Abstract
Species relative abundance (SRA) is an essential attribute of biotic communities, which can provide an accurate description of community structure. However, the sampling method used may have a direct influence on SRA quantification, since the use of attractants (e.g., baits, light, and pheromones) can introduce additional sources of variation in trap performance. We tested how sampling aided by baits affect community data and therefore alter derived metrics. We tested our hypothesis on dung beetles using data from flight interception traps (FITs) as a baseline to evaluate baited pitfall trap performance. Our objective was to assess the effect of bait attractiveness on estimates of SRA and assemblage metrics when sampled by pitfall traps baited with human feces.Dung beetles were sampled at three terra firme primary forest sites in the Brazilian Amazon. To achieve our objective, we (i) identified species with variable levels of attraction to pitfall baited with human feces; (ii) assessed differences in SRA; and (iii) assessed the effect of bait on the most commonly used diversity metrics derived from relative abundance (Shannon and Simpson indices). We identified species less and highly attracted to the baits used, because most attracted species showed greater relative abundances within baited pitfall traps samples compared with our baseline. Assemblages sampled by baited pitfall traps tend to show lower diversity and higher dominance than those sampled by unbaited FITs. Our findings suggest that for ecological questions focused on species relative abundance, baited pitfall traps may lead to inaccurate conclusions regarding assemblage structure. Although tested on dung beetles, we suggest that the same effect could be observed for other insect taxa that are also sampled with baited traps. We highlight a need for further studies on other groups to elucidate any potential effects of using baits.
Keywords: Brazilian Amazon, community structure, flight interception trap, primary forest, terra firme
Assemblages sampled by baited pitfall traps tend to show lower diversity and higher dominance than those sampled by unbaited flight interception traps. Therefore, for ecological questions focused on species relative abundance, baited pitfall traps may lead to inaccurate conclusions regarding assemblage structure.
1. INTRODUCTION
The study of communities allows ecologists to draw inferences about biodiversity (Magurran, 1991), which requires estimates of community attributes (Begon et al., 2006). Although attributes may use data on taxonomic composition (e.g., presence/absence of taxa), considering species abundance patterns provide more detailed description of the community (Peroni & Hernández, 2011). Species relative abundance (SRA) is an essential property of community structure (Holt, 1997), and studies of species commonness or rarity may lead to a better understanding of communities (Anderson et al., 2011). Nevertheless, the choice of sampling method has a direct influence on the community quantification (Campos et al., 2000) because the effectiveness of each sampling method varies among taxa (Katsanevakis et al., 2012; Missa et al., 2009).
Traps, which are widely used to collect a wide range of insects (Juillet, 1963), can be broken into two types: those that capture individuals randomly and those that use some kind of lure to attract insects into the trap (Henderson & Southwood, 2016). For example, flight interception traps (FITs) are a passive method (Matthews & Matthews, 1972) used to collect active flying insects (Campos et al., 2000; Lamarre et al., 2012; Peck & Davies, 1980), providing a random sample of individuals that move through trap height (Ozanne, 2005). In comparison, pitfall traps are a standard method to capture ground‐active insects (Southwood, 1978; Ward et al., 2001) and may incorporate baits to attract insects with any given food preference (Almeida et al., 1998; Woodcock, 2005). Dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae) are a high‐performance indicator group (Gardner et al., 2008), highly suitable for biodiversity monitoring and assessments in tropical forests (Favila & Halffter, 1997; Halffter & Favila, 1993; Lobo et al., 1988). They primarily consume mammal dung (Gill, 1991), but may also feed on carcasses, decaying plant material, and fungi (Bornemissza, 1971; Halffter & Matthews, 1966). Dung beetles are commonly sampled using baited pitfall traps (Doube & Giller, 1990; Raine & Slade, 2019; Silva et al., 2012), whereas FITs are much less commonly used (Da Costa et al., 2009; Puker et al., 2020; Touroult et al., 2017).
The capture effectiveness of pitfall traps for dung beetles has been tested with a wide range of baits including feces from different mammal species (Estrada et al., 1993; Ferreira et al., 2020) or a combination of feces in various proportions (Marsh et al., 2013), as well as decaying meat, and fruits (Beiroz et al., 2016; Silva et al., 2007). These studies show that different dung beetle species are attracted to different bait types (Filgueiras et al., 2009; Silva et al., 2012; Tsuji et al., 2021). It appears that dung beetles are more attracted to feces of omnivorous mammals (Whipple & Hoback, 2012), especially human feces at least in the Neotropical region (Milhomem et al., 2003). The expected species pool represented by pitfall traps baited with human feces is comprised of coprophagous or generalist species, thereby excluding other species with divergent feeding habits. In contrast, FITs may provide a broader inventory of the dung beetle species (Davis et al., 2001), even though the expected species pool for FITs only includes taxa that typically fly at the trap height and excludes flightless species. FITs seem to capture fewer dung beetle individuals and species overall, compared with baited pitfall traps (Audino et al., 2011; Da Silva et al., 2011). However, many studies use a large number of pitfall traps, but allocate limited time and spatial replication to FITs, and thereby hindering comparability in the relative sampling effort between the two methods.
Due to the widespread application of baited pitfall traps in biodiversity studies worldwide, it is essential to quantify possible sampling biases that may affect their performance and limit our interpretation of results from dung beetles surveys. Bait quality (Álvarez et al., 2021; Souza et al., 2015), desiccation resistance (Lucci Freitas et al., 2014; Newton & Peck, 1975), trap size (LeBlanc et al., 2021), and even the position within the pitfall traps can all influence attraction (Lobo et al., 1988). Beyond these factors, there are also idiosyncratic species responses due to food preferences (Almeida et al., 1998; Larsen et al., 2006; Noriega, 2012). The use of baits may therefore result in incomplete or misrepresented information on species abundance patterns, thereby affecting estimates of community structure.
Here, to explore the effects of baited traps on community metrics, we used the dung beetle (Coleoptera: Scarabaeidae: Scarabaeinae) fauna of the western Brazilian Amazon as a model group, and compared the community composition between unbaited FITs and pitfall traps baited with human feces using the former as a baseline. There is no knowledge of flightless dung beetles species in our study area (F. Z. Vaz‐de‐Mello, pers. obs.), and we therefore assume that the expected species pool from pitfall traps is nested within the expected species pool from FITs. We hypothesize that bait affects community data, and therefore alter community metrics. Specifically, we predict that the over‐representation of the most attracted species will alter community metrics, resulting in increased dominance. To test our hypothesis, we aimed to (i) identify species with variable levels of attraction to pitfall traps baited with human feces; (ii) assess differences in SRA between baited pitfalls and unbaited FITs; and (iii) assess the effect of baited traps on dung beetle assemblage metrics.
2. MATERIALS AND METHODS
2.1. Study area
Dung beetles were sampled from October to December 2019 at three localities (Table S1) of lowland terra firme forest—that is, forest areas situated above the flood levels of rivers, streams, and lakes, in the Brazilian Amazon. We sampled at (i) the region of Lago Capanã Grande Extractive Reserve, Amazonas state (hereafter, BR‐319); (ii) the region of Cristalino State Park, Mato Grosso state (hereafter, Cristalino); and (iii) Serra do Divisor National Park, Acre state (hereafter, Serra do Divisor) (Figure 1). At each locality, dung beetles were sampled using FITs, and pitfall traps baited with human feces along three transects (Table S1) of 1000 m.
FIGURE 1.
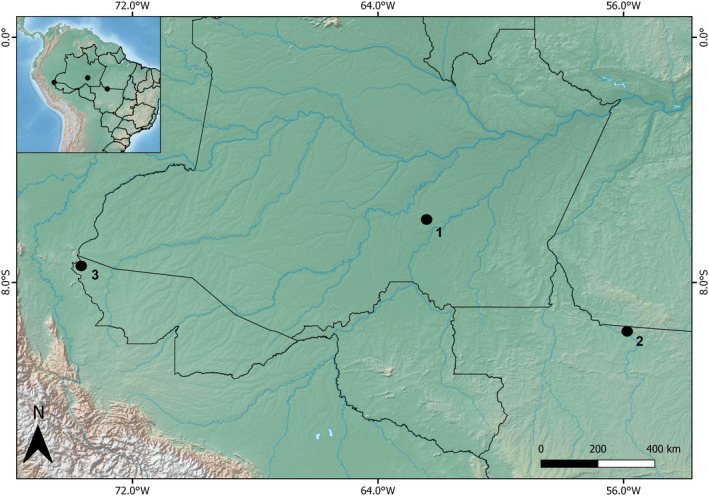
Study region map showing the location of three primary forest areas of terra firme within the Brazilian Amazon (1. BR‐319; 2. Cristalino; and 3. Serra do Divisor) where dung beetles were sampled with flight interception, and baited pitfall traps. Map created in QGIS version 3.8.2.
2.2. Sampling
Four FITs were placed every 250 m along each transect. These were open for 12 days, and checked every 96 h (4 days). Ten pitfall traps were placed every 100 m (Da Silva & Hernández, 2015), with 48 hours of trap exposure in the field (Figure S1). We considered every transect sampled by each method as a sampling unit. Hence, there are nine sampling units for FITs, and nine sampling units for pitfall traps. We assumed spatial independence between all transects, as the minimum distance was 1 km between them (although the mean straight‐line distance between transects is approx. 8 km). There are no temporal differences since pitfalls and FITs were operated simultaneously (Figure S1).
As FITs and pitfall traps were placed at least 50 m apart as suggested by Larsen and Forsyth (2005), we assume that is unlikely that FITs captures were affected by pitfall bait. We also assume that the available dung beetle assemblage was the same for both methods because FITs and pitfall traps were operated simultaneously, and dung beetles are estimated to travel only approximately 90 m during 48 h (at least in the Brazilian Atlantic Forest according to Da Silva & Hernández, 2015). We therefore assume that FITs and pitfall traps within the same transect will sample from the same assemblage, although all traps were spatially independent.
Here, we use FIT data as a baseline to assess the effect of baits on the measure of dung beetle SRA. Although unbaited pitfall traps may seem the obvious baseline to baited pitfall traps, this method captures few individuals (Chong & Hinson, 2015; Frizzas et al., 2020) because dung beetles disperse mainly by flight (Halffter & Edmonds, 1982) and are captured by baited pitfall traps essentially because they are attracted. Furthermore, most of dung beetles appear to fly below two meters in height (Lähteenmäki et al., 2015), and there is no knowledge of flightless species occurring at our three study areas in the Brazilian Amazon (F. Z. Vaz‐de‐Mello, pers. obs.). We assume therefore that the expected species pool for pitfall sampling is nested within the expected species pool for FITs. To support this assumption, we performed a nonmetric multidimensional scaling (NMDS) (“vegan” package; Oksanen et al., 2020) of all dung beetles SRA excluding singletons, and the results showed the expected nesting (Figure S2). As a result, we consider that FITs data provide a feasible baseline for dung beetles, although we are aware that there are other variables influencing both capture for both method (Table S2).
2.3. Identification
Dung beetle species were identified using identification keys, entomological collection for comparison, and taxonomic bibliography (Carvalho De Santana et al., 2019; Cook, 1998, 2000; Cupello & Vaz‐de‐Mello, 2018; Edmonds, 1994; Edmonds & Zídek, 2004, 2010, 2012; Génier, 1996, 2009; Génier & Arnaud, 2016; Rossini & Vaz‐de‐Mello, 2017; Rossini & Vaz‐de‐Mello, 2020; Rossini, Vaz‐de‐Mello, & Zunino, 2018; Rossini, Vaz‐de‐Mello, & Zunino, 2018; Silva & Valois, 2019; Vaz‐de‐Mello et al., 2011). All specimens were deposited at Coleção Entomológica de Mato Grosso Eurides Furtado (CEMT), at Universidade Federal de Mato Grosso, Cuiabá, Mato Grosso, Brazil.
2.4. Data analysis
To compare the sampling effort of FITs and pitfall traps, we used species accumulation curves based on the number of individuals using the vegan package (Oksanen et al., 2020). To assess the effect of bait on the accumulation curves, we only included species captured by baited pitfall traps (Table S3), assuming that these are attracted to bait.
Hotelling's T 2 test was performed to compare the assemblages sampled by FITs and pitfall traps. We used SRA as the response variable and trap (FITs or pitfall traps) as a predictor variable (“Hotelling” package; Curran, 2018). Singletons were excluded from this analysis (Table S3), as the capture of a single individual did not meet our objective. In total, 168 species were considered for Hotelling's T 2.
The indicator value (IndVal) was calculated to identify species associated with FITs or pitfall traps (“indicspecies” package, with 999 permutations; De Cáceres, 2020; De Cáceres & Legendre, 2009). IndVal is the product of two components (specificity and fidelity) multiplied by 100, to yield percentages. These components are calculated based on species abundance and occurrence (Dufrene & Legendre, 1997). Species were categorized as highly (p < .05 and IndVal ≥ 70%), moderately (p < .05, 45% ≤ IndVal < 70%), or weakly associated (when p < .05, IndVal < 45%) to each trapping method (as used by Tonelli et al., 2019; Verdú et al., 2011). Species highly associated were captured almost exclusively by one trap type with great abundance. Species moderately associated were capture exclusively or presented greater abundance in one trap type compared with the other. Species weakly associated were not captured exclusively to one trap type and had low abundance.
Not all dung beetle species are widely distributed across the Brazilian Amazon. To avoid the possibility that association strength between species and traps was altered because of a species not occurring in a particular location, IndVal was calculated for species sampled at all sites and species common to two sites using a pairwise comparison. (Table S3). We also evaluated the correlation between species and trap type using the Point–Biserial Correlation Coefficient. This division is even more relevant to the correlation coefficient, as the absence of species in samples with one type of trap increases the association strength as much as the presence of species in samples with the other trap (De Cáceres & Legendre, 2009).
To test whether the use of bait affects SRA between baited and the unbaited baseline, we used a chi‐squared goodness‐of‐fit test (“chisq.test” function), with standardized residuals (SR) as a post hoc method. Species that contributed to significance were those with p < .05 and SR outside of the range −1.96 to 1.96 (Callegari‐Jacques, 2003). Assuming that unbaited FITs samples reflect dung beetle assemblage structure better than baited pitfall traps, SRA sampled by FITs was used as the expected frequency, while SRA sampled by pitfall traps was used as the observed frequency. We selected species that were present in at least two sites and sampled by both FITs and pitfall traps (Table S3), and chi‐squared was always applied within the same transect.
We evaluated how baited and unbaited traps influences two indices based on SRA: Shannon's entropy index and the inverse of Simpson's concentration index, both used as measures of diversity and based on the Hill Numbers (Chao et al., 2014). We used the “iNEXT” package (Hsieh et al., 2022) for this analysis. Each index was calculated based on the assemblages captured by FITs and pitfall traps separately and the result was compared within the same transect. For these comparisons, we selected only species captured by pitfall traps (Table S3), assuming that these were attracted to the bait. Even if some individuals were not attracted to the bait and yet fell into the pitfall traps as a random event, this is likely to be a rare occurrence and comprise a low number of individuals. These will therefore likely have negligible influence on the overall result.
Analyses were performed on transect‐level data whenever possible, using R version 3.6.2 (R Core Team, 2019).
3. RESULTS
In total, 23,427 dung beetle individuals were sampled belonging to 198 species (Table S3), of which 55 species (27.78%) were captured exclusively by FITs, 35 species (17.68%) were captured exclusively by pitfall traps, and 108 species (54.54%) were collected by both.
Species accumulation curves were elaborated based on attracted species and showed that richness was similar between FITs and baited pitfall, as most confidence intervals were overlapping. Relative abundance differed significantly between FITs and pitfall trap samples (T 2 = 32.07; df: 27/168; p < .001). Five species were weakly associated with FITs (Table 1), and none were highly or moderately associated with this trap type. The same species were all negatively correlated with pitfall traps (p ≤ .016; Table S6). Eight species were highly associated with pitfall traps, while four were moderately associated, and 13 were weakly associated with this trap type. Pitfall‐associated species were positively correlated with pitfall traps (Table S6).
TABLE 1.
IndVal and p‐value of dung beetle species highly, moderately, and weakly associated with unbaited flight interception traps and pitfall traps baited with human feces at three terra firme primary forest sites in the Brazilian Amazon.
| Association strength | Species | IndVal (%) | p |
|---|---|---|---|
| Weakly associated with FITs | Ateuchus aff. frontalis (Boucomont, 1928) | 30.12 | .001 |
| Coprophanaeus telamon (Erichson, 1847) | 27.08 | .035 | |
| Coprophanaeus degallieri (Arnaud, 1997) | 20.62 | .001 | |
| Canthon xanthopus (Blanchard, 1846) | 11.80 | .017 | |
| Dendropaemon angustipennis (Harold, 1869) | 11.11 | .011 | |
| Highly associated with pitfall | Onthophagus aff. rubrescens (Blanchard, 1846) | 97.04 | .001 |
| Onthophagus osculatii (Guérin‐Méneville, 1855) | 94.95 | .001 | |
| Onthophagus aff. onorei (Zunino & Halffter, 1997) | 89.23 | .001 | |
| Eurysternus caribaeus (Herbst, 1789) | 79.6 | .001 | |
| Eurysternus hypocrita (Balthasar, 1939) | 77.67 | .001 | |
| Eurysternus wittmerorum (Martinez, 1988) | 76.03 | .001 | |
| Dichotomius aff. batesi (Harold, 1867) | 73.4 | .001 | |
| Sylvicanthon proseni (Martínez, 1949) | 71.72 | .001 | |
| Moderately associated with pitfall | Canthon luteicollis (Erichson, 1847) | 67.21 | .001 |
| Onthophagus onorei (Zunino & Halffter, 1997) | 65.00 | .001 | |
| Onthophagus aff. osculatii (Guérin‐Méneville, 1855) | 59.83 | .001 | |
| Eurysternus cayennensis (Castelnau, 1840) | 51.93 | .002 | |
| Weakly associated with pitfall | Eurysternus arnaudi (Génier, 2009) | 43.81 | .001 |
| Oxysternon conspicillatum (Weber, 1801) | 41.23 | .001 | |
| Eurysternus hamaticollis (Balthasar, 1939) | 34.83 | .001 | |
| Eurysternus strigilatus (Génier, 2009) | 28.53 | .038 | |
| Deltochilum orbiculare (Lansberge, 1874) | 27.36 | .001 | |
| Dichotomius mamillatus (Felsche, 1901) | 25.37 | .001 | |
| Eurysternus foedus (Guerin‐Meneville, 1844) | 20.25 | .001 | |
| Eurysternus ventricosus (Gill, 1990) | 18.33 | .001 | |
| Canthon rufocoeruleus (Martínez, 1948) | 11.66 | .003 | |
| Dichotomius robustus (Luederwaldt 1935) | 11.64 | .001 | |
| Onthophagus digitifer (Boucomont, 1932) | 10.00 | .011 | |
| Dichotomius melzeri (Luederwaldt, 1922) | 09.66 | .018 |
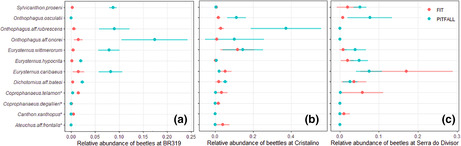
Species relative abundance sampled by FITs and pitfall traps differed significantly (Table S5). FITs‐associated species showed a lower relative abundance in pitfall samples for all localities (Figure 2). Four species highly associated with pitfall traps—E. hypocrita, O. aff. onorei, O. aff. rubrescens, and O. osculatii—presented higher relative abundance in all pitfall trap samples (Figure 2). This pattern was not consistent for other species highly associated with pitfall traps, as in the case of D. aff. batesi, E. caribaeus, E. wittmerorum, and S. proseni (Table S5; Figure 2).
FIGURE 2.
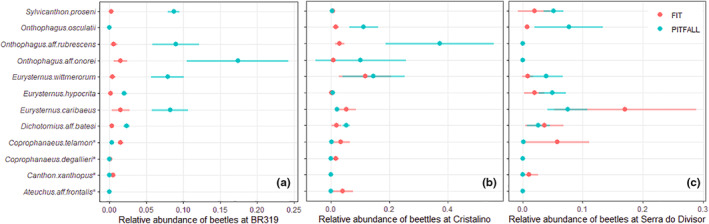
A comparison of the relative abundance of dung beetle species highly associated with pitfall traps and weakly associated with flight interceptions traps (FITs). The comparisons (chi‐squared goodness of fit) were made at transect level to show how species' relative abundance (SRA; mean ± standard deviation) changes when bait is used at three sites (a) BR‐319, Amazonas; (b) Cristalino; (c) Serra do Divisor. Graphics created with “tidyverse” and “ggtext” packages (Wickham et al., 2019; Wilke & Wiernik, 2022). *species associated with fligh interception traps.
In most cases, dung beetle assemblages sampled by pitfall traps showed lower diversity and higher dominance compared with the baseline from FITs (Table 2). We assessed this comparing the confidence intervals (CI) between FITs and pitfall traps within the same transect, when CI is not overlapping the differences are statistically significant. This is clearly observed at Cristalino where there is no CI overlapping, and in all transects, there are higher diversity and lower dominance in the FITs baseline assemblage than for the pitfall traps. The only exceptions were BR‐319 transect A (pitfall traps showed greater diversity and lower dominance); transect B (where there were greater dominance in assemblages sampled by FITs); transect C (where differences were not statistically significant to Shannon index); and Serra do Divisor transect C (where differences were not statistically significant to Shannon index).
TABLE 2.
Shannon and Simpson indices comparing dung beetle assemblage sampled within the same transect by flight interception traps (FITs) an pitfall traps baited with human feces at three terra firme primary forests within the Brazilian Amazon.
| Locality | Transect | Trap type | Shannon CI 95% [L–U] | Simpson CI 95% [L–U] |
|---|---|---|---|---|
| BR‐319 | A | FIT | 14.369 [14.369–16.923] | 8.423 [8.423–9.838] |
| Pitfall | 21.560 [21.560–23.356] | 15.598 [15.598–16.841] | ||
| B | FIT | 13.865 [13.865–17.031] | 5.999 [5.999–7.294] | |
| Pitfall | 14.244 [14.244–15.319] | 9.616 [9.616–10.270] | ||
| C | FIT | 20.364 [20.364–25.318] | 11.193 [11.193–14.902] | |
| Pitfall | 13.802 [13.802–15.111] | 8.916 [8.916–9.647] | ||
| Cristalino | A | FIT | 20.740 [20.74–22.451] | 11.152 [11.152–12.321] |
| Pitfall | 12.470 [12.470–13.454] | 6.288 [6.288–6.697] | ||
| B | FIT | 18.385 [18.385–20.576] | 11.618 [11.618–12.987] | |
| Pitfall | 5.529 [5.529–5.915] | 2.678 [2.678–2.802] | ||
| C | FIT | 21.454 [21.454–27.509] | 16.666 [16.666–21.054] | |
| Pitfall | 8.797 [8.797–9.216] | 5.497 [5.497–5.684] | ||
| Serra do Divisor | A | FIT | 16.033 [16.033–22.623] | 12.402 [12.402–18.794] |
| Pitfall | 9.563 [9.563–10.531] | 4.697 [4.697–5.138] | ||
| B | FIT | 19.393 [19.393–25.148] | 9.589 [9.859–12.790] | |
| Pitfall | 14.200 [14.200–15.560] | 7.145 [7.145–7.861] | ||
| C | FIT | 15.368 [15.368–20.187] | 10.211 [10.211–13.636] | |
| Pitfall | 14.580 [14.580–15.629] | 8.965 [8.965–9.642] |
Note: When confidence intervals (CI) are not overlapping the indices values are statistically different. Confidence interval with 95% [lower limit–upper limit].
4. DISCUSSION
Our results showed that dung beetle species are attracted to a varying extent to traps baited with human feces. We identified species that were greatly attracted (E. hypocrita, O. aff. onorei, O. aff. rubrescens, and O. osculatii) and those that were less so (A. aff. frontalis, Co. degallieri, Co. telamon, and Ca. xanthopus) to this type of bait (Table 1). We showed that FITs and pitfall traps result in different patterns of SRA, which we consider to be an effect of bait attractiveness, as species highly attracted to bait express a greater relative abundance in baited traps. Overall, dung beetle assemblages sampled by baited pitfall traps exhibit lower diversity and greater dominance.
The variable level of attraction to traps baited with human fecal baits can be explained by the known feeding habits of different dung beetle species. Of those species strongly attracted to detrital bait across our samples, E. hypocrita is known to be highly attracted to human and howler monkey (Alouatta spp.) feces, fish, and decaying meat (Génier, 2009). Although most Onthophagus species are generalist coprophages, without a clear preference for specific mammal feces (Pulido‐Herrera & Zunino, 2007), human feces is known to effectively attract O. osculatii (Rossini, Vaz‐de‐Mello, & Zunino, 2018) and O. onorei (Rossini, 2016). Of those species less attracted to baited traps across our samples, Ateuchus species are usually coprophagous, but are not necessarily attracted to human feces (Vaz‐de‐Mello et al., 1998), while Coprophanaeus species are preferentially necrophagous (Edmonds & Zídek, 2010). Canthon xanthopus is possibly a predator or specializes on decaying arthropods such as dead millipedes (Cupello & Vaz‐de‐Mello, 2018). Due to its low abundance in the FITs baseline samples (Table S3), we do not believe that Ca. xanthopus was attracted to decaying arthropods within the traps but was more likely to be captured during dispersal flights.
Baited pitfall traps therefore appear to disproportionally sample Scarabaeinae dung beetle species that are either coprophagous and/or generalists feeders. We confirm that noncoprophagous species or those without preference for human feces were less attracted to human feces bait in our study. This is consistent with other studies that used FIT and baited pitfall traps, which found noncoprophagous species or species with no preference for human feces (Da Silva et al., 2011). In addition, baited pitfall traps tend to capture very small counts of necrophagous (Audino et al., 2011), myrmecophilous, and termitophilous species (Ong et al., 2021), and this method is therefore likely to yield severely biased samples of dung beetle communities, which underestimate overall taxonomic and functional diversity.
Other species in our samples (D. aff. batesi, E. caribaeus, E. wittmerorum, and S. proseni) exhibited wide variation in relative abundance in baited pitfall traps depending on trap location (Table 1). Dichotomius species are coprophagous (F. Z. Vaz‐de‐Mello, pers. obs.); Eurysternus caribaeus is attracted to a wide range of feces, fish, and decaying meat; E. wittmerorum is mainly attracted by human feces and fish (Génier, 2009); and S. proseni is preferentially coprophagous and attracted to human feces (Cupello & Vaz‐de‐Mello, 2018). Since the proportional representation of these coprophagous or generalist species varies across sites, they have a lesser influence on overall dung beetle assemblage metrics. The reasons for this variation across sites are still unclear and may be related to random spatial variation and/or microclimatic factors.
Our results show that dung beetle assemblages sampled by baited pitfall traps tend to have lower diversity and higher dominance than those provided by baseline FITs (Table 2), even where species richness was higher in baited pitfall traps. We suggest that this is due to the attractiveness effect of the bait, with abundance overestimates of strongly attracted species resulting in higher relative abundance and dominance (if not hyperabundance) in our samples (Figure 2). Although this pattern was prevalent in most of our samples (Figure 2), it was not found at BR‐319 (Table 2). However, in most cases, the assemblage structure is likely to be misrepresented due to the inherent biases introduced by the use of baits.
We emphasize that our aim focuses on the use of FITs as a baseline for community metrics and does not involve further comparisons between unbaited FITs and baited pitfall. No sampling method is completely unbiased, and both FITs and pitfall traps come with their own set of advantages and disadvantages (Table S2).
Flight interception traps represent a passive trapping technique (Matthews & Matthews, 1972) that also provides information about flight direction, an important consideration for studies of migratory insects (Henderson & Southwood, 2016) and edge effects (González et al., 2020). However, FITs may not be effective for direct population estimates of beetles as individuals in flight may successfully avoid the trap, and capture success is influenced by light intensity and wind direction (Boiteau, 2000). To optimize the chances of capture, FITs should be installed along trails or open glades (Souza et al., 2015) or along flight paths (Henderson & Southwood, 2016). FITs also represent a more costly method because they are expensive to construct or purchase (Souza et al., 2015) and require greater time investment for field installation (González et al., 2020) compared with pitfalls. FITs are also large and bulky, another potential disadvantage since this increases the likelihood of disturbance by large vertebrates (Missa et al., 2009), potentially introducing additional replacement costs.
In contrast to FITs, pitfall traps are extremely low‐cost, as cheap and widely available containers may be used for trap construction (González et al., 2020; Henderson & Southwood, 2016). They are also easy and quick to operate, ensuring robust spatial replication (Missa et al., 2009). Such considerations are especially relevant in the tropics where there is an even more pressing demand for biodiversity data and financial resources are often limited (Gardner et al., 2008). Baited pitfall traps are an efficient and economic method in terms of labour, which improves the chances of detectability of low‐densities taxa in the field (if they are attracted to the bait in use), and increases the capture success of potentially attracted species (Weinzierl et al., 2005). However, it has been suggested that pitfall traps do not provide reliable estimates of insect density (Topping & Sunderland, 1992) or relative abundance (Woodcock, 2005). Also, some preservation fluids may attract particular taxa (Greenslade & Greenslade, 1971) and their efficiency may be limited when sampling larger insects (Hancock & Legg, 2012; Spence & Niemelä, 1994).
Our results suggest that FITs provide a useful baseline for dung beetle communities, with reduced sampling biases compared with baited pitfall traps. Pitfall traps baited with fecal material clearly remain a valid, useful, and efficient tool for dung beetle surveys, providing reliable capture success for species that feed on feces. Their use in biodiversity surveys and ecological studies has been increasing over the past 30 years (Raine & Slade, 2019), many of which focus on dung beetle assemblage metrics (Bogoni et al., 2019; Chiew et al., 2021; Enari et al., 2018; Fuzessy et al., 2021; Nependa et al., 2021), and dung beetle‐mammal interaction networks (Nichols et al., 2009; Raine et al., 2018). Clearly, it is not our intention to reject a widely established and broadly accepted sampling protocol for the dung beetle field studies. Rather, we suggest that when an ecological question relies on the SRA, baited pitfall traps may often overestimate the relative abundance of species that are more strongly attracted to the bait. The main concern is that such systematic sampling bias may lead to inaccurate conclusions regarding assemblage structure. So we suggest the use of unbaited FITs to access better estimates of relative abundance of coprophagous/generalists species attracted to baits. Although tested here for dung beetles, we suggest that a similar effect could be observed for other groups of insects that are typically sampled with baited traps.
AUTHOR CONTRIBUTIONS
Andressa Bach: Conceptualization (supporting); data curation (lead); formal analysis (lead); investigation (equal); methodology (equal); writing – original draft (lead). Lúcia A. F. Mateus: Formal analysis (supporting); methodology (equal); writing – review and editing (supporting). Carlos A. Peres: Conceptualization (equal); funding acquisition (equal); project administration (equal); supervision (equal); writing – review and editing (equal). Torbjørn Haugaasen: Conceptualization (equal); funding acquisition (equal); project administration (equal); resources (equal); writing – review and editing (equal). Julio Louzada: Investigation (equal); methodology (equal); writing – review and editing (equal). Joseph E. Hawes: Methodology (supporting); writing – review and editing (equal). Renato A. Azevedo: Methodology (supporting); writing – review and editing (supporting). Emanuelly F. Lucena: Methodology (supporting); writing – review and editing (supporting). José Victor A. Ferreira: Methodology (supporting). Fernando Z. Vaz‐de‐Mello: Conceptualization (lead); methodology (equal); supervision (lead); writing – original draft (equal).
CONFLICT OF INTEREST STATEMENT
Authors declare no conflict of interest concerning the publication of this article.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
This work derives from the first author's Master's thesis, developed in the Postgraduate Program in Ecology and Biodiversity Conservation at the Federal University of Mato Grosso, Brazil. We are grateful to the Research Council of Norway (project no. 288086) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (131260/2020‐0, 431760/2018‐7 and 306745/2016‐0) for financial and logistic support. The permanent license for zoological material collection was authorized by Ministério do Meio Ambiente—MMA, Instituto Chico Mendes de Conservação e Biodiversidade—ICMBio and Sistema de Autorização e Informação em Biodiversidade—SISBIO (n. 72874) on November 4, 2019. We are grateful to all members of the Amazon Biodiversity and Carbon (ABC) Expeditions project for logistic and field support. We also thank the residents and managers of our study sites for their support and assistance. We are grateful to Bruna R. Bordin for her assistance in triage and preparation of the specimens. Finally, we thank Maria E. Maldaner, Ricardo R. C. Solar and Thiago J. Izzo for their valuable suggestions and comments on the manuscript. This is publication #01 of the Amazon Biodiversity and Carbon Expeditions.
Bach, A. , Mateus, L. A. F. , Peres, C. A. , Haugaasen, T. , Louzada, J. , Hawes, J. E. , Azevedo, R. A. , Lucena, E. F. , Ferreira, J. V. A. , & Vaz‐de‐Mello, F. Z. (2023). Bait attractiveness changes community metrics in dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Ecology and Evolution, 13, e9975. 10.1002/ece3.9975
DATA AVAILABILITY STATEMENT
Data used in this study are available on Dryad. DOI: https://doi.org/10.5061/dryad.3tx95x6m5.
REFERENCES
- Almeida, L. M. , Ribeiro‐Costa, C. S. , & Marinoni, L. (1998). Manual de Coleta, Conservação, Montagem e Identificação de Insetos (1st ed.). Holos Editora. [Google Scholar]
- Álvarez, C. F. , Clavijo‐Giraldo, A. , Inés Uribe, S. , Pyrcz, T. W. , Agra Iserhard, C. , Lucci Freitas, A. V. , & Marín, M. A. (2021). Sampling performance of bait traps in high Andean fruit‐feeding butterflies. Neotropical Biodiversity, 7(1), 507–513. 10.1080/23766808.2021.2004802 [DOI] [Google Scholar]
- Anderson, M. J. , Crist, T. O. , Chase, J. M. , Valend, M. , Inouye, B. D. , Freestone, A. L. , Sanders, N. J. , Cornell, H. V. , Comita, L. S. , Davies, K. F. , Harrison, S. P. , Kraft, N. J. B. , Stegen, J. C. , & Swenson, N. G. (2011). Navigation the multiple meanings of ß diversity: A roadmap for the practicing ecologist. Ecology Letters, 14(1), 19–28. 10.1111/j.1461-0248.2010.01552.x [DOI] [PubMed] [Google Scholar]
- Audino, L. D. , Da Silva, P. G. , Nogueira, J. M. , De Moraes, L. P. , & Vaz‐de‐Mello, F. Z. (2011). Scarabaeinae (Coleoptera, Scarabaeidae) de um bosque de eucalipto introduzido em uma região originalmente campestre. Iheringia, Série Zoologia, 101(1–2), 121–126. 10.1590/S0073-47212011000100017 [DOI] [Google Scholar]
- Begon, M. , Townsed, C. R. , & Harper, J. L. (2006). Ecology from individuals to ecosystems (4th ed.). Blackwell Publishing. [Google Scholar]
- Beiroz, W. , Slade, E. M. , Barlow, J. , Silveira, J. M. , Louzada, J. , & Sayer, E. (2016). Dung beetle community dynamics in undisturbed tropical forests: Implications for ecological evaluations of land‐use change. Insect Conservation and Diversity, 10(1), 94–106. 10.1111/icad.12206 [DOI] [Google Scholar]
- Bogoni, J. A. , Da Silva, P. G. , & Peres, C. A. (2019). Co‐declining mammal‐dung beetle faunas throughout the Atlantic Forest biome of South America. Ecography, 42(11), 1803–1818. 10.1111/ecog.04670 [DOI] [Google Scholar]
- Boiteau, G. (2000). Efficiency of flight interception traps for adult Colorado potato eetles (Coleoptera: Chrysomelidae). Journal of Economic Entomology, 93(3), 630–635. 10.1603/0022-0493-93.3.630 [DOI] [PubMed] [Google Scholar]
- Bornemissza, G. F. (1971). Mycetophagous breeding in the Australian dung beetle, Onthophagus dunning . Pedobiologia, 11, 133–142. [Google Scholar]
- Callegari‐Jacques, S. M. (2003). Bioestatística Princípios e Aplicações (1st ed.). Artmed. [Google Scholar]
- Campos, W. G. , Pereira, D. B. S. , & Schoereder, J. H. (2000). Comparison of the efficiency of flight‐interception trap models for sampling Hymenoptera and other insects. Anais da Sociedade Entomológica Do Brasil, 29, 381–389. 10.1590/S0301-80592000000300001 [DOI] [Google Scholar]
- Carvalho De Santana, E. C. , Pacheco, T. L. , & Vaz‐de‐Mello, F. Z. (2019). Taxonomic revision of the Canthidium Erichson, 1847 species of the gigas group (Coleoptera, Scarabaeidae, Scarabaeinae). European Journal of Taxonomy, 530, 1–24. 10.5852/ejt.2019.530 [DOI] [Google Scholar]
- Chao, A. , Gotelli, N. J. , Hsieh, T. C. , Sander, E. L. , Ma, K. H. , Colwell, R. K. , & Ellison, A. M. (2014). Rarefaction and extrapolation with hill numbers: A framework for sampling and estimation in species diversity studies. Ecological Monographs, 84(1), 45–67. 10.1890/13-0133.1 [DOI] [Google Scholar]
- Chiew, L. Y. , Hackett, T. D. , Brodie, J. F. , Teoh, S. W. , Burslem, D. F. R. P. , Reynolds, G. , Deere, N. J. , Vairappan, C. S. , & Slade, E. M. (2021). Tropical forest dung beetle‐mammal dung interaction networks remain similar across an environmental disturbance gradient. Journal of Animal Ecology, 91(3), 604–6017. 10.1111/1365-2656.13655 [DOI] [PubMed] [Google Scholar]
- Chong, J. H. , & Hinson, K. R. (2015). A comparison of trap types for assessing diversity of Scarabaeoidea on South Carolina golf courses. Journal of Economic Entomology, 108(5), 2383–2396. 10.1093/jee/tov209 [DOI] [PubMed] [Google Scholar]
- Cook, J. (1998). A revision of the neotropical genus Bdelyrus Harold (Coleoptera: Scarabaeidae). The Canadian Entomologist, 130(5), 631–689. 10.4039/Ent130631-5 [DOI] [Google Scholar]
- Cook, J. (2000). A revision of the neotropical genus Cryptocanthon Balthasar (Coleoptera: Scarabaeidae: Scarabaeinae). The Coleopterists Bulletin, 56(1), 1–96. 10.1649/0010-065X(2002)56[3:AROTNG]2.0.CO,2 [DOI] [Google Scholar]
- Cupello, M. , & Vaz‐de‐Mello, F. Z. (2018). A monographic revision of the neotropical dung beetle genus Sylvicanthon Halffter and Martínez, 1977 (Coleoptera: Scarabaeidae: Scarabaeinae, Deltochilini), including a reappraisal of the taxonomic history of ‘Canthon sensu lato’. European Journal of Taxonomy, 467, 1–205. 10.5852/ejt.2018.467 [DOI] [Google Scholar]
- Curran, J. (2018). Hotelling: Hotelling's T 2 test and variants . R package version 1.0‐5. https://CRAN.R‐project.org/package=Hotelling
- Da Costa, C. M. Q. , Silva, F. A. B. , De Farias, A. I. , & De Moura, R. C. (2009). Diversidade de Scarabaeinae (Coleoptera, Scarabaeidae) coletados com armadilha de interceptação de vôo no refúgio ecológico Charles Darwin, Igarassu‐PE, Brasil. Revista Brasileira de Entomologia, 53(1), 88–64. 10.1590/S0085-56262009000100021 [DOI] [Google Scholar]
- Da Silva, P. G. , Audino, L. D. , Nogueira, J. M. , De Moraes, L. P. , & Vaz‐de‐Mello, F. Z. (2011). Escarabeíneos (Coleoptera: Scarabaeidae: Scarabaeinae) de uma área de campo nativo no bioma Pampa, Rio Grande do Sul, Brasil. Biota Neotropica, 12, 246–253. 10.1590/S1676-06032012000300024 [DOI] [Google Scholar]
- Da Silva, P. G. , & Hernández, M. I. M. (2015). Spatial patterns of movement of dung beetle species in a tropical forest suggest a new trap spacing for dung beetle biodiversity studies. PLoS One, 10(5), e0126112. 10.1371/journal.pone.0126112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, A. J. , Holloway, J. D. , Huijbregts, H. , Krikken, J. , Kirk‐Springgs, A. H. , & Sutton, D. L. (2001). Dung beetles as indicators of change in the forests of northern Borneo. Journal of Applied Ecology, 38(3), 593–616. 10.1046/j.1365-2664.2001.00619.x [DOI] [Google Scholar]
- De Cáceres, M. (2020). How to use the indicspecies package . R package version 1.7.8. http://www2.uaem.mx/r‐mirror/web/packages/indicspecies/vignettes/indicspeciesTutorial.pdf
- De Cáceres, M. , & Legendre, P. (2009). Associations between species and groups of sites: Indices and statistical inference. Ecology, 90(12), 3566–3574. 10.1890/08-1823.1 [DOI] [PubMed] [Google Scholar]
- Doube, B. M. , & Giller, P. S. (1990). A comparison of two types of trap for sampling dung beetle populations (Coleoptera: Scarabaeidae). Bulletin of Entomological Research, 80(3), 259–263. 10.1017/S0007485300050458 [DOI] [Google Scholar]
- Dufrene, M. , & Legendre, P. (1997). Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecological Monographs, 67(3), 345–366. 10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO,2 [DOI] [Google Scholar]
- Edmonds, W. D. (1994). Contributions in science: Revision of Phanaeus Macleay, a New World genus of Scarabaeinae dung beetles (Coleoptera: Scarabaeidae, Scarabaeinae) (1st ed.). Natural History of Museum of Los Angeles County. [Google Scholar]
- Edmonds, W. D. , & Zídek, J. (2004). Revision of the neotropical dung beetle genus Oxysternon (Scarabaeidae: Scarabaeinae: Phanaeini). Folia Heyrovskyana, 11, 1–58. [Google Scholar]
- Edmonds, W. D. , & Zídek, J. (2010). A taxonomic review of the neotropical genus Coprophanaeus Olsoufieff, 1924 (Coleoptera: Scarabaeidae, Scarabaeinae). Insecta Mundi, 0129, 1–111. 10.5281/zenodo.5352924 [DOI] [Google Scholar]
- Edmonds, W. D. , & Zídek, J. (2012). Taxonomy of Phanaeus revisited: Revised keys to and comments on species of the New World dung beetle genus Phanaeus Macleay, 1819 (Coleoptera: Scarabaeidae: Scarabaeinae, Phanaeini). Insecta Mundi, 0274, 1–108. 10.5281/zenodo.5182095 [DOI] [Google Scholar]
- Enari, H. , Koibe, S. , Enari, H. S. , Seki, Y. , Okuda, K. , & Kodera, Y. (2018). Early‐stage ecological influences of population recovery of large mammals on dung beetle assemblages in heavy snow areas. Acta Oecologica, 92, 7–15. 10.1016/j.actao.2018.07.007 [DOI] [Google Scholar]
- Estrada, A. , Halffter, G. , Coates‐Estrada, R. , & Meritt, D. A. (1993). Dung beetles attracted to mammalian herbivore (Alouatta palliata) and omnivore (Nasua narica) dung in the tropical rain forest of los Tuxtlas, Mexico. Journal of Tropical Ecology, 9(1), 45–54. 10.1017/S0266467400006933 [DOI] [Google Scholar]
- Favila, M. E. , & Halffter, G. (1997). The use of indicator groups for measuring biodiversity as related to community structure and function. Acta Zoológica Mexicana (n.s.), 72, 1–25. 10.21829/azm.1997.72721734 [DOI] [Google Scholar]
- Ferreira, K. R. , Puker, A. , & Correa, C. M. A. (2020). The attraction of Amazonian dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae) to the feces of omnivorous mammals is dependent on their diet: Implication for ecological monitoring. Environmental Entomology, 49(6), 1383–1392. 10.1093/ee/nvaa106 [DOI] [PubMed] [Google Scholar]
- Filgueiras, B. K. , Liberal, C. N. , Aguiar, C. D. M. , Hernández, M. I. M. , & Iannuzzi, L. (2009). Attractivity of omnivore, carnivore and herbivore mammalian dung to Scarabaeinae (Coleoptera, Scarabaeidae) in a tropical Atlantic rainforest remnant. Revista Brasileira de Entomologia, 53, 422–427. 10.1590/S0085-56262009000300017 [DOI] [Google Scholar]
- Frizzas, M. R. , Batista, J. L. F. L. , Rocha, M. V. C. , & Oliveira, C. M. (2020). Diversity of Scarabaeinae (Coleoptera: Scarabaeidae) in an urban fragment of Cerrado in Central Brazil. European Journal of Entomology, 117, 273–281. 10.14411/eje.2020.031 [DOI] [Google Scholar]
- Fuzessy, L. F. , Benítez‐López, A. , Slade, E. M. , Bufalo, F. S. , Magro‐de‐Souza, G. C. , Pereira, L. A. , & Culot, L. (2021). Identifying the anthropogenic drivers of declines in tropical dung beetle communities and functions. Biological Conservation, 256, 109063. 10.1016/j.biocon.2021.109063 [DOI] [Google Scholar]
- Gardner, T. A. , Barlow, J. , Araujo, I. S. , Avila‐Pires, T. C. , Bonaldo, A. B. , Costa, J. E. , Esposito, M. C. , Ferreira, L. V. , Hawes, J. , Hernandez, M. I. M. , Hoogmoed, M. S. , Leite, R. N. , Lo‐Man‐Hung, N. F. , Malcolm, J. R. , Martins, M. B. , Mestre, L. A. M. , Miranda‐Santos, R. , Overal, W. L. , Parry, L. , … Peres, C. A. (2008). The cost‐effectiveness of biodiversity surveys in tropical forests. Ecology Letters, 11(2), 139–150. 10.1111/j.1461-0248.2007.01133.x [DOI] [PubMed] [Google Scholar]
- Génier, F. (1996). A revision of the neotropical genus Ontherus Erichson (Coleoptera: Scarabaeidae, Scarabaeinae). The Memoirs of the Entomological Society of Canada, 170(S170), 1–169. 10.4039/entm128170fv [DOI] [Google Scholar]
- Génier, F. (2009). Le genre Eurysternus Dalman, 1824 (Scarabaeidae: Scarabaeinae, Oniticellini), révision taxonomique et clés détermination illustrées (1st ed.). Pensoft Publishers. [Google Scholar]
- Génier, F. , & Arnaud, P. (2016). Dendropaemon Perty, 1830: Taxonomy, systematics and phylogeny of the morphologically most derived phanaeine genus (Coleoptera: Scarabaeidae, Scarabaeinae, Phanaeini). Zootaxa, 4099(1), 1–125. 10.11646/zootaxa.4099.1.1 [DOI] [PubMed] [Google Scholar]
- Gill, B. D. (1991). Dung beetles in tropical American forests. In Hanski I. & Cambefort Y. (Eds.), Dung beetle ecology (pp. 211–229). Princeton University Press. [Google Scholar]
- González, E. , Salvo, A. , & Valladares, G. (2020). Insects moving through forest‐crop edges: A comparison among sampling methods. Journal of Insect Conservation, 24(2), 249–258. 10.1007/s10841-019-00201-6 [DOI] [Google Scholar]
- Greenslade, P. , & Greenslade, P. J. M. (1971). The use of baits and preservatives in pitfall traps. Journal of the Australian Entomological Society, 10(4), 253–260. 10.1111/j.1440-6055.1971.tb00037.x [DOI] [Google Scholar]
- Halffter, G. , & Edmonds, W. D. (1982). The nesting behavior of dung beetles (Scarabaeinae): An ecological and evolutive approach (1st ed.). Instituto de Ecología. [Google Scholar]
- Halffter, G. , & Favila, M. E. (1993). The Scarabaeinae (Insecta: Coleoptera) an animal group for analysing inventorying and monitoring biodiversity in tropical rainforest and modified landscapes. Biology International, 27, 15–21. [Google Scholar]
- Halffter, G. , & Matthews, E. G. (1966). The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera: Scarabaeidae) (1st ed.). Folia Entomologica Mexicana. [Google Scholar]
- Hancock, M. H. , & Legg, C. J. (2012). Pitfall trapping bias and arthropod body mass. Insect Conservation and Diversity, 5(4), 312–318. 10.1111/j.1752-4598.2011.00162.x [DOI] [Google Scholar]
- Henderson, P. A. , & Southwood, T. R. E. (2016). Ecological methods (4th ed.). John Wiley & Sons Ltd. [Google Scholar]
- Holt, R. D. (1997). From metapopulation dynamics to community structure: Some consequences of spatial heterogeneity. In Hanski I. & Gilpin M. E. (Eds.), Metapopulation biology ecology, genetics, and evolution (pp. 149–164). Academic Press. 10.1016/B978-012323445-2/50010-9 [DOI] [Google Scholar]
- Hsieh, T. C. , Ma, K. H. , & Chao, A. (2022). iNEXT: iNterpolation and EXTrapolation for species diversity . R package version 3.0.0. http://chao.stat.nthu.edu.tw/wordpress/software‐download/
- Juillet, J. A. (1963). A comparison of four types of traps used for capturing flying insects. Canadian Journal of Zoology, 41(2), 219–223. 10.1139/z63-023 [DOI] [Google Scholar]
- Katsanevakis, K. , Weber, A. , Pipitone, C. , Leopold, M. , Cronin, M. , Scheidat, M. , Doyle, T. K. , Buhl‐Mortensen, L. , Buhl‐Mortensen, P. , D'anna, G. , De Boois, I. , Dalpadado, P. , Fiorentino, F. , Garofalo, G. , Giacalone, V. M. , Hawley, K. L. , Issaris, Y. , Jansen, J. , Knight, C. M. , … Vöge, S. (2012). Monitoring marine populations and communities: Methods dealing with imperfect detectability. Aquatic Biology, 16(1), 31–52. 10.3354/ab00426 [DOI] [Google Scholar]
- Lähteenmäki, S. , Slade, E. M. , Hardwick, B. , Schiffler, G. , Louzada, J. , Barlow, J. , & Roslin, T. (2015). MESOCLOSURES – Increasing realism in mesocosm studies of ecosystem functioning. Methods in Ecology and Evolution, 6(8), 916–924. 10.1111/2041-210X.12367 [DOI] [Google Scholar]
- Lamarre, G. P. A. , Molto, Q. , Fine, P. V. A. , & Baraloto, C. (2012). A comparison of two common flight interception traps to survey tropical arthropods. ZooKeys, 216, 43–55. 10.3897/zookeys.216.3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, T. H. , & Forsyth, A. (2005). Trap spacing and transect design for dung beetle biodiversity studies. Biotropica, 37(2), 322–325. 10.1111/j.1744-7429.2005.00042.x [DOI] [Google Scholar]
- Larsen, T. H. , Lopera, A. , & Forsyth, A. (2006). Extreme trophic and habitat specialization by peruvian dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). The Coleopterists Bulletin, 60(4), 315–324. 10.1649/0010-065X(2006)60[315:ETAHSB]2.0.CO;2 [DOI] [Google Scholar]
- LeBlanc, K. , Boudreau, D. R. , & Moreau, G. (2021). Small bait traps may not accurately reflect the composition of necrophagous Diptera associated to remains. Insects, 12(261), 83–95. 10.3390/insects12030261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo, J. M. , Martin‐Piera, F. , & Veiga, C. M. (1988). Las trampas pitfall con cebo, sus posibilidades en el estudio de las comunidades coprófagas de Scarabaeoidea (Col.): I. Características determinantes de su capacidad de captura. Revue d'Écologie et de Biologie du Sol, 25, 77–100. [Google Scholar]
- Lucci Freitas, A. V. , Agra Iserhard, C. , Pereira Santos, J. , Oliveira Carreira, J. Y. , Bandini Ribeiro, D. , Alves Melo, D. H. , Batista Rosa, A. H. , Marini‐Filho, O. J. , Mattos Accacio, G. , & Uehara‐Prado, M. (2014). Studies with butterfly bait traps: An overview. Revista Colombiana de Entomologia, 40(2), 203–212. [Google Scholar]
- Magurran, A. E. (1991). Ecological diversity and its measurement (1st ed.). Princeton University Press. [Google Scholar]
- Marsh, C. J. , Louzada, J. , Beiroz, W. , & Ewers, R. M. (2013). Optimising bait for pitfall trapping of Amazonian dung beetles (Coleoptera: Scarabaeinae). PLoS One, 8(8), e73147. 10.1371/journal.pone.0073147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews, R. W. , & Matthews, J. R. (1972). The malaise trap: Its utility and potential for sampling insect populations. The Great Lakes Entomologists, 4(4), 1–6. [Google Scholar]
- Milhomem, M. S. , Vaz‐de‐Mello, F. Z. , & Diniz, I. R. (2003). Técnicas de coleta de besouros copronecrófagos no Cerrado. Pesquisa Agropecuária Brasileira, 38, 1249–1256. 10.1590/S0100-204X2003001100001 [DOI] [Google Scholar]
- Missa, O. , Basset, Y. , Alonso, A. , Miller, S. E. , Curletti, G. , Meyer, M. , Eardley, C. , Mansell, M. W. , & Wagner, T. (2009). Monitoring arthropods in a tropical landscape: Relative effects of sampling methods and habitat types on trap catches. Journal of Insect Conservation, 13(1), 103–118. 10.1007/s10841-007-9130-5 [DOI] [Google Scholar]
- Nependa, H. U. J. , Pryke, J. S. , & Roets, F. (2021). Replacing native mammal assemblages with livestock in African savannas, impacts dung beetle diversity and reduces body size. Biological Conservation, 260, 109211. 10.1016/j.biocon.2021.109211 [DOI] [Google Scholar]
- Newton, A. , & Peck, S. B. (1975). Baited pitfall traps for beetles. The Coleopterists Bulletin, 29(1), 45–46. [Google Scholar]
- Nichols, E. , Gardner, T. A. , Peres, C. A. , Spector, S. , & The Scarabaeinae Research Network . (2009). Co‐declining mammals and dung beetles: An impending ecological cascade. Oikos, 118(4), 481–487. 10.1111/j.1600-0706.2009.17268.x [DOI] [Google Scholar]
- Noriega, J. A. (2012). Dung beetles (Coleoptera: Scarabaeinae) attracted to Lagothrix lagotricha (Humboldt) and Alouatta seniculus (Linnaeus) (Primates: Atelidae) dung in a Colombian Amazon forest. Psyche: A Journal of Entomology, 2012, 1–7. 10.1155/2012/437589 [DOI] [Google Scholar]
- Oksanen, J. , Blanchet, F. G. , Friendly, M. , Kindt, R. , Legendre, P. , McGlin, D. , Minchin, P. R. , O'Hara, R. B. , Simpson, G. L. , Solymos, P. , Stevens, H. H. , Szoecs, E. , & Wagner, H. (2020). Vegan: Community ecology package . R package version 2.5‐7. https://CRAN.R‐project.org/package=vegan
- Ong, X. R. , Hemprich‐Bennet, D. , Gray, C. L. , Kemp, V. , Chung, A. Y. C. , & Slade, E. M. (2021). Trap type affects dung beetle taxonomic and functional diversity in Bornean tropical forests. Austral Ecology, 47(1), 68–78. 10.1111/aec.13124 [DOI] [Google Scholar]
- Ozanne, C. M. P. (2005). Sampling methods for forest understory vegetation. In Leather S. R. (Ed.), Insect sampling in forest ecosystems (pp. 58–76). Blackwell Publishing. [Google Scholar]
- Peck, A. B. , & Davies, A. E. (1980). Collecting small beetles with large‐area “window” traps. The Coleopterists Bulletin, 34(2), 237–239. [Google Scholar]
- Peroni, N. , & Hernández, M. I. M. (2011). Ecologia de Populações e Comunidades (1st ed.). Universidade Federal de Santa Catarina. [Google Scholar]
- Puker, A. , Silva, K. K. G. , Santos, D. C. , Correa, C. M. A. , & Vaz‐de‐Mello, F. Z. (2020). Dung beetles collected using flight intercept traps in an Amazon rainforest fragments and adjacent agroecosystems. International Journal of Tropical Insect Science, 40(4), 1085–1092. 10.1007/s42690-020-00132-9 [DOI] [Google Scholar]
- Pulido‐Herrera, L. A. , & Zunino, M. (2007). Catálogo preliminar de los Onthophagini de América (Coleoptera: Scarabaeine). In Zunino M. & Melic A. (Eds.), Escarabajos, diversidad y conservación biológica. Ensayos en homenaje a Gonzalo Halffter (pp. 93–129). Sociedad Entomológica Aragonesa. [Google Scholar]
- R Core Team . (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing; version 3.6.2. https://www.R‐project.org/ [Google Scholar]
- Raine, E. H. , Mikich, S. B. , Lewis, O. T. , Riordan, P. , Vaz‐de‐Mello, F. Z. , & Slade, E. M. (2018). Extinctions of interactions: Quantifying a dung beetle‐mammal network. Ecosphere, 9(11), e02491. 10.1002/ecs2.2491 [DOI] [Google Scholar]
- Raine, E. H. , & Slade, E. M. (2019). Dung beetles‐mammal associations: Methods, research trends and future directions. Proceedings of the Royal Society B, 286(1897), 20182002. 10.1098/rspb.2018.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini, M. (2016). Taxonomic revision of the American Onthohagus Latreille, 1802 of the “hirculus” group (Coleoptera: Scarabaeidae: Scarabaeinae) (doctoral thesis). Universitá Degli Studi Di Urbino Carlo Bo, Urino, Italy.
- Rossini, M. , & Vaz‐de‐Mello, F. Z. (2017). A taxonomic review of the genus Isocopris Pereira and Martínez, 1960 (Coleoptera: Scarabaeidae, Scarabaeinae), with description of a new Brazilian species. Journal of Natural History, 51(19–20), 1091–1117. 10.1080/00222933.2017.1319517 [DOI] [Google Scholar]
- Rossini, M. , Vaz‐de‐Mello, F. Z. , & Zunino, M. (2018). Toward a comprehensive taxonomic revision of the “hirculus” group of American Onthophagus Latreille, 1802 (Coleoptera, Scarabaeidae, Scarabaeinae). European Journal of Taxonomy, 432, 1–21. 10.5852/ejt.2018.432 [DOI] [Google Scholar]
- Rossini, M. , & Vaz‐de‐Mello, F. Z. (2020). Taxonomic review of the Dichotomius mamillatus group (Coleoptera: Scarabaeidae), with a description of a new species, Dichotomius (Dichotomius) gandinii sp.nov., from western Amazonia. Austral Entomology, 59(1), 52–73. 10.1111/aen.12443 [DOI] [Google Scholar]
- Rossini, M. , Vaz‐de‐Mello, F. Z. , & Zunino, M. (2018). A taxonomic revision of the New World Onthophagus Latreille, 1802 (Coleoptera: Scarabaeidae: Scarabaeinae) of the osculatii species‐complex, with description of two new species from South America. Journal of Natural History, 52(9–10), 541–586. 10.1080/00222933.2018.1437230 [DOI] [Google Scholar]
- Silva, F. A. B. , Hernández, M. I. M. , Ide, S. , & De Moura, R. C. (2007). Comunidade de escarabeíneos (Coleoptera, Scarabaeidae) copro‐necrófagos da região de Brejo Novo, Caruaru, Pernambuco, Brasil. Revista Brasileira de Entomologia, 51(2), 228–233. 10.1590/S0085-56262007000200014 [DOI] [Google Scholar]
- Silva, F. A. B. , & Valois, M. (2019). A taxonomic revision of the genus Scybalocanthon Martínez, 1948 (Coleoptera: Scarabaeidae: Scarabaeinae: Deltochilini). Zootaxa, 4629(3), 301–341. 10.11646/zootaxa.4629.3.1 [DOI] [PubMed] [Google Scholar]
- Silva, P. G. , Vaz‐de‐Mello, F. Z. , & Di Mare, R. A. (2012). Attractiveness of different bait to the Scarabaeinae (Coleoptera: Scarabaeidae) in forest fragments in extreme southern Brazil. Zoological Studies, 51(4), 429–441. [Google Scholar]
- Southwood, T. R. E. (1978). Ecological methods with particular reference to the study of insect populations (2nd ed.). ELSB Publisher. [Google Scholar]
- Souza, M. M. , Perillo, L. N. , Barbosa, B. C. , & Prezoto, F. (2015). Use of flight interception traps of malaise type and attractive traps for social wasps record (Vespidae: Polistinae). Sociobiology, 62(3), 450–456. 10.13102/sociobiology.v62i3.708 [DOI] [Google Scholar]
- Spence, J. R. , & Niemelä, J. K. (1994). Sampling carabid assemblages with pitfall traps: The madness and the method. The Canadian Entomologist., 126(3), 881–894. 10.4039/Ent126881-3 [DOI] [Google Scholar]
- Tonelli, M. , Verdú, J. R. , & Zunino, M. (2019). Grazing abandonment and dung beetle assemblage composition: Reproductive behavior has something to say. Ecological Indicators, 96, 361–367. 10.1016/j.ecolind.2018.09.010 [DOI] [Google Scholar]
- Topping, J. , & Sunderland, K. D. (1992). Limitations to the use of pitfall traps in ecological studies exemplified by a study of spiders in a field of winter wheat. Journal of Applied Ecology, 29, 485–491. [Google Scholar]
- Touroult, J. , Dalens, P. H. , Giuglaris, J. L. , Lapèze, J. , & Boilly, O. (2017). Structure des communautés de Phanaeini (Coleoptera: Scarabaeidae) de Guyane: étude par échantillonnage massif au piège d'interception. Annales de la Société Entomologique de France (NS), 53(3), 143–161. 10.1080/00379271.2017.1319294 [DOI] [Google Scholar]
- Tsuji, Y. , Shiraishi, K. S. T. , Matsubara, M. , & Kosugi, J. (2021). Differential attraction of large and small tunneling dung beetles (Coleoptera: Geotrupidae and Scarabaeidae) to native mammal dung in Satoyama Forest in Central Japan. The Coleopterists Bulletin, 75(2), 376–381. 10.1649/0010-065X-75.2.376 [DOI] [Google Scholar]
- Vaz‐de‐Mello, F. Z. , Edmonds, W. D. , Ocampo, F. C. , & Schoolmeesters, P. (2011). A multilingual key to the genera and subgenera of the subfamily Scarabaeinae of the New World (Coleoptera: Scarabaeidae). Zootaxa, 2854(1), 1–73. 10.11646/zootaxa.2854.1.1 [DOI] [Google Scholar]
- Vaz‐de‐Mello, F. Z. , Louzada, J. N. C. , & Schoereder, J. H. (1998). New data ad comments on Scarabaeidae (Coleoptera: Scarabaeoidea) associated with Attini (Hymenoptera: Formicidae). The Coleopterists Bulletin, 52(3), 209–216. https://www.jstor.org/stable/4009354 [Google Scholar]
- Verdú, J. R. , Numa, C. , & Hernández‐Cuba, O. (2011). The influence of landscape structure on ants and dung beetles diversity in a Mediterranean savanna‐forest ecosystem. Ecological Indicators, 11(3), 831–839. 10.1016/j.ecolind.2010.10.011 [DOI] [Google Scholar]
- Ward, D. F. , New, T. R. , & Yen, A. L. (2001). Effects of pitfall trap spacing on the abundance, richness and composition of invertebrates catches. Journal of Insect Conservation, 5(1), 41–53. 10.1023/A:1011317423622 [DOI] [Google Scholar]
- Weinzierl, R. , Henn, T. , Koehler, P. G. , & Tucker, C. L. (2005). Insect attractants and traps. Alternatives in insect management, ENY‐277 . Available from: http://ufdcimages.Uflib.Ufl.Edu/IR/00/00/27/94/00001/IN08000
- Whipple, S. D. , & Hoback, W. W. (2012). A comparison of dung beetle (Coleoptera: Scarabaeidae) attraction to native and exotic mammal dung. Environmental Entomology, 41(2), 238–244. 10.1603/EN11285 [DOI] [PubMed] [Google Scholar]
- Wickham, H. , Averick, M. , Bryan, J. , Chang, W. , McGowan, L. D. , François, R. , Groelemund, A. H. , Henry, L. , Kuhn, M. , Pedersen, T. L. , Miller, E. , Bache, S. M. , Müller, K. , Ooms, D. R. , Seidel, D. P. , Spinu, V. , Takahashi, K. , Vaughan, D. , Wilke, C. , … Yutani, H. (2019). Welcome to the tidyverse. Journal of Open Source Software, 4(43), 1–6. 10.21105/joss.01686 [DOI] [Google Scholar]
- Wilke, C. O. , & Wiernik, B. M. (2022). Package ‘ggtext: Improved text rendering support for 'ggplot2 version 0.1.2 . Available from: https://wilkelab.org/ggtext/
- Woodcock, B. A. (2005). Pitfall trapping in ecological studies. In Leather S. R. (Ed.), Insect sampling in forest ecosystems (pp. 37–57). Blackwell Publishing. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Data used in this study are available on Dryad. DOI: https://doi.org/10.5061/dryad.3tx95x6m5.


