randomized trials demonstrated that in these patients adding additional treatment after surgery - in case of TNBC capecitabine and in case of HER2-positive breast cancer TDM-1 - improved disease-free survival (DFS) and/or overall survival6,7. Although pCR rates have significantly increased with the implementation of targeted treatments and patients with pCR have a better long-term outcome than those without pCR, still 15–20% of patients with a pCR will relapse within the first 5 years. A better understanding of which patients will relapse despite a pCR may guide further treatment following surgery especially in patients with more aggressive tumors (like triple-negative or HER2-positive disease). Thus, we aimed to identify factors predicting relapse in patients with a pCR.
The presented study is a pooled analysis of the five neoadjuvant GBG/AGO-B trials GeparTrio, GeparQuattro, GeparQuinto, GeparSixto, and GeparSepto8–21 investigating factors predicting a relapse despite a pCR in the entire cohort and in subgroups according to tumor types.
Results
Patient characteristics
Between 2002 and 2013, a total of 7933 patients were recruited within the GeparTrio, GeparQuattro, GeparQuinto, GeparSixto, and GeparSepto trial, of whom 2066 (26%) had a pCR. The median age in patients with a pCR was 48 (range 21–75) years, 55% (n = 1125) were cN0 and 19% (n = 385) had a more advanced cT3/4 tumor at diagnosis. Most patients had a non-lobular histological tumor type (97%, n = 1997) while 40% (n = 805) and 39% (n = 780) of the patients had TNBC or HER2-positive disease, respectively. 1,41 (62%) patients had a high-grade tumor (G3) (Table 1).
Table 1.
Patient, tumor and treatment characteristics.
| Parameter | Category | GeparTrio N = 298 | GeparQuattro N = 370 | GeparQuinto N = 636 | GeparSixto N = 296 | GeparSepto N = 466 | All patients N = 2066 |
|---|---|---|---|---|---|---|---|
| N (valid %) | N (valid %) | N (valid %) | N (valid %) | N (valid %) | N (valid %) | ||
| Age, years | Median (min, max) | 45 (25–74) | 49 (22–70) | 48 (21–74) | 46 (21–73) | 49 (22–75) | 48 (21–75) |
| <30 | 9 (3.0) | 8 (2.2) | 27 (4.2) | 13 (4.4) | 17 (3.6) | 74 (3.6) | |
| 30−40 | 80 (26.8) | 49 (13.2) | 91 (14.3) | 55 (18.6) | 68 (14.6) | 343 (16.6) | |
| 40−50 | 107 (35.9) | 138 (37.3) | 253 (39.8) | 109 (36.8) | 166 (35.6) | 773 (37.4) | |
| 50−60 | 64 (21.5) | 106 (28.6) | 175 (27.5) | 82 (27.7) | 120 (25.8) | 547 (26.5) | |
| 60−70 | 35 (11.7) | 66 (17.8) | 79 (12.4) | 31 (10.5) | 80 (17.2) | 291 (14.1) | |
| 70+ | 3 (1.0) | 3 (0.8) | 11 (1.7) | 6 (2.0) | 15 (3.2) | 38 (1.8) | |
| BMI | <25 kg/m2 | 169 (56.9) | 196 (53.0) | 321 (50.5) | 166 (56.1) | 243 (52.1) | 1095 (53.0) |
| 25–<30 kg/m2 | 93 (31.3) | 116 (31.4) | 202 (31.8) | 93 (31.4) | 134 (28.8) | 638 (30.9) | |
| ≥30 kg/m2 | 35 (11.8) | 58 (15.7) | 113 (17.8) | 37 (12.5) | 89 (19.1) | 332 (16.1) | |
| missing | 1 | 0 | 0 | 0 | 0 | 1 | |
| Menopausal status | premenopausal | 181 (61.1) | 217 (58.6) | 378 (60.1) | 199 (67.2) | 267 (57.3) | 1242 (60.4) |
| postmenopausal | 115 (38.9) | 153 (41.4) | 251 (39.9) | 97 (32.8) | 199 (42.7) | 815 (39.6) | |
| missing | 2 | 0 | 7 | 0 | 0 | 9 | |
| cT | cT1 | 5 (1.7) | 18 (4.9) | 127 (20.0) | 98 (33.3) | 181 (39.0) | 429 (20.8) |
| cT2 | 228 (76.8) | 275 (74.3) | 359 (56.4) | 156 (53.1) | 229 (49.4) | 1247 (60.5) | |
| cT3 | 42 (14.1) | 32 (8.6) | 78 (12.3) | 29 (9.9) | 28 (6.0) | 209 (10.1) | |
| cT4 | 22 (7.4) | 45 (12.2) | 72 (11.3) | 11 (3.7) | 26 (5.6) | 176 (8.5) | |
| missing | 1 | 0 | 0 | 2 | 2 | 5 | |
| cN | cN0 | 145 (49.2) | 162 (43.8) | 318 (50.3) | 186 (64.1) | 314 (68.4) | 1125 (55.0) |
| cN1 | 137 (46.4) | 189 (51.1) | 275 (43.5) | 89 (30.7) | 136 (29.6) | 826 (40.4) | |
| cN2 | 8 (2.7) | 18 (4.9) | 31 (4.9) | 13 (4.5) | 5 (1.1) | 75 (3.7) | |
| cN3 | 5 (1.7) | 1 (0.3) | 8 (1.3) | 2 (0.7) | 4 (0.9) | 20 (1.0) | |
| missing | 3 | 0 | 4 | 6 | 7 | 20 | |
| ER/PgR | Both ER, PgR negative | 177 (65.6) | 215 (58.1) | 396 (62.3) | 236 (79.7) | 191 (41.0) | 1215 (59.6) |
| ER and/or PgR positive | 93 (34.4) | 155 (41.9) | 240 (37.7) | 60 (20.3) | 275 (59.0) | 823 (40.4) | |
| missing | 28 | 0 | 0 | 0 | 0 | 28 | |
| HER2 status | Negative | 244 (100) | 202 (54.6) | 425 (66.8) | 157 (53.0) | 204 (43.8) | 1232 (61.2) |
| Positive | 0 (0.0) | 168 (45.4) | 211 (33.2) | 139 (47.0) | 262 (56.2) | 780 (38.8) | |
| missing | 54 | 0 | 0 | 0 | 0 | 54 | |
| Biological subtype | HER2-/HR + | 84 (36.4) | 80 (21.6) | 154 (24.2) | 0 (0.0) | 96 (20.6) | 414 (20.7) |
| TNBC | 147 (63.6) | 122 (33.0) | 271 (42.6) | 157 (53.0) | 108 (23.2) | 805 (40.3) | |
| HER2 + /HR + | 0 (0.0) | 75 (20.3) | 86 (13.5) | 60 (20.3) | 179 (38.4) | 400 (20.0) | |
| HER2 + /HR- | 0 (0.0) | 93 (25.1) | 125 (19.7) | 79 (26.7) | 83 (17.8) | 380 (19.0) | |
| missing | 67 | 0 | 0 | 0 | 0 | 67 | |
| Tumor grading | Grade 1 | 5 (2.0) | 6 (1.7) | 5 (0.8) | 5 (1.7) | 7 (1.5) | 28 (1.4) |
| Grade 2 | 94 (36.7) | 156 (44.2) | 236 (37.3) | 83 (28.0) | 165 (35.4) | 734 (36.6) | |
| Grade 3 | 157 (61.3) | 191 (54.1) | 391 (61.9) | 208 (70.3) | 294 (63.1) | 1241 (62.0) | |
| missing | 42 | 17 | 4 | 0 | 0 | 63 | |
| Histological tumor type | Lobular subtype | 19 (6.4) | 17 (4.6) | 22 (3.5) | 1 (0.3) | 8 (1.7) | 67 (3.2) |
| Non-lobular | 278 (93.6) | 353 (95.4) | 613 (96.5) | 295 (99.7) | 458 (98.3) | 1997 (96.8) | |
| missing | 1 | 0 | 1 | 0 | 0 | 2 | |
| Ki-67, % | Median (min, max) | 46.5 (1.5−97.5) | 40.0 (0-90.0) | 40 (1.0-100) | 40.0 (3.0−95.0) | 40.0 (3.0−95.0) | 40.0 (0.0−100) |
| ≤20% | 25 (15.2) | 22 (25.3) | 44 (18.8) | 52 (17.6) | 101 (21.7) | 244 (19.6) | |
| >20% | 139 (84.8) | 65 (74.7) | 190 (81.2) | 244 (82.4) | 365 (78.3) | 1003 (80.4) | |
| missing | 134 | 283 | 402 | 0 | 0 | 819 | |
|
Number of cycles of chemo-therapy scheduled |
≤6 | 160 (53.7) | 0 (0.0) | 0 (0.0) | 296 (100) | 0 (0.0) | 456 (22.1) |
| >6 | 138 (46.3) | 370 (100) | 636 (100) | 0 (0.0) | 466 (100) | 1610 (77.9) | |
| Clinical response after 2–4 cycles | Complete response | 87 (29.3) | 61 (16.5) | 118 (18.8) | 40 (13.8) | 93 (20.8) | 399 (19.6) |
| Partial response | 195 (65.7) | 270 (73.0) | 471 (75.0) | 203 (70.0) | 288 (64.4) | 1427 (70.2) | |
| Stable disease | 15 (5.1) | 39 (10.5) | 38 (6.1) | 42 (14.5) | 51 (11.4) | 185 (9.1) | |
| Progress | 0 (0.0) | 0 (0.0) | 1 (0.2) | 5 (1.7) | 15 (3.4) | 21 (1.0) | |
| missing | 1 | 0 | 8 | 6 | 19 | 34 | |
| Residual CIS | ypT0 | 254 (85.2) | 273 (73.8) | 501 (78.8) | 237 (80.1) | 407 (87.3) | 1672 (80.9) |
| ypTis | 44 (14.8) | 97 (26.2) | 135 (21.2) | 59 (19.9) | 59 (12.7) | 394 (19.1) |
Data are N and valid % unless otherwise stated; BMI body mass index, CIS carcinoma in situ, ER estrogen receptor, PgR progesterone receptor, HER2 human epidermal growth factor receptor 2, HR hormone receptor, TNBC triple-negative breast cancer.
After a median follow-up of 58.0 months (IQR 47.0–73.6), 269 events for DFS, 184 for DDFS, and 118 for OS were observed. Survival curves showing patients at risk by one year increment are provided in Supplementary Figure 1a–c. Residual in situ disease was associated with a significantly worse DFS, but not DDFS or OS. Median follow-up for the most recent studies GeparSixto and GeparSepto was shorter (48.4 and 49.7 months, respectively), thus an estimation of 4-year survival rates was reported in the following.
Association of potential risk factors and outcome
1892 patients with a pCR and non-missing risk factors were included in multivariate Cox regression analyses of potential risk factors. In the entire cohort, DFS, DDFS, and OS were significantly worse for patients with a positive cN status at baseline (cN+ vs cN0 hazard ratio 1.94, 95%CI 1.48–2.54, p < 0.001 for DFS; hazard ratio 2.29, 95%CI 1.64–3.19, p < 0.001 for DDFS; hazard ratio 1.98, 95%CI 1.31–2.98, p = 0.001 for OS) (Fig. 1a–c). A worse DDFS and OS was seen in patients with lobular tumor type (lobular vs other tumor type hazard ratio 1.95, 95% CI 1.02–3.70, p = 0.043 for DDFS; hazard ratio 2.47, 95%CI 1.19–5.10, p = 0.015 for OS) (Fig 1b, c). The results for the various biological subtypes were inconsistent for DFS, DDFS, and OS (Fig. 1a–c). Patients with TNBC patients and patients with HER2 + /HR- tumors had a significantly shorter DFS compared to the rest (TNBC vs HER2-/HR + hazard ratio 1.49, 95%CI 1.03–2.17, p = 0.036 and HER2 + /HR- vs HER2-/HR + hazard ratio 1.61, 95%CI 1.06–2.45, p = 0.027). OS was significantly worse in TNBC patients compared to HR + /HER2- patients (TNBC vs HER2-/HR + hazard ratio 1.84, 95%CI 1.06–3.21, p = 0.031).
Fig. 1. Multivariate Cox regression models for disease-free survival (a), distant disease-free survival (b) and overall survival (c) for the total population. Error bars represent the 95%CI.
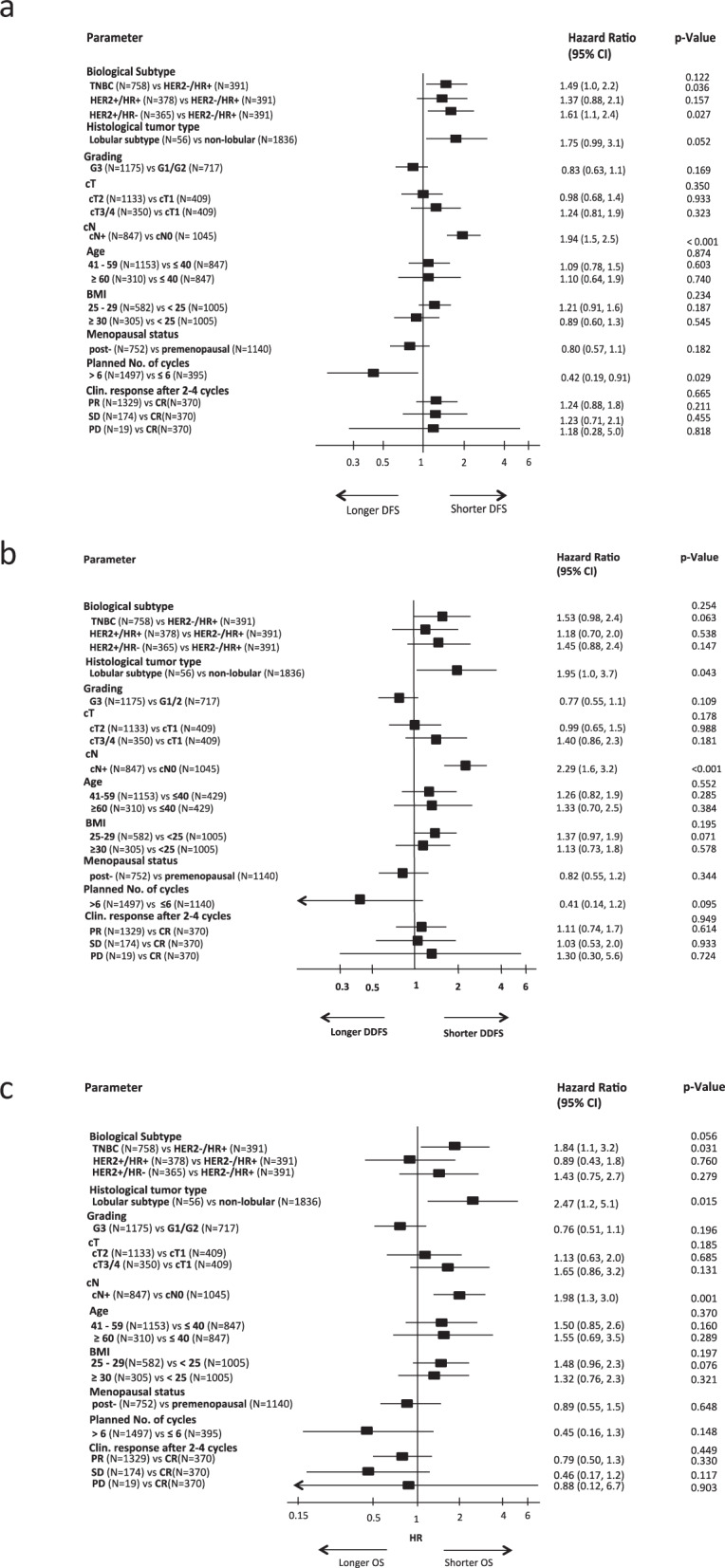
HR hazard ratio, CI confidence interval, HER2 human epidermal growth factor receptor 2, TNBC triple-negative breast cancer.
Potential risk factors and outcome in TNBC
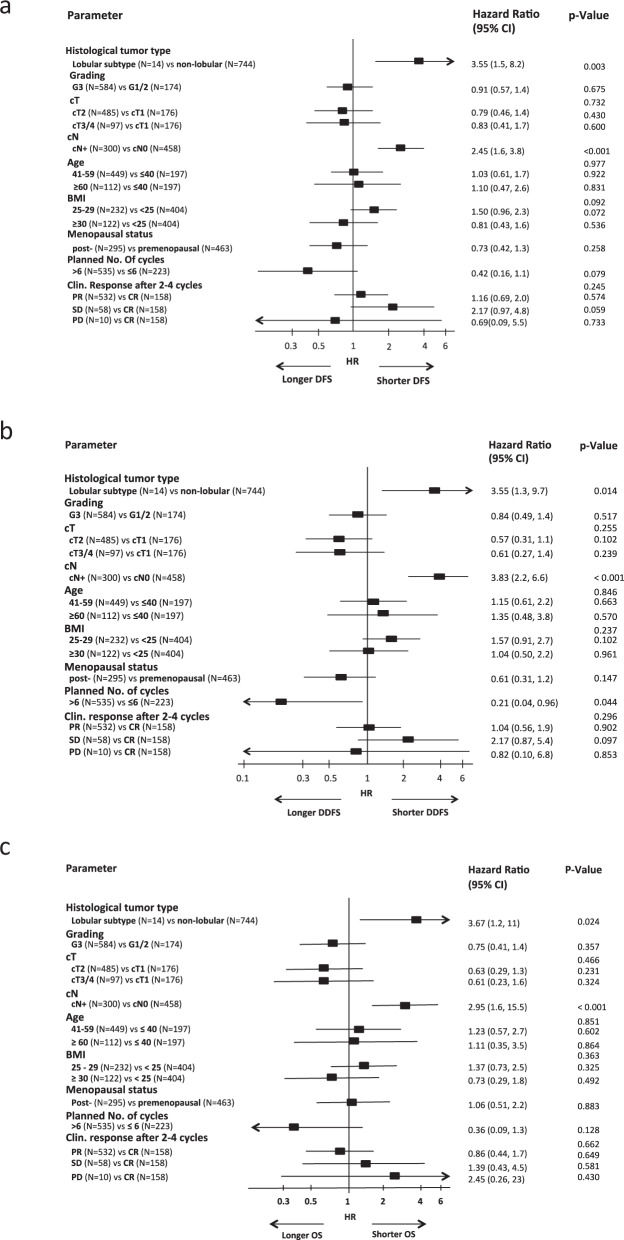
Multivariate Cox regression analyses in biological tumor subtypes revealed that TNBC patients with a pCR were at higher risk for a DFS, DDFS, and OS event in case of lobular histology (DFS hazard ratio 3.55, 95%CI 1.53–8.23, p = 0.003, DDFS hazard ratio 3.55, 95%CI 1.30–9.73, p = 0.014 and OS hazard ratio 3.67, 95%CI 1.19–11.4, p = 0.024). Clinically positive lymph nodes at baseline were also associated with a higher risk for a shorter DFS, DDFS, and OS (DFS hazard ratio 2.45, 95%CI 1.59–3.79, p < 0.001; DDFS hazard ratio 3.83, 95%CI 2.22–6.61, p < 0.001 and OS hazard ratio 2.95, 95%CI 1.58–5.50, p < 0.001) (Fig. 2a–c). Interestingly, patients with >6 scheduled cycles of neoadjuvant treatment had a longer DDFS (hazard ratio 0.21, 95%CI 0.04–0.96, p = 0.044) (Fig. 2b) compared to patients with a shorter therapy.
Fig. 2. Multivariate Cox regression models for disease-free survival (a), distant disease-free survival (b) and overall survival (c) in TNBC cohort. Error bars represent the 95%CI.
HR hazard ratio, CI confidence interval, HER2 human epidermal growth factor receptor 2, TNBC triple-negative breast cancer.
Potential risk factors and outcome in patients with HER2 + disease
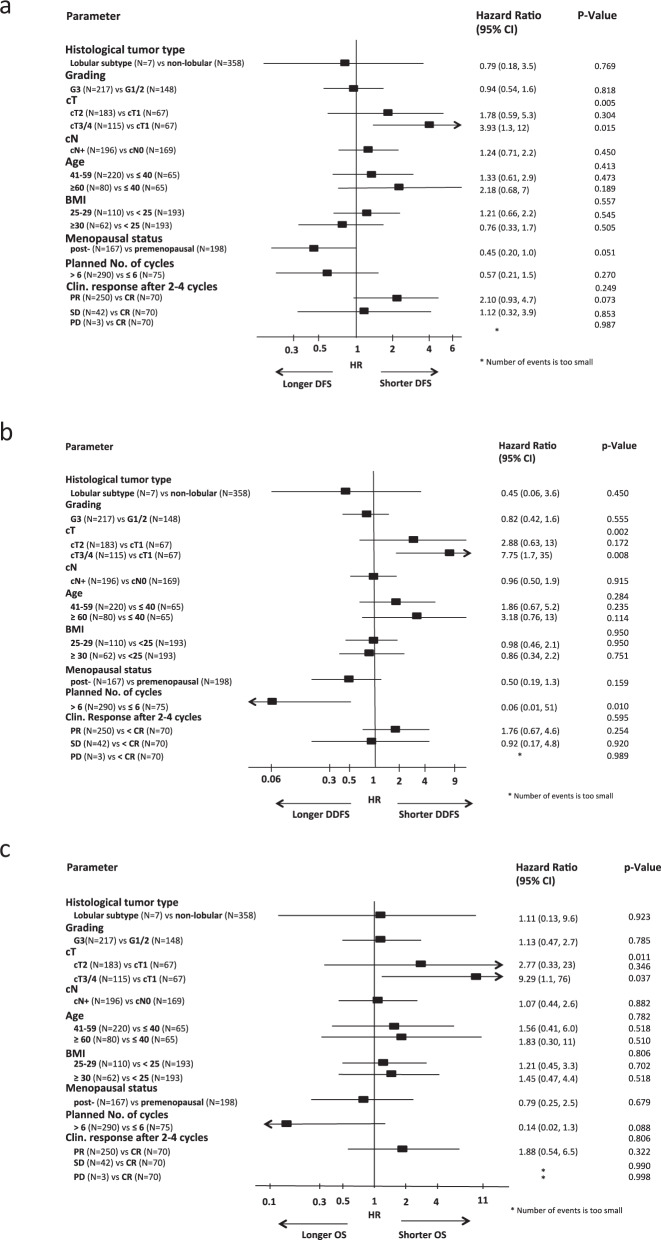
Patients with HER2-positive cT3/4 tumors were at a significantly higher risk for a DFS, DDFS, and OS event compared to HER2-positive cT1 tumors (DFS hazard ratio 2.07, 95%CI 1.06–4.03, p = 0.033; DDFS hazard ratio 3.28, 95%CI 1.38–7.83, p = 0.007; OS hazard ratio 3.42, 95%CI 1.08–10.8, p = 0.036; multivariate Cox regression analyses). This effect was mainly seen in the HER2 + /HR- subtype (DFS hazard ratio 3.93, 95%CI 1.30–11.8, p = 0.015; DDFS: hazard ratio 7.75, 95%CI 1.70–35.2, p = 0.008; OS: hazard ratio 9.29, 95%CI 1.14–75.7, p = 0.037; multivariate Cox regression analyses) (Fig. 3a–c). The risk for a DDFS event was lower in patients with HER2 + /HR- tumors receiving >6 cycles of planned treatment (hazard ratio 0.06, 95%CI 0.01–0.51; p = 0.010; multivariate Cox regression analyses). This was not the case for DFS or OS.
Fig. 3. Multivariate Cox regression models for disease-free survival (a), distant disease-free survival (b) and overall survival (c) in HER2 + /HR- cohort. Error bars represent the 95%CI.
HR hazard ratio, CI confidence interval, HER2 human epidermal growth factor receptor 2, TNBC triple-negative breast cancer.
In the subgroup of HER2 + /HR + breast cancer, none of the potential risk factors significantly predisposed patients to a DFS or OS event (Fig. 4a–c). Only a positive nodal status at baseline significantly increased the risk for a DDFS event in all pts (hazard ratio 2.28, 95%CI 1.02–5.12, p = 0.046; multivariate Cox regression analyses).
Fig. 4. Multivariate Cox regression models for disease-free survival (a), distant disease-free survival (b) and overall survival (c) in HER2 + /HR + cohort. Error bars represent the 95%CI.
HR hazard ratio, CI confidence interval, HER2 human epidermal growth factor receptor 2, TNBC triple-negative breast cancer.
In the subgroup of HER2-/HR + breast cancer receiving neoadjuvant chemotherapy, pCR patients with cN+ at baseline had a higher risk for a DFS event than cN0 patients (hazard ratio 2.24, 95%CI 1.18–4.25, p = 0.013; multivariate Cox regression analyses) but none of the other potential risk factors significantly increased the risk for a DDFS or OS event.
4-year DFS, DDFS, and OS rates overall and in subgroups
The 4-year DFS, DDFS, and OS rates are presented in Table 2. At 4 years DFS rates were 91.9% for HER2-/HR + , 90.2% for TNBC, 89.0% for HER2 + /HR + and 87.9% for HER2 + /HR- patients. Lobular compared to other histological subtypes was associated with worse DFS, DDFS, and OS rates. Lower DFS, DDFS, and OS rates at 4 years were also detected for cT3/4 tumors compared to cT1 or cT2 tumors and for cN+ compared to cN0 status at baseline. 4-year survival rates of breast cancer subtypes according to HER2 and HR status are presented in detail in Table 3. Of clinical interest, the risk of having a DFS event within 4 years was > 10% in HER2 + patients with cT3/4 tumors or nodal involvement at primary diagnosis.
Table 2.
Four-year rates for DFS, DDFS, and OS according to subgroups.
| DFS rate (95% CI) | DDFS rate (95% CI) | OS rate (95% CI) | |
|---|---|---|---|
| Overall | 89.6% (88.1%, 90.9%) | 92.5% (91.2%, 93.6%) | 95.2% (94.2%, 96.1%) |
| Subgroups | |||
| cT1 | 91.2% (87.9%, 93.6%) | 93.9% (91.0%, 95.8%) | 96.1% (93.7%, 97.7%) |
| cT2 | 90.2% (88.3%, 91.8%) | 93.4% (91.7%, 94.7%) | 96.0% (94.6%, 97.0%) |
| cT3/4 | 85.7% (81.5%, 89.0%) | 88.0% (84.1%, 91.0%) | 91.8% (88.4%, 94.3%) |
| cN0 | 92.2% (90.4%, 93.7%) | 95.0% (93.4%, 96.2%) | 96.9% (95.6%, 97.8%) |
| cN+ | 86.5% (83.9%, 88.6%) | 89.5% (87.3%, 91.4%) | 93.3% (91.4%, 94.8%) |
| Lobular tumor type | 82.6% (70.7%, 90.0%) | 87.4% (76.4%, 93.5%) | 90.6% (80.3%, 95.7%) |
| Non-lobular tumor type | 89.8% (88.3%, 91.1%) | 92.6% (91.3%, 93.8%) | 95.4% (94.3%, 96.3%) |
| HER2-/HR + | 91.9% (88.6%, 94.3%) | 94.7%(91.8%, 96.5%) | 97.0%(94.7%, 98.3%) |
| TNBC | 90.2% (87.8%, 92.2%) | 92.9%(90.8%, 94.5%) | 94.6%(92.6%, 96.0%) |
| HER2 + /HR + | 89.0% (85.2%, 91.8%) | 91.4%(88.0%, 93.9%) | 96.8%(94.3%, 98.2%) |
| HER2 + /HR- | 87.9% (84.0%, 90.9%) | 91.2%(87.6%, 93.7%) | 94.2%(91.2%, 96.2%) |
| Ki-67 ≤ 20% | 89.9%(85.0%, 93.2%) | 91.9%(87.4%, 94.8%) | 96.5%(93.1%, 98.2%) |
| Ki-67 > 20% | 89.9%(87.8%, 91.7%) | 92.9%(91.1%, 94.4%) | 95.5%(93.9%, 96.7%) |
| Grade 1/2 | 88.1%(85.4%, 90.3%) | 91.0%(88.6%, 92.9%) | 95.1%(93.2%, 96.5%) |
| Grade 3 | 90.6%(88.7%, 92.2%) | 93.1%(91.5%, 94.4%) | 95.2%(93.7%, 96.3%) |
| Age ≤ 40 years | 90.1%(86.9%, 92.6%) | 93.6%(90.9%, 95.5%) | 95.6%(93.3%, 97.2%) |
| Age 41–59 years | 89.1%(87.1%, 90.8%) | 92.1%(90.3%, 93.5%) | 95.2%(93.8%, 96.3%) |
| Age ≥ 60 years | 90.8%(86.9%, 93.5%) | 92.4%(88.8%, 94.9%) | 94.7%(91.5%, 96.7%) |
| BMI < 25 | 90.4%(88.3%, 92.1%) | 94.0%(92.3%, 95.3%) | 95.9%(94.5%, 97.0%) |
| BMI 25–29 | 87.7%(84.7%, 90.1%) | 89.9%(87.1%, 92.1%) | 94.2%(91.9%, 95.8%) |
| BMI ≥ 30 | 90.7%(86.8%, 93.5%) | 92.5%(88.9%, 94.9%) | 95.0%(91.8%, 97.0%) |
| Premenopausal | 88.7%(86.7%, 90.4%) | 92.3%(90.6%, 93.8%) | 95.3%(93.9%, 96.4%) |
| Postmenopausal | 90.8%(88.5%, 92.7%) | 92.6%(90.5%, 94.3%) | 95.1%(93.3%, 96.5%) |
| ≤6 planned CT cycles | 89.1%(85.7%, 91.8%) | 91.5%(88.4%, 93.9%) | 93.7%(90.8%, 95.7%) |
| >6 planned CT cycles | 89.7%(88.1%, 91.2%) | 92.8%(91.3%, 94.0%) | 95.7%(94.5%, 96.6%) |
| Complete response after 2–4 cycles | 91.7%(88.4%, 94.2%) | 93.4%(90.4%, 95.6%) | 94.2%(91.3%, 96.2%) |
| Partial response after 2–4 cycles | 89.0%(87.1%, 90.6%) | 92.2%(90.6%, 93.5%) | 95.1%(93.8%, 96.2%) |
| Stable disease after 2–4 cycles | 90.0% (84.1%, 93.8%) | 93.3%(88.2%, 96.2%) | 97.5% (93.5%, 99.1%) |
| Progress after 2–4 cycles | 84.7%(59.7%, 94.8%) | 84.1%(58.3%, 94.6%) | 94.7%(68.1%, 99.2%) |
| ypT0 | 90.8% (89.2%, 92.2%) | 93.0% (91.6%, 94.1%) | 95.4% (94.2%, 96.3%) |
| ypTis | 84.5% (80.3%, 87.9%) | 90.5% (86.9%, 93.1%) | 94.8% (91.8%, 96.7%) |
BMI body mass index, DDFS distant disease-free survival, DFS disease-free survival, ER estrogen receptor, PgR progesterone receptor, HER2 human epidermal growth factor receptor 2, HR hormone receptor, OS overall survival, TNBC triple-negative breast cancer
Table 3.
Four-year rates for DFS, DDFS, and OS in subgroups by biological subtype.
| HER2-/HR + | TNBC | HER2 + /HR + | HER2 + /HR- | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroups | DFS rate (95% CI) | DDFS rate (95% CI) | OS rate (95% CI) | DFS rate (95% CI) | DDFS rate (95% CI) | OS rate (95% CI) | DFS rate (95% CI) | DDFS rate (95% CI) | OS rate (95% CI) | DFS rate (95% CI) | DDFS rate (95% CI) | OS rate (95% CI) |
| cT1 |
92.7% (83.4%, 96.9%) |
95.6% (86.9%, 98.6%) |
98.6% (90.4%, 99.8%) |
87.8% (81.7%, 92.0%) |
91.1% (85.6%, 94.5%) |
93.8% (88.8%, 96.6%) |
92.3% (84.6%, 96.3%) |
94.5% (87.2%, 97.7%) |
96.7% (90.3%, 98.9%) |
97.0% (88.4%, 99.2%) |
98.5% (89.6%, 99.8%) |
98.4% (89.4%, 99.8%) |
| cT2 |
91.6% (87.3%, 94.5%) |
94.6% (90.9%, 96.8%) |
97.1% (94.0%, 98.6%) |
91.3% (88.4%, 93.6%) |
94.2% (91.6%, 96.0%) |
95.6% (93.3%, 97.2%) |
88.5% (83.1%, 92.2%) |
91.5% (86.7%, 94.7%) |
96.8% (92.9%, 98.5%) |
89.7% (84.2%, 93.4%) |
93.2% (88.4%, 96.1%) |
96.6% (92.5%, 98.4%) |
| cT3/4 |
92.1% (82.0%, 96.6%) |
93.8% (84.3%, 97.6%) |
95.3% (86.0%, 98.4%) |
88.7% (80.6%, 93.6%) |
89.7% (81.6%, 94.3%) |
90.6% (82.7%, 95.0%) |
85.8% (75.2%, 92.1%) |
87.2% (76.8%, 93.1%) |
96.9% (88.0%, 99.2%) |
79.3% (70.2%, 85.9%) |
83.2% (74.5%, 89.1%) |
87.6% (79.6%, 92.6%) |
| cN0 |
93.9% (89.5%, 96.5%) |
95.9% (92.0%, 97.9%) |
99.0% (96.0%, 99.7%) |
93.3% (90.4%, 95.3%) |
96.1% (93.8%, 97.6%) |
96.7% (94.6%, 98.1%) |
92.9% (88.2%, 95.7%) |
96.1% (92.3%, 98.0%) |
97.5% (94.2%, 99.0%) |
88.4% (82.2%, 92.6%) |
91.0% (85.2%, 94.6%) |
95.6% (91.1%, 97.9%) |
| cN+ |
89.5% (83.8%, 93.3%) |
93.1% (88.1%, 96.0%) |
94.7% (90.1%, 97.2%) |
85.6% (81.0%, 89.2%) |
88.0% (83.6%, 91.3%) |
91.5% (87.5%, 94.2%) |
84.5% (77.9%, 89.3%) |
86.1% (79.6%, 90.7%) |
95.7% (90.7%, 98.1%) |
87.4% (81.8%, 91.4%) |
91.3% (86.3%, 94.5%) |
93.0% (88.3%, 95.9%) |
| Lobular tumor type |
83.6% (64.9%, 92.8%) |
87.1% (69.1%, 94.9%) |
93.5% (76.5%, 98.3%) |
70.1% (42.3%, 86.3%) |
82.4% (54.7%, 93.9%) |
82.4% (54.7%, 93.9%) |
100% (100%, 100%) |
100% (100%, 100%) |
100% (100%, 100%) |
87.5% (38.7%, 98.1%) |
87.5% (38.7%, 98.1%) |
87.5% (38.7%, 98.1%) |
| Non-lobular tumor type |
92.7% (89.3%, 95.0%) |
95.3% (92.5%, 97.1%) |
97.3% (94.9%, 98.6%) |
90.7% (88.3%, 92.6%) |
93.1% (91.0%, 94.8%) |
94.8% (92.9%, 96.3%) |
88.8% (85.0%, 91.6%) |
91.3% (87.8%, 93.8%) |
96.7% (94.1%, 98.2%) |
87.9% (83.9%, 91.0%) |
91.2% (87.7%, 93.8%) |
94.4% (91.3%, 96.4%) |
| Ki-67 ≤ 20% |
92.0% (80.0%, 96.9%) |
96.0% (84.9%, 99.0%) |
98.0% (86.6%, 99.7%) |
90.9% (68.1%, 97.7%) |
90.9% (68.1%, 97.7%) |
96.0% (74.8%, 99.4%) |
87.8% (78.4%, 93.3%) |
89.4% (80.5%, 94.3%) |
97.6% (90.8%, 99.4%) |
90.5% (80.0%, 95.6%) |
92.2% (82.1%, 96.7%) |
94.0% (84.7%, 97.7%) |
| Ki-67 > 20% |
91.3% (85.5%, 94.9%) |
92.0% (86.3%, 95.4%) |
95.1% (89.9%, 97.6%) |
90.3% (86.9%, 92.8%) |
93.8% (90.9%, 95.8%) |
95.7% (93.1%, 97.3%) |
90.8% (85.8%, 94.1%) |
94.4% (90.0%, 96.8%) |
97.4% (93.8%, 98.9%) |
86.7% (80.6%, 91.0%) |
90.3% (84.8%, 93.8%) |
93.6% (88.8%, 96.4%) |
| Grade 1/2 |
90.8% (85.6%, 94.2%) |
94.1% (89.6%, 96.7%) |
97.3% (93.7%, 98.9%) |
87.1% (80.9%, 91.4%) |
89.7% (83.9%, 93.5%) |
92.5% (87.1%, 95.7%) |
87.0% (81.3%, 91.1%) |
89.6% (84.2%, 93.3%) |
96.5% (92.4%, 98.4%) |
87.0% (80.1%, 91.6%) |
90.6% (84.3%, 94.4%) |
94.1% (88.5%, 97.0%) |
| Grade 3 |
93.2% (88.4%, 96.1%) |
94.9% (90.4%, 97.3%) |
96.5% (92.3%, 98.4%) |
91.2% (88.5%, 93.3%) |
93.8% (91.4%, 95.5%) |
95.1% (92.9%, 96.7%) |
90.5% (85.0%, 94.1%) |
93.0% (88.0%, 96.0%) |
97.0% (92.8%, 98.7%) |
88.4% (83.2%, 92.1%) |
91.4% (86.7%, 94.5%) |
94.3% (90.1%, 96.7%) |
| Age ≤ 40 years |
94.6% (87.6%, 97.7%) |
96.7% (90.2%, 98.9%) |
98.9% (92.7%, 99.8%) |
90.1% (84.9%, 93.6%) |
92.8% (88.2%, 95.7%) |
94.8% (90.6%, 97.2%) |
91.0% (80.9%, 95.9%) |
95.4% (86.3%, 98.5%) |
98.2% (87.8%, 99.7%) |
88.3% (77.0%, 94.3%) |
93.2% (82.9%, 97.4%) |
94.9% (84.9%, 98.3%) |
| Age 41–59 years |
89.9% (85.1%, 93.1%) |
93.1% (88.9%, 95.7%) |
96.0% (92.5%, 97.9%) |
90.8% (87.6%, 93.1%) |
93.1% (90.2%, 95.1%) |
94.6% (92.0%, 96.4%) |
88.0% (83.0%, 91.6%) |
90.7% (86.1%, 93.9%) |
97.2% (93.9%, 98.8%) |
86.9% (81.5%, 90.7%) |
90.8% (86.0%, 93.9%) |
94.3% (90.2%, 96.7%) |
| Age ≥ 60 years |
96.1% (85.2%, 99.0%) |
98.0% (86.9%, 99.7%) |
98.0% (86.9%, 99.7%) |
88.4% (80.4%, 93.2%) |
92.3% (85.1%, 96.1%) |
94.1% (87.2%, 97.3%) |
89.8% (79.6%, 95.0%) |
89.8% (79.6%, 95.0%) |
94.0% (84.7%, 97.7%) |
90.6% (81.3%, 95.4%) |
90.6% (81.3%, 95.4%) |
93.3% (84.8%, 97.2%) |
| Premenopausal |
92.6% (88.4%, 95.4%) |
95.2% (91.5%, 97.3%) |
97.8% (94.9%, 99.1%) |
89.6% (86.3%, 92.1%) |
92.6% (89.8%, 94.7%) |
94.7% (92.2%, 96.5%) |
89.0% (84.1%, 92.5%) |
92.7% (88.3%, 95.5%) |
97.0% (93.4%, 98.7%) |
84.4% (78.3%, 88.9%) |
89.9% (84.5%, 93.4%) |
94.0% (89.3%, 96.6%) |
| Postmenopausal |
90.7% (84.5%, 94.5%) |
93.8% (88.3%, 96.7%) |
95.7% (90.6%, 98.0%) |
91.1% (87.1%, 93.9%) |
93.2% (89.5%, 95.6%) |
94.2% (90.7%, 96.4%) |
88.8% (82.3%, 93.0%) |
89.5% (83.2%, 93.5%) |
96.4% (91.5%, 98.5%) |
91.9% (86.4%, 95.2%) |
92.5% (87.2%, 95.7%) |
94.4% (89.5%, 97.0%) |
| ypT0 |
91.7% (87.8%, 94.4%) |
94.7% (91.3%, 96.8%) |
97.2% (94.4%, 98.6%) |
91.1% (88.6%, 93.1%) |
93.2% (91.0%, 94.9%) |
95.0% (93.0%, 96.4%) |
91.0% (87.0%, 93.9%) |
92.7% (88.9%, 95.2%) |
96.6% (93.5%, 98.2%) |
90.6% (86.4%, 93.5%) |
92.1% (88.1%, 94.8%) |
94.6% (91.1%, 96.8%) |
| ypTis |
92.4% (84.7%, 96.3%) |
94.6% (87.5%, 97.7%) |
96.7% (90.0%, 98.9%) |
83.8% (74.2%, 90.1%) |
90.6% (82.1%, 95.2%) |
91.6% (83.0%, 95.9%) |
82.0% (71.9%, 88.8%) |
87.0% (77.8%, 92.6%) |
97.4% (90.1%, 99.4%) |
79.7% (69.2%, 86.9%) |
88.2% (79.1%, 93.5%) |
93.1% (85.4%, 96.9%) |
BMI body mass index, DDFS distant disease-free survival, DFS disease-free survival, ER estrogen receptor, PgR progesterone receptor, HER2 human epidermal growth factor receptor 2, HR hormone receptor, OS overall survival, TNBC triple-negative breast cancer
Discussion
This analysis identified potential clinical risk factors in women with a pCR after neoadjuvant chemotherapy combined with anti-HER2 therapy in cases of HER2-positive disease. Overall, nodal involvement at diagnosis,cT3/4 tumors as well as lobular histology were identified being the most adverse factors in patients with a pCR, underlining that tumor burden at the time of diagnosis is important. In patients with TNBC, an initial positive nodal status and lobular histology were predicting a significantly higher risk of relapse or death. In patients with HER2-positive disease tumors initially classified as cT3/4 tumors indicated a higher risk of relapse and death, again very consistently for DFS, DDFS, and OS. This effect was strongest in the HER2-positive hormone receptor-negative subgroup. Four-year DFS and DDFS rates for patients included in this pooled analysis were lowest in those patients with lobular histology, cN+, and cT3/4 tumors.
Several individual trials and meta-analyses showed that a pCR following neoadjuvant chemotherapy improves long-term outcomes in terms of DFS, DDFS, and OS and this was in particular seen in patients with more aggressive tumor types like triple-negative or HER2-positive tumors3–5,22. However, up to 20% of patients with pCR will eventually relapse.
In individual studies long-term outcome is mainly driven by pCR vs no pCR, but also tumor size and nodal involvement assessed before neoadjuvant therapy resulted in an inferior outcome23,24. This is also reflected by the fact that these factors are included in the clinical pathological stage (CPS) score with poorer outcome in case of a more advanced tumor extent in HR positive BC25–27. However, none of these studies analyzed data of patients with and without pCR separately due to limitations in the number of patients and events. Several neoadjuvant trials have shown that patients with small tumors or no nodal involvement at diagnosis are more likely to have a pCR23,28,29. It is of interest that in patients diagnosed with a more advanced clinical stage before neoadjuvant treatment a pCR was less likely and in case a pCR was present the relapse rates were still higher compared to those patients with a pCR and initially small tumors or negative nodes. An explanation might be that these patients may have more systemic disseminated tumor cells at diagnosis, which are resistant to treatment. In fact, trials investigating the role of circulating tumor cells (CTCs) in neoadjuvantly treated patients demonstrated that the presence of CTCs before and not after neoadjuvant therapy negatively influenced the outcome in terms of OS, DDFS, and locoregional relapse-free survival30,31. The number of CTCs before the start of neoadjuvant therapy was also important with the worst outcome seen in patients with the highest CTC counts. In the meta-analysis of Bidard et al., the detection of CTCs at diagnosis was significantly associated with greater tumor size30. A retrospective analysis on tissues from the primary and the metastases from patients with a recurrent disease despite pCR and relapse matched to controls of pts with a pCR but no relapse showed the potential of transcriptomic analyses in this understudied cohort32.
Earlier analyses demonstrated that patients with lobular subtypes have lower pCR rates after neoadjuvant chemotherapy compared with invasive ductal carcinomas33. However, if they had no pCR their outcome was better compared to the non-lobular types. The low responses of lobular histology can be explained at least in part by their particular biologic profile with low proliferation rates, positive hormone receptors, and low grade34. Thus, patients with pure lobular histology (G2 and HR + /HER2-) are usually not candidates for the use of neoadjuvant chemotherapy and in fact only 3–4% of the patients included in the five GBG neoadjuvant trials were diagnosed with an invasive lobular subtype. From the GeparSepto study onwards patients with tumors of pure lobular histology were excluded from trial participation. However, there are also subtypes of lobular histology being high grade, hormone receptor-negative, and HER2-positive and in those lobular tumors with more aggressive biological features pCR rates were significantly higher with up to 20%33. In our investigation relapse rates were significantly higher in the non-classical lobular cohort (TNBC or HER2-positive) which may confirm that this histologic subtype is different from the pure lobular cancers. These high-risk lobular types may be enriched with HER2 mutations35,36.
A pooled analysis has several limitations. One of them is that the treatment regimens were quite different since the included trials were conducted between 2002 and 2013. However, the chemotherapy backbone in every trial was an anthracycline-taxane-based regimen, which can still be considered the standard of care, except for the paclitaxel and liposomal doxorubicin treatment applied in the GeparSixto trial. However, as this study focuses on patients with a pCR after neoadjuvant treatment, it was considered justifiable to also include patients from this trial as an anthracycline was administered. Taking the chemotherapy backbone in consideration, the findings may not be extrapolated to patients achieving a pCR with different treatment regimens. The limitation could only be overcome by conducting this analysis in patients that were treated with the same chemotherapy regimen. As relapses in breast cancer can frequently be seen beyond 5 years, especially in those of HR + subtype, our median follow-up time of nearly 5 years may be still too short. However, our trial population was enriched with biologically aggressive tumors, where typically most of the relapses occur during the first 5 years37,38, therefore our follow-up time most likely covers the majority of relapses. To overcome this limitation a longer follow-up would be necessary and this would implement that the analysis should be repeated in the future to confirm the conclusions. The strength of our analysis is that we could include almost 2,000 patients with a pCR in this pooled analysis. Our analysis was based on five prospectively randomized phase 3 neoadjuvant trials of a study collaboration (GBG/AGO-B), ensuring a very homogenous assessment of all study procedures like pre-treatment tumor assessments, central review of histopathological reports, and definition of pCR but also of additional study procedures including further post-surgical treatments which are usually not part of neoadjuvant trials. Furthermore, lymph node status before treatment was consistently assessed throughout all trials not only clinically but also with ultrasound.
Taken together this is a pooled analysis identifying factors predicting a relapse after pathological complete response following neoadjuvant chemotherapy. In our analysis lobular histology, larger tumor size and initially involved lymph nodes indicated a higher risk for DFS, DDFS, and OS events after a pCR following neoadjuvant treatment. The importance of these risk factors varied by intrinsic subtype, with nodal status and lobular histology as predictors in the triple-negative cohort, and the tumor size in the HER2-positive cohort. Thus, factors predicting a higher risk of relapse after a pCR were tumor extent before therapy and histological type. These data might become of clinical relevance, especially after validation, as treatments are available now for patients at high risk after neoadjuvant therapy, namely those with no pCR, i.e. T-DM1 for HER2 + , capecitabine, and pembrolizumab for TNBC and most recently, PARP inhibitors for patients with BRCA1/2 mutations and CDK 4/6 inhibitors for HER2-/HR + patients. So far, most trials recruiting in the postneoadjuvant setting focus on patients that did not experience a pCR. Taking the results of the here presented analysis into consideration there is also a group of patients with pCR that have a higher risk of relapse and should therefore be considered for an additional treatment despite pCR in postneoadjuvant studies to improve long-term outcome of this otherwise neglected patient population.
Patients and methods
Patients
Data from the five neoadjuvant trials GeparTrio (NCT00544765) (ethics committee of the University of Frankfurt), GeparQuattro (NCT00288002) (ethics committee of the University of Frankfurt), GeparQuinto (NCT00567554) (ethics committee of the University of Frankfurt), GeparSixto (NCT01426880) (ethics committee Nordrhein, Duesseldorf) and GeparSepto (NCT01583426) (ethics committee Berlin) conducted between 2002 and 2013 were pooled and only patients with a pCR (defined as no microscopic evidence of residual invasive tumor cells in any resected specimens of the breast and axillary nodes with in-situ residuals being allowed (ypT0/ypTis, ypN0)) were considered. Individual results and study designs of these studies have previously been reported8–21. All trials were approved by the respective ethics committees and patients had given written informed consent for study participation and data collection. All trials had comparable main eligibility criteria and used an anthracycline-taxane-based chemotherapy backbone (Supplementary table 1). The GeparSixto study enrolled only patients with triple-negative and HER2-positive breast cancer. Patients with HER2-positive disease received anti-HER2 treatment as part of their neoadjuvant therapy within the GeparQuattro, GeparQuinto, GeparSixto, and GeparSepto study. In the GeparTrio trial patients with HER2-positive tumors did not receive any anti-HER2 therapy as part of their neoadjuvant or adjuvant treatment since this was the clinical practice when the study was conducted between 2002 and 2005. Patients with HER2-positive disease from GeparTrio were therefore excluded from the analysis. After surgery, anti-HER2 therapy, endocrine therapy in patients with hormone receptor-positive breast cancer, and radiotherapy were given according to current national guidelines. The tumor subtype was centrally tested.
Objectives and endpoints
The primary objective of this pooled analysis was to characterize patients at higher risk for relapse despite a pCR after neoadjuvant chemotherapy for early breast cancer. Therefore, the influence of predefined potential risk factors as biological subtype (HER2-negative/Hormone receptor (HR)-positive, TNBC, HER2-positive/HR-positive, HER2-positive/HR negative), histological tumor type (lobular subtype, other), tumor grade (G1/G2, G3), tumor stage at baseline (cT1, cT2, cT3/4), nodal stage at baseline (cN0, cN + ), age (≤40, 41–59, ≥ 60 years), BMI ( < 25, 25–29, ≥ 30), menopausal status (pre-, postmenopausal), scheduled number of chemotherapy cycles (≤6, >6) and clinical response after 2–4 cycles (stable disease, partial response, complete response, progressive disease) on DFS was analysed. Secondary objectives were to assess the influence of the same risk factors on distant disease-free survival (DDFS) and overall survival (OS). Ki-67 (≤20% vs higher) was considered as a covariate in preliminary analyses but was excluded due to too many missing values in GeparTrio, GeparQuattro, and GeparQuinto where Ki-67 was not centrally assessed.
DFS was defined as the time in months from randomization to first relapse (local or distant), secondary malignancy, or death from any cause, whichever occurred first39. DDFS was defined as the time in months from randomization to any distant recurrence of disease, any secondary malignancy, or death due to any cause, whichever occurred first; OS as the time in months from randomization to death due to any cause. Patients without an event were censored at the date of the last contact.
Statistical analysis
Multivariate (including all potential risk factors and study) Cox proportional hazards models were used to report hazard ratios with 95% confidence intervals (CIs), adjusted for study to account for possible heterogeneity between the trials. Patients with missing values in risk factors were excluded from multivariate Cox regression models. Patients with missing values for the variable defining the subgroup were excluded from the analyses in this subgroup.
For every potential risk factor, 4-year DFS, DDFS, and OS rates and the corresponding 95% CIs were estimated using the Kaplan–Meier method.
All endpoints were additionally analyzed in the subgroups according to biological subtype.
All reported p-values with p < 0.05 were considered statistically significant. No adjustment for multiple testing was performed. SAS versions 9.2 and 9.4 under SAS Enterprise Guide 4.3 and 8.5 were used to perform the analyses.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We would like to thank all patients, investigators and study personnel who supported the trials. This analysis was funded by GBG.
Presented in part at the 2018 San Antonio Breast Cancer Symposium, San Antonio, TX, December 4–8, 2018, and at the 2019 ESMO Breast Cancer Congress, Berlin, Germany, May 2–4, 2019.
Author contributions
J.H. and M.v.M. contributed equally and are co-first authors. The trial was designed and the protocol was written by the members of the neoadjuvant subcommittee of the German Breast Group (J.H., M.U., C.J., A.S., C.D., P.A.F., C.H., J.-U.B., K.R., T.L., C.S. and S.L.). J.H., M.v.M., M.U., C.J., A.S., C.D., P.A.F., C.H., J-UB, K.R., T.L., C.S. and S.L. contributed to data acquisition. F.S. and V.N. have analysed the data. F.S. and V.N. had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors interpreted the data. The manuscript was written by B.L., M.v.M.and J.H. The decision to submit the manuscript for publication was made by all authors. All authors contributed to the review of the manuscript and are accountable for all aspects of the work. No persons other than the listed authors contributed to the writing of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
Individual participant data that underlie the results reported in this article, after final analysis and publication of all secondary efficacy endpoints, study protocol and statistical report will be available upon request. All relevant data are within the paper and its supporting information files. The data underlying the results presented in the study are available from GBG. Some restrictions apply due to confidentiality of patient data. Since these data are derived from a prospective clinical trial with ongoing follow up collection there are legal and ethical restrictions to share sensitive patient related data publicly. Interested groups may use the “Cooperation Proposal Form” on https://www.gbg.de/en/research/trafo.php. Data can be requested in context of a translational research project by sending the form to trafo@gbg.de. Translational research proposals are approved by the GBG scientific boards
Data can be requested in context of a translational research project by sending the form to trafo@gbg.de. Translational research proposals are approved by the GBG scientific boards.
Competing interests
J.H. reports receiving speakers honoraria from Lilly, Novartis, Roche, Pfizer, AstraZeneca, MSD, Celgene, and Eisai, travel expenses from Roche, Pfizer, Novartis, Celgene, and Daiichi, consulting fees from Lilly, Novartis, Roche, Pfizer, Hexal, AstraZeneca, MSD, and Celgene, and research grants from Celgene and Novartis. M.v.M. reports receiving honoraria from Amgen, AstraZeneca, Daiichi Sankyo, GenomicHealth, Gilead, GSK, Lilly, Molecular Health, Mylan, Novartis, Pfizer, PierreFabre, Roche, Seagen, travel expenses from Lilly, Gilead and Daiichi Sankyo. A.S. reports receiving honoraria from Roche, Celgene, Pfizer, AstraZeneca, Novartis, MSD, Tesaro, and Lilly, research funding from Celgene, Roche, AbbVie, and Molecular Partner, expert testimony from Roche and AstraZeneca, and travel expenses from Celgene and Roche. J.-U.B. reports receiving honoraria from Amgen, AstraZeneca, GenomicHealth, MSD Oncology, Myriad Genetics, Novartis, Pfizer, Roche, and Sonoscape. C.D. was a cofounder and shareholder of Sividon Diagnostics (now Myriad), he reports receiving speakers honoraria and travel support from Teva, Celgene, Roche, Pfizer, Novartis, Astra Zeneca, he is a consultant for MSD and Daichii Sankyo and an inventor on patent EP18209672 and patent EP20150702464. H.T. reports receiving honoraria and consulting fees from Pfizer, Novartis, Roche, Lilly, and AstraZeneca, and travel expenses from Pfizer, and Lilly. C.H. reports receiving consulting fees from Roche, Novartis, Pfizer, AstraZeneca, Celgene, and Lilly Pharma. K.R. reports receiving honoraria from AstraZeneca, Pfizer, Tesaro, and Roche, consulting fees from Tesaro and Pfizer, and travel expenses from Pfizer, Tesaro, and Roche. CSo reports personal fees and non-financial support from Olympus Europa, MYLAN, and Medtronic, and personal fees from Roche, Pfizer, and AstraZeneca. P.A.F. reported grants from Novartis, Biontech, Novartis, Roche, Pfizer, Celgene, Daiichi-Sankyo, Teva, Astra Zeneca, Merck Sharp and Dohme, Myelo Therapeutics, Macrogenics, Eisai, Puma, and Cepheid. C.J. reports receiving honoraria from Roche, Sanofi Aventis, AstraZeneca, and Novartis, consulting fees from Roche and Celgene, and travel expenses from Roche, AstraZeneca, and Novartis. T.L. reports receiving honoraria from Pfizer, Novartis, TEVA, Tesaro, MSD, Roche, and Amgen, consulting fees from Amgen, Roche, Tesaro, and travel expenses from Daiichi Sankyo, Pharma Mar, Roche, and Pfizer. S.L. reported grants and honoraria to institution from Abbvie, Amgen, AstraZeneca, BMS, Celgene, Cepheid, Immunomedics, Myriad Genetics, Novartis, Lilly, MSD, Pfizer, Roche, and Daiichi Sankyo, and patent EP14153692.0. M.U. reports receiving honoraria to institution from Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Lilly, MSD Merck, Mundipharma, Myriad Genetics, Novartis, Pfizer, PUMA, Roche, Sanofi Aventis, and TEVA, and consulting fees from Abbvie, Amgen, AstraZeneca, Celgene, Lilly, Daiichi Sankyo, MSD, Novartis, Odonate, Pfizer, PUMA, Roche, and Sanofi Aventis. F.S., B.L., K.M. and V.N. declares to be GBG Forschungs GmbH employee. GBG Forschungs GmbH received funding for research grants from Abbvie, AstraZeneca, BMS, Daiichi-Sankyo, Gilead, Novartis, Pfizer and Roche (paid to the institution); other (non-financial/medical writing) from Daiichi-Sankyo, Gilead, Novartis, Pfizer, Roche and Seagen (paid to the institution). GBG Forschungs GmbH has following royalties/patents: EP14153692.0, EP21152186.9, EP15702464.7, EP19808852.8 and VM Scope GmbH. All other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jens Huober, Marion van Mackelenbergh.
Contributor Information
Marion van Mackelenbergh, Email: marion.vanmackelenbergh@gbg.de.
Sibylle Loibl, Email: sibylle.loibl@gbg.de.
Supplementary information
The online version contains supplementary material available at 10.1038/s41523-023-00525-2.
References
- 1.Huober J, von Minckwitz G. Neoadjuvant therapy—what have we achieved in the last 20 years? Breast Care. 2011;6:419–426. doi: 10.1159/000335347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) BBarlow W. et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27–39. doi: 10.1016/S1470-2045(17)30777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortazar P, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (London, England). 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 4.Broglio KR, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016;2:751–760. doi: 10.1001/jamaoncol.2015.6113. [DOI] [PubMed] [Google Scholar]
- 5.von Minckwitz G, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 6.von Minckwitz G, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 2018;380:617–28. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 7.Masuda N, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med. 2017;376:2147–59. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 8.Untch M, et al. NAB-paclitaxel improves disease-free survival in early breast cancer: GBG 69–GeparSepto. J. Clin. Oncol. 2019;37:2226–2234. doi: 10.1200/JCO.18.01842. [DOI] [PubMed] [Google Scholar]
- 9.Loibl S, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19:497–509. doi: 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
- 10.Loibl S, et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response—final results from GeparSixto. Ann. Oncol. 2018;29:2341–2347. doi: 10.1093/annonc/mdy460. [DOI] [PubMed] [Google Scholar]
- 11.von Minckwitz G, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 12.Untch M, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 2012;13:135–144. doi: 10.1016/S1470-2045(11)70397-7. [DOI] [PubMed] [Google Scholar]
- 13.Gerber B, et al. Neoadjuvant bevacizumab and anthracycline-taxane-based chemotherapy in 678 triple-negative primary breast cancers; results from the geparquinto study (GBG 44) Ann. Oncol. 2013;24:2978–2984. doi: 10.1093/annonc/mdt361. [DOI] [PubMed] [Google Scholar]
- 14.von Minckwitz G, et al. Survival after neoadjuvant chemotherapy with or without bevacizumab or everolimus for HER2-negative primary breast cancer (GBG 44–GeparQuinto)†. Ann. Oncol. 2014;25:2363–2372. doi: 10.1093/annonc/mdu455. [DOI] [PubMed] [Google Scholar]
- 15.Huober J, et al. Neoadjuvant chemotherapy with paclitaxel and everolimus in breast cancer patients with non-responsive tumours to epirubicin/cyclophosphamide (EC)±bevacizumab—Results of the randomised GeparQuinto study (GBG 44) Eur. J. Cancer. 2013;49:2284–2293. doi: 10.1016/j.ejca.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 16.von Minckwitz G, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N. Engl. J. Med. 2012;366:299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- 17.von Minckwitz G, et al. Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: Phase III GeparQuattro Study. J. Clin. Oncol. 2010;28:2015–2023. doi: 10.1200/JCO.2009.23.8303. [DOI] [PubMed] [Google Scholar]
- 18.von Minckwitz G, et al. Survival after adding capecitabine and trastuzumab to neoadjuvant anthracycline-taxane-based chemotherapy for primary breast cancer (GBG 40—GeparQuattro) Ann. Oncol. 2014;25:81–89. doi: 10.1093/annonc/mdt410. [DOI] [PubMed] [Google Scholar]
- 19.von Minckwitz G, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J. Clin. Oncol. 2013;31:3623–30. doi: 10.1200/JCO.2012.45.0940. [DOI] [PubMed] [Google Scholar]
- 20.von Minckwitz G, et al. Intensified neoadjuvant chemotherapy in early-responding breast cancer: Phase III randomized GeparTrio study. J. Natl. Cancer Inst. 2008;100:552–562. doi: 10.1093/jnci/djn089. [DOI] [PubMed] [Google Scholar]
- 21.von Minckwitz G, et al. Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: Phase III randomized GeparTrio trial. J. Natl. Cancer Inst. 2008;100:542–551. doi: 10.1093/jnci/djn085. [DOI] [PubMed] [Google Scholar]
- 22.Huober J, et al. Survival outcomes of the NeoALTTO study (BIG 1–06): updated results of a randomised multicenter phase III neoadjuvant clinical trial in patients with HER2-positive primary breast cancer. Eur. J. Cancer. 2019;118:169–177. doi: 10.1016/j.ejca.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 23.Goorts B, et al. Clinical tumor stage is the most important predictor of pathological complete response rate after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res. Treat. 2017;163:83–91. doi: 10.1007/s10549-017-4155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fayanju OM, et al. The clinical significance of breast-only and node-only pathologic Complete Response (pCR) After Neoadjuvant Chemotherapy (NACT) Ann. Surg. 2018;268:591–601. doi: 10.1097/SLA.0000000000002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marmé F, et al. Utility of the CPS+EG staging system in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer treated with neoadjuvant chemotherapy. Eur. J. Cancer. 2016;53:65–74. doi: 10.1016/j.ejca.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Jeruss JS, et al. Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy. J. Clin. Oncol. 2008;26:246–252. doi: 10.1200/JCO.2007.11.5352. [DOI] [PubMed] [Google Scholar]
- 27.Mittendorf EA, et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J. Clin. Oncol. 2011;29:1956–1962. doi: 10.1200/JCO.2010.31.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi HJ, et al. Nomogram for accurate prediction of breast and axillary pathologic response after neoadjuvant chemotherapy in node positive patients with breast cancer. Ann. Surg. Treat. Res. 2014;96:169. doi: 10.4174/astr.2019.96.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NWAOGU IY, et al. Predictors of pathological complete response to neoadjuvant chemotherapy in stage II and III breast cancer: the impact of chemotherapeutic regimen. Mol. Clin. Oncol. 2015;3:1117–1122. doi: 10.3892/mco.2015.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bidard F-C, et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J. Natl. Cancer Inst. 2018;110:560–567. doi: 10.1093/jnci/djy018. [DOI] [PubMed] [Google Scholar]
- 31.Riethdorf S, et al. Prognostic impact of circulating tumor cells for breast cancer patients treated in the neoadjuvant “Geparquattro” trial. Clin. Cancer Res. 2017;23:5384–5393. doi: 10.1158/1078-0432.CCR-17-0255. [DOI] [PubMed] [Google Scholar]
- 32.Bruzas S, et al. Gene signatures in patients with early breast cancer and relapse despite pathologic complete response. NPJ Breast Cancer. 2022;8:42. doi: 10.1038/s41523-022-00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huober J, et al. Effect of neoadjuvant anthracycline–taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res. Treat. 2010;124:133–140. doi: 10.1007/s10549-010-1103-9. [DOI] [PubMed] [Google Scholar]
- 34.Katz A, et al. Primary systemic chemotherapy of invasive lobular carcinoma of the breast. Lancet Oncol. 2007;8:55–62. doi: 10.1016/S1470-2045(06)71011-7. [DOI] [PubMed] [Google Scholar]
- 35.Christgen M, et al. ERBB2 mutation frequency in lobular breast cancer with pleomorphic histology or high-risk characteristics by molecular expression profiling. Genes Chromosom. Cancer. 2019;58:175–185. doi: 10.1002/gcc.22716. [DOI] [PubMed] [Google Scholar]
- 36.Ross JS, et al. Relapsed Classic E-Cadherin (CDH1)-mutated invasive lobular breast cancer shows a high frequency of HER2 (ERBB2) gene mutations. Clin. Cancer Res. 2013;19:2668–2676. doi: 10.1158/1078-0432.CCR-13-0295. [DOI] [PubMed] [Google Scholar]
- 37.Prat A, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24:S26–S35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Pan H, et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 2017;377:1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hudis CA, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J. Clin. Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data that underlie the results reported in this article, after final analysis and publication of all secondary efficacy endpoints, study protocol and statistical report will be available upon request. All relevant data are within the paper and its supporting information files. The data underlying the results presented in the study are available from GBG. Some restrictions apply due to confidentiality of patient data. Since these data are derived from a prospective clinical trial with ongoing follow up collection there are legal and ethical restrictions to share sensitive patient related data publicly. Interested groups may use the “Cooperation Proposal Form” on https://www.gbg.de/en/research/trafo.php. Data can be requested in context of a translational research project by sending the form to trafo@gbg.de. Translational research proposals are approved by the GBG scientific boards
Data can be requested in context of a translational research project by sending the form to trafo@gbg.de. Translational research proposals are approved by the GBG scientific boards.