Abstract
Quantifying habitat quality is dependent on measuring a site's relative contribution to population growth rate. This is challenging for studies of waterbirds, whose high mobility can decouple demographic rates from local habitat conditions and make sustained monitoring of individuals near‐impossible. To overcome these challenges, biologists have used many direct and indirect proxies of waterbird habitat quality. However, consensus on what methods are most appropriate for a given scenario is lacking. We undertook a structured literature review of the methods used to quantify waterbird habitat quality, and provide a synthesis of the context‐dependent strengths and limitations of those methods. Our search of the Web of Science and Scopus databases returned a sample of 666 studies, upon which our review was based. The reviewed studies assessed habitat quality by either measuring habitat attributes (e.g., food abundance, water quality, vegetation structure), or measuring attributes of the waterbirds themselves (e.g., demographic parameters, body condition, behavior, distribution). Measuring habitat attributes, although they are only indirectly related to demographic rates, has the advantage of being unaffected by waterbird behavioral stochasticity. Conversely, waterbird‐derived measures (e.g., body condition, peck rates) may be more directly related to demographic rates than habitat variables, but may be subject to greater stochastic variation (e.g., behavioral change due to presence of conspecifics). Therefore, caution is needed to ensure that the measured variable does influence waterbird demographic rates. This assumption was usually based on ecological theory rather than empirical evidence. Our review highlighted that there is no single best, universally applicable method to quantify waterbird habitat quality. Individual project specifics (e.g., time frame, spatial scale, funding) will influence the choice of variables measured. Where possible, practitioners should measure variables most directly related to demographic rates. Generally, measuring multiple variables yields a better chance of accurately capturing the relationship between habitat characteristics and demographic rates.
Keywords: environmental proxies, habitat condition, habitat selection, population growth rate, wetland monitoring
Our review of studies that measured waterbird habitat quality found that practitioners typically use proxies for habitat quality that focus on some aspect of the habitat or some aspect of the waterbirds themselves. The former may be relatively insensitive to waterbird behavioral stochasticity, whereas the latter may be more directly linked to waterbird demographic rates. We recommend that practitioners base their choice of variable to measure on the ecology of their study species, and preferentially measure variables most directly linked to waterbird demographic rates.
1. INTRODUCTION
A core aim of conservation management is optimizing habitat quality for focal species (Johnson, 2005; McComb, 2016). For management to be truly optimized, a measurable understanding of what constitutes habitat quality is required (Marzluff et al., 2000). The term habitat has diverse meanings in the literature. For the purposes of this review, we follow the definition provided by Kearney (2006) whereby habitat is a spatially and temporally explicit description of the physical features, including abiotic and biotic elements, of a place where an organism either actually or potentially lives. The ultimate measure of the quality of a habitat for an individual is the individual's relative contribution to the growth rate of the population when inhabiting a given habitat (Johnson, 2007). There are two components of this measure: survival and reproduction. By defining habitat quality in terms of population growth rate, habitat quality can be assessed on a continuous temporal scale. For example, habitat quality can be measured instantaneously or as a life‐time measure of habitat quality akin to the individual's fitness. There are many components that combine to influence survival and reproductive output including food availability, predation risk, habitat structure and configuration, and the presence of disturbances (e.g., human foot traffic; Johnson, 2007). Moreover, different species will experience habitat in different ways because of the species‐specific mechanistic ways in which they interact with features of the habitat (i.e., their respective niches as per the definition of Kearney (2006)) and habitat quality is therefore species‐specific.
Quantifying demographic rates (survival and reproductive output) is a challenging task (Stephens et al., 2015), as it requires sustained monitoring of individuals of known identity. Studies that do achieve this are often conducted either on sessile organisms (e.g., Ma et al., 2014; Wang et al., 2012; Zhao et al., 2006) or large‐bodied organisms that are restricted to a small geographic area (e.g., islands: Kruuk et al., 1999, Richard et al., 2014; natal colony: Baker & Thompson, 2007; Le Boeuf et al., 2019). Demographic rates are also financially costly to measure (Knutson et al., 2006; Pidgeon et al., 2006), and the long timeframes for data collection can mean that research extends beyond typical funding cycles and research project lifetimes, particularly for research on long‐lived species (Le Boeuf et al., 2019). Despite these challenges, there have been studies that successfully monitor survival (Valdez‐Juarez et al., 2019) and reproductive performance (Pérot & Villard, 2009; Pidgeon et al., 2006; Zanette, 2001) of birds in relation to habitat quality. Outputs from these studies are often very applied with actionable recommendations for conservation decision‐makers.
Waterbirds are a particularly challenging group to obtain habitat quality estimates for because multiple factors can confound the relationship between site habitat conditions and resultant demographic rates. The distribution of many waterbird species is influenced by social attraction (Gawlik & Crozier, 2007). As a result, areas of favorable habitat may go unused because waterbirds newly arriving in an area are drawn to sites with existing waterbird presence (Gawlik & Crozier, 2007). Moreover, many waterbirds are highly dispersive and track ephemeral habitat conditions at local, regional, or even continental scales (Cumming et al., 2012; Pedler et al., 2014; Roshier et al., 2006), creating the potential for mismatches between the scale of monitoring and the scale at which demographic processes are governed.
Dispersive behavior can also mean that the conditions at a particular wetland may be conducive for survival and reproduction, yet waterbirds do not capitalize on these favorable conditions because other wetlands in the broader landscape are also providing favorable conditions (behavioral choice impacts) (Cumming et al., 2012). This poses a challenge for management because under the theoretical definition of habitat quality, unused sites are not conferring anything to an individual's relative contribution to population growth rate and so the wetland would be deemed low‐quality habitat. Yet, from a management perspective, the site is being managed to provide conditions consistent with high‐quality habitat. Similarly, this scenario also highlights the role of density in habitat quality when habitat quality is assessed at the level of the population rather than the individual. Although a site may support only modest population growth from an individual's perspective, if the site has a high population density, then the population growth rate may be relatively high. Conversely, if a site has only a small number of resources but these are high‐quality resources (e.g., optimum nest sites), the per capita contribution to population growth rate at the site may be high, but the site has low habitat quality from a population perspective because the density of individuals supported by the site is low.
Many waterbirds are also migratory. Consequently, demographic parameters in one part of the range may be decoupled from the habitat conditions experienced at that time due to carry‐over effects from previous seasons (Aharon‐Rotman, Bauer, & Klaassen, 2016; Sedinger & Alisauskas, 2014; Swift et al., 2020). For example, survival during the breeding period and breeding success may be higher in individuals that depart their nonbreeding grounds in better condition (Swift et al., 2020). Furthermore, breeding performance in one part of the range may influence parameters including abundance and population age structure on the nonbreeding grounds, irrespective of the local conditions on the nonbreeding grounds (Rogers & Gosbell, 2006). In addition to carryover effects, survival data may be particularly sensitive to pinch points of low‐quality habitat along the migratory flyway (Piersma et al., 2016; Studds et al., 2017).
Due to the difficulties of obtaining waterbird demographic data in a given area, an array of methods have been used as proxies to measure habitat quality (Ma et al., 2010). The use of proxies also helps to overcome budget limitations of management agencies by allowing snapshot estimates of habitat quality to be made without the need for extended periods of data collection in space and time (Osborn et al., 2017). However, the many different options available for measuring habitat quality can be bewildering for research scientists and conservation practitioners (Pidgeon et al., 2006). There is little consensus on which method, or combination of methods, produces the most meaningful estimate of waterbird habitat quality, and in some cases, it is unclear as to whether particular proxies meaningfully reflect underlying habitat quality from the perspective of direct impact on population processes (Johnson, 2005, 2007; Stephens et al., 2015; Van Horne, 1983). For example, density of individuals may not reflect underlying habitat quality if the population does not follow the ideal free distribution (Van Horne, 1983), and time spent foraging may not reflect underlying habitat quality if individuals are constrained by prey handling time or digestive bottlenecks (Van Gils et al., 2005). Furthermore, the spatial scale at which proxies are measured may have implications for their relevance to managers (Guisan & Thuiller, 2005; Pidgeon et al., 2006; Stephens et al., 2015). The dynamic nature of wildlife populations also means that meta‐population processes (e.g., local extinction and recolonization, source‐sink dynamics), including those of competitors and predators, may confound proxy measures for habitat quality because these proxies typically assume the study population is in equilibrium (Guisan & Thuiller, 2005; Pulliam, 2000).
In this review, we seek to catalogue the methods that have been used to quantify waterbird habitat quality and provide a synthesis of the conditions under which each may provide meaningful measures of habitat quality in future waterbird studies. Outputs from this review are intended to guide environmental managers on the types of data they should be collecting when attempting to quantify waterbird habitat quality. This will ensure that decisions on how to manage habitat to optimize habitat quality are based on meaningful information. Outputs are also expected to be useful for conservation practitioners in terrestrial settings because the factors governing the relationship between a proxy and underlying habitat quality are founded on general ecological principles. Therefore, support for a method in a waterbird context is likely to mean that the same data type would provide a useful habitat quality proxy in a terrestrial setting. Furthermore, the open habitat structure occupied by many waterbird species (e.g., bare mudflats) facilitates prolonged periods of observation of individuals relative to those from terrestrial settings such as forests and scrublands. Likewise, visual methods for assessing body condition have been developed for waterbirds (Féret et al., 2005; Wiersma & Piersma, 1995), whereas capture is required to collect the same data for terrestrial birds (e.g., Milenkaya et al., 2015). Therefore, waterbirds provide a novel test‐case to quantify the relationship between some purported habitat quality proxies and the expected outcome (e.g., influence of body condition on survival and reproduction) where evidence from terrestrial settings remains unclear (Barnett et al., 2015; Milenkaya et al., 2015).
2. METHODS
For the purposes of this review, we followed the definition of waterbirds used by Wetlands International (2012). This covers all species within 32 bird families that are ecologically dependent on wetlands. The most familiar of these families are the Anatidae (ducks, geese, and swans), Laridae (gulls and terns), Ardeidae (herons and egrets), Scolopacidae (sandpipers), and Charadriidae (plovers). Other representatives include the Rallidae (rails and crakes), Podicipedidae (grebes), Threskiornithidae (ibises and spoonbills), and Recurvirostridae (stilts and avocets).
Systematic reviews require defining the question elements ‘subject’, ‘intervention’, ‘outcome’, and ‘comparator’ (Pullin & Stewart, 2006). Very few studies of habitat quality involve manipulative experiments (Stephens et al., 2015), so for the majority of studies there is a lack of a clearly defined intervention. Furthermore, for data to be pooled for meta‐analysis, it is important to consider the heterogeneity of the input data, with high levels of heterogeneity making data pooling inappropriate (Crowther et al., 2010). For example, it is important that only data with similar interventions and measures of outcomes are combined (Crowther et al., 2010). Among the diverse waterbird habitat quality literature, there are clear differences in terms of the experimental design, comparator group used (e.g., some use neighboring populations as comparators whereas others use previous points in time, or a comparator is lacking completely), and the measured outcome (e.g., some relate changes in a measured variable to change in abundance whereas for others the response is changes in reproductive rate). Consequently, pooling data to conduct a formal meta‐analysis would have been inappropriate in this review. Hence, we used the ‘narrative synthesis’ approach recommended by Haddaway et al. (2020) for synthesizing heterogeneous literature. To obtain a representative sample of the literature for synthesis, we used a structured approach to identify relevant information sources (published literature, reports, and gray literature) and use these sources to make qualitative assessments of the various methods that have been used for measuring waterbird habitat quality.
We searched the Web of Science (all databases) and Scopus on September 1, 2022 to obtain a set of papers on which to base this review. When searching the Web of Science we used the search string TS = (waterbird* OR shorebird* OR wader* OR “wading bird*” OR waterfowl) AND TS = (“habitat quality” OR “habitat condition” OR “environment* quality” OR “environment* condition” OR “wetland quality” OR “wetland condition”), where TS means ‘Topic Search’.
When searching the Scopus database we specified two search fields. The first contained the search string waterbird* OR shorebird* OR wader* OR “wading bird*” OR waterfowl and the second contained the search string “habitat quality” OR “habitat condition” OR “environment* quality” OR “environment* condition” OR “wetland quality” OR “wetland condition”. In both cases, the search was restricted to include only information appearing in the article title, abstract, keywords.
These searches returned a sample of 1085 results once duplicates and records pertaining to datasets (e.g., publication supporting information lodged with Dryad or Figshare) had been removed. Further screening removed an additional 158 papers that did not relate to waterbirds, 155 papers in which no habitat quality assessment was made, 25 studies that related to toxicology, and 52 review articles. It was not possible to obtain a copy of the full text in 29 cases and these records were also excluded from the synthesis. This resulted in a final sample of 666 papers upon which our synthesis was based (see Appendix S1 for a spreadsheet of search results and inclusion status). For cases where the returned paper was in a language other than English or French, Google Translate was used to extract the relevant information. Our aim in conducting this review was to catalogue the spectrum of methods that have been used for waterbird habitat quality assessments. Therefore, we did not appraise the quality of individual studies when determining whether they should be included in the review or not. Rather we included any study that purported to measure habitat quality and used evidence from within that study or others in the returned set to contextualize whether the method was likely to provide an accurate estimate of habitat quality, as well as outlining any caveats as to the settings under which the method is likely to provide useful data. It was intended that this would help readers make their own assessment as to whether a particular habitat quality metric is useful for their own needs. We also avoid ‘vote counting’ (i.e., tallying how many studies used a particular method) as a way of assessing how well a given habitat quality assessment method performs because there are many factors that can contribute to a particular method being used (e.g., ease of data collection), or the likelihood of the research being published (e.g., sample size needed for statistical power) that are unrelated to the performance of that method.
3. SYNTHESIS OF REVIEWED STUDIES
Our structured search returned studies that undertook waterbird habitat quality assessments in two main ways: studies that measured some biophysical attribute(s) of the habitat; and studies that measured some attribute(s) of waterbirds themselves to infer underlying habitat quality (Table 1). Studies that measured attributes of waterbirds themselves could be further broken down into four subcategories: studies that directly measured waterbird demographic characteristics; studies that measured waterbird body condition; studies that measured waterbird behavior; and studies that measured waterbird distribution (Table 1). There were also studies that used methods from a combination of these categories.
TABLE 1.
Catalogue of methods used to assess waterbird habitat quality in studies reviewed as part of the structured literature review. For each method, examples of studies that used the method are given along with an indication of the support or lack thereof for the given method.
| Method | Metrics | Supporting evidence | Contradictory evidence | Relevant spatial and temporal scales |
|---|---|---|---|---|
| Direct habitat measures | ||||
| Food availability |
|
|
Site/region—instantaneous/within season/annual | |
| Primary productivity |
|
|
Site/region/flyway—instantaneous/within season/annual | |
| Predation pressure |
|
|
|
Site/region—instantaneous/within season/annual |
| Vegetation structure |
|
|
|
Site/region—instantaneous/within season/annual |
| Wetland spatial attributes |
|
|
Site/region—instantaneous/within season/annual | |
| Water level |
|
Site/region—instantaneous/within season/annual | ||
| Disturbance |
|
|
Site/region—instantaneous/within season/annual | |
| Foraging substrate |
|
Site/region—instantaneous/within season/annual | ||
| Land use |
|
|
Site/region—instantaneous/within season/annual | |
| Water chemistry |
|
|
Site/region—instantaneous/within season/annual | |
| Climate and weather |
|
|
|
|
| Terrain features |
|
|
||
| Bird‐derived estimates | ||||
| Demographic measures | ||||
| Reproduction |
|
|
Site/region—instantaneous/within season/annual | |
| Survival |
|
|
Site/region—instantaneous/within season/annual | |
| Density or abundance |
|
|
|
Site/region—instantaneous/within season/annual |
| Age at first breeding |
|
|
||
| Distributional measures | ||||
| Phenology |
|
|
Site/region—within season/annual | |
| Age class distribution |
|
|
|
Site/region—instantaneous/within season |
| Hunting records |
|
|
— | Region—annual |
| Individual condition measures | ||||
| Morphological variables |
|
|
Site/region—within season/annual | |
| Physiological variables |
|
|
Site/region –Within season/annual | |
| Parasite burden |
|
|
Site/region –Within season/annual | |
| Ptilochronology |
|
|
— | Site/region –Within season/annual |
| Behavioral measures | ||||
| Foraging parameters |
|
|
Site/region – Instantaneous/within season/annual | |
| Time budgets |
|
Site/region – Instantaneous/within season/annual | ||
| Anti‐predator behaviors |
|
|
Site/region – Instantaneous/within season/annual | |
| Individual movements |
|
|
Site/region – Instantaneous/within season/annual | |
| Flight speeds |
|
|
Site/region – Instantaneous/within season/annual | |
| Parental investment |
|
|||
Note: A ‘—’ symbol in the supporting evidence and contradictory evidence columns indicates that no data for these cells were found in the reviewed papers. The spatial (site, region, and flyway) and temporal (instantaneous, within‐season, annual) scales that data collection pertains to are also given.
In many cases, the proxies used assumed a simplistic relationship between the measured attribute and habitat quality that ignored other potential influences on true habitat quality. For example, studies that measured prey intake rate assumed that higher prey intake rates equated to better quality habitat without explicitly considering factors such as predation risk or the physiological costs of inhabiting sites with profitable foraging conditions. This is an important consideration that practitioners must evaluate when determining which variables they will measure and the reliability of their findings.
The reviewed studies often presented evidence for a hierarchy in terms of how relevant each proxy was for assessing habitat quality. Some proxies were used more often as response variables whereas others were used consistently as predictor variables. Proxies often used as response variables (e.g., measures of reproductive output, survival, abundance, body condition) were typically more directly linked to demographic parameters, whereas proxies relating to physico‐chemical properties of wetlands (e.g., vegetation structure, primary productivity, nutrient concentrations) seldom featured as response variables. We interpret this as an indication that managers seeking to assess habitat quality are best equipped if they are able to measure proxies most directly related to demographic parameters (Figure 1).
FIGURE 1.
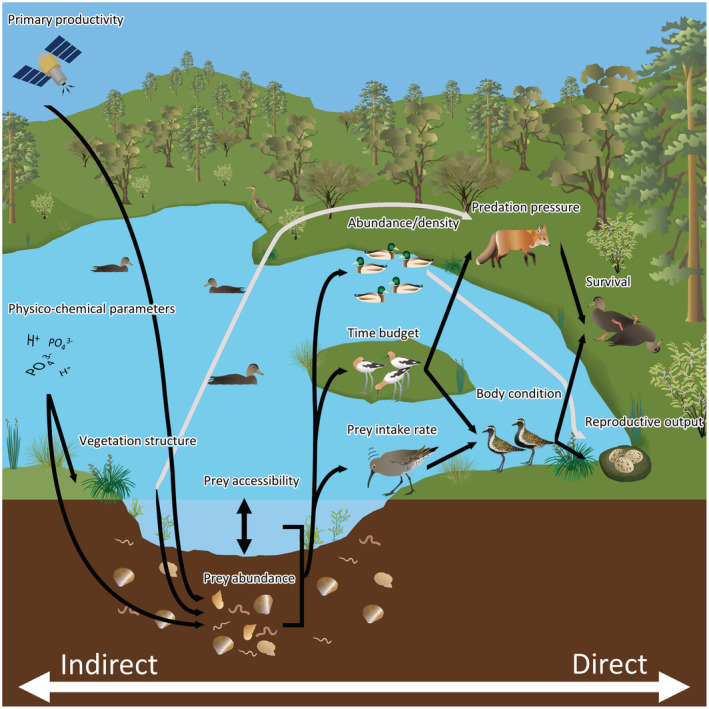
An example of the hierarchy among a selection of proxy methods for assessing waterbird habitat quality. The horizontal axis shows differences in terms of how directly or indirectly a selected habitat quality proxy relates to the ultimate determinants of habitat quality: reproductive output and survival probability. Arrows point in the direction of the presumed relationship from causal factor to outcome. Arrows originating from a line linking two proxy measures together indicate that the outcome results from the interaction between the two linked factors. Gray arrows depict relationships where the link likely manifests itself via another pathway in the ecological web. For example, greater waterbird abundance/density does not necessarily increase the reproductive output of an individual. Rather, more individuals are probably drawn to sites with high prey abundance and accessibility, where prey intake rates and thus waterbird body condition and then reproductive output can be maximized. Artworks for some proxy measures were obtained from the Integration and Application Network Media Library (ian.umces.edu/media‐library), with contributions from artists Tracey Saxby, Kim Kraeer, Lucy Van Essen‐Fishman, Dieter Tracey (Marine Botany UQ), Joanna Woerner, Emily Nastase, Jason C. Fisher (University of California Los Angeles), Jane Hawkey, and Jane Thomas.
3.1. Methods of habitat quality assessment
3.1.1. Measuring demographic parameters
By definition, the quantification of habitat quality depends on estimating a site's contribution to survival and reproduction. Therefore, any method that directly measures survival or reproductive output will produce a quantitative estimate of the respective parameter that is free from error propagation caused by imperfect correlation between a measured attribute and these variables. Consequently, studies that directly measure both of these parameters are likely to provide the most accurate quantification of habitat quality with the fewest assumptions about the direction and strength of correlations between the measured attribute and population growth rate. However, cautious interpretation is still required when only one of these attributes is measured, because sites with similar reproductive output can have divergent population trajectories if the population size is governed by adult survival, and vice versa (Cohen et al., 2009). Similarly, emigration or immigration at a site may also obscure the signal arising from measures of reproduction and survival (Cohen et al., 2009). Caution must also be used if the measure of reproductive output reflects an early stage in the reproductive cycle (e.g., number of breeding pairs, clutch size) because the number of individuals that recruit into the breeding population from these nesting attempts may not be perfectly correlated with this early season data. For example, density‐dependent effects may mean that the number of pairs increases but the number of chicks fledged per pair declines (Kwon et al., 2021). Measuring demographic rates can be a lengthy, costly, and logistically challenging process. In waterbird research, directly measuring a site's contribution to survival and reproduction may be unachievable owing to the mobility of waterbird populations. Although our structured search returned examples of studies that did quantify survival (e.g., Alves et al., 2013; Rice et al., 2007; Swift et al., 2020) and/or reproduction (e.g., Hunt et al., 2017; Powell & Powell, 1986; Swift et al., 2020), most studies used proxies for one or both of these measures.
3.1.2. Estimating food abundance and availability
Many of the proxies in the reviewed studies assumed that a high‐quality habitat provided waterbird individuals with a high net energy intake rate. The corollary assumption was that high net energy intake results in increased survival and reproductive performance. Methods used to infer net energy intake rate included measures of prey abundance, prey accessibility, and waterbird physiology or morphology as an indicator of past foraging returns (Table 1). Habitat quality assessments that are based on habitat attributes are appealing because results are independent of variation in bird behavior caused by factors unrelated to local habitat quality (e.g., current wind and rain conditions can determine which sites waterbirds use at very local scales (Kelly, 2001) and these short term changes are not typically useful for managers). For this reason, measuring the abundance or biomass of food was used widely in the reviewed studies to assess waterbird habitat quality. There is support for this method being an appropriate proxy for habitat quality because waterbirds preferentially forage at sites with the highest prey biomass and density (Guerra et al., 2016; Rose & Nol, 2010). Moreover, prey availability has a positive influence on reproductive performance and survival (Herring et al., 2010; Holopainen et al., 2014; Swift et al., 2020). However, there are also situations where prey biomass at a site can be a poor indicator of habitat quality. For example, sites with high prey biomass are not always favored foraging sites (Hagy & Kaminski, 2015), and although these sites might have high occupancy, they do not necessarily support high waterbird abundance (Gillespie & Fontaine, 2017). This suggests that factors such as predation risk, forager condition, and prey accessibility modulate the effect of prey biomass on habitat quality (Hagy & Kaminski, 2015). Such a relationship is also dependent on waterbirds having a perfect knowledge of the distribution of prey resources (Reurink et al., 2015), which may not always be the case (Lewis et al., 2010), and relies on researchers correctly identifying dietary preferences and requirements of focal species.
The presence of suitable water levels and variation in water levels was also used as a proxy for habitat quality in the reviewed studies. These habitat attributes can influence accessibility of prey and foraging energetics (Herring & Gawlik, 2013; Kang & King, 2014). In some cases, only a small proportion of a wetland provides suitable water levels for waterbirds to access prey (Collazo et al., 2002). This suggests that there is value in quantifying either prey biomass or the amount of suitable habitat through water level measurements. However, the two attributes will interact to influence the net rate of energy intake possible at a site meaning studies that measure both variables may have a greater likelihood of teasing apart meaningful habitat quality relationships and informing appropriate management (Herring & Gawlik, 2013). Similarly, remotely sensed measures of primary productivity (e.g., Normalized Difference Vegetation Index) are expected to be correlated with prey abundance. Yet, the relationship between net energy intake rate and primary productivity is dependent on changes in primary productivity causing changes in prey abundance (e.g., invertebrates, seeds, tubers) as well as those prey items being available to feeding waterbirds (Guan et al., 2016; Zhang et al., 2017). This suggests there is a hierarchy in the ability of proxies to provide precise habitat quality estimates based on how direct the link between the variable being measured and net energy intake rates is (Figure 1). In addition to this hierarchy, the precision of the proxy used may also be dependent on other factors, such as predation risk, that are not encompassed within the scope of the proxy measure but do influence true habitat quality.
3.1.3. Estimating food intake rate
The behavior and habitat use patterns of waterbirds themselves were often used in the reviewed studies to infer underlying patterns of habitat quality (Table 1). Indicators of prey intake rate (be it current, past or expected future foraging returns) were frequently used metrics of habitat quality. Variables including peck rate, capture success rate, and the proportion of time a bird spent foraging were commonly measured to assess the current rate of energy intake supported by a habitat. Defecation rate is significantly correlated with peck rate in a visually foraging shorebird, supporting the assumption that peck rate represents a valid indicator of intake rate (Rose & Nol, 2010). Likewise, sites with a higher peck rate or probe rate had a higher rate of successful prey captures in a study where capture success could be visually verified (Kuwae et al., 2010). However, different prey items have different energy content and different processing costs within the digestive system (Dugger et al., 2007; Jorde et al., 1995). This means that the net rate of energy intake will depend on the prey type consumed. This may not be an issue in studies of diet specialists, but it may confound the interpretation of peck rate and capture success data for diet generalists. In situations where the diet of the population being studied is not well understood, investigating the prey community composition to determine prey encounter rates, or dietary studies (e.g., metabarcoding of prey DNA sequences in fecal samples) will inform whether differences in peck rate between sites or across time genuinely reflect changes in energy returns.
Intake rates over the recent and more distant past were inferred from a variety of variables including body condition, blood metabolites, and indicators of feather growth rate. These have the advantage that they reflect assimilated energy rather than gross intake including energy lost via excretion or through processing costs. However, the longer timeframe of integration meant that studies using these methods were rarely site‐specific, rather they tended to assess habitat quality at regional scales (e.g., Aharon‐Rotman, Buchanan, et al., 2016). In cases where individuals use only a small geographic area (e.g., when nesting constrains movements, or individuals have strong residency patterns) these measures may provide insights into site‐specific habitat quality. For example, Swift et al. (2020) found that visually scored body condition of nonbreeding Hudsonian Godwits Limosa haemastica was correlated with pecking rate at individual nonbreeding sites. This suggests that these birds were resident at sites long enough to integrate site‐specific habitat quality information in the form of body condition. Importantly, birds with higher body condition had higher survival and reproductive output the following breeding season, indicating that body condition reliably influenced demographic rates (Swift et al., 2020). Conversely, body condition may not be a sensitive enough parameter in some cases to truly reflect underlying changes in habitat quality. For example, Gunness et al. (2001) detected no seasonal change in duckling survival for mothers with high body condition, but broods of mothers with low body condition did have reduced survival late in the season. This suggests that there is some buffering capacity for females with high body condition to moderate the effects of seasonal declines in habitat quality. Furthermore, individuals inhabiting sites with variable prey availability may store more energy reserves than individuals with consistent access to prey because they must maintain enough energy reserves to see them through periods of resource scarcity (Ruthrauff et al., 2013). For this reason, it may be challenging to infer patterns of habitat quality from body condition alone.
3.1.4. Predation pressure
Given the direct link between predation pressure and survival, it was surprising that predation pressure was estimated relatively infrequently in the reviewed studies. This is perhaps reflective of the difficulties of censusing predator populations due to predators of waterbirds typically occurring at low density and predation events on adult waterbirds being rare. Where predation pressure was quantified, these studies often focused on nest predation (e.g., Kenow et al., 2009; Pehlak & Lõhmus, 2008; Trinder et al., 2009). Most studies that inferred an influence of predation pressure on habitat quality assumed that the abundance of predators was correlated with predation rate without explicitly testing this assumption, which may be problematic when generalist predators are involved. Some studies also assessed predation pressure by using vigilance or escape behaviors of waterbirds (Fernández & Lank, 2010; Gunness et al., 2001). This has the advantage of integrating information on the degree of lost foraging time as a result of predation pressure because lost foraging opportunities will affect reproductive performance as well as survival (Castillo‐Guerrero et al., 2009).
3.1.5. Physical habitat attributes
Many of the reviewed studies measured various physical and/or chemical attributes of waterbird habitats to infer habitat quality. The attributes measured were purported to influence habitat quality via their contribution to supporting viable prey populations (e.g., water pH, water conductivity, sediment grain size), enabling access to sufficient quantities of food (e.g., water area, pond density in the local area and vegetation composition, as well as water level which we discussed previously), or providing shelter from predators (e.g., vegetation structure). In most cases, these environment attributes are linked indirectly to demographic rates (Figure 1) and the mechanisms governing their effects may be difficult to disentangle (Raquel et al., 2016). Nonetheless, physical attributes of the habitat may provide waterbirds with visual cues as to the quality of a site and play a role in determining patterns of site use, which can have flow‐on effects on demographic rates (Buderman et al., 2020).
3.1.6. Species richness
Among the reviewed studies, some used species richness as an indicator of habitat quality (e.g., Arzel et al., 2015; Dugger & Feddersen, 2009; Hickman, 1994). As established above (Section 1), habitat quality is a species‐specific parameter. Hence, species richness does not necessarily align with the definition of habitat quality unless it is viewed as an emergent property of a system that is able to provide high‐quality habitat for an array of species. There are some circumstances whereby the presence of heterospecific individuals may enhance habitat quality for certain species (Arzel et al., 2015). For example, the abundance of gulls (Larus spp.) was correlated with better waterbird brood success, probably because gulls provided defense against predators (Arzel et al., 2015; but see Broyer (2009) where no such effect of gulls was found). However, facilitative interactions will not occur in all species pairings and some interactions will have a negative impact on habitat quality for one species, such as aggression by Mute Swans (Cygnus olor) leading to a reduction in breeding pair density of Mallards (Anas platyrhynchos; Broyer, 2009).
3.1.7. Climate, weather, and terrain attributes
A feature of many of the reviewed studies was the inclusion of climate, weather, and terrain attributes. These variables generally cannot be influenced by wetland managers, so on face value they may seem less important for the study of habitat quality from a management perspective. Nonetheless, they can play a key role in wetland availability (e.g., rainfall) and can also directly influence energy budgets (e.g., temperature and wind effects on thermoregulation), which have important ramifications for whether a site or region represents suitable habitat (Krijgsveld et al., 2003; McKinney & McWilliams, 2005; Sorenson et al., 1998). Furthermore, these parameters can provide contextual information that helps explain some of the variation in relationships between other parameters of management interest. For example, survival and fecundity of Eurasian Oystercatchers (Haematopus ostralegus) with a bill morphology suited to generalist foraging is high in most years, but in years with a harsh winter their survival is reduced relative to individuals with a bill morphology suited to specialized foraging strategies (van de Pol, Brouwer, et al., 2010). The addition of climate information to van de Pol, Brouwer, et al. (2010) study provides insight into why measurements of short‐term prey intake rates do not align with long‐term fitness outcomes for individuals. As a consequence of the influence of climate, weather, and terrain variables on habitat quality, they may provide information relevant to management prioritization decisions. For example, species that are restricted to a narrow elevation range may be more sensitive to changes in habitat quality and may benefit most from management to improve habitat conditions (Xu et al., 2019). Given the ready availability of datasets relating to climate, weather, and terrain in most cases (e.g., global climate models, local weather stations, and high resolution digital elevation models) the ability to include these variables as covariates provide researchers with additional insights to disentangle the complex interactions influencing waterbird habitat quality.
3.1.8. Other methods
A variety of other methods were used infrequently in the reviewed studies (Table 1). These included estimates of levels of human disturbance, individual movement data (e.g., home range size), and the spatial distribution of individuals in different age classes. Despite their infrequent use, these methods may provide meaningful habitat quality information. Factors such as the cost of obtaining the data or the difficulty of obtaining the data (e.g., challenges distinguishing between age classes in the field) probably contributed to their infrequent use.
3.1.9. Combination of methods
Many of the reviewed studies recorded data on multiple proxies for habitat quality. Multiple lines of evidence allowed researchers to tease apart complex relationships among various parameters in their respective study systems and provide powerful insight to conservation managers (Cohen et al., 2009; Hunt et al., 2017; Swift et al., 2020). In these studies, it was often possible to pinpoint factors that were limiting habitat quality, providing managers with priorities to address in order to improve habitat quality. For example, Cohen et al. (2009) recommended that restoring Piping Plover, Charadrius melodus, habitat adjacent to bayside intertidal flats would improve habitat quality by increasing the number of breeding pairs that could occupy a site. However, this action must be carried out in conjunction with predator management in order to achieve the desired increase in reproductive output.
Applications
A feature of many of the reviewed studies was the use of chosen habitat quality proxy variable(s) as inputs into sophisticated ecological analyses, such as habitat suitability models (e.g., Guan et al., 2016; Hsu et al., 2014; Tang et al., 2016; Wen et al., 2016), carrying capacity estimates (e.g., Collazo et al., 2002; Mu et al., 2022; Stillman et al., 2000), population viability analyses (e.g., Mattsson et al., 2012; Saunders et al., 2021; Weegman et al., 2022), or to predict the outcome of management interventions on waterbird vital rates (e.g., Beerens et al., 2015; Toral et al., 2012). Where the predictor variables for these analytical approaches covered large spatial extents or could be sourced from extensive temporal archives, knowledge gained from site‐level studies could be extrapolated in space and/or time to provide a far‐reaching evaluation of habitat quality. Accordingly, many of the studies that produced a habitat suitability model used remotely sensed data sources. For example, Mokany et al. (2022) used satellite‐derived water cover data to predict habitat quality for waterbirds during two time periods across a study area spanning 1.06 million km2, and Wen et al. (2016) used waterbird presence and Normalized Difference Vegetation Index data to predict changes in waterbird distribution in response to drought‐breaking rains across a 810,000 km2 study area.
3.2. Factors influencing the choice of variables to measure
3.2.1. Staying within the project's scope
Our synthesis of the habitat quality literature indicates that there is a hierarchy of data quality from directly monitoring demographic rates to measuring parameters that are increasingly indirectly linked to demography. Yet, practitioners typically face a trade‐off between the need for accuracy of the habitat quality estimate and their particular study's aims and constraints. If it is feasible, measuring demographic rates directly generally involves extended field time, individually marked birds, limited spatial scale, and substantial costs (Buderman et al., 2020). Other factors may also influence the suitability of a proxy for the habitat quality assessment at hand including ethical considerations (Hunt et al., 2013), and the availability of appropriately trained personnel. Physiological and morphological measurements used in the reviewed studies typically required birds to be handled (but see the abdominal profile index method; Swift et al., 2020), which imposes stress on the study subjects (Karlíková et al., 2018), and capturing a large sample size of birds can be time consuming. This may mean that methods requiring birds to be handled, including individually marking birds for quantifying demographic rates, are not feasible within the scope of a project.
Spatial and temporal scales of assessments
Another consideration that must be made prior to implementing a study on habitat quality is whether the habitat quality measure being used returns data at a relevant spatial and/or temporal scale. For example, prey abundance measures typically provide very local scale (both spatial and temporal) information on habitat conditions, but may not be representative of habitat quality across the entire wetland or extended timeframes (e.g., the entire nonbreeding period). For example, Fonseca and Navedo (2020) reported a 43% reduction in invertebrate prey biomass as a result of shorebird foraging in study plots over the course of 3 days. Consequently, habitat quality assessments either side of this three‐day period could yield vastly different inferences about local habitat quality and neither may be representative of habitat quality over an extended timeframe. The accuracy of these methods in terms of returning habitat quality data at timescales meaningful for management will therefore be increased by repeated sampling (Murray et al., 2010). This was reflected in a number of the reviewed studies, especially those aimed at specifying management regimes, repeating sampling both spatially, and intra‐ and inter‐annually (e.g., Gillespie & Fontaine, 2017). Whereas methods that relied on measuring attributes of the habitat typically provided snapshot estimates of habitat quality, methods reliant on waterbird body condition or physiology (e.g., abdominal profile index or red blood cell heat shock protein concentrations) often provide information integrated over longer timeframes (Herring & Gawlik, 2013). They may therefore be unsuitable for site‐specific and/or instantaneous habitat quality questions, but may be applied to questions informing management of a regional wetland complex over broader timeframes. Similarly, remotely sensed measures of primary productivity offer the potential to rapidly and cost‐effectively monitor habitat conditions at large spatial and temporal scales. For example, Wen et al. (2016) used remotely sensed primary productivity data to inform an assessment of waterbird habitat quality across a 810,000 km2 study area in multiple years.
There is no rule that governs whether the spatial or temporal scale of a particular proxy is appropriate for a particular application because even labour intensive or costly methods that return site‐specific information may be suitable for large‐scale projects if the budget enables sufficiently widespread sampling (e.g., sites and time points). We provide some recommendations as to the spatial and temporal scales that methods for habitat quality assessments are typically carried out at (Table 1). Readers may also find papers such as Behney et al. (2014) guide to determining the optimum number of benthic core samples to collect useful for planning how much field effort is likely to be involved when planning a sampling regime.
3.3. What makes for a good habitat quality assessment?
Measuring habitat quality enables conservation managers to assess the need for or effectiveness of management actions (e.g., Schultz et al., 2020). The ultimate objective of conservation management is to influence demographic parameters of conservation targets to improve conservation status. Therefore, assessments of habitat quality inherently must determine a site's contribution to survival probability and/or reproductive output. This requires there to be a link between the variable, or combination of variables, used to measure habitat quality and demographic rates (Figure 1). Before commencing an assessment of habitat quality, the researcher must carefully consider whether the selected measure does actually influence demographic rates. For example, quantifying the time budgets of waterbirds is a commonly used method for inferring differences in habitat quality (Dugger & Feddersen, 2009; van der Kolk et al., 2019). However, the inferences derived from time budget comparisons may not actually reflect changes in underlying habitat quality. Time budgets can be flexible to buffer intrinsic changes in requirements (Mallory et al., 1999). For example, this may be due to individuals dedicating more time to foraging to meet the metabolic demands of producing a clutch of eggs (Mallory et al., 1999), or dedicating more time to feeding to fatten up for migration (Castillo‐Guerrero et al., 2009). That is not to say that time budgets are unsuitable for quantifying habitat quality, but care must be taken to ensure that appropriate comparison groups are being used (e.g., sampling at the same time of year; Pezzanite et al., 2005). Similarly, care must be taken to ensure that the appropriate response variable is measured. For example, management may result in better nesting habitat, but no advantage may be conferred on subsequent chick survival (Smart et al., 2013). In this case, management may result in more chicks recruiting to the breeding population because there are more nests, but if managers were measuring the probability of a given chick fledging there would be no signal to indicate the improvement in population growth.
Researchers must also be aware that inferences made about populations that are not at equilibrium may depart from theoretical relationships underpinning many habitat quality proxies. For example, populations that have been reduced below carrying capacity by historical or offsite factors may not show any temporal differences in various local habitat quality proxies (e.g., foraging success, stress markers, body condition, time budgets) because individuals are easily able to meet their resource requirements even if local habitat quality is declining. Similarly, there may be differences in the relevance of some habitat quality proxies depending on whether the conservation target is a resident population, or a dispersive or migratory population (Loewenthal et al., 2015). Abundance and density are clearly linked to local habitat quality for resident populations, but may not be truly reflective of local habitat quality for populations that undertake large‐scale movements exposing individuals to factors that limit population size elsewhere in the range. For example, Jia et al. (2018) reported declines in abundance of migratory shorebirds at a migratory staging site, but none of the measured proxy variables for habitat quality could explain these declines. They suggest that factors in other parts of the migratory range may be responsible for driving the observed declines in abundance rather than changes in habitat quality at their study site.
Many of the habitat quality proxies identified in this review assume individuals have perfect knowledge of the resource distribution at a site and behave such that the net rate of energy gain is being maximized at any given time (Reurink et al., 2015). Several factors can result in waterbirds using their habitat in ways that do not conform to these assumptions (Stillman et al., 2005). The choice of foraging site for many waterbirds is strongly influenced by conspecific attraction (Gawlik & Crozier, 2007; Herring et al., 2015; Smith, 1995). This is also true for the selection of nest sites (Sebastián‐González, Sánchez‐Zapata, Botella, & Otso, 2010). Furthermore, fidelity to areas that have provided favorable habitat conditions in the past may decouple patterns of waterbird habitat use from current habitat conditions (O'Neil et al., 2014). Waterbird habitat requirements may also change with breeding stage (Holopainen et al., 2014), and during less energetically demanding parts of the annual cycle, such as the nonbreeding period, individuals may be less selective in their habitat use decisions (Sebastián‐González, Sánchez‐Zapata, & Botella, 2010).
Most of the reviewed studies provided a relative assessment of habitat quality (i.e., they compared waterbird habitat quality at a site to previous points in time, or made comparisons between sites). These studies allow researchers to determine habitat quality trends or identify the best and worst sites in a landscape, but do not enable managers to determine whether the habitat quality is sufficient to maintain viable waterbird populations. There were some studies that sought to determine whether the habitat quality at a site was sufficient to support population growth or whether the site represented a sink habitat (e.g., Roy et al., 2019; Sabatier et al., 2010). These studies do enable managers to determine whether management intervention is necessary rather than arbitrarily setting a reference site as the standard against which to decide whether management is warranted. In particular, studies seeking to identify whether a site had sufficient habitat quality to support population growth tended to focus directly on reproductive output or survival data (Roy et al., 2019; Weiser et al., 2018a), or in some cases focused on energetic demands relative to prey resources (West et al., 2005).
Together, the potentially confounding factors mean that there is no universally applicable habitat quality proxy. Yet, with careful consideration and a detailed understanding of the ecology of the study system, waterbird researchers and management practitioners can derive meaningful measures of habitat quality.
3.4. Limitations of the review
We used only English language search terms. This could have resulted in some methods that are used in only a subset of countries that are non‐English‐speaking being overlooked by our research. Overlooking some methods should not bias the inferences we have drawn about methods that we did catalogue because evidence within the reviewed studies supporting or contradicting the use of a particular method remains valid. Furthermore, the mobility of many waterbirds and the frequency with which they cross international borders also promotes international collaboration along flyways as evidenced by groups such as the Atlantic Flyway Shorebird Initiative and the East Asian‐Australasian Flyway Partnership. These groups share knowledge among partners in countries flyway‐wide, so it is unlikely that a method that is useful in one part of the flyway would not be used throughout the flyway. Given that seven of the nine flyways recognized by Wetlands International (https://wpp.wetlands.org/background/WAF) include English‐speaking nations, we feel that this is unlikely to have resulted in us overlooking regionally specific methods to a large degree.
4. CONCLUSIONS
This review of the literature comprising more than 600 articles strongly suggests that there is no one broadly accepted method for assessing waterbird habitat quality. Directly measuring breeding success and survival rate are the most reliable measures, but it is unfeasible to obtain these data in many cases. A variety of proxy measures are available, but their interpretation requires substantive contextualization and a good understanding of their appropriateness to a specific project aim.
In general, if it is not possible to measure direct demographic parameters, projects should consider the suite of available proxy measures (Table 1) and consider which are most suitable to their site, budget and timeframe. Often, developing a protocol based on multiple proxies will increase confidence in results over the use of a single proxy. For example, studies investigating the comparative habitat quality of multiple sites could use a combination of waterbird abundance, behavior and body condition coupled with a measure of prey availability to gain insight into which site(s) are providing better food resources. Studies assessing if a single site is profitable for waterbirds from an energy perspective (i.e., habitat quality is sufficiently high to support population growth) could use a combination of waterbird behavior and available energy density to assess whether daily energy requirements are being met at the site. All studies using proxy measures should be mindful of the potential for interactions between features of the habitat (e.g., prey abundance and prey accessibility) to influence the direction of the relationship between habitat conditions and resultant demographic rates.
AUTHOR CONTRIBUTIONS
Rowan Mott: Conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); writing – original draft (lead); writing – review and editing (equal). Thomas A. A. Prowse: Conceptualization (equal); funding acquisition (equal); investigation (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Micha V. Jackson: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Daniel J. Rogers: Funding acquisition (equal); writing – original draft (equal); writing – review and editing (equal). Jody A. O'Connor: Funding acquisition (equal); writing – original draft (equal); writing – review and editing (equal). Justin D. Brookes: Funding acquisition (equal); writing – original draft (equal); writing – review and editing (equal). Phillip Cassey: Funding acquisition (equal); writing – original draft (equal); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
This research was funded by the South Australian Government's Healthy Coorong, Healthy Basin Program, which is jointly funded by the Australian and South Australian governments. We thank our project advisory committee and partners within the Goyder Institute for Water Research and Department for Environment and Water for feedback on drafts of this review.
Mott, R. , Prowse, T. A. A. , Jackson, M. V. , Rogers, D. J. , O’Connor, J. A. , Brookes, J. D. , & Cassey, P. (2023). Measuring habitat quality for waterbirds: A review. Ecology and Evolution, 13, e09905. 10.1002/ece3.9905
DATA AVAILABILITY STATEMENT
Data are available in supplementary material.
REFERENCES
- Aharon‐Rotman, Y. , Bauer, S. , & Klaassen, M. (2016). A chain is as strong as its weakest link: Assessing the consequences of habitat loss and degradation in a long‐distance migratory shorebird. Emu – Austral Ornithology, 116, 199–207. [Google Scholar]
- Aharon‐Rotman, Y. , Buchanan, K. L. , Clark, N. J. , Klaassen, M. , & Buttemer, W. A. (2016). Why fly the extra mile? Using stress biomarkers to assess wintering habitat quality in migratory shorebirds. Oecologia, 182, 385–395. [DOI] [PubMed] [Google Scholar]
- Alves, J. A. , Gunnarsson, T. G. , Hayhow, D. B. , Appleton, G. F. , Potts, P. M. , Sutherland, W. J. , & Gill, J. A. (2013). Costs, benefits, and fitness consequences of different migratory strategies. Ecology, 94, 11–17. [DOI] [PubMed] [Google Scholar]
- Arzel, C. , Rönkä, M. , Tolvanen, H. , Aarras, N. , Kamppinen, M. , & Vihervaara, P. (2015). Species diversity, abundance and brood numbers of breeding waterbirds in relation to habitat properties in an agricultural watershed. Annales Zoologici Fennici, 52, 17–32. [Google Scholar]
- Atiénzar, F. , Antón‐Pardo, M. , Armengol, X. , & Barba, E. (2012). Distribution of the white‐headed duck Oxyura leucocephala is affected by environmental factors in a Mediterranean wetland. Zoological Studies, 51, 783–792. [Google Scholar]
- Aubry, L. M. , Rockwell, R. F. , Cooch, E. G. , Brook, R. W. , Mulder, C. P. H. , & Koons, D. N. (2013). Climate change, phenology, and habitat degradation: Drivers of gosling body condition and juvenile survival in lesser snow geese. Global Change Biology, 19, 149–160. [DOI] [PubMed] [Google Scholar]
- Austin, J. E. , Buhl, T. K. , Guntenspergen, G. R. , Norling, W. , & Sklebar, H. T. (2001). Duck populations as indicators of landscape condition in the prairie pothole region. Environmental Monitoring and Assessment, 69, 29–48. [DOI] [PubMed] [Google Scholar]
- Austin, J. E. , O'Neil, S. T. , & Warren, J. M. (2017). Habitat selection by postbreeding female diving ducks: Influence of habitat attributes and conspecifics. Journal of Avian Biology, 48, 295–308. [Google Scholar]
- Baker, J. D. , & Thompson, P. M. (2007). Temporal and spatial variation in age‐specific survival rates of a long‐lived mammal, the Hawaiian monk seal. Proceedings of the Royal Society B: Biological Sciences, 274, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barati, A. , Ataei, F. , Esfandabad, B. S. , Shabanian, N. , & Etezadifar, F. (2011). Variations in nest‐site parameters in breeding waterbirds at Lake Zarivar, western Iran. Avian Biology Research, 4, 87–92. [Google Scholar]
- Barnett, C. A. , Suzuki, T. N. , Sakaluk, S. K. , & Thompson, C. F. (2015). Mass‐based condition measures and their relationship with fitness: in what condition is condition? Journal of zoology (London, England: 1987), 296, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerens, J. M. , Frederick, P. C. , Noonburg, E. G. , & Gawlik, D. E. (2015). Determining habitat quality for species that demonstrate dynamic habitat selection. Ecology and Evolution, 5, 5685–5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behney, A. C. , O'shaughnessy, R. , Eichholz, M. W. , & Stafford, J. D. (2014). Influence of item distribution pattern and abundance on efficiency of benthic core sampling. Wetlands, 34, 1109–1121. [Google Scholar]
- Benedict, R. J. , & Hepp, G. R. (2000). Wintering waterbird use of two aquatic plant habitats in a southern reservoir. The Journal of Wildlife Management, 64, 269–278. [Google Scholar]
- Blums, P. , Nichols, J. D. , Hines, J. E. , & Mednis, A. (2002). Sources of variation in survival and breeding site fidelity in three species of European ducks. Journal of Animal Ecology, 71, 438–450. [Google Scholar]
- Boyer, R. A. , Coluccy, J. M. , Montgomery, R. A. , Redilla, K. M. , & Winterstein, S. R. (2018). The effect of habitat on the breeding season survival of mallards (Anas platyrhynchos) in the Great Lakes region. Canadian Journal of Zoology, 96, 700–706. [Google Scholar]
- Broyer, J. (2009). Compared distribution within a disturbed fishpond ecosystem of breeding ducks and bird species indicators of habitat quality. Journal of Ornithology, 150, 761–768. [Google Scholar]
- Bucher, E. H. , & Curto, E. (2012). Influence of long‐term climatic changes on breeding of the Chilean flamingo in mar Chiquita, Córdoba, Argentina. Hydrobiologia, 697, 127–137. [Google Scholar]
- Buderman, F. E. , Devries, J. H. , & Koons, D. N. (2020). Changes in climate and land use interact to create an ecological trap in a migratory species. Journal of Animal Ecology, 89, 1961–1977. [DOI] [PubMed] [Google Scholar]
- Buehler, D. M. , Irene Tieleman, B. , & Piersma, T. (2009). Age and environment affect constitutive immune function in red knots (Calidris canutus). Journal of Ornithology, 150, 815–825. [Google Scholar]
- Burton, N. H. K. , Armitage, M. J. S. , Musgrove, A. J. , & Rehfisch, M. M. (2002). Impacts of man‐made landscape features on numbers of estuarine waterbirds at low tide. Environmental Management, 30, 857–864. [DOI] [PubMed] [Google Scholar]
- Bustnes, J. O. , Erikstad, K. E. , & Bjorn, T. H. (2002). Body condition and brood abandonment in common eiders breeding in the high arctic. Waterbirds, 25, 63–66. [Google Scholar]
- Castillo‐Guerrero, J. A. , Fernández, G. , Arellano, G. , & Mellink, E. (2009). Diurnal abundance, foraging behavior and habitat use by non‐breeding marbled godwits and willets at Guerrero Negro, Baja California Sur, México. Waterbirds, 32, 400–407. [Google Scholar]
- Cheng, K. , Ayjan, Y. , Li, F. F. , & Zong, C. (2018). Behavioral responses of breeding common coots (Fulica atra) to recreational disturbancein the Anbang river nature reserve. Shengtai Xuebao, 38, 485–492. [Google Scholar]
- Chyb, A. , & Minias, P. (2022). Complex associations of weather conditions with reproductive performance in urban population of a common waterbird. International Journal of Biometeorology, 66, 1163–1172. [DOI] [PubMed] [Google Scholar]
- Cleasby, I. R. , Bodey, T. W. , Vigfusdottir, F. , Mcdonald, J. L. , Mcelwaine, G. , Mackie, K. , Colhoun, K. , & Bearhop, S. (2017). Climatic conditions produce contrasting influences on demographic traits in a long‐distance Arctic migrant. Journal of Animal Ecology, 86, 285–295. [DOI] [PubMed] [Google Scholar]
- Cohen, J. B. , Houghton, L. M. , & Fraser, J. D. (2009). Nesting density and reproductive success of piping plovers in response to storm‐ and human‐created habitat changes. Wildlife Monographs, 173, 1–24. [Google Scholar]
- Collazo, J. A. , Dawn, A. O. H. , & Kelly, C. A. (2002). Accessible habitat for shorebirds: Factors influencing its availability and conservation implications. Waterbirds, 25, 13–24. [Google Scholar]
- Conner England, J. , Levengood, J. M. , Osborn, J. M. , Yetter, A. P. , Suski, C. D. , Cole, R. A. , & Hagy, H. M. (2018). Associations of intestinal helminth infections with health parameters of spring‐migrating female lesser scaup (Aythya affinis) in the upper Midwest, USA. Parasitology Research, 117, 1877–1890. [DOI] [PubMed] [Google Scholar]
- Cope, D. R. (2003). Variation in daily and seasonal foraging routines of non‐breeding barnacle geese (Branta leucopsis): Working harder does not overcome environmental constraints. Journal of Zoology, 260, 65–71. [Google Scholar]
- Crowther, M. , Lim, W. , & Crowther, M. A. (2010). Systematic review and meta‐analysis methodology. Blood, 116, 3140–3146. [DOI] [PubMed] [Google Scholar]
- Cumming, G. S. , Paxton, M. , King, J. , & Beuster, H. (2012). Foraging guild membership explains variation in waterbird responses to the hydrological regime of an arid‐region flood‐pulse river in Namibia. Freshwater Biology, 57, 1202–1213. [Google Scholar]
- Custer, C. M. , Suárez, S. A. , & Olsen, D. A. (2004). Feeding habitat characteristics of the great blue heron and great egret nesting along the upper Mississippi River, 1995‐1998. Waterbirds, 27, 454–468. [Google Scholar]
- Davis, C. A. , & Smith, L. M. (1998). Ecology and management of migrant shorebirds in the Playa Lakes region of Texas. Wildlife Monographs, 140, 3–45. [Google Scholar]
- Deboelpaep, E. , Coenegracht, T. , De Wolf, L. , Libert, A. , Vanschoenwinkel, B. , & Koedam, N. (2020). Bio‐energetic data show weak spatial but strong seasonal differences in wetland quality for waders in a Mediterranean migration bottleneck. Freshwater Biology, 65, 1529–1542. [Google Scholar]
- Dickson, D. L. (1992). The red‐throated loon as an indicator of environmental quality . Occasional Paper – Canadian Wildlife Service, 73.
- Dugger, B. D. , & Feddersen, J. C. (2009). Using river flow management to improve wetland habitat quality for waterfowl on the Mississippi River, USA. Wild, 59, 62–74. [Google Scholar]
- Dugger, B. D. , Moore, M. L. , Finger, R. S. , & Petrie, M. J. (2007). True metabolizable energy for seeds of common moist‐soil plant species. Journal of Wildlife Management, 71, 1964–1967. [Google Scholar]
- Duncan, P. , Hewison, A. J. M. , Houte, S. , Rosoux, R. , Tournebize, T. , Dubs, F. , Burel, F. , & Bretagnolle, V. (1999). Long‐term changes in agricultural practices and wildfowling in an internationally important wetland, and their effects on the guild of wintering ducks. Journal of Applied Ecology, 36, 11–23. [Google Scholar]
- Féret, M. , Bêty, J. , Gauthier, G. , Giroux, J.‐F. , & Picard, G. (2005). Are abdominal profiles useful to assess body condition of spring staging greater snow geese? The Condor, 107, 694–702. [Google Scholar]
- Fernández, G. , & Lank, D. B. (2006). Sex, age, and body size distributions of western sandpipers during the nonbreeding season with respect to local habitat. The Condor, 108, 547–557. [Google Scholar]
- Fernández, G. , & Lank, D. B. (2010). Do sex and habitat differences in antipredator behavior of Western sandpipers Calidris mauri reflect cumulative or compensatory processes? Journal of Ornithology, 151, 665–672. [Google Scholar]
- Fleskes, J. P. , Jarvis, R. L. , & Gilmer, D. S. (2002). September‐march survival of female northern pintails radiotagged in San Joaquin Valley, California. Journal of Wildlife Management, 66, 901–911. [Google Scholar]
- Fletcher, K. , Aebischer, N. J. , Baines, D. , Foster, R. , & Hoodless, A. N. (2010). Changes in breeding success and abundance of ground‐nesting moorland birds in relation to the experimental deployment of legal predator control. Journal of Applied Ecology, 47, 263–272. [Google Scholar]
- Fonseca, J. , & Navedo, J. G. (2020). Shorebird predation on benthic invertebrates after shrimp‐pond harvesting: Implications for semi‐intensive aquaculture management. Journal of Environmental Management, 262, 110290. [DOI] [PubMed] [Google Scholar]
- Ganzevles, W. , & Bredenbeek, J. (2005). Waders and waterbirds in the floodplains of the Logone, Cameroon and Chad, February 2000 . WIWO report 82. Beek‐Ubbergen, The Netherlands: Working Group of International Wader and Waterfowl Research (WIWO).
- Gawlik, D. E. , & Crozier, G. E. (2007). A test of cues affecting habitat selection by wading birds. The Auk, 124, 1075–1082. [Google Scholar]
- Ge, Z. M. , Wang, T. H. , Zhou, X. , Wang, K. Y. , & Shi, W. Y. (2007). Changes in the spatial distribution of migratory shorebirds along the Shanghai shoreline, China, between 1984 and 2004. Emu, 107, 19–27. [Google Scholar]
- Gillespie, C. R. , & Fontaine, J. J. (2017). Shorebird stopover habitat decisions in a changing landscape. The Journal of Wildlife Management, 81, 1051–1062. [Google Scholar]
- Godfrey, J. D. , Bryant, D. M. , & Williams, M. (2003). Energetics of blue ducks in rivers of differing physical and biological characteristics. Science for Conservation, 214, 35–68. [Google Scholar]
- Guan, L. , Lei, J. , Zuo, A. , Zhang, H. , Lei, G. , & Wen, L. (2016). Optimizing the timing of water level recession for conservation of wintering geese in Dongting Lake, China. Ecological Engineering, 88, 90–98. [Google Scholar]
- Guerra, A. S. , Micheli, F. , & Wood, C. L. (2016). Ecology of a vulnerable shorebird across a gradient of habitat alteration: Bristle‐thighed curlews (Numenius tahitiensis)(Aves: Charadriiformes) on Palmyra atoll. Pacific Science, 70, 159–174. [Google Scholar]
- Guisan, A. , & Thuiller, W. (2005). Predicting species distribution: Offering more than simple habitat models. Ecology Letters, 8, 993–1009. [DOI] [PubMed] [Google Scholar]
- Gunness, M. A. , Clark, R. G. , & Weatherhead, P. J. (2001). Counterintuitive parental investment by female dabbling ducks in response to variable habitat quality. Ecology, 82, 1151–1158. [Google Scholar]
- Haddaway, N. R. , Bethel, A. , Dicks, L. V. , Koricheva, J. , Macura, B. , Petrokofsky, G. , Pullin, A. S. , Savilaakso, S. , & Stewart, G. B. (2020). Eight problems with literature reviews and how to fix them. Nature Ecology & Evolution, 4, 1582–1589. [DOI] [PubMed] [Google Scholar]
- Hagy, H. M. , & Kaminski, R. M. (2015). Determination of foraging thresholds and effects of application on energetic carrying capacity for waterfowl. PLoS One, 10, e0118349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza, F. , Hammouda, A. , & Selmi, S. (2015). Species richness patterns of waterbirds wintering in the Gulf of Gabès in relation to habitat and anthropogenic features. Estuarine, Coastal and Shelf Science, 165, 254–260. [Google Scholar]
- Hario, M. , & Rintala, J. (2009). Age of first breeding in the common eider somateria m. mollissima population in the northern Baltic Sea. Ornis Fennica, 86, 81–88. [Google Scholar]
- Haukos, D. A. , & Neaville, J. (2003). Spatial and temporal changes in prevalence of a cloacal cestode in wintering waterfowl along the Gulf coast of Texas. Journal of Wildlife Diseases, 39, 152–160. [DOI] [PubMed] [Google Scholar]
- Hazlitt, S. L. (2001). Territory quality and reproductive success of black oystercatchers in British Columbia. Wilson Bulletin, 113, 404–409. [Google Scholar]
- He, C.‐G. , Cui, L.‐J. , Ishikawa, T. , Sheng, L.‐X. , & Jiang, H.‐B. (2009). Study on the water regulation for preserving the nesting habitat of red‐crowned crane (Grus japonensis (Muller)). Journal of Northeast Normal University Natural Sciences Edition, 41, 105–111. [Google Scholar]
- Herring, G. , & Collazo, J. A. (2005). Habitat use, movements and home range of wintering lesser Scaup in Florida. Waterbirds, 28, 71–78. [Google Scholar]
- Herring, G. , & Gawlik, D. E. (2013). Differential physiological responses to prey availability by the great egret and white ibis. The Journal of Wildlife Management, 77, 58–67. [Google Scholar]
- Herring, G. , Gawlik, D. E. , Cook, M. I. , & Beerens, J. M. (2010). Sensitivity of nesting great egrets (Ardea alba) and white ibises (Eudocimus albus) to reduced prey availability. The Auk, 127, 660–670. [Google Scholar]
- Herring, G. , Herring, H. K. , & Gawlik, D. E. (2015). Social cues and environmental conditions influence foraging flight distances of breeding wood storks (Mycteria americana). Waterbirds, 38, 30–39. [Google Scholar]
- Hickman, S. (1994). Improvement of habitat quality for nesting and migrating birds at the Des Plaines River wetlands demonstration project. Ecological Engineering, 3, 485–494. [Google Scholar]
- Hierl, L. A. , Loftin, C. S. , Longcore, J. R. , Mcauley, D. G. , & Urban, D. L. (2007). A multivariate assessment of changes in wetland habitat for waterbirds at Moosehorn National Wildlife Refuge, Maine, USA. Wetlands, 27, 141–152. [Google Scholar]
- Holopainen, S. , Nummi, P. , & Pöysä, H. (2014). Breeding in the stable boreal landscape: Lake habitat variability drives brood production in the teal (Anas crecca). Freshwater Biology, 59, 2621–2631. [Google Scholar]
- Hsu, C.‐B. , Hwang, G.‐W. , Lu, J.‐F. , Chen, C.‐P. , Tao, H.‐H. , & Hsieh, H.‐L. (2014). Habitat characteristics of the wintering common teal in the Huajiang wetland, Taiwan. Wetlands, 34, 1207–1218. [Google Scholar]
- Hu, C.‐S. , Song, X. , Ding, C.‐Q. , Ye, Y.‐X. , Qing, B.‐P. , & Wang, C. (2016). The size of winter‐flooded paddy fields no longer limits the foraging habitat use of the endangered crested ibis (Nipponia nippon) in winter. Zoological Science, 33, 345–351. [DOI] [PubMed] [Google Scholar]
- Hunt, K. L. , Catlin, D. H. , Felio, J. H. , & Fraser, J. D. (2013). Effect of capture frequency on the survival of piping plover chicks. Journal of Field Ornithology, 84, 299–303. [Google Scholar]
- Hunt, K. L. , Fraser, J. D. , Karpanty, S. M. , & Catlin, D. H. (2017). Body condition of piping plovers (Charadrius melodus) and prey abundance on flood‐created habitat on the Missouri River, USA. The Wilson Journal of Ornithology, 129, 754–764. [Google Scholar]
- Jackson, D. B. (1994). Breeding dispersal and site‐fidelity in three monogamous wader species in the Western isles, U.K. Ibis, 136, 463–473. [Google Scholar]
- Jia, Q. , Wang, X. , Zhang, Y. , Cao, L. , & Fox, A. D. (2018). Drivers of waterbird communities and their declines on Yangtze River floodplain lakes. Biological Conservation, 218, 240–246. [Google Scholar]
- Johnson, M. D. (2005). Habitat quality: a brief review for wildlife biologists. Transactions of the Western Section of the Wildlife Society, 41, 31–41. [Google Scholar]
- Johnson, M. D. (2007). Measuring habitat quality: A review. The Condor, 109, 489–504. [Google Scholar]
- Jorde, D. G. , Haramis, G. M. , Bunck, C. M. , & Pendleton, G. W. (1995). Effects of diet on rate of body mass gain by wintering canvasbacks. Journal of Wildlife Management, 59, 31–39. [Google Scholar]
- Jourdan, C. , Fort, J. , Pinaud, D. , Delaporte, P. , Gernigon, J. , Guenneteau, S. , Jomat, L. , Lelong, V. , Lemesle, J.‐C. , Robin, F. , Rousseau, P. , & Bocher, P. (2021). Highly diversified habitats and resources influence habitat selection in wintering shorebirds. Journal of Ornithology, 162, 823–838. [Google Scholar]
- Kang, S.‐R. , & King, S. L. (2014). Suitability of coastal marshes as whooping crane (Grus americana) foraging habitat in Southwest Louisiana, USA. Waterbirds, 37, 254–263. [Google Scholar]
- Karlíková, Z. , Kejzlarová, T. , & Šálek, M. (2018). Breath rate patterns in precocial northern lapwing (Vanellus vanellus) chicks in the wild. Journal of Ornithology, 159, 555–563. [Google Scholar]
- Kearney, M. (2006). Habitat, environment and niche: What are we modelling? Oikos, 115, 186–191. [Google Scholar]
- Kelly, J. P. (2001). Hydrographic correlates of winter dunlin abundance and distribution in a temperate estuary. Waterbirds, 24, 309–322. [Google Scholar]
- Kenow, K. P. , Kapfer, J. M. , & Korschgen, C. E. (2009). Predation of radio‐marked mallard (Anas platyrhynchos) ducklings by eastern snapping turtles (Chelydra serpentina serpentina) and Western Fox snakes (Pantherophis vulpinus) on the upper Mississippi River. Journal of Herpetology, 43, 154–158. [Google Scholar]
- Khan, T. N. (2010). Temporal changes to the abundance and community structure of migratory waterbirds in Santragachhi Lake, West Bengal, and their relationship with water hyacinth cover. Current Science, 99, 1570–1577. [Google Scholar]
- Klaassen, M. , Hahn, S. , Korthals, H. , & Madsen, J. (2017). Eggs brought in from afar: Svalbard‐breeding pink‐footed geese can fly their eggs across the Barents Sea. Journal of Avian Biology, 48, 173–179. [Google Scholar]
- Knutson, M. G. , Powell, L. A. , Hines, R. K. , Friberg, M. A. , & Niemi, G. J. (2006). An assessment of bird habitat quality using population growth rates. Condor, 108, 301–314. [Google Scholar]
- Koivula, K. , & Rönkä, A. (1998). Habitat deterioration and efficiency of antipredator strategy in a meadow‐breeding wader, Temminck's stint (Calidris temminckii). Oecologia, 116, 348–355. [DOI] [PubMed] [Google Scholar]
- Kosztolányi, A. , Székely, T. , Cuthill, I. C. , Yilmaz, K. T. , & Berberoǧlu, S. (2006). Ecological constraints on breeding system evolution: The influence of habitat on brood desertion in Kentish plover. Journal of Animal Ecology, 75, 257–265. [DOI] [PubMed] [Google Scholar]
- Krijgsveld, K. L. , Reneerkens, J. W. H. , Mcnett, G. D. , & Ricklefs, R. E. (2003). Time budgets and body temperatures of American Golden‐plover chicks in relation to ambient temperature. Condor, 105, 268–278. [Google Scholar]
- Kruuk, L. E. B. , Clutton‐Brock, T. H. , Rose, K. E. , & Guinness, F. E. (1999). Early determinants of lifetime reproductive success differ between the sexes in Red Deer. Proceedings: Biological Sciences, 266, 1655–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwae, T. , Miyoshi, E. , Sassa, S. , & Watabe, Y. (2010). Foraging mode shift in varying environmental conditions by dunlin Calidris alpina . Marine Ecology Progress Series, 406, 281–289. [Google Scholar]
- Kwon, E. , Robinson, S. , Weithman, C. E. , Catlin, D. H. , Karpanty, S. M. , Altman, J. , Simons, T. R. , & Fraser, J. D. (2021). Contrasting long‐term population trends of beach‐nesting shorebirds under shared environmental pressures. Biological Conservation, 260, 109178. [Google Scholar]
- Lantz, S. M. , Gawlik, D. E. , & Cook, M. I. (2011). The effects of water depth and emergent vegetation on foraging success and habitat selection of wading birds in the Everglades. Waterbirds, 34, 439–447. [Google Scholar]
- Le Boeuf, B. , Condit, R. , & Reiter, J. (2019). Lifetime reproductive success of northern elephant seals (Mirounga angustirostris). Canadian Journal of Zoology, 97, 1203–1217. [Google Scholar]
- Le Fer, D. , Fraser, J. D. , & Kruse, C. D. (2008). Piping plover chick foraging, growth, and survival in the Great Plains. Journal of Wildlife Management, 72, 682–687. [Google Scholar]
- Lebeuf, A. P. , & Giroux, J.‐F. (2014). Density‐dependent effects on nesting success of temperate‐breeding Canada geese. Journal of Avian Biology, 45, 600–608. [Google Scholar]
- Lecomte, N. , Gauthier, G. , & Giroux, J. F. (2008). Breeding dispersal in a heterogeneous landscape: The influence of habitat and nesting success in greater snow geese. Oecologia, 155, 33–41. [DOI] [PubMed] [Google Scholar]
- Legagneux, P. , Harms, N. J. , Gauthier, G. , Chastel, O. , Gilchrist, H. G. , Bortolotti, G. , Bêty, J. , & Soos, C. (2013). Does feather corticosterone reflect individual quality or external stress in arctic‐nesting migratory birds? PLoS One, 8, e82644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, T. , Flint, P. L. , Schmutz, J. A. , & Derksen, D. V. (2010). Pre‐moult patterns of habitat use and moult site selection by Brent geese Branta bernicla nigricans: Individuals prospect for moult sites. Ibis, 152, 556–568. [Google Scholar]
- Li, X. , Hou, X. , Song, Y. , Shan, K. , Zhu, S. , Yu, X. , & Mo, X. (2019). Assessing changes of habitat quality for shorebirds in stopover sites: A case study in Yellow River Delta, China. Wetlands, 39, 67–77. [Google Scholar]
- Liu, S.‐L. , Cai, Y.‐J. , Pang, S.‐L. , Qiu, F.‐C. , He, C.‐G. , & Song, Y.‐J. (2006). The influence of water resource condition on rare waterfowl in Zhalong National Nature Reserve. Journal of Northeast Normal University Natural Sciences Edition, 38, 105–108. [Google Scholar]
- Loewenthal, D. , Paijmans, D. M. , & Hockey, P. A. R. (2015). Year‐round territoriality in long‐lived birds: Rethinking the concept of carrying capacity. Ostrich, 86, 23–34. [Google Scholar]
- Lok, T. , Overdijk, O. , Tinbergen, J. M. , & Piersma, T. (2011). The paradox of spoonbill migration: Most birds travel to where survival rates are lowest. Animal Behaviour, 82, 837–844. [Google Scholar]
- Lourenço, P. M. , Granadeiro, J. P. , & Palmeirim, J. M. (2005). Importance of drainage channels for waders foraging on tidal flats: Relevance for the management of estuarine wetlands. Journal of Applied Ecology, 42, 477–486. [Google Scholar]
- Lovvorn, J. R. , North, C. A. , Grebmeier, J. M. , Cooper, L. W. , & Kolts, J. M. (2018). Sediment organic carbon integrates changing environmental conditions to predict benthic assemblages in shallow Arctic seas. Aquatic Conservation: Marine and Freshwater Ecosystems, 28, 861–871. [Google Scholar]
- Lyons, J. E. , Collazo, J. A. , & Guglielmo, C. G. (2008). Plasma metabolites and migration physiology of semipalmated sandpipers: Refueling performance at five latitudes. Oecologia, 155, 417–427. [DOI] [PubMed] [Google Scholar]
- Ma, L. , Chen, C. , Shen, Y. , Wu, L.‐F. , Huang, Z.‐L. , & Cao, H.‐L. (2014). Determinants of tree survival at local scale in a sub‐tropical forest. Ecological Research, 29, 69–80. [Google Scholar]
- Ma, Z. , Cai, Y. , Li, B. , & Chen, J. (2010). Managing wetland habitats for waterbirds: An international perspective. Wetlands, 30, 15–27. [Google Scholar]
- Machín, P. , Fernández‐Elipe, J. , Hungar, J. , Angerbjörn, A. , Klaassen, R. H. G. , & Aguirre, J. I. (2019). The role of ecological and environmental conditions on the nesting success of waders in sub‐Arctic Sweden. Polar Biology, 42, 1571–1579. [Google Scholar]
- Mallory, M. L. , Mcnicol, D. K. , & Weatherhead, P. J. (1994). Habitat quality and reproductive effort of common goldeneyes nesting near Sudbury, Canada. The Journal of Wildlife Management, 58, 552–560. [Google Scholar]
- Mallory, M. L. , Walton, R. A. , & Mcnicol, D. K. (1999). Influence of intraspecific competition and habitat quality on diurnal activity budgets of breeding common goldeneyes. Écoscience, 6, 481–486. [Google Scholar]
- Mander, L. , Marie‐Orleach, L. , & Elliott, M. (2013). The value of wader foraging behaviour study to assess the success of restored intertidal areas. Estuarine, Coastal and Shelf Science, 131, 1–5. [Google Scholar]
- Manton, M. , & Angelstam, P. (2021). Macroecology of north european wet grassland landscapes: Habitat quality, waders, avian predators and nest predation. Sustainability (Switzerland), 13, 8138. [Google Scholar]
- Markkola, J. A. , & Karvonen, R. T. (2020). Changing environmental conditions and structure of a breeding population of the threatened lesser white‐fronted goose (Anser erythropus L.). Ornis Fennica, 97, 113–130. [Google Scholar]
- Marmillot, V. , Gauthier, G. , Cadieux, M. C. , & Legagneux, P. (2016). Plasticity in moult speed and timing in an arctic‐nesting goose species. Journal of Avian Biology, 47, 650–658. [Google Scholar]
- Marzluff, J. M. , Raphael, M. G. , & Sallabanks, R. (2000). Understanding the effects of forest management on avian species. Wildlife Society Bulletin, 28, 1132–1143. [Google Scholar]
- Mascitti, V. (2001). Habitat changes in Laguna de Pozuelos, Jujuy, Argentina: Implication for south American flamingo populations. Waterbirds, 24, 16–21. [Google Scholar]
- Maslo, B. , Burger, J. , & Handel, S. N. (2012). Modeling foraging behavior of piping plovers to evaluate habitat restoration success. The Journal of Wildlife Management, 76, 181–188. [Google Scholar]
- Master, T. L. , Leiser, J. K. , Bennett, K. A. , Bretsch, J. K. , & Wolfe, H. J. (2005). Patch selection by snowy egrets. Waterbirds, 28, 220–224. [Google Scholar]
- Mattsson, B. J. , Runge, M. C. , Devries, J. H. , Boomer, G. S. , Eadie, J. M. , Haukos, D. A. , Fleskes, J. P. , Koons, D. N. , Thogmartin, W. E. , & Clark, R. G. (2012). A modeling framework for integrated harvest and habitat management of North American waterfowl: Case‐study of northern pintail metapopulation dynamics. Ecological Modelling, 225, 146–158. [Google Scholar]
- McComb, B. C. (2016). Wildlife habitat management: Concepts and applications in forestry. CRC Press. [Google Scholar]
- McKinney, R. A. , & McWilliams, S. R. (2005). A new model to estimate daily energy expenditure for wintering waterfowl. Wilson Bulletin, 117, 44–55. [Google Scholar]
- McKinnon, L. , Schmaltz, L. , Aubry, Y. , Rochepault, Y. , Buidin, C. , & Juillet, C. (2022). Female migration phenology and climate conditions explain juvenile red knot (Calidris canutus rufa) counts during fall migration. Avian Conservation and Ecology, 17. 10.5751/ACE-02021-170109 [DOI] [Google Scholar]
- Meltofte, H. (2006). Wader populations at Zackenberg, high‐arctic Northeast Greenland, 1996–2005. Dansk Ornitologisk Forenings Tidsskrift, 100, 16–28. [Google Scholar]
- Merendino, M. T. , & Ankney, C. D. (1994). Habitat use by mallards and American black ducks breeding in Central Ontario. The Condor, 96, 411–421. [Google Scholar]
- Merendino, M. T. , Dennis, D. G. , & Ankney, C. D. (1992). Mallard harvest data: An index of wetland quality for breeding waterfowl. Wildlife Society Bulletin, 20, 171–175. [Google Scholar]
- Merrill, L. , Levengood, J. M. , England, J. C. , Osborn, J. M. , & Hagy, H. M. (2018). Blood parasite infection linked to condition of spring‐migrating lesser Scaup (Aythya affinis). Canadian Journal of Zoology, 96, 1145–1152. [Google Scholar]
- Meyer, N. , Bollache, L. , Galipaud, M. , Moreau, J. , Dechaume‐Moncharmont, F. X. , Afonso, E. , Angerbjörn, A. , Bêty, J. , Brown, G. , Ehrich, D. , Gilg, V. , Giroux, M. A. , Hansen, J. , Lanctot, R. , Lang, J. , Latty, C. , Lecomte, N. , McKinnon, L. , Kennedy, L. , … Gilg, O. (2021). Behavioural responses of breeding arctic sandpipers to ground‐surface temperature and primary productivity. Science of the Total Environment, 755, 142485. [DOI] [PubMed] [Google Scholar]
- Milenkaya, O. , Catlin, D. H. , Legge, S. , & Walters, J. R. (2015). Body condition indices predict reproductive success but not survival in a sedentary, tropical bird. PLoS ONE, 10, e0136582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minias, P. , & Janiszewski, T. (2016). Territory selection in the city: Can birds reliably judge territory quality in a novel urban environment? Journal of Zoology, 300, 120–126. [Google Scholar]
- Mokany, K. , Ware, C. , Harwood, T. D. , Schmidt, R. K. , & Ferrier, S. (2022). Habitat‐based biodiversity assessment for ecosystem accounting in the Murray–Darling Basin. Conservation Biology, 36, e13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney, P. D. , Gormley, A. M. , Toop, S. D. , Flesch, J. S. , Forsyth, D. M. , Ramsey, D. S. L. , & Hampton, J. O. (2022). Bayesian modelling reveals differences in long‐term trends in the harvest of native and introduced species by recreational hunters in Australia. Wildlife Research, 49, 673–685. 10.1071/WR21138 [DOI] [Google Scholar]
- Mu, T. , Cai, S. , Peng, H. B. , Hassell, C. J. , Boyle, A. , Zhang, Z. , Piersma, T. , & Wilcove, D. S. (2022). Evaluating staging habitat quality to advance the conservation of a declining migratory shorebird, red knot Calidris canutus . Journal of Applied Ecology, 59, 2084–2093. [Google Scholar]
- Murray, D. L. , Anderson, M. G. , & Steury, T. D. (2010). Temporal shift in density dependence among North American breeding duck populations. Ecology, 91, 571–581. [DOI] [PubMed] [Google Scholar]
- Nam, H. K. , Choi, Y. S. , Choi, S. H. , & Yoo, J. C. (2015). Distribution of waterbirds in Rice fields and their use of foraging habitats. Waterbirds, 38, 173–183. [Google Scholar]
- Neuman, K. K. , Henkel, L. A. , & Page, G. W. (2008). Shorebird use of sandy beaches in Central California. Waterbirds, 31, 115–121. [Google Scholar]
- Nolet, B. A. , De Boer, T. , & De Vries, P. P. (2007). Habitat quality estimated from head‐dipping time in trampling swans. Israel Journal of Ecology and Evolution, 53, 317–328. [Google Scholar]
- Nolet, B. A. , & Klaassen, M. (2009). Retrodicting patch use by foraging swans in a heterogeneous environment using a set of functional responses. Oikos, 118, 431–439. [Google Scholar]
- Ntiamoa‐Baidu, Y. , Nuoh, A. A. , Reneerkens, J. , & Piersma, T. (2014). Population increases in non‐breeding sanderlings in Ghana indicate site preference. Ardea, 102, 131–137. [Google Scholar]
- Nyman, J. A. , & Chabreck, R. H. (1996). Some effects of 30 years of weir‐management on coastal marsh aquatic vegetation and implications to waterfowl management. Gulf of Mexico Science, 14, 16–25. [Google Scholar]
- O'Neal, B. J. , Stafford, J. D. , & Larkin, R. P. (2012). Stopover duration of fall‐migrating dabbling ducks. The Journal of Wildlife Management, 76, 285–293. [Google Scholar]
- O'Neil, S. T. , Warren, J. M. , Takekawa, J. Y. , De La Cruz, S. E. W. , Cutting, K. A. , Parker, M. W. , & Yee, J. L. (2014). Behavioural cues surpass habitat factors in explaining prebreeding resource selection by a migratory diving duck. Animal Behaviour, 90, 21–29. [Google Scholar]
- Osborn, J. M. , Hagy, H. M. , Mcclanahan, M. D. , & Gray, M. J. (2017). Temporally robust models for predicting seed yield of moist‐soil plants. Wildlife Society Bulletin, 41, 157–161. [Google Scholar]
- Owen, T. M. , & Pierce, A. R. (2014). Productivity and chick growth rates of royal tern (Thalasseus maximus) and sandwich tern (Thalasseus sandvicensis) on the isles Dernieres Barrier Island refuge, Louisiana. Waterbirds, 37, 245–253. [Google Scholar]
- Parks, M. A. , Collazo, J. A. , Colón, J. A. , Álvarez, K. R. R. , & Díaz, O. (2016). Change in numbers of resident and migratory shorebirds at the Cabo Rojo salt flats, Puerto Rico, USA (1985–2014). Waterbirds, 39, 209–214. [Google Scholar]
- Pedler, R. D. , Ribot, R. F. H. , & Bennett, A. T. D. (2014). Extreme nomadism in desert waterbirds: Flights of the banded stilt. Biology Letters, 10, 20140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehlak, H. , & Lõhmus, A. (2008). An artificial nest experiment indicates equal nesting success of waders in coastal meadows and mires. Ornis Fennica, 85, 66–71. [Google Scholar]
- Pérot, A. , & Villard, M.‐A. (2009). Putting density back into the habitat‐quality equation: Case study of an open‐nesting forest bird. Conservation Biology, 23, 1550–1557. [DOI] [PubMed] [Google Scholar]
- Peters, K. A. , & Otis, D. L. (2007). Shorebird roost‐site selection at two temporal scales: Is human disturbance a factor? Journal of Applied Ecology, 44, 196–209. [Google Scholar]
- Pezzanite, B. , Rockwell, R. F. , Davies, J. C. , Loonen, M. J. J. E. , & Seguin, R. J. (2005). Has habitat degradation affected foraging behaviour and reproductive success of lesser snow geese (Chen caerulescens caerulescens)? Ecoscience, 12, 439–446. [Google Scholar]
- Pidgeon, A. M. , Radeloff, V. C. , & Mathews, N. E. (2006). Contrasting measures of fitness to classify habitat quality for the black‐throated sparrow (Amphispiza bilineata). Biological Conservation, 132, 199–210. [Google Scholar]
- Pierce, A. K. , Dinsmore, S. J. , & Wunder, M. B. (2019). Decreased nest survival associated with low temperatures in a high‐elevation population of mountain plover (Charadrius montanus). Wilson Journal of Ornithology, 131, 502–513. [Google Scholar]
- Piersma, T. , Lok, T. , Chen, Y. , Hassell, C. J. , Yang, H.‐Y. , Boyle, A. , Slaymaker, M. , Chan, Y.‐C. , Melville, D. S. , Zhang, Z.‐W. , & Ma, Z. (2016). Simultaneous declines in summer survival of three shorebird species signals a flyway at risk. Journal of Applied Ecology, 53, 479–490. [Google Scholar]
- Powell, G. V. N. , & Powell, A. H. (1986). Reproduction by great white herons Ardea herodias in Florida bay as an indicator of habitat quality. Biological Conservation, 36, 101–113. [Google Scholar]
- Pöysä, H. , Sjöberg, K. , Elmberg, J. , & Nummi, P. (2001). Pair formation among experimentally introduced mallards Anas platyrhynchos reflects habitat quality. Annales Zoologici Fennici, 38, 179–184. [Google Scholar]
- Pulliam, H. R. (2000). On the relationship between niche and distribution. Ecology Letters, 3, 349–361. [Google Scholar]
- Pullin, A. S. , & Stewart, G. B. (2006). Guidelines for systematic review in conservation and environmental management. Conservation Biology, 20, 1647–1656. [DOI] [PubMed] [Google Scholar]
- Quan, R.‐C. , Wen, X. , & Yang, X. (2002). Effects of human activities on migratory waterbirds at Lashihai Lake, China. Biological Conservation, 108, 273–279. [Google Scholar]
- Raquel, A. J. , Devries, J. H. , Howerter, D. W. , Alisauskas, R. T. , Leach, S. W. , & Clark, R. G. (2016). Timing of nesting of upland‐nesting ducks in the Canadian Prairies and its relation to spring wetland conditions. Canadian Journal of Zoology, 94, 575–581. [Google Scholar]
- Reed, E. T. , Gauthier, G. , Pradel, R. , & Lebreton, J. D. (2003). Age and environmental conditions affect recruitment in greater snow geese. Ecology, 84, 219–230. [Google Scholar]
- Reurink, F. , Hentze, N. , Rourke, J. , & Ydenberg, R. (2015). Site‐specific flight speeds of nonbreeding Pacific dunlins as a measure of the quality of a foraging habitat. Behavioral Ecology, 27, 803–809. [Google Scholar]
- Reynolds, M. H. , Hatfield, J. S. , Laniawe, L. P. , Vekasy, M. S. , Klavitter, J. L. , Berkowitz, P. , Crampton, L. H. , & Walters, J. R. (2012). Influence of space use on fitness and the reintroduction success of the Laysan teal. Animal Conservation, 15, 305–317. [Google Scholar]
- Rice, S. M. , Collazo, J. A. , Alldredge, M. W. , Harrington, B. A. , & Lewis, A. R. (2007). Local annual survival and seasonal residency rates of semipalmated sandpipers (Calidris pusilla) in Puerto Rico. The Auk, 124, 1397–1406. [Google Scholar]
- Richard, E. , Simpson, S. E. , Medill, S. A. , & Mcloughlin, P. D. (2014). Interacting effects of age, density, and weather on survival and current reproduction for a large mammal. Ecology and Evolution, 4, 3851–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecke, T. V. , Conway, W. C. , Haukos, D. A. , Moon, J. A. , & Comer, C. E. (2019). Nest survival of black‐necked stilts (Himantopus mexicanus) on the upper Texas coast, USA. Waterbirds, 42, 261–271. [Google Scholar]
- Ringelman, K. M. , Walker, J. , Ringelman, J. K. , & Stephens, S. E. (2018). Temporal and multi‐spatial environmental drivers of duck nest survival. Auk, 135, 486–494. [Google Scholar]
- Rizzolo, D. J. , Schmutz, J. A. , & Speakman, J. R. (2015). Fast and efficient: Postnatal growth and energy expenditure in an Arctic‐breeding waterbird, the red‐throated loon (Gavia stellata). Auk, 132, 657–670. [Google Scholar]
- Rogers, K. G. , & Gosbell, K. (2006). Demographic models for red‐necked stint and curlew sandpiper in Victoria. Stilt, 50, 205–214. [Google Scholar]
- Rose, M. , & Nol, E. (2010). Foraging behavior of non‐breeding Semipalmated plovers. Waterbirds, 33, 59–69. [Google Scholar]
- Roshier, D. , Klomp, N. , & Asmus, M. (2006). Movements of a nomadic waterfowl, grey teal Anas gracilis, across inland Australia–results from satellite telemetry spanning fifteen months. Ardea, 94, 461–475. [Google Scholar]
- Ross, B. E. , Haukos, D. A. , & Walther, P. (2018). Quantifying changes and influences on mottled duck density in Texas. Journal of Wildlife Management, 82, 374–382. [Google Scholar]
- Roy, C. L. , Berdeen, J. B. , & Clark, M. (2019). A demographic model for ring‐necked ducks breeding in Minnesota. Journal of Wildlife Management, 83, 1720–1734. [Google Scholar]
- Russell, I. A. , Randall, R. M. , & Hanekom, N. (2014). Spatial and temporal patterns of waterbird assemblages in the wilderness lakes complex, South Africa. Waterbirds, 37, 1–18. [Google Scholar]
- Ruthrauff, D. R. , Dekinga, A. , Gill, R. E., Jr. , Summers, R. W. , & Piersma, T. (2013). Ecological correlates of variable organ sizes and fat loads in the most northerly wintering shorebirds. Canadian Journal of Zoology, 91, 698–705. [Google Scholar]
- Sabatier, R. , Doyen, L. , & Tichit, M. (2010). Modelling trade‐offs between livestock grazing and wader conservation in a grassland agroecosystem. Ecological Modelling, 221, 1292–1300. [Google Scholar]
- Saunders, S. P. , Piper, W. , Farr, M. T. , Bateman, B. L. , Michel, N. L. , Westerkam, H. , & Wilsey, C. B. (2021). Interrelated impacts of climate and land‐use change on a widespread waterbird. Journal of Animal Ecology, 90, 1165–1176. [DOI] [PubMed] [Google Scholar]
- Schaffer‐Smith, D. , Swenson, J. J. , Reiter, M. E. , & Isola, J. E. (2018). Quantifying shorebird habitat in managed wetlands by modeling shallow water depth dynamics. Ecological Applications, 28, 1534–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlägel, U. E. , & Mädlow, W. (2022). All‐season space use by non‐native resident mandarin ducks (Aix galericulata) in northeastern Germany. Journal of Ornithology, 163, 71–82. [Google Scholar]
- Schultz, R. , Straub, J. , Kaminski, M. , & Ebert, A. (2020). Floristic and macroinvertebrate responses to different wetland restoration techniques in southeastern Wisconsin. Wetlands, 40, 2025–2040. [Google Scholar]
- Schummer, M. L. , Kaminski, R. M. , Raedeke, A. H. , & Graber, D. A. (2010). Weather‐related indices of autumn–winter dabbling duck abundance in middle North America. Journal of Wildlife Management, 74, 94–101. [Google Scholar]
- Sebastián‐González, E. , Botella, F. , Sempere, R. A. , & Sánchez‐Zapata, J. A. (2010). An empirical demonstration of the ideal free distribution: Little grebes Tachybaptus ruficollis breeding in intensive agricultural landscapes. Ibis, 152, 643–650. [Google Scholar]
- Sebastián‐González, E. , Sánchez‐Zapata, J. A. , & Botella, F. (2010). Agricultural ponds as alternative habitat for waterbirds: Spatial and temporal patterns of abundance and management strategies. European Journal of Wildlife Research, 56, 11–20. [Google Scholar]
- Sebastián‐González, E. , Sánchez‐Zapata, J. A. , Botella, F. , & Otso, O. (2010). Testing the heterospecific attraction hypothesis with time‐series data on species co‐occurrence. Proceedings of the Royal Society B: Biological Sciences, 277, 2983–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedinger, J. S. , & Alisauskas, R. T. (2014). Cross‐seasonal effects and the dynamics of waterfowl populations. Wild, 4, 277–304. [Google Scholar]
- Simpson, J. W. , Yerkes, T. , Nudds, T. D. , & Smith, B. D. (2007). Effects of habitat on mallard duckling survival in the Great Lakes region. Journal of Wildlife Management, 71, 1885–1891. [Google Scholar]
- Sjöberg, K. , Pöysä, H. , Elmberg, J. , & Nummi, P. (2000). Response of mallard ducklings to variation in habitat quality: An experiment of food limitation. Ecology, 81, 329–335. [Google Scholar]
- Smart, J. , Bolton, M. , Hunter, F. , Quayle, H. , Thomas, G. , & Gregory, R. D. (2013). Managing uplands for biodiversity: Do Agri‐environment schemes deliver benefits for breeding lapwing Vanellus vanellus? Journal of Applied Ecology, 50, 794–804. [Google Scholar]
- Smart, J. , & Gill, J. A. (2003). Non‐intertidal habitat use by shorebirds: A reflection of inadequate intertidal resources? Biological Conservation, 111, 359–369. [Google Scholar]
- Smith, J. P. (1995). Foraging sociability of nesting wading birds (Ciconiiformes) at Lake Okeechobee, Florida. The Wilson Bulletin, 107, 437–451. [Google Scholar]
- Sorenson, L. G. , Goldberg, R. , Root, T. L. , & Anderson, M. G. (1998). Potential effects of global warming on waterfowl populations breeding in the northern Great Plains. Climatic Change, 40, 343–369. [Google Scholar]
- Souchay, G. , Gauthier, G. , Lefebvre, J. , & Pradel, R. (2015). Absence of difference in survival between two distant breeding sites of greater snow geese. Journal of Wildlife Management, 79, 570–578. [Google Scholar]
- Steen, V. , Skagen, S. K. , & Noon, B. R. (2018). Preparing for an uncertain future: Migrating shorebird response to past climatic fluctuations in the prairie potholes: Migrating. Ecosphere, 9, e02095. [Google Scholar]
- Stephens, P. A. , Pettorelli, N. , Barlow, J. , Whittingham, M. J. , & Cadotte, M. W. (2015). Management by proxy? The use of indices in applied ecology. Journal of Applied Ecology, 52, 1–6. [Google Scholar]
- Stillman, R. A. , Goss‐Custard, J. D. , West, A. D. , Dit Durell, S. E. L. V. , Caldow, R. W. G. , Mcgrorty, S. , & Clarke, R. T. (2000). Predicting mortality in novel environments: Tests and sensitivity of a behaviour‐based model. Journal of Applied Ecology, 37, 564–588. [Google Scholar]
- Stillman, R. A. , West, A. D. , Goss‐Custard, J. D. , Mcgrorty, S. , Frost, N. J. , Morrisey, D. J. , Kenny, A. J. , & Drewitt, A. L. (2005). Predicting site quality for shorebird communities: A case study on the Humber estuary, UK. Marine Ecology Progress Series, 305, 203–217. [Google Scholar]
- Stolen, E. D. , Collazo, J. A. , & Percival, H. F. (2012). Group‐foraging effects on capture rate in wading birds. The Condor, 114, 744–754. [Google Scholar]
- Studds, C. E. , Kendall, B. E. , Murray, N. J. , Wilson, H. B. , Rogers, D. I. , Clemens, R. S. , Gosbell, K. , Hassell, C. J. , Jessop, R. , Melville, D. S. , Milton, D. A. , Minton, C. D. T. , Possingham, H. P. , Riegen, A. C. , Straw, P. , Woehler, E. J. , & Fuller, R. A. (2017). Rapid population decline in migratory shorebirds relying on Yellow Sea tidal mudflats as stopover sites. Nature Communications, 8, 14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift, R. J. , Rodewald, A. D. , Johnson, J. A. , Andres, B. A. , & Senner, N. R. (2020). Seasonal survival and reversible state effects in a long‐distance migratory shorebird. Journal of Animal Ecology, 89, 2043–2055. [DOI] [PubMed] [Google Scholar]
- Tang, X. , Li, H. , Xu, X. , Yang, G. , Liu, G. , Li, X. , & Chen, D. (2016). Changing land use and its impact on the habitat suitability for wintering Anseriformes in China's Poyang Lake region. Science of the Total Environment, 557–558, 296–306. [DOI] [PubMed] [Google Scholar]
- Tavernia, B. G. , & Reed, J. M. (2012). The impact of exotic purple loosestrife (Lythrum salicaria) on wetland bird abundances. The American Midland Naturalist, 168, 352–363. [Google Scholar]
- Thibault, J. M. , Sanders, F. J. , & Jodice, P. G. R. (2010). Parental attendance and brood success in American oystercatchers in South Carolina. Waterbirds, 33, 511–517. [Google Scholar]
- Thomas, N. E. , & Swanson, D. L. (2013). Plasma metabolites and creatine kinase levels of shorebirds during fall migration in the prairie pothole region. The Auk, 130, 580–590. [Google Scholar]
- Tombre, I. M. , Tømmervik, H. , & Madsen, J. (2005). Land use changes and goose habitats, assessed by remote sensing techniques, and corresponding goose distribution, in Vesterålen, northern Norway. Agriculture, Ecosystems & Environment, 109, 284–296. [Google Scholar]
- Toral, G. M. , Stillman, R. A. , Santoro, S. , & Figuerola, J. (2012). The importance of rice fields for glossy ibis (Plegadis falcinellus): Management recommendations derived from an individual‐based model. Biological Conservation, 148, 19–27. [Google Scholar]
- Townsend, S. , Pearlstine, E. , Mazzotti, F. , & Deren, C. (2006). Wading birds, shorebirds, and waterfowl in rice fields within the Everglades agricultural area. Florida Field Naturalist, 34, 9–20. [Google Scholar]
- Trinder, M. N. , Hassell, D. , & Votier, S. (2009). Reproductive performance in arctic‐nesting geese is influenced by environmental conditions during the wintering, breeding and migration seasons. Oikos, 118, 1093–1101. [Google Scholar]
- Valdez‐Juarez, S. O. , Krebs, E. A. , Drake, A. E. , & Green, D. J. (2019). Assessing the effect of seasonal agriculture on the condition and winter survival of a migratory songbird in Mexico. Conservation Science and Practice, 1, e19. [Google Scholar]
- van de Pol, M. , Brouwer, L. , Ens, B. J. , Oosterbeek, K. , & Tinbergen, J. M. (2010). Fluctuating selection and the maintenance of individual and sex‐specific diet specialization in free‐living oystercatchers. Evolution, 64, 836–851. [DOI] [PubMed] [Google Scholar]
- van de Pol, M. , Ens, B. J. , Heg, D. , Brouwer, L. , Krol, J. , Maier, M. , Exo, K. M. , Oosterbeek, K. , Lok, T. , Eising, C. M. , & Koffijberg, K. (2010). Do changes in the frequency, magnitude and timing of extreme climatic events threaten the population viability of coastal birds? Journal of Applied Ecology, 47, 720–730. [Google Scholar]
- van der Kolk, H. J. , Ens, B. J. , Oosterbeek, K. , Bouten, W. , Allen, A. M. , Frauendorf, M. , Lameris, T. K. , Oosterbeek, T. , Deuzeman, S. , De Vries, K. , Jongejans, E. , & van de Pol, M. (2019). Shorebird feeding specialists differ in how environmental conditions alter their foraging time. Behavioral Ecology, 31, 371–382. [Google Scholar]
- Van Gils, J. A. , Dekinga, A. , Spaans, B. , Vahl, W. K. , & Piersma, T. (2005). Digestive bottleneck affects foraging decisions in red knots Calidris canutus. II. Patch choice and length of working day. Journal of Animal Ecology, 74, 120–130. [Google Scholar]
- Van Horne, B. (1983). Density as a misleading indicator of habitat quality. The Journal of Wildlife Management, 47, 893–901. [Google Scholar]
- Walsh, K. A. , Halliwell, D. R. , Hines, J. E. , Fournier, M. A. , Czarnecki, A. , & Dahl, M. F. (2006). Effects of water quality on habitat use by lesser scaup (Aythya affinis) broods in the boreal Northwest Territories, Canada. Hydrobiologia, 567, 101–111. [Google Scholar]
- Wang, T. , & So, S. (2003). Waterbirds spending the boreal summer at Dalai Nur National Nature Reserve, Inner Mongolia, China. Bulletin of the Oriental Bird Club, 38, 58–61. [Google Scholar]
- Wang, X. , Comita, L. S. , Hao, Z. , Davies, S. J. , Ye, J. , Lin, F. , & Yuan, Z. (2012). Local‐scale drivers of tree survival in a temperate forest. PLoS One, 7, e29469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock, N. , Haig, S. M. , & Oring, L. W. (1998). Monitoring species richness and abundance of shorebirds in the western Great Basin. Condor, 100, 589–600. [Google Scholar]
- Weegman, M. D. , Alisauskas, R. T. , Kellett, D. K. , Zhao, Q. , Wilson, S. , & Telenský, T. (2022). Local population collapse of Ross's and lesser snow geese driven by failing recruitment and diminished philopatry. Oikos, 2022, 1–13. [Google Scholar]
- Weiser, E. L. , Brown, S. C. , Lanctot, R. B. , Gates, H. R. , Abraham, K. F. , Bentzen, R. L. , Bêty, J. , Boldenow, M. L. , Brook, R. W. , Donnelly, T. F. , English, W. B. , Flemming, S. A. , Franks, S. E. , Gilchrist, H. G. , Giroux, M.‐A. , Johnson, A. , Kendall, S. , Kennedy, L. V. , Koloski, L. , … Sandercock, B. K. (2018a). Effects of environmental conditions on reproductive effort and nest success of Arctic‐breeding shorebirds. Ibis, 160, 608–623. [Google Scholar]
- Weiser, E. L. , Brown, S. C. , Lanctot, R. B. , Gates, H. R. , Abraham, K. F. , Bentzen, R. L. , Bêty, J. , Boldenow, M. L. , Brook, R. W. , Donnelly, T. F. , English, W. B. , Flemming, S. A. , Franks, S. E. , Gilchrist, H. G. , Giroux, M. A. , Johnson, A. , Kennedy, L. V. , Koloski, L. , Kwon, E. , … Sandercock, B. K. (2018b). Life‐history tradeoffs revealed by seasonal declines in reproductive traits of Arctic‐breeding shorebirds. Journal of Avian Biology, 49, jav.01531. [Google Scholar]
- Wen, L. , Saintilan, N. , Reid, J. R. W. , & Colloff, M. J. (2016). Changes in distribution of waterbirds following prolonged drought reflect habitat availability in coastal and inland regions. Ecology and Evolution, 6, 6672–6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, A. D. , Goss‐Custard, J. D. , Dit Durell, S. E. L. V. , & Stillman, R. A. (2005). Maintaining estuary quality for shorebirds: Towards simple guidelines. Biological Conservation, 123, 211–224. [Google Scholar]
- Wetlands International . (2012). Waterbird population estimates, fifth edition. Summary report. Wetlands International. [Google Scholar]
- Wiersma, P. , & Piersma, T. (1995). Scoring abdominal profiles to characterize migratory cohorts of shorebirds: An example with red knots. Journal of Field Ornithology, 66, 88–98. [Google Scholar]
- Williams, B. R. , Benson, T. J. , Yetter, A. P. , Lancaster, J. D. , & Hagy, H. M. (2019). Stopover duration of spring migrating dabbling ducks in the Wabash River valley. Wildlife Society Bulletin, 43, 590–598. [Google Scholar]
- Xu, F. , & Si, Y. (2019). The frost wave hypothesis: How the environment drives autumn departure of migratory waterfowl. Ecological Indicators, 101, 1018–1025. [Google Scholar]
- Xu, Y. , Si, Y. , Yin, S. , Zhang, W. , Grishchenko, M. , Prins, H. H. T. , Gong, P. , & De Boer, W. F. (2019). Species‐dependent effects of habitat degradation in relation to seasonal distribution of migratory waterfowl in the East Asian–Australasian Flyway. Landscape Ecology, 34, 243–257. [Google Scholar]
- Yu, C. , Zhou, L. , Mahtab, N. , Fan, S. , & Song, Y. (2020). Microhabitat variables explain patch switching by wintering Bewick's swans through giving‐up net energy intake rates. Environmental Science and Pollution Research, 27, 18843–18852. [DOI] [PubMed] [Google Scholar]
- Zanette, L. (2001). Indicators of habitat quality and the reproductive output of a forest songbird in small and large fragments. Journal of Avian Biology, 32, 38–46. [Google Scholar]
- Zhang, W. , Li, X. , Yu, L. , & Si, Y. (2018). Multi‐scale habitat selection by two declining east Asian waterfowl species at their core spring stopover area. Ecological Indicators, 87, 127–135. [Google Scholar]
- Zhang, Y. , Wang, Z. , Ren, C. , Yu, H. , Dong, Z. , Lu, C. , & Mao, D. (2017). Changes in habitat suitability for waterbirds of the Momoge nature Reserve of China during 1990–2014. Journal of Environmental Engineering and Landscape Management, 25, 367–378. [Google Scholar]
- Zhao, D. , Borders, B. , Wilson, M. , & Rathbun, S. L. (2006). Modeling neighborhood effects on the growth and survival of individual trees in a natural temperate species‐rich forest. Ecological Modelling, 196, 90–102. [Google Scholar]
- Zharikov, Y. , Elner, R. W. , Shepherd, P. C. F. , & Lank, D. B. (2009). Interplay between physical and predator landscapes affects transferability of shorebird distribution models. Landscape Ecology, 24, 129–144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Data are available in supplementary material.


