PURPOSE
Idecabtagene vicleucel (ide-cel) is an autologous B-cell maturation antigen–directed chimeric antigen receptor T-cell therapy approved for relapsed/refractory multiple myeloma (RRMM) on the basis of the phase II pivotal KarMMa trial, which demonstrated best overall and ≥ complete response rates of 73% and 33%, respectively. We report clinical outcomes with standard-of-care (SOC) ide-cel under the commercial Food and Drug Administration label.
METHODS
Data were retrospectively collected from patients with RRMM who underwent leukapheresis as of February 28, 2022, at 11 US institutions with intent to receive SOC ide-cel. Toxicities were graded per American Society for Transplantation and Cellular Therapy guidelines and managed according to each institution's policies. Responses were graded on the basis of the International Myeloma Working Group response criteria.
RESULTS
One hundred fifty-nine of 196 leukapheresed patients received ide-cel by data cutoff. One hundred twenty (75%) infused patients would have been ineligible for participation in the KarMMa clinical trial because of comorbidities at the time of leukapheresis. Any grade and grade ≥ 3 cytokine release syndrome and neurotoxicity occurred in 82/3% and 18/6%, respectively. Best overall and ≥ complete response rates were 84% and 42%, respectively. At a median follow-up of 6.1 months from chimeric antigen receptor T infusion, the median progression-free survival was 8.5 months (95% CI, 6.5 to not reached) and the median overall survival was 12.5 months (95% CI, 11.3 to not reached). Patients with previous exposure to B-cell maturation antigen–targeted therapy, high-risk cytogenetics, Eastern Cooperative Oncology Group performance status ≥ 2 at lymphodepletion, and younger age had inferior progression-free survival on multivariable analysis.
CONCLUSION
The safety and efficacy of ide-cel in patients with RRMM in the SOC setting were comparable with those in the phase II pivotal KarMMa trial despite most patients (75%) not meeting trial eligibility criteria.
INTRODUCTION
Patients with multiple myeloma refractory to immunomodulatory drugs (IMiD), proteasome inhibitors (PIs), and anti-CD38 monoclonal antibodies have dismal outcomes with a median progression-free survival (PFS) of 3 to 4 months and a median overall survival (OS) of 8-9 months.1-4 In March 2021, idecabtagene vicleucel (ide-cel), an autologous B-cell maturation antigen (BCMA)–directed chimeric antigen receptor (CAR) T-cell therapy, was approved for the treatment of relapsed or refractory multiple myeloma (RRMM) after at least four prior lines of therapy.5 In the pivotal phase II KarMMa trial of ide-cel, the overall response rate (ORR), ≥ complete response (CR), and minimal residual disease (MRD) negative rates were 73%, 33%, and 26% respectively.6,7 Cytokine release syndrome (CRS) was observed in 84% (grade ≥ 3: 5%), and neurotoxicity (NT) was observed in 18% (grade ≥ 3: 3%) of patients.6 The median PFS was 8.8 months, and the estimated median OS was 19.4 months.6,7
CONTEXT
Key Objective
This multicenter retrospective study at 11 US academic medical centers evaluated safety and efficacy of standard-of-care idecabtagene vicleucel, an autologous anti–B-cell maturation antigen (BCMA) chimeric antigen receptor T-cell therapy for treatment of relapsed/refractory multiple myeloma.
Knowledge Generated
The study population included 196 patients who underwent apheresis and 159 patients who had received ide-cel infusion by study cutoff. The patient population differed from the pivotal KarMMa clinical trial as 75% of apheresed patients in our study would not have met trial eligibility criteria. The rate of manufacturing failure was also higher with a 6% failure rate with first manufacturing attempt. Despite this, we observed comparable safety and efficacy outcomes with standard-of-care ide-cel as reported in the pivotal phase II KarMMa trial. We also identified patient and disease characteristics associated with outcomes. Patients with prior exposure to BCMA-targeted therapy had lower response rates and shorter progression-free survival with ide-cel.
Relevance (S. Lentzsch)
-
The efficacy and safety profile of standard-of-care ide-cel is comparable with that observed in the KarMMa trial although most of the patients would not have met clinical trial eligibility criteria. If BCMA chimeric antigen receptor-T-cell treatment is planned, prior exposure to BCMA-targeted therapy should be avoided.*
*Relevance section written by JCO Associate Editor, Suzanne Lentzsch, MD.
The KarMMa trial had stringent eligibility criteria, and patients treated on this trial are likely not representative of patients treated with ide-cel as a standard-of-care (SOC) treatment, as patients might have comorbidities that would have made them ineligible for the trial. The goal of this study was to evaluate safety and efficacy of SOC ide-cel for the treatment of RRMM in a real-world population.
METHODS
This was a retrospective multicenter observational study of patients planned for SOC ide-cel for RRMM from 11 US medical centers. Each center obtained independent institutional review board approval and informed consent per institutional requirements.
Patients
All patients with RRMM who had received ≥ 4 prior lines of therapy and underwent leukapheresis from April 1, 2021, until February 28, 2022, with intent to manufacture commercial ide-cel were included. If the CAR T product did not meet release criteria, patients were treated under an expanded access protocol.
Treatment and Clinical Assessment
After leukapheresis, patients could receive bridging chemotherapy and/or radiation at the discretion of the treating physician. Cyclophosphamide 300 mg/m2 and fludarabine were used once daily for lymphodepleting chemotherapy on days –5, –4, and –3 before CAR T infusion. Fludarabine dose was adjusted on the basis of creatinine clearance per institutional protocols. Hematologic toxicity was graded on the basis of the Common Terminology Criteria for Adverse Events version 5.0, whereas CRS and NT were assessed on the basis of the American Society for Transplantation and Cellular Therapy criteria.8,9 Treatment of CRS and NT was per institutional guidelines, as were infectious disease prophylaxis and use of growth factors. Response was assessed on the basis of the International Myeloma Working Group Criteria (IMWG),10 per investigator discretion, but because of the retrospective nature of our study, all the IMWG criteria were not required to be fulfilled. Confirmatory testing and imaging to confirm CR in the case of extramedullary disease were not mandated. Patients who died because of toxicity or had missing data with sufficient follow-up are included in the response assessment and considered as nonresponders. MRD was determined by either clonoSEQ or flow cytometry at a sensitivity of at least 10−5 nucleated cells. High-risk cytogenetics were defined by the presence of del (17p), t(4;14), and t(14;16) at any time point before CAR T-cell infusion.
Statistical Analyses
The distribution of patient characteristics was examined by severe CRS (< grade 3, ≥ grade 3), severe NT (< grade 2, ≥ grade 2), best response of ≥ CR by day 90 (CR or better, < CR), and best ORR by day 90 (partial response [PR] or better, < PR) using chi-square or Fisher's exact tests for categorical variables or Kruskal-Wallis rank sum tests for continuous variables. We performed multivariable logistic regression to examine the association of a priori selected patient characteristics, which are given in the Appendix 1 (online only).
Definitions of OS, PFS, and duration of response (DOR) are given in the Appendix 1. Kaplan-Meier survival curves were used to estimate OS, PFS, and DOR, and the log-rank test was used to compare survival among groups on the basis of prior use of BCMA-targeted therapy (TT), meeting KarMMA trial eligibility criteria, and high-risk cytogenetics. Multivariable Cox proportional hazard regression models were used to examine the association of aforementioned a priori selected patient characteristics with PFS. The proportional hazard assumption was tested using covariate × time interaction terms individually and collectively. No violations of proportional hazards were observed. All analyses were conducted using R (Version 4.1.2).
Ethics Approval
This multicenter study was approved by respective institutions' Institutional Review Board.
RESULTS
Patient Characteristics and Disposition
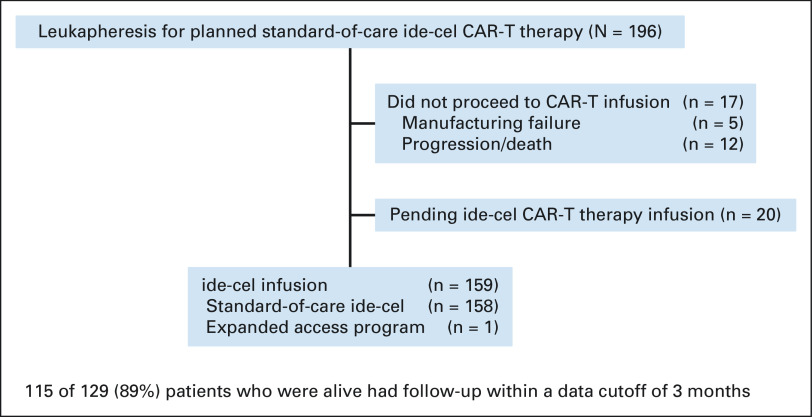
As of February 28, 2022, 196 patients completed leukapheresis with intent to manufacture and receive commercial ide-cel at 11 US medical centers (Fig 1 and Appendix Table A1, online only). Manufacturing failure was seen in 12 patients (6%), and of these, 7 (58%) manufactured successfully with repeat apheresis. A total of 159 patients received ide-cel, 17 did not proceed to CAR T because of progression/manufacturing failure, and 20 were pending infusion at data cutoff, indicating that 90% of patients (159 of 176) were able to be successfully administered ide-cel.
FIG 1.
Flow diagram. CAR, chimeric antigen receptor; ide-cel, idecabtagene vicleucel.
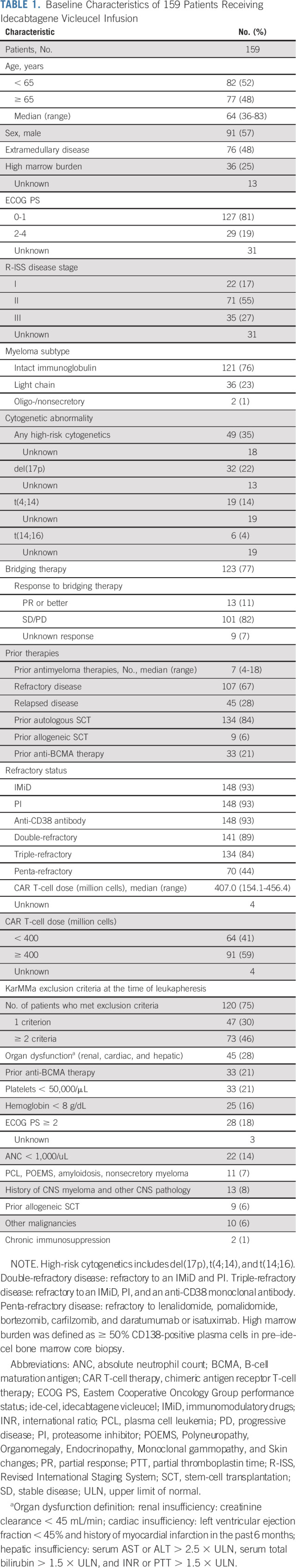
Table 1 shows the patient characteristics. The median age was 64 years, and 35% of patients had high-risk cytogenetics. The median number of prior lines of therapy was seven (range, 4-18), and 44% of patients had penta-refractory disease. At leukapheresis, 129 of 159 (75%) infused patients had comorbidities that would have made them ineligible for participation in the KarMMa clinical trial (ClinicalTrials.gov identifier: NCT03361748). The most common reasons for trial ineligibility included inadequate organ function in 45 patients (28% in total and 13% with renal dysfunction), prior use of BCMA-TT in 33 patients (21%), cytopenias (absolute neutrophil count < 1,000/μL in 22 [14%], hemoglobin < 8 g/dL in 25 [16%], and platelet count < 50,000/μL in 33 [21%] of patients, respectively), and an Eastern Cooperative Oncology Group performance status (ECOG PS) ≥ 2 in 28 patients (18%). In addition, relative to KarMMa trial, this real-world cohort had more patients with extramedullary and penta-refractory disease at 48% versus 39% and 44% versus 26%, respectively. The median time from leukapheresis to ide-cel infusion was 47 days (range, 34-91 days).
TABLE 1.
Baseline Characteristics of 159 Patients Receiving Idecabtagene Vicleucel Infusion
After conditioning chemotherapy with cyclophosphamide and fludarabine, 159 patients (90%) received ide-cel (Fig 1) with the median follow-up of 6.1 months (range, 0.0-13.1 months) and 8.0 months (range, 1.7-14.3 months) from infusion and leukapheresis, respectively. Of these, 158 (99.4%) received commercial ide-cel and one (0.6%) was treated under an expanded access protocol.
Safety
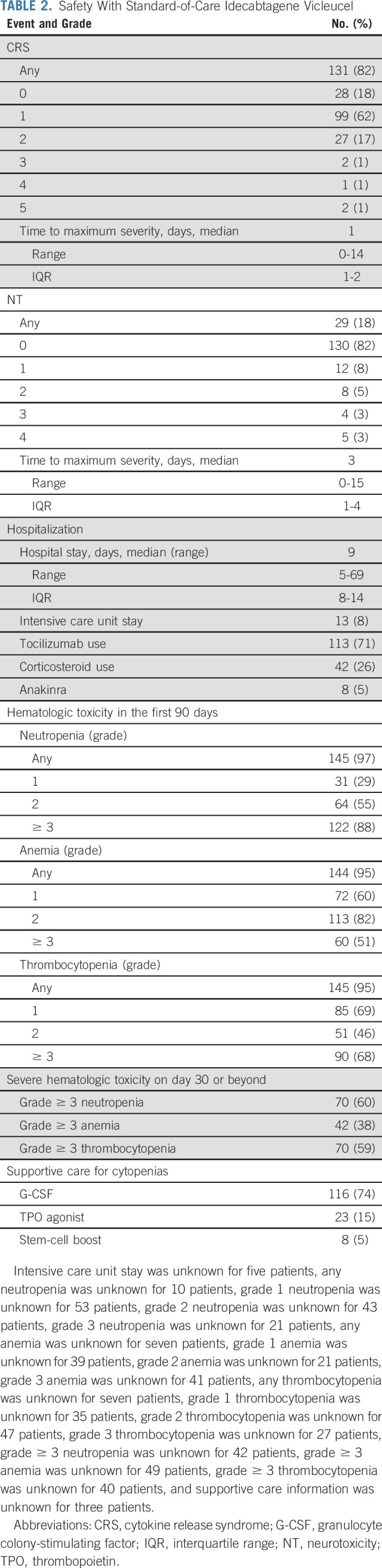
Median hospital stay was 9 days (range, 5-69 days). Any grade, grade ≥ 2, and grade ≥ 3 CRS occurred in 82%, 20%, and 3% of patients, respectively. Any grade, grade ≥ 2, and grade ≥ 3 NT occurred in 18%, 11%, and 6% of patients, respectively (Table 2). Seventy-one percent of patients received tocilizumab, 5% received anakinra, and 26% received glucocorticoids for CRS, NT, or both; 8% were transferred to an intensive care unit. Of these, one patient (0.6%) required hemodialysis. Any grade (and grade ≥ 3) neutropenia, anemia, and thrombocytopenia occurred in 97% (88%), 95% (51%), and 95% (68%) of patients, respectively (Table 2). Grade ≥ 3 hematologic toxicity persisted ≥ 30 days after infusion as follows: neutropenia in 60%, anemia in 38%, and thrombocytopenia in 59% of patients. Seventy-four percent of patients received granulocyte colony-stimulating factor, 15% received a thrombopoietin agonist, and 5% received an autologous stem-cell boost. Infections occurred in 52 (34%) patients, with 31 (20%) experiencing bacterial, 24 (16%) viral, and 2 (1%) fungal infections.
TABLE 2.
Safety With Standard-of-Care Idecabtagene Vicleucel
Thirty (19%) patients who received commercial ide-cel have died by last follow-up: 20 deaths were attributed to myeloma progression, eight were a result of nonrelapse mortality (5%), and cause of death was unknown in two patients. Causes of nonrelapse mortality included ide-cel–related toxicity (n = 3; two with grade 5 CRS, one with hemophagocytic lymphohistiocytosis who also had concomitant grade 5 CRS, and one with other NT in the form of progressive ascending weakness without evidence of central nervous system myeloma involvement), COVID-19 disease (n = 3), and cardiomyopathy (n = 2; Appendix Table A2, online only).
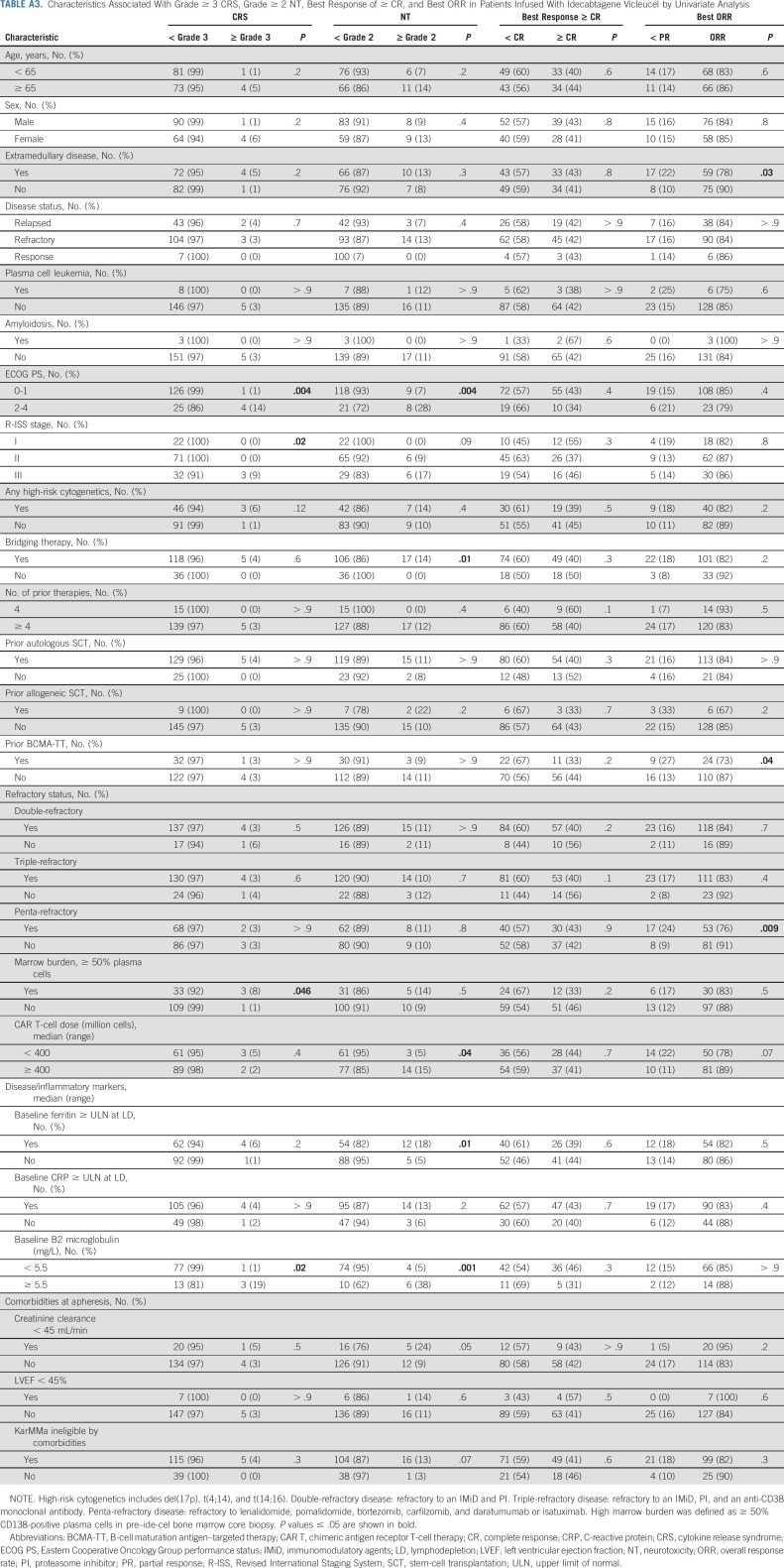
Univariable analysis was performed to determine the association of patient and disease characteristics with the risk of grade ≥ 3 CRS and grade ≥ 2 NT (Appendix Table A3, online only). Patients with a poor ECOG PS of ≥ 2 (P = .004), a higher Revised International Staging System (R-ISS) stage (P = .02), and a high marrow burden defined as ≥ 50% CD138-positive plasma cells in the pre–ide-cel bone marrow core biopsy (P = .046) were more likely to experience grade ≥ 3 CRS. Grade ≥ 2 NT was associated with a poor ECOG PS of ≥ 2 (P = .004), elevated baseline ferritin > upper limit of normal (P = .010), elevated baseline B2 microglobulin > 5.5 mg/L (P = .001), use of bridging chemotherapy (P = .014), and a higher cell dose of ≥ 400 × 106 CAR T cells (P = .036). Multivariable analysis for toxicity was not performed because of the small number of higher-grade CRS and NT events.
Response to Therapy
Day 30 and best overall responses were assessed in 159 patients, and day 90 response was assessed in 149 patients as 10 patients who were in active follow-up had not reached this time point. Patients who were not evaluable for any reason including death because of toxicity or missing data with sufficient follow-up were considered nonresponders. The best ORR and ≥ CR rate after commercial ide-cel were 84% and 42%, respectively (Fig 2 and Appendix Table A4, online only). In the CR/stringent complete response population, 72% of patients achieved MRD-negative status (at 10−5 nucleated cells). Day 30 and day 90 ORR rates were 78% and 72% with corresponding ≥ CR rates being 30% and 38%, respectively, among evaluable patients. The median time for both the response and ≥ CR was 30 days. The median DOR was 8.6 months (95% CI, 6.6 months to not reached; Fig 2).
FIG 2.
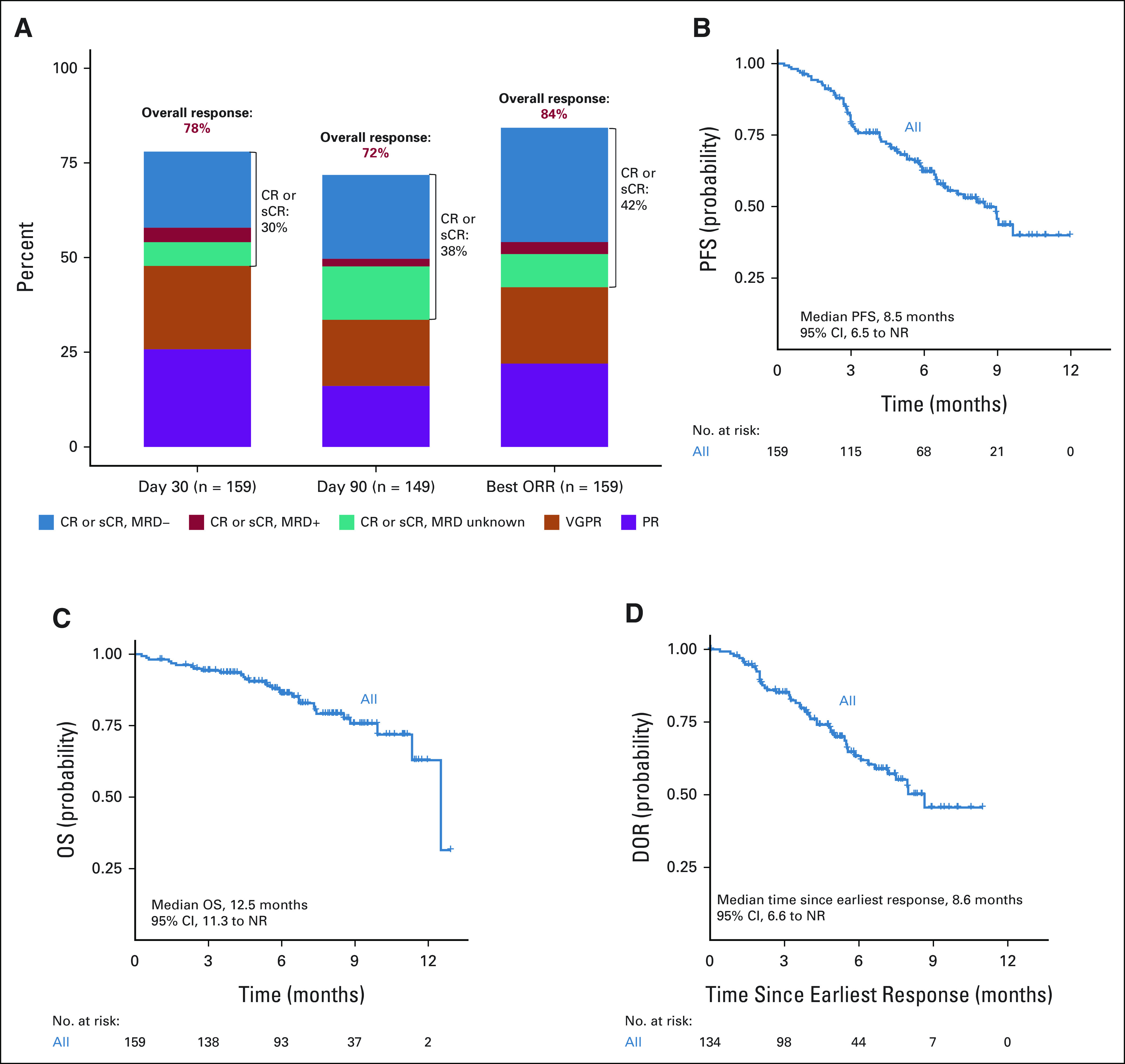
Tumor responses, DOR, PFS, and OS estimates. (A) Day 30, day 90, and best overall tumor responses. (B) PFS from ide-cel infusion. (C) OS from ide-cel infusion. (D) DOR in ide-cel responders. CR, complete response; DOR, Duration of response; ide-cel, idecabtagene vicleucel; MRD, minimal residual disease; NR, not reached; OS, overall survival; PFS, progression-free survival; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
To examine how responses changed over time, we evaluated patients with a day 30 response of stable disease, PR, or very good partial response and a day 90 response assessment (n = 88). Among the 32 patients with a very good partial response and 39 with a PR, 40% and 10% improved to ≥ CR at day 90, respectively. Of the 17 patients with stable disease, no patients achieved ≥ CR response. Patients with light chain–only disease were noted to achieve ≥ CR earlier compared with those with intact immunoglobulin disease. CR or better rates at day 30 were 27% versus 47%, and at day 90, they were 39% versus 57%, respectively.
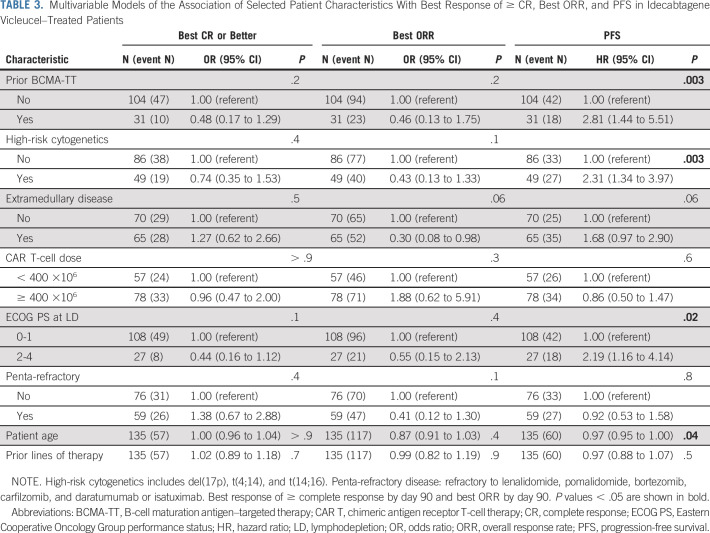
Univariable (Appendix Table A3) and multivariable analyses (Table 3) were performed to determine baseline characteristics associated with best response of ≥ CR and best ORR. In univariable analysis, the following factors were associated with inferior ORR: extramedullary disease (P = .03), prior use of BCMA-TT (P = .04), and penta-refractory status (P = .009). No associations were identified by best response of ≥ CR on univariable and by both best response of ≥ CR and ORR on multivariable analyses. Response rates by prior BCMA-TT are shown in Figure 3. Rate of ≥ CR and best ORR in patients with and without prior BCMA-TT exposure were 33% versus 44% (P = .2) and 73% versus 87% (P = .04), respectively.
TABLE 3.
Multivariable Models of the Association of Selected Patient Characteristics With Best Response of ≥ CR, Best ORR, and PFS in Idecabtagene Vicleucel–Treated Patients
FIG 3.
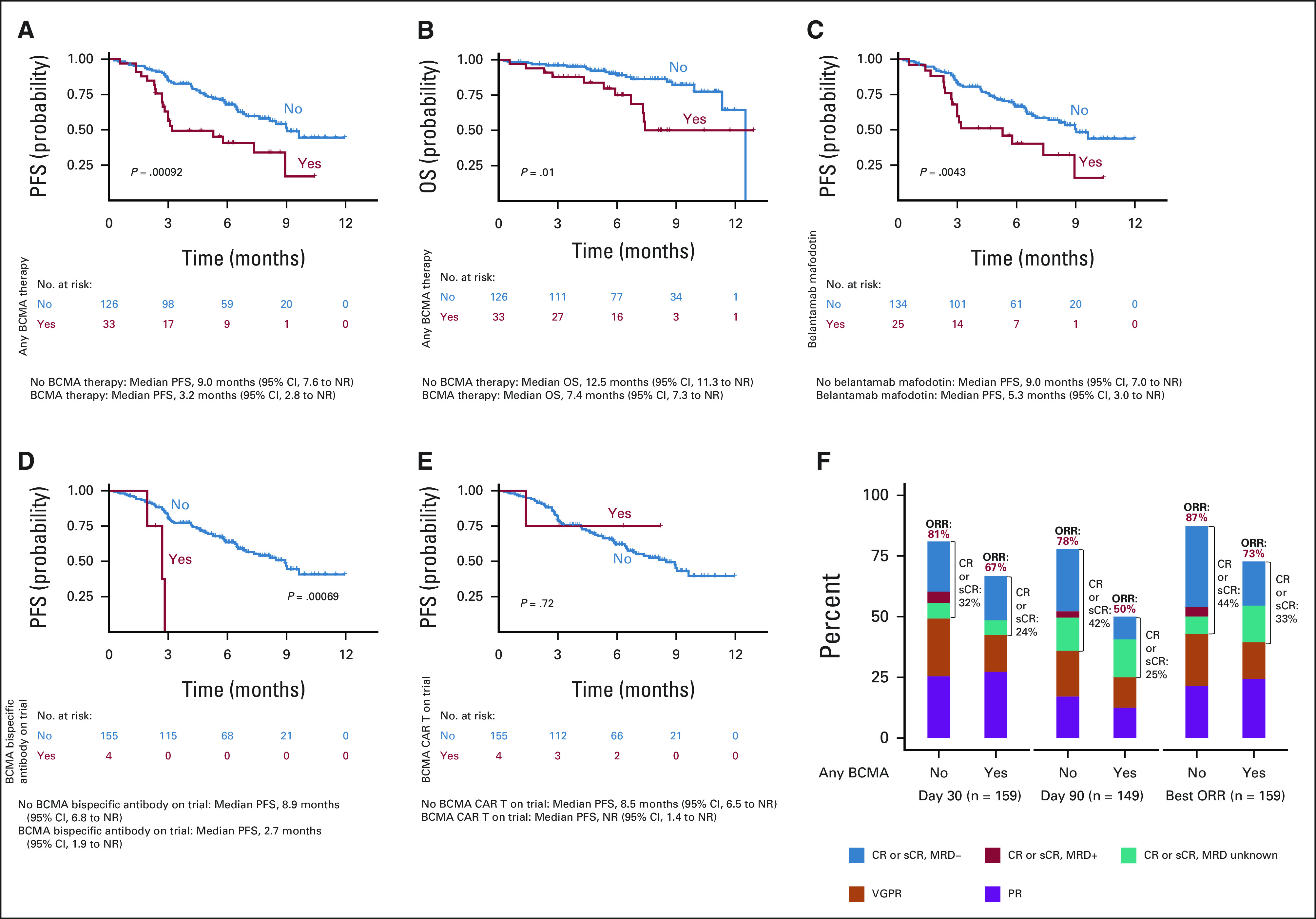
PFS and OS estimates by prior BCMA exposure. (A) PFS by any prior BCMA-TT. (B) OS by any prior BCMA-TT. (C) PFS by prior belantamab mafodotin. (D) PFS by BCMA bispecific T-cell recruiting antibody on clinical trial. (E) PFS by prior BCMA CAR T on clinical trial. (F) Tumor responses by any prior BCMA-TT. BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; CR, complete response; MRD, minimal residual disease; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; sCR, stringent complete response; TT, targeted therapy; VGPR, very good partial response.
PFS and OS
The median follow-up was 6.1 months from CAR T infusion. In this cohort, the median PFS and OS from CAR T infusion were 8.5 months (95% CI, 6.5 months to not reached) and 12.5 months (95% CI, 11.3 months to not reached), respectively (Fig 2). Multivariable analysis identified baseline characteristics associated with inferior PFS to include prior use of BCMA-TT (hazard ratio [HR], 2.81; 95% CI, 1.44 to 5.51; P = .003), high-risk cytogenetics (HR, 2.31; 95% CI, 1.34 to 3.97; P = .003), an ECOG PS of 2-4 at lymphodepletion (HR, 2.19; 95% CI, 1.16 to 4.14; P = .016), and younger age (HR, 0.97; 95% CI, 0.95 to 1.00; P = .043; Table 3).
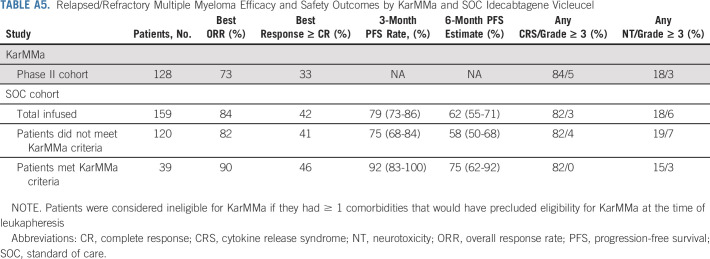
Compared with patients who did not receive prior BCMA-TT, patients who received any prior BCMA-TT (n = 33) had inferior PFS (median PFS of 9.0 v 3.2 months, respectively; P = .00092) and OS (median OS of 12.5 v 7.4 months, respectively; P = .01). When evaluating each type of prior BCMA-TT, inferior PFS was observed in patients who received (n = 25) versus did not receive belantamab mafodotin (median PFS of 5.3 v 9.0 months, P = .0043) and BCMA bispecific antibody on clinical trial (n = 4, median PFS of 2.7 v 8.9 months, P = .00069). Median PFS was not reached in patients who received (n = 4) versus did not receive prior BCMA CAR T-cell therapy on clinical trial (median PFS not reached v 8.5 months, P = .72; Fig 3). Patients with high-risk cytogenetics had a trend for inferior PFS (P = .072), but there was no difference in OS (Appendix Fig A1, online only). Infused patients who were ineligible for KarMMa trial had a trend for inferior PFS (median PFS 7.6 v 9.0 months, P = .19) but no difference in OS relative to eligible patients (Appendix Fig A2, online only). Ineligibility for KarMMa trial was not included as a variable in the multivariable model because of collinearity with other factors in the model. Efficacy and safety outcomes were comparable with those in KarMMa trial as listed in Appendix Table A5 (online only).
DISCUSSION
To our knowledge, this large multicenter study is the first real-world report of clinical outcomes in patients with RRMM receiving SOC ide-cel CAR T. Overall, we observed comparable efficacy and toxicity with ide-cel as reported in the KarMMa trial although 75% of our patients would not have met KarMMa eligibility criteria. The most common reasons for trial ineligibility included inadequate organ function, prior exposure to BCMA-TT, cytopenias, and poor performance status. Despite this, 90% of eligible patients were administered ide-cel, which is comparable with 91% in the KarMMa trial. Our data indicate that CAR T administration in the real world is feasible, safe, and effective, even among patients with comorbidities.
The safety profile of ide-cel in our real-world cohort was comparable with those in the KarMMa trial, with similar rates of CRS, NT, infections, and persistent cytopenias. NT in the current study was defined on the basis of the consensus American Society for Transplantation and Cellular Therapy criteria,8 which are different from the National Cancer Institute Common Terminology Criteria for Adverse Events criteria used for NT assessment on the KarMMa trial. Despite this, we observed a comparably lower rate of NT (18% in both), including grade ≥ 3 NT (6% v 3%) in our cohort versus trial patients, respectively. Grade 3 or higher persistent cytopenias beyond 1 month were seen in 38%-60% of patients in our cohort, which is comparable with those in the KarMMa trial (neutropenia: 41%; thrombocytopenia: 48%).
Successful and timely manufacture of CAR T cells is an essential component of delivering this complex therapy to patients. Manufacturing failure with first apheresis occurred in 6% (n = 12 of 196 who underwent apheresis) of our patients although more than half of them (n = 7) subsequently successfully manufactured CAR T cells. The manufacturing failure rate in real-world patients is higher than that seen in KarMMa where only 1 of the 140 enrolled patients had a manufacturing failure. Higher rate of manufacturing failure with SOC ide-cel could be due to poor bone marrow reserve among our patients, as reflected by the high rate of baseline cytopenias. This may be related to prior treatment, including alkylators, which result in T-cell depletion and are associated with poor manufacturing.11 Future efforts should focus on investigating factors associated with poor CAR T manufacturing to allow for optimal patient selection.
Efficacy of ide-cel in our cohort was comparable with that in the KarMMa trial population. The response and CR rates with SOC ide-cel were 84% and 42%, which are comparable with 73% and 33% noted in the KarMMa trial overall and in patients receiving 450 million CAR T-cell dose at 81% and 39%, respectively. The difference in CR and ORR between the current study and the KarMMa trial may be related to the fact that KarMMa responses were determined by an independent response assessment board, whereas the current study results were obtained by investigator determination and confirmatory testing/imaging was not mandated. Similarly, the high CR/ORR observed in the SOC setting are likely due to patients receiving a higher CAR T-cell dose with a median of 407 million CAR T cells and the fact that there were no limitations in the duration and type of bridging chemotherapy that patients received compared with the KarMMa clinical trial. The median DOR was 8.6 months in our study versus 10.7 months in the KarMMa trial. CAR T-cell therapy results in rapid responses with a median time to response of 1 month in our cohort, similar to that reported in clinical trials with ide-cel and other CAR T constructs.6,12-14 The median PFS of 8.5 months in our cohort is similar to that observed in KarMMa. Not surprisingly, PFS was lower in patients who would not have met KarMMa eligibility criteria. Our relatively large sample size allowed us to conduct a multivariable analysis, indicating that prior use of BCMA-TT, high-risk cytogenetics, ECOG PS ≥ 2 at lymphodepletion, and younger age were independent predictors of inferior PFS.
Despite advances in therapy, patients with RRMM eventually relapse. There are several other BCMA-TT available commercially or in advanced clinical development such as antibody drug conjugates (belantamab mafodotin),15,16 other CAR Ts (such as ciltacabtagene autoleucel and others),12,13,17 and bispecific T cell recruiting antibodies (teclistamab, elranatamab, and others).18-22 Efficacy of sequential treatment with different BCMA-TT remains an unanswered question in the treatment of RRMM. Twenty one percent of our cohort was previously treated with BCMA-TT, and although responses were observed in these patients, response and ≥ CR rates were lower and prior BCMA-TT was an independent predictor of inferior PFS. This finding has important implications for clinical practice. It is important to note that most patients received prior belantamab mafodotin although responses were seen after prior BCMA CAR T and other investigational therapies on trial. Given the responses seen with ide-cel after relapse after other BCMA-TT, we hypothesize that BCMA expression is retained or regained in most patients even after relapse on these therapies although these data are not available for patients in our cohort. Whether BCMA expression at relapse varies on the basis of the type of BCMA-TT is unknown and should be explored in future studies. In the KarMMa trial, BCMA expression was retained in around 95% of patients at relapse.6 CAR T and bispecific antibodies against non-BCMA targets such as GPRC5D23-26 and FcRH527 have shown promising early activity, including in patients treated with prior BCMA-TT.25-27 Clinical trials with these therapies can be considered at relapse after ide-cel, as these therapies are not commercially available yet.
Strengths of this study include a large multi-institutional cohort of patients treated with ide-cel over a short period of time. Limitations of our study include its retrospective design, limited follow-up, and heterogeneity in institutional standards for toxicity management across different centers. In addition, P values were not adjusted for multiple comparisons but provide first of its kind data on factors associated with efficacy and safety of ide-cel in patients with RRMM. Another limitation of our study is that response assessment was per investigator discretion, and there was no independent review committee. Confirmatory testing and imaging to confirm CR in the case of extramedullary disease were also not mandated. These are limitations of the retrospective design of the study, and data should be interpreted in this context. Despite these limitations, the observed PFS in our study was remarkably similar to that observed in the KarMMa trial. As response rates are closely related to PFS in multiple myeloma, this similarity in PFS is reassuring that response assessment is representative of changes in disease burden. Future studies with standard-of-care CAR T in RRMM should focus on mechanisms of relapse, long-term outcomes including risk of infections, other toxicities and durability of response, and comparative safety and efficacy of different SOC CAR T constructs in myeloma.
In summary, the efficacy and safety profile with SOC ide-cel is comparable with that observed in the KarMMa trial although the majority of patients would not have met clinical trial eligibility criteria. It is feasible to administer ide-cel as SOC, with high response rates and low incidence of severe CRS and NT, although persistent cytopenias remain an ongoing issue.
APPENDIX 1. Supplementary Methods
A uniform data collection form with embedded data dictionary was provided to all participating centers by the coordinating center, along with an example on guidelines for data collection. All sites returned data to the coordinating center. A quality control check was done by the coordinating center, and queries were issued for missing data or data that did not follow the format specified in the data collection form.
Overall survival was calculated as time between the date of infusion and date of death from any cause or last contact, and progression-free survival was calculated as time between the date of infusion and date of progression, death, or last contact. DOR was calculated as time between first response of partial response or better and date of progression, death, or last contact. We performed multivariable logistic regression to examine the association of a priori selected patient characteristics (prior B-cell maturation antigen–targeted therapy [yes, no], high-risk cytogenetics [yes, no], extramedullary disease [yes, no], cell dose [< 400, ≥ 400 million CAR-T cells], Eastern Cooperative Oncology Group performance status [0-1, 2-4], penta-refractory disease [yes, no], number of prior lines of therapy [continuous, number of lines], and age at infusion [continuous, years]) with outcomes of best response of ≥ complete response by day 90 and best overall response rate by day 90.
TABLE A1.
Baseline Characteristics of 196 Patients Who Underwent Apheresis With Intent to Manufacture Idecabtagene Vicleucel
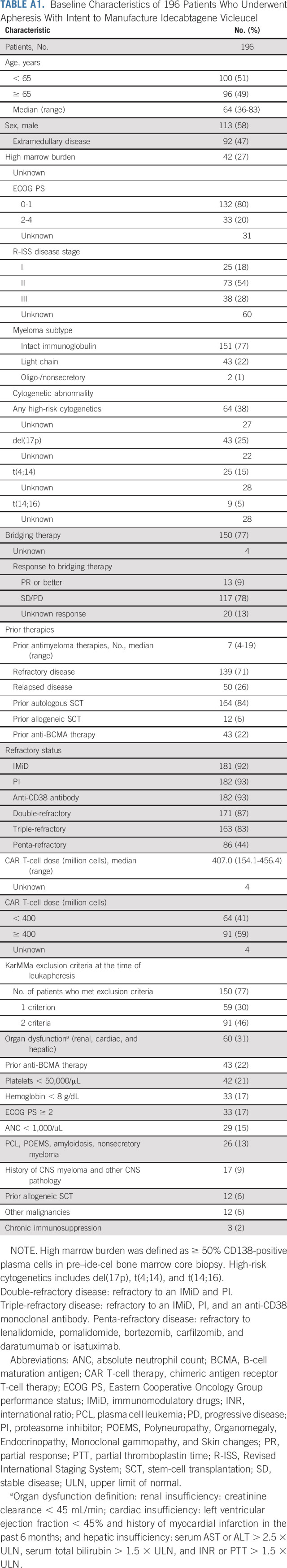
TABLE A2.
Cause of Death in Patients Who Received Idecabtagene Vicleucel
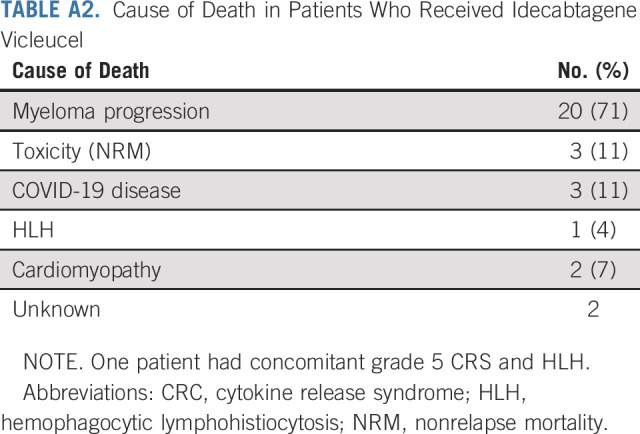
TABLE A3.
Characteristics Associated With Grade ≥ 3 CRS, Grade ≥ 2 NT, Best Response of ≥ CR, and Best ORR in Patients Infused With Idecabtagene Vicleucel by Univariate Analysis
TABLE A4.
Patients' Response to Idecabtagene Vicleucel
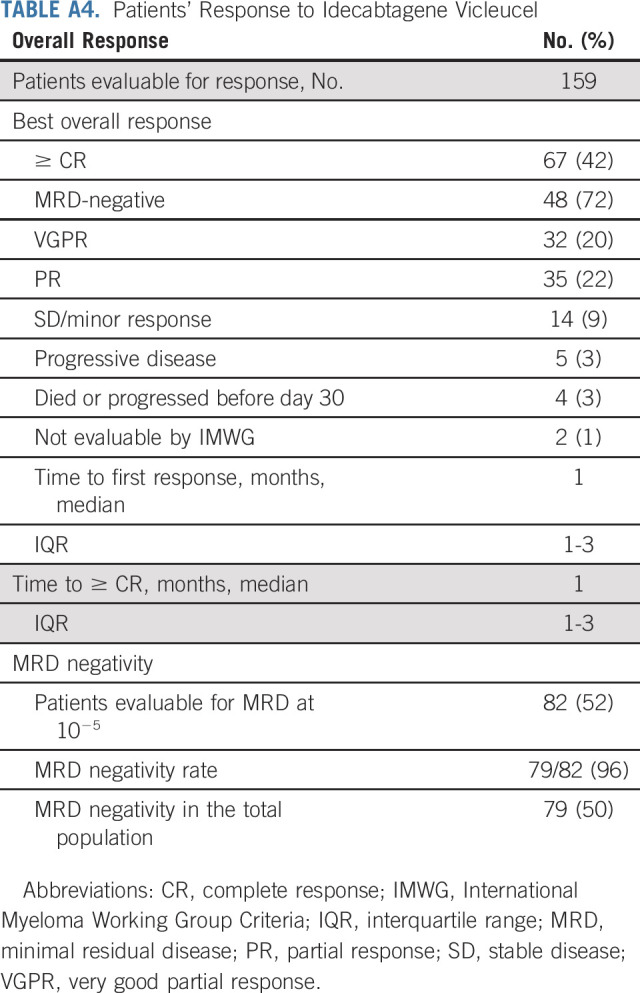
TABLE A5.
Relapsed/Refractory Multiple Myeloma Efficacy and Safety Outcomes by KarMMa and SOC Idecabtagene Vicleucel
FIG A1.
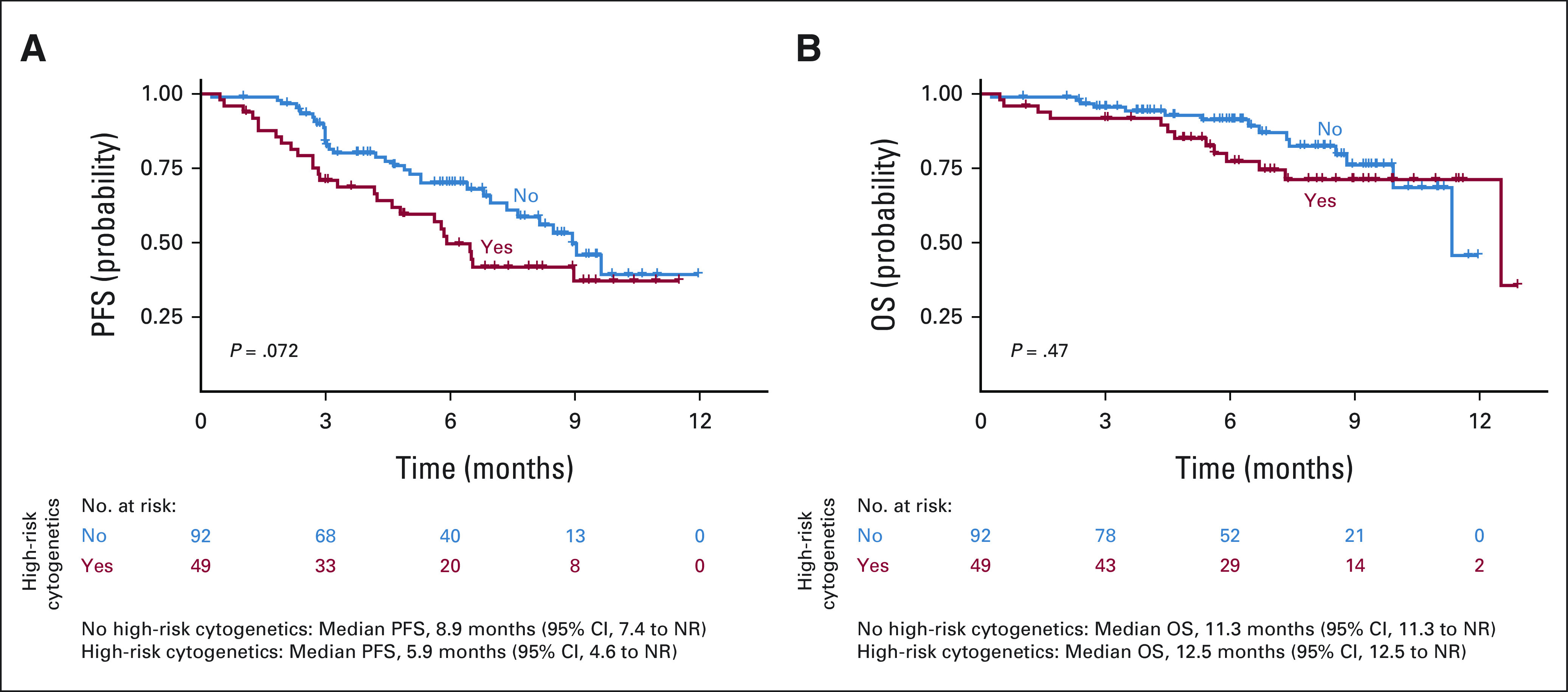
(A) PFS and (B) OS by high-risk cytogenetics. NR, not reached; OS, overall survival; PFS, progression-free survival.
FIG A2.
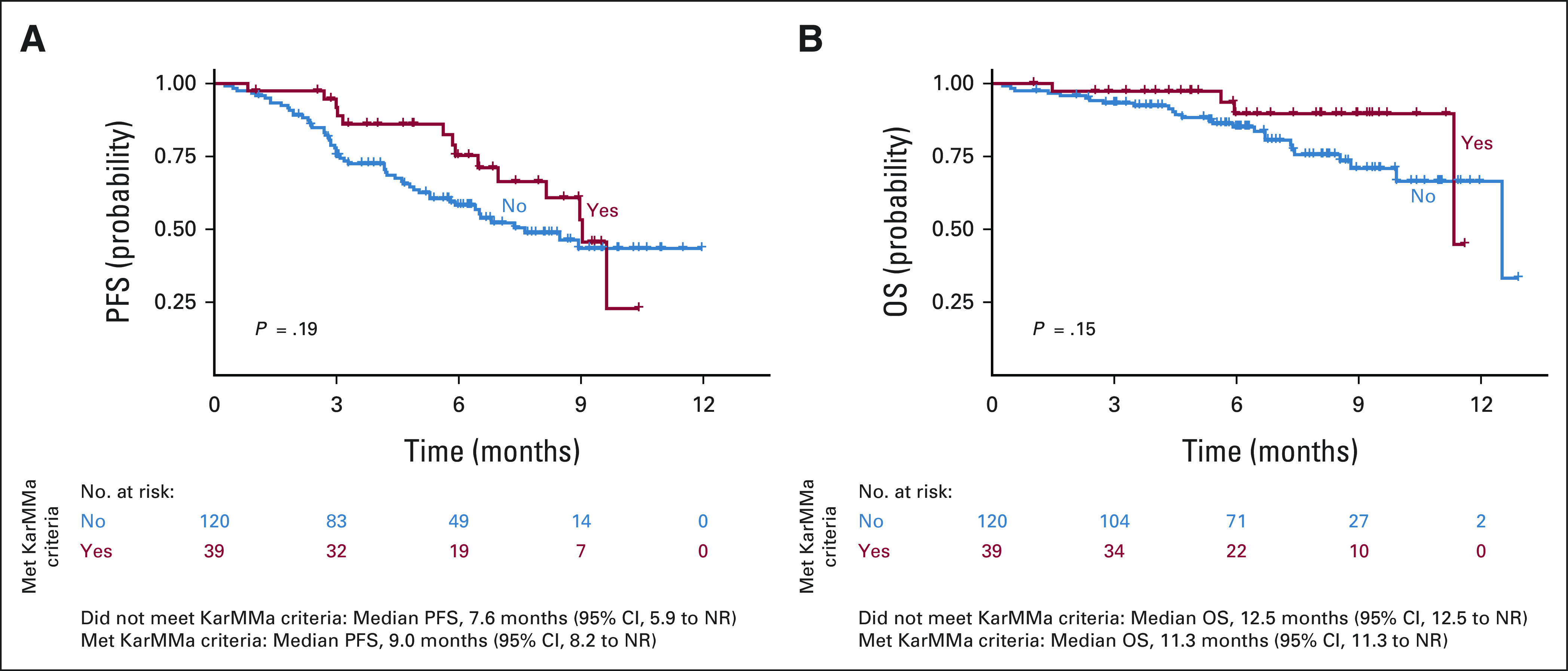
(A) PFS and (B) OS by presence or absence of KarMMa exclusion criteria. NR, not reached; OS, overall survival; PFS, progression-free survival.
Doris K. Hansen
Consulting or Advisory Role: Bristol Myers Squibb Foundation
Research Funding: Bristol Myers Squibb/Celgene
Surbhi Sidana
Consulting or Advisory Role: Janssen, Bristol Myers Squibb/Celgene, Oncopeptides, Magenta Therapeutics, Sanofi
Research Funding: Janssen (Inst), Magenta Therapeutics (Inst), Allogene Therapeutics (Inst), Bristol Myers Squibb/Celgene (Inst)
Lauren C. Peres
Research Funding: Bristol Myers Squibb Foundation (Inst)
Douglas W. Sborov
Consulting or Advisory Role: Sanofi, GlaxoSmithKline, Bristol Myers Squibb/Celgene, Legend Biotech, Janssen, Skyline Diagnostics, AbbVie, Pfizer
Charlotte Wagner
Employment: AbbVie
Consulting or Advisory Role: Pfizer
Hamza Hashmi
Consulting or Advisory Role: Sanofi, Bristol Myers Squibb/Celgene, Janssen
Mehmet H. Kocoglu
Research Funding: BMS (Inst), Genentech (Inst), GlaxoSmithKline (Inst), AbbVie/Genentech (Inst), Janssen (Inst), Poseida (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/366351
Shebli Atrash
Honoraria: Janssen
Speakers’ Bureau: Celgene, Janssen
Research Funding: GlaxoSmithKline, Amgen, Karyopharm Therapeutics, Janssen Oncology (Inst), Celgene/Bristol Myers Squibb (Inst)
Gary Simmons
Speakers’ Bureau: Kite, a Gilead company
Christopher Ferreri
Stock and Other Ownership Interests: Affimed Therapeutics
Consulting or Advisory Role: Sanofi
Ankit Kansagra
Employment: Johnson and Johnson
Consulting or Advisory Role: Janssen Oncology, Celgene, Sanofi, Karyopharm Therapeutics, GlaxoSmithKline, Pfizer, Takeda, Pharmacyclics, Alnylam, Oncopeptides
Peter Voorhees
Consulting or Advisory Role: AbbVie/Genentech, Karyopharm Therapeutics, Bristol Myers Squibb, Pfizer, Sanofi, Janssen, GlaxoSmithKline
Research Funding: AbbVie (Inst), Janssen (Inst), GlaxoSmithKline (Inst), TeneoBio (Inst)
Travel, Accommodations, Expenses: Sanofi
Uncompensated Relationships: GlaxoSmithKline
Rachid Baz
Consulting or Advisory Role: Karyopharm Therapeutics, BMS, AbbVie, Oncopeptides, Shattuck Labs, Sanofi, Janssen
Research Funding: Karyopharm Therapeutics (Inst), Celgene (Inst), Merck Sharp & Dohme (Inst), Takeda (Inst), Signal Genetics (Inst), AbbVie (Inst), Sanofi (Inst), Janssen (Inst), BMS (Inst)
Other Relationship: GlaxoSmithKline
Melissa Alsina
Honoraria: Janssen
Consulting or Advisory Role: BMS, Janssen Oncology
Speakers’ Bureau: Janssen Oncology
Joseph McGuirk
Honoraria: Kite, a Gilead company, AlloVir, Magenta Therapeutics, Nektar, Sana Biotechnology
Consulting or Advisory Role: Kite, a Gilead company, Juno Therapeutics, AlloVir, Magenta Therapeutics, EcoR1 Capital, CRISPR therapeutics
Speakers’ Bureau: Kite/Gilead
Research Funding: Novartis (Inst), Fresenius Biotech (Inst), Astellas Pharma (Inst), Bellicum Pharmaceuticals (Inst), Novartis (Inst), Gamida Cell (Inst), Pluristem Therapeutics (Inst), Kite, a Gilead company (Inst), AlloVir (Inst)
Travel, Accommodations, Expenses: Kite, a Gilead company
Frederick L. Locke
Consulting or Advisory Role: Novartis, Celgene, Calibr, Alimera Sciences, Gerson Lehrman Group, EcoR1 Capital, Amgen, Bluebird Bio, Bristol Myers Squibb, Iovance Biotherapeutics, Legend Biotech, Cowen, Kite, a Gilead company, Umoja Biopharma, Takeda, Sana Biotechnology, Daiichi Sankyo/UCB Japan, Bristol Myers Squibb/Celgene, Janssen, A2 Biotherapeutics, Miltenyi Biotec, Caribou Biosciences, Takeda, Umoja Biopharma
Research Funding: Kite, a Gilead company (Inst), Alimera Sciences (Inst), Novartis (Inst), Bluebird Bio (Inst), Bristol Myers Squibb/Celgene (Inst)
Patents, Royalties, Other Intellectual Property: Double Mutant Survivin Vaccine. US010414810B2 (Inst), CAR T Cells with Enhanced Metabolic Fitness. Serial No.: 62/939,727 (Inst), Methods of Enhancing CAR T Cell Therapies. Serial No.: 62/892,292 (Inst), Evolutionary Dynamics of Non-Hodgkin Lymphoma CAR-T cell therapy. Serial No.: 62/879,534 (Inst)
Travel, Accommodations, Expenses: Kite, a Gilead company, A2 Biotherapeutics
Krina K. Patel
Consulting or Advisory Role: Celgene, Bristol Myers Squibb, Janssen, Pfizer, Arcellx, Karyopharm Therapeutics
Research Funding: Celgene/Bristol Myers Squibb, Poseida, Takeda, Janssen, Cellectis, Nektar, AbbVie/Genentech, Precision Biosciences, Allogene Therapeutics
Travel, Accommodations, Expenses: Bristol Myers Squibb
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the ASCO Annual Meeting and Exposition, Chicago, IL, June 3-7, 2022; the International Myeloma Society Annual Meeting, Los Angeles, CA, August 25-27, 2022.
D.K.H. and S.S. are cofirst authors with equal contribution; J.M., F.L.L., and K.K.P. are cosenior authors.
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
AUTHOR CONTRIBUTIONS
Conception and design: Doris K. Hansen, Surbhi Sidana, Lauren C. Peres, Leyla Shune, Rachid Baz, Melissa Alsina, Frederick L. Locke, Krina K. Patel
Administrative support: Frederick L. Locke, Krina K. Patel
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: Doris K. Hansen, Surbhi Sidana, Lauren C. Peres, Christelle Colin Leitzinger, Peter Voorhees, Rachid Baz, Melissa Alsina, Joseph McGuirk, Frederick L. Locke, Krina K. Patel
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Idecabtagene Vicleucel for Relapsed/Refractory Multiple Myeloma: Real-World Experience from the Myeloma CAR T Consortium
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Doris K. Hansen
Consulting or Advisory Role: Bristol Myers Squibb Foundation
Research Funding: Bristol Myers Squibb/Celgene
Surbhi Sidana
Consulting or Advisory Role: Janssen, Bristol Myers Squibb/Celgene, Oncopeptides, Magenta Therapeutics, Sanofi
Research Funding: Janssen (Inst), Magenta Therapeutics (Inst), Allogene Therapeutics (Inst), Bristol Myers Squibb/Celgene (Inst)
Lauren C. Peres
Research Funding: Bristol Myers Squibb Foundation (Inst)
Douglas W. Sborov
Consulting or Advisory Role: Sanofi, GlaxoSmithKline, Bristol Myers Squibb/Celgene, Legend Biotech, Janssen, Skyline Diagnostics, AbbVie, Pfizer
Charlotte Wagner
Employment: AbbVie
Consulting or Advisory Role: Pfizer
Hamza Hashmi
Consulting or Advisory Role: Sanofi, Bristol Myers Squibb/Celgene, Janssen
Mehmet H. Kocoglu
Research Funding: BMS (Inst), Genentech (Inst), GlaxoSmithKline (Inst), AbbVie/Genentech (Inst), Janssen (Inst), Poseida (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/366351
Shebli Atrash
Honoraria: Janssen
Speakers’ Bureau: Celgene, Janssen
Research Funding: GlaxoSmithKline, Amgen, Karyopharm Therapeutics, Janssen Oncology (Inst), Celgene/Bristol Myers Squibb (Inst)
Gary Simmons
Speakers’ Bureau: Kite, a Gilead company
Christopher Ferreri
Stock and Other Ownership Interests: Affimed Therapeutics
Consulting or Advisory Role: Sanofi
Ankit Kansagra
Employment: Johnson and Johnson
Consulting or Advisory Role: Janssen Oncology, Celgene, Sanofi, Karyopharm Therapeutics, GlaxoSmithKline, Pfizer, Takeda, Pharmacyclics, Alnylam, Oncopeptides
Peter Voorhees
Consulting or Advisory Role: AbbVie/Genentech, Karyopharm Therapeutics, Bristol Myers Squibb, Pfizer, Sanofi, Janssen, GlaxoSmithKline
Research Funding: AbbVie (Inst), Janssen (Inst), GlaxoSmithKline (Inst), TeneoBio (Inst)
Travel, Accommodations, Expenses: Sanofi
Uncompensated Relationships: GlaxoSmithKline
Rachid Baz
Consulting or Advisory Role: Karyopharm Therapeutics, BMS, AbbVie, Oncopeptides, Shattuck Labs, Sanofi, Janssen
Research Funding: Karyopharm Therapeutics (Inst), Celgene (Inst), Merck Sharp & Dohme (Inst), Takeda (Inst), Signal Genetics (Inst), AbbVie (Inst), Sanofi (Inst), Janssen (Inst), BMS (Inst)
Other Relationship: GlaxoSmithKline
Melissa Alsina
Honoraria: Janssen
Consulting or Advisory Role: BMS, Janssen Oncology
Speakers’ Bureau: Janssen Oncology
Joseph McGuirk
Honoraria: Kite, a Gilead company, AlloVir, Magenta Therapeutics, Nektar, Sana Biotechnology
Consulting or Advisory Role: Kite, a Gilead company, Juno Therapeutics, AlloVir, Magenta Therapeutics, EcoR1 Capital, CRISPR therapeutics
Speakers’ Bureau: Kite/Gilead
Research Funding: Novartis (Inst), Fresenius Biotech (Inst), Astellas Pharma (Inst), Bellicum Pharmaceuticals (Inst), Novartis (Inst), Gamida Cell (Inst), Pluristem Therapeutics (Inst), Kite, a Gilead company (Inst), AlloVir (Inst)
Travel, Accommodations, Expenses: Kite, a Gilead company
Frederick L. Locke
Consulting or Advisory Role: Novartis, Celgene, Calibr, Alimera Sciences, Gerson Lehrman Group, EcoR1 Capital, Amgen, Bluebird Bio, Bristol Myers Squibb, Iovance Biotherapeutics, Legend Biotech, Cowen, Kite, a Gilead company, Umoja Biopharma, Takeda, Sana Biotechnology, Daiichi Sankyo/UCB Japan, Bristol Myers Squibb/Celgene, Janssen, A2 Biotherapeutics, Miltenyi Biotec, Caribou Biosciences, Takeda, Umoja Biopharma
Research Funding: Kite, a Gilead company (Inst), Alimera Sciences (Inst), Novartis (Inst), Bluebird Bio (Inst), Bristol Myers Squibb/Celgene (Inst)
Patents, Royalties, Other Intellectual Property: Double Mutant Survivin Vaccine. US010414810B2 (Inst), CAR T Cells with Enhanced Metabolic Fitness. Serial No.: 62/939,727 (Inst), Methods of Enhancing CAR T Cell Therapies. Serial No.: 62/892,292 (Inst), Evolutionary Dynamics of Non-Hodgkin Lymphoma CAR-T cell therapy. Serial No.: 62/879,534 (Inst)
Travel, Accommodations, Expenses: Kite, a Gilead company, A2 Biotherapeutics
Krina K. Patel
Consulting or Advisory Role: Celgene, Bristol Myers Squibb, Janssen, Pfizer, Arcellx, Karyopharm Therapeutics
Research Funding: Celgene/Bristol Myers Squibb, Poseida, Takeda, Janssen, Cellectis, Nektar, AbbVie/Genentech, Precision Biosciences, Allogene Therapeutics
Travel, Accommodations, Expenses: Bristol Myers Squibb
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gandhi UH, Cornell RF, Lakshman A, et al. : Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 33:2266-2275, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikhael J: Treatment options for triple-class refractory multiple myeloma. Clin Lymphoma Myeloma Leuk 20:1-7, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Usmani S, Ahmadi T, Ng Y, et al. : Analysis of real-world data on overall survival in multiple myeloma patients with ≥3 prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory drug (ImiD), or double refractory to a PI and an ImiD. Oncologist 21:1355-1361, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chari A, Vogl DT, Gavriatopoulou M, et al. : Oral selinexor–dexamethasone for triple-class refractory multiple myeloma. N Engl J Med 381:727-738, 2019 [DOI] [PubMed] [Google Scholar]
- 5.BMS Pharma : ABECMA (idecabtagene vicleucel) [package insert]. https://packageinserts.bms.com/pi/pi_abecma.pdf [Google Scholar]
- 6.Munshi NC, Anderson LD Jr, Shah N, et al. : Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 384:705-716, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Raje N, Berdeja J, Lin Y, et al. : Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med 380:1726-1737, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DW, Santomasso BD, Locke FL, et al. : ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 25:625-638, 2019 [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute : Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. U.S. Department of Health and Human Services, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_ 5x7.pdf [Google Scholar]
- 10.Kumar S, Paiva B, Anderson KC, et al. : International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 17:e328-e346, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Rytlewski J, Madduri D, Fuller J, et al. : Effects of prior alkylating therapies on preinfusion patient characteristics and starting material for CAR T cell product manufacturing in late-line multiple myeloma. Blood 136:7-8, 2020. (suppl 1) [Google Scholar]
- 12.Berdeja JG, Madduri D, Usmani SZ, et al. : Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 398:314-324, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Martin T, Usmani SZ, Berdeja JG, et al. : Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol 41:1265-1274, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsina M, Shah N, Raje NS, et al. : Updated results from the phase I CRB-402 study of anti-Bcma CAR-T cell therapy bb21217 in patients with relapsed and refractory multiple myeloma: Correlation of expansion and duration of response with T cell phenotypes. Blood 136:25-26, 2020. (suppl 1) [Google Scholar]
- 15.Lonial S, Lee HC, Badros A, et al. : Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol 21:207-221, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Lonial S, Lee HC, Badros A, et al. : Longer term outcomes with single-agent belantamab mafodotin in patients with relapsed or refractory multiple myeloma: 13-month follow-up from the pivotal DREAMM-2 study. Cancer 127:4198-4212, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mailankody S, Matous JV, Liedtke M, et al. : Universal: An allogeneic first-in-human study of the anti-Bcma ALLO-715 and the anti-CD52 ALLO-647 in relapsed/refractory multiple myeloma. Blood 136:24-25, 2020. (suppl 1)32430494 [Google Scholar]
- 18.Usmani SZ, Garfall AL, van de Donk NWCJ, et al. : Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): A multicentre, open-label, single-arm, phase 1 study. Lancet 398:665-674, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Sebag M, Raje NS, Bahlis NJ, et al. : Elranatamab (PF-06863135), a B-cell maturation antigen (BCMA) targeted CD3-engaging bispecific molecule, for patients with relapsed or refractory multiple myeloma: Results from magnetismm-1. Blood 138:895, 2021. (suppl 1) [Google Scholar]
- 20.Hosoya H, Sidana S: Antibody-based treatment approaches in multiple myeloma. Curr Hematol Malig Rep 16:183-191, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S, D’Souza A, Shah N, et al. : A phase 1 first-in-human study of Tnb-383B, a BCMA x CD3 bispecific T-cell redirecting antibody, in patients with relapsed/refractory multiple myeloma. Blood 138:900, 2021. (suppl 1) [Google Scholar]
- 22.Zonder JA, Richter J, Bumma N, et al. : Early, deep, and durable responses, and low rates of cytokine release syndrome with REGN5458, a BCMAxCD3 bispecific monoclonal antibody, in a phase ½ first-in-human study in patients with relapsed/refractory multiple myeloma (RRMM). Blood 138:160, 2021. (suppl 1)33831168 [Google Scholar]
- 23.Smith EL, Harrington K, Staehr M, et al. : GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med 11:eaau7746, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pillarisetti K, Edavettal S, Mendonça M, et al. : A T-cell-redirecting bispecific G-protein-coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood 135:1232-1243, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chari A, Berdeja JG, Oriol A, et al. : A phase 1, first-in-human study of talquetamab, a G protein-coupled receptor family C group 5 member D (GPRC5D) x CD3 bispecific antibody, in patients with relapsed and/or refractory multiple myeloma (RRMM). Blood 136:40-41, 2020. (suppl 1) [Google Scholar]
- 26.Mailankody S, Diamonte C, Fitzgerald L, et al. : Phase I first-in-class trial of MCARH109, a G protein coupled receptor class C group 5 member D (GPRC5D) targeted CAR T cell therapy in patients with relapsed or refractory multiple myeloma. Blood 138:827, 2021. (suppl 1)34075408 [Google Scholar]
- 27.Trudel S, Cohen AD, Krishnan AY, et al. : Cevostamab monotherapy continues to show clinically meaningful activity and manageable safety in patients with heavily pre-treated relapsed/refractory multiple myeloma (RRMM): Updated results from an ongoing phase I study. Blood 138:157, 2021. (suppl 1) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.