Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
PURPOSE
The phase III ADAURA (ClinicalTrials.gov identifier: NCT02511106) primary analysis demonstrated a clinically significant disease-free survival (DFS) benefit with adjuvant osimertinib versus placebo in EGFR-mutated stage IB-IIIA non–small-cell lung cancer (NSCLC) after complete tumor resection (DFS hazard ratio [HR], 0.20 [99.12% CI, 0.14 to 0.30]; P < .001). We report an updated exploratory analysis of final DFS data.
METHODS
Overall, 682 patients with stage IB-IIIA (American Joint Committee on Cancer/Union for International Cancer Control, seventh edition) EGFR-mutated (exon 19 deletion/L858R) NSCLC were randomly assigned 1:1 (stratified by stage, mutational status, and race) to receive osimertinib 80 mg once-daily or placebo for 3 years. The primary end point was DFS by investigator assessment in stage II-IIIA disease analyzed by stratified log-rank test; following early reporting of statistical significance in DFS, no further formal statistical testing was planned. Secondary end points included DFS in stage IB-IIIA, overall survival, and safety. Patterns of recurrence and CNS DFS were prespecified exploratory end points.
RESULTS
At data cutoff (April 11, 2022), in stage II-IIIA disease, median follow-up was 44.2 months (osimertinib) and 19.6 months (placebo); the DFS HR was 0.23 (95% CI, 0.18 to 0.30); 4-year DFS rate was 70% (osimertinib) and 29% (placebo). In the overall population, DFS HR was 0.27 (95% CI, 0.21 to 0.34); 4-year DFS rate was 73% (osimertinib) and 38% (placebo). Fewer patients treated with osimertinib had local/regional and distant recurrence versus placebo. CNS DFS HR in stage II-IIIA was 0.24 (95% CI, 0.14 to 0.42). The long-term safety profile of osimertinib was consistent with the primary analysis.
CONCLUSION
These updated data demonstrate prolonged DFS benefit over placebo, reduced risk of local and distant recurrence, improved CNS DFS, and a consistent safety profile, supporting the efficacy of adjuvant osimertinib in resected EGFR-mutated NSCLC.
INTRODUCTION
Until recently, the standard of care for patients with resectable non–small-cell lung cancer (NSCLC) has been surgical resection followed by postoperative adjuvant chemotherapy, when indicated.1-4 However, recurrence is common and increases with disease stage.5,6 Recently, epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) and immunotherapies have demonstrated improvements in disease-free survival (DFS) in the adjuvant setting.7-9
CONTEXT
Key Objective
ADAURA is an ongoing phase III trial assessing efficacy and safety of osimertinib versus placebo in patients with completely resected stage IB-IIIA non–small-cell lung cancer, with/without adjuvant chemotherapy. After 2 years of additional follow-up, we report updated analyses of final disease-free survival (DFS) data, recurrence patterns, and long-term safety.
Knowledge Generated
These updated data, in which all patients had the opportunity to receive 3 years of planned treatment, were consistent with the primary analysis and demonstrated prolonged DFS benefit with adjuvant osimertinib versus placebo: stage II-IIIA DFS hazard ratio, 0.23; 95% CI, 0.18 to 0.30; stage IB-IIIA DFS hazard ratio, 0.27; 95% CI, 0.21 to 0.34. Adjuvant osimertinib reduced the risk of local and distant recurrence, and improved CNS DFS. The long-term safety profile of osimertinib was consistent with the primary analysis.
Relevance (T.E. Stinchcombe)
-
The final DFS analysis demonstrated that adjuvant osimertinib improved DFS in resected EGFR-mutant non–small-cell lung cancer, and in the patient subgroups analyzed.*
*Relevance section written by JCO Associate Editor Thomas E. Stinchcombe, MD.
Osimertinib is a third-generation EGFR-TKI with demonstrated efficacy in NSCLC, including in the CNS.10-15 The primary DFS analysis of the phase III ADAURA trial of adjuvant osimertinib was reported 2 years earlier than planned following Independent Data Monitoring Committee recommendation because of an efficacy benefit. Osimertinib demonstrated a significant DFS benefit versus placebo in patients with EGFR-mutated stage IB-IIIA NSCLC after complete tumor resection with or without adjuvant chemotherapy.11 The DFS hazard ratio (HR) for patients with stage II-IIIA was 0.17 (99.06% CI, 0.11 to 0.26); P < .001; DFS HR for stage IB-IIIA was 0.20 (99.12% CI, 0.14 to 0.30); P < .001, with a median treatment duration of 22.5 months (range, 0-38 months) for the osimertinib group and 18.7 months (range, 0-36 months) for the placebo group.11 On the basis of these data, osimertinib was the first targeted therapy approved in many countries as an adjuvant treatment for resected EGFR-mutated stage IB-IIIA NSCLC16-18 and is recommended by international treatment guidelines.2,4
Whether recurrences are local or distant following surgery can impact postrecurrence survival.19 CNS metastases are common among patients with NSCLC, and are a poor prognostic factor associated with deterioration in quality of life.20,21 In the ADAURA primary analysis, patients treated with osimertinib had a lower incidence of metastatic disease and the CNS DFS HR was 0.18 (95% CI, 0.10 to 0.33).11
Here, we report updated DFS data at the protocol-specified maturity of approximately 50% and recurrence patterns after 2 years of further follow-up. We also report a post hoc exploratory analysis of DFS by disease stage according to the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) eighth edition cancer staging manual.22
METHODS
Trial Design and Patients
Full details of ADAURA methodology have been published previously11,23 and are available in the Protocol (online only); the study design is shown in the Data Supplement (online only). Eligible patients age ≥ 18 years (≥ 20 years in Japan and Taiwan) had NSCLC of postsurgical pathologic stage IB (T2a tumors > 3 cm and ≤ 5 cm in size), II, or IIIA (AJCC/UICC seventh edition), centrally confirmed EGFR mutation (exon 19 deletion [Ex19del]/L858R), and WHO performance status of 0 or 1. Complete surgical resection of primary NSCLC, and adjuvant chemotherapy (if required, per physician and patient choice) took place before random assignment. A computed tomography (CT) or magnetic resonance imaging (MRI) brain scan at baseline before surgery or random assignment was required. Patients were stratified by EGFR mutation (Ex19del/L858R), disease stage (IB/II/IIIA), and race (Asian/non-Asian) and randomly assigned 1:1 to receive oral osimertinib 80 mg once-daily or placebo until disease recurrence, meeting a treatment discontinuation criterion, or up to 3 years of treatment.
The protocol and amendments were approved by the relevant ethics committees. The trial was conducted in accordance with the International Conference for Harmonisation Good Clinical Practice guidelines, applicable regulatory requirements, and the trial sponsor's policy on bioethics and human samples. All patients provided written informed consent.
End Points and Assessments
The primary end point was DFS by investigator assessment in patients with stage II-IIIA disease. Secondary end points included DFS in the overall population (stage IB-IIIA), overall survival, health-related quality of life, and safety. Assessment of site(s) of recurrence (including the CNS; Data Supplement) and time to CNS disease recurrence or death by any cause (CNS DFS) were prespecified exploratory end points. Brain scans were not mandated at regular follow-up assessments; these were performed on the basis of symptoms that may have occurred at follow-up visits, or between visits. However, at the time of recurrence, MRI or contrast CT brain scans were required, per the protocol, to fully capture all sites of recurrence, in line with clinical guidelines. Post hoc analyses of competing risk of CNS recurrence and DFS according to AJCC/UICC eighth edition staging were performed.
Statistical Analysis
Per the protocol, the planned primary DFS analysis was to take place after approximately 247 DFS events (50% maturity) in stage II-IIIA had occurred. As the primary DFS analysis was completed in 2020 ahead of plan,11 the statistical analysis plan was updated to account for a final exploratory DFS analysis at the original protocol-specified maturity of approximately 50% in stage II-IIIA. Given that the primary end point was tested and reached statistical significance at the time of the primary analysis, no further formal statistical testing of DFS was planned; consequently, P values are not reported. The data cutoff date for this analysis was April 11, 2022. DFS was analyzed using a log-rank test stratified by disease stage (AJCC/UICC seventh edition II/IIIA or IB/II/IIIA), EGFR mutational status (Ex19del/L858R), and race (Asian/non-Asian), the same analysis method applied to the primary analysis.
A competing risk analysis for CNS recurrence was performed and the probability of observing CNS recurrence, conditional on the patient not experiencing a competing risk of non-CNS recurrence or death by time t, was calculated using a Fine and Gray model. As no further scans were required once patients experienced DFS events, non-CNS disease recurrence events precluded the observation of the event of interest (CNS recurrence).
The final OS analysis is planned to take place at approximately 20% maturity in the stage II-IIIA population.
Study populations are defined in the Data Supplement.
RESULTS
Patients and Treatment
From November 2015 to February 2019, 682 eligible patients were randomly assigned: 339 to receive osimertinib and 343 to receive placebo (Data Supplement). At data cutoff, all patients had completed the planned treatment duration or prematurely discontinued treatment. Sixty-six percent (osimertinib) and 41% (placebo) of patients completed the planned treatment duration of 3 years. Cumulative total exposure is shown in the Data Supplement. Median total treatment exposure was 35.8 months (range, 0-38 months) in the osimertinib group and 25.1 months (range, 0-39 months) in the placebo group.
Baseline characteristics have been reported previously (Data Supplement) and were balanced between the two treatment groups.11 Use of brain CT and MRI scans at baseline before surgery or random assignment was balanced across groups and modalities, with 49% of patients receiving brain CT and 51% receiving brain MRI overall (Data Supplement).
Disease-Free Survival
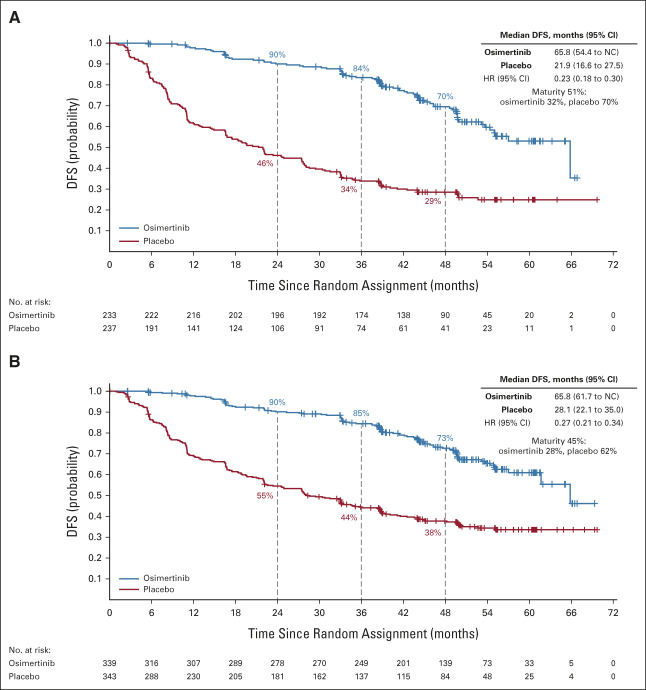
In patients with stage II-IIIA disease, the median duration of follow-up for DFS was 44.2 months (range, 0-67 months) among the 233 patients on osimertinib and 19.6 months (range, 0-70 months) among the 237 patients on placebo. In the osimertinib group, 75 DFS events occurred (32% maturity) and 167 events occurred in the placebo group (70% maturity); overall DFS maturity was 51%. The DFS HR was 0.23 (95% CI, 0.18 to 0.30; Fig 1A). Median DFS was longer for the osimertinib group at 65.8 months (95% CI, 54.4 to not calculable [NC]) than the placebo group at 21.9 months (95% CI, 16.6 to 27.5). The percentage of patients alive and disease-free at 48 months was 70% (95% CI, 62 to 76) for osimertinib and 29% (95% CI, 23 to 35) for placebo.
FIG 1.
DFS per investigator assessment. Kaplan-Meier estimates of duration of (A) DFS in patients with stage II-IIIA disease and (B) in the overall population (stage IB-IIIA) by seventh edition staging per the protocol (full analysis set). Tick marks indicate censored data. An HR < 1 favors osimertinib. DFS, disease-free survival; HR, hazard ratio; NC, not calculated.
In the overall population of 682 patients with stage IB-IIIA disease, 94 patients in the osimertinib group (28% maturity) and 211 in the placebo group had DFS events (62% maturity; overall maturity 45%). The DFS HR was 0.27 (95% CI, 0.21 to 0.34; Fig 1B). Median DFS was 65.8 months (95% CI, 61.7 to NC) for osimertinib compared with 28.1 months (95% CI, 22.1 to 35.0) for placebo. At 48 months, the percentage of patients alive and disease-free was 73% (95% CI, 67 to 78) for osimertinib and 38% (95% CI, 32 to 43) for placebo.
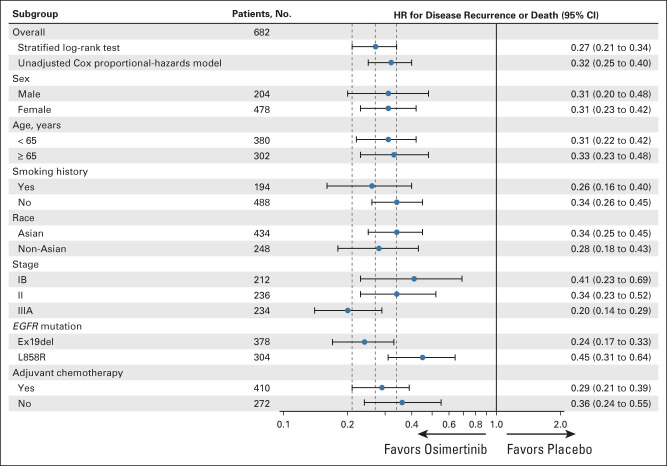
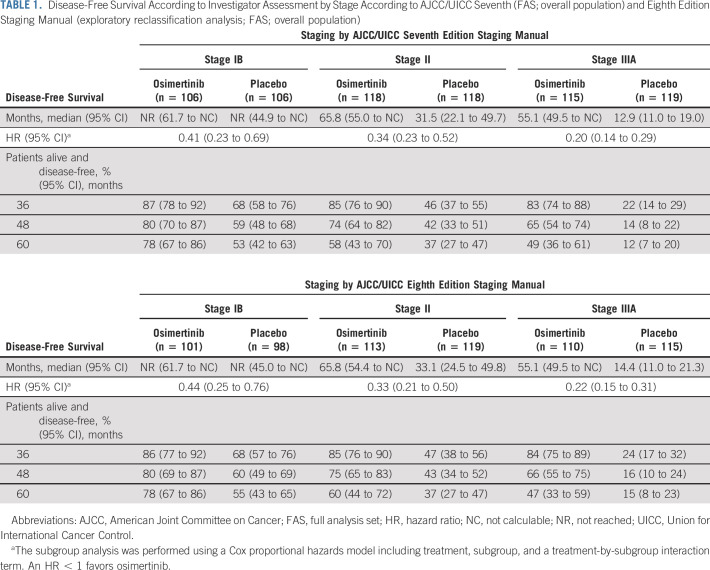
DFS benefit with osimertinib in the overall population was consistent across all predefined subgroups (Fig 2) including patients who did and did not receive prior adjuvant chemotherapy, by age (<65 years/≥65 years), race (Asian/non-Asian), smoking history (yes/no), and disease stage (IB/II/IIIA [AJCC/UICC, seventh edition]; see Data Supplement for Kaplan-Meier curves for stages IB, II, and IIIA). Among patients with stage IB disease, the percentages alive and disease-free at 48 months were 80% (95% CI, 70 to 87) for osimertinib and 59% (95% CI, 48 to 68) for placebo; the DFS HR was 0.41 (95% CI, 0.23 to 0.69; Table 1). Among those with stage II disease, these percentages were 74% (95% CI, 64 to 82) and 42% (95% CI, 33 to 51), respectively (DFS HR, 0.34; 95% CI, 0.23 to 0.52); among those with stage IIIA disease, these percentages were 65% (95% CI, 54 to 74) and 14% (95% CI, 8 to 22), respectively (DFS HR, 0.20; 95% CI, 0.14 to 0.29; Table 1). Treatment status at recurrence is shown in the Data Supplement.
FIG 2.
Disease-free survival subgroup analysis per investigator assessment (full analysis set; overall population). The subgroup analysis was performed using a Cox proportional hazards model including treatment, subgroup, and a treatment-by-subgroup interaction term. An HR < 1 favors osimertinib. EGFR, epidermal growth factor receptor; HR, hazard ratio.
TABLE 1.
Disease-Free Survival According to Investigator Assessment by Stage According to AJCC/UICC Seventh (FAS; overall population) and Eighth Edition Staging Manual (exploratory reclassification analysis; FAS; overall population)
DFS by Disease Stage According to AJCC/UICC Eighth Edition Staging Manual
Following restaging to AJCC/UICC, eighth edition staging manual on the basis of data captured during follow-up, per investigator assessment, 656/682 patients had stage IB-IIIA disease. Of the remaining 26 patients, three patients were classified as stage IA, 18 were stage IIIB, one was stage IV, and four were missing. The proportion in each stage group was 29% (IB), 34% (II), and 33% (IIIA; Data Supplement). There was a DFS benefit across stages IB-IIIA among patients after restaging (Table 1; see Data Supplement for Kaplan-Meier curves for stages IB, II, and IIIA). Landmark DFS rates were consistent with those for patients classified using the seventh edition manual (Table 1).
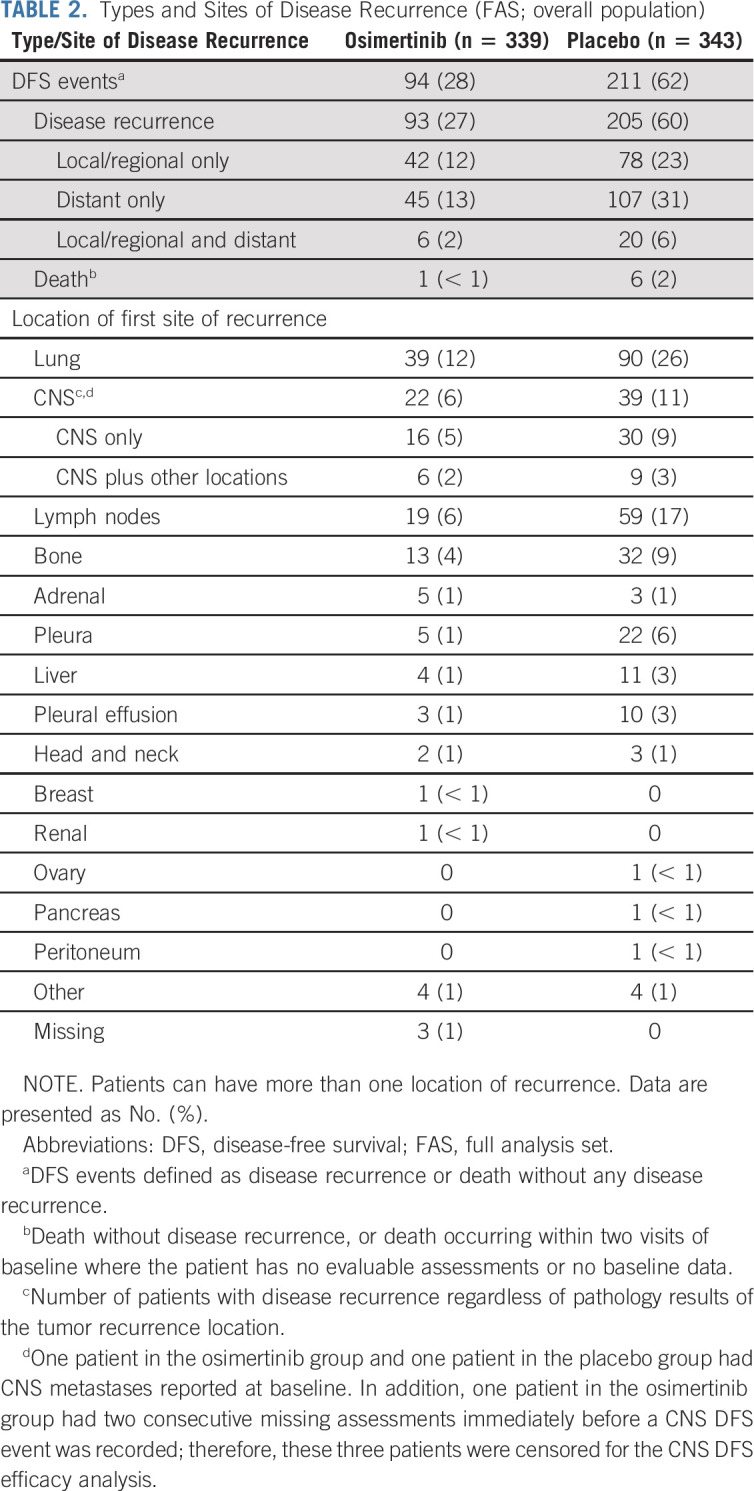
Patterns of Disease Recurrence
In the overall population, fewer patients had a disease recurrence with osimertinib versus placebo (93/339; 27% v 205/343; 60%). In the osimertinib group, 45/339 (13%) had distant metastases only, 42/339 (12%) had local/regional only, and 6/339 (2%) had local/regional and distant recurrences. In the placebo group, 107/343 (31%) had distant metastases only, 78/343 (23%) had local/regional only, and 20/343 (6%) had local/regional and distant recurrences (Table 2). Fewer patients had disease recurrence on osimertinib than placebo across the most common first sites of recurrence: lung, CNS, lymph nodes, and bone (Table 2).
TABLE 2.
Types and Sites of Disease Recurrence (FAS; overall population)
CNS Recurrence
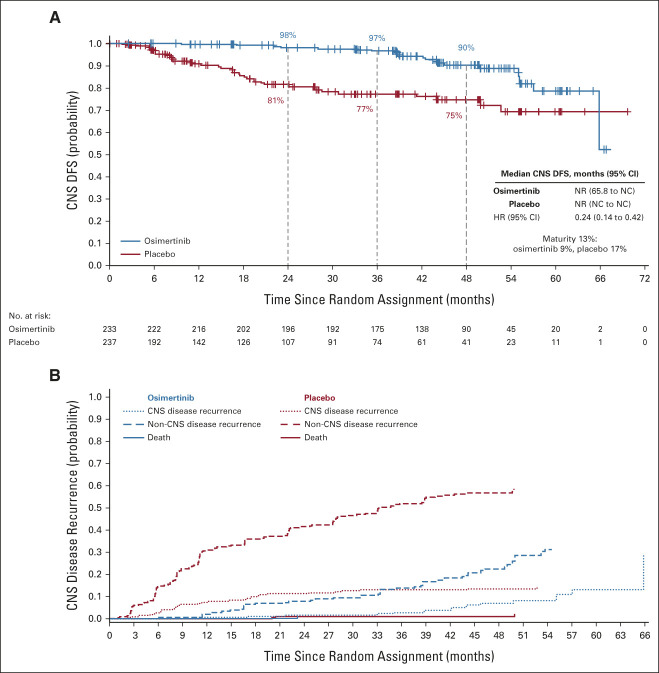
In patients with stage II-IIIA disease, CNS DFS events, defined as CNS disease recurrence or death by any cause, occurred in 22/233 (9%) and 41/237 (17%) patients in the osimertinib and placebo groups, respectively, of whom 18 (8%; osimertinib) and 32 (14%; placebo) patients had CNS recurrences. In the overall population, CNS DFS events occurred in 25/339 (7%) and 50/343 (15%) patients in the osimertinib and placebo groups; of these, 20 patients (6%; osimertinib) and 38 (11%; placebo) had CNS recurrences.
In patients with stage II-IIIA disease, CNS DFS HR was 0.24 (95% CI, 0.14 to 0.42; Fig 3A). In the overall population, CNS DFS HR was 0.36 (95% CI, 0.23 to 0.57; Data Supplement). For both the stage II-IIIA and overall populations, median CNS DFS was not reached in the osimertinib group (95% CI, 65.8 to NC) or the placebo group (95% CI, NC to NC). In stage II-IIIA disease, at 48 months, 90% (95% CI, 85 to 94) of patients with osimertinib and 75% (95% CI, 67 to 81) with placebo were alive and CNS disease-free. In the overall population, at 48 months, 92% (95% CI, 88 to 95) of patients with osimertinib and 81% (95% CI, 75 to 85) with placebo were alive and CNS disease-free.
FIG 3.
CNS analyses (full analysis set; stage II-IIIA). Kaplan-Meier estimates of duration of (A) CNS DFS per investigator assessment in patients with stage II-IIIA disease. Tick marks indicate censored data. An HR < 1 favors osimertinib. (B) Conditional probability of observing CNS and non-CNS recurrence. The graph shows the estimated probability of observing CNS recurrence event, conditional on the patient not experiencing a competing risk event (non-CNS recurrence and death by any cause) by time t. Cumulative incidence was calculated using a Fine and Gray model. CNS disease recurrence includes patients who have disease recurrence in the CNS alone or in the CNS in addition to other anatomies at the same overall visit. Non-CNS recurrence includes disease recurrence outside the CNS only. Death was defined as death occurring without confirmed CNS or non-CNS recurrence. DFS, disease-free survival; HR, hazard ratio; NC, not calculated; NR, not reached.
In the stage II-IIIA population, 15/18 CNS recurrences in the osimertinib group occurred following completion or discontinuation of treatment compared with 3/32 CNS recurrences in the placebo group. Treatment status at CNS recurrence and timing of CNS recurrence by baseline imaging modality are shown in the Data Supplement.
In stage II-IIIA, the estimated probability of observing CNS recurrence (in the absence of non-CNS recurrence or death) at 36 months was 2% (95% CI, 0.86 to 5.03) with osimertinib versus 13% (95% CI, 8.52 to 18.48) with placebo. The cumulative incidence of CNS recurrence was consistently lower in the osimertinib group than in the placebo group (Fig 3B). Estimated probability data for the overall population are shown in the Data Supplement.
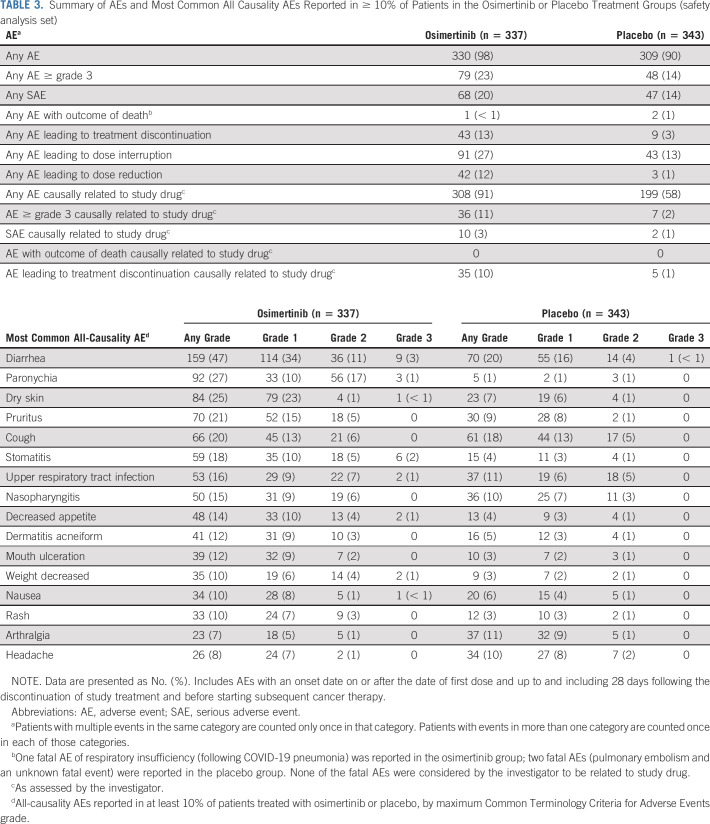
Safety
The safety analysis set included 680 patients (osimertinib: n = 337; placebo: n = 343). Adverse events (AEs) were reported in 330 (98%) and 309 (90%) patients in the osimertinib and placebo groups, respectively (Table 3). Incidence of Common Terminology Criteria for Adverse Events (version 4) grade ≥ 3 AEs were 23% in the osimertinib group and 14% in the placebo group; AEs considered by the investigator to be possibly causally related to study drug (all grades) were 91% (osimertinib) and 58% (placebo; Data Supplement). Incidence rates of serious AEs were 20% (osimertinib) and 14% (placebo; Data Supplement). One fatal AE was reported in the osimertinib group; two fatal AEs were reported in the placebo group; none were considered to be causally related to study drug. Dose interruptions, dose reductions, and discontinuations of study drug because of AEs were 27%, 12%, and 13% in the osimertinib group and 13%, 1%, and 3% in the placebo group. The most commonly reported AEs (of all causality) were diarrhea (osimertinib 47% v placebo 20%), paronychia (27% v 1%), and dry skin (25% v 7%; Table 3). Interstitial lung disease (grouped term) was reported in 11 (3%) and zero patients in the osimertinib and placebo groups, respectively; all AEs were grades 1 or 2 and none were fatal. Cardiac effects (grouped term) were reported in 19 (6%) and nine (3%) patients in the osimertinib and placebo groups, respectively. Electrocardiogram QT prolonged was reported in 30 (9%) patients in the osimertinib group and eight (2%) patients in the placebo group (Data Supplement).
TABLE 3.
Summary of AEs and Most Common All Causality AEs Reported in ≥ 10% of Patients in the Osimertinib or Placebo Treatment Groups (safety analysis set)
DISCUSSION
In this updated analysis of the final, mature DFS data, all 682 patients had the opportunity of 3 years of treatment; 66% and 41% patients completed 3 years of planned adjuvant osimertinib and placebo treatment, respectively. Adjuvant osimertinib demonstrated a sustained clinically meaningful DFS benefit, consistent with primary reporting11 (DFS HR, 0.23; 95% CI, 0.18 to 0.30 in stage II-IIIA; DFS HR, 0.27; 95% CI, 0.21 to 0.34 in the overall population). Consistent with the primary analysis,11,18,24 DFS benefit was observed across all subgroups and stages defined, including by the AJCC/UICC eighth edition manual.
The early separation in the Kaplan-Meier curves reported in the primary analysis was sustained to the last observed date in this updated analysis. There was an observed trend toward an increased DFS event rate beyond 36 months compared with the previous 36 months in the osimertinib group; however, the benefit of osimertinib treatment is clearly maintained as the curves remain separated beyond the 3-year treatment period. This observation suggests that some patients may benefit from adjuvant osimertinib beyond 3 years; molecular profiling and monitoring of minimal residual disease may help inform optimal treatment duration.
Overall recurrences, including locoregional only and distant recurrence, were lower with osimertinib compared with placebo. In both treatment groups, the majority of recurrences involved a distant recurrence, albeit with a smaller proportion of patients with distant recurrence in the osimertinib group. The increased maturity of these data provides a robust assessment of recurrence patterns.
Importantly, CNS DFS data from ADAURA demonstrate clear CNS efficacy with osimertinib. CNS DFS was improved with osimertinib in the stage II-IIIA (HR, 0.24; 95% CI, 0.14 to 0.42) and overall population (HR, 0.36; 95% CI, 0.23 to 0.57). The majority of CNS recurrences in the osimertinib group occurred after treatment was completed. In the CNS DFS analysis (stage II-IIIA), only three of 18 patients in the osimertinib group had CNS as their first site of recurrence while on treatment, compared with 29 of 32 patients in the placebo group. The conditional probability of CNS recurrence was consistently lower with osimertinib compared with placebo. The use of CT or MRI as baseline brain imaging in ADAURA was similar between groups and unlikely to have affected CNS efficacy assessment (Data Supplement).
Previous trials of adjuvant first-generation EGFR-TKIs have reported CNS efficacy results. The ADJUVANT/CTONG1104 trial reported a reduced risk of extracranial metastases with gefitinib, although a high incidence of CNS metastases was observed among all treated patients (adjuvant gefitinib 29/106 [27%]; adjuvant chemotherapy, 21/87 [24%]).9,25 Higher rates of CNS metastases over comparator were also seen among patients who experienced recurrence in the IMPACT26 and RADIANT trials.27 Although these trials differ with respect to patient population and follow-up time, these results in the adjuvant setting are consistent with findings that first- and second-generation EGFR-TKIs have less CNS efficacy compared with osimertinib in advanced NSCLC.13,14 These updated ADAURA data demonstrate osimertinib to be a compelling therapeutic option to reduce the risk of developing CNS metastases in resected EGFR-mutated NSCLC.
The safety profile observed for this extended treatment duration was consistent with the primary analysis. No new safety concerns were reported.
These updated data highlight the importance of routine EGFR testing at diagnosis to ensure that patients have the opportunity for optimal treatment. Future data of interest from ADAURA include long-term safety, subsequent treatment patterns, and overall survival. Tumor and circulating tumor DNA molecular profiling for analyses of minimal residual disease and acquired resistance may provide important information on persistence and resistance mechanisms to optimize treatment strategies in this setting.
Other ongoing studies will inform the efficacy and safety of osimertinib as adjuvant treatment for resected EGFR-mutated stage IA2-IA3 NSCLC (ADAURA2; ClinicalTrials.gov identifier: NCT05120349), as 5-year adjuvant treatment for EGFR-mutated stage II-IIIB (TARGET; ClinicalTrials.gov identifier: NCT05526755), as neoadjuvant treatment for EGFR-mutated stage II-IIIB N2 NSCLC (NeoADAURA; ClinicalTrials.gov identifier: NCT04351555), and as maintenance therapy in unresectable EGFR-mutated stage III NSCLC (LAURA; ClinicalTrials.gov identifier: NCT03521154).
Other EGFR-TKIs are being investigated in the adjuvant setting.7,28-34 The adjuvant treatment setting is also expanding beyond EGFR-TKIs to include immunotherapies.35,36 However, the benefit of immunotherapy has not been established for patients with EGFR-mutated disease, and patient numbers in prospective immunotherapy trials to date are limited.
In conclusion, the prolonged DFS benefit over placebo, magnitude of DFS benefit, reduced risk of local and distant recurrence, improved CNS DFS, and consistent safety profile observed in this updated analysis support adjuvant osimertinib as a highly effective treatment in patients with resected EGFR-mutated stage IB-IIIA NSCLC.
ACKNOWLEDGMENT
The authors thank all the patients and their families, as well as the staff and investigators at all study sites. The authors would like to acknowledge Ajlan Atasoy for her valuable contributions to the study. The authors would like to acknowledge Sally Cotterill, PhD, CMPP, of Ashfield MedComms, Macclesfield, United Kingdom, an Inizio Company, for medical writing support that was funded by AstraZeneca in accordance with Good Publications Practice (GPP) guidelines (https://www.ismpp.org/gpp-2022).
Roy S. Herbst
Leadership: Junshi Pharmaceuticals, Immunocore
Consulting or Advisory Role: AstraZeneca, Genentech/Roche, Merck, Pfizer, AbbVie, Biodesix, Bristol-Myers Squibb, Lilly, EMD Serono, Heat Biologics, Junshi Pharmaceuticals, Loxo, Nektar, NextCure, Novartis, Sanofi, Seattle Genetics, Shire, Spectrum Pharmaceuticals, Symphogen, Tesaro, Neon Therapeutics, Infinity Pharmaceuticals, ARMO Biosciences, Genmab, Halozyme, Tocagen, Bolt Biotherapeutics, I-Mab, Mirati Therapeutics, Takeda, Cybrexa Therapeutics, eFFECTOR Therapeutics, Candel Therapeutics, Oncternal Therapeutics, STCube Pharmaceuticals Inc, WindMIL, Xencor, Bayer, Checkpoint Therapeutics, DynamiCure Biotechnology, Foundation Medicine, Gilead/Forty Seven, HiberCell, Immune-Onc Therapeutics, Johnson & Johnson, Ocean Biomedical, OncoCyte, Refactor Health, Ribon Therapeutics, Ventana Medical Systems
Research Funding: AstraZeneca, Merck, Lilly, Genentech/Roche
Yi-Long Wu
Honoraria: AstraZeneca, Lilly, Roche, Pfizer, Boehringer Ingelheim, MSD Oncology, Bristol Myers Squibb/China, Hengrui Pharmaceutical, BeiGene Beijing
Consulting or Advisory Role: AstraZeneca, Roche, Boehringer Ingelheim, Takeda
Research Funding: Boehringer Ingelheim (Inst), Roche (Inst), Pfizer (Inst), Bristol‐Myers Squibb (Inst)
Thomas John
Honoraria: AstraZeneca/MedImmune, Roche/Genentech, Bristol Myers Squibb, MSD Oncology
Consulting or Advisory Role: AstraZeneca, Pfizer, AstraZeneca/MedImmune, Roche/Genentech, Ignyta, Boehringer Ingelheim, Novartis, MSD Oncology, Merck KGaA, Bristol Myers Squibb, Amgen (Inst), PharmaMar (Inst), Specialised Therapeutics, Gilead Sciences
Travel, Accommodations, Expenses: Boehringer Ingelheim, Roche, AstraZeneca, Bristol Myers Squibb, Roche, Merck Sharp & Dohme
Christian Grohe
Honoraria: Boehringer Ingelheim, AstraZeneca, Lilly, Roche, Novartis, MSD Oncology, Takeda
Consulting or Advisory Role: MSD Oncology, AstraZeneca, Boehringer Ingelheim
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: Roche, Boehringer Ingelheim, Bristol Myers Squibb
Margarita Majem
Consulting or Advisory Role: AstraZeneca, Roche, Bristol Myers Squibb, Merck Sharp & Dohme, Pfizer, Boehringer Ingelheim, Novartis, Tesaro, Helsinn Therapeutics, Takeda, Sanofi, Janssen Oncology, Pierre Fabre
Research Funding: Bristol‐Myers Squibb (Inst), AstraZeneca (Inst), Roche (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Roche, Lilly
Terufumi Kato
Employment: Lilly (I)
Honoraria: Chugai Pharma, Ono Pharmaceutical, Lilly, AstraZeneca, Taiho Pharmaceutical, Pfizer, Merck Sharp & Dohme, Novartis, Takeda, Daiichi Sankyo, GlaxoSmithKline, Amgen, Merck KGaA
Consulting or Advisory Role: AstraZeneca, MSD, Lilly, Pfizer, Merck Serono
Research Funding: Chugai Pharma (Inst), Merck Sharp & Dohme (Inst), Pfizer (Inst), AstraZeneca (Inst), Lilly (Inst), AbbVie (Inst), Regeneron (Inst), Novartis (Inst), Amgen (Inst), Merck KGaA (Inst), Takeda (Inst), Haihe Biopharma (Inst), Blueprint Medicines (Inst), Turning Point Therapeutics (Inst)
Jonathan W. Goldman
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Lilly, Pfizer, AbbVie, Genentech
Research Funding: Lilly (Inst), Genentech/Roche (Inst), Bristol Myers Squibb (Inst), AstraZeneca/MedImmune (Inst), AbbVie (Inst), Corvus Pharmaceuticals (Inst), Spectrum Pharmaceuticals (Inst), Advaxis (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Open Payments Link: https://openpaymentsdata.cms.gov/physician/124819
Shun Lu
Consulting or Advisory Role: AstraZeneca, Pfizer, Boehringer Ingelheim, Hutchison MediPharma, Simcere Diagnostics, Zai Lab, GenomiCare, Yuhan, Prime Oncology, Roche
Speakers' Bureau: AstraZeneca, Roche, Hansoh Pharma, Hengrui Therapeutics
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), Bristol‐Myers Squibb (Inst), Hengrui Therapeutics (Inst), BeiGene (Inst), Roche (Inst), Hansoh (Inst), Lilly Suzhou Pharmaceutical Co Ltd (Inst)
Kye Young Lee
Employment: Exosignal Inc
Stock and Other Ownership Interests: Exosignal Inc
Research Funding: MSD Oncology
Charuwan Akewanlop
Speakers' Bureau: Merck Sharp & Dohme, Novartis, Amgen, AstraZeneca/MedImmune, Roche
Filippo de Marinis
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Bristol Myers Squibb, Roche/Genentech, Pfizer, Novartis, Takeda
Laura Bonanno
Consulting or Advisory Role: AstraZeneca, Roche/Genentech, Novartis, Bristol Myers Squibb/Medarex, MSD Oncology
Speakers' Bureau: Novartis/Ipsen, Bristol Myers Squibb/Medarex, AstraZeneca/MedImmune
Manuel Domine
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Pfizer, Roche, Takeda
Frances A. Shepherd
Stock and Other Ownership Interests: Lilly, AstraZeneca
Honoraria: AstraZeneca, Merck Serono, Takeda, Daiichi Sankyo
Consulting or Advisory Role: AstraZeneca, Merck Serono
Research Funding: Lilly (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), AstraZeneca/MedImmune (Inst), Roche Canada (Inst)
Damien Urban
Honoraria: Merck Sharp & Dohme, Roche, Bristol Myers Squibb, Takeda, AstraZeneca, Merck Serono
Consulting or Advisory Role: Merck Sharp & Dohme, Takeda, Roche Israel, Nucleai, Rhenium/Oncotest
Xiangning Huang
Employment: AstraZeneca/MedImmune
Stock and Other Ownership Interests: AstraZeneca/MedImmune
Ana Bolanos
Employment: AstraZeneca Canada, Bayer (I)
Stock and Other Ownership Interests: Bayer (I)
Marta Stachowiak
Employment: AstraZeneca/MedImmune
Masahiro Tsuboi
Honoraria: AstraZeneca Japan, Chugai Pharma, Taiho Pharmaceutical, Johnson & Johnson, Novartis, MSD K.K, Ono Pharmaceutical, Bristol Myers Squibb Japan, Medtronic, Lilly Japan, Daiichi-Sankyo
Consulting or Advisory Role: AstraZeneca Japan, Chugai Pharma, MSD, Novartis, AstraZeneca
Research Funding: Boehringer Ingelheim (Inst), Merck (Inst), AstraZeneca Japan (Inst), Ono Pharmaceutical (Inst), Bristol Myers Squibb KK (Inst), Novartis (Inst), MiRXES Japan (Inst), BMG KK (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the European Society for Medical Oncology (ESMO) annual meeting, Paris, France, September 9-13, 2022.
SUPPORT
The study (ClinicalTrials.gov identifier: NCT02511106) was supported by AstraZeneca, the manufacturer of osimertinib.
CLINICAL TRIAL INFORMATION
R.S.H. and Y.-L.W. contributed equally to this work.
DATA SHARING STATEMENT
Provision of standard data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
AUTHOR CONTRIBUTIONS
Conception and design: Roy S. Herbst, Yi-Long Wu, Thomas John, Christian Grohe, Sang-We Kim, Manuel Domine, Frances A. Shepherd, Masahiro Tsuboi
Provision of study materials or patients: Roy S. Herbst, Yi-Long Wu, Thomas John, Margarita Majem, Jie Wang, Jonathan W. Goldman, Sang-We Kim, Chong-Jen Yu, Shun Lu, Guzel Mukhametshina, Charuwan Akewanlop, Laura Bonanno, Manuel Domine, Frances A. Shepherd, Damien Urban, Masahiro Tsuboi
Collection and assembly of data: Roy S. Herbst, Yi-Long Wu, Thomas John, Christian Grohe, Margarita Majem, Jie Wang, Terufumi Kato, Jonathan W. Goldman, Konstantin Laktionov, Sang-We Kim, Chong-Jen Yu, Huu Vinh Vu, Shun Lu, Kye Young Lee, Guzel Mukhametshina, Charuwan Akewanlop, Filippo de Marinis, Laura Bonanno, Frances A. Shepherd, Damien Urban, Masahiro Tsuboi
Data analysis and interpretation: Roy S. Herbst, Yi-Long Wu, Thomas John, Christian Grohe, Margarita Majem, Terufumi Kato, Jonathan W. Goldman, Konstantin Laktionov, Sang-We Kim, Chong-Jen Yu, Filippo de Marinis, Damien Urban, Xiangning Huang, Ana Bolanos, Marta Stachowiak, Masahiro Tsuboi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Adjuvant Osimertinib for Resected EGFR-Mutated Stage IB-IIIA Non–Small-Cell Lung Cancer: Updated Results From the Phase III Randomized ADAURA Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Roy S. Herbst
Leadership: Junshi Pharmaceuticals, Immunocore
Consulting or Advisory Role: AstraZeneca, Genentech/Roche, Merck, Pfizer, AbbVie, Biodesix, Bristol-Myers Squibb, Lilly, EMD Serono, Heat Biologics, Junshi Pharmaceuticals, Loxo, Nektar, NextCure, Novartis, Sanofi, Seattle Genetics, Shire, Spectrum Pharmaceuticals, Symphogen, Tesaro, Neon Therapeutics, Infinity Pharmaceuticals, ARMO Biosciences, Genmab, Halozyme, Tocagen, Bolt Biotherapeutics, I-Mab, Mirati Therapeutics, Takeda, Cybrexa Therapeutics, eFFECTOR Therapeutics, Candel Therapeutics, Oncternal Therapeutics, STCube Pharmaceuticals Inc, WindMIL, Xencor, Bayer, Checkpoint Therapeutics, DynamiCure Biotechnology, Foundation Medicine, Gilead/Forty Seven, HiberCell, Immune-Onc Therapeutics, Johnson & Johnson, Ocean Biomedical, OncoCyte, Refactor Health, Ribon Therapeutics, Ventana Medical Systems
Research Funding: AstraZeneca, Merck, Lilly, Genentech/Roche
Yi-Long Wu
Honoraria: AstraZeneca, Lilly, Roche, Pfizer, Boehringer Ingelheim, MSD Oncology, Bristol Myers Squibb/China, Hengrui Pharmaceutical, BeiGene Beijing
Consulting or Advisory Role: AstraZeneca, Roche, Boehringer Ingelheim, Takeda
Research Funding: Boehringer Ingelheim (Inst), Roche (Inst), Pfizer (Inst), Bristol‐Myers Squibb (Inst)
Thomas John
Honoraria: AstraZeneca/MedImmune, Roche/Genentech, Bristol Myers Squibb, MSD Oncology
Consulting or Advisory Role: AstraZeneca, Pfizer, AstraZeneca/MedImmune, Roche/Genentech, Ignyta, Boehringer Ingelheim, Novartis, MSD Oncology, Merck KGaA, Bristol Myers Squibb, Amgen (Inst), PharmaMar (Inst), Specialised Therapeutics, Gilead Sciences
Travel, Accommodations, Expenses: Boehringer Ingelheim, Roche, AstraZeneca, Bristol Myers Squibb, Roche, Merck Sharp & Dohme
Christian Grohe
Honoraria: Boehringer Ingelheim, AstraZeneca, Lilly, Roche, Novartis, MSD Oncology, Takeda
Consulting or Advisory Role: MSD Oncology, AstraZeneca, Boehringer Ingelheim
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: Roche, Boehringer Ingelheim, Bristol Myers Squibb
Margarita Majem
Consulting or Advisory Role: AstraZeneca, Roche, Bristol Myers Squibb, Merck Sharp & Dohme, Pfizer, Boehringer Ingelheim, Novartis, Tesaro, Helsinn Therapeutics, Takeda, Sanofi, Janssen Oncology, Pierre Fabre
Research Funding: Bristol‐Myers Squibb (Inst), AstraZeneca (Inst), Roche (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Roche, Lilly
Terufumi Kato
Employment: Lilly (I)
Honoraria: Chugai Pharma, Ono Pharmaceutical, Lilly, AstraZeneca, Taiho Pharmaceutical, Pfizer, Merck Sharp & Dohme, Novartis, Takeda, Daiichi Sankyo, GlaxoSmithKline, Amgen, Merck KGaA
Consulting or Advisory Role: AstraZeneca, MSD, Lilly, Pfizer, Merck Serono
Research Funding: Chugai Pharma (Inst), Merck Sharp & Dohme (Inst), Pfizer (Inst), AstraZeneca (Inst), Lilly (Inst), AbbVie (Inst), Regeneron (Inst), Novartis (Inst), Amgen (Inst), Merck KGaA (Inst), Takeda (Inst), Haihe Biopharma (Inst), Blueprint Medicines (Inst), Turning Point Therapeutics (Inst)
Jonathan W. Goldman
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Lilly, Pfizer, AbbVie, Genentech
Research Funding: Lilly (Inst), Genentech/Roche (Inst), Bristol Myers Squibb (Inst), AstraZeneca/MedImmune (Inst), AbbVie (Inst), Corvus Pharmaceuticals (Inst), Spectrum Pharmaceuticals (Inst), Advaxis (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Open Payments Link: https://openpaymentsdata.cms.gov/physician/124819
Shun Lu
Consulting or Advisory Role: AstraZeneca, Pfizer, Boehringer Ingelheim, Hutchison MediPharma, Simcere Diagnostics, Zai Lab, GenomiCare, Yuhan, Prime Oncology, Roche
Speakers' Bureau: AstraZeneca, Roche, Hansoh Pharma, Hengrui Therapeutics
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), Bristol‐Myers Squibb (Inst), Hengrui Therapeutics (Inst), BeiGene (Inst), Roche (Inst), Hansoh (Inst), Lilly Suzhou Pharmaceutical Co Ltd (Inst)
Kye Young Lee
Employment: Exosignal Inc
Stock and Other Ownership Interests: Exosignal Inc
Research Funding: MSD Oncology
Charuwan Akewanlop
Speakers' Bureau: Merck Sharp & Dohme, Novartis, Amgen, AstraZeneca/MedImmune, Roche
Filippo de Marinis
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Bristol Myers Squibb, Roche/Genentech, Pfizer, Novartis, Takeda
Laura Bonanno
Consulting or Advisory Role: AstraZeneca, Roche/Genentech, Novartis, Bristol Myers Squibb/Medarex, MSD Oncology
Speakers' Bureau: Novartis/Ipsen, Bristol Myers Squibb/Medarex, AstraZeneca/MedImmune
Manuel Domine
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Pfizer, Roche, Takeda
Frances A. Shepherd
Stock and Other Ownership Interests: Lilly, AstraZeneca
Honoraria: AstraZeneca, Merck Serono, Takeda, Daiichi Sankyo
Consulting or Advisory Role: AstraZeneca, Merck Serono
Research Funding: Lilly (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), AstraZeneca/MedImmune (Inst), Roche Canada (Inst)
Damien Urban
Honoraria: Merck Sharp & Dohme, Roche, Bristol Myers Squibb, Takeda, AstraZeneca, Merck Serono
Consulting or Advisory Role: Merck Sharp & Dohme, Takeda, Roche Israel, Nucleai, Rhenium/Oncotest
Xiangning Huang
Employment: AstraZeneca/MedImmune
Stock and Other Ownership Interests: AstraZeneca/MedImmune
Ana Bolanos
Employment: AstraZeneca Canada, Bayer (I)
Stock and Other Ownership Interests: Bayer (I)
Marta Stachowiak
Employment: AstraZeneca/MedImmune
Masahiro Tsuboi
Honoraria: AstraZeneca Japan, Chugai Pharma, Taiho Pharmaceutical, Johnson & Johnson, Novartis, MSD K.K, Ono Pharmaceutical, Bristol Myers Squibb Japan, Medtronic, Lilly Japan, Daiichi-Sankyo
Consulting or Advisory Role: AstraZeneca Japan, Chugai Pharma, MSD, Novartis, AstraZeneca
Research Funding: Boehringer Ingelheim (Inst), Merck (Inst), AstraZeneca Japan (Inst), Ono Pharmaceutical (Inst), Bristol Myers Squibb KK (Inst), Novartis (Inst), MiRXES Japan (Inst), BMG KK (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kris MG, Gaspar LE, Chaft JE, et al. : Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario clinical practice guideline update. J Clin Oncol 35:2960-2974, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Pisters K, Kris MG, Gaspar LE, et al. : Adjuvant systemic therapy and adjuvant radiation therapy for stage I-IIIA completely resected non-small-cell lung cancer: ASCO guideline rapid recommendation update. J Clin Oncol 40:1127-1129, 2022 [DOI] [PubMed] [Google Scholar]
- 3.Postmus PE, Kerr KM, Oudkerk M, et al. : Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv1-iv21, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Remon J, Soria JC, Peters S, et al. : Early and locally advanced non-small-cell lung cancer: An update of the ESMO clinical practice guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol 32:1637-1642, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Pignon JP, Tribodet H, Scagliotti GV, et al. : Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol 26:3552-3559, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Peters S, Weder W, Dafni U, et al. : Lungscape: Resected non-small-cell lung cancer outcome by clinical and pathological parameters. J Thorac Oncol 9:1675-1684, 2014 [DOI] [PubMed] [Google Scholar]
- 7.He J, Su C, Liang W, et al. : Icotinib versus chemotherapy as adjuvant treatment for stage II-IIIA EGFR-mutant non-small-cell lung cancer (EVIDENCE): A randomised, open-label, phase 3 trial. Lancet Respir Med 9:1021-1029, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Felip E, Altorki N, Zhou C, et al. : Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 398:1344-1357, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Zhong WZ, Wang Q, Mao WM, et al. : Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): A randomised, open-label, phase 3 study. Lancet Oncol 19:139-148, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Cross DA, Ashton SE, Ghiorghiu S, et al. : AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 4:1046-1061, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y-L, Tsuboi M, He J, et al. : Osimertinib in resected EGFR-mutated non–small-cell lung cancer. N Engl J Med 383:1711-1723, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Mok TS, Wu YL, Ahn MJ, et al. : Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 376:629-640, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reungwetwattana T, Nakagawa K, Cho BC, et al. : CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non–small-cell lung cancer. J Clin Oncol 36:3290-3297, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Soria JC, Ohe Y, Vansteenkiste J, et al. : Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113-125, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Wu YL, Ahn MJ, Garassino MC, et al. : CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: Data from a randomized phase III trial (AURA3). J Clin Oncol 36:2702-2709, 2018 [DOI] [PubMed] [Google Scholar]
- 16.European Medicines Agency : TAGRISSO (osimertinib) Summary of Product Characteristics, 2021. https://www.ema.europa.eu/en/documents/product-information/tagrisso-epar-product-information_en.pdf [Google Scholar]
- 17.US Food and Drug Administration : TAGRISSO (osimertinib) Highlights of Prescribing Information. Silver Spring, MD, Food and Drug Administration (FDA), 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf [Google Scholar]
- 18.Koch AL, Vellanki PJ, Drezner N, et al. : FDA approval summary: Osimertinib for adjuvant treatment of surgically resected non-small cell lung cancer, a collaborative project orbis review. Clin Cancer Res 27:6638-6643, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Sekihara K, Hishida T, Yoshida J, et al. : Long-term survival outcome after postoperative recurrence of non-small-cell lung cancer: Who is “cured” from postoperative recurrence? Eur J Cardiothorac Surg 52:522-528, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Peters S, Bexelius C, Munk V, et al. : The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev 45:139-162, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Preusser M, Winkler F, Valiente M, et al. : Recent advances in the biology and treatment of brain metastases of non-small cell lung cancer: Summary of a multidisciplinary roundtable discussion. ESMO Open 3:e000262, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rami-Porta R: Staging Manual in Thoracic Oncology. North Fort Myers, FL, Editorial Rx Press, 2016 [Google Scholar]
- 23.Wu YL, Herbst RS, Mann H, et al. : ADAURA: Phase III, double-blind, randomized study of osimertinib versus placebo in EGFR mutation-positive early-stage NSCLC after complete surgical resection. Clin Lung Cancer 19:e533-e536, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Wu YL, John T, Grohe C, et al. : Postoperative chemotherapy use and outcomes from ADAURA: Osimertinib as adjuvant therapy for resected EGFR-mutated NSCLC. J Thorac Oncol 17:423-433, 2022 [DOI] [PubMed] [Google Scholar]
- 25.Xu ST, Xi JJ, Zhong WZ, et al. : The unique spatial-temporal treatment failure patterns of adjuvant gefitinib therapy: A post hoc analysis of the ADJUVANT trial (CTONG 1104). J Thorac Oncol 14:503-512, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Tada H, Mitsudomi T, Misumi T, et al. : Randomized phase III study of gefitinib versus cisplatin plus vinorelbine for patients with resected stage II-IIIA non-small-cell lung cancer with EGFR mutation (IMPACT). J Clin Oncol 40:231-241, 2022 [DOI] [PubMed] [Google Scholar]
- 27.Kelly K, Altorki NK, Eberhardt WE, et al. : Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): A randomized, double-blind, phase III trial. J Clin Oncol 33:4007-4014, 2015 [DOI] [PubMed] [Google Scholar]
- 28.ClinicalTrials.gov : NCT02193282: Erlotinib hydrochloride in treating patients with stage IB-IIIA non-small cell lung cancer that has been completely removed by surgery (an ALCHEMIST treatment trial), 2022 [Google Scholar]
- 29.ClinicalTrials.gov : NCT03381066: A phase III, randomized, multi-center study to determine the efficacy of the intercalating combination treatment of chemotherapy and gefitinib or chemotherapy as adjuvant treatment in NSCLC with common EGFR mutations, 2019 [Google Scholar]
- 30.ClinicalTrials.gov : NCT01996098: Icotinib following chemotherapy versus chemotherapy as adjuvant therapy in stage IIA-IIIA NSCLC with EGFR mutation (ICTAN), 2018 [Google Scholar]
- 31.ClinicalTrials.gov : NCT02125240: Icotinib versus placebo as adjuvant therapy in EGFR-mutant lung adenocarcinoma (ICWIP), 2018 [Google Scholar]
- 32.ClinicalTrials.gov : NCT04853342: To assess the efficacy and safety of furmonertinib versus placebo, in patients with epidermal growth factor receptor mutation positive stage II-IIIA non-small cell lung carcinoma, following complete tumour resection with or without adjuvant chemotherapy (FORWARD), 2021 [Google Scholar]
- 33.ClinicalTrials.gov : NCT04687241: Almonertinib versus placebo as adjuvant therapy in resected stage II-IIIB non-small cell lung cancer with EGFR-sensitive mutations, 2020 [Google Scholar]
- 34.ClinicalTrials.gov : NCT04762459: Efficacy and safety of almonertinib combined with or without chemotherapy as an adjuvant treatment for stage II-IIIA non-small cell lung carcinoma following complete tumour resection (APEX), 2022 [Google Scholar]
- 35.US Food and Drug Administration : TECENTRIQ (atezolizumab) Prescribing Information. Silver Spring, MD, Food and Drug Administration (FDA), 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761034s047lbl.pdf [Google Scholar]
- 36.Paz-Ares L, O'Brien MER, Mauer M, et al. : VP3-2022: Pembrolizumab (pembro) versus placebo for early-stage non-small cell lung cancer (NSCLC) following complete resection and adjuvant chemotherapy (chemo) when indicated: Randomized, triple-blind, phase III EORTC-1416-LCG/ETOP 8-15—PEARLS/KEYNOTE-091 study. Ann Oncol 33:451-453, 2022 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Provision of standard data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.