PURPOSE
Monoclonal antibodies directed against insulin-like growth factor-1 receptor (IGF-1R) have shown activity in patients with relapsed Ewing sarcoma. The primary objective of Children's Oncology Group trial AEWS1221 was to determine if the addition of the IGF-1R monoclonal antibody ganitumab to interval-compressed chemotherapy improves event-free survival (EFS) in patients with newly diagnosed metastatic Ewing sarcoma.
METHODS
Patients were randomly assigned 1:1 at enrollment to standard arm (interval-compressed vincristine/doxorubicin/cyclophosphamide alternating once every 2 weeks with ifosfamide/etoposide = VDC/IE) or to experimental arm (VDC/IE with ganitumab at cycle starts and as monotherapy once every 3 weeks for 6 months after conventional therapy). A planned sample size of 300 patients was projected to provide 81% power to detect an EFS hazard ratio of 0.67 or smaller for the experimental arm compared with the standard arm with a one-sided α of .025.
RESULTS
Two hundred ninety-eight eligible patients enrolled (148 in standard arm; 150 in experimental arm). The 3-year EFS estimates were 37.4% (95% CI, 29.3 to 45.5) for the standard arm and 39.1% (95% CI, 31.3 to 46.7) for the experimental arm (stratified EFS-event hazard ratio for experimental arm 1.00; 95% CI, 0.76 to 1.33; 1-sided, P = .50). The 3-year overall survival estimates were 59.5% (95% CI, 50.8 to 67.3) for the standard arm and 56.7% (95% CI, 48.3 to 64.2) for the experimental arm. More cases of pneumonitis after radiation involving thoracic fields and nominally higher rates of febrile neutropenia and ALT elevation were reported on the experimental arm.
CONCLUSION
Ganitumab added to interval-compressed chemotherapy did not significantly reduce the risk of EFS event in patients with newly diagnosed metastatic Ewing sarcoma, with outcomes similar to prior trials without IGF-1R inhibition or interval compression. The addition of ganitumab may be associated with increased toxicity.
INTRODUCTION
Patients with newly diagnosed, metastatic Ewing sarcoma have poor outcomes, with most studies reporting 3-year event-free survival (EFS) rates < 50%.1-5 Studies restricted to patients with isolated pulmonary metastatic disease have reported more favorable outcomes, although still only approximately half of such patients are expected to remain disease-free.2,6 Although outcomes have improved in recent decades for patients with localized Ewing sarcoma,1,7 there has been no demonstrable improvement for patients with metastatic Ewing sarcoma despite addition of ifosfamide/etoposide, addition of topotecan/cyclophosphamide, augmentation of alkylator and anthracycline doses, or use of high-dose chemotherapy with autologous stem-cell rescue.1-3,8 Although interval-compressed chemotherapy administered once every 2 weeks (a successful strategy in localized Ewing sarcoma7) had not previously been investigated in patients with metastatic disease, prior failures of chemotherapy intensification in this population argue for incorporation of novel agents.
CONTEXT
Key Objective
Does the addition of an insulin-like growth factor-1 receptor monoclonal antibody improve event-free survival in patients with newly diagnosed metastatic Ewing sarcoma?
Knowledge Generated
In this randomized phase III clinical trial, patients with metastatic Ewing sarcoma randomly assigned to receive ganitumab plus interval-compressed chemotherapy had similar event-free survival (3-year estimate of 39.1%) compared with patients randomly assigned to receive interval-compressed chemotherapy (3-year estimate of 37.4%). These poor outcomes in both arms of the trial highlight the need for new approaches for this high-risk patient population.
Relevance (S. Bhatia)
-
The lack of benefit from the addition of ganitumab to interval-compressed chemotherapy coupled with possibly higher risk of toxicity suggest the need to identify new approaches for patients with metastatic Ewing sarcoma.*
*Relevance section written by JCO Associate Editor Smita Bhatia, MD, MPH, FASCO.
The insulin-like growth factor-1 (IGF-1) and its receptor (IGF-1R) have long been implicated in the pathogenesis of Ewing sarcoma. These tumors overexpress IGF-1R, and the canonical oncogenic translocation most commonly seen in this disease (EWSR1/FLI1) has been shown to repress expression of an endogenous negative regulator (IGFBP3), thereby leading to pathway activation.9-11 A large body of preclinical work has shown efficacy of small molecules and monoclonal antibodies targeting IGF-1R against Ewing sarcoma.9,11-16 These findings motivated phase I and II trials of IGF-1R–directed monoclonal antibodies for relapsed Ewing sarcoma. Collectively, these trials showed an objective response rate of approximately 10% with a favorable toxicity profile.17-24 For example, a phase II trial of the IGF-1R monoclonal antibody ganitumab showed that 11% of patients with relapsed Ewing sarcoma had partial response or prolonged stable disease with this agent.17 No prior trials have evaluated an IGF-1R monoclonal antibody in combination with chemotherapy for patients with Ewing sarcoma, although trials in other indications have demonstrated feasibility of this approach.25,26
In this context, we conducted a phase III, randomized, open-label trial with the primary objective to determine if the addition of ganitumab to interval-compressed chemotherapy reduces the risk of EFS event for patients with newly diagnosed, metastatic Ewing sarcoma. Key secondary and exploratory clinical objectives included evaluation of overall survival (OS), response, toxicity, and ganitumab trough concentrations. This report represents the primary analysis of the trial.
METHODS
Patient Eligibility
Patients age < 50 years with newly diagnosed, metastatic Ewing sarcoma or primitive neuroectodermal tumor (according to institutional histologic diagnosis; molecular confirmation was not required) were eligible if they had adequate renal, hepatic, and cardiac function according to protocol definitions (Protocol, online only). Patients were required to have normal blood glucose, no known diabetes mellitus, and not be receiving chronic pharmacologic doses of corticosteroids. Patients were excluded if they met any of these criteria: primary tumors arising in the intradural soft tissues; regional node involvement as only site of disease outside primary site; prior chemotherapy or radiotherapy; or pregnant or breastfeeding.
The National Cancer Institute's (NCI) Pediatric Central Institutional Review Board (IRB) approved AEWS1221. Participating sites either relied upon the central IRB or obtained IRB approval locally. All patients (or legal guardians for minor subjects) provided written informed consent before enrollment.
Study Design and Interventions
AEWS1221 (ClinicalTrials.gov identifier: NCT02306161) was a prospective, randomized, open-label, phase III trial for patients with newly diagnosed, metastatic Ewing sarcoma. Patients were randomly assigned 1:1 at enrollment to interval-compressed chemotherapy analogous to AEWS0031 Regimen B,7 or to that same chemotherapy plus the addition of ganitumab (Fig 1). Random assignment was stratified according to age at enrollment (< 21 years v ≥ 21 years) and metastatic pattern (isolated pulmonary metastasis v all others).
FIG 1.
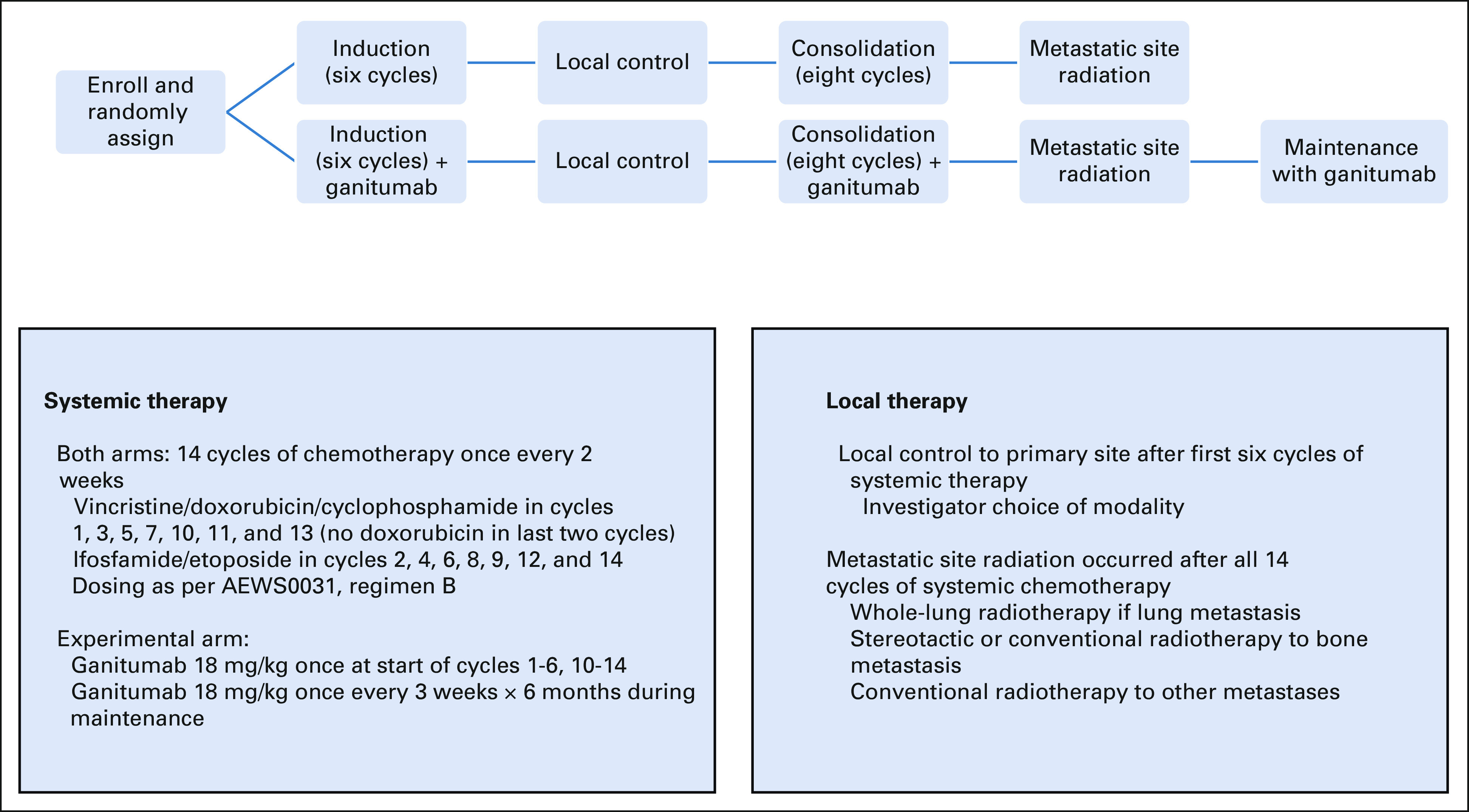
Study schema.
Patients in both arms were to receive 14 chemotherapy cycles (Fig 1). In addition, local control of the primary tumor was planned after the first six cycles, using surgery, radiation, or surgery plus radiation according to investigator choice. After completing 14 cycles, patients were to receive radiotherapy to metastatic sites, including whole-lung radiation for patients with pulmonary metastasis at diagnosis and radiation (conventional or stereotactic) to bone metastasis. The intent was to treat all bone metastases present at diagnosis, recognizing that some patients have too many sites to treat feasibly. Patients with thoracic primary tumors and lung metastases received whole-lung radiation concurrent with primary site radiation. Surgical management of soft tissue metastatic sites was allowed at investigator discretion. See full protocol for local control guidelines.
Patients randomly assigned to the experimental arm received ganitumab 18 mg/kg/dose intravenously once on day 1 of cycles 1-6 and 10-14. Ganitumab was omitted during cycles 7-9 regardless of local control modality. After completing metastatic site radiation, patients on the experimental arm received ganitumab 18 mg/kg/dose once every 3 weeks for eight doses as monotherapy maintenance. Ganitumab was administered over 30-120 minutes depending upon dose and prior infusion-related reactions.
End Points and Evaluations
The primary outcome measure was EFS, defined as the time from enrollment to event (disease progression, biopsy-proven marrow involvement at end-consolidation, diagnosis of a second malignancy [SMN], or death regardless of cause) or last patient contact, whichever occurred first. Patients without event were censored at last contact. OS and toxicity were secondary end points. OS was defined as the time from enrollment to death regardless of cause or last patient contact. A patient who was alive at last contact was considered censored. Toxicity was assessed using NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4. Exploratory end points reported herein included bone marrow response, maximum standardized uptake values (SUV) at the primary site, and ganitumab trough concentrations.
Patients underwent primary site imaging (computed tomography [CT] or magnetic resonance imaging [MRI]), chest CT, fluorodeoxyglucose-positron emission tomography (FDG-PET), and imaging of bone and/or soft tissue metastasis at study entry and then after 6, 10, and 14 cycles. Thereafter, patients underwent primary site imaging (CT or MRI), chest CT, and FDG-PET every 3 months for the first year after chemotherapy followed by surveillance radiographs of primary site and chest unless symptoms or tumor site warranted additional imaging. Bilateral bone marrow aspirates and biopsies were required at study entry. Patients with bone marrow metastasis were to repeat testing after cycle 3, 6, and 10 until negative, and then again after 14 cycles even if previously cleared.
Progression of primary bone tumors was defined as > 40% increase in tumor volume compared with smallest volume since study entry. Progression of soft tissue primary or metastatic tumors was defined according to RECIST version 1.1.27 Progression of bone metastasis was defined as any of the following: new bone metastasis at a previously uninvolved site; soft tissue mass > 1 cm at a prior bone metastasis either without a prior soft tissue mass or that previously had a soft tissue mass < 1 cm; > 20% increase in axial measurement of soft tissue mass for bone metastasis with known soft tissue mass > 1 cm; or viable tumor detected at least 8 weeks after radiation to a prior bone metastasis. Progression of bone marrow metastasis was defined as new marrow involvement in patients without prior marrow involvement or in patients who had prior clearance of marrow disease.
The first 10 patients < 21 years randomly assigned to the experimental arm provided serum samples before first, second, third, and sixth ganitumab doses, and then again before first, third, fourth, and sixth ganitumab monotherapy doses during the maintenance phase. Ganitumab serum concentrations were measured as previously described.24
Statistical Considerations
AEWS1221 was originally opened as a randomized phase II study. The expected enrollment rate was 3.5 patients/mo. The primary analysis was planned after 3 years of enrollment and 1 year of follow-up. Given the original planning parameters, this would provide 126 patients and 82 EFS events. At that time, we were to test the hypothesis of no reduction in risk of EFS event using a stratified relative risk regression model whose only variable was the randomized treatment assignment. If one-sided log-rank test with an alternative of reduced relative hazard ratio (RHR) associated with the experimental regimen obtained a P value of .20 or less, we were to consider this evidence that the experimental regimen should be considered for further evaluation.
Twenty months after enrollment began and before any interim analyses, we observed that the enrollment rate was approximately 5 patients/mo, providing an unexpected opportunity to address definitively the role of IGF-1R inhibition in this population in a feasible timeline. After consultation with NCI and without review of trial outcome data, we modified the study design to enroll 300 patients over 5 years and continue follow-up for 1.5 years after the last patient was enrolled. At that time, we expected to observe 196 EFS events if the RHR associated with the experimental regimen was 0.67. We planned to assess the efficacy of the experimental regimen by testing the hypothesis of no reduction in risk of EFS event using a stratified relative risk regression model whose only variable in the model was a term for the randomized treatment assignment. If 1-sided log-rank test with an alternative of reduced RHR associated with the experimental regimen obtained a P value of .025 or less, we considered this evidence of a significant reduction in EFS risk associated with the experimental regimen. This testing procedure provided 81% power if the experimental regimen RHR was 0.67. Detailed statistical properties of the design are described in the Data Supplement (online only).
EFS events were classified into one of five categories: (1) primary site only relapse; (2) distant site relapse; (3) combined distant and primary site relapse; (4) SMN; or (5) death. The cumulative incidence of a particular event type was estimated and equality of cumulative incidence between the two arms was tested using the method of Gray.28
As detailed below, the Data and Safety Monitoring Committee (DSMC) mandated crossover from experimental to standard regimen at the second interim analysis. To assess the effect of this unanticipated crossover for a subset of patients, we fitted a stratified time-dependent covariate relative hazards model (Data Supplement).
The equality of distributions of continuous variables, such as time to complete induction and time to complete consolidation, was assessed using the Kruskal-Wallis test.29 Each patient's outcome was associated with their randomized treatment assignment.
Risk for EFS event was assessed using a one-sided P value; all other quoted P values are two-sided, with two-sided P values of .05 considered to indicate a statistically significant result.
RESULTS
Patient Characteristics
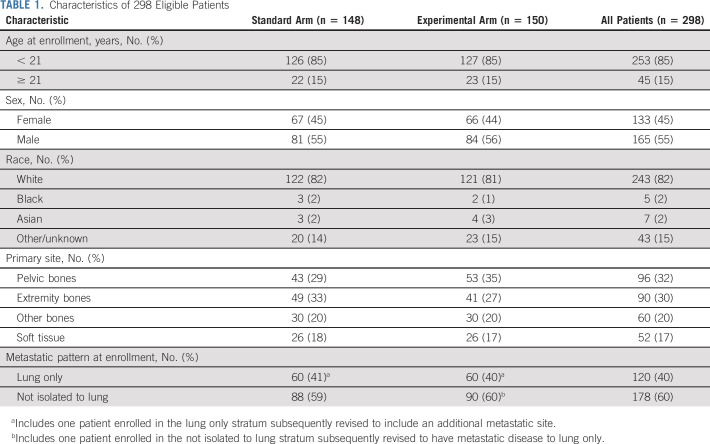
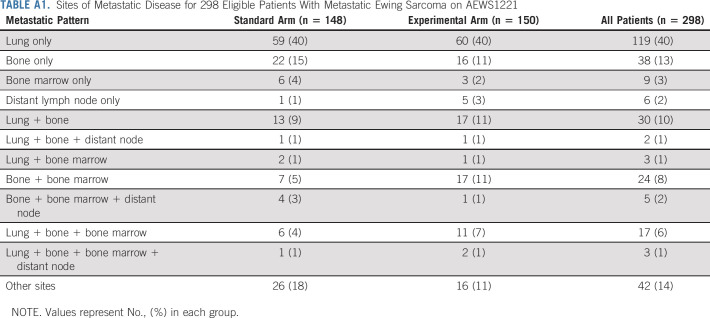
Three hundred twelve patients were enrolled from December 2014 to March 2019, of whom 14 were ineligible (Fig 2). Characteristics of the 298 eligible patients were similar between the two randomized arms (Table 1). Forty percent of patients had isolated pulmonary metastasis. The 60% of patients with metastasis not confined to the lung most commonly had bone and/or bone marrow involvement (Appendix Table A1, online only). Most patients (77.1%) received definitive radiotherapy for primary site local control (Appendix Table A2, online only).
FIG 2.
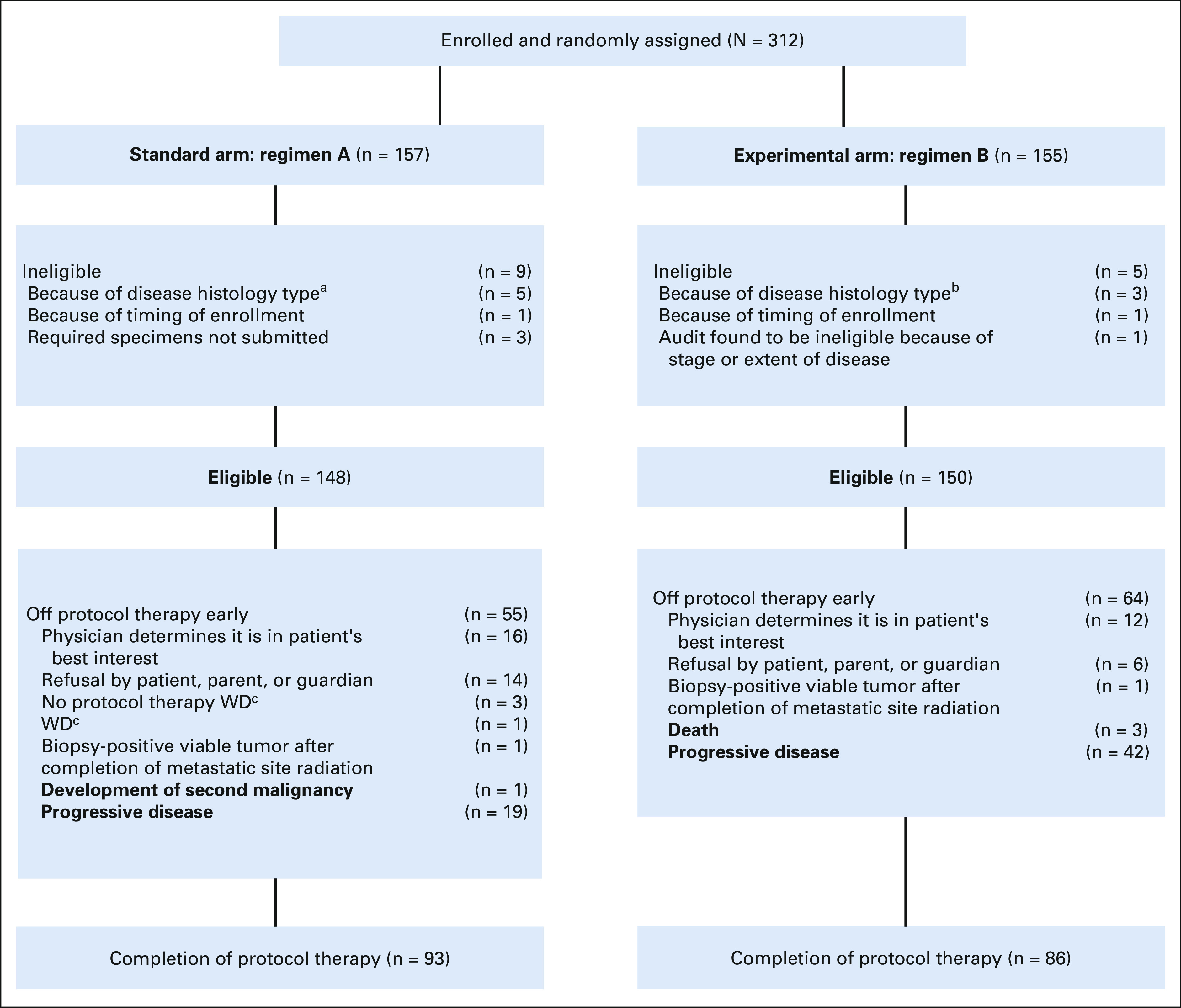
CONSORT diagram of patient enrollment and eligibility. aRound cell sarcoma (n = 2); desmoplastic small round cell tumor; NUT carcinoma; BCOR-rearranged sarcoma; bMesenchymal chondrosarcoma; rhabdomyosarcoma; lymphoblastic lymphoma; cWD, withdrawal of consent for any further data submission.
TABLE 1.
Characteristics of 298 Eligible Patients
Toxicity
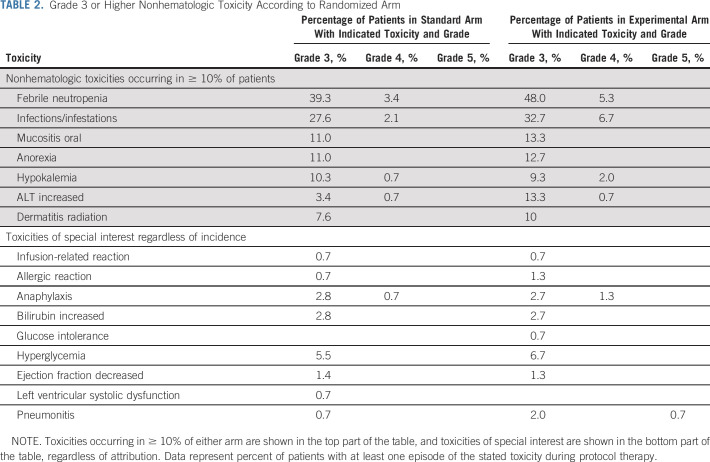
Rates of grade 3+ nonhematologic toxicity were broadly similar between randomized arm, although rates of febrile neutropenia and increased ALT were each approximately 10% greater in patients randomly assigned to the experimental arm (Table 2). Given prior experience with IGF-1R monoclonal antibodies, we also evaluated rates of grade 3+ toxicities of special interest, including allergic/infusion reactions, hyperbilirubinemia, hyperglycemia, cardiac dysfunction, and pneumonitis (Table 2). All of these toxicities occurred in < 10% of patients on both arms. Only the rates of pneumonitis appeared higher in the experimental arm (0.7% v 2.7%), including the only grade 5 event. All four patients with grade 3+ pneumonitis on the experimental arm had received radiotherapy to the thorax.
TABLE 2.
Grade 3 or Higher Nonhematologic Toxicity According to Randomized Arm
We also investigated whether the addition of ganitumab increased time to complete standard chemotherapy. For the 276 patients who began consolidation, the median time to complete induction was 102.5 (range, 83-170) days on the standard arm versus 105 (range, 85-191) days on the experimental arm (P = .16). For the 158 patients who began metastatic site radiation, the median time to complete consolidation was 156 (range, 105-250) days on the standard arm versus 170 (range, 125-243) days on the experimental arm (P = .012).
Efficacy
At the second DSMC interim monitoring point reflecting 50.5% observed information, the EFS hazard ratio for experimental arm compared with standard arm was 0.95 (95% CI, 0.65 to 1.39; Data Supplement). Although the protocol-defined inefficacy boundary was not yet crossed at this second interim analysis, the DSMC recommended early closure and early discontinuation of ganitumab in March 2019 based upon the observed outcome data and the aforementioned potential increased risk of pneumonitis. The study team accepted this recommendation. Patients were followed for an additional three years from time of early closure for this final primary analysis.
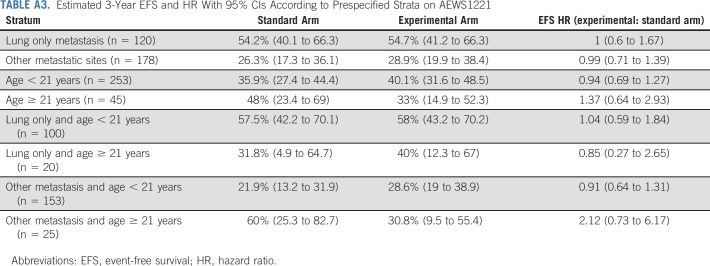
As of the March 31, 2022, data cutoff for this final analysis, 195 events were reported: 180 relapses/progressions (including one patient with bone marrow involvement at end-consolidation); 10 SMNs; and five deaths as first events. The 3-year EFS estimate was 38.3 (95% CI, 32.7 to 43.9). Risk for EFS event did not differ significantly between randomized arms (3-year EFS 37.4% [95% CI, 29.3 to 45.5] for standard arm versus 39.1% [95% CI, 31.3 to 46.9], for experimental arm; stratified hazard ratio [HR] for experimental arm 1.00; 95% CI, 0.76 to 1.33; 1-sided P = .50; Figure 3A). EFS estimates within predefined strata were similar between randomized arms (Appendix Table A3, online only). The failure patterns (primary v metastatic v combined primary + metastatic) were predominantly metastatic and were broadly similar between randomized arms, although no patients on the experimental arm had combined failure (Appendix Table A4, online only). Risk for death did not differ significantly between randomized arms (3-year OS 59.5% [95% CI, 50.8 to 67.3] for standard arm v 56.7% [95% CI, 48.3 to 64.2], for experimental arm; stratified HR for experimental arm 1.03; 95% CI, 0.75 to 1.42; P = .99; Figure 3B).
FIG 3.
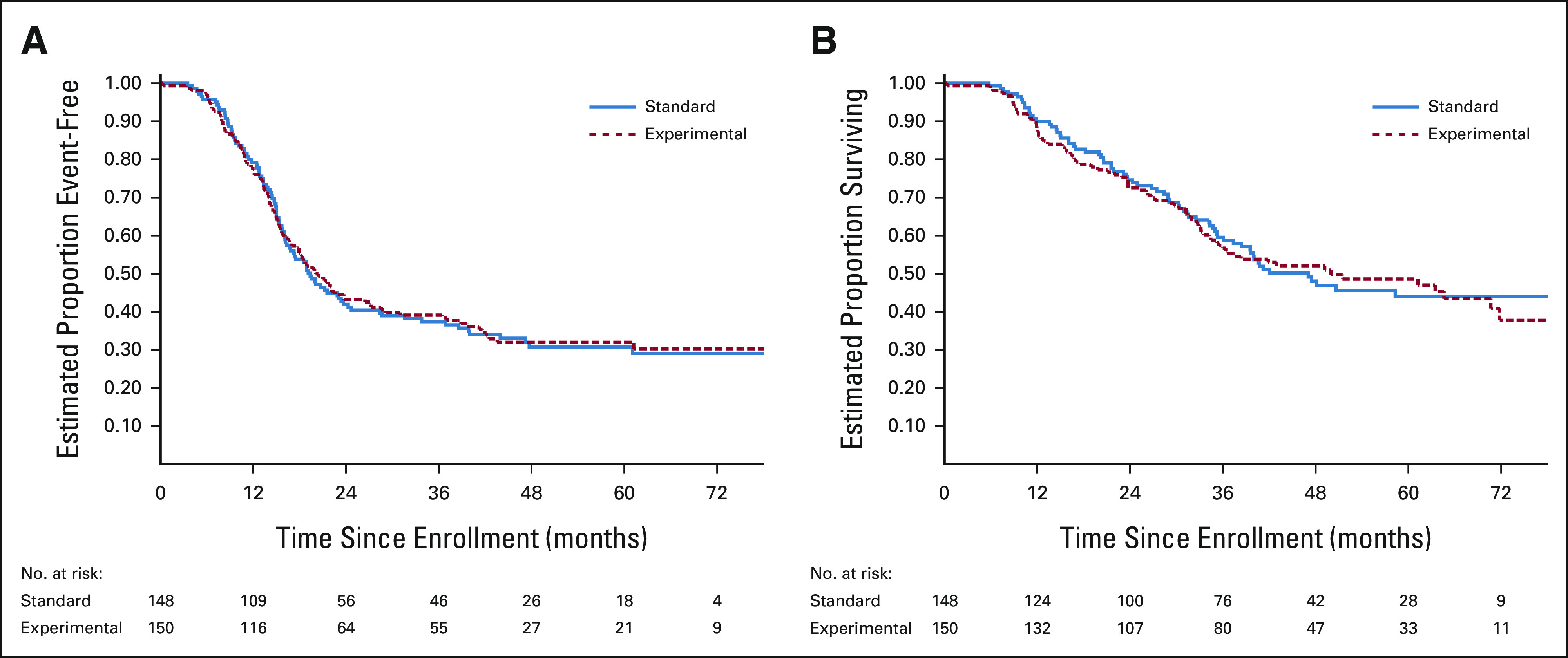
Kaplan-Meier curves of (A) EFS and (B) OS from time of random assignment according to randomized arm. EFS, event-free survival; OS, overall survival.
Since ganitumab was discontinued early in 45 patients after interim analysis, we completed a post hoc analysis to evaluate the possible impact of this unanticipated crossover from experimental arm to standard arm. Time-dependent covariates accounting for these crossovers were not significantly associated with risk for event or death, indicating no significant crossover effect on EFS or OS (Data Supplement).
Among the 69 patients with bone marrow metastatic disease at study entry who had follow-up marrow evaluations, 49/69 (71%) cleared after first three cycles, 18/69 (26.1%) cleared after first six cycles, and 2/69 (2.9%) remained involved after first six cycles. There were similar rates of clearance between randomized arms (69% [22/32] and 31% [10/32] cleared after three and six cycles on standard arm v 73% [27/37] and 22% [8/37] cleared after three and six cycles on experimental arm, respectively).
Paired data for primary site FDG-PET maximum SUV at baseline and end-induction were available for 172 patients. The median relative SUV (reflecting end-induction SUV/baseline SUV) for the full cohort was 0.34 (range, 0.063-2.7), with no significant difference in median relative SUV between randomized arms (median of 0.34 on both arms; P = .94).
Ganitumab Trough Concentrations
Ganitumab trough concentrations for prespecified time points are shown in Appendix Table A5 (online only). Based upon prior testing,24 the goal trough concentration was a level > 10,000 ng/mL. After the first and second doses, 9/10 and 10/10 patients met this benchmark, respectively. During maintenance with ganitumab administered once every 3 weeks instead of once every 2 weeks, all patients with evaluable data met this benchmark.
DISCUSSION
In this trial, the largest ever, to our knowledge, conducted by the Children's Oncology Group (COG) for patients with metastatic Ewing sarcoma, the IGF-1R–directed monoclonal antibody ganitumab did not reduce risk of EFS event or death when added to conventional therapy. Outcomes for patients treated with interval-compressed chemotherapy on the standard arm were similar to those reported in prior trials in this population,1-3 adding to accumulating data that chemotherapy intensification is unlikely to improve outcomes in these patients. Even in the most favorable stratum of this trial (younger patients with isolated pulmonary metastasis), 3-year EFS was < 60%. These results argue strongly for new strategies to improve outcomes in this population.
Our results add to existing literature that demonstrate the failure of IGF-1R–directed monoclonal antibodies to improve outcomes when added to conventional chemotherapy across a range of cancers. Ganitumab added to gemcitabine did not improve OS for patients with metastatic pancreatic cancer.25 Likewise, figitumumab added to paclitaxel/carboplatin did not improve OS for patients with advanced non–small-cell lung cancer.26 As with our study, these and other trials were motivated in part by laboratory data demonstrating that blocking IGF-1R signaling could improve the efficacy of conventional chemotherapy.30-32 The reasons these preclinical findings have not translated into clinical benefit are not clear.
There are several potential reasons for failure of ganitumab to improve outcomes in this trial. Fundamentally, it is possible that the IGF-1R pathway is not as critical to growth and survival of Ewing sarcoma as decades of preclinical data have suggested, although objective responses in patients with relapsed disease argue against this possibility. Although blood levels of ganitumab exceeded goal concentrations, it is possible that intratumoral concentrations were inadequate. Patients randomly assigned to the experimental arm required slightly more time to complete consolidation, and this reduction in treatment intensity may have contributed to the current null finding. Early discontinuation of ganitumab following DSMC review could have diluted any beneficial effects, although our post hoc analysis does not support this possibility. Since local control strategies of the primary and metastatic sites were at investigator choice, it is also possible that unplanned differences in local control approaches between arms may have affected observed outcomes. Finally, there is no international consensus on response criteria for patients with metastatic bone sarcoma. We acknowledge that the response criteria reflected expert consensus at the time, although these criteria were applied uniformly in both arms of the trial, mitigating any impact on trial outcomes.
Lack of patient selection may have contributed to our observed findings in at least three ways. First, eligibility criteria did not require molecular confirmation of diagnosis and centralized molecular testing was not performed. A prior analysis of institutional translocation testing from this trial showed that 14% of patients lacked molecular data supporting the diagnosis.33 As such, it is possible that patients with histologic mimics of Ewing sarcoma without an established role for IGF-1R inhibition may have enrolled, further diluting any beneficial effect of ganitumab in bona fide Ewing sarcoma (ie, tumors with FET/ETS translocations). Second, there are no validated biomarkers predictive of benefit from IGF-1R inhibition and therefore no enrichment could be performed in our trial. Correlative biology work, including potential genomic predictors, is ongoing from specimens obtained from AEWS1221 participants and will be reported in subsequent manuscripts. Third, it is possible that evaluating IGF-1R inhibition in a more favorable population without initial metastatic disease might lead to a different outcome.
In conclusion, the addition of ganitumab to interval-compressed VDC/IE did not improve outcomes in patients with newly diagnosed metastatic Ewing sarcoma. Our results cannot speak to other strategies that may target the IGF-1R axis in Ewing sarcoma, such as novel-novel combinations targeting this pathway or agents that target IGF-1 and IGF-2 ligands. Nevertheless, the lack of benefit in the current trial raises concerns about these other strategies in this disease and calls for additional preclinical studies and predictive biomarkers in this treatment-resistant disease. This outcome is particularly sobering as a prior analysis from the COG highlighted IGF-1R monoclonal antibodies as a drug class that satisfied all criteria to advance to a frontline randomized trial,34 suggesting that a higher level of activity in the relapse setting may be needed before embarking on frontline trials. These findings underscore the urgent need for novel strategies for these patients.
ACKNOWLEDGMENT
The authors gratefully acknowledge the patients and families who participated in this trial, the local study teams who treated participants, the study nurses, pharmacists, protocol coordinators, research coordinators, the team at the Imaging and Radiation Oncology Core—Rhode Island, and Anthony Welch at the Developmental Therapeutics Program, NCI Division of Cancer Treatment and Diagnosis, who contributed to the trial conduct, as well as Paul Manning for administrative support.
APPENDIX
TABLE A1.
Sites of Metastatic Disease for 298 Eligible Patients With Metastatic Ewing Sarcoma on AEWS1221
TABLE A2.
Number of Patients (%) According to Mode of Primary Site Local Control for Patients With Metastatic Ewing Sarcoma on AEWS1221
TABLE A3.
Estimated 3-Year EFS and HR With 95% CIs According to Prespecified Strata on AEWS1221
TABLE A4.
Cumulative Incidence Rate at 3 Years With 95% CIs for Each Type of Failure According to Randomized Arm
TABLE A5.
Ganitumab Serum Concentrations for the First 10 Patients With Metastatic Ewing Sarcoma Age < 21 Years on AEWS1221
Steven G. DuBois
Consulting or Advisory Role: Bayer, Amgen, Jazz Pharmaceuticals
Research Funding: Merck (Inst), Roche/Genentech (Inst), Lilly (Inst), Curis (Inst), Loxo (Inst), BMS (Inst), Eisai (Inst), Pfizer (Inst), Turning Point Therapeutics (Inst), Bayer (Inst), Salarius Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Salarius Pharmaceuticals
Uncompensated Relationships: Ymabs Therapeutics Inc
Mark D. Krailo
Consulting or Advisory Role: Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Julia Glade-Bender
Consulting or Advisory Role: Jazz Pharmaceuticals
Research Funding: Eisai (Inst), Lilly (Inst), Loxo (Inst), Roche/Genentech (Inst), Bayer (Inst), Jazz Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Patent on a T lympboblastic lymphoma cell line, CUTLL1
Travel, Accommodations, Expenses: Amgen (Inst)
Uncompensated Relationships: SpringWorks Therapeutics, Bristol Myers Squibb, Merck, Eisai
Open Payments Link: https://openpaymentsdata.cms.gov/physician/708514
Nadia Laack
Research Funding: Bristol Myers Squib (Inst)
Travel, Accommodations, Expenses: Google Health
R. Lor Randall
Honoraria: Biomet, Daiichi Sankyo, Onkos Surgical, Onclive, SpringWorks Therapeutics
Travel, Accommodations, Expenses: Biomet, Daiichi Sankyo/Lilly, SpringWorks Therapeutics
Matthew Boron
Employment: AstraZeneca/MedImmune
Stock and Other Ownership Interests: AstraZeneca/MedImmune
Travel, Accommodations, Expenses: AstraZeneca/MedImmune
Christine Hill-Kayser
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Joel M. Reid
Consulting or Advisory Role: Elucida Oncology, Inc
Lisa Teot
Patents, Royalties, Other Intellectual Property: Royalties for Springer monograph Pediatric Cytopathology
Dinesh Rakheja
Consulting or Advisory Role: ClearNano Inc
Open Payments Link: https://openpaymentsdata.cms.gov/physician/591421
Carola Arndt
Consulting or Advisory Role: Merck
Stephen L. Lessnick
Stock and Other Ownership Interests: Salarius Pharmaceuticals
Consulting or Advisory Role: Salarius Pharmaceuticals
Patents, Royalties, Other Intellectual Property: 1. United States Patent No. US 7,939,253 B2, “Methods and compositions for the diagnosis and treatment of Ewing's Sarcoma,” Stephen L. Lessnick, Assigned to The University of Utah Research Foundation, Salt Lake City, UT, filed on May 9, 2007, awarded on May 10, 2011. 2. United States Patent No. US 8,557,532, “Diagnosis and treatment of drug-resistant Ewing's sarcoma,” Stephen L. Lessnick and Wen Luo, Assigned to The University of Utah Research Foundation, Salt Lake City, UT, filed on August 30, 2010, awarded on October 15, 2013
Other Relationship: Multiple pharmaceutical, biotech, device, and similar companies
Brian D. Crompton
Employment: Acceleron Pharma, Generate Biomedicines
Stock and Other Ownership Interests: Acceleron Pharma
Consulting or Advisory Role: PetDx, Animal Cancer Foundation, Osteosarcoma Institute (Foundation)
Research Funding: Gradalis
E. Anders Kolb
Travel, Accommodations, Expenses: Roche/Genentech
Heike Daldrup-Link
Leadership: Monasteria Press LLC
Research Funding: MegaPo Biomedical
Patents, Royalties, Other Intellectual Property: WO 2015014756: Activatable theranostic nanoparticles
Damon R. Reed
Consulting or Advisory Role: Eisai, Pfizer, SpringWorks Therapeutics
Katherine A. Janeway
Honoraria: Foundation Medicine, Takeda
Consulting or Advisory Role: Bayer, Ipsen
Travel, Accommodations, Expenses: Bayer
Richard G. Gorlick
Research Funding: Eisai (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the CTOS 2019 Annual Meeting, Tokyo, Japan, November 13-16, 2019.
SUPPORT
Supported by NIH/NCI through NCTN Operations Center Grant No. U10CA180886 and NCTN Statistics and Data Center Grant No. U10CA180899 to the Children's Oncology Group, St Baldrick's Foundation, and the QuadW Foundation. S.G.D. was supported by Alex's Lemonade Stand Foundation. J.G.B. was supported by NIH/NCI CA008748. J.M.R. was supported in part by NCI P30 CA015083.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Steven G. DuBois, Mark D. Krailo, Julia Glade-Bender, R. Lor Randall, Helen X. Chen, Nita L. Seibel, Matthew Boron, Stephanie Terezakis, Christine Hill-Kayser, Andrea Hayes, Joel M. Reid, Dinesh Rakheja, Richard Womer, Stephen L. Lessnick, E. Anders Kolb, Damon R. Reed, Katherine A. Janeway, Richard G. Gorlick
Financial support: Katherine A. Janeway
Administrative support: Matthew Boron, Damon R. Reed, Katherine A. Janeway
Provision of study materials or patients: Steven G. DuBois, Julia Glade-Bender, Matthew Boron, Richard Womer, Damon R. Reed
Collection and assembly of data: Steven G. DuBois, Mark D. Krailo, Julia Glade-Bender, Allen Buxton, R. Lor Randall, Christine Hill-Kayser, Andrea Hayes, Brian D. Crompton, E. Anders Kolb, Eric Eutsler, Damon R. Reed
Data analysis and interpretation: Steven G. DuBois, Mark D. Krailo, Julia Glade-Bender, Allen Buxton, Nadia Laack, R. Lor Randall, Helen X. Chen, Nita L. Seibel, Stephanie Terezakis, Andrea Hayes, Joel M. Reid, Lisa Teot, Richard Womer, Carola Arndt, Stephen L. Lessnick, E. Anders Kolb, Heike Daldrup-Link, Damon R. Reed, Katherine A. Janeway
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase III Trial of Ganitumab With Interval-Compressed Chemotherapy for Patients With Newly Diagnosed Metastatic Ewing Sarcoma: A Report From the Children's Oncology Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Steven G. DuBois
Consulting or Advisory Role: Bayer, Amgen, Jazz Pharmaceuticals
Research Funding: Merck (Inst), Roche/Genentech (Inst), Lilly (Inst), Curis (Inst), Loxo (Inst), BMS (Inst), Eisai (Inst), Pfizer (Inst), Turning Point Therapeutics (Inst), Bayer (Inst), Salarius Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Salarius Pharmaceuticals
Uncompensated Relationships: Ymabs Therapeutics Inc
Mark D. Krailo
Consulting or Advisory Role: Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Julia Glade-Bender
Consulting or Advisory Role: Jazz Pharmaceuticals
Research Funding: Eisai (Inst), Lilly (Inst), Loxo (Inst), Roche/Genentech (Inst), Bayer (Inst), Jazz Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Patent on a T lympboblastic lymphoma cell line, CUTLL1
Travel, Accommodations, Expenses: Amgen (Inst)
Uncompensated Relationships: SpringWorks Therapeutics, Bristol Myers Squibb, Merck, Eisai
Open Payments Link: https://openpaymentsdata.cms.gov/physician/708514
Nadia Laack
Research Funding: Bristol Myers Squib (Inst)
Travel, Accommodations, Expenses: Google Health
R. Lor Randall
Honoraria: Biomet, Daiichi Sankyo, Onkos Surgical, Onclive, SpringWorks Therapeutics
Travel, Accommodations, Expenses: Biomet, Daiichi Sankyo/Lilly, SpringWorks Therapeutics
Matthew Boron
Employment: AstraZeneca/MedImmune
Stock and Other Ownership Interests: AstraZeneca/MedImmune
Travel, Accommodations, Expenses: AstraZeneca/MedImmune
Christine Hill-Kayser
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Joel M. Reid
Consulting or Advisory Role: Elucida Oncology, Inc
Lisa Teot
Patents, Royalties, Other Intellectual Property: Royalties for Springer monograph Pediatric Cytopathology
Dinesh Rakheja
Consulting or Advisory Role: ClearNano Inc
Open Payments Link: https://openpaymentsdata.cms.gov/physician/591421
Carola Arndt
Consulting or Advisory Role: Merck
Stephen L. Lessnick
Stock and Other Ownership Interests: Salarius Pharmaceuticals
Consulting or Advisory Role: Salarius Pharmaceuticals
Patents, Royalties, Other Intellectual Property: 1. United States Patent No. US 7,939,253 B2, “Methods and compositions for the diagnosis and treatment of Ewing's Sarcoma,” Stephen L. Lessnick, Assigned to The University of Utah Research Foundation, Salt Lake City, UT, filed on May 9, 2007, awarded on May 10, 2011. 2. United States Patent No. US 8,557,532, “Diagnosis and treatment of drug-resistant Ewing's sarcoma,” Stephen L. Lessnick and Wen Luo, Assigned to The University of Utah Research Foundation, Salt Lake City, UT, filed on August 30, 2010, awarded on October 15, 2013
Other Relationship: Multiple pharmaceutical, biotech, device, and similar companies
Brian D. Crompton
Employment: Acceleron Pharma, Generate Biomedicines
Stock and Other Ownership Interests: Acceleron Pharma
Consulting or Advisory Role: PetDx, Animal Cancer Foundation, Osteosarcoma Institute (Foundation)
Research Funding: Gradalis
E. Anders Kolb
Travel, Accommodations, Expenses: Roche/Genentech
Heike Daldrup-Link
Leadership: Monasteria Press LLC
Research Funding: MegaPo Biomedical
Patents, Royalties, Other Intellectual Property: WO 2015014756: Activatable theranostic nanoparticles
Damon R. Reed
Consulting or Advisory Role: Eisai, Pfizer, SpringWorks Therapeutics
Katherine A. Janeway
Honoraria: Foundation Medicine, Takeda
Consulting or Advisory Role: Bayer, Ipsen
Travel, Accommodations, Expenses: Bayer
Richard G. Gorlick
Research Funding: Eisai (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Grier HE, Krailo MD, Tarbell NJ, et al. : Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 348, 694-701, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Miser JS, Krailo MD, Tarbell NJ, et al. : Treatment of metastatic Ewing's sarcoma or primitive neuroectodermal tumor of bone: Evaluation of combination ifosfamide and etoposide—A Children's Cancer Group and Pediatric Oncology Group study. J Clin Oncol 22, 2873-2876, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Miser JS, Goldsby RE, Chen Z, et al. : Treatment of metastatic Ewing sarcoma/primitive neuroectodermal tumor of bone: Evaluation of increasing the dose intensity of chemotherapy—A report from the Children's Oncology Group. Pediatr Blood Cancer 49, 894-900, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Ladenstein R, Potschger U, Le Deley MC, et al. : Primary disseminated multifocal Ewing sarcoma: Results of the Euro-EWING 99 trial. J Clin Oncol 28, 3284-3291, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Bernstein ML, Devidas M, Lafreniere D, et al. : Intensive therapy with growth factor support for patients with Ewing tumor metastatic at diagnosis: Pediatric Oncology Group/Children's Cancer Group phase II study 9457—A report from the Children's Oncology Group. J Clin Oncol 24, 152-159, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Dirksen U, Brennan B, Le Deley MC, et al. : High-dose chemotherapy compared with standard chemotherapy and lung radiation in Ewing sarcoma with pulmonary metastases: Results of the European Ewing Tumour Working Initiative of National Groups, 99 trial and EWING 2008. J Clin Oncol 37, 3192-3202, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Womer RB, West DC, Krailo MD, et al. : Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children's Oncology Group. J Clin Oncol 30, 4148-4154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch R, Gelderblom H, Haveman L, et al. : High-dose treosulfan and melphalan as consolidation therapy versus standard therapy for high-risk (metastatic) Ewing sarcoma. J Clin Oncol:40, 2022, 2307, 2320 [DOI] [PubMed] [Google Scholar]
- 9.Yee D, Favoni RE, Lebovic GS, et al. : Insulin-like growth factor I expression by tumors of neuroectodermal origin with the t(11;22) chromosomal translocation. A potential autocrine growth factor. J Clin Invest 86, 1806-1814, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prieur A, Tirode F, Cohen P, et al. : EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol 24, 7275-7283, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scotlandi K, Benini S, Sarti M, et al. : Insulin-like growth factor I receptor-mediated circuit in Ewing's sarcoma/peripheral neuroectodermal tumor: A possible therapeutic target. Cancer Res 56, 4570-4574, 1996 [PubMed] [Google Scholar]
- 12.van Valen F, Winkelmann W, Jurgens H: Type I and type II insulin-like growth factor receptors and their function in human Ewing's sarcoma cells. J Cancer Res Clin Oncol 118, 269-275, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Scotlandi K, Benini S, Nanni P, et al. : Blockage of insulin-like growth factor-I receptor inhibits the growth of Ewing's sarcoma in athymic mice. Cancer Res 58, 4127-4131, 1998 [PubMed] [Google Scholar]
- 14.Houghton PJ, Morton CL, Gorlick R, et al. : Initial testing of a monoclonal antibody (IMC-A12) against IGF-1R by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer 54, 921-926, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolb EA, Gorlick R, Houghton PJ, et al. : Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr Blood Cancer 50, 1190-1197, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Beltran PJ, Chung YA, Moody G, et al. : Efficacy of ganitumab (AMG 479), alone and in combination with rapamycin, in Ewing's and osteogenic sarcoma models. J Pharmacol Exp Ther 337, 644-654, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Tap WD, Demetri G, Barnette P, et al. : Phase II study of ganitumab, a fully human anti-type-1 insulin-like growth factor receptor antibody, in patients with metastatic Ewing family tumors or desmoplastic small round cell tumors. J Clin Oncol 30, 1849-1856, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Pappo AS, Patel SR, Crowley J, et al. : R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: Results of a phase II Sarcoma Alliance for Research through Collaboration Study. J Clin Oncol 29, 4541-4547, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weigel B, Malempati S, Reid JM, et al. : Phase 2 trial of cixutumumab in children, adolescents, and young adults with refractory solid tumors: A report from the Children's Oncology Group. Pediatr Blood Cancer 61, 452-456, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malempati S, Weigel B, Ingle AM, et al. : Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: A report from the Children's Oncology Group. J Clin Oncol 30, 256-262, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olmos D, Postel-Vinay S, Molife LR, et al. : Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751, 871) in patients with sarcoma and Ewing's sarcoma: A phase 1 expansion cohort study. Lancet Oncol 11, 129-135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juergens H, Daw NC, Geoerger B, et al. : Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol 29, 4534-4540, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atzori F, Tabernero J, Cervantes A, et al. : A phase I pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 17, 6304-6312, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Tolcher AW, Sarantopoulos J, Patnaik A, et al. : Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol 27, 5800-5807, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Fuchs CS, Azevedo S, Okusaka T, et al. : A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: The GAMMA trial. Ann Oncol 26, 921-927, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langer CJ, Novello S, Park K, et al. : Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J Clin Oncol 32, 2059-2066, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45, 228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 29.Kruskal WH, Wallis WA: Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47, 583-621, 1952 [Google Scholar]
- 30.Martins AS, Mackintosh C, Martin DH, et al. : Insulin-like growth factor I receptor pathway inhibition by ADW742, alone or in combination with imatinib, doxorubicin, or vincristine, is a novel therapeutic approach in Ewing tumor. Clin Cancer Res 12, 3532-3540, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Benini S, Manara MC, Baldini N, et al. : Inhibition of insulin-like growth factor I receptor increases the antitumor activity of doxorubicin and vincristine against Ewing's sarcoma cells. Clin Cancer Res 7, 1790-1797, 2001 [PubMed] [Google Scholar]
- 32.Scotlandi K, Manara MC, Nicoletti G, et al. : Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res 65, 3868-3876, 2005 [DOI] [PubMed] [Google Scholar]
- 33.DuBois SG, Krailo MD, Buxton A, et al. : Patterns of translocation testing in patients enrolling in a cooperative group trial for newly diagnosed metastatic Ewing sarcoma. Arch Pathol Lab Med 145, 1564-1568, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey K, Cost C, Davis I, et al. : Emerging novel agents for patients with advanced Ewing sarcoma: A report from the Children's Oncology Group (COG) new agents for Ewing Sarcoma Task Force. F1000Res 8:493, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]