PURPOSE
To report the efficacy and safety of postoperative adjuvant hepatic arterial infusion chemotherapy (HAIC) with 5-fluorouracil and oxaliplatin (FOLFOX) in hepatocellular carcinoma (HCC) patients with microvascular invasion (MVI).
PATIENTS AND METHODS
In this randomized, open-label, multicenter trial, histologically confirmed HCC patients with MVI were randomly assigned (1:1) to receive adjuvant FOLFOX-HAIC (treatment group) or routine follow-up (control group). The primary end point was disease-free survival (DFS) by intention-to-treat (ITT) analysis while secondary end points were overall survival, recurrence rate, and safety.
RESULTS
Between June 2016 and August 2021, a total of 315 patients (ITT population) at five centers were randomly assigned to the treatment group (n = 157) or the control group (n = 158). In the ITT population, the median DFS was 20.3 months (95% CI, 10.4 to 30.3) in the treatment group versus 10.0 months (95% CI, 6.8 to 13.2) in the control group (hazard ratio, 0.59; 95% CI, 0.43 to 0.81; P = .001). The overall survival rates at 1 year, 2 years, and 3 years were 93.8% (95% CI, 89.8 to 98.1), 86.4% (95% CI, 80.0 to 93.2), and 80.4% (95% CI, 71.9 to 89.9) for the treatment group and 92.0% (95% CI, 87.6 to 96.7), 86.0% (95% CI, 79.9 to 92.6), and 74.9% (95% CI, 65.5 to 85.7) for the control group (hazard ratio, 0.64; 95% CI, 0.36 to 1.14; P = .130), respectively. The recurrence rates were 40.1% (63/157) in the treatment group and 55.7% (88/158) in the control group. Majority of the adverse events were grade 0-1 (83.8%), with no treatment-related death in both groups.
CONCLUSION
Postoperative adjuvant HAIC with FOLFOX significantly improved the DFS benefits with acceptable toxicities in HCC patients with MVI.
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for 90% cases of primary liver cancers, of which 70% of patients are ineligible for curative treatments.1,2 At present, surgical resection remains as the mainstay of curative treatment option.3 However, the recurrence rate after surgical resection in patients with HCC could be 70%-80%.2 The incidence of microvascular invasion (MVI) in HCC is about 30%-50%, and the expected 1- and 2-year disease-free survival (DFS) of patients with MVI positive is about 50%-60% and 30%-40%, respectively.4-6 Besides, multiple retrospective studies substantiated MVI as a key risk factor in the early recurrence of HCC after surgical resection and a better predictor for DFS and overall survival (OS).6-8 Despite the availability of various adjuvant therapies to reduce recurrence and prolong OS, there is no global consensus on the recommendation of adjuvant therapies for HCC after surgical resection. Moreover, the overall outcomes of these interventions are variable, and rendering the improvement of prognosis for these patients is a major challenge.9
CONTEXT
Key Objective
To our knowledge, no standard treatment has been proposed as the adjuvant therapy for the hepatocellular carcinoma (HCC) patients with microvascular invasion, and our study is the first phase III trial to evaluate the value of hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin (hepatic arterial infusion chemotherapy with 5-fluorouracil and oxaliplatin) as the adjuvant therapy in this population.
Knowledge Generated
Hepatic arterial infusion chemotherapy with 5-fluorouracil and oxaliplatin significantly improved the disease-free survival (20.3 v 10.0 months, P = .001) compared with routine follow-up in HCC patients with microvascular invasion. There was no significant difference in the incidence of operation-related adverse events between the two groups (P = .597).
Relevance (E.M. O'Reilly)
-
These data are intriguing and provide ongoing support for the continued investigation of hepatic artery infusional therapy in patients with HCC.*
*Relevance section written by JCO Associate Editor Eileen M. O'Reilly, MD.
Although several studies substantiated that hepatic arterial infusion chemotherapy (HAIC) has a higher response rate than systemic chemotherapy with longer OS and tolerable toxicity in patients with advanced HCC, only the Japanese guidelines recommend HAIC as a treatment option for advanced HCC.1 In addition, studies comparing HAIC with 5-fluorouracil and oxaliplatin (FOLFOX) regimen either alone or in combination with sorafenib evidenced an improvement in the prognosis in patients with intermediate and advanced HCC.10-12 Although there was no direct comparison between HAIC and the current standard first-line treatment, such as combination of atezolizumab and bevacizumab, given that the overall response rate of atezolizumab and bevacizumab in IMbrave 150 study was only 27.3%,13 previous studies suggested that the response rate of HAIC in advanced HCC was significantly better. Although it was not possible to directly compare the results of different studies, these data still demonstrated the potential efficacy of HAIC. Recently, we reported our preliminary findings of phase III, randomized controlled trial where adjuvant HAIC after hepatectomy may be associated with survival benefits in HCC patients with MVI.14 In this study, we report the updated efficacy and safety data with an extended follow-up.
PATIENTS AND METHODS
Study Design and Participants
Details on study design, inclusion criteria, and exclusion criteria were described previously in our preliminary report.14 Briefly, a phase III, multicenter, prospective, open-label, randomized controlled clinical trial was conducted in China at the following five centers: Sun Yat-sen University Cancer Center (SYSUCC), Guangzhou, China; the First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China; the First People's Hospital of Foshan, Foshan, China; Zhujiang Hospital of Southern Medical University, Guangzhou, China; and the First Affiliated Hospital of Jinan University, Guangzhou, China. The following details are considered for inclusion: patients age 18 years and older to younger than and 75 years with histologically confirmed HCC with MVI; treatment-naive; Eastern Cooperative Oncology Group performance score of ≤ 2; absence of macrovascular invasion, distant metastasis, and intrahepatic or extrahepatic recurrence at radiological follow-up (4-6 weeks after surgery); and adequate hematologic, hepatic, and renal functions (details are in the study Protocol [online only] and the Data Supplement [online only]). Furthermore, patients with histologically proven positive resection margin (R1 resection); severe functional impairment of organs (heart, brain, lung, kidney, and liver); allergy to related drugs or intolerance to HAIC; previous or concomitant antitumor therapy; and a history of organ transplantation, neurologic, or psychiatric diseases, human immunodeficiency virus infection, esophageal or gastric variceal bleeding, hepatic encephalopathy, or cardio-cerebrovascular events within 30 days of random assignment were excluded.
The implementation of this clinical study complies with all local laws and regulations and is implemented in accordance with the ethical principles of the Declaration of Helsinki. Before the study, all patients provided their written informed consent. The study protocol was approved by the Institutional Review Board and Institutional Ethics Committee of SYSUCC (Institutional Review Board Approval No.: B2017-006-01). The study has been registered at ClinicalTrials.gov (identifier: NCT03192618). Furthermore, this study was reported as per the Consolidated Standards of Reporting Trials reporting guidelines.
Trial Design and Treatment
Surgical resection procedures were described in previously reported studies.15,16 All resection margins were negative. All patients had at least seven paraffin-embedded tissue blocks, with a mean of 7.2 (median, 8; range, 7-10) blocks per tumor available for pathologic examination. Slides were re-examined to solve the discrepancy with a double-headed microscope, and a consensus was reached. The presence of MVI was defined as a tumor within a vascular space lined by the endothelium that was visible only via microscopy.4,5 After surgery (4-6 weeks), all patients were randomly assigned to receive either one to two cycles of adjuvant HAIC (treatment group) or routine follow up without any adjuvant treatment (control group) in a 1:1 ratio by using a simple random assignment method. Random assignment was performed using a computer-generated random assignment sequence at the Clinical Trial Center of SYSUCC. Details of the random allocations were provided in sequentially numbered, opaque, sealed envelopes prepared by a statistician (Li Jibin), who participated in the statistical analysis and data review. The random assignment and allocation concealment were conducted according to practical guidance.17 HAIC procedure was performed as per previously reported studies.11,12,14,18 After successful percutaneous femoral artery puncture and catheterization, superior mesenteric arteriography and hepatic arteriography were performed. After confirming that the patients met the inclusion criteria according to the results of arteriography, the hepatic artery was intubated to the predetermined position, and patients with indwelling catheter were shifted to the ward. Any implanted port system was not applied. The catheter was connected to the injection pump in the ward, and the following chemotherapeutic agents were continuously pumped: oxaliplatin, 85 mg/m2 from 0 to 3 hours once on day 1; leucovorin, 400 mg/m2 from 3 to 4.5 hours once on day 1; fluorouracil, 400 mg/m2 from 4.5 to 6.5 hours once on day 1; and fluorouracil, 2,400 mg/m2 once over 46 hours from days 1 to 3. The patient was bedridden during chemotherapy. When chemotherapy ended, the catheter was pulled out, and the patient was discharged after complete hemostasis at the puncture site. The time interval between two cycles of HAIC was set at 4-5 weeks. In the control group, patients with recurrence confirmed by imaging have received hepatic arteriography and subsequent transarterial chemoembolization (TACE).
End Points and Follow-Up
The primary end point was DFS, defined as interval between random assignment and first documented diagnosis of HCC recurrence or death due to all causes depending on which occurred first. The secondary end point was OS, defined as the duration from the date of random assignment to the death due to all causes. Patients who had not experienced recurrence or death at the time of data analysis were censored as alive and event-free at the date of last follow-up. Recurrence rate (on the basis of angiographic or/and radiologic findings) and safety assessment included continuous assessment of adverse events (AEs) throughout the trial and were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.19 More specifically, AEs were evaluated twice a day during hospitalization. During the home-stay period, patients can contact the investigators over phone if they have serious AEs. Other AEs were documented at the time of scheduled review. All patients were followed up at an interval of 2-3 months per our previous studies.16,20 At each follow-up visit, physical examination, blood test (serum levels of alpha-fetoprotein and liver function), and enhanced abdominal computed tomography or magnetic resonance imaging scans were performed. Once suspicious recurrence/metastasis was detected, further examinations including hepatic arteriography or biopsy were conducted. Recurrence/metastasis was confirmed based on the cytologic/histologic evidence or the noninvasive diagnostic criteria for HCC by the European Association for the Study of Liver. Patients with recurrence in both the groups received subsequent treatment according to the decision of the multidisciplinary team of each center.
Statistical Analysis
The sample size estimation was based on assumptions that a median DFS of the control group was 12.0 months, and adjuvant HAIC could improve the median DFS of treatment group to 18.0 months. To detect this difference with a power of 90% and a two-sided α of .05, we estimated that the required number of events would be observed if 131 patients were enrolled in each group with an enrollment period of 24 months and a follow-up period of 24 months.
Clinical and pathologic differences in the distribution of baseline characteristics between the treatment and control groups were compared using the Pearson's χ2 test or Fisher's exact test (categorical variable). For normally distributed and non-normally distributed values, the variable distributions were described using the mean ± standard deviation and median and range, respectively. Depending on data normality, Student's t test or Mann-Whitney test was used to assess the difference in continuous variables between the two groups.
The efficacy analyses were performed in the intention-to-treat (ITT) population, which included all randomly assigned patients, and in the per-protocol (PP) population, which included patients who completed two cycles of adjuvant HAIC. Safety analyses about AEs associated with HAIC were conducted among those who received at least one dose of the trial regimen. The cumulative survival probabilities were estimated using the Kaplan-Meier curve method, and the group differences were compared using log-rank tests in the ITT population and in the PP population. We calculated hazard ratios (HRs) using the Cox proportional hazards model. The proportional hazards assumption was confirmed based on Schoenfeld residuals.21 The exploratory subgroup analyses were conducted according to the prognostic factors including age, tumor number, tumor diameter, tumor distribution, Milan criteria, alpha-fetoprotein, HBV-DNA, cirrhosis, and Edmondson-Steiner grade. The treatment effects in each subgroup were evaluated using an unadjusted Cox proportional hazards model. The interaction effect was evaluated by adding interaction terms to Cox proportional hazards models.
All the analyses were performed using the SPSS software, version 24.0 (SPSS Inc, Chicago, IL). A two-tailed P < .05 was considered statistically significant.
RESULTS
Patient Characteristics and Treatment Administration
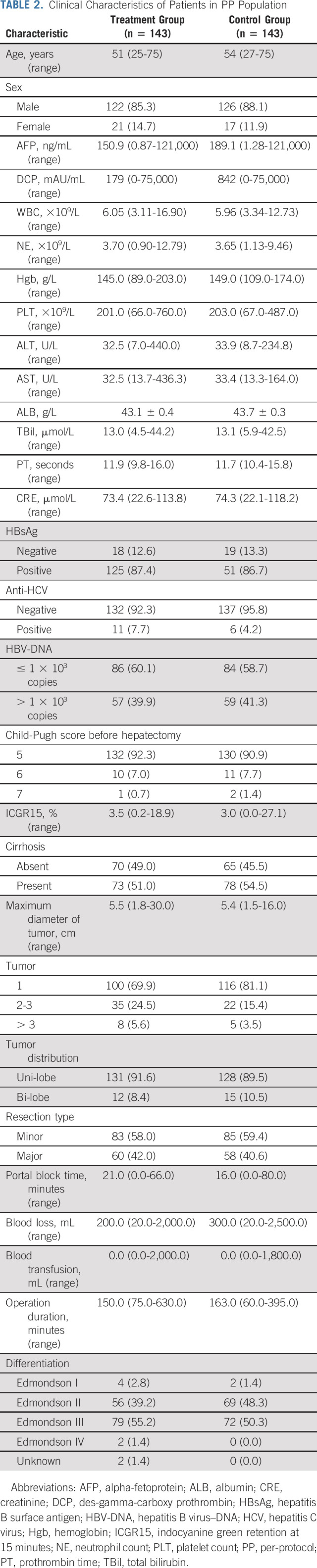
Between June 2016 and August 2021, a total of 351 patients were screened and 315 patients were randomly assigned to receive adjuvant FOLFOX-HAIC (treatment group, n = 157) or to follow up without any adjuvant treatment (control group, n = 158) and were included in the ITT population. Among them, 14 patients from the treatment group and 15 patients from the control group were excluded from the PP population. The reasons for the exclusion and the patient disposition process are summarized in Figure 1. Overall, 148 patients in treatment group underwent at least one cycle of HAIC, and these patients were included in safety analyses. The baseline demographics and the clinical characteristics were comparable between the two groups (Tables 1 and 2).
FIG 1.
CONSORT diagram. AHGUCM, The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China; FAHJNU, The First Affiliated Hospital of Jinan University, Guangzhou, China; FPHF, The First People's Hospital of Foshan, Foshan, China; HAIC, hepatic arterial infusion chemotherapy; ITT, intention-to-treat; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand-1; PP, per-protocol; SYSUCC, Sun Yat-sen University Cancer Center, Guangzhou, China; ZJHSMU, Zhujiang Hospital of Southern Medical University, Guangzhou, China.
TABLE 1.
Clinical Characteristics of Patients in ITT Population
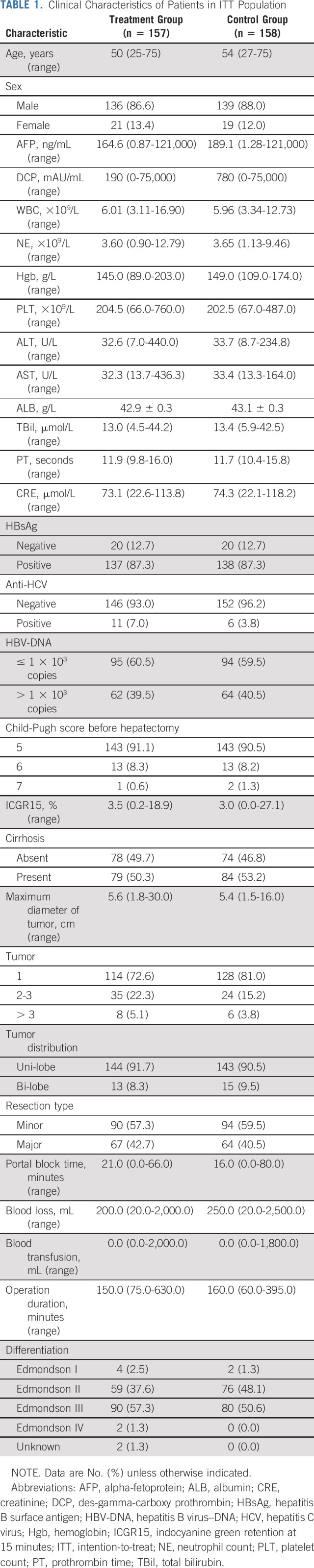
TABLE 2.
Clinical Characteristics of Patients in PP Population
Finally, there were 24 patients (15.3%) who received only one cycle of HAIC in the ITT population. In the PP population, 124 patients (86.7%) completed the planned two cycles of HAIC and 18 patients (12.6%) received only one cycle of HAIC. Among these 18 patients, 14 patients (9.8%) refused to accept the second cycle of HAIC due to their personal reasons and four patients (2.8%) were diverted to accept TACE since intrahepatic recurrence was found during the hepatic arteriography of the second cycle of HAIC. In addition, one patient (0.7%) did not undergo HAIC as planned but was diverted to accept TACE since intrahepatic recurrence was found during the hepatic arteriography of the first cycle of HAIC.
Those patients who were diagnosed with recurrent HCC through hepatic arteriography were included in survival analysis, as hepatic arteriography was not given to the patients in the control group. Moreover, as tumor recurrence was confirmed in the aforementioned five patients, they were treated with TACE with epirubicin, lobaplatin, and lipiodol, instead of HAIC.
Efficacy Analysis
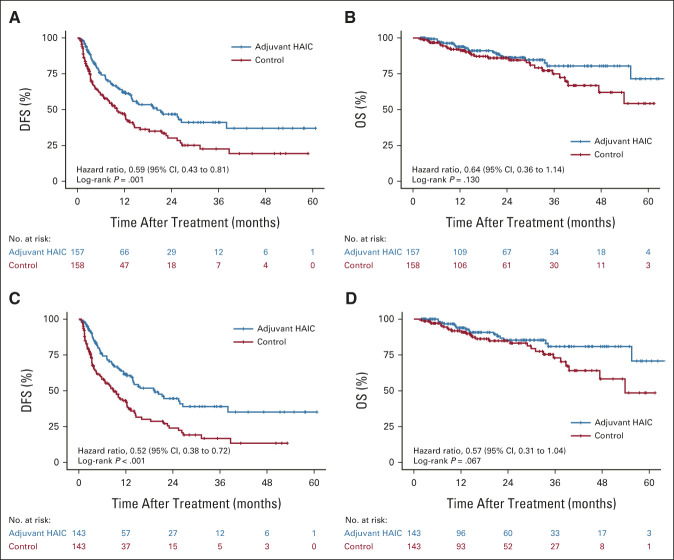
The study was censored on September 30, 2021. The median follow-up period was 23.7 months (95% CI, 21.0 to 26.5) for the treatment group and 21.5 months (95% CI, 17.6 to 25.4) for the control group in the ITT population, whereas in the PP population, it was 23.2 months (95% CI, 17.9 to 28.5) and 20.5 months (95% CI, 15.4 to 25.5) for the treatment and the control groups, respectively. At the time of last follow-up, there were 151 recurrences (63 in the treatment group v 88 in the control group) and 47 deaths (19 v 28) in the ITT population and 144 recurrences (58 v 86) and 44 deaths (17 v 27) in the PP population. Only five patients had no observable recurrence before death. The median DFS of the treatment and control groups was 20.3 months (95% CI, 10.4 to 30.3) and 10.0 months (95% CI, 6.8 to 13.2), respectively, in the ITT population (HR, 0.59; 95% CI, 0.43 to 0.81; P = .001; Fig 2A), whereas it was 19.3 months (95% CI, 12.2 to 26.4) and 8.9 months (95% CI, 5.9 to 11.8), respectively, in the PP population (HR, 0.52; 95% CI, 0.38 to 0.72; P < .001; Fig 2C). The DFS rates at 1 year, 2 years, and 3 years were 62.2% (95% CI, 54.2 to 71.3), 46.8% (95% CI, 38.0 to 57.6), and 41.1% (31.8 to 53.0) in the treatment group and 47.2% (95% CI, 39.2 to 56.7), 30.1% (95% CI, 22.1 to 41.0), and 22.6% (95% CI, 14.8 to 34.5) in the control group, respectively, in the ITT population; whereas the rates were 61.6% (95% CI, 53.2 to 71.4), 44.6% (95% CI, 35.4 to 56.2), and 39.0% (95% CI, 29.6 to 51.5) in the treatment group and 43.2% (95% CI, 35.0 to 53.4), 24.0% (95% CI, 16.3 to 35.2), and 16.8% (95% CI, 9.9 to 28.5) in the control group, respectively, in the PP population. During the same follow-up period, in the ITT population, the OS rates at 1 year, 2 years, and 3 years were 93.8% (95% CI, 89.8 to 98.1), 86.4% (95% CI, 80.0 to 93.2), and 80.4% (95% CI, 71.9 to 89.9), respectively, for the treatment group and 92.0% (95% CI, 87.6 to 96.7), 86.0% (95% CI, 79.9 to 92.6), and 74.9% (95% CI, 65.5 to 85.7), respectively, for the control group. The results showed that no significant difference in OS in both the ITT population and the PP population. The estimated HR was 0.64 (95% CI, 0.36 to 1.14; P = .130; Fig 2B). In the PP population, the OS rates at 1 year, 2 years, and 3 years were 93.9% (95% CI, 89.6 to 98.4), 85.3% (95% CI, 78.4 to 93.0), and 80.9% (95% CI, 72.3 to 90.6), respectively, for the treatment group and 91.8% (95% CI, 87.1 to 96.8), 84.9% (95% CI, 78.1 to 92.2), and 72.9% (95% CI, 62.8 to 84.6), respectively, for the control group. The estimated HR was 0.57 (95% CI, 0.31 to 1.04; P = .067; Fig 2D).
FIG 2.
Kaplan-Meier curves demonstrating (A) DFS in the ITT population, (B) OS in the ITT population, (C) DFS in the PP population, and (D) OS in the PP population. DFS, disease-free survival; HAIC, hepatic arterial infusion chemotherapy; ITT, intention-to-treat; OS, overall survival; PP, per-protocol.
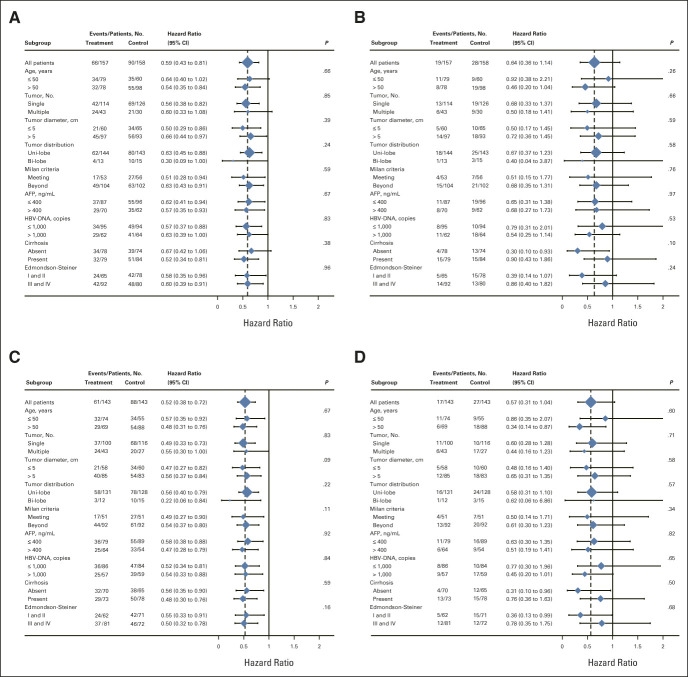
The Cox proportional hazards model was examined as applicable (on the basis of Schoenfeld residuals, P = .19 for DFS and P = .54 for OS analyses in the ITT population; P = .23 for DFS and P = .45 for OS analyses in the PP population). The results of subgroup analyses were consistent with those in the whole enrolled patients. This indicated that almost all patients could have better DFS benefits from adjuvant HAIC in the ITT population (P < .001; Fig 3A) and in the PP population (P < .001; Fig 3C). Those patients without liver cirrhosis could benefit from adjuvant HAIC in terms of OS both in the ITT population (P = .038; Fig 3B) and in the PP population (P = .043; Fig 3D).
FIG 3.
Forest plots by subgroup: (A) DFS in the ITT population, (B) OS in the ITT population, (C) DFS in the PP population, and (D) OS in the PP population. Unadjusted Cox model was used to estimate HRs with 95% CIs and to test for interactions among subgroups using two-sided P values. AFP, alpha-fetoprotein; DFS, disease-free survival; HBV-DNA, hepatitis B virus–DNA; HR, hazard ratio; ITT, intention-to-treat; OS, overall survival; PP, per-protocol.
Among the patients who had recurrence, 48 (76.2%) patients in the treatment group and 59 (67.0%) patients in the control group underwent subsequent antitumor therapies (Data Supplement). The patterns of recurrence were similar between the two groups (Data Supplement).
Safety Analysis
The overall incidence of operation-related AEs is summarized in the Data Supplement. There was no significant difference in the incidence of operation-related AEs between the two groups (P = .597). Among these, majority are grade 1 AEs (treatment group, n = 110 [70.1%]; control group, n = 117 [74.1%]). Grade 3 AEs include elevated alanine transaminase level, anemia, hyperbilirubinemia, and thrombocytopenia. Furthermore, grade 4 severe infection was observed in one patient in the control group. Moreover, no patient died of AEs during hospitalization.
Overall, AEs associated with HAIC are presented in the Data Supplement. Majority of the AEs of patients, who received adjuvant HAIC treatment, were grade 0-1 (n = 124 [83.8%]). There were no incidences of death due to HAIC or surgery.
DISCUSSION
Intrahepatic recurrence of HCC after hepatectomy is more frequent due to intrahepatic dissemination or micrometastases of primary cancer cells,22 and MVI is recognized as a risk factor for recurrence.16,23 Considering the high risk of recurrence, local adjuvant therapy may offer better survival benefits than systemic adjuvant therapy in patients with HCC recurrence. Although adjuvant TACE has shown survival benefits in HCC patients with MVI after curative resection, complications caused by embolization limited its applicability.16,24 Moreover, there is no universally accepted adjuvant therapy for HCC patients with MVI. At this juncture, the results from our current study substantiated that adjuvant HAIC with FOLFOX provided acceptable survival benefits. In addition, our study suggests that FOLFOX-HAIC has acceptable safety profiles and was well-tolerated.
The results of EACH study confirmed the value of systemic chemotherapy with FOLFOX regimen in the treatment of advanced HCC.25 Recently, several retrospective studies and a few randomized trials substantiated the survival benefits of FOLFOX-HAIC either alone or in combination with sorafenib in patients with advanced HCC with and without MVI.10,11 Earlier, Lyu et al10 conducted a retrospective study involving the comparison of survival outcomes in patients with advanced HCC undergoing FOLFOX-HAIC with and without sorafenib and reported that FOLFOX-HAIC improved survival benefits when compared with sorafenib in a large number of patients. Furthermore, in this study, a trend of superior OS seemed to be demonstrated in the treatment group compared with that in the control group. However, the 1-, 2-, and 3-year rates of OS and the overall regression comparisons showed no significant difference. We believe that a longer follow-up period might reveal the benefits of adjuvant HAIC in terms of OS.
In this study, most patients benefit from DFS in the early 2 years. The main reason for the early recurrence of HCC after surgery was the existence of small metastases in residual liver, which is the high-risk outcome of MVI in HCC.26,27 The continuous infusion of FOLFOX drugs has the potential to eliminate the micrometastasis in liver parenchyma and blood circulation. Therefore, HAIC mainly reduces early recurrence, which is in accordance with the treatment principle and investigators' expectations.
The role of chemotherapy drugs in locoregional therapy is undoubtedly important. A study has shown that the chemotherapeutic drugs, rather than embolization, played a dominant role in TACE treatment.28 The continuous infusion of chemotherapeutic drugs can ensure the adequate local drug concentration in the liver so that the efficacy was not inferior to that of TACE. At the same time, it can avoid the complications due to embolization and reduce the damage of liver function. Besides, we performed arterial catheterization in every cycle rather than using an implanted port system to avoid port-related complications such as local infection, thrombosis, and toxicities caused due to leakage of chemotherapeutic drugs.29
Although this study is a complete multicenter, prospective, randomized controlled study, our study has certain limitations. First, MVI scale was not used as a randomized stratification factor in the initial design of the study, and most centers have begun to grade MVI (M1 or M2) in the last 1 to 2 years. Therefore, it was not possible to evaluate the MVI scale for the early enrolled cases. As a result, MVI that affects the prognosis was not included in the analysis of this study. However, we will add this factor to subsequent clinical and basic research to design and analyze research data. Second, although the incidence of grade 3 or higher AEs was quite low, the proportion of patients who refused to complete two cycles of HAIC because of various reasons was relatively high (9.8%, 14/143), suggesting that adjuvant HAIC still had a certain impact on the quality of life of patients. Third, since all patients enrolled in this study are Chinese, the value of adjuvant HAIC in HCC patients with different ethnic groups and hepatitis backgrounds needs to be further studied. Finally, the current HAIC plan requires patients to stay in bed (> 50 hours), which will indeed affect the patient's treatment compliance and further necessitates the optimization of the trial protocol or chemotherapy regimen.
In conclusion, this study evidenced that postoperative adjuvant HAIC with FOLFOX significantly improved the DFS benefits with acceptable toxicities in HCC patients with MVI.
PRIOR PRESENTATION
Presented in part at the Gastrointestinal Cancer session of the 2022 ASCO annual meeting, Chicago, IL, June 5, 2022.
SUPPORT
Supported by the National Natural Science Foundation of China (No. 81871985), Natural Science Foundation of Guangdong Province (No. 2018A0303130098 and No. 2017A030310203), Science and Technology Planning Project of Guangdong Province (No. 2017A020215112), Medical Scientific Research Foundation of Guangdong Province (No. A2017477), Science and Technology Planning Project of Guangzhou (No. 201903010017 and No. 201904010479), Clinical Trials Project (5010 Project) of Sun Yat-sen University (No. 5010-2017009), and Clinical Trials Project (308 Project) of Sun Yat-sen University Cancer Center (No. 308-2015-014).
CLINICAL TRIAL INFORMATION
S.-H.L., J.M., Y.C., and Q.L. contributed equally to this work. H.-W.C., C.Z., W.W., and R.-P.G. are co-senior authors and contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Shao-Hua Li, Ji-Bin Li, Lie Zheng, Huan-Wei Chen, Chong Zhong, Wei Wei
Financial support: Shao-Hua Li, Huan-Wei Chen, Wei Wei
Administrative support: Jing-Wen Zou, Yu-Hua Wen, Lie Zheng, Huan-Wei Chen, Wei Wei
Provision of study materials or patients: Yuan Cheng, Qiao-Xuan Wang, Chong-Kai Fang, Qiu-Cheng Lei, Ming-Rong Cao, Jing-Duo Deng, Yu-Chuan Jiang, Rong-Ce Zhao, Huan-Wei Chen, Wei Wei
Collection and assembly of data: Shao-Hua Li, Jie Mei, Yuan Cheng, Qiang Li, Qiao-Xuan Wang, Chong-Kai Fang, Qiu-Cheng Lei, Hua-Kun Huang, Ming-Rong Cao, Rui Luo, Jing-Duo Deng, Yu-Chuan Jiang, Rong-Ce Zhao, Jing-Wen Zou, Min Deng, Ren-Guo Guan, Yu-Hua Wen, Zhi-Xing Guo, Yi-Hong Ling, Huan-Wei Chen, Chong Zhong, Wei Wei
Data analysis and interpretation: Shao-Hua Li, Jie Mei, Ming-Rong Cao, Yu-Chuan Jiang, Rong-Ce Zhao, Liang-He Lu, Wen-Ping Lin, Ji-Bin Li, Huan-Wei Chen, Chong Zhong, Wei Wei, Rong-Ping Guo
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Postoperative Adjuvant Hepatic Arterial Infusion Chemotherapy With FOLFOX in Hepatocellular Carcinoma With Microvascular Invasion: A Multicenter, Phase III, Randomized Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Hsu SJ, Xu X, Chen MP, et al. : Hepatic arterial infusion chemotherapy with modified FOLFOX as an alternative treatment option in advanced hepatocellular carcinoma patients with failed or unsuitability for transarterial chemoembolization. Acad Radiol 28:S157-S166, 2021. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 2.Saito A, Toyoda H, Kobayashi M, et al. : Prediction of early recurrence of hepatocellular carcinoma after resection using digital pathology images assessed by machine learning. Mod Pathol 34:417-425, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Govalan R, Lauzon M, Luu M, et al. : Comparison of surgical resection and systemic treatment for hepatocellular carcinoma with vascular invasion: National cancer database analysis. Liver Cancer 10:407-418, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roayaie S, Blume IN, Thung SN, et al. : A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 137:850-855, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodríguez-Perálvarez M, Luong TV, Andreana L, et al. : A systematic review of microvascular invasion in hepatocellular carcinoma: Diagnostic and prognostic variability. Ann Surg Oncol 20:325-339, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Lim KC, Chow PK, Allen JC, et al. : Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg 254:108-113, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Llovet JM, Miceli R, et al. : Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol 10:35-43, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Guo Y, Zhong J, et al. : The clinical significance of microvascular invasion in the surgical planning and postoperative sequential treatment in hepatocellular carcinoma. Sci Rep 11:2415, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Wang Y, Guo X, et al. : Comparative effectiveness of adjuvant treatment for resected hepatocellular carcinoma: A systematic review and network meta-analysis. Front Oncol 11:709278, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyu N, Kong Y, Mu L, et al. : Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol 69:60-69, 2018 [DOI] [PubMed] [Google Scholar]
- 11.He M, Li Q, Zou R, et al. : Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial. JAMA Oncol 5:953-960, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He MK, Le Y, Li QJ, et al. : Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: A prospective non-randomized study. Chin J Cancer 36:83, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finn RS, Qin S, Ikeda M, et al. : Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382:1894-1905, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Li S, Mei J, Wang Q, et al. : Postoperative adjuvant transarterial infusion chemotherapy with FOLFOX could improve outcomes of hepatocellular carcinoma patients with microvascular invasion: A preliminary report of a phase III, randomized controlled clinical trial. Ann Surg Oncol 27:5183-5190, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Shi M, Guo RP, Lin XJ, et al. : Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: A prospective randomized trial. Ann Surg 245:36-43, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei W, Jian PE, Li SH, et al. : Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: A randomized clinical trial of efficacy and safety. Cancer Commun (Lond) 38:61, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doig GS, Simpson F: Randomization and allocation concealment: A practical guide for researchers. J Crit Care 20:187-191, 2005; discussion 191-193 [DOI] [PubMed] [Google Scholar]
- 18.Li S, Mei J, Wang Q, et al. : Transarterial infusion chemotherapy with FOLFOX for advanced hepatocellular carcinoma: A multi-center propensity score matched analysis of real-world practice. Hepatobiliary Surg Nutr 10:631-645, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou WP, Lai EC, Li AJ, et al. : A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg 249:195-202, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Shaohua L, Qiaoxuan W, Peng S, et al. : Surgical strategy for hepatocellular carcinoma patients with portal/hepatic vein tumor thrombosis. PLoS One 10:e0130021, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wileyto EP, Li Y, Chen J, et al. : Assessing the fit of parametric cure models. Biostatistics 14:340-350, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, He XD, Yao N, et al. : A meta-analysis of adjuvant therapy after potentially curative treatment for hepatocellular carcinoma. Can J Gastroenterol 27:351-363, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau WY, Lai EC, Lau SH: The current role of neoadjuvant/adjuvant/chemoprevention therapy in partial hepatectomy for hepatocellular carcinoma: A systematic review. Hepatobiliary Pancreat Dis Int 8:124-133, 2009 [PubMed] [Google Scholar]
- 24.Sun JJ, Wang K, Zhang CZ, et al. : Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann Surg Oncol 23:1344-1351, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Qin S, Bai Y, Lim HY, et al. : Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 31:3501-3508, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Xing H, Sun LY, Yan WT, et al. : Repeat hepatectomy for patients with early and late recurrence of hepatocellular carcinoma: A multicenter propensity score matching analysis. Surgery 169:911-920, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Hu Z, Zhou J, Li Z, et al. : Time interval to recurrence as a predictor of overall survival in salvage liver transplantation for patients with hepatocellular carcinoma associated with hepatitis B virus. Surgery 157:239-248, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Shi M, Lu LG, Fang WQ, et al. : Roles played by chemolipiodolization and embolization in chemoembolization for hepatocellular carcinoma: Single-blind, randomized trial. J Natl Cancer Inst 105:59-68, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Schwartz JD, Schwartz M, Mandeli J, et al. : Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: Review of the randomised clinical trials. Lancet Oncol 3:593-603, 2002 [DOI] [PubMed] [Google Scholar]