Abstract
Recent studies have identified a novel programmed cell death based on copper, named cuproptosis. However, as an anti-cuproptosis gene, the functional roles, definite mechanisms and prognostic value of CDKN2A in pan-cancer are largely unclear. The GEPIA2, cancer genome atlas (TCGA), the tumor immune estimation resource 2.0 and CPTAC databases were performed to validate the differential expression of CDKN2A in 33 tumors. The clinical features and survival prognosis analysis were conducted by GEPIA2 and UALCAN web tool. Genetic alteration analysis of CDKN2A in pan-cancer was also evaluated. Furthermore, the functional roles of CDKN2A were explored via DNA methylation analysis, tumor microenvironment, infiltration of immune cells, enrichment analysis and gene co-expression associated with cuproptosis and immune regulation. The CDKN2A expression, both at the transcriptional and translational level, was obviously upregulated in most cancer patients, which might lead to poor survival in certain cancer types. CDKN2A expression was significantly associated with tumor pathological stages in some cancer types. In adrenocortical carcinoma (ACC) and kidney renal clear cell carcinoma (KIRC), DNA methylation of CDKN2A was explored to induce poor clinical outcomes. Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analysis indicated that CDKN2A expression was closely related to several cancer-associated signaling pathways, such as the p53 signaling pathway, Cellular senescence, DNA replication and Cell cycle signaling pathways. Gene set enrichment analysis (GSEA) analysis suggested that aberrantly expressed CDKN2A took part in the cell cycle regulation, immune regulation and mitochondrial signaling pathways in certain cancer patients. In addition, aberrant CDKN2A expression was closely correlated to immune infiltration and the levels of immune-regulatory genes. The study deeply defined the concrete roles of cuproptosis-related gene CDKN2A in tumorigenesis. The results provided new insights and pieces of evidence for treatment.
Keywords: cuproptosis, CDKN2A, pan-cancer, immunotherapy, prognosis
1. Introduction
Due to its rapidly increased incidence and mortality, cancer is a major public health problem and a barrier to prolonging life expectancy.[1,2] The highly intricate process of tumorigenesis and poor prognosis pose a great threat to cancer therapy.[1] Therefore, it is urgent to explore in-depth the definite mechanisms and elucidate the expression of relevant genes related to tumor occurrence so that the results provided new directions for diagnosis and treatment.
Programmed cell death is vital to the homeostasis of body tissues.[3] At present, more types of cell death, such as necroptosis, pyroptosis and ferroptosis, have been found and identified.[4–6] It is reported that cell death is tightly involved in tumorigenesis and prognosis.[7] Therefore, striking cell death might be a latent strategy for tumor therapy. Cuproptosis, arising from copper-mediated mitochondrial proteotoxic stress, is a newly identified form of regulatory cell death.[8] The process was verified that the lipoyl moiety of increased lipoylated TCA enzyme bound to copper, which induced proteotoxic stress characterized by lipoylated protein aggregation, decreased Fe-S cluster–containing proteins and increased HSP70.[8] Several studies have recognized the crucial roles of copper and cuproptosis modulated by copper in living things, such as the liver,[9] lung,[10] and other tissues.[8,11] A total of 12 genes were verified to take part in the cuproptosis pathway, consisting of 7 pro-cuproptosis genes (ferredoxin 1, dihydrolipoamide dehydrogenase, dihydrolipoamide S-Acetyltransferase, lipoic acid synthetase, homo sapiens lipoyltransferase 1, pyruvate dehydrogenase E1 subunit Alpha 1 and pyruvate dehydrogenase E1 subunit beta), 3 anti-cuproptosis genes (CDKN2A, glutaminase and metal regulatory transcription factor 1), and 2 transporters for copper-solute carrier family 31 member 1 and ATPase copper transporting beta.[12]
Cyclin dependent kinase inhibitor 2A (CDKN2A), serving as a tumor suppressor and cell cycle regulator, has been elucidated in some cancers, such as glioblastoma (GBM),[13] follicular lymphoma,[14] and colorectal cancer (CRC).[15] Disruption of CDKN2A (deletion or methylation) has been reported to be a frequent event in tumorigenesis, which affects the clinical characteristics and patient outcomes.[14–16] A meta-analysis from Xing showed that CDKN2A hypermethylation was significantly associated with unfavorable prognosis in CRC patients.[15] Alhejaily and his colleagues revealed that silence of CDKN2A by deletion or methylation was correlated with worse clinical outcome in follicular lymphoma.[14] Similar results were also found in pancreatic ductal adenocarcinoma (PDAC), thymic carcinoma, head and neck squamous cell carcinoma (HNSC) and muscle invasive bladder cancer (MIBC).[16–19] Although the tumorigenic effects of disruption of CDKN2A were well confirmed, unexpectedly high CDKN2A indicated a poor clinical outcome in some cancer, including Colon adenocarcinoma (COAD), Bladder Urothelial Carcinoma (BLCA) and Liver hepatocellular carcinoma (LIHC).[17,20–22] The mechanism by which CDKN2A serves as a tumor suppressor but results in unfavorable prognosis is speculated that CDKN2A may involve in cuproptosis activity.[23–25] Nevertheless, as an anti-cuproptosis gene, the roles or signatures and the regulatory mechanisms of CDKN2A in pan-cancer have not yet been explored in depth.
In this study, a comprehensive analysis of CDKN2A expression in 33 cancer types was performed. In detail, aberrantly expressed genes and protein analysis, survival rate, methylation analysis and enrichment analysis were carried out. The correlation between CDKN2A expression and immune infiltration was followed explored. Lastly, the association between CDKN2A expression and gene related to immune regulation or cuproptosis was studied. The results explored the predictive value of CDKN2A and provided new insight into cancer therapy.
2. Methods
2.1. CDKN2A differential expression analysis
The Tumor Immune Estimation Resource (https://cistrome.shinyapps.io/timer/)[26] and Gene Expression Profiling Interactive Analysis (GEPIA2, http://gepia2.cancer-pku.cn/#analysis)[27] were applied to compare the CDKN2A expression in pan-cancer and their corresponding paracancerous tissue samples with the threshold of P value < .05 and |Log2FC| > 1. The total protein level of CDKN2A between normal tissues and ten cancer samples (kidney renal clear cell carcinoma [KIRC], uterine corpus endometrial carcinoma [UCEC], lung adenocarcinoma [LUAD], HNSC, LIHC, breast cancer, ovarian cancer, COAD and pancreatic adenocarcinoma [PAAD]) was analyzed by the “CPTAC analysis” module in the UALCAN database (http://ualcan.path.uab.edu)[28] and immunohistochemical (IHC) staining downloaded from “The Human Protein Atlas” database. The GEPIA2 database was performed to investigate the clinic correlation of CDKN2A expression.
2.2. Survival analysis in pan-cancer
The prognostic value of CDKN2A, including overall survival (OS), disease-free survival (DFS), disease-specific survival and progress-free interval, were determined by GEPIA and Xiantao bioinformatics toolbox (https://www.xiantao.love) tool according to the Kaplan–Meier analysis between high-expression and low-expression groups. We performed GEPIA2 to examine the relationship between CDKN2A expression and OS and DFS. The heatmap data and survival curve were displayed. Xiantao toolbox was carried out to analyze TCGA data of pan-cancer. Forest plots and Kaplan–Meier curves were drawn to explore the effect of CDKN2A expression on disease progression. Furthermore, the frequency, type, and site information related to mutation of CDKN2A in pan-cancer were analyzed by cBioPortal (https://www.cbioportal.org/).[29]
2.3. Genetic alteration analysis of CDKN2A in pan-cancer
The genetic alteration, including mutation type, alteration frequency and the copy number alteration (CNA), of CDKN2A was performed by cBioPortal tool. The mutation site with highest change frequency was visualized in the 3D structure of CDKN2A protein.
2.4. Immune infiltration analysis
The association between CDKN2A expression and immune infiltration in pan-cancer was explored by using the “Immune” module of TIMER (http://timer.cistrome.org/).[26] We applied 7 algorithms, exactly, MCPCOUNTER, CIBERSORT, CIBERSORT-ABS, EPIC, QUANTISEQ and XCELL, to analyze the infiltration of tumor-infiltration immune cells, such as macrophages (including M0, M1 and M2) and cancer-associated fibroblasts (CAF). Heat maps and scatter plots were displayed according to the TCGA data.
2.5. Methylation analysis
The effect of DNA methylation on CDKN2A expression in some cancers was explored based on TCGA databases by the “Methylation” module of the xiantao bioinformatics toolbox. The “MethSurv” web tool (https://biit.cs.ut.ee/methsurv/)[30] was carried out to further excavate the correlation between CDKN2A methylation and patients’ survival. Kaplan–Meier plots were drawn for visualization.
2.6. Enrichment analysis of CDKN2A-related genes
The Protein-Protein Network Interaction network was constructed based on the STRING website, and the top 20 experimental identified CDKN2A-binding molecules were analyzed by Cytoscape. Gene ontology (GO) and KEGG (Kyoto Encyclopedia of Genes and Genomes) enrichment analysis about selected genes were performed by the xiantao bioinformatics toolbox. GO [including biological process, cellular component (CC) and molecular function], and KEGG analysis were visualized by the xiantao bioinformatics toolbox and bioinformatics website (http://www.bioinformatics.com.cn/). GEPIA2 tool was carried out to explore similar genes related to CDKN2A. TCGA data in pan-cancer were downloaded. Gene Set Enrichment Analysis (GSEA) and correlation analysis was performed via the xiantao bioinformatics toolbox to uncover relevant pathways affected by CDKN2A expression in pan-cancer. |NES| >1, adjust P value < .05 and FDR < 0.25 were considered as obviously enriched.
2.7. Statistical analysis
In TIMER 2.0, the statistical significance calculated by the Wilcoxon test is annotated by the number of stars. The ANOVA method was carried out to compare the tumor and all normal samples. The Kaplan–Meier method was utilized to assess the relationship between prognosis versus CDKN2A expression, mutation or methylation levels. The Pearson rank correlation coefficient was used to explore the association between the 2 groups. A P value <.05 was considered statistically significant.
3. Results
3.1. Aberrant expression of CDKN2A in pan-cancers
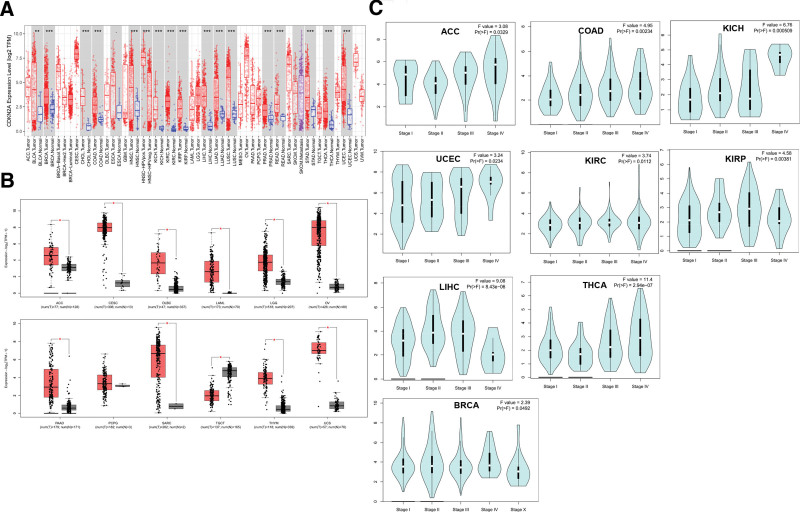
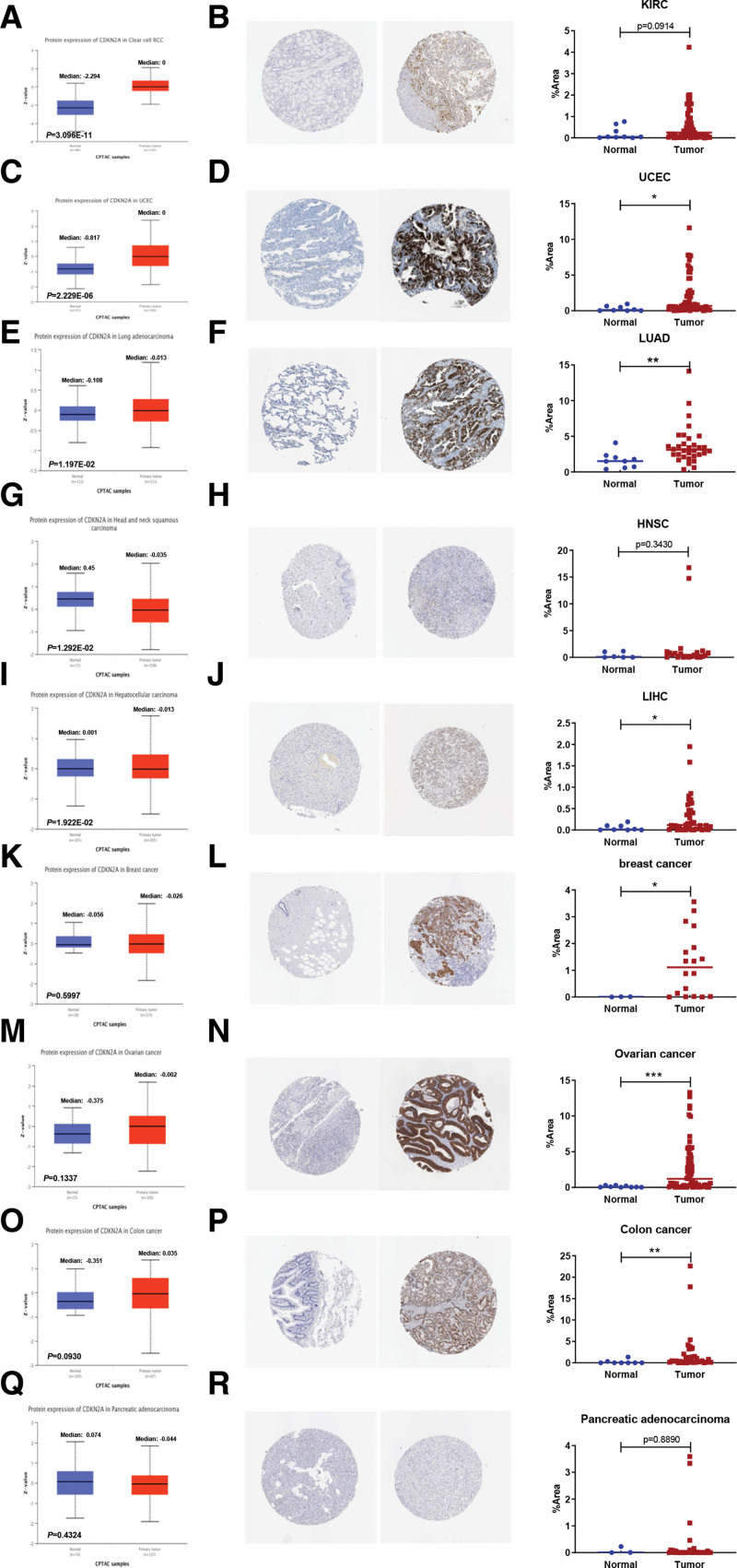
The CDKN2A expression in pan-cancer and paracancerous tissue samples was analyzed by the TIMER database. As displayed in Figure 1A, CDKN2A was dramatically higher expressed in cancer tissues than in paracancerous tissue samples, such as BLCA, Breast invasive carcinoma (BRCA), Cholangiocarcinoma, COAD, HNSC, Kidney Chromophobe (KICH), KIRC, Kidney renal papillary cell carcinoma (KIRP), LIHC, LUAD, Lung squamous cell carcinoma, Prostate adenocarcinoma (PRAD), Rectum adenocarcinoma, Stomach adenocarcinoma, Thyroid carcinoma (THCA) and UCEC. Furthermore, the TCGA and GTEx data from GEPIA2 were integrated to analyze CDKN2A expression in some cancers lacking paracancerous normal tissues. The results indicated that the CDKN2A expression in cancer samples, respectively, Adrenocortical carcinoma (ACC), Cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), Lymphoid Neoplasm Diffuse Large B-cell Lymphoma, Acute Myeloid Leukemia, Brain Lower Grade Glioma, Ovarian serous cystadenocarcinoma (OV), PAAD, Pheochromocytoma and Paraganglioma (PCPG), Sarcoma, Thymoma (THYM) and Uterine Carcinosarcoma was obviously upregulated (Fig. 1B). Instead, CDKN2A was significantly downregulated in Testicular Germ Cell Tumors (TGCT) tissues (Fig. 1B). We further accessed the DCKN2A protein levels and IHC staining in various cancers. The “CPTAC analysis” results showed that the total expression of CDKN2A was dramatically elevated in KIRC (Fig. 2A), UCEC (Fig. 2C) and LUAD (Fig. 2E), and decreased in HNSC (Fig. 2G) and LIHC (Fig. 2I). There was no significant change in Breast cancer (Fig. 2K), Ovarian cancer (Fig. 2M), Colon cancer (Fig. 2O) and PAAD samples (Fig. 2Q). Consistent with the “CPTAC analysis” results, stronger IHC staining was discovered in UCEC (Fig. 2D) and LUAD (Fig. 2F). Contrary to above protein expression results, IHC staining showed that CDKN2A expression was increased in LIHC tissues (Fig. 2J), with no statistical significance in KIRC (Fig. 2B), HNSC (Fig. 2H) and PAAD (Fig. 2R) tissues. Besides, IHC staining results also indicated the increased expression in breast cancer (Fig. 2L), Ovarian cancer (Fig. 2N) and Colon cancer (Fig. 2P).
Figure 1.
Differential expression of CDKN2A in pan-cancers. (A) mRNA levels of CDKN2A in pan-cancers and their corresponding normal samples performed by TIMER2 database. (B) CDKN2A expression from GEPIA2 database. (C) Correlation analysis of CDKN2A expression and tumor pathological stage. CDKN2A = cyclin dependent kinase inhibitor 2A, GEPIA2 = gene expression profiling interactive analysis.
Figure 2.
CDKN2A protein expression in pan-cancer. Protein levels of CDKN2A between normal samples and KIRC (A), UCEC (C), LUAD (E), HNSC (G), LIHC (I), breast cancer (K), ovarian cancer (M), Colon cancer (O) and PAAD (Q) samples were identified by “CPTAC analysis” module in the UALCAN database. Immunohistochemical (IHC) staining of CDKN2A protein in pan-cancer was downloaded from “The Human Protein Atlas” database. IHC staining of CDKN2A and their quantification in KIRC (B), UCEC (D), LUAD (F), HNSC (H), LIHC (j), breast cancer (L), ovarian cancer (N), Colon cancer (P) and PAAD (R) samples tissues were displayed. CDKN2A = cyclin dependent kinase inhibitor 2A, KIRC = kidney renal clear cell carcinoma, HNSC = head and neck squamous cell carcinoma, LIHC = liver hepatocellular carcinoma, LUAD = lung adenocarcinoma, PAAD = pancreatic adenocarcinoma, UCEC = uterine corpus endometrial carcinoma.
3.2. Correlation between CDKN2A expression and tumor pathological stage in pan-cancer
The GEPIA2 tool was used to elucidate the relationship between CDKN2A and clinical information. Figure 1C indicated that 9 cancers had a stage-specific change of CDKN2A, including ACC, COAD, KICH, UCEC, BRCA, KIRC, KIRP, LIHC, and THCA. In other tumor types, there was no obvious correlation between CDKN2A and pathological stage (see Figure S1, Supplemental Digital Content 1, http://links.lww.com/MD/I754, Supplemental Content, which demonstrates the CDKN2A expression through different pathological stages in some cancers).
3.3. Prognostic value of CDKN2A in cancer patients
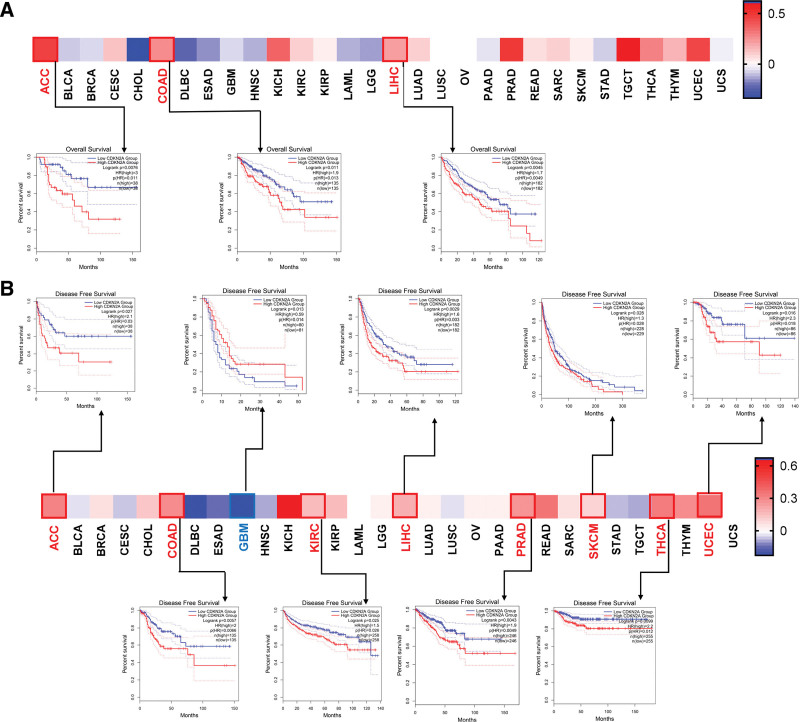
The potential role of CDKN2A in prognosis was assessed by the GEPIA2 tool. As displayed in Figure 3A, the results indicated that CDKN2A expression was negatively related to OS of ACC (P = .011), COAD (P = .013) and LIHC (P = .0049). Moreover, high expression of CDKN2A predicted poor DFS in ACC (P = .03, Fig. 3B), COAD (P = .0066, Fig. 3B), KIRC (P = .026, Fig. 3B), LIHC (P = .003, Fig. 3B), PRAD (P = .0049, Fig. 3B), skin cutaneous melanoma (SKCM) (P = .028, Fig. 3B), THCA (P = .012, Fig. 3B) and UCEC (P = .018, Fig. 3B), while high CDKN2A predicted better DFS in GBM (P = .014, Fig. 3B).
Figure 3.
Prognostic assessment of CDKN2A level in OS and DFS. Kaplan–Meier analysis of OS (A) and DFS (B) in patients with low and high CDKN2A. The low-expression and high-expression cohorts were divided according to the thresholds with low cutoff (50%) and high cutoff (50%) values. P value < .01 and | logFC| > 1 were cutoff values. CDKN2A = cyclin dependent kinase inhibitor 2A, DFS = disease free survival, OS = overall survival.
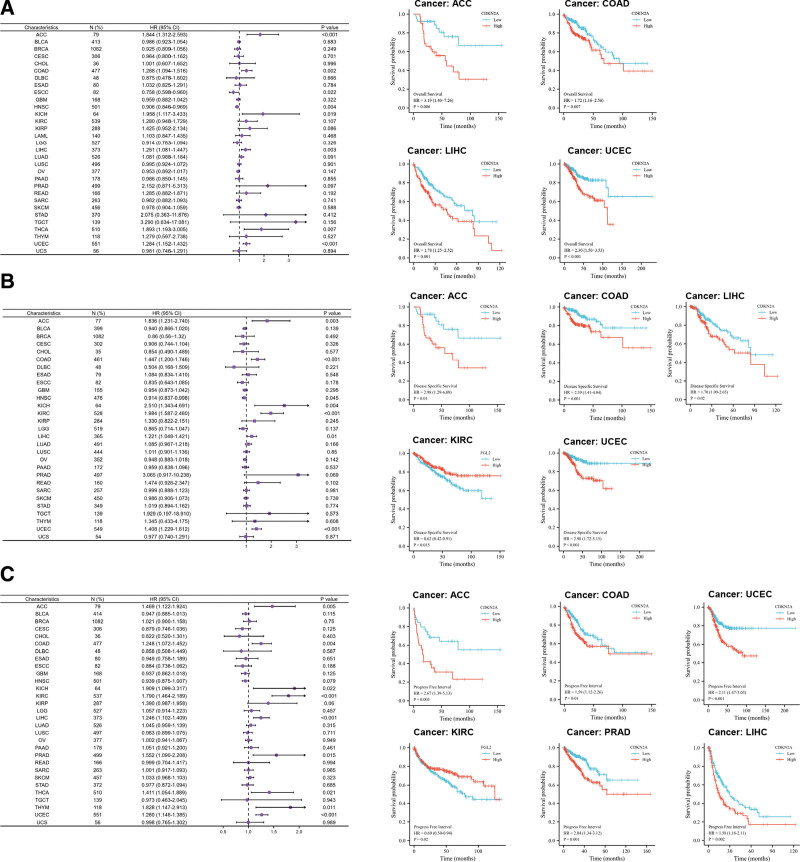
The results of Cox regression model indicated that CDKN2A expression may play adverse roles in the OS of ACC (P < .001, Fig. 4A), COAD (P = .002, Fig. 4A), KICH (P = .019, Fig. 4A), LIHC (P = .003, Fig. 4A), THCA (P = .007, Fig. 4A) and UCEC (P < .001, Fig. 4A), but protective roles in HNSC (P = .004, Fig. 4A) and ESCC (Esophageal Squamous Cell Carcinoma, P = .022, Fig. 4A). Consistent with the Cox regression results, Kaplan–Meier curve showed that CDKN2A expression was negatively related to OS in ACC (P = .006, Fig. 4A), COAD (P = .007, Fig. 4A), LIHC (P = .001, Fig. 4A), and UCEC (P < .001, Fig. 4A). There was no clear correlation between CDKN2A levels and the outcomes of KICH, THCA, HNSC, and ESCC patients (see Figure S2A, Supplemental Digital Content 2, http://links.lww.com/MD/I755, Supplemental Content, which indicated the effect of CDKN2A on disease outcomes). For disease-specific survival, in accord with Cox regression, Kaplan–Meier analysis showed that high level of CDKN2A predicted poor outcome in ACC (P = .01, Fig. 4B), COAD (P = .001, Fig. 4B), KIRC (P = .015, Fig. 4B), LIHC (P = .02, Fig. 4B) and UCEC (P < .001, Fig. 4B). For HNSC and KICH patients, the survival time was similar between high and low CDKN2A (see Figure S2B, Supplemental Digital Content 2, http://links.lww.com/MD/I755, Supplemental Content, which indicated the effect of CDKN2A on disease outcomes). Forest plot indicated that high level of CDKN2A portended shorten progress-free interval in ACC (P = .005, Fig. 4C), COAD (P = .004, Fig. 4C), KICH (P = .022, Fig. 4C), KIRC (P < .001, Fig. 4C), LIHC (P < .001, Fig. 4C), PRAD (P = .015, Fig. 4C), THYM (P = .011, Fig. 4C) and UCEC (P < .001, Fig. 4C). However, the Kaplan–Meier curve found that KICH and THYM are not statistically significant (see Figure S2C, Supplemental Digital Content 2, http://links.lww.com/MD/I755 Supplemental Content, which indicated the effect of CDKN2A on disease outcomes).
Figure 4.
Prognostic value of CDKN2A expression in pan-cancer. The Cox regression and Kaplan–Meier analysis of CDKN2A expression in OS (A), DSS (B), and PFI (C). 0 < HR (95% CI) < 1 means that CDKN2A may play a protective role in cancer, and HR (95% CI) > 1 indicates CDKN2A may play an adverse role in pan-cancer. CDKN2A = cyclin dependent kinase inhibitor 2A, DSS = disease specific survival, OS = overall survival, PFI = progress free interval.
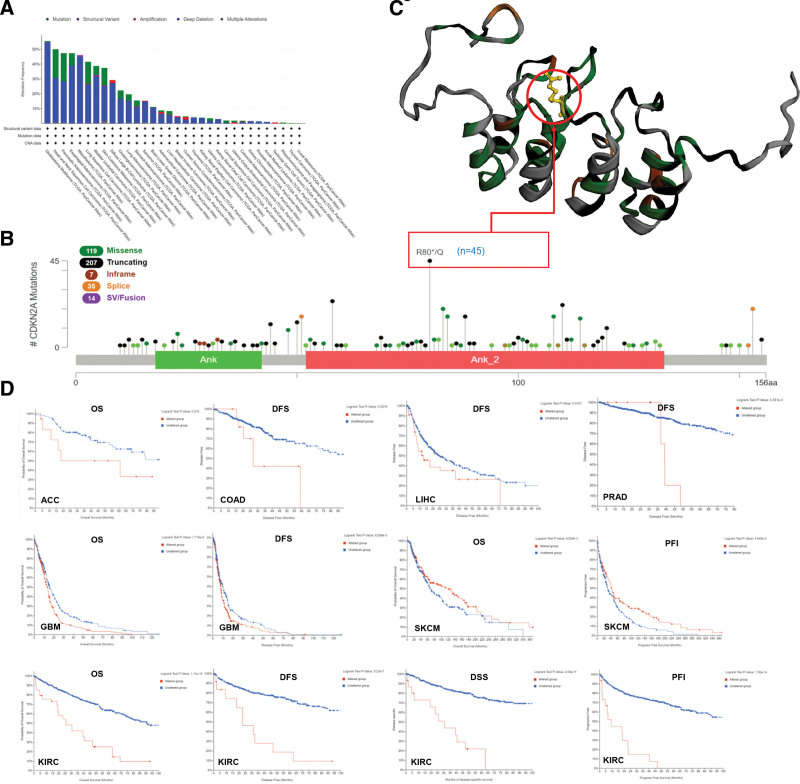
3.4. CDKN2A genetic alteration analysis
CDKN2A genetic alteration in pan-cancer was explored to elucidate the possible mechanisms that affected the expression. Figure 5A indicated that the highest alteration frequency of CDKN2A (>50%) happened in GBM patients, of which “Deep Deletion” was the primary alteration type. HNSC patients had the highest “mutation” frequency (almost 20%). As presented in Figure 5B, 382 mutations were found in the CDKN2A sequence, of which truncating seems to be the main mutation type. Moreover, most of the mutation was located in the “Ank_2” (Ankyrin repeats, 52–133) domain. The R80*/Q alteration with the highest alteration frequency was detected in 45 cases of carcinoma, and its mutation site was presented in the 3D structure of CDKN2A protein (Fig. 5C). The survival analysis showed that the CDKN2A alteration resulted in a poor prognosis in ACC, COAD, LIHC, PRAD, GBM, SKCM, and KIRC patients (Fig. 5D).
Figure 5.
Mutation feature of CDKN2A in pan-cancer performed by cBioPortal tool. The mutation status (A) and mutation types of CDKN2A (B) were displayed. The mutation site with the highest change frequency (R80*Q) was visualized in the 3D structure of CDKN2A protein (C). The correlation between CDKN2A alteration and patient prognosis, including OS, DFS, DSS and PFI, was represented (D). CDKN2A = cyclin dependent kinase inhibitor 2A, DFS = disease-free survival, DSS = disease specific survival, OS = overall survival, PFI = progress free interval.
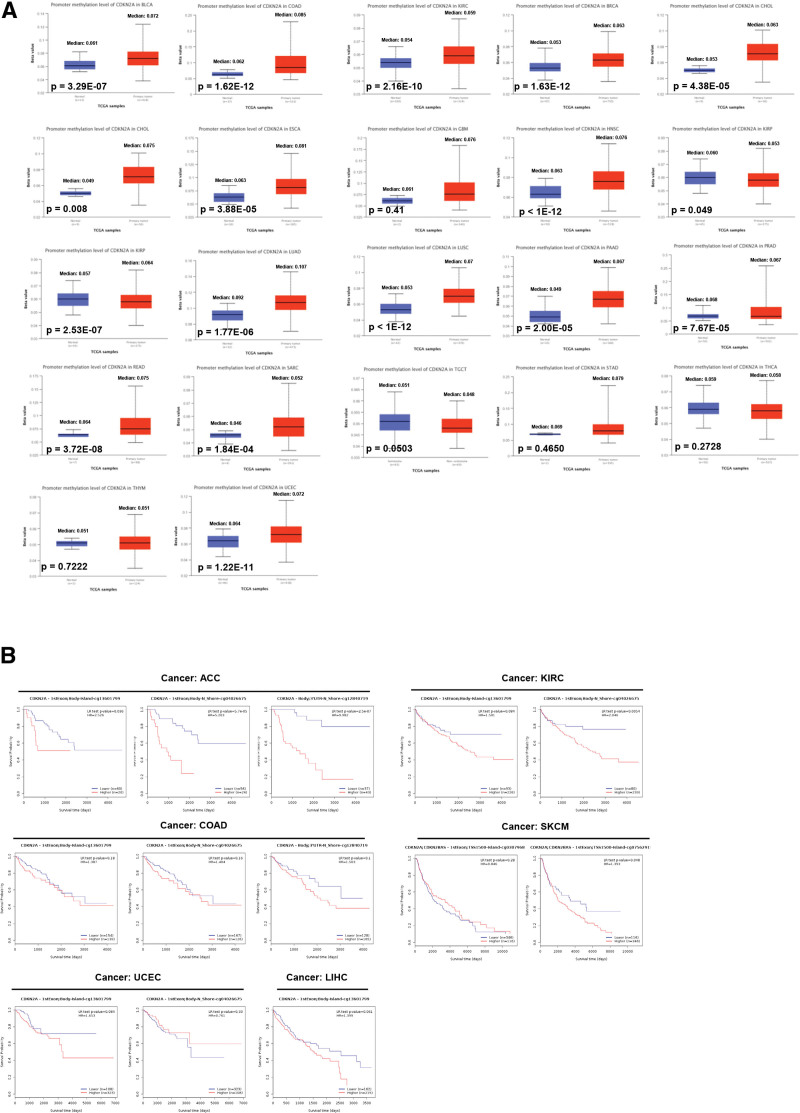
3.5. DNA methylation analysis
Numerous types of research have indicated that DNA methylation, as an epigenetic modification, contributed to regulating the expression of cancer-related genes.[31] Thus, the promoter methylation level of CDKN2A between tumors and normal tissues was explored via the “CPTAC” dataset. As displayed in Figure 6A, compared with normal samples, the promoter methylation level of CDKN2A was dramatically increased in most cancers (e.g., BLCA, COAD, KIRC, LIHC, and so on), except KIRP with decreased methylation level. No obvious change in methylation was found in TGCT, THCA, stomach adenocarcinoma, and THYM. Then, the “MethSurv” web tool was performed to further excavate the effect of CDKN2A methylation on survival. Figure 6B indicated that higher CDKN2A methylation (both island and N_shore region) led to poor prognosis in ACC patients. For KIRC, DNA methylation in the N_shore region induced decreased survival probability. There is no significant survival difference between the lower versus higher CDKN2A methylation group in COAD, SKCM, UCEC, and LIHC.
Figure 6.
Methylation analysis in tumors. (A) The promoter methylation level of CDKN2A in pan-cancer. (B) Survival analysis of CDKN2A methylation in ACC, COAD, KIRC, SKCM, UCEC and LIHC. ACC = adrenocortical carcinoma, CDKN2A = cyclin dependent kinase inhibitor 2A, COAD = colon adenocarcinoma, KIRC = kidney renal clear cell carcinoma, LIHC = liver hepatocellular carcinoma, SKCM = skin cutaneous melanoma, UCEC = uterine corpus endometrial carcinoma.
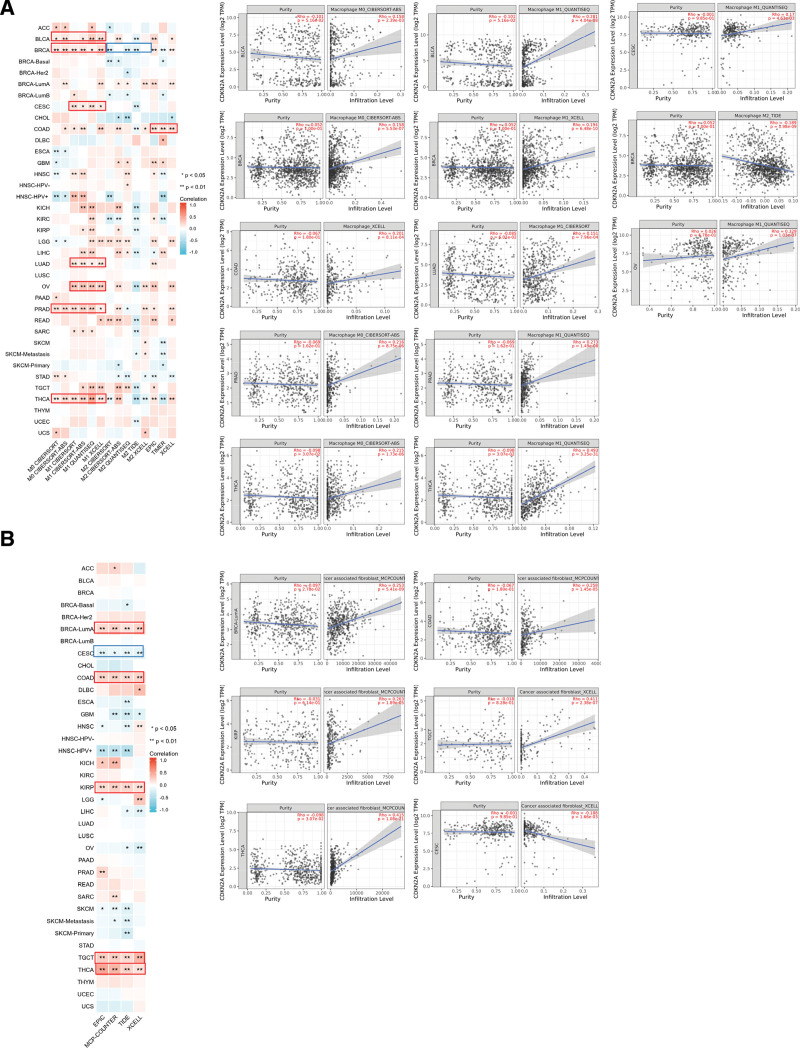
3.6. The correlation analysis between CDKN2A expression and immune infiltration
As we all know, the tumor microenvironment affects tumor occurrence and development, in which the infiltration of immune cells, especially macrophages and CAF, plays crucial roles. So, here we explored the relationship between CDKN2A expression and immune infiltration in TCGA tumors by the “Immune” module of TIMER web serve. Heatmap data and Scatter plot data indicated a significantly positive association between CDKN2A expression and the infiltration of macrophages (M0 and M1) in BLCA, PARD and THCA. For BRCA, we found a positive relationship between CDKN2A level and the infiltration of M0 and M1 cell but noted a negative association in M2 cell infiltration. Moreover, the CDKN2A expression in CESC, LUAD, and OV was positively correlated with the estimated infiltration of M1 macrophages based on all algorithms (Fig. 7A). Besides, the expression of CDKN2A in BRCA-lumA, COAD, KIRP, TGCT, and THCA was positively associated with the CAF infiltration, while CESC was negatively correlated (Fig. 7B).
Figure 7.
Correlation analysis between CDKN2A expression and immune infiltration in pan-cancer. (A) A positive correlation between immune infiltration of macrophages and CDKNA2 expression in BLCA, CESC, BRCA, COAD, LUAD, OV, PRAD and THCA. (B) CDKN2A expression was positively correlated with the infiltration of cancer-associated fibroblasts in BLCA-Luma, COAD, KIRP, TGCT and THCA, but negatively correlated in CESC. The relationship was displayed in different algorithms. A positive correlation was labeled as red color, while negative correlation was blue. * P < .05, ** P < .01. BLCA = bladder urothelial carcinoma, BRCA = breast invasive carcinoma, CESC = cervical squamous cell carcinoma and endocervical adenocarcinoma, CDKN2A = cyclin dependent kinase inhibitor 2A, CESC = cervical squamous cell carcinoma and endocervical adenocarcinoma, COAD = colon adenocarcinoma, LUAD = lung adenocarcinoma, OV = ovarian serous cystadenocarcinoma, PRAD = prostate adenocarcinoma, THCA = thyroid carcinoma.
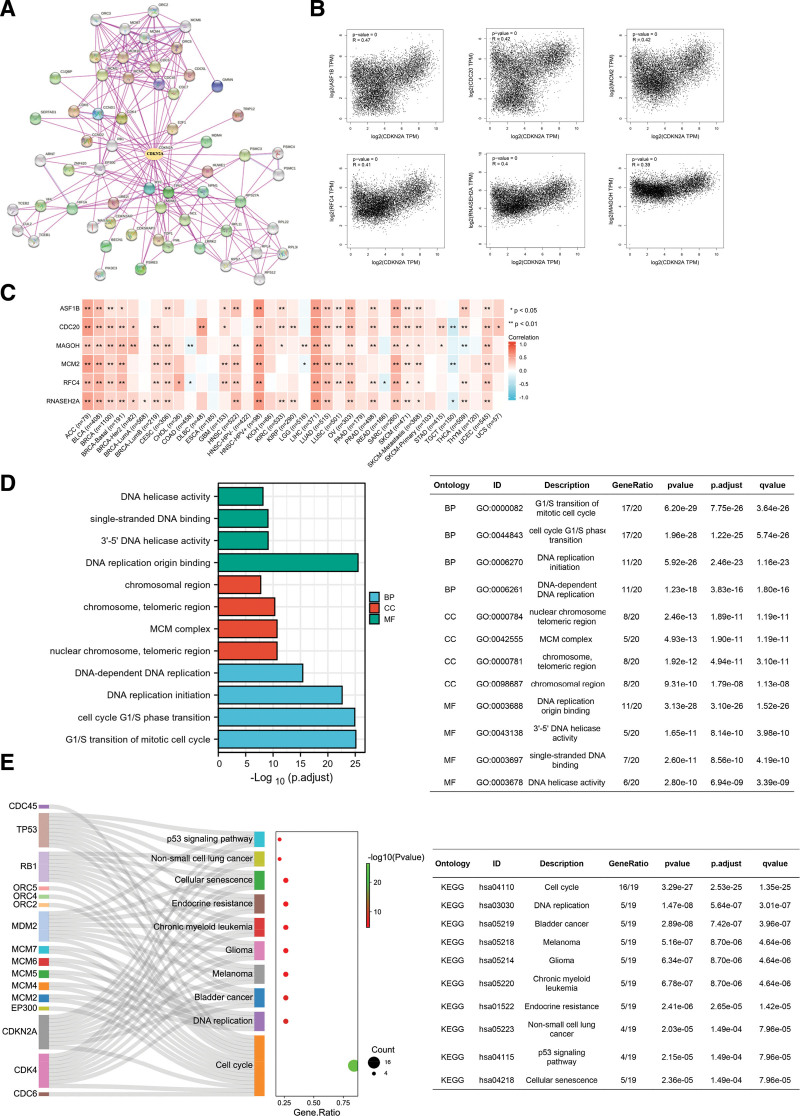
3.7. Enrichment and co-expression analysis of CDKN2A
To elucidate the function of CDKN2A, GEPIA2, and xiantao bioinformatic toolbox were carried out. At the gene expression level, GEPIA2 was used to find similar genes. As represented in Figure 8B, the CDKN2A expression was positively correlated with identical genes, including ASF, CDC20, MCM2, replication factor C subunit 4, RNSSEH2A and Mago homolog, exon junction complex subunit. The correlation analysis suggested that genes-ASF, CDC20, MCM2, replication factor C subunit 4, RNSSEH2A and Mago homolog, exon junction complex subunit- were positively related to most cancer, especially ACC, BLCA, HNSC-HPV+, SARC, and LIHC (Fig. 8C). However, the above genes seemed to play a weakly negative role in TGCT (Fig. 8C, blue square). Moreover, Protein-Protein Network Interaction (Fig. 8A) was established to display identified CDKN2A-binding molecules intuitively. The top 20 molecules (core molecules) were chosen for further GO and KEGG enrichment analysis. Go enrichment analysis results indicated that CDKN2A and the core molecules mainly involved biological process, such as G1/S transition of mitotic cell cycle, cell cycle G1/S phase transition, DNA replication initiation, and DNA-dependent DNA replication (Fig. 8D, blue columns). For CC analysis, core molecules were mostly involved in the chromosomal region, chromosome, telomeric region, MCM complex and nuclear chromosome, and telomeric region (Fig. 8D, red columns). The molecular function showed that the core molecules were mainly involved in DNA helicase activity, single-stranded DNA binding, 3’-5’ DNA helicase activity and DNA replication origin binding (Fig. 8D, green columns). KEGG analysis figured out that core molecules mainly participated in the p53 signaling pathway, non-small cell lung cancer, Cellular senescence, Endocrine resistance, Chronic myeloid leukemia, Glioma, Melanoma, Bladder cancer, DNA replication and Cell cycle signaling pathways (Fig. 8E). Moreover, single-gene GSEA was performed to analyze CDKN2A-relevant pathways in ACC, COAD, KIRC, LIHC, PRAD, SKCM, THCA, and UCEC. The top 5 most frequently enriched pathways were visualized (see Figure S3A–H, Supplemental Digital Content 3, http://links.lww.com/MD/I756, Supplemental Content, which suggested CDKN2A-relevant pathways in pan-cancer).
Figure 8.
Co-expression and enrichment analysis of CDKN2A in pan-cancer. (A) Experimentally identified CDKN2A binding molecules were exhibited in PPI networks established by STRING. (B) Core CDKN2A-related genes, including ASF, CDC20, MCM2, RFC4, RNSSEH2A and MAGOH, were analyzed by GEPIA2. (C) The correlation between CDKN2A expression and selected CDKN2A-related genes (ASF, CDC20, MCM2, RFC4, RNSSEH2A and MAGOH) in pan-cancer was represented via heatmap. GO (D) and KEGG (E) enrichment for the top 20 experimental identified CDKN2A binding molecules from PPI network. BP = biological process, CC = cellular component, CDKN2A = cyclin dependent kinase inhibitor 2A, GEPIA2 = gene expression profiling interactive analysis, GO = gene ontology, KEGG = Kyoto encyclopedia of genes and genomes, MF = molecular function.
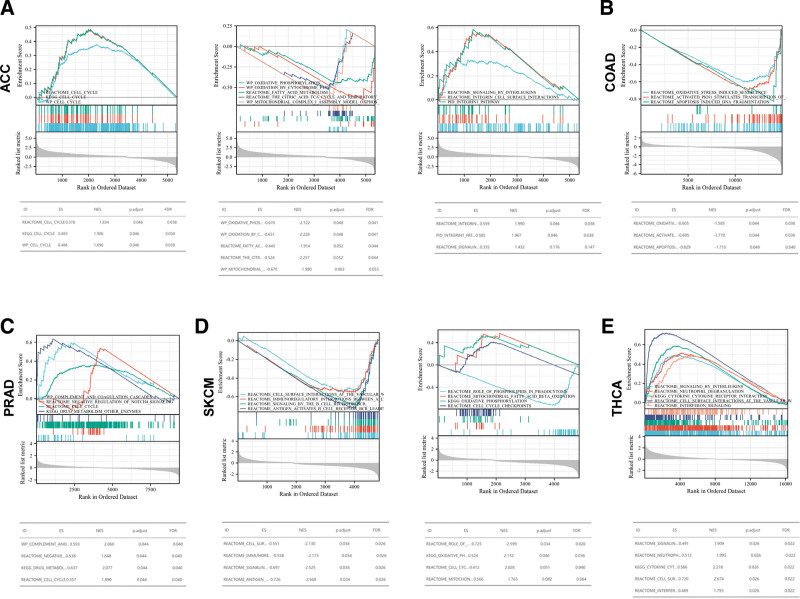
As we all know, the cell cycle, immune response and metabolism affect the occurrence and development of pan-cancer. The relevant pathways related to cell cycle, immune regulation (e.g., signaling by interleukins, integrin cell surface interactions, integrin-1 pathway and so on) and metabolisms relevant to oxidative stress, mitochondrial activity and fatty acid were conducted. GSEA results showed that CDKN2A was positively enriched in the cell cycle in ACC and PRAD (Fig. 9A and C). Furthermore, CDKN2A was found to be positively associated with the pathways related to oxidative response, fatty acid metabolism and mitochondrial metabolism in ACC (Fig. 9A), COAD (Fig. 9B), PRAD (Fig. 9C), and SKCM (Fig. 9D). Moreover, CDKN2A was positively related to immune-related pathways in ACC (Fig. 9A) and PRAD (Fig. 9E). Conversely, the aforementioned pathways in SKCM and THCA were negatively regulated. For KIRC, LIHC and UCEC, the pathways mentioned above were not dramatically enriched (see Figure S4A–C, Supplemental Digital Content 4, http://links.lww.com/MD/I757, Supplemental Content, which indicated CDKN2A-relavant pathways related to cell cycle, immune and metabolisms in KIRC, LIHC, and UCEC). All of the above results indicated that the CDKN2A expression was linked to some critical pathways in cancer formation, occurrence and metastases.
Figure 9.
Gene set enrichment analysis. The roles of CDKN2A in ACC (A), COAD (B), PRAD (C), SKCM (D), THCA (E) by performing Gene Set Enrichment Analysis (GSEA). ACC = adrenocortical carcinoma, CDKN2A = cyclin dependent kinase inhibitor 2A, COAD = colon adenocarcinoma, PRAD = prostate adenocarcinoma, SKCM = skin cutaneous melanoma, THCA = thyroid carcinoma.
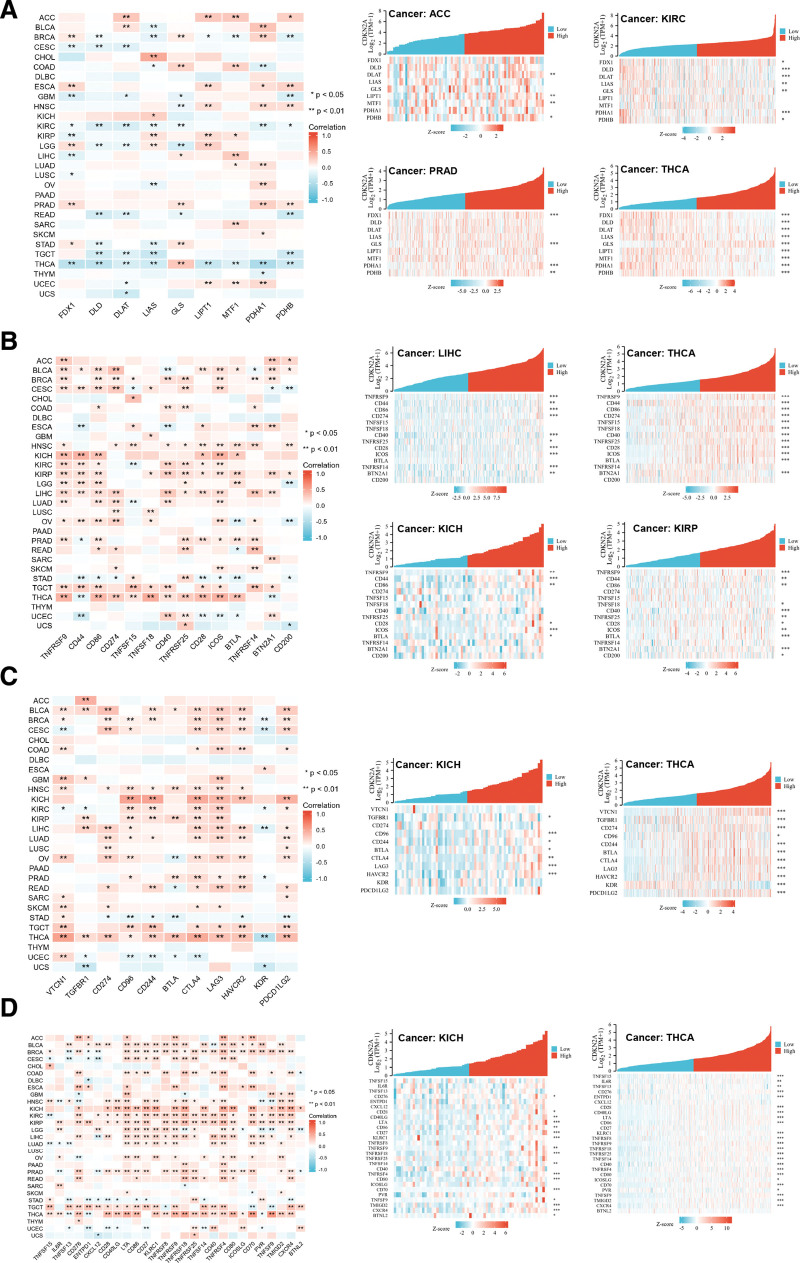
3.8. CDKN2A correlated with the majority of cuproptosis-related genes and immune-regulatory genes
To further validate the roles of CDKN2A in different cancers, the co-expression analysis related to cuproptosis, immune checkpoint and immune regulation was also conducted. As presented in Figure 10A, cuproptosis-related genes in ACC were positively correlated with CDKN2A expression while negatively related in KIRC, PRAD, and THCA. Moreover, the correlation analysis of immune checkpoints showed that most genes were significantly positively associated with CDKN2A in BLCA, BRCA, HNSC, KICH, KIRP, LIHC, TGCT, and THCA. Conversely, some immune checkpoint genes in UCEC were negatively associated with CDKN2A (Fig. 10B). In addition, the correlation analysis of immune-regulatory genes results was simultaneous with the previous ones (Fig. 10C and D). Consequently, CDKN2A might play a pivotal role in pan-cancer copper metabolism and immune infiltration.
Figure 10.
Gene correlation analysis. The association between CDKN2A expression and genes related to cuproptosis (A), immune checkpoint (B), immune-suppressive status (C) and immune-activation (D) was presented by heatmaps. Each square represented the correlation between CDKN2A and other genes. Red suggested positive correlation, and blue represented negative correlation. * P < .05, ** P < .01. CDKN2A = cyclin dependent kinase inhibitor 2A.
4. Discussion
CDKN2A, a tumor suppressor and cell cycle regulator, was verified to take part in the cell cycle and p53 signaling pathway.[13] CDKN2A encoded 2 proteins, named p16INK4a and p14ARF.[32] It is reported that p14ARF regulated the cell cycle by preventing p53 inactivation, while p16INK4a prevented the phosphorylation of Rb proteins.[32,33] Previous studies have revealed the suppressive roles of CDKN2A in some cancer types.[20,34–36] Disruption of CDKN2A (deletion or methylation) has been reported to be a frequent event in tumorigenesis, which affected the clinical characteristics and patient outcomes.[14–16] Alhejaily and his colleagues revealed that silence of CDKN2A by deletion or methylation was correlated with worse clinical outcome in follicular lymphoma.[14] A meta-analysis from Xing showed that CDKN2A hypermethylation was significantly associated with unfavorable prognosis in CRC patients.[15] Similar results were also discovered in PDAC, thymic carcinoma, HNSC and MIBC.[16–19] Although the tumorigenic effects of disruption of CDKN2A were well confirmed, unexpectedly high CDKN2A indicated a poor clinical outcome in some cancer, including COAD, BLCA and LIHC.[17,20–22] The mechanism by which CDKN2A serves as a tumor suppressor but results in unfavorable prognosis is speculated that CDKN2A may involve in tumor initiation and progression as an anti-cuproptosis gene by performing genome-wide CRISPR/Cas9 knock-out screens.[8,23–25] However, as an anti-cuproptosis gene, the roles or signatures and the regulatory mechanisms of CDKN2A in pan-cancer have not yet been explored in depth. Thus, we conducted this pan-cancer analysis for CDKN2A.
In this study, overexpressed CDKN2A was observed in the majority of cancer tissues (e.g., ACC, BLCA, CESC, lymphoid neoplasm diffuse large B-cell lymphoma, esophageal carcinoma, UCEC and so on), while downregulated CDKN2A was found in TGCT tissues by the assessment of CDKN2A mRNA level. These results indicated that CDKN2A might involve in different pathways in cancer initiation and progression. Consistent with the transcriptional level, an augmented translational level of CDKN2A was found in KIRC, UCEC, LUAD, and LIHC samples. Curiously, the protein expression of CDKN2A in HNSC was not consistent, which might be due to metabolism or posttranscriptional protein modification. Moreover, it is verified that the expression of CDKN2A was related to the tumor pathological stages in ACC, COAD, KICH, UCEC, KIRC, KIRP, LIHC, and THCA, which suggested the potential of CDKN2A as a biomarker for the clinical stage.
The Cox regression and Kaplan–Meier analysis revealed that upregulated CDKN2A predicted a poor prognosis for ACC, COAD, KIRC, LIHC, PRAD, SKCM, THCA, and UCEC. Concordant with this, recent convincing research also discovered that overexpressed CDKN2A was correlated with the poor prognosis in CRC.[21,34,37] These results revealed the potential of CDKN2A as an original prognostic predictor for some cancer types.
Deneka et al[38] found the relationship between CDKN2A mutation and tumor mutation burden. Liu and his colleagues demonstrated that the presence of CDKN2A deletion might induce the progression of ESCC.[39] In T-cell acute lymphoblastic leukemia, CDKN2A deletion was an independent poor prognostic factor.[40] Similar to the above findings, deep deletion happened in most cancer types. Besides, CDKN2A mutation played important roles in certain cancers. Our results showed that the CDKN2A amplification has arisen in ACC, OV, UCEC and TGCT, and the alteration of CDKN2A implicated inferior outcomes in cancer such as ACC, COAD, LIHC, KIRC, and so on. These results were seemly contradictory to the aforementioned CDKN2A expression levels. Possible reasons for these conflicting results are as follows: different change types occurred in tumors. For instance, compared with deep deletion, a mutation might have a greater impact on tumorigenesis in certain human tumors, which resulted in a loss of function. Then compensatory overexpression of CDKN2A was measured. Moreover, amplification might occupy center stage in some cancers, leading to the upregulated expression.
As an epigenetic modification, DNA methylation contributes to regulating the expression of cancer-related genes.[31] A meta-analysis performed by Zhou pointed out that CDKN2A methylation had a crucial role in the occurrence of esophageal cancer.[41] However, Cao et al indicated that CDKN2A methylation was not significantly correlated with the progression of PRAD.[42] This study explored that obviously increased promoter methylation level of CDKN2A happened in most cancers, which led to poor outcomes in ACC and KIRC. Therefore, we hypothesized that aberrant methylation of CDKN2A might take part in tumor progression and prognosis. Furthermore, enrichment analysis results suggested CDKN2A was closely correlated to cell cycle, immune responses and DNA replication. Especially, CDKN2A was positively enriched in cell cycle, oxidative response, fatty acid metabolism and mitochondrial metabolisms and immune-related pathways in ACC and PRAD. Thus, the results conformed to the existing mechanisms of cancer formation, occurrence and metastases.[43] Recent studies have revealed the indispensable and integral roles of tumor microenvironment (TME) in tumor physiology.[44,45] Growing evidence discovered that immune cells, transformed ECM and soluble factors presented in TME gave rise to tumor progression and metastasis.[44,46,47] Macrophages, the most abundant immune cells residing in TME, reflected the Th1/Th2 paradigm via antigen presentation and pathogen phagocytosis, which played crucial roles in immune homeostasis.[44] Tumor-associated macrophages (TAMs) were found to be correlated with shorter survival in cancer patients.[48,49] The analysis from Luo et al revealed that CDKN2A was positively related to the infiltration of macrophages in LIHC,[22] which indicated its value for prognosis or immunotherapy.[50] Xiao and his team discovered that high-risk PDAC group had higher CDKN2A mutations with mounted infiltration of macrophages M0 and M2.[51] CAF, as the most essential components of the TME, have also been verified to have a critical role in tumorigenesis and progression.[52] CAFs interact with immune cells by the secretion of growth factors, cytokines and other molecules, and induce an immunosuppressive TME that facilitates the growth of tumor cells.[52–54] Similar to the previous studies, the immune infiltration analysis in our study suggested that CDKN2A expression was positively associated with the infiltration of macrophages or CAFs in pan-cancer, which exposed the important roles of CDKN2A in tumor immunology. It is noteworthy that more macrophages M1 infiltration was found in some CDKN2A overexpressed samples, which seemly contradictory to the aforementioned prognostic evaluation of CDKN2A. These conflicting results may be due to the double-edged roles of CDKN2A in pan-cancer. Furthermore, our study also analyzes the relationship between CDKN2A expression and immune-related genes, including the immune checkpoint, immunosuppressive and immune activated genes. Fascinatingly, consistent with enrichment analysis results, a strong correlation between CDKN2A and immune-related genes was found in diverse cancers. These exciting results offered new insights and orientations for cancer treatment. Moreover, CDKN2A associated with the majority of cuproptosis-related genes in ACC, KIRC, PRAD and THCA, which implied the vital roles of CDKN2A in copper metabolism.
Nevertheless, there are some limitations in this study. Firstly, this study had not yet explored the definite mechanisms of CDKN2A expression in copper metabolisms. How CDKN2A expression affected immune infiltration and tumor progression needs to be verified in later research. Secondly, we explored the double-edged roles of CDKN2A as tumor suppressors or oncogenes in pan-cancer, and it might be induced by the diverse origin of cancers and tumor heterogeneity. In addition, more Vivo and Vitro experiments are further needed for verification.
In summary, our study pointed out the potential of CDKN2A as a predictor and biomarker associated with prognosis. The results provided new insights and orientations for novel anti-cancer treatments via facilitating tumor silence or regulating cuproptosis.
Acknowledgments
We would like to thank the Tianjin Union Medical Center and Tianjin Institute of Coloproctology for the support. In addition, we thank all tools used in this study for data analysis and visualization.
Author contributions
Conceptualization: Xipeng Zhang.
Data curation: Di Zhang.
Formal analysis: Di Zhang, Xipeng Zhang.
Investigation: Di Zhang, Tao Wang.
Methodology: Yi Zhou.
Project administration: Xipeng Zhang.
Resources: Di Zhang, Yi Zhou.
Software: Tao Wang.
Supervision: Xipeng Zhang.
Validation: Xipeng Zhang.
Visualization: Di Zhang, Tao Wang.
Writing – original draft: Di Zhang.
Writing – review & editing: Xipeng Zhang.
Supplementary Material
Abbreviations:
- ACC
- adrenocortical carcinoma
- BLCA
- bladder urothelial carcinoma
- BRCA
- breast invasive carcinoma
- CAF
- cancer-associated fibroblasts
- CC
- cellular component
- CDKN2A
- cyclin dependent kinase inhibitor 2A
- CESC
- cervical squamous cell carcinoma and endocervical adenocarcinoma
- COAD
- colon adenocarcinoma
- DFS
- disease-free survival
- GBM
- glioblastoma multiforme
- GEPIA2
- gene expression profiling interactive analysis
- GO
- gene ontology
- GSEA
- gene set enrichment analysis
- HNSC
- head and neck squamous cell carcinoma
- KEGG
- Kyoto encyclopedia of genes and genomes
- KICH
- kidney chromophobe
- KIRC
- kidney renal clear cell carcinoma
- KIRP
- kidney renal papillary cell carcinoma
- LIHC
- liver hepatocellular carcinoma
- LUAD
- lung adenocarcinoma
- OS
- overall survival
- OV
- ovarian serous cystadenocarcinoma
- PAAD
- pancreatic adenocarcinoma
- SKCM
- skin cutaneous melanoma
- TGCT
- testicular germ cell tumors
- THCA
- thyroid carcinoma
- THYM
- thymoma
- UCEC
- uterine corpus endometrial carcinoma
The datasets generated during and/or analyzed during the current study are publicly available.
Supplemental Digital Content is available for this article.
The authors have no funding and conflicts of interest to disclose.
The study protocol complied with the Declaration of Helsinki. The work described has not been submitted elsewhere for publication, in whole or in part, and all authors listed have approved the manuscript that in enclosed.
How to cite this article: Zhang D, Wang T, Zhou Y, Zhang X. Comprehensive analyses of cuproptosis-related gene CDKN2A on prognosis and immunologic therapy in human tumors. Medicine 2023;102:14(e33468).
Contributor Information
Di Zhang, Email: zhxp0813@163.com.
Tao Wang, Email: 739347208@qq.com.
Yi Zhou, Email: cathyfly1006@163.com.
References
- [1].Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- [2].Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029–30. [DOI] [PubMed] [Google Scholar]
- [3].Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, et al. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–47. [DOI] [PubMed] [Google Scholar]
- [4].Zhou J, Guo H, Liu L, et al. Pyroptosis patterns of colon cancer could aid to estimate prognosis, microenvironment and immunotherapy: evidence from multi-omics analysis. Aging (Milano). 2022;14:7547–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hua L, Lei P, Hu Y. Construction and validation model of necroptosis-related gene signature associates with immunity for osteosarcoma patients. Sci Rep. 2022;12:15893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fujihara KM, Zhang BZ, Jackson TD, et al. Eprenetapopt triggers ferroptosis, inhibits NFS1 cysteine desulfurase, and synergizes with serine and glycine dietary restriction. Sci Adv. 2022;8:eabm9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Qin X, Ma D, Tan YX, et al. The role of necroptosis in cancer: a double-edged sword? Biochim Biophys Acta Rev Cancer. 2019;1871:259–66. [DOI] [PubMed] [Google Scholar]
- [8].Tsvetkov P, Coy S, Petrova B, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Keswani T, Mitra S, Bhattacharyya A. Copper-induced immunotoxicity involves cell cycle arrest and cell death in the liver. Environ Toxicol. 2015;30:411–21. [DOI] [PubMed] [Google Scholar]
- [10].Jian Z, Guo H, Liu H, et al. Oxidative stress, apoptosis and inflammatory responses involved in copper-induced pulmonary toxicity in mice. Aging (Milano). 2020;12:16867–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–85. [DOI] [PubMed] [Google Scholar]
- [12].Liu H. Pan-cancer profiles of the cuproptosis gene set. Am J Cancer Res. 2022;12:4074–81. [PMC free article] [PubMed] [Google Scholar]
- [13].Kreuger IZM, Slieker RC, van Groningen T, et al. Therapeutic strategies for targeting CDKN2A loss in melanoma. J Invest Dermatol. 2023;143:18–25. [DOI] [PubMed] [Google Scholar]
- [14].Alhejaily A, Day AG, Feilotter HE, et al. Inactivation of the CDKN2A tumor-suppressor gene by deletion or methylation is common at diagnosis in follicular lymphoma and associated with poor clinical outcome. Clin Cancer Res. 2014;20:1676–86. [DOI] [PubMed] [Google Scholar]
- [15].Xing X, Cai W, Shi H, et al. The prognostic value of CDKN2A hypermethylation in colorectal cancer: a meta-analysis. Br J Cancer. 2013;108:2542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Oshima M, Okano K, Muraki S, et al. Immunohistochemically detected expression of 3 major genes (CDKN2A/p16, TP53, and SMAD4/DPC4) strongly predicts survival in patients with resectable pancreatic cancer. Ann Surg. 2013;258:336–46. [DOI] [PubMed] [Google Scholar]
- [17].Worst TS, Weis C-A, Stöhr R, et al. CDKN2A as transcriptomic marker for muscle-invasive bladder cancer risk stratification and therapy decision-making. Sci Rep. 2018;8:14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ryu HJ, Kim EK, Heo SJ, et al. Architectural patterns of p16 immunohistochemical expression associated with cancer immunity and prognosis of head and neck squamous cell carcinoma. APMIS. 2017;125:974–84. [DOI] [PubMed] [Google Scholar]
- [19].Aesif SW, Aubry MC, Yi ES, et al. Loss of p16(INK4A) expression and homozygous CDKN2A deletion are associated with worse outcome and younger age in thymic carcinomas. J Thorac Oncol. 2017;12:860–71. [DOI] [PubMed] [Google Scholar]
- [20].Li CH, Haider S, Boutros PC. Age influences on the molecular presentation of tumours. Nat Commun. 2022;13:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kang N, Xie X, Zhou X, et al. Identification and validation of EMT-immune-related prognostic biomarkers CDKN2A, CMTM8 and ILK in colon cancer. BMC Gastroenterol. 2022;22:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Luo JP, Wang J, Huang JH. CDKN2A is a prognostic biomarker and correlated with immune infiltrates in hepatocellular carcinoma. Biosci Rep. 2021;41:BSR20211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhou Z, Zhou Y, Liu D, et al. Prognostic and immune correlation evaluation of a novel cuproptosis-related genes signature in hepatocellular carcinoma. Front Pharmacol. 2022;13:1074123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bian Z, Fan R, Xie L. A novel cuproptosis-related prognostic gene signature and validation of differential expression in clear cell renal cell carcinoma. Genes. 2022;13:851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen Y. Identification and validation of cuproptosis-related prognostic signature and associated regulatory axis in uterine corpus endometrial carcinoma. Front Genet. 2022;13:912037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li T, Fan J, Wang B, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77:e108–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tang Z, Kang B, Li C, et al. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Modhukur V, Iljasenko T, Metsalu T, et al. MethSurv: a web tool to perform multivariable survival analysis using DNA methylation data. Epigenomics. 2018;10:277–88. [DOI] [PubMed] [Google Scholar]
- [31].Liu D, Li L, Wang L, et al. Recognition of DNA methylation molecular features for diagnosis and prognosis in gastric cancer. Front Genet. 2021;12:758926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Adib E, Nassar AH, Akl EW, et al. CDKN2A alterations and response to immunotherapy in solid tumors. Clin Cancer Res. 2021;27:4025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chan SH, Chiang J, Ngeow J. CDKN2A germline alterations and the relevance of genotype-phenotype associations in cancer predisposition. Hered Cancer Clin Pract. 2021;19:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang QQ, Zhou Y-C, Zhou Ge Y-J, et al. Comprehensive proteomic signature and identification of CDKN2A as a promising prognostic biomarker and therapeutic target of colorectal cancer. World J Clin Cases. 2022;10:7686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cheng T, Wu Y, Liu Z, et al. CDKN2A-mediated molecular subtypes characterize the hallmarks of tumor microenvironment and guide precision medicine in triple-negative breast cancer. Front Immunol. 2022;13:970950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lee CC, Kuo Y-C, Hu J-M, et al. MTNR1B polymorphisms with CDKN2A and MGMT methylation status are associated with poor prognosis of colorectal cancer in Taiwan. World J Gastroenterol. 2021;27:5737–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bruneval F, Dattani N, van Setten MJ. The GW miracle in many-body perturbation theory for the ionization potential of molecules. Front Chem. 2021;9:749779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Deneka AY, Baca Y, Serebriiskii IG, et al. Association of TP53 and CDKN2A mutation profile with tumor mutation burden in head and neck cancer. Clin Cancer Res. 2022;28:1925–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liu M, Liu Y, Zhou R, et al. Absence of NOTCH1 mutation and presence of CDKN2A deletion predict progression of esophageal lesions. J Pathol. 2022;258:38–48. [DOI] [PubMed] [Google Scholar]
- [40].Wang HP, Zhou Y-L, Huang X, et al. CDKN2A deletions are associated with poor outcomes in 101 adults with T-cell acute lymphoblastic leukemia. Am J Hematol. 2021;96:312–9. [DOI] [PubMed] [Google Scholar]
- [41].Zhou C, Li J, Li Q. CDKN2A methylation in esophageal cancer: a meta-analysis. Oncotarget. 2017;8:50071–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cao Z, Wei L, Zhu W, et al. Meta-analysis of CDKN2A methylation to find its role in prostate cancer development and progression, and also to find the effect of CDKN2A expression on disease-free survival (PRISMA). Medicine (Baltimore). 2018;97:e0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pang B, Xu X, Lu Y, et al. Prediction of new targets and mechanisms for quercetin in the treatment of pancreatic cancer, colon cancer, and rectal cancer. Food Funct. 2019;10:5339–49. [DOI] [PubMed] [Google Scholar]
- [44].Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nakamura K, Smyth MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol. 2020;17:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Petitprez F, Meylan M, de Reynies A, et al. The tumor microenvironment in the response to immune checkpoint blockade therapies. Front Immunol. 2020;11:784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vitale I, Manic G, Coussens LM, et al. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019;30:36–50. [DOI] [PubMed] [Google Scholar]
- [48].DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17:887–904. [DOI] [PubMed] [Google Scholar]
- [50].Liu H, Jia S, Guo K, et al. INK4 cyclin-dependent kinase inhibitors as potential prognostic biomarkers and therapeutic targets in hepatocellular carcinoma. Biosci Rep. 2022;42:BSR20221082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Xiao Z, Li J, Yu Q, et al. An inflammatory response related gene signature associated with survival outcome and gemcitabine response in patients with pancreatic ductal adenocarcinoma. Front Pharmacol. 2021;12:778294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Barrett R, Pure E. Cancer-associated fibroblasts: key determinants of tumor immunity and immunotherapy. Curr Opin Immunol. 2020;64:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Barrett RL, Pure E. Cancer-associated fibroblasts and their influence on tumor immunity and immunotherapy. eLife. 2020;9:e57243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].An Y, Liu F, Chen Y, et al. Crosstalk between cancer-associated fibroblasts and immune cells in cancer. J Cell Mol Med. 2020;24:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]