PURPOSE
Chemotherapy has not demonstrated benefit over adjuvant endocrine therapy alone for postmenopausal patients with node-positive breast cancer with a 21-gene breast recurrence score (RS) of 25 or below (RS ≤ 25). We tested whether combined results from RS and the sensitivity to endocrine therapy (SET2,3) index of endocrine-related transcription (SETER/PR) adjusted for baseline prognostic index (BPI) improve prognostic assessment, and whether SET2,3 predicted benefit from anthracycline-based chemotherapy.
METHODS
A blinded retrospective clinical validation of SET2,3 in two randomized treatment arms from the SWOG S8814 trial comparing adjuvant anthracycline-based chemotherapy followed by tamoxifen endocrine therapy for 5 years, versus tamoxifen alone. SET2,3 assay was calibrated and measured using whole-transcriptome RNA sequence of tumor samples already tested for RS. The primary end point was disease-free survival (DFS).
RESULTS
There were 106 events in 283 patients over a median follow-up of 8.99 years. Proportional hazards assumptions were met during the first 5 years only. SET2,3 index and RS were not correlated (r = –0.04) and were independently prognostic (SET2,3: hazard ratio [HR], 0.48 per unit; 95% CI, 0.34 to 0.68; P < .001; RS: HR, 1.28 per 10 units; 95% CI, 1.14 to 1.44; P < .001). SET2,3 index did not predict chemotherapy benefit (interaction P = .77). SET2,3 was high in 93/175 (53%) patients with RS ≤ 25 (concordant low-risk), with 5-year DFS 97%. SET2,3 was low in 55/108 (51%) patients with RS > 25 (concordant high-risk), with 5-year DFS 53%. Both components of SET2,3 index were prognostic after adjustment for RS: SETER/PR (HR, 0.65; 95% CI, 0.46 to 0.92) and BPI (HR, 0.45; 95% CI, 0.31 to 0.64).
CONCLUSION
SET2,3 index was not correlated with RS, demonstrated additive prognostic performance, and was not chemopredictive in this subset of patients from S8814. The SETER/PR and BPI components of SET2,3 each added prognostic information to RS.
INTRODUCTION
It remains an important clinical challenge to identify which patients with estrogen receptor–positive (ER+), node-positive breast cancer will have very low risk of disease recurrence after adjuvant endocrine therapy alone, and therefore do not benefit from chemotherapy.1 Ideally, selection criteria should combine predictions of favorable prognostic risk, negligible benefit from chemotherapy, and high sensitivity to endocrine therapy. We considered that two test results combined might refine this approach for patients with node-positive disease, if their results were independent and complementary.
CONTEXT
Key Objective
To determine whether an index measuring endocrine receptor–related transcription to predict sensitivity to endocrine therapy adjusted for baseline prognosis (SET2,3) improves the prognostic information from 21-gene breast recurrence score (RS) and whether cancers predicted to have low sensitivity to endocrine therapy preferentially benefit from adjuvant anthracycline-based chemotherapy.
Knowledge Generated
SET2,3 and RS were independent from each other and provided additive prognostic information when combined. SET2,3 index did not predict benefit from anthracycline-based chemotherapy in the S8814 trial.
Relevance (G.F. Fleming)
-
The combination of two independent tests (SET2,3 and RS) added meaningfully to the prognostic assessment of postmenopausal patients with node-positive breast cancer, independent of anthracycline chemotherapy. There are multiple genomic assays already used in breast cancer. Different genomic assays can provide additive information. Further work is needed to determine when use of multiple assays might be appropriate.*
*Relevance section written by JCO Associate Editor Gini F. Fleming, MD.
Oncotype Dx recurrence score (RS) has been shown to be both prognostic and predictive of chemotherapy benefit in women with node-negative and node-positive ER+ breast cancer.2,3 Nevertheless, nodal status still contributes independently to prognosis.1,4,5 RS was prognostic in the recent RxPONDER trial that evaluated the contribution of chemotherapy to adjuvant endocrine therapy for patients with 1-3 involved lymph nodes and RS of 25 or below (RS ≤ 25).1 No significant benefit from chemotherapy was observed in postmenopausal women, although the trial was not powered to establish noninferiority.1
The sensitivity to endocrine therapy (SET2,3) index is a customized assay for formalin-fixed paraffin-embedded tumor tissues, which yields reproducible results within and between different laboratories, offering prognostic information for patients receiving endocrine therapy.6-8 It combines the SET index of transcription related to estrogen and progesterone receptors (SETER/PR) with a baseline prognostic index (BPI) derived from pathologic tumor size, nodal involvement, and molecular subtype by RNA4 (ESR1, PGR, ERBB2, and AURKA).7-9 Each component, SETER/PR index and BPI, added independent prognostic information in prior studies8,10 and multivariable Cox models of SET2,3 demonstrated that the RNA4 within the BPI could be substituted by contemporary prognostic signatures, but the SETER/PR index could not be substituted.8 High SET2,3 scores are associated with endocrine sensitivity and more favorable prognosis, and low SET2,3 scores with endocrine therapy resistance and less-favorable outcomes.8-10
Two arms of the SWOG S8814 trial in postmenopausal patients with node-positive ER+ breast cancer compared anthracycline-based chemotherapy (cyclophosphamide, doxorubicin, and fluorouracil [CAF]) before 5 years of tamoxifen endocrine therapy, versus tamoxifen alone.11 RS was previously tested on 367 of these primary tumor samples and predicted benefit from CAF chemotherapy in patients with high RS (> 31).3 Our analysis addressed two hypotheses: (1) that SET2,3 adds prognostic information to RS and (2) that cancers with lower SET2,3 would benefit from CAF chemotherapy. We also explored prognostic contributions from each component index of SET2,3 (SETER/PR index and BPI).
METHODS
Study Materials and Design
Genomic Health (Exact Sciences, Madison, WI) performed whole-transcriptome RNA sequencing (wtRNAseq) using residual RNA after completion of the RS test, with 283 of 367 samples yielding high-quality data (Fig 1).3,12 Briefly, DNA libraries were prepared by depletion of ribosomal RNA from total RNA, then reverse transcription and amplification with random sequence primers (Illumina, San Diego, CA) and sequenced on an Illumina HiSeq instrument.12 Separately, the MD Anderson Cancer Center laboratory followed this Illumina sequencing procedure using KAPA kit (KAPA Biosystems, Wilmington, DE) and the same computational pipeline to calibrate SET2,3 and its component indices from wtRNAseq to the QuantigenePlex hybridization platform for SET2,3 (QGP, Thermo Fisher, Woburn, MA) in an independent cohort of 106 matched formalin-fixed paraffin-embedded samples (Appendix Fig A1, online only). Thereafter, SET2,3 values were calculated from the S8814 wtRNAseq data and analyzed by the clinical trial statistician at the SWOG Statistical Center (W.E.B.).
FIG 1.
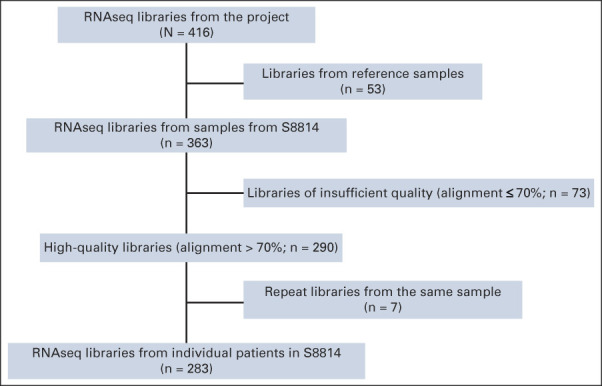
REMARK diagram of samples evaluated in this study. alignment, proportion of sequencing reads that align to the reference human genome, that is, known human DNA sequence; RNAseq, RNA sequencing; S8814, SWOG S8814 clinical trial.
Analysis Plan and Statistical Methods
RS was evaluated as a continuous index (per 10 units) and categorically using the predefined cutpoint for the RxPONDER trial (RS > 25 is high, RS ≤ 25 is low).1 SET2,3 index was evaluated as a continuous index (per unit) and categorically using a predefined cutpoint (SET2,3 > 2.10 is high, SET2,3 ≤ 2.10 is low). Because these were from surgical specimens, the published cutpoint for the neoadjuvant setting (using clinical tumor [T] stage and clinical node [N] stage) was calibrated to postsurgical specimens using pathologic information (pT and pN) using linear regression in a cohort of surgical specimens with known clinical and pathologic information about T and N.8 SETER/PR and BPI were also evaluated as continuous indices with exploratory cutpoint at median value (high if above median). Distribution of scores and cutoff values are presented in Appendix Figure A2 (online only). Kaplan-Meier plots with log-rank test and multivariate Cox models of disease-free survival (DFS) were adjusted for treatment arm only, unless it specifically states additional adjustment by RS. The predefined significance level was a two-sided α < .05. Tumor size, nodal status, and ER status and subtype were part of the SET2,3 index score calculation, so they were not included as adjustment variables.
RESULTS
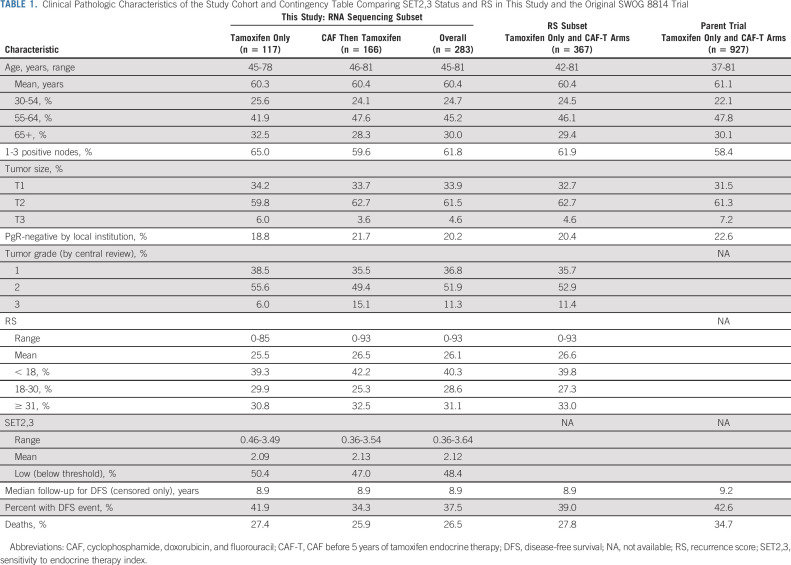
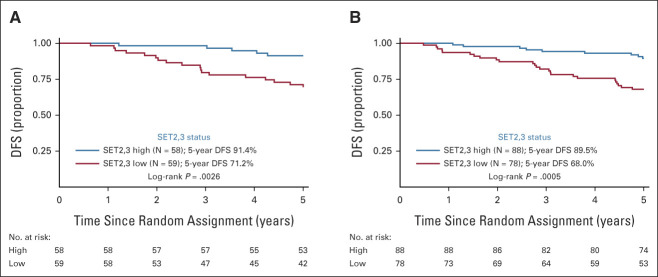
There were 106 events over a median follow-up of 9.1 years in the 283 patients in this study. Clinical characteristics of the 283 patients in this analysis are compared with a larger published subset of 367 patients with known RS result, and to the 927 patients overall in the two treatment arms from the parent S8814 trial in Table 1. There were no significant differences between those with SET2,3 values and those with RS only (Table 1). As the proportional hazards assumption only held during the initial 5 years of follow-up, the primary analyses were restricted to years 0-5 with 57 events. Five years was chosen since it is the end of recommended endocrine therapy. Continuous SET2,3 index was independently prognostic, with hazard ratio (HR, 0.46) and 95% CI (0.32 to 0.66; P < .001) adjusted for treatment arm. However, it did not predict chemotherapy benefit (interaction P = .77). High SET2,3 in 52% (146/283) of patients appeared to have prognostic gain only during the early follow-up (Fig 2). SET2,3 was highly prognostic within each treatment arm, with a 5-year DFS of 91.4% (95% CI, 80.5 to 96.3) in SET2,3 high patients and 71.2% (95% CI, 57.8 to 81.0) in SET2,3 low patients only receiving tamoxifen (P = .0026, Appendix Fig A3, online only). Similarly, 5-year DFS was 89.5% (95% CI, 80.8 to 94.4) in high SET2,3 patients and 68.0% (95% CI, 56.4 to 77.1) in low SET2,3 patients receiving chemotherapy and tamoxifen (P = .0005, Appendix Fig A3).
TABLE 1.
Clinical Pathologic Characteristics of the Study Cohort and Contingency Table Comparing SET2,3 Status and RS in This Study and the Original SWOG 8814 Trial
FIG 2.
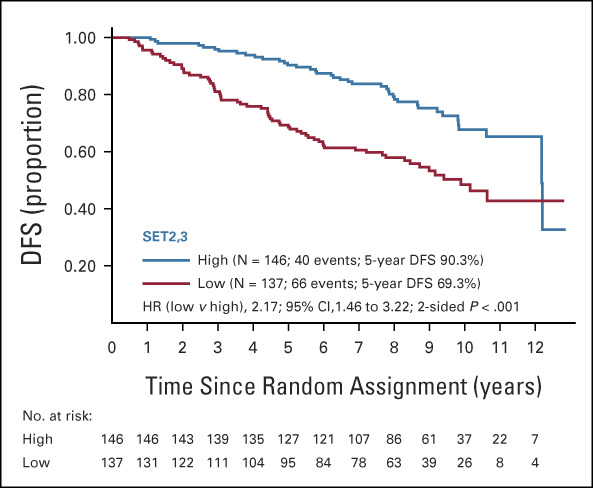
Kaplan-Meier plot of DFS by SET2,3 status according to the predefined cutpoint (high > 2.10, low ≤ 2.10). Prognostic separation continued throughout the first 5 years of follow-up (duration of adjuvant endocrine therapy). DFS, disease-free survival; HR, hazard ratio; SET2,3, sensitivity to endocrine therapy index.
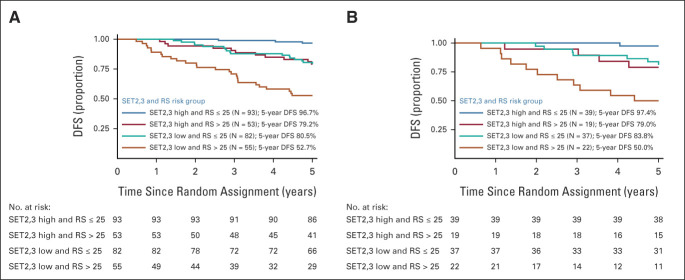
Continuous SET2,3 and RS were not correlated as continuous variables (r = –0.04) or categories (Table 2), yet both RS (HR [10 units], 1.28; 95% CI, 1.14 to 1.44; P < .001) and SET2,3 (HR, 0.48; 95% CI, 0.34 to 0.68; P < .001) were independently prognostic in multivariable analysis adjusting for treatment (Table 3). High SET2,3 status was observed in 53% (93/175) of cancers with RS ≤ 25 and 49% (53/108) of cancers with RS > 25 (Table 2). Overall, similar outcomes by RS and SET2,3 were observed in the overall SET2,3 study population (Fig 3A) and in the patients who received tamoxifen alone (Fig 3B). SET2,3 index was prognostic in the subset of 175 patients with lower-risk RS ≤ 25 (HR, 0.34; 95% CI, 0.18 to 0.62; P < .001) and the subset of 108 patients with higher-risk RS > 25 (HR, 0.53; 95% CI, 0.33 to 0.84; P = .007; Fig 3A). When both RS and SET2,3 results were favorable (93/283 [33%] patients), there were only rare DFS events observed in the overall study population and in those treated with tamoxifen alone (97% 5-year DFS; Figs 3A and 3B). When both results were unfavorable (55/283; 19%), 5-year DFS was approximately 50%, identifying a group at particularly high risk of disease recurrence (Figs 3A and 3B).
TABLE 2.
Contingency Table Comparing SET2,3 Status and RS Status
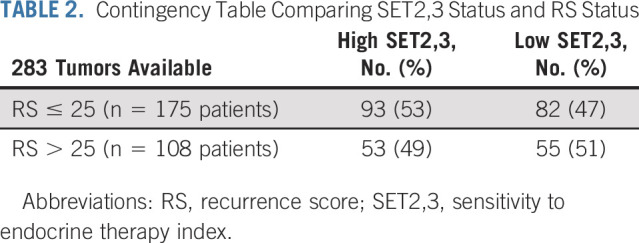
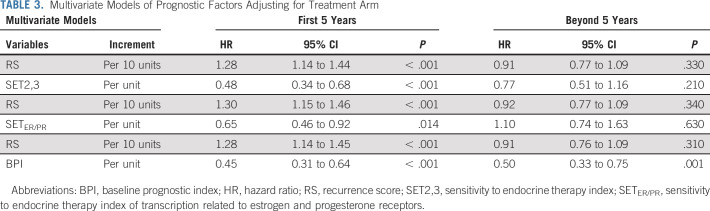
TABLE 3.
Multivariate Models of Prognostic Factors Adjusting for Treatment Arm
FIG 3.
Kaplan-Meier plot of DFS by the combination of SET2,3 status (high > 2.10, low ≤ 2.10) and RS status (low ≤ 25, high > 25) in (A) all patients and (B) patients treated with endocrine therapy alone. During the first 5 years, the complementary prognostic effect from combined SET2,3 and RS appeared identical in both A and B. DFS, disease-free survival; RS, recurrence score; SET2,3, sensitivity to endocrine therapy index.
Since the BPI component of SET2,3 includes pathologic tumor size and number of involved nodes, we separately analyzed the BPI and SETER/PR index to explore the prognostic contribution from each. Therefore, we evaluated multivariable Cox models of RS with BPI, and separately, RS with SETER/PR index while adjusting for treatment. In both models, RS was independently prognostic during years 0-5 (Table 3). BPI was independently prognostic from RS during years 0-5 (HR, 0.45; P < .001) and after 5 years (HR, 0.50; P = .001). In the other model, SETER/PR index was independently prognostic from RS during years 0-5 (HR, 0.65; P = .014), but neither RS nor SETER/PR index was prognostic after 5 years (Table 3).
DISCUSSION
The universal use of chemotherapy in women with node-positive, ER+ breast cancer leads to overtreatment of a significant number of women who neither need nor benefit from such treatment. Genomic test results and clinicopathologic information can guide these recommendations for chemotherapy, but the utility of SET2,3 index in this patient population has not yet been evaluated. Our results demonstrated that SET2,3 added complementary prognostic information to RS results in the S8814 trial for postmenopausal patients with node-positive disease treated with tamoxifen, but did not predict benefit from CAF chemotherapy. Interestingly, the independent prognostic contribution from BPI was during the full 10 years of follow-up, perhaps because BPI combines purely prognostic information from tumor size, number of involved lymph nodes, and molecular subtype. We might expect the risk associated with a purely prognostic biomarker to persist during the full natural history of the disease. However, both the SETER/PR index and RS contributed to prognosis during the 5 years of adjuvant treatment (possibly also a brief carryover period until years 6-7; Fig 2). This might be expected from a predictive biomarker if the discrimination between patient outcomes only lasts the duration of the benefit from the treatment itself.
Unlike other prognostic biomarkers, SET2,3 was not correlated with RS.13,14 The correlation coefficient for RS with SET2,3 was –0.04 in our analysis of 283 ER+ cancers from S8814, whereas the reported correlation coefficients of RS with PAM50 risk of recurrence (ROR) score, EndoPredict score, and Breast Cancer Index were 0.32, 0.63, and 0.35, respectively, in 785 ER+ cancers from the TransATAC trial.15 Visual inspection of the published scatterplots from the TransATAC analysis illustrated that few cancers with RS > 25 have low-risk PAM50 ROR, EndoPredict, or Breast Cancer Index status.15 However, we observed that 49% of tumors with RS > 25 were classified as high SET2,3 index (high sensitivity to endocrine therapy adjusted for baseline prognosis) and those patients had better DFS than patients with RS > 25 and low SET2,3, even in the tamoxifen-alone treatment arm. This has potential relevance for contemporary treatments for patients with high-risk ER+ cancer that are combined with endocrine therapy, for example, combining a cyclin-dependent kinase 4/6 inhibitor with endocrine therapy. We think it would be informative to evaluate both RS and SET2,3 in that treatment setting.
The TransATAC analysis of biomarkers also concluded that RS had more correlation with endocrine-related transcription and less correlation with proliferation than PAM50 ROR, EndoPredict, or Breast Cancer Index.15 Interestingly, we have demonstrated that the SETER/PR index, the component of SET2,3 that was designed to measure endocrine-related transcriptional output that is not directly related to proliferation, by itself added independent prognostic information to RS. We interpret this as added information about endocrine activity in the tumor. Overall, we can interpret the added prognostic contribution from SET2,3 to be from both the inclusion of tumor size and nodal burden in the BPI, and the additional measure of endocrine activity from the SETER/PR index. Hence, combination of SET2,3 and RS could improve prediction of sensitivity to endocrine therapy by RS alone, since these biomarkers are not correlated and SET2,3 alone predicted early response and long-term survival following neoadjuvant endocrine therapy in the Z1031 trial.10
Caution is advised if considering our results from S8814 in the postmenopausal population in RxPONDER. These populations differed in the proportion of tumors with RS > 25 (38% in S8814 [Table 1], v 11% of patients screened for RxPONDER), number of involved lymph nodes (38% with ≥ 4 nodes involved in S8814, v only 1-3 nodes involved in RxPONDER, with only one node involved in 66% and including micrometastasis only), tumor stage (33% with pT1, v 59%), proportion with progesterone receptor–negative status (21% v 8%), likely human epidermal growth factor receptor 2 (HER2)–positive status (12% estimated from gene expression level, v 0%), patients who received chemotherapy containing a taxane (0% v 96%), and the type of endocrine therapy (all tamoxifen, v 86% aromatase inhibitor).1,3 Essentially, the populations of S8814 and RxPONDER represent different eras in the management of node-positive ER+ breast cancer. Nevertheless, it would be important to study this combination of biomarkers in samples from RxPONDER.
The combined results from two complementary tests could also be useful for patients with node-positive ER+ breast cancer of lower risk (such as RS ≤ 25), if they strengthen the prognostic basis for relying on endocrine therapy alone. In the recent RxPONDER trial, postmenopausal women with RS ≤ 25 did not benefit from chemotherapy, but RS remained prognostic in each treatment arm.1 Although the S8814 population had higher risk and received less effective adjuvant treatment than the patients in RxPONDER, patients from S8814 whose cancer had RS ≤ 25 and high SET2,3 had 5-year DFS of 97%, even in the tamoxifen alone treatment arm. We did observe that relapse after 5 years was associated with disease burden and molecular subtype (BPI), but not RS or SETER/PR index. However, contemporary endocrine therapy is more effective and of longer duration, and this might influence the duration of their prognostic effect. Patients with node-positive ER+ breast cancer have increased prognostic risk and two tests may be reasonable to support a decision to de-escalate adjuvant treatment, if the tests are additive and independent. Our results suggest that combining SET2,3 and RS might improve prognostic assessment for node-positive patients with RS ≤ 25, to increase confidence in endocrine therapy alone. This needs to be confirmed in a second clinical trial.
A limitation of our study is that SET2,3 was interpreted from wtRNAseq data, rather than tested on samples, albeit with calibration of the highly correlated measurements from these techniques using linear regression.16 Additionally, treatments in S8814 are no longer contemporary: chemotherapy did not include a taxane and endocrine therapy did not include aromatase inhibition. Additionally, S8814 predates HER2 testing and targeted therapy that might influence outcomes in this cohort. We estimate that 12% of samples might be from HER2-positive cancers, on the basis of RS measurements of ERBB2 expression, with most of those having RS > 25 and low SET2,3.3
SET2,3 index testing is already being performed in a Clinical Laboratory Improvement Amendments–certified laboratory in the context of ongoing clinical trials under investigational device exemption from the US Food and Drug Administration (I-SPY, ClinicalTrials.gov identifier: NCT01042379). It adds independent prognostic information to response to neoadjuvant chemotherapy and has demonstrated ability to identify patients for whom neoadjuvant endocrine therapy would be appropriate.8,10 Added value of this test in the future would probably derive from its accurate measurement of endocrine transcriptional activity in the tumor to predict sensitivity to endocrine therapy, since that information is relevant to a patient's overall endocrine treatment strategy.
In summary, the prognostic, chemopredictive, and endocrine predictive qualities of RS and SET2,3 provided clinically meaningful assessment of prognosis during the first 5 years of follow-up. Their combined results were stronger than either biomarker alone and were similar in both treatment arms. Thus, we conclude that SET2,3 is a biomarker with potential to improve the clinical performance of genomic testing of stage II-III breast cancer and deserves further evaluation of clinical utility.
APPENDIX
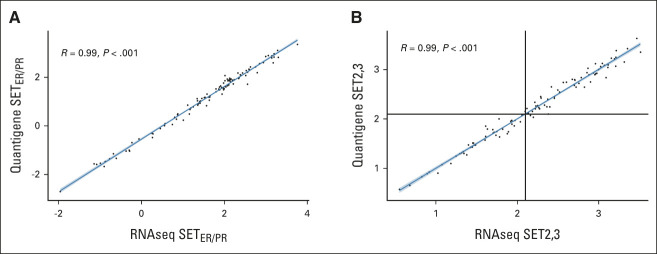
FIG A1.
Independent calibration of SETER/PR and SET2,3 indices from the diagnostic assay platform (Quantigene) to whole-transcriptome RNAseq in 106 formalin-fixed paraffin-embedded tumor samples of breast cancer: (A) correlation of the SETER/PR index before calibration and (B) correlation of the SET2,3 index after calibration using linear regression. Black horizontal and vertical lines at 2.1 indicate the threshold between high and low SET2,3. R, Pearson correlation coefficient; RNAseq, RNA sequencing; SET2,3, sensitivity to endocrine therapy index; SETER/PR, sensitivity to endocrine therapy index of transcription related to estrogen and progesterone receptors.
FIG A2.
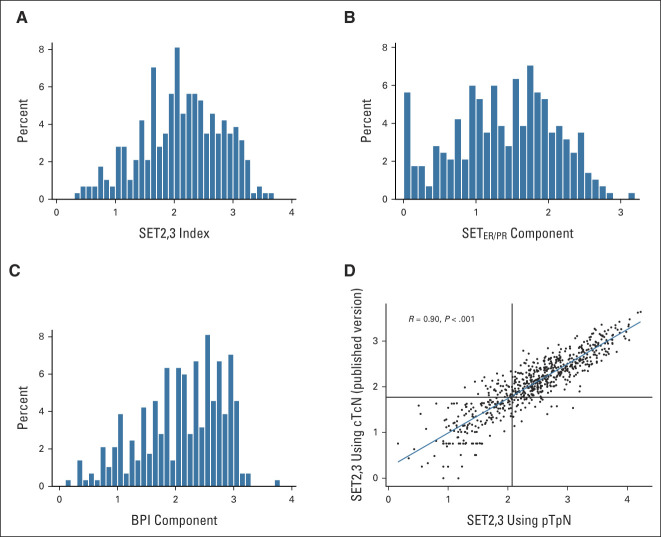
(A) Distribution of SET2,3 scores across the entire cohort; median, 2.15; IQR, 1.65-2.64. (B) Distribution of the estrogen receptor/progesterone receptor component of SET2,3; SETER/PR median, 1.45; IQR, 0.92-1.92 and (C) the BPI component; median, 2.18; IQR, 1.64-2.67. (D) Independent calibration of SET2,3 indices in the diagnostic assay platform (Quantigene) with 807 tumor samples of hormone receptor–positive/human epidermal growth factor receptor 2–negative breast cancer: correlation of the SET2,3 index between using pT with pathologic number of pN and published version of SET2,3 using cT with cN. Black vertical line at 2.10 for pathologic SET2,3 and horizontal line at 1.77 for clinical SET2,3 indicate the threshold between high and low SET2,3. BPI, baseline prognostic index; cN, clinical node stage; cT, clinical tumor stage; IQR, interquartile range; pN, positive nodes; pT, pathologic tumor size; SET2,3, sensitivity to endocrine therapy index; SETER/PR, sensitivity to endocrine therapy index of transcription related to estrogen and progesterone receptors.
FIG A3.
Kaplan-Meier plots of DFS according to SET2,3 status (high, low) during the first 5 years in each treatment arm: (A) tamoxifen only and (B) CAF chemotherapy followed by tamoxifen. CAF, cyclophosphamide, doxorubicin, and fluorouracil; DFS, disease-free survival; SET2,3, sensitivity to endocrine therapy index.
Corey W. Speers
Consulting or Advisory Role: Exact Sciences
Patents, Royalties, Other Intellectual Property: Compositions and Methods for the Analysis of Radiosensitivity, UM-33550/US-1, Coinventor, Submitted on 09/2013, Methods and Genomic Classifiers for Prognosis of Breast Cancer and Predicting Benefit from Adjuvant Radiotherapy, Application No. 61/205,279, Co-inventor
W. Fraser Symmans
Stock and Other Ownership Interests: ISIS Pharmaceuticals, Delphi Diagnostics, Eiger BioPharmaceuticals
Consulting or Advisory Role: Merck, AstraZeneca
Research Funding: Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Co-inventor, US Patent No. 11,459,617 “Targeted Measure of Transcriptional Activity Related to Hormone Receptors,” issued on October 4, 2022 (applicant proprietor: University of Texas MD Anderson Cancer Center)
Uncompensated Relationships: Delphi Diagnostics
Open Payments Link: https://openpaymentsdata.cms.gov/physician/256534
William E. Barlow
Research Funding: Merck (Inst), AstraZeneca (Inst)
Steven Shak
Employment: Exact Sciences, Roche (I)
Leadership: Exact Sciences
Stock and Other Ownership Interests: Exact Sciences
Consulting or Advisory Role: Exact Sciences, Genomic Life
Patents, Royalties, Other Intellectual Property: Filed Oncotype DX patents (Inst)
Rick Baehner
Employment: Exact Sciences
Leadership: Exact Sciences
Stock and Other Ownership Interests: Exact Sciences
Priyanka Sharma
Stock and Other Ownership Interests: Amgen Astellas BioPharma (I)
Consulting or Advisory Role: Novartis, Merck, AstraZeneca, Pfizer, Gilead Sciences, GlaxoSmithKline, Sanofi
Research Funding: Novartis (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Gilead Sciences
Patents, Royalties, Other Intellectual Property: UpToDate
Lajos Pusztai
Honoraria: bioTheranostics, Natera, OncoCyte, Athenex
Consulting or Advisory Role: H3 Biomedicine, Merck, Novartis, Seattle Genetics, Syndax, AstraZeneca, Roche/Genentech, Bristol Myers Squibb, Clovis Oncology, Immunomedics, Eisai, Almac Diagnostics, Pfizer
Research Funding: Merck (Inst), Genentech (Inst), Seattle Genetics (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Uncompensated Relationships: NanoString Technologies, Foundation Medicine.
Open Payments Link: https://openpaymentsdata.cms.gov/physician/110878
Gabriel N. Hortobagyi
Consulting or Advisory Role: Novartis, Seagan, Blueprint Medicines, AstraZeneca
Research Funding: Novartis (Inst)
Daniel F. Hayes
Stock and Other Ownership Interests: InBiomotion
Honoraria: Tempus
Consulting or Advisory Role: Cepheid, Freenome, Epic Sciences, Cellworks, BioVica, Oncocyte, Turnstone Bio, Predictus Biosciences, Guardant Health, L-Nutra, Macrogenics, Tempus, Xilis, Exact Sciences
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Menarini Silicon Biosystems (Inst), Cepheid/Danaher (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from licensed technology, Diagnosis and Treatment of Breast Cancer. Patent No. US 8,790,878 B2. Date of Patent: July 29, 2014. Applicant Proprietor: University of Michigan. Dr Daniel F. Hayes is designated as inventor/coinventor, Circulating Tumor Cell Capturing Techniques and Devices. Patent No.: US 8,951,484 B2. Date of Patent: February 10, 2015. Applicant Proprietor: University of Michigan. Dr Daniel F. Hayes is designated as inventor/coinventor, Title: A method for predicting progression free and overall survival at each follow-up timepoint during therapy of metastatic breast cancer patients using circulating tumor cells. Patent no. 05725638.0-1223-US2005008602
Other Relationship: Menarini, UpToDate
Uncompensated Relationships: UpToDate
Kathy S. Albain
Honoraria: Encore Medical Education
Research Funding: Seattle Genetics (Inst), Quantum Leap Healthcare Collaborative (Inst)
Other Relationship: Seattle Genetics
Andrew Godwin
Stock and Other Ownership Interests: Clara Biotech
Honoraria: Sinochips Kansas
Consulting or Advisory Role: Biovica
Research Funding: BioFluidica (Inst), VITRAC Therapeutics (Inst), Clara Biotech (Inst), Predicine (Inst)
Patents, Royalties, Other Intellectual Property: Provisional Patent application titled—WJMSC-Derived Small Extracellular Vesicle (sEVs) Enhance T Cell Suppression Through Checkpoint PD-L1—Dr Joseph McGuirk (applicant) (Inst)
Alastair Thompson
Employment: Lilly (I)
Stock and Other Ownership Interests: Lilly (I)
No other potential conflicts of interest were reported.
See accompanying editorial on page 1816
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented in part at the San Antonio Breast Cancer Symposium, San Antonio, TX, December 9, 2021.
SUPPORT
Supported by the NIH/NCI through Grant Nos. U10CA180888 and U10CA180819; and in part by Genomic Health Inc (now Exact Sciences Corporation) by RNA sequencing of residual tumor RNA samples. Also supported in part by a SWOG/Hope Foundation Impact Award, Cancer Prevention and Research Institute of Texas Grant Award No. RP#180712, and by Breast Cancer Research Foundation Grant Award No. BCRF-158. Study S8814 was sponsored by the National Cancer Institute, was led by SWOG, and was conducted by the NIH-funded National Clinical Trials Network (NCTN).
CLINICAL TRIAL INFORMATION
C.W.S. and W.F.S. contributed equally to this work as co-first authors.
AUTHOR CONTRIBUTIONS
Conception and design: Corey W. Speers, W. Fraser Symmans, William E. Barlow, Steven Shak, Rick Baehner, Priyanka Sharma, Lajos Pusztai, Daniel F. Hayes, Kathy S. Albain, Alastair Thompson
Financial support: Corey W. Speers, James M. Rae
Administrative support: Corey W. Speers, Lajos Pusztai, Gabriel N. Hortobagyi, Andrew Godwin
Provision of study materials or patients: Corey W. Speers, W. Fraser Symmans, James M. Rae, Rick Baehner, Lajos Pusztai, Daniel F. Hayes, Kathy S. Albain
Collection and assembly of data: Corey W. Speers, W. Fraser Symmans, William E. Barlow, James M. Rae
Data analysis and interpretation: Corey W. Speers, William E. Barlow, Alex Trevarton, Stephanie The, Lili Du, James M. Rae, Steven Shak, Lajos Pusztai, Gabriel N. Hortobagyi, Kathy S. Albain, Andrew Godwin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Evaluation of the Sensitivity to Endocrine Therapy Index and 21-Gene Breast Recurrence Score in the SWOG S8814 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Corey W. Speers
Consulting or Advisory Role: Exact Sciences
Patents, Royalties, Other Intellectual Property: Compositions and Methods for the Analysis of Radiosensitivity, UM-33550/US-1, Coinventor, Submitted on 09/2013, Methods and Genomic Classifiers for Prognosis of Breast Cancer and Predicting Benefit from Adjuvant Radiotherapy, Application No. 61/205,279, Co-inventor
W. Fraser Symmans
Stock and Other Ownership Interests: ISIS Pharmaceuticals, Delphi Diagnostics, Eiger BioPharmaceuticals
Consulting or Advisory Role: Merck, AstraZeneca
Research Funding: Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Co-inventor, US Patent No. 11,459,617 “Targeted Measure of Transcriptional Activity Related to Hormone Receptors,” issued on October 4, 2022 (applicant proprietor: University of Texas MD Anderson Cancer Center)
Uncompensated Relationships: Delphi Diagnostics
Open Payments Link: https://openpaymentsdata.cms.gov/physician/256534
William E. Barlow
Research Funding: Merck (Inst), AstraZeneca (Inst)
Steven Shak
Employment: Exact Sciences, Roche (I)
Leadership: Exact Sciences
Stock and Other Ownership Interests: Exact Sciences
Consulting or Advisory Role: Exact Sciences, Genomic Life
Patents, Royalties, Other Intellectual Property: Filed Oncotype DX patents (Inst)
Rick Baehner
Employment: Exact Sciences
Leadership: Exact Sciences
Stock and Other Ownership Interests: Exact Sciences
Priyanka Sharma
Stock and Other Ownership Interests: Amgen Astellas BioPharma (I)
Consulting or Advisory Role: Novartis, Merck, AstraZeneca, Pfizer, Gilead Sciences, GlaxoSmithKline, Sanofi
Research Funding: Novartis (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Gilead Sciences
Patents, Royalties, Other Intellectual Property: UpToDate
Lajos Pusztai
Honoraria: bioTheranostics, Natera, OncoCyte, Athenex
Consulting or Advisory Role: H3 Biomedicine, Merck, Novartis, Seattle Genetics, Syndax, AstraZeneca, Roche/Genentech, Bristol Myers Squibb, Clovis Oncology, Immunomedics, Eisai, Almac Diagnostics, Pfizer
Research Funding: Merck (Inst), Genentech (Inst), Seattle Genetics (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Uncompensated Relationships: NanoString Technologies, Foundation Medicine.
Open Payments Link: https://openpaymentsdata.cms.gov/physician/110878
Gabriel N. Hortobagyi
Consulting or Advisory Role: Novartis, Seagan, Blueprint Medicines, AstraZeneca
Research Funding: Novartis (Inst)
Daniel F. Hayes
Stock and Other Ownership Interests: InBiomotion
Honoraria: Tempus
Consulting or Advisory Role: Cepheid, Freenome, Epic Sciences, Cellworks, BioVica, Oncocyte, Turnstone Bio, Predictus Biosciences, Guardant Health, L-Nutra, Macrogenics, Tempus, Xilis, Exact Sciences
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Menarini Silicon Biosystems (Inst), Cepheid/Danaher (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from licensed technology, Diagnosis and Treatment of Breast Cancer. Patent No. US 8,790,878 B2. Date of Patent: July 29, 2014. Applicant Proprietor: University of Michigan. Dr Daniel F. Hayes is designated as inventor/coinventor, Circulating Tumor Cell Capturing Techniques and Devices. Patent No.: US 8,951,484 B2. Date of Patent: February 10, 2015. Applicant Proprietor: University of Michigan. Dr Daniel F. Hayes is designated as inventor/coinventor, Title: A method for predicting progression free and overall survival at each follow-up timepoint during therapy of metastatic breast cancer patients using circulating tumor cells. Patent no. 05725638.0-1223-US2005008602
Other Relationship: Menarini, UpToDate
Uncompensated Relationships: UpToDate
Kathy S. Albain
Honoraria: Encore Medical Education
Research Funding: Seattle Genetics (Inst), Quantum Leap Healthcare Collaborative (Inst)
Other Relationship: Seattle Genetics
Andrew Godwin
Stock and Other Ownership Interests: Clara Biotech
Honoraria: Sinochips Kansas
Consulting or Advisory Role: Biovica
Research Funding: BioFluidica (Inst), VITRAC Therapeutics (Inst), Clara Biotech (Inst), Predicine (Inst)
Patents, Royalties, Other Intellectual Property: Provisional Patent application titled—WJMSC-Derived Small Extracellular Vesicle (sEVs) Enhance T Cell Suppression Through Checkpoint PD-L1—Dr Joseph McGuirk (applicant) (Inst)
Alastair Thompson
Employment: Lilly (I)
Stock and Other Ownership Interests: Lilly (I)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kalinsky K, Barlow WE, Gralow JR, et al. : 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med 385:2336-2347, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paik S, Tang G, Shak S, et al. : Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726-3734, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Albain KS, Barlow WE, Shak S, et al. : Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol 11:55-65, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sparano JA, Gray RJ, Makower DF, et al. : Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373:2005-2014, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparano JA, Gray RJ, Makower DF, et al. : Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379:111-121, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau R, Du L, Chen E, et al. : Technical validity of a customized assay of sensitivity to endocrine therapy using sections from fixed breast cancer tissue. Clin Chem 66:934-945, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Bossuyt V, Lau R, Young B, et al. : Intra- and interlaboratory reproducibility of the sensitivity to endocrine therapy assay for stage II/III breast cancer. Clin Chem 67:1240-1248, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Du L, Yau C, Brown-Swigart L, et al. : Predicted sensitivity to endocrine therapy for stage II-III hormone receptor-positive and HER2-negative (HR+/HER2-) breast cancer before chemo-endocrine therapy. Ann Oncol 32:642-651, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Sinn BV, Fu C, Lau R, et al. : SET-ER/PR—A robust 18-gene predictor for sensitivity to endocrine therapy for metastatic breast cancer. NPJ Breast Cancer 5:16, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suman VJ, Du L, Hoskin TL, et al. : Evaluation of sensitivity to endocrine therapy index (SET2,3) for response to neoadjuvant endocrine therapy (NET) and longer-term breast cancer patient outcomes. Clin Cancer Res 28:3287-3295, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albain KS, Barlow WE, Ravdin PM, et al. : Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: A phase 3, open-label, randomised controlled trial. Lancet 374:2055-2063, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherbavaz DB, Hayes DF, Qu K, et al. : Abstract P5-07-01: Successful whole transcriptome analysis of 25-year-old breast tumor samples from the phase III trial SWOG-8814 by next generation sequencing (NGS): Standardized analytical methods for exploratory and validation studies. Cancer Res 76, 2016. (4 suppl; abstr P5-07-01) [Google Scholar]
- 13.Fan C, Oh DS, Wessels L, et al. : Concordance among gene-expression-based predictors for breast cancer. N Engl J Med 355:560-569, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Sotiriou C, Pusztai L: Gene-expression signatures in breast cancer. N Engl J Med 360:790-800, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Buus R, Sestak I, Kronenwett R, et al. : Molecular drivers of Oncotype DX, Prosigna, EndoPredict, and the Breast Cancer Index: A TransATAC study. J Clin Oncol 39:126-135, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suman VJ, Du L, Hoskin T, et al. : Evaluation of sensitivity to endocrine therapy index (SET2,3) for response to neoadjuvant endocrine therapy and longer-term breast cancer patient outcomes (Alliance Z1031). Clin Cancer Res 28:3287-3295, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]