Objective:
To investigate the outcome of conversion surgery in patients with metastatic pancreatic cancer (mPDAC) and to identify patients who may benefit from this approach.
Background:
The role of conversion surgery in patients with mPDAC and exceptional response to chemotherapy remains unclear.
Methods:
Patients who underwent surgical exploration for mPDAC following chemotherapy between 2006 and 2019 were included. Data on demographics, oncologic treatment, pathology, and postoperative outcomes were analyzed. Univariate and multivariate survival analyses were performed.
Results:
Some 173 patients received preoperative chemotherapy and underwent surgical exploration. Ninety-three patients underwent resection of the primary tumor and metastatic sites, 80 patients underwent exploration only. In the resection subgroup, 45 patients had complete pathological response of metastases (ypM0) and 48 patients had residual metastases (ypM1). ypM0 status was associated with lower carcinoembryonic antigen levels and lower ypN stage. Overall survival after resection was 25.5 months in ypM0, 10.7 months in ypM1, and 8.1 months in patients without resection (P<0.001). Additional adjuvant chemotherapy was significantly associated with prolonged survival in resected patients (29.0 vs 14.8 mo, P=0.024) as well as in ypM0 (29.1 vs 19.2 mo, P=0.047). Multivariable analysis identified conversion surgery, carbohydrate antigen 19-9 (CA19-9) and time of resection as independent prognostic markers for the entire cohort. CA19-9, ypM0 and adjuvant treatment were independent predictors of survival in the resection subgroup.
Conclusion:
In patients with mPDAC and ypM0 status after chemotherapy, surgical resection is associated with encouraging survival. mPDAC patients with exceptional response to chemotherapy may be candidates for exploration and for resection in ypM0. Adjuvant chemotherapy may provide an additional survival advantage.
Keywords: pancreatic cancer, PDAC, metastatic pancreatic cancer, mPDAC, conversion surgery, metastasectomy, survival
Over the past decade, the perioperative surgical and long-term oncological outcomes of patients with resectable pancreatic ductal adenocarcinoma (PDAC) have substantially improved.1 With modern surgery and adjuvant chemotherapy, observed 5-year survival rates are around 20%, and even higher in prognostically favorable subgroups.2 Survival is expected to increase further with the use of novel multiagent therapies.3,4 Similarly, patients with previously unresectable locally advanced PDAC have become candidates for resection following neoadjuvant chemotherapy, with an up to 60% chance of eligibility for conversion surgery.5–7 The neoadjuvant strategy incorporates both biological selection of individuals with response to systemic chemotherapy and downstaging of the primary tumor with improved local resectability and higher likelihood of achieving R0 resection.8,9
In patients with metastatic pancreatic cancer (mPDAC), current guidelines recommend FOLFIRINOX or gemcitabine plus nab-paclitaxel as the regimens for first-line palliative chemotherapy in individuals with good performance status.10 These strategies have shown higher response rates and longer overall survival than classical monotherapies in large randomized trials.11,12 Some patients experience favorable radiological and biological responses to chemotherapy as assessed by cross-sectional imaging and carbohydrate antigen 19-9 (CA19-9) levels, respectively, and may qualify for conversion surgery.13 However, data on the survival outcomes of conversion surgery in mPDAC are sparse. The reported overall survival times after chemotherapy and resection in case series with heterogenous and highly selected study populations range between 18 and 56 months.14 Moreover, the surgical approaches to metastatic sites are poorly reported or differ between studies. It therefore remains unclear which patients, based on survival, might actually be candidates for conversion surgery for mPDAC.15–17
The aim of the present study of a large cohort in patients with preoperative chemotherapy for mPDAC was to clarify the survival outcome that can be expected after conversion surgery and to assess factors that are associated with survival and may, therefore, be helpful for patient selection and treatment decisions in this novel scenario.
METHODS
Study Design and Patient Selection
This comparative cohort study was approved by the local ethics committee in Heidelberg (project number S-226/2017) and was designed in accordance with the STROBE criteria for observational studies (Suppl. Table 1, Supplemental Digital Content 1, http://links.lww.com/SLA/D959).18 Adult patients who had undergone surgical exploration for PDAC after preoperative chemotherapy or chemoradiation between January 2006 and May 2019 were identified from a prospectively maintained institutional database at the Department of General, Visceral and Transplantation Surgery, Heidelberg University Hospital. The inclusion criteria were histologically proven PDAC with distant metastasis at diagnosis, good performance status, and systemic preoperative chemotherapy. The exclusion criteria were other malignant tumor entities of the pancreas and periampullary region, including neuroendocrine carcinoma, acinar cell carcinoma, and distal cholangiocarcinoma, as well as preoperative chemotherapy in the absence of distant metastasis.
Multimodal Oncological Management
Preoperative Chemotherapy
Because of the referral patterns to our tertiary hospital, the majority of patients presented with favorable response to chemotherapy after previous treatment at other centers. Gemcitabine-based regimens with or without nab-paclitaxel or FOLFIRINOX (folinic acid, fluorouracil, irinotecan, and oxaliplatin) were administered as first-line treatment according to the contemporary guidelines for mPDAC.10,19 Patients usually received at least 6 cycles of chemotherapy and restaging was performed every 3 months with multidisciplinary team evaluations. Patients qualified for surgical exploration when stable disease, partial or complete response of the primary tumor, and partial or complete response of metastatic lesions in cross sectional imaging based on the RECIST criteria20 were observed in combination with a biological tumor response [decrease in CA19-9 and carcinoembryonic antigen (CEA) levels]. In selected patients with unclear lesions, positron emission tomography–computed tomography was performed in addition to conventional cross-sectional imaging. Furthermore, the original sites of metastatic disease needed to be limited and technically resectable. If these criteria were not initially met, chemotherapy was continued until they were achieved and patients underwent surgery. Patients not meeting the criteria for surgery continued on palliative therapy and were not evaluated for this study. There was no general recommendation for additional therapy, and adjuvant chemotherapy was administered based on individualized decisions based on the extent of preoperative chemotherapy and the patient’s performance status.
Surgical Approach
Surgery was performed in all cases where the above-mentioned criteria were fulfilled and patients were both fit and willing to undergo operative intervention. Exploration started with careful examination of the liver, including intraoperative ultrasound. Peritoneal metastases were assessed by systematic exploration of the abdominal cavity. Any suspicious lesions were excised and assessed by means of intraoperative frozen sections. To evaluate the resectability of the primary tumor, an artery-first approach was performed before any irreversible surgery was carried out.21 If both the primary tumor and (former) metastatic sites were judged to be resectable, conversion surgery with radical resection of the primary tumor with standard lymphadenectomy in combination with resection of the metastatic sites was performed with the aim of achieving R0 status.22–24
Outcome Parameters and Data Collection
The primary outcome was overall postoperative survival in resected patients with complete pathological response (ypM0) compared with those with residual metastases (ypM1). Secondary outcomes included overall postoperative survival in resected patients compared with those undergoing exploration only, as well as prognostic factors associated with prolonged survival.
Multiple perioperative variables were extracted from the prospectively maintained institutional pancreatic database, including patient’s age, sex, body mass index, American Society of Anesthesiologists (ASA) classification, type and duration of preoperative chemotherapy regimens, preoperative CA19-9 and CEA levels, resectability status of primary tumor, metastatic sites, vascular infiltration, and type and extent of primary and metastatic resection. The perioperative outcomes included major surgical morbidity according to the Clavien-Dindo Classification (CDC), duration of postoperative hospital stay, and 90-day mortality. Pathological assessment of the specimens was performed in accordance with the 8th edition of the AJCC Cancer Staging Manual and included tumor size, T-stage, N-stage, and M-stage. Resection margin status was based on a strict 1mm rule.24,25 Follow-up was last updated in April 2020, with patients followed up until their last oncological surveillance or until death. To reduce risk of detection bias, the investigator responsible for the assessment of data was blinded.
Statistical Analysis
Categorical variables are expressed as absolute numbers (relative percentages), continuous data as median and interquartile range (IQR). The nonparametric Mann-Whitney U test and the Kruskal-Wallis test were used to compare quantitative parameters among subgroups. Categorical parameters were compared among subgroups using the χ2 test, if appropriate, or the Fisher exact test. Overall survival was calculated using the Kaplan-Meier method and was defined as the time from conversion surgery to either death or last follow-up. Patients alive at the last follow-up were censored. Disease-free survival was defined as the time from the date of resection to either disease recurrence or last follow-up. Patients who either died or were lost to follow-up within 90 days after surgery were excluded from analysis of disease-free survival. The 2YSR and 3YSR and the median survival time are presented. The log-rank test was used to compare survival curves among subgroups. Univariable and multivariable proportional hazard regression (Cox model) analyses of the prognostic value of appropriate parameters were performed. The parameters with P<0.1 results in univariable analysis were subsequently used for multivariable survival analysis. Hazard ratios (HR) are reported with 95% confidence intervals (CI). Because of the exploratory character of the analyses performed, all P values were interpreted descriptively. A 2-sided P value of 0.05 was considered significant. Missing data were rare; therefore, no imputation was performed. For all statistical computations SAS software (release 9.4, SAS Institute Inc., Cary, NC) was used.
RESULTS
Characteristics of the Study Cohort
In total, 750 patients with preoperative chemotherapy for (non-) metastatic PDAC underwent surgical exploration at the Department of General, Visceral and Transplantation Surgery, Heidelberg University Hospital, during the study period (Suppl. Fig. 1, Supplemental Digital Content 2, http://links.lww.com/SLA/D960). Of these patients, 577 individuals (76.9%) with nonmetastatic borderline resectable or locally advanced PDAC did not meet the inclusion criteria and were ineligible for the study. This left 173 patients with confirmed mPDAC who met the inclusion criteria and were available for analysis; their median age was 61.2 years, and 97 of them were men (56.1%). Of these 173 patients, 104 (60.1%) received FOLFIRINOX, 55 patients (31.8%) received gemcitabine-based regimens, 12 patients (6.9%) received FOLFIRINOX followed by gemcitabine or vice versa, and the remaining 2 patients (1.2%) received other regimens. The most common metastatic sites at the time of diagnosis were liver [116 (67.1%)], peritoneum [32 (18.5%)], and distant lymph nodes [11 (6.4%)]. In 80 patients (46.2%) the disease was deemed unresectable upon surgical exploration. The remaining 93 patients (53.8%) underwent resection of the pancreatic primary tumor with simultaneous resection of metastatic sites (Table 1). The median duration of postoperative follow-up was 8.4 months (IQR: 3.7–15.5 months) for the study cohort and 12.5 months for the resection cohort (IQR: 6.9–25.1 months).
TABLE 1.
Demographic, Clinicopathological, and Surgical Characteristics in mPDAC Patients
| Exploration (M1) N=80 (%) | Resection (M1/ypM0) N=93 (%) | P * | Resection (M1) N=48 (%) | Resection (ypM0) N=45 (%) | P † | |
|---|---|---|---|---|---|---|
| Age (y), median (IQR) | 61 (53–68) | 61 (52–67) | 0.672 | 62 (50–67) | 61 (54–67) | 0.599 |
| <50 | 14 (17.5) | 17 (18.3) | 0.964 | 13 (27.1) | 4 (8.9) | 0.063 |
| 50–69 | 54 (67.5) | 61 (65.6) | 27 (56.2) | 34 (75.6) | ||
| ≥70 | 12 (15.0) | 15 (16.1) | 8 (16.7) | 7 (15.5) | ||
| Sex ratio (m:f) | 53:27 (66:34) | 44:49 (47:53) | 0.012 | 22:26 (46:54) | 22:23 (49:51) | 0.768 |
| BMI (kg/m2), median (IQR) | 24 (22–26) | 23 (21–26) | 0.248 | 23 (21–25) | 24 (22–26) | 0.076 |
| ASA classification >2 | 41 (51.2) | 34 (37.4) | 0.162 | 15 (31.9) | 19 (43.2) | 0.603 |
| Site of distant metastasis‡ | 0.014 | 0.252 | ||||
| Hepatic | 49 (61.2) | 67 (72.0) | 36 (75.0) | 31 (68.9) | ||
| Peritoneal | 20 (25.0) | 12 (12.9) | 4 (8.3) | 8 (17.8) | ||
| Hepatic+peritoneal | 5 (6.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Lymphatic | 3 (3.8) | 8 (8.6) | 6 (12.5) | 2 (4.4) | ||
| Other | 3 (3.8) | 6 (6.5) | 2 (4.2) | 4 (8.9) | ||
| Vascular involvement‡ | 0.060 | 0.083 | ||||
| No vascular involvement | 37 (46.2) | 56 (60.2) | 32 (66.7) | 24 (53.4) | ||
| Venous involvement | 15 (18.7) | 21 (22.6) | 6 (12.5) | 15 (33.3) | ||
| Venous+arterial involvement | 21 (26.3) | 11 (11.8) | 6 (12.5) | 5 (11.1) | ||
| Arterial involvement | 7 (8.8) | 5 (5.4) | 4 (8.3) | 1 (2.2) | ||
| Neoadjuvant chemotherapy | 0.214 | 0.828 | ||||
| FOLFIRINOX (only) | 43 (53.8) | 61 (65.6) | 31 (64.6) | 30 (66.7) | ||
| Gemcitabine alone/other | 28 (35.0) | 27 (29.0) | 15 (31.2) | 12 (26.7) | ||
| Gemcitabine+FOLFIRINOX | 7 (8.7) | 5 (5.4) | 2 (4.2) | 3 (6.6) | ||
| Other | 2 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Time diagnosis to operation (mo) | 6.9 (4.7–8.4) | 6.2 (4.8–9.4) | 0.691 | 5.9 (4.4–9.0) | 8.1 (5.0–9.5) | 0.172 |
| Preoperative CA19-9, median (IQR) | 161 (50–571) | 44 (11–193) | <0.001 | 44 (10–272) | 42 (12–134) | 0.245 |
| <37 U/ml | 19 (23.7) | 42 (46.1) | <0.001 | 22 (46.8) | 20 (45.5) | 0.068 |
| 37–<400 U/ml | 35 (43.8) | 38 (41.8) | 16 (34.0) | 22 (50.0) | ||
| ≥400 U/ml | 26 (32.5) | 11 (12.1) | 9 (19.2) | 2 (4.5) | ||
| Preoperative CEA, median (IQR) | 2.9 (1.2–5.0) | 2.4 (1.2–4.5) | 0.582 | 3.0 (1.2–5.8) | 2.2 (1.2–3.5) | 0.113 |
| <2.5 µg/L | 33 (42.3) | 47 (52.2) | 0.403 | 20 (43.5) | 27 (61.4) | 0.030 |
| 2.5–<5 µg/L | 25 (32.1) | 22 (24.5) | 10 (21.7) | 12 (27.3) | ||
| ≥5 µg/L | 20 (25.6) | 21 (23.3) | 16 (34.8) | 5 (11.3) | ||
| Surgical procedure | <0.001 | 0.034 | ||||
| Pancreatoduodenectomy | 0 (0.0) | 41 (44.1) | 15 (31.2) | 26 (57.8) | ||
| Distal pancreatectomy | 0 (0.0) | 37 (39.8) | 24 (50.0) | 13 (28.9) | ||
| Total pancreatectomy | 0 (0.0) | 15 (16.1) | 9 (18.8) | 6 (13.3) | ||
| Explorative laparotomy | 80 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Hospital stay (d), median (IQR) | 5 (4–7) | 12 (9–19) | <0.001 | 14 (10–20) | 12 (9–15) | 0.143 |
| ypT stage | 0.240 | |||||
| T0 | — | 5 (5.4) | 2 (4.2) | 3 (6.7) | ||
| T1 | — | 21 (22.6) | 8 (16.7) | 13 (28.9) | ||
| T2 | — | 47 (50.5) | 24 (50.0) | 23 (51.1) | ||
| T3 | — | 17 (18.3) | 11 (22.9) | 6 (13.3) | ||
| T4 | — | 3 (3.2) | 3 (6.2) | 0 (0.0) | ||
| ypN stage | 0.020 | |||||
| N0 | — | 45 (48.4) | 19 (39.6) | 26 (57.8) | ||
| N1 | — | 26 (28.0) | 12 (25.0) | 14 (31.1) | ||
| N2 | — | 22 (23.6) | 17 (35.4) | 5 (11.1) | ||
| R classification | 0.482 | |||||
| R0 | — | 38 (40.8) | 17 (35.4) | 21 (46.7) | ||
| R1 (<1 mm) | — | 30 (32.3) | 16 (33.3) | 14 (31.1) | ||
| R1 (direct) | — | 25 (26.9) | 15 (31.3) | 10 (22.2) |
Bold indicates statistically significant P-value.
Comparison of exploration and resection.
Comparison of resection groups yM1 and ypM0.
Radiological status before start of chemotherapy.
BMI indicates body mass index.
Factors Associated With Resection
Table 1 summarizes the demographics and clinicopathological characteristics of patients undergoing resection and exploration only. The sex ratio differed significantly between these 2 groups, with a higher proportion of male patients in the exploration group but equal distribution of sex in the resection subgroup. Resection status was associated with different site of distant metastasis including a higher proportion of hepatic and fewer peritoneal metastases (HEP: 72% vs 61.2%, PER: 12.9% vs 25.0%; P=0.014). In addition, patients with resection had significantly lower preoperative CA19-9 levels (44.1 vs 161.4 U/mL; P<0.001) than patients with exploration only.
Among the 93 patients who underwent resection, surgical procedures of the primary tumor included partial pancreatoduodenectomy, distal pancreatectomy, and total pancreatectomy in 44.1%, 39.8%, and 16.1% of cases, respectively. In addition, hepatic resections were performed in 67 (72.0%) individuals with predominantly atypical liver resections (87.5%), followed by anatomical segmental resections (9.7%) and right-sided/left-sided hemihepatectomy (2.8%). Other procedures included localized resection of peritoneal metastases (12.9%) and extended lymph node dissection (8.6%). The median length of hospital stay was 12 days after resection (IQR: 9–19), compared with 5 days after exploration only (IQR: 4–7). Major surgical morbidity (CDC≥3) was detected in 19.4% of the 93 patients, and the 90-day mortality after resection was 3.2%.
ypM0 Status and Associated Factors
The pathological tumor stages of the resected group are summarized in Table 1. Most tumors were still in the category ypT2 or greater (ypT2: 50.5%; ypT3: 18.3%), and more than half of the patients had positive lymph nodes (yN1: 28.0%; ypN2: 23.7%). However, final pathology revealed complete response of metastases (ypM0) in 45 individuals (48.4%), while 48 individuals (51.6%) had residual active metastasis in the resected specimens (ypM1). As shown in Table 1, ypM0 status was associated with significantly lower CEA levels after stratification into different cut-off levels, while the type of chemotherapy and the site of distant metastases were comparable between the 2 groups. Furthermore, patients with ypM0 status had significantly different types of resections of the primary tumor, including more pancreatic head resections and fewer distal pancreatectomies, compared with patients with ypM1 status (PD: 57.8% vs 31.2%, DP: 28.9% vs 50%; P=0.034). ypM0 status was associated with more favorable posttreatment pathological parameters of the primary tumor, with a trend toward lower ypT stage and significantly lower ypN stage (ypN0: 57.8% vs 39.6%; P=0.020), pointing to an overall better response to preoperative chemotherapy in the ypM0 subgroup.
Survival Outcome After Conversion Surgery for mPDAC and Prognostic Factors
The median overall survival after resection was 25.5 months in ypM0 patients, 10.7 months in ypM1 patients, and 8.1 months in individuals without resection (Fig. 1; P<0.001). The corresponding 3YSR survival rates were 32.1%, 9.0%, and 0%, respectively. Complete pathological response (ypT0yN0yM0) was observed in only 3 patients. One of these patients died 2 months after surgery due to postoperative complications, while the other 2 patients were still alive after 20 and 48 months. Further subgroup analysis demonstrated no survival differences in the resection subgroups according to the site of distant metastasis (Suppl. Table 2, Supplemental Digital Content 3, http://links.lww.com/SLA/D961).
FIGURE 1.
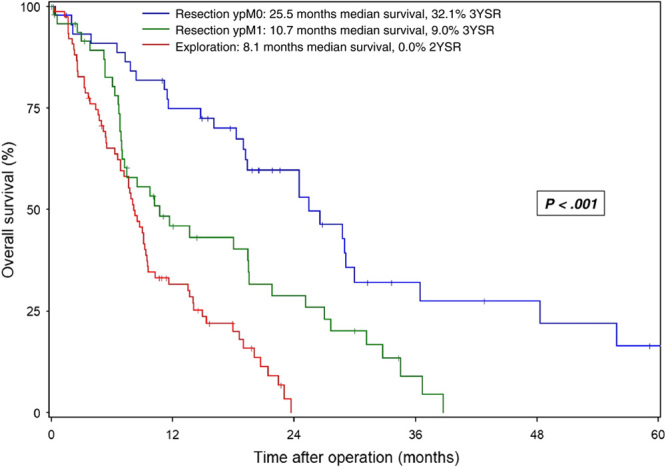
Overall survival in patients with mPDAC undergoing conversion surgery after preoperative chemotherapy. Survival data are calculated starting from the date of the operation. Patients alive at the last follow-up are censored (I).
In univariable analyses for the entire cohort, conversion surgery and administration of preoperative FOLFIRINOX were positive prognostic factors for overall survival, whereas higher ASA classification, preoperative CA19-9 levels ≥400 U/mL, CEA levels ≥5 µg/L, timing of resection after initial diagnosis (<5 or ≥9 months), and arterial infiltration of the primary tumor were negative prognostic factors (Table 2).
TABLE 2.
Univariable Analyses of Prognostic Factors for Overall Survival
| Variable | Category | N | Events | HR | 95% CI | P |
|---|---|---|---|---|---|---|
| Age | <50 y | 31 | 28 | 1.34 | 0.90–2.15 | 0.134 |
| ≥50–<70 y | 115 | 83 | 1 | — | ||
| ≥70 y | 27 | 20 | 1.36 | 0.83–2.23 | 0.225 | |
| Sex | Male | 97 | 73 | 0.82 | 0.58–1.16 | 0.263 |
| Female | 97 | 73 | 1 | |||
| BMI (kg/m2) | <18.5 | 8 | 7 | 1.12 | 0.52–2.40 | 0.778 |
| ≥18.5–<30 | 154 | 1 | 1 | — | ||
| ≥30 | 8 | 4 | 0.63 | 0.23–1.71 | 0.365 | |
| ASA classification | ASA 1–2 | 96 | 68 | 1 | – | |
| ASA 3–4 | 75 | 62 | 1.62 | 1.15–2.30 | 0.007 | |
| CA19-9 | <37 U/mL | 61 | 41 | 1 | – | |
| ≥37–<400 U/mL | 73 | 58 | 1.17 | 0.78–1.75 | 0.445 | |
| ≥400 U/mL | 37 | 31 | 3.76 | 2.30–6.15 | <0.001 | |
| CEA | <2.5 µg/L | 80 | 62 | 1 | — | |
| ≥2.5–<5 g/L | 47 | 34 | 1.23 | 0.80–1.88 | 0.341 | |
| ≥5 g/L | 41 | 32 | 1.65 | 1.07–2.54 | 0.024 | |
| Lymph node metastasis | No | 152 | 113 | 1 | — | |
| Yes | 21 | 18 | 1.44 | 0.87–2.37 | 0.157 | |
| Hepatic metastasis | No | 52 | 38 | 1 | — | |
| Yes | 121 | 93 | 1.12 | 0.77–1.64 | 0.560 | |
| Peritoneal metastasis | No | 136 | 104 | 1 | — | |
| Yes | 37 | 27 | 1.02 | 0.66–1.56 | 0.943 | |
| Vascular involvement | No | 93 | 66 | 1 | — | |
| Venous | 36 | 28 | 1.26 | 0.81–1.96 | 0.310 | |
| Arterial | 44 | 37 | 1.48 | 0.98–2.24 | 0.059 | |
| Timing of resection* | 5–<9 mo | 81 | 57 | 1 | — | |
| <5 mo | 50 | 40 | 1.52 | 1.01–2.29 | 0.043 | |
| ≥9 mo | 41 | 34 | 1.56 | 1.02–2.40 | 0.041 | |
| Neoadjuvant therapy | G/GF/O | 69 | 51 | 1 | — | |
| F | 104 | 80 | 0.72 | 0.50–1.02 | 0.063 | |
| Surgery | Exploration | 80 | 65 | 1 | — | |
| Resection ypM1 | 48 | 38 | 0.52 | 0.34–0.80 | 0.003 | |
| Resection ypM0 | 45 | 28 | 0.23 | 0.14–0.38 | <0.001 |
Bold indicates statistically significant P-value.
Time from diagnosis to surgery.
G indicates gemcitabine; GF, gemcitabine + FOLFIRINOX; O, other; F, FOLFIRINOX.
Multivariable analysis revealed that conversion surgery (ypM0: HR: 0.27, P<0.001; ypM1: HR: 0.59, P=0.023), timing of resection (<5 months: HR: 1.51, P=0.063; ≥9 mo: HR: 1.82, P=0.005), and CA19-9 level (HR: 2.31; P<0.001) were independently associated with overall survival for the entire cohort (Table 3). Subgroup analysis identified adjuvant chemotherapy (HR: 0.44; P=0.003), ypM1 status (HR: 1.99; P=0.011), and CA19-9 level (HR: 6.89; P<0.001) as independent prognostic factors in the resection subgroup (Table 4).
TABLE 3.
Multivariable Analysis of Prognostic Factors for Overall Survival in the Entire Cohort
| Variable | Category | HR | 95% CI | P |
|---|---|---|---|---|
| Likelihood ratio: χ2 58.23, 5 DF, P<0.0001 | ||||
| Conversion surgery | Resection ypM1 vs. exploration | 0.59 | 0.38–0.93 | 0.023 |
| Resection ypM0 vs. exploration | 0.27 | 0.16–0.47 | <0.001 | |
| CA19-9 | ≥400 U/mL vs. <400 U/mL | 2.31 | 1.46–3.67 | <0.001 |
| Timing of operation* | <5 mo vs. 5–<9 mo | 1.51 | 0.98–2.32 | 0.063 |
| ≥9 mo vs. 5–<9 mo | 1.82 | 1.20–2.77 | 0.005 | |
| Not included: | ||||
| Vascular involvement | Arterial/venous vs. no | — | — | 0.792 |
| CEA | ≥5 µg/L vs. <5 µg/L | — | — | 0.398 |
| Neoadjuvant therapy | F vs. G/GF/O | — | — | 0.525 |
| ASA classification | ASA 3–4 vs. ASA 1–2 | — | — | 0.064 |
Bold indicates statistically significant P-value.
Time from diagnosis to surgery.
F indicates FOLFIRINOX; G, gemcitabine; GF, gemcitabine + FOLFIRINOX; O, other.
TABLE 4.
Multivariable Analysis of Prognostic Factors for Overall Survival in the Resection Cohort
| Variable | Category | HR | 95% CI | P |
|---|---|---|---|---|
| Likelihood Rario: χ2 33.35, 5 DF, P<0.0001 | ||||
| Conversion surgery | Resection ypM1 vs. ypM0 | 1.99 | 1.17–3.39 | 0.011 |
| CA19-9 | ≥400 U/mL vs. <400 U/mL | 6.89 | 2.96–16.03 | <0.001 |
| Adjuvant chemotherapy | Yes vs. no | 0.44 | 0.26–0.75 | 0.003 |
| Not included | ||||
| Neoadjuvant therapy | F vs. G/GF/O | — | — | 0.895 |
| Vascular involvement | Arterial/venous vs. no | — | — | 0.624 |
| Timing of operation* | <5/≥9 mo vs. 5–<9 mo | — | — | 0.289 |
| ASA classification | ASA 3–4 vs. ASA 1–2 | — | — | 0.075 |
Bold indicates a statistically significant P-value.
Time from diagnosis to surgery.
F indicates FOLFIRINOX; G, gemcitabine, GF, gemcitabine + FOLFIRINOX; O, other.
Role of Adjuvant Therapy After Preoperative Chemotherapy and Conversion Surgery
Administration of additional adjuvant chemotherapy was associated with overall survival after resection (Fig. 2A). Patients who received adjuvant treatment had median overall survival of 29.0 months, compared with 14.8 months for patients without adjuvant treatment (P=0.024). This survival advantage was also demonstrated in the ypM0 and ypM1 subgroups: ypM0 patients receiving adjuvant treatment had median overall survival of 29.1 months versus 19.2 months without adjuvant therapy (Fig. 2B; P=0.047). ypM1 patients had median overall survival of 19.4 months with adjuvant treatment versus 7.5 months without additional treatment (Fig. 2C; P=0.285).
FIGURE 2.
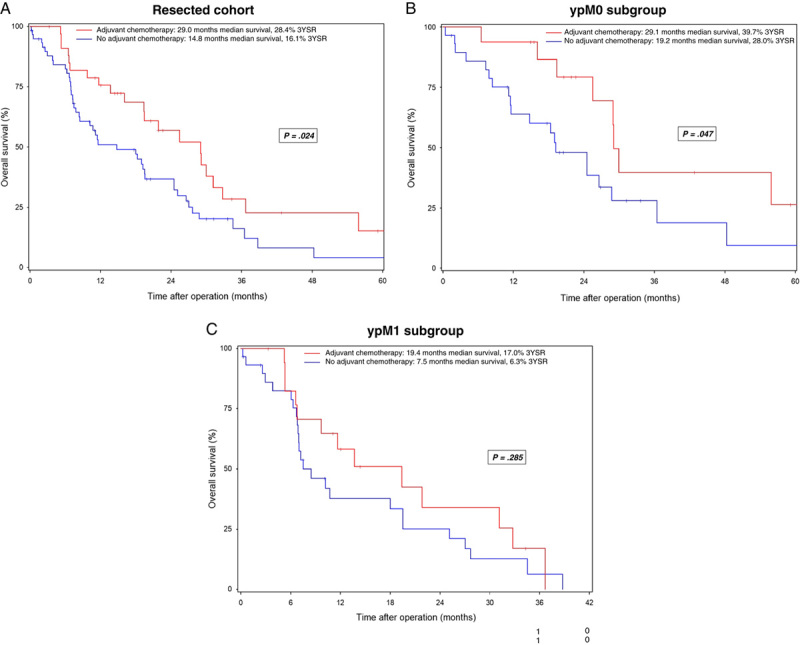
Overall survival in patients with mPDAC undergoing conversion surgery after preoperative chemotherapy with or without adjuvant chemotherapy. Resected cohort (A), ypM0 subgroup (B), ypM1 subgroup (C). Survival data are calculated starting from the date of the operation. Patients alive at the last follow-up are censored (I).
Disease-free Survival After Conversion Surgery
Median disease-free survival was significantly longer in ypM0 patients than in ypM1 patients (8.7 and 5.2 months, respectively; P<0.001) (Fig. 3), and administration of adjuvant chemotherapy was associated with significantly prolonged disease-free survival in all patients with resection (9.0 vs 5.3 months; P=0.008) (Fig. 4A) as well as in the ypM0 and ypM1 subgroups (ypM0: 12.2 vs. 6.8 months, P=0.037; ypM1: 8.0 vs 3.6 months, P=0.047) (Fig. 4B,C).
FIGURE 3.
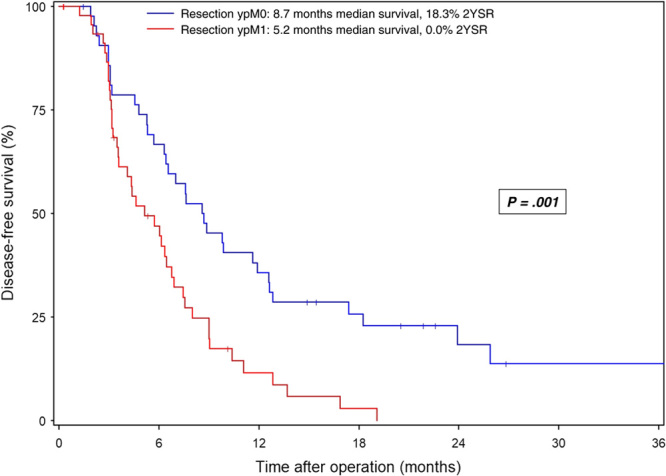
Disease-free survival in patients with mPDAC undergoing conversion surgery after preoperative chemotherapy. Survival data are calculated starting from the date of the operation. Patients alive at the last follow-up are censored (I).
FIGURE 4.
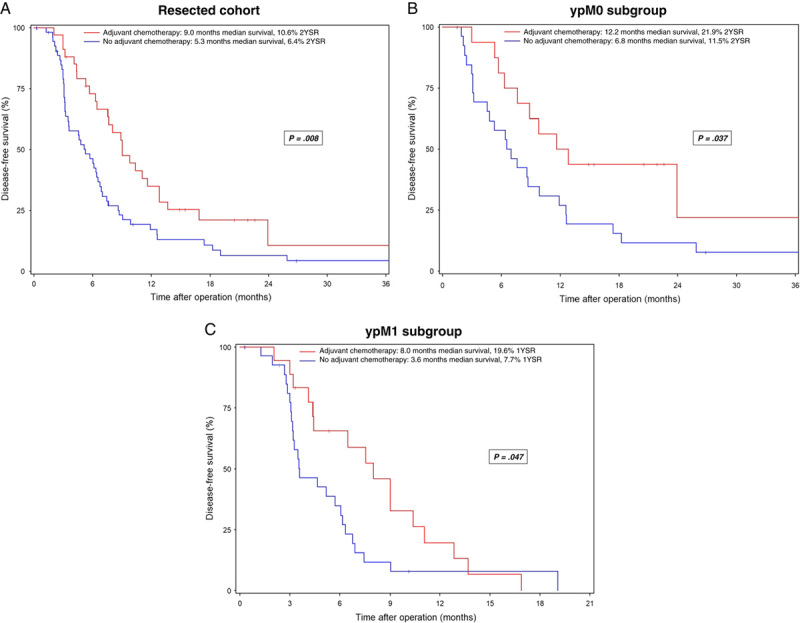
Disease-free survival in patients with mPDAC undergoing conversion surgery after preoperative chemotherapy with or without adjuvant chemotherapy. Resected cohort (A), ypM0 subgroup (B), ypM1 subgroup (C). Survival data are calculated starting from the date of the operation. Patients alive at the last follow-up are censored (I).
DISCUSSION
This study, the largest investigating the oncological outcomes of conversion surgery, has identified several prognostic factors in mPDAC patients with exceptional response to preoperative systemic chemotherapy. The results show that conversion surgery is associated with postresection overall survival of 25.5 months in ypM0 patients, compared with postresection overall survival of only 10.7 months in ypM1 patients and 8.1 months after exploration only. The median duration from diagnosis to surgery of 8.1 months in resected ypM0 patients, 5.9 months in resected ypM1 patients, and 6.9 months after exploration only resulted in overall survival from the time of diagnosis of 33.6, 16.6, and 15 months, respectively, with this multimodal concept. Univariable and multivariable analyses confirmed conversion surgery as an independent prognostic parameter in this cohort of mPDAC patients with response to preoperative chemotherapy.
Only a few, much smaller series of conversion surgery in mPDAC have previously been published. Crippa and colleagues reported a median survival time of 39 months in 11 patients with mPDAC and metastases confined to the liver who underwent conversion surgery after preoperative multiagent chemotherapy.15 Only 3 of these 11 patients underwent synchronous liver resection during pancreatic surgery, with the presence of fibrosis on final pathology, while the other 8 patients had no evidence of metastasis intraoperatively. Another study reported overall survival of 56 months after diagnosis in 24 mPDAC patients undergoing resection after complete radiological response of liver metastases to preoperative chemotherapy.16 However, only patients without detectable liver metastasis by intraoperative ultrasound were eligible for conversion surgery. A 2-center study reported overall survival of 18.2 months from the time of surgery in 23 patients with good response to preoperative chemotherapy who underwent resection of the primary tumor and metastatic sites including liver, lung, and peritoneum.17 The heterogeneity of survival times in these previous series and in the present study can be attributed mainly to different selection criteria of mPDAC patients for conversion surgery.
The extent of pathological response and especially complete pathological response of synchronous metastases after preoperative chemotherapy is an important predictor of survival in stage IV colorectal and gastric cancer.26,27 In borderline-resectable and locally advanced PDAC, ypTNM stage and major pathological response of the primary tumor after preoperative chemotherapy have been established as important predictors of postresection survival.9,28,29 This study demonstrates for the first time a significant survival benefit of complete pathological response of metastases (ypM0) compared with residual metastases (ypM1) in mPDAC patients undergoing resection after preoperative chemotherapy. While resection status was significantly associated with lower CA19-9 levels and a lower proportion of peritoneal metastases, ypM0 patients also presented with a higher frequency of ypN0 status and lower CEA levels. In line with these findings, lower CA19-9 and timing of resection between 5 and 9 months after diagnosis were confirmed as independent predictors of longer overall survival in the entire cohort. Frigerio et al defined radiological disappearance of metastases and normalization of CA19-9 as important selection criteria for resection after chemotherapy in mPDAC and reported impressive overall survival times in the few resected patients.16 Together, these data suggest close observation of potential candidates to assess the potential of major radiological and biological (CA19-9 and CEA) response as predictors of ypM0 status, which was confirmed as an independent predictor of survival in the resection cohort, and to determine the optimal time for surgical exploration. However, the best treatment regimen and duration of preoperative chemotherapy remain to be determined in future studies.14
Before the era of preoperative chemotherapy with modern multiagent regimens, several studies investigated the role of surgery for mPDAC in patients with synchronous or metachronous metastases with heterogenous oncological outcomes.30–32 The largest study, from Heidelberg, investigated radical resection in selected patients with synchronous and metachronous metastasis and found that median overall survival after surgery was only 12 months but the 5-year survival rate was almost 10%, suggesting that selected patients may benefit from resection.33 A recent multicenter study of 25 patients undergoing liver resection for PDAC with metachronous liver metastases reported median overall survival of 36.8 months after liver resection compared with 9.2 months in the control group with no resection.34 The authors attributed the favorable oncological outcomes to the use of preoperative chemotherapy in their study cohort. In line with this, the present study highlights encouraging survival times in the subset of patients with excellent tumor response to preoperative systemic treatment, identifying ypM0 patients as suitable candidates for conversion surgery with resection of the primary tumor and simultaneous metastasectomy. Our study investigates the role of adjuvant chemotherapy after chemotherapy and resection in mPDAC and demonstrates prolonged overall and disease-free survival in both ypM0 and ypM1 patients. Adjuvant chemotherapy was confirmed as an independent predictor of postresection survival in patients undergoing conversion surgery. This is consistent with a recent multicenter analysis of patients with locally advanced PDAC which demonstrated that additional adjuvant chemotherapy is beneficial after preoperative chemotherapy with FOLFIRINOX and surgery in node-positive disease (as in the majority of patients in our study).35 After all, advanced PDAC has to be considered a systemic disease requiring a treatment strategy that includes effective systemic therapy.
In contrast to previous studies investigating the role of conversion surgery in mPDAC with liver metastasis only,15,16 the present study also included patients with other metastatic sites, such as peritoneal (21%) and distant lymph node (6%) metastases. Peritoneal carcinosis was associated with a higher probability of undergoing exploration only, whereas the survival after resection was comparable between patients with hepatic or peritoneal metastasis. Moreover, a recent prospective multicenter trial from Japan reported encouraging outcomes in mPDAC patients with peritoneal carcinosis who underwent conversion surgery after systemic and intraperitoneal chemotherapy.36 The conversion rate was 20%, with an associated median overall survival of 32.5 months.36 Further prospective trials investigating the role of conversion and intraoperative chemotherapy in patients with peritoneal carcinosis are needed to improve the definition of suitable patients and refine the treatment algorithms.
This study features limitations. First, the observational nature of the study design bears the inherent risk of bias, primarily selection bias resulting from the high-volume and tertiary referral center characteristics of the authors’ institution, attracting a selected cohort of responders to previous treatments. Because an intention-to-treat analysis was therefore not feasible, any conclusions on preoperative treatment and selection criteria for conversion surgery have to be considered with caution. Second, the heterogeneity due to changing standard regimens for palliative and adjuvant therapy during the study period must be borne in mind and owing to the monocentric trial design, generalizability of data is limited. Future studies on preoperative chemotherapy and conversion surgery for mPDAC should ideally be prospective and investigate intention-to-treat populations.
In summary, this is the largest study to date assessing the outcomes of conversion surgery after preoperative chemotherapy for mPDAC. Median overall survival after conversion surgery was 25.5 months in patients with complete pathological response of metastasis (ypM0) but only 10.7 months in patients with resected active metastasis (ypM1). On the basis of these observed survival outcomes, the concept of conversion surgery should be evaluated further in patients with mPDAC and good response to chemotherapy, and resection may be recommended for patients in whom complete pathological response of metastasis appears likely. Conversion surgery for mPDAC should remain reserved to clinical studies in high volume settings. Adjuvant chemotherapy may confer an additional survival benefit in this context.
Supplementary Material
Footnotes
T.H. and U.K. contributed equally.
All authors made substantial contributions to the conception and design, and/or acquisition of data, analysis and interpretation of data. All authors participated in drafting the article and revising it critically for important intellectual content and gave final approval of the version to be published.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
REFERENCES
- 1.Strobel O, Neoptolemos J, Jager D, et al. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11–26. [DOI] [PubMed] [Google Scholar]
- 2.Strobel O, Lorenz P, Hinz U, et al. Actual five-year survival after upfront resection for pancreatic ductal adenocarcinoma: who beats the odds? Ann Surg. 2022;275:962–971. [DOI] [PubMed] [Google Scholar]
- 3.Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. [DOI] [PubMed] [Google Scholar]
- 5.Hackert T, Sachsenmaier M, Hinz U, et al. Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg. 2016;264:457–463. [DOI] [PubMed] [Google Scholar]
- 6.Gemenetzis G, Groot VP, Blair AB, et al. Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg. 2019;270:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michelakos T, Pergolini I, Castillo CF, et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. 2019;269:733–740. [DOI] [PubMed] [Google Scholar]
- 8.Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5:1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J, Blair AB, Groot VP, et al. Is a pathological complete response following neoadjuvant chemoradiation associated with prolonged survival in patients with pancreatic cancer? Ann Surg. 2018;268:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1028–1061. [DOI] [PubMed] [Google Scholar]
- 11.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, Heckler M, Mihaljevic AL, et al. CT response of primary tumor and CA19-9 predict resectability of metastasized pancreatic cancer after FOLFIRINOX. Eur J Surg Oncol. 2019;45:1453–1459. [DOI] [PubMed] [Google Scholar]
- 14.Hank T, Strobel O. Conversion surgery for advanced pancreatic cancer. J Clin Med. 2019;8:1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crippa S, Bittoni A, Sebastiani E, et al. Is there a role for surgical resection in patients with pancreatic cancer with liver metastases responding to chemotherapy? Eur J Surg Oncol. 2016;42:1533–1539. [DOI] [PubMed] [Google Scholar]
- 16.Frigerio I, Regi P, Giardino A, et al. Downstaging in stage IV pancreatic cancer: a new population eligible for surgery? Ann Surg Oncol. 2017;24:2397–2403. [DOI] [PubMed] [Google Scholar]
- 17.Wright GP, Poruk KE, Zenati MS, et al. Primary tumor resection following favorable response to systemic chemotherapy in stage IV pancreatic adenocarcinoma with synchronous metastases: a bi-institutional analysis. J Gastrointest Surg. 2016;20:1830–1835. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 19.Sohal DPS, Kennedy EB, Cinar P, et al. Metastatic pancreatic cancer: ASCO Guideline Update. J Clin Oncol. 2020:JCO2001364. [DOI] [PubMed] [Google Scholar]
- 20.Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749–5756. [DOI] [PubMed] [Google Scholar]
- 21.Weitz J, Rahbari N, Koch M, et al. The “artery first” approach for resection of pancreatic head cancer. J Am Coll Surg. 2010;210:e1–e4. [DOI] [PubMed] [Google Scholar]
- 22.Tol JA, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 2014;156:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strobel O, Hinz U, Gluth A, et al. Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann Surg. 2015;261:961–969. [DOI] [PubMed] [Google Scholar]
- 24.Strobel O, Hank T, Hinz U, et al. Pancreatic cancer surgery: the new R-status counts. Ann Surg. 2017;265:565–573. [DOI] [PubMed] [Google Scholar]
- 25.Hank T, Hinz U, Tarantino I, et al. Validation of at least 1 mm as cut-off for resection margins for pancreatic adenocarcinoma of the body and tail. Br J Surg. 2018;105:1171–1181. [DOI] [PubMed] [Google Scholar]
- 26.Chan G, Hassanain M, Chaudhury P, et al. Pathological response grade of colorectal liver metastases treated with neoadjuvant chemotherapy. HPB (Oxford). 2010;12:277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beom SH, Choi YY, Baek SE, et al. Multidisciplinary treatment for patients with stage IV gastric cancer: the role of conversion surgery following chemotherapy. BMC Cancer. 2018;18:1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klaiber U, Schnaidt ES, Hinz U, et al. Prognostic factors of survival after neoadjuvant treatment and resection for initially unresectable pancreatic cancer. Ann Surg. 2021;273:154–162. [DOI] [PubMed] [Google Scholar]
- 29.Perri G, Prakash L, Wang H, et al. Radiographic and serologic predictors of pathologic major response to preoperative therapy for pancreatic cancer. Ann Surg. 2021;273:806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunschede F, Will L, von Langsdorf C, et al. Treatment of metachronous and simultaneous liver metastases of pancreatic cancer. Eur Surg Res. 2010;44:209–213. [DOI] [PubMed] [Google Scholar]
- 31.Zanini N, Lombardi R, Masetti M, et al. Surgery for isolated liver metastases from pancreatic cancer. Updates Surg. 2015;67:19–25. [DOI] [PubMed] [Google Scholar]
- 32.Tachezy M, Gebauer F, Janot M, et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery. 2016;160:136–144. [DOI] [PubMed] [Google Scholar]
- 33.Hackert T, Niesen W, Hinz U, et al. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol. 2017;43:358–363. [DOI] [PubMed] [Google Scholar]
- 34.Schwarz C, Fitschek F, Primavesi F, et al. Metachronous hepatic resection for liver only pancreatic metastases. Surg Oncol. 2020;35:169–173. [DOI] [PubMed] [Google Scholar]
- 35.van Roessel S, van Veldhuisen E, Klompmaker S, et al. Evaluation of adjuvant chemotherapy in patients with resected pancreatic cancer after neoadjuvant FOLFIRINOX treatment. JAMA Oncol. 2020;6:1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada S, Fujii T, Yamamoto T, et al. Conversion surgery in patients with pancreatic cancer and peritoneal metastasis. J Gastrointest Oncol. 2021;12:S110–S117. [DOI] [PMC free article] [PubMed] [Google Scholar]


