PURPOSE
To present primary and final analyses from the randomized, double-blind, placebo-controlled, phase III iNTEGRATE study, which evaluated the safety and efficacy of ibrutinib with prednisone in previously untreated patients with chronic graft-versus-host disease (cGVHD).
METHODS
Patients (age ≥ 12 years) with newly diagnosed moderate or severe cGVHD, requiring systemic corticosteroid therapy, and with no prior systemic treatment for cGVHD were randomly assigned 1:1 to receive ibrutinib 420 mg once daily plus prednisone, starting at 1 mg/kg once daily or placebo plus prednisone. The primary end point was response rate at 48 weeks according to 2014 National Institutes of Health Consensus Development Project Criteria. Other end points included event-free survival, duration of response, time to withdrawal of immunosuppressants, improvement in Lee cGVHD Symptom Scale score, overall survival (OS), and safety.
RESULTS
Ninety-five and 98 patients enrolled in the ibrutinib-prednisone and placebo-prednisone arms, respectively. At 48 weeks, response rates were 41% (ibrutinib-prednisone) and 37% (placebo-prednisone; P = .54). At 33 months of follow-up, median duration of response was 19 months (ibrutinib-prednisone) and 10 months (placebo-prednisone; P = .10). Median event-free survival was 15 months (ibrutinib-prednisone) and 8 months (placebo-prednisone; hazard ratio, 0.76; 95% CI, 0.54 to 1.1; P = .11). Improvement in overall Lee cGVHD Symptom Scale was 43% (ibrutinib-prednisone) and 31% (placebo-ibrutinib; P = .07). Median OS was not reached in either arm. The 24-month Kaplan-Meier OS estimates were 80% for both arms (hazard ratio, 1.06; 95% CI, 0.59 to 1.90). Grade ≥ 3 serious adverse events occurred in 49% (ibrutinib-prednisone) and 47% (placebo-prednisone) of patients.
CONCLUSION
There was no statistical difference observed in the primary and secondary end points with ibrutinib-prednisone treatment. No new safety signals were observed with ibrutinib treatment in previously untreated patients with cGVHD. The primary end point of iNTEGRATE was not met.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (allo-HCT) is potentially curative for various malignant and nonmalignant hematologic conditions. However, chronic graft-versus-host disease (cGVHD) can limit allo-HCT effectiveness.1 cGVHD, a leading cause of late nonrelapse mortality in patients receiving allo-HCT, contributes to patient morbidity and reduced quality of life.2-4 Up to 70% of patients who undergo allo-HCT develop cGVHD, and approximately 30%-40% require systemic treatment for cGVHD.5 Corticosteroids have been the standard first-line treatment for cGVHD but their long-term use contributes to morbidity,5,6 and there are no approved alternative therapies for previously untreated cGVHD. A need remains for non-steroid or steroid-sparing treatment options to improve cGVHD outcomes.
CONTEXT
Key Objective
Chronic graft-versus-host disease (cGVHD) is a potentially life-threatening complication of allogeneic hematopoietic cell transplantation. Corticosteroids have been the standard first-line treatment for cGVHD, but their long-term use contributes to morbidity. An unmet need for non–steroid- or steroid-sparing treatment options to improve cGVHD patient outcomes remains since there are no approved alternative therapies for previously untreated cGVHD.
Knowledge Generated
To our knowledge, the iNTEGRATE study represents the first prospective, randomized, double-blind, placebo-controlled phase III therapeutics trial in previously untreated cGVHD using objective response criteria but demonstrated no benefit of ibrutinib-prednisone therapy in this clinical setting.
Relevance (C.F. Craddock)
-
This study confirms the feasibility of delivering a randomized trial in untreated cGVHD using objective response criteria but failed to identify any improvement in outcomes using ibrutinib-prednisone therapy. Novel agents with potential to improve outcomes in this area of unmet medical need are required.*
*Relevance section written by JCO Associate Editor Charles F. Craddock, MD.
Clinical manifestations of cGVHD result from complex immune pathology involving both B and T cells.7-11 Ibrutinib is a once-daily Bruton's tyrosine kinase (BTK) inhibitor approved in the United States for adult patients with cGVHD after failure of ≥ 1 line of systemic therapy.11,12 The BTK signaling pathway is triggered by B-cell receptor activation, which regulates B-cell survival.13 Ibrutinib also inhibits interleukin-2–inducible T-cell kinase (ITK); immune reactivity toward healthy tissues is driven in part by ITK-mediated activation of T-cell subsets.14,15 By inhibiting BTK and ITK, ibrutinib can be beneficial for cGVHD, with previous studies demonstrating that ibrutinib is tolerable and efficacious in patients with relapsed chronic lymphatic leukemia after allo-HCT.15
The phase III, randomized, double-blind, placebo-controlled iNTEGRATE study (PCYC-1140; ClinicalTrials.gov identifier: NCT02959944) evaluated the safety and efficacy of ibrutinib in combination with corticosteroids in previously untreated patients with cGVHD. Here, we present the primary and final analyses from the randomized arms of the iNTEGRATE study.
METHODS
Study Design
iNTEGRATE was a phase III, double-blind, randomized, placebo-controlled, multicenter, international study designed to assess the safety and efficacy of ibrutinib-prednisone versus placebo-prednisone in patients with new-onset cGVHD. Patients age ≥ 12 years with moderate or severe cGVHD (defined by 2014 National Institutes of Health [NIH] Consensus Development Project criteria16) and a need for systemic treatment with corticosteroids were eligible. Patients with prior systemic treatment for cGVHD were ineligible, but patients who had received other prophylaxis immunosuppressants or treatment of acute graft-versus-host disease were eligible. Full inclusion and exclusion criteria are in the Data Supplement. Patients were randomly assigned 1:1 to receive ibrutinib 420 mg once daily plus prednisone starting at 1 mg/kg once daily or placebo plus prednisone starting at 1 mg/kg once daily. Random assignment between arms was stratified according to age group (12 to < 22 years v ≥ 22 years), per NIH Global Severity grade (moderate v severe), and by ongoing use of systemic immunosuppressants initiated for treatment or prophylaxis of acute graft-versus-host disease. Ibrutinib or placebo was administered until cGVHD progression, relapse of underlying malignancy, initiation of another systemic cGVHD treatment, or unacceptable toxicity. Prednisone was administered until unacceptable toxicity or tapered as clinically indicated with a suggested 6-month taper schedule (Data Supplement). Primary analysis was conducted after the last patient enrolled completed 48 weeks of follow-up; final analysis was conducted when all patients had ≥ 2 years of follow-up. See the Data Supplement for additional methods. The Protocol (online only) was approved by participating institutions' institutional review boards or independent ethics committees. The study was conducted according to the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines from the International Conference on Harmonisation. All patients provided written informed consent before screening.
End Points and Assessments
The primary end point was response rate (complete response [CR] or partial response [PR]; improvement in ≥ 1 organ or site without progression in any other organ or site) at 48 weeks per 2014 NIH Consensus Development Project Criteria.16 Patients were not considered responders at 48 weeks if, at or before the 48-week response assessment, they started second-line systemic therapy for cGVHD, had evidence of underlying malignancy progression (indication for transplant), or withdrew from the study/response assessments. Other end points included event-free survival (EFS; survival without cGVHD progression, relapse of underlying disease, or start of subsequent cGVHD therapy); failure-free survival (FFS; survival without relapse of underlying disease, or start of subsequent cGVHD therapy); duration of response (DOR; time of initial CR or PR until progression of cGVHD, relapse of underlying disease or start of subsequent cGVHD treatment, or death); time to withdrawal of corticosteroids; time to withdrawal of immunosuppressants, excluding and including ibrutinib; and improvement of Lee cGVHD Symptom Scale (LSS) score (≥ 7-point decrease on ≥ 2 consecutive visits), overall survival (OS), and safety. EFS was analyzed (in addition to FFS) to address regulatory agency requests for objective measures of response in evaluation of efficacy. All patients completed the LSS assessments at screening and at each cGVHD assessment and response follow-up visit.
Statistical Analysis
The study was powered to detect a 20% difference in response rate at 48 weeks between arms at a two-sided alpha level of 5%, assuming a 30% response rate at 48 weeks for the placebo-prednisone arm, with a sample size of 186 randomly assigned patients. A chi-square test was used to compare response rates between the treatment arms. On the basis of a serial gatekeeping testing strategy, the P values for all secondary end point analyses were considered nominal because of a statistically insignificant result from the primary end point analysis. Efficacy was analyzed in the intent-to-treat population, defined as all randomly assigned patients. Safety was assessed in the safety population, which included patients who received ≥ 1 dose of study drug (ibrutinib or placebo). See the Data Supplement for additional methods.
RESULTS
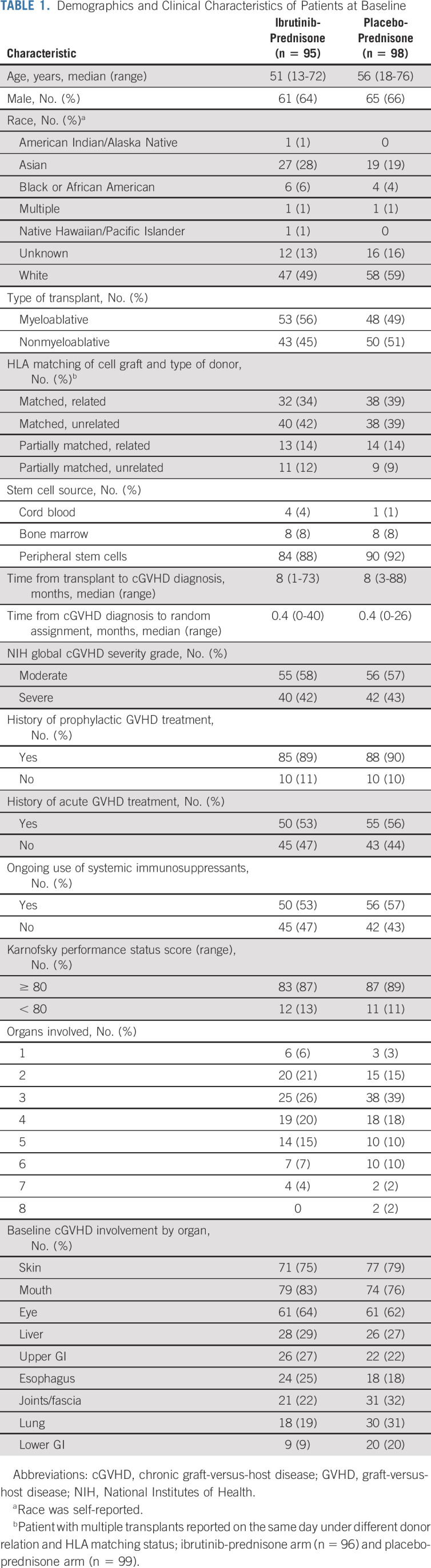
One hundred ninety-three patients were enrolled, with 95 and 98 patients in the ibrutinib-prednisone and placebo-prednisone arms, respectively. Baseline demographics and disease characteristics were generally well balanced between the arms and are shown in Table 1 (Data Supplement).
TABLE 1.
Demographics and Clinical Characteristics of Patients at Baseline
Disposition
At final analysis, median follow-up was 33 months (range, 0.03-47.20 months). All patients had discontinued ibrutinib or placebo per protocol. The most common reasons for discontinuing ibrutinib or placebo were progressive cGVHD (n = 22 [23%] and n = 30 [31%]), adverse events (AEs) not related to progressive cGVHD (n = 19 [20%] and n = 16 [16%]), and investigator decision (which included unblinding at primary analysis; n = 22 [23%] and n = 26 [27%]; Data Supplement, Fig 1). Fifteen patients (8%) discontinued ibrutinib (n = 13) or placebo (n = 2) because of study closure. Six patients (3%) discontinued study ibrutinib and continued ibrutinib treatment on a long-term access study. Patients in the ibrutinib-prednisone arm received 4.9 g (mean; median, 4.2 g; range, 0-18 g) of prednisone on study; patients in the placebo-prednisone arm received 5.4 g (mean; median, 4.1 g; range, 0-22 g) on study.
FIG 1.
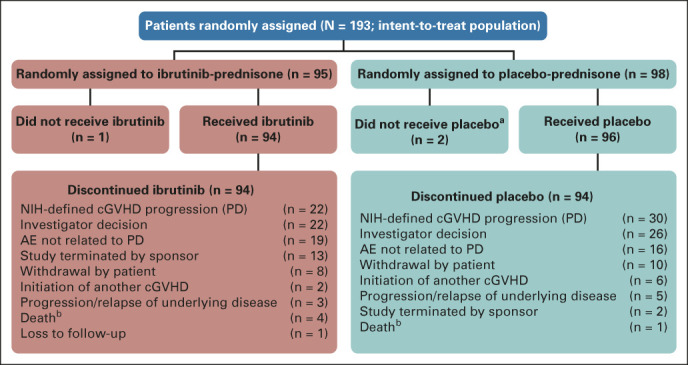
CONSORT diagram of ibrutinib disposition. aReasons for not receiving the study drug were hyperbilirubinemia, relapse of malignant disease, and withdrawal of consent. bDeaths are listed as reasons for discontinuation of study drug. AE, adverse event; cGVHD, chronic graft-versus-host disease; ibr, ibrutinib; NIH, National Institutes of Health; PD, progressive disease.
Response
Response rate after 48 weeks (primary end point) was 41% for ibrutinib-prednisone and 37% for placebo-prednisone (P = .54; Table 2). Among responders, nine (9%) and six (6%) patients achieved a CR in the ibrutinib-prednisone and placebo-prednisone arms, respectively. Additional follow-up at 96 weeks demonstrated response rates of 27% (ibrutinib-prednisone) and 22% (placebo-prednisone; P = .43); 13 patients (14%) and nine patients (9%), respectively, reported a CR at 96 weeks (Data Supplement). The Data Supplement shows responses by organ involvement. Although organ-specific responses were not prespecified end points and this study was not designed to assess them comprehensively, a reduced best overall response was observed in liver and lower gastrointestinal tract in patients treated with ibrutinib-prednisone.
TABLE 2.
Response Rates at 48 Weeks (intent-to-treat population)
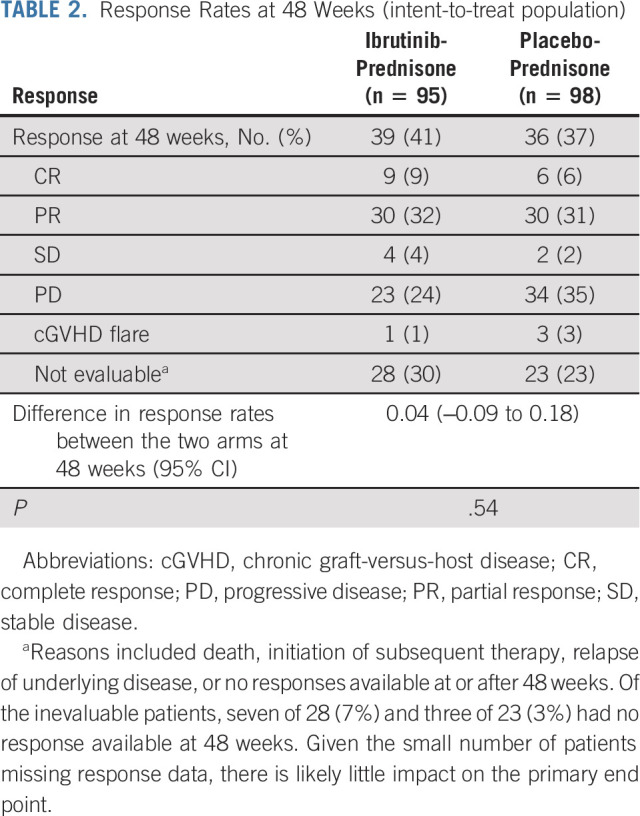
EFS and FFS
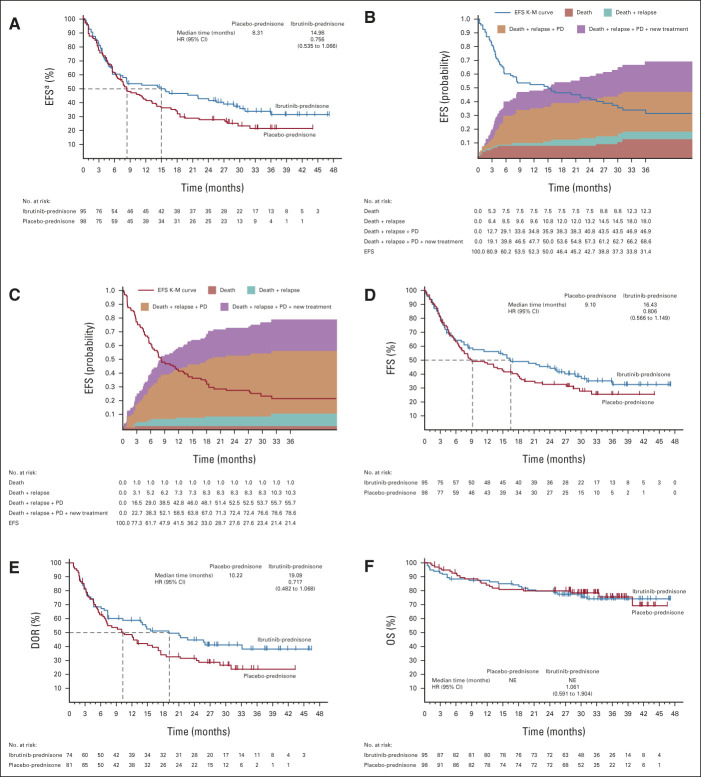
Median EFS was 15 months (95% CI, 6 to 27) for ibrutinib-prednisone and 8 months (95% CI, 6 to 13) for placebo-prednisone (hazard ratio [HR], 0.76; 95% CI, 0.54 to 1.07; P = .11; Fig 2A). For EFS analysis, the earliest event for each patient was captured and included cGVHD progression (n = 26 [27%] v 43 [44%]), initiation of subsequent cGVHD treatment (n = 18 [19%] v n = 21 [21%]), death (n = 10 [11%] v n = 1 [1%]), and relapse of underlying malignancy (n = 5 [5%] v n = 8 [8%]) for ibrutinib-prednisone and placebo-prednisone, respectively. The 24-month EFS estimates for ibrutinib-prednisone and placebo-prednisone were 43% (95% CI, 32 to 53) and 28% (95% CI, 19 to 37), respectively (Figs 2B and 2C).
FIG 2.
(A) EFS for all patients, (B) EFS and cumulative incidence of competing events in the ibrutinib-prednisone arm, (C) EFS and cumulative incidence of competing events in the placebo-prednisone arm, and (D) FFS, (E) DOR, and (F) OS in the two treatment arms. End points evaluated with 6 months of additional follow-up after primary analysis (median follow-up, 33 months [range, 0.03-47.20]). aA total of 18 fatal AE events were reported, and 11 total deaths (including AEs and non-AEs) were events for the EFS analysis. Nine of the 18 fatal AE events (ibrutinib-prednisone n = 4; placebo-prednisone n = 5) were not captured as death events in the EFS analysis because they occurred after other preceding events (such as relapse, initiation of subsequent cGVHD treatment, or cGVHD progression). Conversely, of the 11 total EFS death events, two (in the ibrutinib-prednisone arm) were not considered fatal AE events because they occurred after the prespecified AE treatment-emergent period but were not preceded by any other EFS event. AE, adverse event; cGVHD, chronic graft-versus-host disease; DOR, duration of response; EFS, event-free survival; FFS, failure-free survival; HR, hazard ratio; K-M, Kaplan-Meier; NE, not estimable; OS, overall survival; PD, progressive disease.
Median FFS was 16 months (95% CI, 8 to 29) for ibrutinib-prednisone and 9 months (95% CI, 7 to 18) for placebo-prednisone. The 12-month FFS estimates were 56% (95% CI, 45 to 66) and 47% (95% CI, 37 to 57), respectively. The 24-month FFS estimate was 45% (95% CI, 35 to 55) for ibrutinib-prednisone and 33% (95% CI, 23 to 42) for placebo-prednisone (Fig 2D).
Duration of Response
In patients with a CR or PR at any time during the study, median DOR was 19 months (95% CI, 7 to not evaluable) for ibrutinib-prednisone and 10 months (95% CI, 6.5 to 17) for placebo-prednisone (P = .10). The 24-month DOR estimate was 45% (95% CI, 33 to 56) and 32% (95% CI, 22 to 42), respectively (Fig 2E).
Overall Survival
The estimated 24-month OS rates were similar between treatments (80% in each arm; Fig 2F); median OS was not reached in either arm (HR, 1.06; 95% CI, 0.59 to 1.90). At final analysis, 23 patients (24%) in the ibrutinib-prednisone arm and 22 patients (22%) in the placebo-prednisone arm had died.
Improvement of LSS Scores
Over time, LSS scores improved from baseline in both arms. The proportion of patients with ≥ 7-point score improvement on ≥ 2 consecutive visits was 43% (n = 41) in the ibrutinib-prednisone arm and 31% (n = 30) in the placebo-prednisone arm (P = .07). Although unblinding (after final patient reached 48-week response evaluation) is a potential study limitation, assessment of the LSS end point, which is most potentially subject to this limitation (rates of LSS score improvement), showed similarity before unblinding (39% ibrutinib-prednisone; 27% placebo-prednisone) with rates after unblinding.
Time to Withdrawal of Immunosuppressants and Corticosteroids
Among patients receiving ibrutinib-prednisone, withdrawal of corticosteroids was observed in 47% compared with 39% of patients receiving placebo-prednisone (P = .28). In the ibrutinib-prednisone and placebo-prednisone arms, 41% and 46% of patients, respectively, had a reduction in prednisone to < 0.15 mg/kg/d at 24 weeks that was sustained for ≥ 30 days. Withdrawal of all immunosuppressants, excluding ibrutinib or placebo, was observed in 39% and 31% of patients receiving ibrutinib-prednisone and placebo-prednisone, respectively (P = .22). Withdrawal of all immunosuppressants, including ibrutinib or placebo, was observed in 17 (18%) and eight (8%) patients in the ibrutinib-prednisone and placebo-prednisone arms, respectively (P = .03). At 24 months, cumulative incidence of withdrawal of all immunosuppressants, including ibrutinib, was 0.17% (95% CI, 0.10 to 0.26) in the ibrutinib-prednisone arm and 0.08% (95% CI, 0.03 to 0.14) in the placebo-prednisone arm. Kaplan-Meier curves show a trend toward faster withdrawal of immunosuppressants and corticosteroids (Figs 3A-3C).
FIG 3.
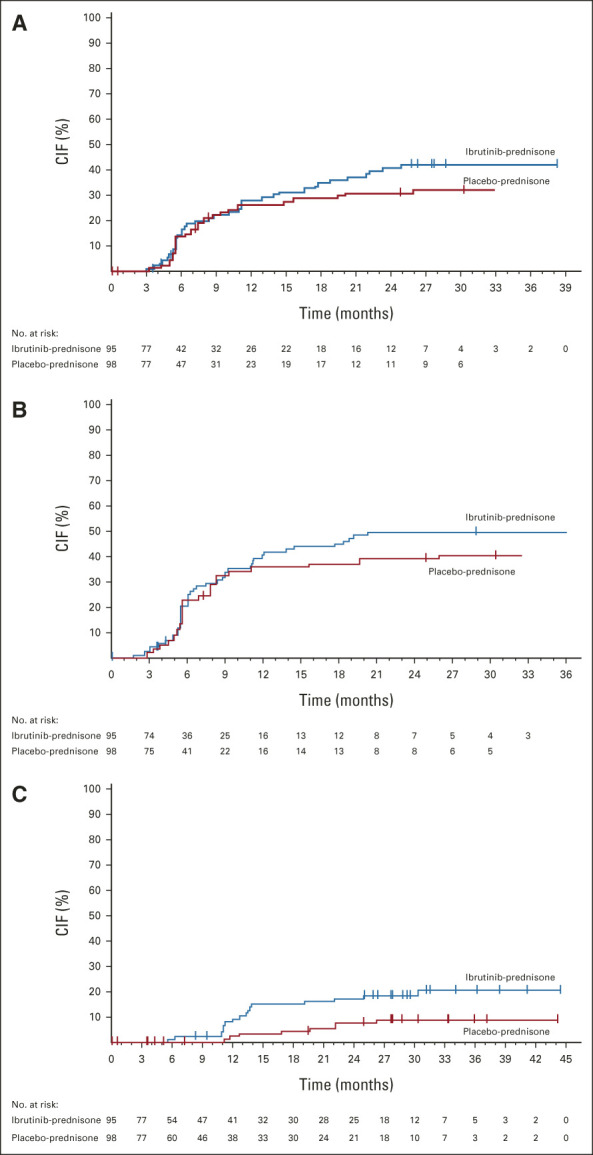
Time to withdrawal of (A) all immunosuppressants, excluding ibrutinib, (B) all corticosteroids, and (C) all immunosuppressants. End point evaluated with 6 months of additional follow-up after primary analysis (median follow-up, 33 months [range, 0.03-47.20]). CIF, cumulative incidence function.
Safety
At the time of data cutoff, median treatment duration for ibrutinib-prednisone was 5 months (range, 0-44 months) for ibrutinib and 5 months (range, 0-34 months) for prednisone. For placebo-prednisone, median treatment duration was 6 months (range, 0-36 months) for placebo and 6 months (range, 0-44 months) for prednisone. The median relative ibrutinib dose intensity was 100%, defined as total cumulative dose administered divided by total dose expected. Over the prednisone treatment duration, 4 g of prednisone (median total dose) was received in both arms (range in ibrutinib-prednisone arm, 0-18 g; range in placebo-prednisone arm, 0-22 g).
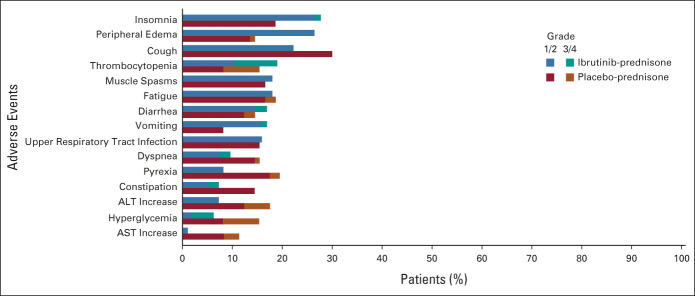
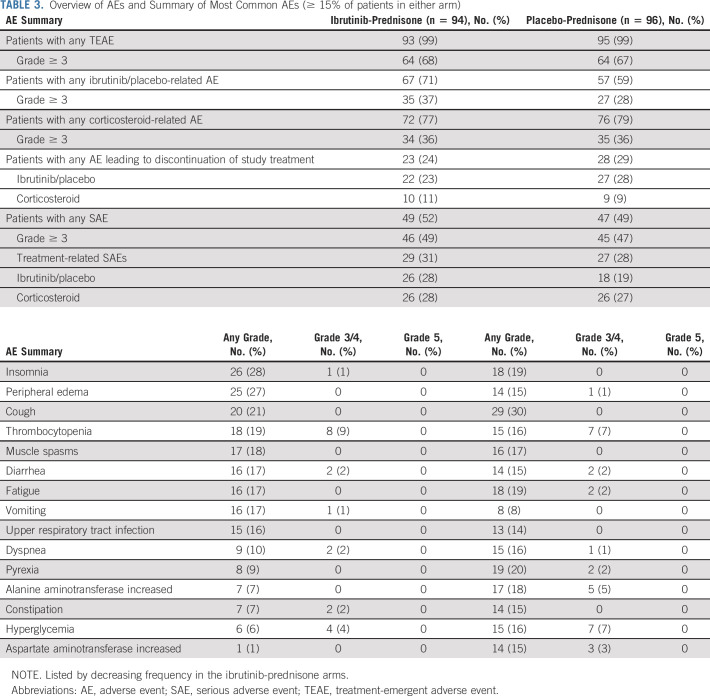
In the safety population, 99% of patients in each arm experienced treatment-emergent AEs (TEAEs) of any grade (ibrutinib-prednisone, n = 93/94; placebo-prednisone, n = 95/96). Grade ≥ 3 TEAEs occurred in 64 patients in each arm (68% ibrutinib-prednisone; 67% placebo-prednisone). The most common any-grade TEAEs in each arm included insomnia (28% and 19%), peripheral edema (27% and 15%), and cough (21% and 30%). TEAE data are summarized in Figure 4 and Table 3. TEAEs of clinical interest (grade ≥ 3) that occurred in each arm included opportunistic infections (6% and 2%), hyperglycemia (4% and 7%), hypertension (5% and 5%), major hemorrhage (3% and 4%), atrial fibrillation (2% and 2%), and cardiac arrest (1% and 1%; Data Supplement).
FIG 4.
Most common any-grade treatment-emergent adverse events (occurring in ≥ 15% of patients in the ibrutinib-prednisone or placebo-prednisone arm).
TABLE 3.
Overview of AEs and Summary of Most Common AEs (≥ 15% of patients in either arm)
Serious AEs occurred in 49 patients (52%) in the ibrutinib-prednisone arm and 47 patients (49%) in the placebo-prednisone arm. Twenty-six patients in each arm experienced serious AEs that were considered by the investigator to be corticosteroid-related (28% ibrutinib-prednisone; 27% placebo-prednisone).
In the ibrutinib-prednisone arm, nine (10%) patients experienced an AE leading to ibrutinib dose reduction. In the placebo-prednisone arm, 11 patients (11%) experienced an AE leading to placebo dose reduction. Twenty-two patients (23%) experienced an AE leading to discontinuation of ibrutinib. In the placebo-prednisone arm, 27 patients (28%) experienced an AE leading to discontinuation of placebo. AEs leading to dose reduction or discontinuation are summarized in the Data Supplement. Fatal AE events were reported for 12 patients (13%) in the ibrutinib-prednisone arm and six patients (6%) in the placebo-prednisone arm (Data Supplement).
DISCUSSION
Several phase III studies have evaluated treatment of new-onset cGVHD17-23; of these, only two were randomized and double-blinded,17,21 and neither used objective response criteria. In the randomized controlled trial reported by Martin et al,21 mycophenolate mofetil plus steroids showed statistically insignificant benefit (treatment success rate of 23% v 18% in control arm, determined by withdrawal of systemic immunosuppression and resolution of reversible cGVHD manifestations) and an estimated HR of death of 1.99. To our knowledge, the iNTEGRATE study represents the first prospective, randomized, double-blind, placebo-controlled phase III trial of a therapeutic agent in previously untreated cGVHD using objective response criteria. The response rate at 48 weeks was not different in patients receiving ibrutinib-prednisone compared with placebo-prednisone. Secondary end points of EFS, DOR, and improvement in overall LSS scores in ≥ 2 consecutive visits also did not differ. Withdrawal of all immunosuppressants, including ibrutinib or placebo, was observed in significantly more patients treated with ibrutinib-prednisone than in those treated with placebo-prednisone. OS was similar between the two study arms. Safety was consistent with the known profiles of ibrutinib, corticosteroids, and the underlying condition of cGVHD; no new safety signals were identified.
Response rate at 48 weeks has been identified as the most reliable indicator of long-term treatment success.24 Compared with the 1-year FFS reported in Martin et al21 (estimated at approximately 15%), the 12-month FFS in the current study (ibrutinib-prednisone, 56%; placebo-prednisone, 47%) was higher in both arms, as was the response at 48 weeks (ibrutinib-prednisone, 41%; placebo-prednisone, 37%). This may be accounted for by differences in trial design or consistency of follow-up. The FFS reported here is consistent with that reported by Inamoto et al25 (54% at 12 months), and is slightly higher in both arms compared with that reported in BMT-CTN-0801 (46.2%-48.6% at 2 years).26 By contrast, a recent single-center retrospective study of ibrutinib in adults with steroid-refractory cGVHD reported a median FFS of 4.5 months and a 2-year FFS rate of 9%.27 The disparities in FFS between studies are likely attributable to various factors, such as differences in patient population and trial design; the inclusion of a placebo control is a particular strength of this study that adds context for interpretation of these results.
OS at 2 years was similar in both arms to the BMT-CTN-0801 trial, which also included patients with mild cGVHD26; although not directly comparable; OS was also consistent with previously reported 5-year rates of 67%-72%.20
Unblinding of the study after the last subject reached 48 weeks is a limitation that prevents late time point analysis but was necessary as the primary end point was not met. We note that 24-month landmark estimate for EFS at primary analysis was 43.3% for ibrutinib-prednisone and 23.8% for placebo-prednisone (P = .08). At final analysis, these estimates were 42.7% versus 27.6% (P = .11). The P value for DOR was 0.10 at both primary analysis and final analysis, and OS remains a valid end point not subject to bias related to therapy changes or AE reporting. Perhaps, future cGVHD studies should test primary end points determined after longer periods of treatment.
Because individual organ response assessments were truncated at the time of progression of any evaluated organ per the NIH definition of overall progressive disease, the reduced best overall response observed in liver and lower gastrointestinal tract in patients in the ibrutinib-prednisone arm likely reflects initial responses to high dose corticosteroids. Therefore, the relative contribution of ibrutinib is not clear.
In relapsed/refractory cGVHD settings, ibrutinib has demonstrated sustained single-agent efficacy, safety, and improvements in patient-reported outcomes in long-term follow-up of 2 years.28 Additional agents being evaluated in this setting include belumosudil, an inhibitor of Rho-associated coiled-coil-containing protein kinase-2, which was recently approved in the United States for adult and pediatric patients age ≥ 12 years after failure of ≥ 2 lines of systemic therapy.29 The Janus kinase inhibitor ruxolitinib was recently approved in the United States for cGVHD treatment on the basis of the results of an open-label randomized phase III trial in patients with corticosteroid-refractory cGVHD.30,31 Ruxolitinib has demonstrated improvements in overall response rate compared with best available therapies in patients with steroid-resistant cGVHD. Direct comparisons of efficacy across trials are not possible because of significant differences in study design, as demonstrated by the large difference in CR rates obtained with ruxolitinib (6.7% at 24 weeks) compared with ibrutinib (21% at 1 year).28,30 The disparity between response rates to ibrutinib reported in REACH3 versus the phase Ib/II PCYC-1129 study (22% v 67%) highlights the importance of using appropriate placebo controls when relying on relatively subjective response criteria such as the 2014 NIH Consensus Development Project Criteria.16,30 To our knowledge, no therapy has yet been proven to be superior to standard of care in a placebo-controlled trial, as first- or second-line treatment for cGVHD. Additional studies are needed to identify patients who may benefit from specific targeted therapies and further refine the best use of new steroid-sparing agents in the course of disease. For example, given the ability of ibrutinib to inhibit both BTK and ITK (in contrast to other BTK inhibitors actively under study for cGVHD [ClinicalTrials.gov identifier: NCT04198922]), earlier use of ibrutinib may interrupt the adaptive immunity feedback loop that contributes to cGVHD development. Future studies of ibrutinib could use alternative study designs aimed at quickly reducing corticosteroid therapy and/or providing steroid-sparing therapy. A mandated steroid taper challenge might elucidate potential clinical benefits.
The safety of combining immunosuppressants in an already immunocompromised host was of primary interest in this study. The safety data generated in this randomized controlled trial may be clinically applicable to the use of ibrutinib in relapsed/refractory cGVHD. Ibrutinib-prednisone and placebo-prednisone had similar safety profiles, with similar proportions of patients experiencing grade ≥ 3 AEs. Although the rates of fatal TEAEs were higher in the ibrutinib-prednisone arm compared with the placebo-prednisone arm, rates of opportunistic infection, hypertension, atrial fibrillation, cardiac arrest, and OS were similar in each arm. Overall, the safety profile of ibrutinib in cGVHD is consistent with that observed in RESONATE-2, a long-term study in chronic lymphatic leukemia that demonstrated a decline in rates of most AEs over long-term follow-up.32,33
cGVHD remains a challenging disease to study because of its protean, often subjective manifestations and assessment methods. This placebo-controlled trial, one of the most rigorous ever conducted in cGVHD, demonstrated no difference in NIH-graded response at 48 weeks. Nonetheless, positive trends in other end points, for example, withdrawal of immunosuppression and patient-reported outcomes, support the refinement of the tools used to assess the efficacy of interventions for cGVHD. Overlap between nominal score categories using the NIH Consensus Project Criteria16 may have a limited ability to discern smaller-scale improvements during the first year of treatment. Similarly, improvements over a prolonged period require larger subject size and longitudinal comparison of symptoms compared with baseline, which can be challenging to perform accurately and objectively in clinical practice, especially in the setting of corticosteroids. Coadministration of prednisone may have affected study outcomes. The 9-month steroid taper schedule could potentially have been shortened to a 6-month challenge to better assess the effect of ibrutinib versus placebo. Finally, the end point of EFS, which incorporates NIH-defined progression into the existing FFS approach, may represent a useful and clinically relevant end point for evaluation of efficacy in future cGVHD studies. A head-to-head trial comparing ibrutinib with prednisone could elucidate beneficial effects of ibrutinib as a single agent in the treatment of cGVHD, potentially reducing the toxic effects of corticosteroids. Additionally, future studies may elucidate the clinical and laboratory markers that predict response to therapy, and the impacts of timing and/or therapeutic combinations on efficacy and safety outcomes.
ACKNOWLEDGMENT
The authors thank the patients who participated in the study and their supportive families; and the investigators, study coordinators, study team, and nurses who cared for the patients and the iNTEGRATE Study Group for contributing to this study.
David Bernard Miklos
Honoraria: Janssen, Fosun Kite Biotechnology
Consulting or Advisory Role: Adaptive Biotechnologies, Juno/Celgene, Pharmacyclics LLC, an AbbVie Company, Janssen
Research Funding: Pharmacyclics LLC, an AbbVie Company, Novartis, Roche/Genentech, Kite, a Gilead Company, Adaptive Biotechnologies, Alimera Sciences, Precision Biosciences, Adicet Bio
Patents, Royalties, Other Intellectual Property: Patent held with Pharmacyclics LLC, an AbbVie Company, supporting Ibrutinib for cGVHD (no royalty claim)
Mohammad Abu Zaid
Stock and Other Ownership Interests: Pieris Pharmaceuticals
Consulting or Advisory Role: Syndax, Ossium Health
Research Funding: Syndax (Inst), Pharmacyclics LLC, an AbbVie Company, (Inst), Janssen (Inst), Incyte (Inst), AlloVir (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1350343
Mary Flowers
Honoraria: Astellas Pharma, Mallinckrodt
Research Funding: Pharmacyclics LLC, an AbbVie Company, Incyte
Alan P. Skarbnik
Consulting or Advisory Role: Alexion Pharmaceuticals, AstraZeneca, AbbVie, BeiGene, Epizyme, Genentech, Janssen, Genmab, Curio Science, Novartis, Kite, a Gilead Company, MorphoSys, Pharmacyclics LLC, an AbbVie Company, TG Therapeutics, Lilly, Elsevier
Speakers' Bureau: AstraZeneca, ADC Therapeutics, AbbVie, BeiGene, Celgene, Genentech, Kite, a Gilead Company, Jazz Pharmaceuticals, Janssen, Pharmacyclics LLC, an AbbVie Company, TG Therapeutics
Travel, Accommodations, Expenses: AstraZeneca, Epizyme, AbbVie, Pharmacyclics LLC, an AbbVie Company/Janssen
Ibrahim Yakoub-Agha
Honoraria: Celgene, Novartis, Gilead Sciences, Biotest, Janssen, Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Gilead Sciences
Bor-Sheng Ko
Leadership: AHEAD Medicine Ltd (I)
Stock and Other Ownership Interests: AHEAD Medicine Ltd (I)
Honoraria: Novartis, AbbVie, Roche, Amgen, Johnson & Johnson/Janssen, Gilead Sciences, Chugai Pharma, Pfizer
Benedetto Bruno
Honoraria: Novartis
Edmund K. Waller
Employment: Cambium Medical Technologies
Leadership: Cambium Medical Technologies
Stock and Other Ownership Interests: Cambium Medical Technologies, Cerus, Chimerix
Honoraria: Novartis, Partners, Verastem, Kite, a Gilead Company, Pharmacyclics LLC, an AbbVie Company, Karyopharm Therapeutics
Consulting or Advisory Role: Novartis, Verastem, Pharmacyclics LLC, an AbbVie Company, Karyopharm Therapeutics, Partners Healthcare, Kite, a Gilead Company
Research Funding: Novartis, Amgen, Juno Therapeutics, Verastem, Partners Healthcare
Patents, Royalties, Other Intellectual Property: Receive Royalties from patent on preparing platelet lysate that has been licensed to Cambium Medical Technologies
Travel, Accommodations, Expenses: Pharmacyclics LLC, an AbbVie Company
Jean Yared
Consulting or Advisory Role: Kadmon, Omeros
Claude-Eric Bulabois
Honoraria: Novartis, Kite, a Gilead Company, Astellas Pharma
Takanori Teshima
Honoraria: Merck Sharp & Dohme, Kyowa Kirin, Takeda, Pfizer, Bristol Myers Squibb
Consulting or Advisory Role: Takeda, Merck Sharp & Dohme, Novartis
Research Funding: Kyowa Kirin, Novartis, Chugai Pharma, Sanofi, Astellas Pharma, Teijin Pharma, Fuji Pharma, Nippon Shinyaku
Other Relationship: Janssen
Hildegard Greinix
Consulting or Advisory Role: Novartis, Gilead, Celgene, Sanofi
Speakers' Bureau: Therakos Novartis, Celgene, Gilead, Sanofi, Amgen
Ahmad Mokatrin
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Justin T. Wahlstrom
Employment: Atara Biotherapeutics, AbbVie/Pharmacyclics LLC, an AbbVie Company
Stock and Other Ownership Interests: AbbVie, Atara Biotherapeutics
Lori Styles
Employment: Summit Therapeutics, AbbVie/Pharmacyclics LLC, an AbbVie Company
Stock and Other Ownership Interests: AbbVie, Summit Therapeutics
Gerard Socie
Consulting or Advisory Role: Xenokos, Novartis
Research Funding: Alexion Pharmaceuticals (Inst)
No other potential conflicts of interest were reported.
See accompanying Oncology Grand Rounds on page 1820
PRIOR PRESENTATION
The data included in this manuscript were presented in part at EHA 2021 Virtual Congress June 9-17, 2021; abstract S235.
SUPPORT
Supported by Pharmacyclics LLC, an AbbVie Company. Study investigators and their research teams collected the data. The sponsor confirmed data accuracy and performed analysis of the data. Medical writing support was funded by the sponsor. Editorial support was provided by Cindi A. Hoover, PhD, and funded by Pharmacyclics LLC, an AbbVie Company.
CLINICAL TRIAL INFORMATION
NCT02959944; EudraCT: 2016-003286-26
DATA SHARING STATEMENT
Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
AUTHOR CONTRIBUTIONS
Conception and design: David Bernard Miklos, Hildegard Greinix, Ahmad Mokatrin, Yihua Lee, Justin T. Wahlstrom, Lori Styles, Gerard Socie
Provision of study materials or patients: David Bernard Miklos, Mohammad Abu Zaid, Mary Flowers, Ibrahim Yakoub-Agha, Bor-Sheng Ko, Sang Kyun Sohn, Claude-Eric Bulabois, Hildegard Greinix, Gerard Socie
Collection and assembly of data: David Bernard Miklos, Mohammad Abu Zaid, Julian P. Cooney, Jörn C. Albring, Mary Flowers, Alan P. Skarbnik, Ibrahim Yakoub-Agha, Bor-Sheng Ko, Benedetto Bruno, Edmund K. Waller, Takanori Teshima, Hildegard Greinix, Ahmad Mokatrin, Yihua Lee, Justin T. Wahlstrom, Lori Styles
Data analysis and interpretation: David Bernard Miklos, Mohammad Abu Zaid, Julian P. Cooney, Mary Flowers, Alan P. Skarbnik, Ibrahim Yakoub-Agha, Bor-Sheng Ko, Benedetto Bruno, Edmund K. Waller, Jean Yared, Sang Kyun Sohn, Claude-Eric Bulabois, David Jacobsohn, Hildegard Greinix, Yihua Lee, Justin T. Wahlstrom, Lori Styles
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Ibrutinib for First-Line Treatment of Chronic Graft-Versus-Host Disease: Results From the Randomized Phase III iNTEGRATE Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David Bernard Miklos
Honoraria: Janssen, Fosun Kite Biotechnology
Consulting or Advisory Role: Adaptive Biotechnologies, Juno/Celgene, Pharmacyclics LLC, an AbbVie Company, Janssen
Research Funding: Pharmacyclics LLC, an AbbVie Company, Novartis, Roche/Genentech, Kite, a Gilead Company, Adaptive Biotechnologies, Alimera Sciences, Precision Biosciences, Adicet Bio
Patents, Royalties, Other Intellectual Property: Patent held with Pharmacyclics LLC, an AbbVie Company, supporting Ibrutinib for cGVHD (no royalty claim)
Mohammad Abu Zaid
Stock and Other Ownership Interests: Pieris Pharmaceuticals
Consulting or Advisory Role: Syndax, Ossium Health
Research Funding: Syndax (Inst), Pharmacyclics LLC, an AbbVie Company, (Inst), Janssen (Inst), Incyte (Inst), AlloVir (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1350343
Mary Flowers
Honoraria: Astellas Pharma, Mallinckrodt
Research Funding: Pharmacyclics LLC, an AbbVie Company, Incyte
Alan P. Skarbnik
Consulting or Advisory Role: Alexion Pharmaceuticals, AstraZeneca, AbbVie, BeiGene, Epizyme, Genentech, Janssen, Genmab, Curio Science, Novartis, Kite, a Gilead Company, MorphoSys, Pharmacyclics LLC, an AbbVie Company, TG Therapeutics, Lilly, Elsevier
Speakers' Bureau: AstraZeneca, ADC Therapeutics, AbbVie, BeiGene, Celgene, Genentech, Kite, a Gilead Company, Jazz Pharmaceuticals, Janssen, Pharmacyclics LLC, an AbbVie Company, TG Therapeutics
Travel, Accommodations, Expenses: AstraZeneca, Epizyme, AbbVie, Pharmacyclics LLC, an AbbVie Company/Janssen
Ibrahim Yakoub-Agha
Honoraria: Celgene, Novartis, Gilead Sciences, Biotest, Janssen, Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Gilead Sciences
Bor-Sheng Ko
Leadership: AHEAD Medicine Ltd (I)
Stock and Other Ownership Interests: AHEAD Medicine Ltd (I)
Honoraria: Novartis, AbbVie, Roche, Amgen, Johnson & Johnson/Janssen, Gilead Sciences, Chugai Pharma, Pfizer
Benedetto Bruno
Honoraria: Novartis
Edmund K. Waller
Employment: Cambium Medical Technologies
Leadership: Cambium Medical Technologies
Stock and Other Ownership Interests: Cambium Medical Technologies, Cerus, Chimerix
Honoraria: Novartis, Partners, Verastem, Kite, a Gilead Company, Pharmacyclics LLC, an AbbVie Company, Karyopharm Therapeutics
Consulting or Advisory Role: Novartis, Verastem, Pharmacyclics LLC, an AbbVie Company, Karyopharm Therapeutics, Partners Healthcare, Kite, a Gilead Company
Research Funding: Novartis, Amgen, Juno Therapeutics, Verastem, Partners Healthcare
Patents, Royalties, Other Intellectual Property: Receive Royalties from patent on preparing platelet lysate that has been licensed to Cambium Medical Technologies
Travel, Accommodations, Expenses: Pharmacyclics LLC, an AbbVie Company
Jean Yared
Consulting or Advisory Role: Kadmon, Omeros
Claude-Eric Bulabois
Honoraria: Novartis, Kite, a Gilead Company, Astellas Pharma
Takanori Teshima
Honoraria: Merck Sharp & Dohme, Kyowa Kirin, Takeda, Pfizer, Bristol Myers Squibb
Consulting or Advisory Role: Takeda, Merck Sharp & Dohme, Novartis
Research Funding: Kyowa Kirin, Novartis, Chugai Pharma, Sanofi, Astellas Pharma, Teijin Pharma, Fuji Pharma, Nippon Shinyaku
Other Relationship: Janssen
Hildegard Greinix
Consulting or Advisory Role: Novartis, Gilead, Celgene, Sanofi
Speakers' Bureau: Therakos Novartis, Celgene, Gilead, Sanofi, Amgen
Ahmad Mokatrin
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Justin T. Wahlstrom
Employment: Atara Biotherapeutics, AbbVie/Pharmacyclics LLC, an AbbVie Company
Stock and Other Ownership Interests: AbbVie, Atara Biotherapeutics
Lori Styles
Employment: Summit Therapeutics, AbbVie/Pharmacyclics LLC, an AbbVie Company
Stock and Other Ownership Interests: AbbVie, Summit Therapeutics
Gerard Socie
Consulting or Advisory Role: Xenokos, Novartis
Research Funding: Alexion Pharmaceuticals (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Jaglowski SM, Blazar BR: How ibrutinib, a B-cell malignancy drug, became an FDA-approved second-line therapy for steroid-resistant chronic GVHD. Blood Adv 2:2012-2019, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SJ, Klein JP, Barrett AJ, et al. : Severity of chronic graft-versus-host disease: Association with treatment-related mortality and relapse. Blood 100:406-414, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Fraser CJ, Bhatia S, Ness K, et al. : Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: A report from the Bone Marrow Transplant Survivor Study. Blood 108:2867-2873, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora M, Klein JP, Weisdorf DJ, et al. : Chronic GVHD risk score: A Center for International Blood and Marrow Transplant Research analysis. Blood 117:6714-6720, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garnett C, Apperley JF, Pavlu J: Treatment and management of graft-versus-host disease: Improving response and survival. Ther Adv Hematol 4:366-378, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flowers ME, Martin PJ: How we treat chronic graft-versus-host disease. Blood 125:606-615, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn R, Du J, Veenstra RG, et al. : Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood 123:3988, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miklos DB, Kim HT, Miller KH, et al. : Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood 105:2973-2978, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Gao Q, Feng Y, et al. : Developing role of B cells in the pathogenesis and treatment of chronic GVHD. Br J Haematol 184:323-336, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Socie G, Ritz J: Current issues in chronic graft-versus-host disease. Blood 124:374-384, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miklos D, Cutler CS, Arora M, et al. : Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 130:2243-2250, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IMBRUVICA® (Ibrutinib) Prescribing Information. South San Francisco, CA, Pharmacyclics LLC, an AbbVie Company, 2020 [Google Scholar]
- 13.Shinners NP, Carlesso G, Castro I, et al. : Bruton's tyrosine kinase mediates NF-kappa B activation and B cell survival by B cell-activating factor receptor of the TNF-R family. J Immunol 179:3872-3880, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Dubovsky JA, Beckwith KA, Natarajan G, et al. : Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 122:2539-2549, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan CE, Sahaf B, Logan AC, et al. : Ibrutinib efficacy and tolerability in patients with relapsed chronic lymphocytic leukemia following allogeneic HCT. Blood 128:2899-2908, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jagasia MH, Greinix HT, Arora M, et al. : National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 21:389-401.e1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan KM, Witherspoon RP, Storb R, et al. : Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-v-host disease: Prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood 72:546-554, 1988 [PubMed] [Google Scholar]
- 18.Koc S, Leisenring W, Flowers ME, et al. : Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood 96:3995-3996, 2000 [PubMed] [Google Scholar]
- 19.Arora M, Wagner JE, Davies SM, et al. : Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant 7:265-273, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Koc S, Leisenring W, Flowers ME, et al. : Therapy for chronic graft-versus-host disease: A randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood 100:48-51, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Martin PJ, Storer BE, Rowley SD, et al. : Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood 113:5074-5082, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilman AL, Schultz KR, Goldman FD, et al. : Randomized trial of hydroxychloroquine for newly diagnosed chronic graft-versus-host disease in children: A Children's Oncology Group study. Biol Blood Marrow Transplant 18:84-91, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpenter PA, Logan BR, Lee SJ, et al. : Prednisone (PDN)/Sirolimus (SRL) compared to PDN/SRL/calcineurin inhibitor (CNI) as treatment for chronic graft-versus-host-disease (cGVHD): A randomized phase ii study from the blood and marrow transplant clinical trials network. Bio Blood Marrow Transplant 22:S50-S52, 2016 [Google Scholar]
- 24.Martin PJ, Storer BE, Inamoto Y, et al. : An endpoint associated with clinical benefit after initial treatment of chronic graft-versus-host disease. Blood 130:360-367, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inamoto Y, Flowers ME, Sandmaier BM, et al. : Failure-free survival after initial systemic treatment of chronic graft-versus-host disease. Blood 124:1363-1371, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carpenter PA, Logan BR, Lee SJ, et al. : A phase II/III randomized, multicenter trial of prednisone/sirolimus versus prednisone/sirolimus/calcineurin inhibitor for the treatment of chronic graft-versus-host disease: BMT CTN 0801. Haematologica 103:1915-1924, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chin KK, Kim HT, Inyang EA, et al. : Ibrutinib in steroid-refractory chronic graft-versus-host disease, a single-center experience. Transplant Cell Ther 27:990.e1-990.e7, 2021 [DOI] [PubMed] [Google Scholar]
- 28.Waller EK, Miklos D, Cutler C, et al. : Ibrutinib for chronic graft-versus-host disease after failure of prior therapy: 1-Year update of a phase 1b/2 study. Biol Blood Marrow Transplant 25:2002-2007, 2019 [DOI] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration : FDA approves belumosudil for chronic graft-versus-host disease [press release]. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-belumosudil-chronic-graft-versus-host-disease
- 30.Zeiser R, Polverelli N, Ram R, et al. : Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med 385:228-238, 2021 [DOI] [PubMed] [Google Scholar]
- 31.US Food and Drug Administration : FDA approves ruxolitinib for chronic graft-versus-host disease [press release]. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ruxolitinib-chronic-graft-versus-host-disease#:∼:text=On%20September%2022%2C%202021%2C%20the,patients%2012%20years%20and%20older
- 32.Barr PM, Owen C, Robak T, et al. : Up to seven years of follow-up in the RESONATE-2 study of first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. J Clin Oncol 39, 2021. (15 suppl; abstr 7523) [Google Scholar]
- 33.Ghia P, Owen C, Robak T, et al. : Ibrutinib treatment in the first-line setting for patients with chronic lymphocytic leukemia: Up to 7 years of follow-up in the resonate-2 study. Presented at EHA 2021, Virtual, June 11, 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.