Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
We report 5-year efficacy and safety outcomes from the phase III KEYNOTE-407 study (ClinicalTrials.gov identifier: NCT02775435). Eligible patients with previously untreated, metastatic squamous non–small-cell lung cancer (NSCLC) were randomly assigned 1:1 to pembrolizumab 200 mg or placebo plus carboplatin and paclitaxel/nab-paclitaxel once every 3 weeks for four cycles, followed by pembrolizumab or placebo for up to 35 cycles. Primary end points were overall survival (OS) and progression-free survival (PFS) per RECIST version 1.1 by blinded independent central review (BICR). Five hundred fifty-nine patients were randomly assigned in the intention-to-treat population (pembrolizumab plus chemotherapy, n = 278; placebo plus chemotherapy, n = 281). The median time from random assignment to data cutoff was 56.9 (range, 49.9-66.2) months. OS and PFS were improved with pembrolizumab plus chemotherapy versus placebo plus chemotherapy (hazard ratio [95% CI], 0.71 [0.59 to 0.85] and 0.62 [0.52 to 0.74]), with 5-year OS rates of 18.4% versus 9.7%, respectively. Toxicity was manageable. Among 55 patients who completed 35 cycles of pembrolizumab, the objective response rate was 90.9% and the 3-year OS rate after completion of 35 cycles (approximately 5 years after random assignment) was 69.5%. Pembrolizumab plus chemotherapy maintained an OS and PFS benefit versus placebo plus chemotherapy in previously untreated, metastatic squamous NSCLC and is a standard-of-care first-line treatment option for metastatic squamous NSCLC regardless of programmed death ligand 1 expression.
INTRODUCTION
The primary analysis of the global, randomized, phase III KEYNOTE-407 study demonstrated significantly improved overall survival (OS) and progression-free survival (PFS) with pembrolizumab, an anti–programmed death 1 (anti–PD-1) monoclonal antibody, in combination with carboplatin and paclitaxel or nab-paclitaxel chemotherapy versus placebo plus chemotherapy in patients with previously untreated, metastatic squamous non–small-cell lung cancer (NSCLC; OS hazard ratio [HR], 0.64 [95% CI, 0.49 to 0.85], P < .001; PFS HR, 0.56 [95% CI, 0.45 to 0.70], P < .001).1 Pembrolizumab plus chemotherapy continued to show a clinically meaningful improvement in OS (HR, 0.71; 95% CI, 0.58 to 0.88) and PFS (HR, 0.57; 95% CI, 0.47 to 0.69) versus placebo plus chemotherapy in the protocol-specified final analysis.2 We report efficacy outcomes and safety from KEYNOTE-407 with an approximately 5-year follow-up.
CONTEXT
Key Objective
This exploratory analysis of the phase III KEYNOTE-407 study evaluated whether patients with previously untreated, metastatic squamous non–small-cell lung cancer (NSCLC) treated with pembrolizumab plus carboplatin and paclitaxel or nab-paclitaxel chemotherapy had improved long-term survival outcomes versus patients treated with placebo plus chemotherapy after a 5-year follow-up.
Knowledge Generated
With an approximately 5-year follow-up, pembrolizumab plus chemotherapy continued to provide a clinically meaningful survival benefit compared with placebo plus chemotherapy with manageable safety in patients with metastatic squamous NSCLC. Five-year overall survival rates were 18.4% with pembrolizumab plus chemotherapy versus 9.7% with placebo plus chemotherapy in the intention-to-treat population.
Relevance (T.E. Stinchcombe)
-
The 5-year follow-up demonstrates durable benefit for the combination of carboplatin and paclitaxel or nab-paclitaxel and pembrolizumab and the benefit in the patient subsets based on tumor programmed death ligand 1 expression. In the future, the landmark analyses of progression-free survival or overall survival at 3 or 5 years may be used to assess the long-term benefit of novel immunotherapies.*
*Relevance section written by JCO Associate Editor Thomas E. Stinchcombe, MD.
METHODS
Study Design and Patients
The design for this study has been previously described.1,2 Patients provided written informed consent.
Eligible patients had previously untreated, histologically/cytologically confirmed stage IV squamous NSCLC, measurable disease per RECIST version 1.1, and provided tumor tissue for determination of programmed death ligand 1 (PD-L1) status. Patients were randomly assigned 1:1 to pembrolizumab 200 mg or placebo once every 3 weeks plus carboplatin area under the curve 6 mg/mL/min plus paclitaxel 200 mg/m2 or nab-paclitaxel 100 mg/m2 on days 1, 8, and 15 once every 3 weeks for four cycles, followed by pembrolizumab 200 mg or placebo per initially assigned treatment until completion of 35 cycles, progressive disease (PD), unacceptable adverse event (AE), or patient withdrawal. Eligible patients in the placebo plus chemotherapy group with confirmed PD per blinded independent central review (BICR) could cross over to pembrolizumab monotherapy for up to 35 cycles. Patients were eligible to receive second-course pembrolizumab monotherapy for 17 cycles (approximately 1 year) on PD after either completing 35 cycles of pembrolizumab with a best overall response of stable disease (SD) or better or achieving a confirmed complete response (CR) per investigator assessment after receiving eight or more cycles of pembrolizumab and having received two or more cycles beyond the initial CR assessment.
End Points and Statistical Analysis
The dual primary end points were OS and PFS per RECIST version 1.1 by BICR. Secondary end points included objective response rate (ORR) and duration of response (DOR) per RECIST version 1.1 by BICR and safety. PFS2 (time from random assignment to subsequent PD after next line of treatment or death from any cause) was an exploratory end point. After documented PD or the start of new anticancer treatment, patients were followed up for survival once every 12 weeks. Statistical methods have been previously reported.1,2 No alpha was assigned to this analysis.
RESULTS
Patients
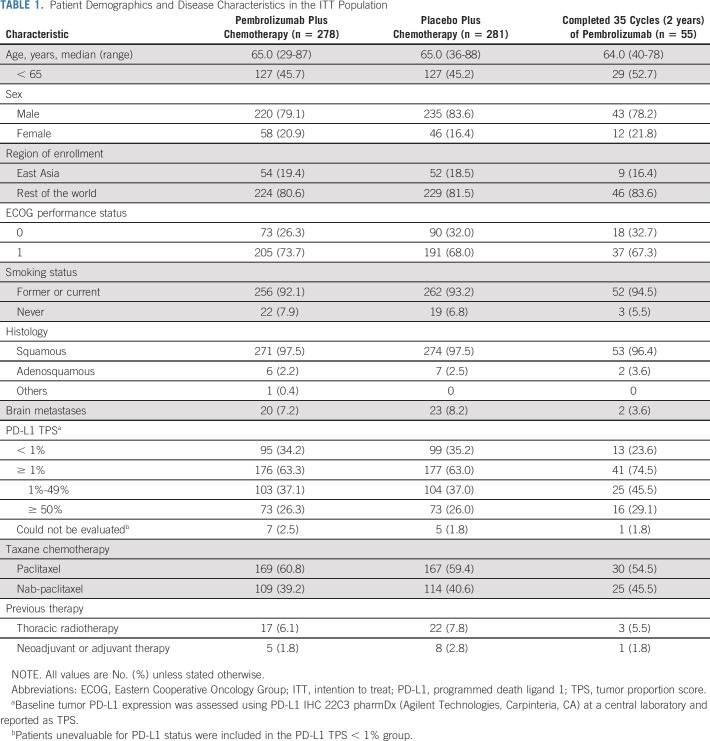
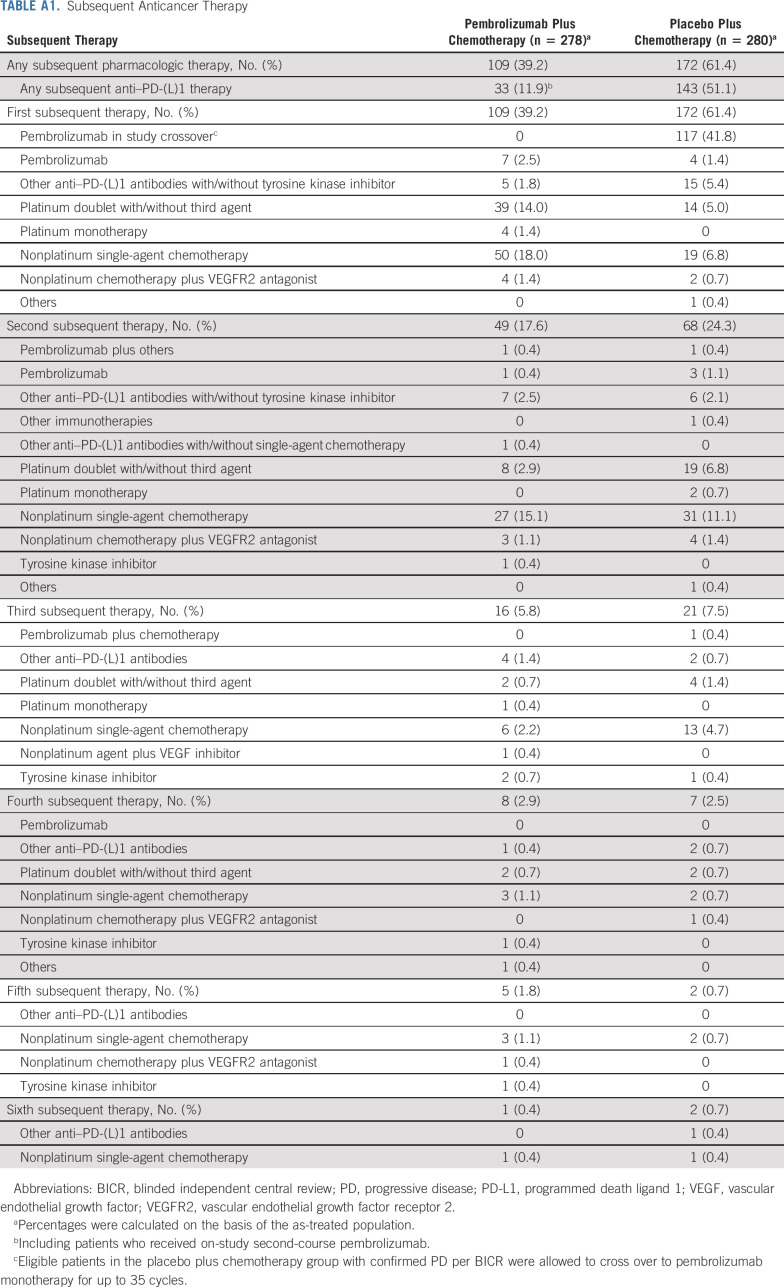
There were 559 patients in the intention-to-treat (ITT) population (pembrolizumab plus chemotherapy, n = 278; placebo plus chemotherapy, n = 281). Baseline characteristics are summarized in Table 1. Subsequent anticancer therapy was received by 109 patients in the pembrolizumab plus chemotherapy group (33 received anti‒PD-[L]1 therapy, including 12 who received on-study second-course pembrolizumab). In the placebo plus chemotherapy group, 172 patients received subsequent therapy. Of these, 117 patients crossed over to pembrolizumab monotherapy on-study and an additional 26 had received subsequent anti‒PD-(L)1 therapy outside the study for an effective crossover rate of 50.9% (Appendix Table A1, online only). Fifty-five patients in the pembrolizumab plus chemotherapy group completed 35 cycles of pembrolizumab, and 12 began a second course of pembrolizumab.
TABLE 1.
Patient Demographics and Disease Characteristics in the ITT Population
Efficacy Outcomes
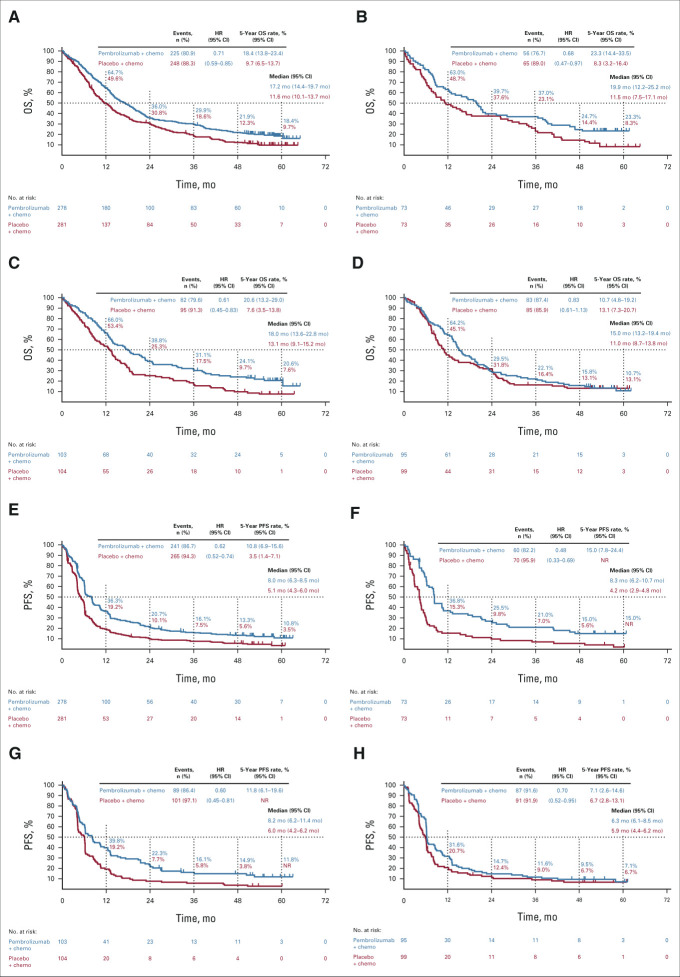
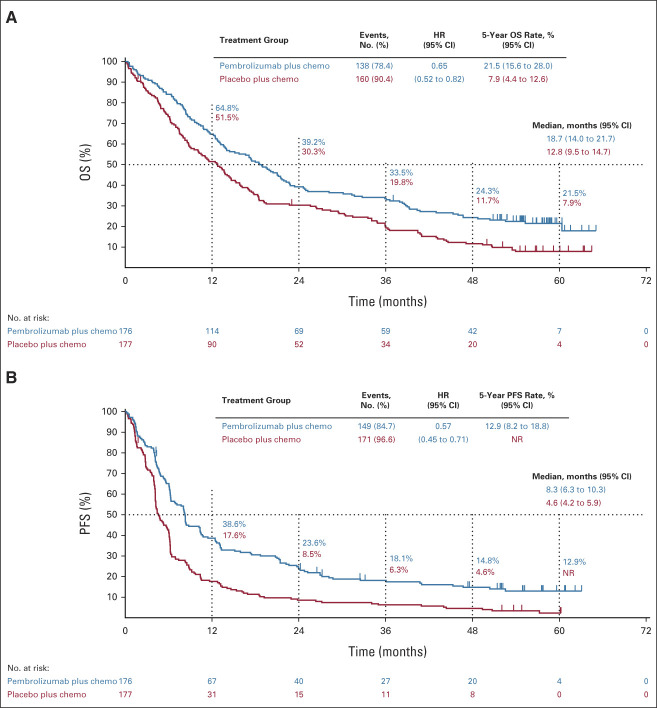
The median time from random assignment to database cutoff (February 23, 2022) was 56.9 (range, 49.9-66.2) months. OS was improved with pembrolizumab plus chemotherapy versus placebo plus chemotherapy (HR, 0.71; 95% CI, 0.59 to 0.85; Fig 1). Estimated 5-year OS rates were 18.4% versus 9.7%. PFS was improved with pembrolizumab plus chemotherapy versus placebo plus chemotherapy (HR, 0.62; 95% CI, 0.52 to 0.74; Fig 1). Estimated 5-year PFS rates were 10.8% versus 3.5%. HRs for OS and PFS favored pembrolizumab plus chemotherapy across all PD-L1 tumor proportion score (TPS) subgroups (Fig 1; Appendix Fig A1, online only).
FIG 1.
Kaplan-Meier estimates of OS and PFS in the (A and E) ITT population, (B and F) patients with PD-L1 TPS ≥ 50%, (C and G) patients with PD-L1 TPS 1%-49%, and (D and H) patients with PD-L1 TPS < 1%. chemo, chemotherapy; HR, hazard ratio; ITT, intention to treat; NR, not reached; OS, overall survival; PD-L1, programmed death ligand 1; PFS, progression-free survival; TPS, tumor proportion score.
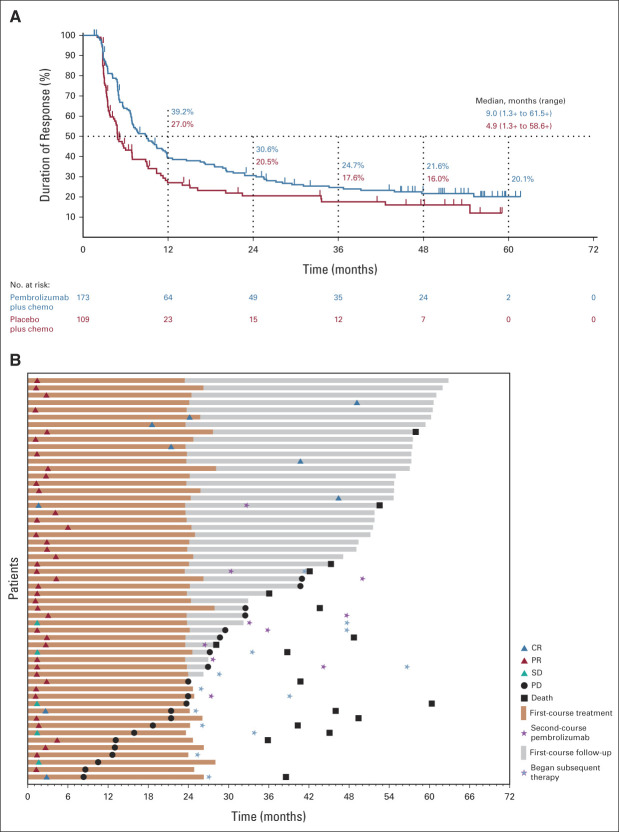
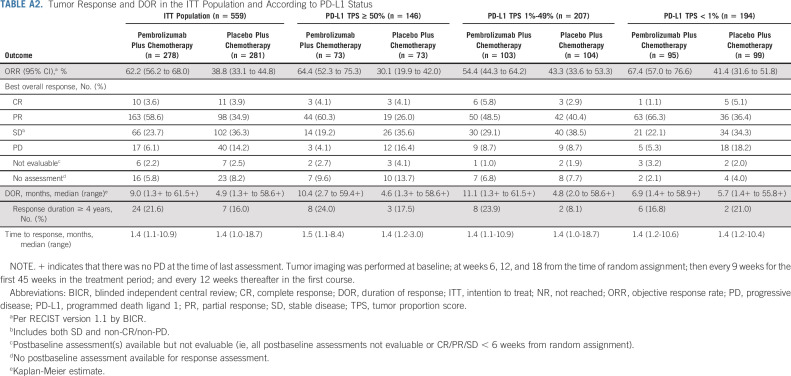
The ORR (95% CI) was 62.2% (56.2 to 68.0) and 38.8% (33.1 to 44.8) with pembrolizumab plus chemotherapy versus placebo plus chemotherapy, respectively (Appendix Table A2, online only). The median (range) DOR was 9.0 (1.3+ to 61.5+) months and 4.9 (1.3+ to 58.6+) months, respectively (Fig 2). A higher ORR and longer DOR were observed in the pembrolizumab plus chemotherapy group in all PD-L1 TPS subgroups (Appendix Table A2).
FIG 2.
Patient response to pembrolizumab plus chemotherapy and placebo plus chemotherapy. (A) DOR in the ITT population and (B) time to response and DOR in patients who completed 35 cycles of pembrolizumab. Median PFS was NR (95% CI, 21.2 months to NR) among patients who completed 35 cycles. The PFS rate 3 years after completion of 35 cycles was 58.4% (95% CI, 39.8 to 73.0). chemo, chemotherapy; CR, complete response; DOR, duration of response; ITT, intention to treat; NR, not reached; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
Median PFS2 was longer for pembrolizumab plus chemotherapy versus placebo plus chemotherapy (HR, 0.60; 95% CI, 0.50 to 0.72). Five-year PFS2 rates were 18.1% (95% CI, 13.6 to 23.1) versus 7.1% (95% CI, 4.4 to 10.7).
Safety
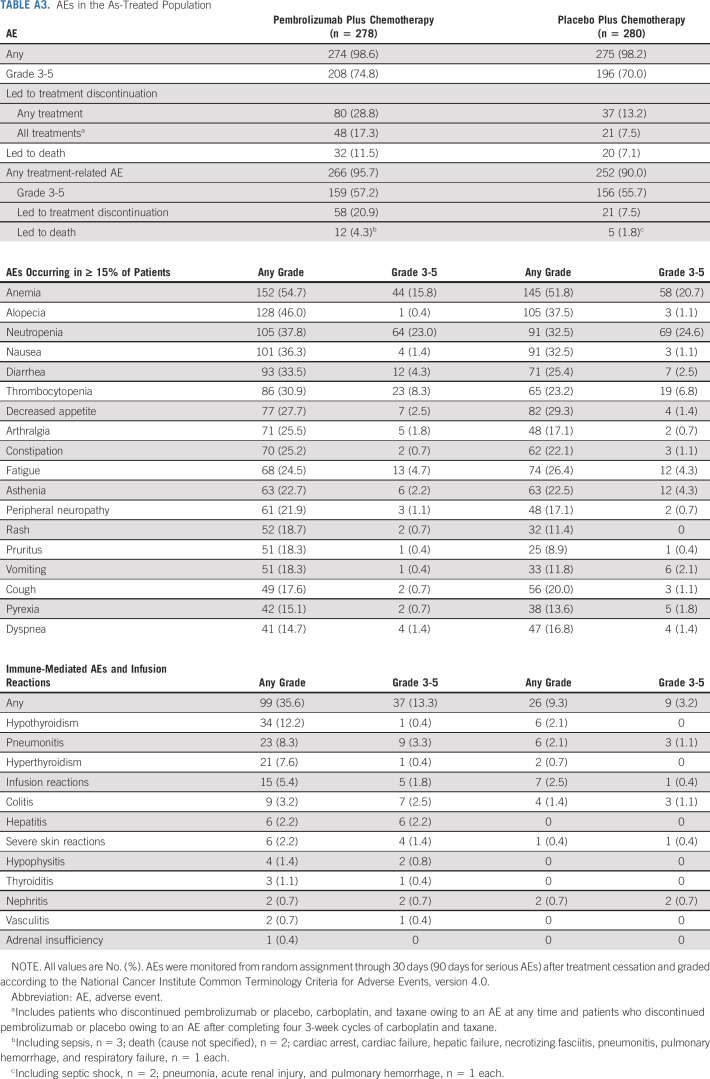
In the as-treated population (pembrolizumab plus chemotherapy, n = 278; placebo plus chemotherapy, n = 280), 98.6% and 98.2% experienced an AE (grade 3-5, 74.8% v 70.0%). No new treatment-related deaths were reported since the protocol-specified final analysis.2 Immune-mediated AEs and infusion reactions occurred in 35.6% (grade 3-5, 13.3%) and 9.3% (grade 3-5, 3.2%) of patients, respectively (Appendix Table A3, online only).
Patients Who Completed 35 Cycles of Pembrolizumab
Among patients randomly assigned to pembrolizumab plus chemotherapy, 55 (19.8%) completed 35 cycles of pembrolizumab. Baseline characteristics are provided in Table 1. The ORR was 90.9%; nine patients (16.4%) had CR, 41 (74.5%) had partial response (PR), and an additional five (9.1%) had SD. Median DOR was not reached (range, 7.1-61.5+ months). At data cutoff, 38 patients (69.1%) were alive and 24 (43.6%) were alive without PD or subsequent therapy (Fig 2). The 3-year OS rate after completion of 35 cycles of pembrolizumab (ie, approximately 5 years after random assignment) was 69.5% (95% CI, 54.8 to 80.2). All 55 patients had an AE (all treatment-related), and 35 (63.6%) had grade 3/4 AEs (no deaths). Immune-mediated AEs and infusion reactions occurred in 21 patients (38.2%; one grade 3 event).
DISCUSSION
In this 5-year analysis of KEYNOTE-407, first-line pembrolizumab plus chemotherapy provided clinically meaningful improvements in OS and PFS compared with placebo plus chemotherapy in patients with metastatic squamous NSCLC, regardless of PD-L1 TPS. Five-year OS rates were approximately doubled with pembrolizumab plus chemotherapy over placebo plus chemotherapy, although there were a limited number of patients at risk at 5 years. Toxicity was manageable and consistent with prior reports from KEYNOTE-407.1,2 These findings are consistent with those reported for the phase III KEYNOTE-189 study of first-line pembrolizumab plus pemetrexed-platinum versus placebo plus pemetrexed-platinum in metastatic nonsquamous NSCLC.3
Sustained improvements in OS were observed despite an effective crossover rate of 50.9% of patients in the placebo plus chemotherapy group to subsequent anti–PD-(L)1 therapy. This high crossover rate likely provides an explanation for the flattening of the Kaplan-Meier curve observed with placebo plus chemotherapy, particularly among patients with PD-L1 TPS ≥ 1%, which was not observed in historical chemotherapy trials before the introduction of immunotherapy.4 This crossover rate and plateauing of the Kaplan-Meier curve in the placebo plus chemotherapy group likely attenuated the differences in the OS HR between treatment groups observed in later analyses compared with the first reported analysis.1 Improvement in PFS2 with pembrolizumab plus chemotherapy versus placebo plus chemotherapy indicated that the benefit of pembrolizumab plus chemotherapy was maintained after initial PD, further supporting first-line use. Notably, the HR for OS favored the pembrolizumab group across subgroups defined by baseline PD-L1 TPS, although the treatment effect was less pronounced among patients with PD-L1 TPS ≤ 1% and the 95% CI contained 1.
Among patients who completed 35 cycles of pembrolizumab, responses were durable, with the majority of patients (69.0%) alive at data cutoff (ie, approximately 5 years after random assignment). These findings provide evidence of long-term benefit in the first-line setting and support the duration of treatment for pembrolizumab (35 cycles [approximately 2 years]); similar benefit was observed among patients with PD-L1‒positive disease receiving pembrolizumab monotherapy.5,6
In conclusion, pembrolizumab plus chemotherapy provided OS and PFS benefit versus placebo plus chemotherapy in patients with previously untreated, metastatic squamous NSCLC. As reported in the final analysis,2 toxicity was manageable. These data support pembrolizumab plus chemotherapy as a standard-of-care first-line treatment option for metastatic squamous NSCLC, regardless of PD-L1 expression.
ACKNOWLEDGMENT
We thank the patients and their families and caregivers for participating in this study, along with all investigators and site personnel. Additional study support was provided by Greg Lubiniecki, MD, and Kumar Rajagopalan, MD, of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ. Medical writing assistance was provided by Christabel Wilson, MSc, of ICON plc (Blue Bell, PA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ.
APPENDIX
TABLE A1.
Subsequent Anticancer Therapy
TABLE A2.
Tumor Response and DOR in the ITT Population and According to PD-L1 Status
TABLE A3.
AEs in the As-Treated Population
FIG A1.
Kaplan-Meier estimates of (A) OS and (B) PFS in patients with PD-L1 TPS ≥ 1%. chemo, chemotherapy; HR, hazard ratio; NR, not reached; OS, overall survival; PD-L1, programmed death ligand 1; PFS, progression-free survival; TPS, tumor proportion score.
Silvia Novello
Consulting or Advisory Role: Sanofi
Speakers' Bureau: AstraZeneca, MSD, Bristol Myers Squibb, Roche, Pfizer, Lilly, Takeda, AbbVie, Boehringer Ingelheim, Bayer, Amgen, BeiGene, Novartis, Janssen
Dariusz M. Kowalski
Consulting or Advisory Role: Bristol Myers Squibb, Boehringer Ingelheim, Merck Serono, Roche/Genentech, AstraZeneca, MSD, Pfizer, Amgen, Johnson & Johnson/Janssen, Takeda
Mahmut Gümüş
Honoraria: MSD Oncology (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Novartis (Inst)
Consulting or Advisory Role: Roche, Lilly (Inst), Amgen (Inst), Gen (Inst), Novartis (Inst), Takeda (Inst), Gilead Sciences (Inst)
Speakers' Bureau: Roche (Inst), MSD Oncology (Inst), Novartis (Inst), Polipharma (Inst), Amgen (Inst)
Research Funding: Amgen (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Pfizer
David Vicente
Honoraria: AstraZeneca
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Roche/Genentech, Pfizer, AstraZeneca, Boehringer Ingelheim, Gilead/Forty Seven
Travel, Accommodations, Expenses: AstraZeneca
Julien Mazières
Consulting or Advisory Role: Novartis, Roche/Genentech, Pfizer, Bristol Myers Squibb, Lilly/ImClone, MSD, AstraZeneca, Pierre Fabre, Blueprint Medicines, Hengrui Therapeutics
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pierre Fabre (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, Bristol Myers Squibb
Jeronimo Rodríguez-Cid
Consulting or Advisory Role: Roche, Bristol Myers Squibb (Mexico), MSD Oncology, Takeda, Bayer
Speakers' Bureau: MSD Oncology, Bristol Myers Squibb (Mexico), Roche, Boehringer Ingelheim, Novartis, Bayer, Lilly, AstraZeneca
Research Funding: MSD, Bristol Myers Squibb (Mexico), Roche, Celltrion, Lilly, BeiGene, AstraZeneca
Travel, Accommodations, Expenses: Roche, MSD Oncology, AstraZeneca, Boehringer Ingelheim
Ki Hyeong Lee
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Pfizer, Lilly
Research Funding: Merck
Shunichi Sugawara
Honoraria: AstraZeneca, Chugai Pharma, Nippon Boehringer Ingelheim, Taiho Pharmaceutical, Pfizer, Lilly, Novartis, Bristol Myers Squibb, Ono Pharmaceutical, MSD K.K, Yakult Honsha, Kyowa Kirin, Towa Pharmaceutical, Takeda, Nippon Kayaku, Otsuka, Merck, Amgen, Thermo Fisher Scientific, AbbVie
Andrew G. Robinson
Honoraria: Merck
Consulting or Advisory Role: AstraZeneca, Merck, Amgen
Research Funding: AstraZeneca (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Roche Canada (Inst)
Balazs Halmos
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Genentech/Roche, Pfizer, Takeda, Novartis, Merck, Bristol Myers Squibb, Turning Point Therapeutics, Apollomics, Janssen Oncology, Veracyte, BeiGene, Arcus Biosciences
Research Funding: Merck (Inst), AstraZeneca (Inst), Mirati Therapeutics (Inst), Boehringer Ingelheim (Inst), Roche/Genentech (Inst), Pfizer (Inst), Takeda (Inst), AbbVie (Inst), Bristol Myers Squibb (Inst), GlaxoSmithKline (Inst), Blueprint Medicines (Inst), Novartis (Inst), Advaxis (Inst), Janssen Oncology (Inst), Elevation Oncology (Inst), Amgen (Inst), BeiGene (Inst), Daiichi Sankyo/Astra Zeneca (Inst)
Erin Jensen
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck Sharp & Dohme LLC
Paul Schwarzenberger
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck Sharp & Dohme LLC
Travel, Accommodations, Expenses: AstraZeneca
M. Catherine Pietanza
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck Sharp & Dohme LLC
Luis Paz-Ares
Leadership: Genomica, ALTUM Sequencing
Honoraria: Roche/Genentech, Lilly, Pfizer, Bristol Myers Squibb, MSD, AstraZeneca, Merck Serono, PharmaMar, Novartis, Amgen, Sanofi, Bayer, Takeda, Mirati Therapeutics, Daiichi Sankyo, Hutchmed, BeiGene, GlaxoSmithKline, Janssen, Medscape, Regeneron
Speakers' Bureau: MSD Oncology, BMS, Roche/Genentech, Pfizer, Lilly, AstraZeneca, Merck Serono
Research Funding: BMS (Inst), AstraZeneca (Inst), PharmaMar (Inst), Kura Oncology (Inst), MSD (Inst), Pfizer (Inst)
Other Relationship: Novartis, Ipsen, Pfizer, SERVIER, Sanofi, Roche, Amgen, Merck, Roche
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at European Society for Medical Oncology, Paris, France, September 9-13, 2022.
SUPPORT
Supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and the European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
AUTHOR CONTRIBUTIONS
Conception and design: David Vicente, Paul Schwarzenberger, M. Catherine Pietanza, Luis Paz-Ares
Provision of study materials or patients: Ying Cheng, Ki Hyeong Lee, Alexander Golf, Shunichi Sugawara, Andrew G. Robinson, Balazs Halmos, Luis Paz-Ares
Collection and assembly of data: Silvia Novello, Dariusz M. Kowalski, Alexander Luft, Mahmut Gümüş, Julien Mazières, Jeronimo Rodríguez-Cid, Ali Tafreshi, Ying Cheng, Ki Hyeong Lee, Alexander Golf, Shunichi Sugawara, Andrew G. Robinson, Balazs Halmos, Paul Schwarzenberger, M. Catherine Pietanza, Luis Paz-Ares
Data analysis and interpretation: Silvia Novello, Dariusz M. Kowalski, Mahmut Gümüş, Julien Mazières, Ki Hyeong Lee, Andrew G. Robinson, Balazs Halmos, Erin Jensen, Paul Schwarzenberger, M. Catherine Pietanza, Luis Paz-Ares
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pembrolizumab Plus Chemotherapy in Squamous Non–Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Silvia Novello
Consulting or Advisory Role: Sanofi
Speakers' Bureau: AstraZeneca, MSD, Bristol Myers Squibb, Roche, Pfizer, Lilly, Takeda, AbbVie, Boehringer Ingelheim, Bayer, Amgen, BeiGene, Novartis, Janssen
Dariusz M. Kowalski
Consulting or Advisory Role: Bristol Myers Squibb, Boehringer Ingelheim, Merck Serono, Roche/Genentech, AstraZeneca, MSD, Pfizer, Amgen, Johnson & Johnson/Janssen, Takeda
Mahmut Gümüş
Honoraria: MSD Oncology (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Novartis (Inst)
Consulting or Advisory Role: Roche, Lilly (Inst), Amgen (Inst), Gen (Inst), Novartis (Inst), Takeda (Inst), Gilead Sciences (Inst)
Speakers' Bureau: Roche (Inst), MSD Oncology (Inst), Novartis (Inst), Polipharma (Inst), Amgen (Inst)
Research Funding: Amgen (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Pfizer
David Vicente
Honoraria: AstraZeneca
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Roche/Genentech, Pfizer, AstraZeneca, Boehringer Ingelheim, Gilead/Forty Seven
Travel, Accommodations, Expenses: AstraZeneca
Julien Mazières
Consulting or Advisory Role: Novartis, Roche/Genentech, Pfizer, Bristol Myers Squibb, Lilly/ImClone, MSD, AstraZeneca, Pierre Fabre, Blueprint Medicines, Hengrui Therapeutics
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pierre Fabre (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, Bristol Myers Squibb
Jeronimo Rodríguez-Cid
Consulting or Advisory Role: Roche, Bristol Myers Squibb (Mexico), MSD Oncology, Takeda, Bayer
Speakers' Bureau: MSD Oncology, Bristol Myers Squibb (Mexico), Roche, Boehringer Ingelheim, Novartis, Bayer, Lilly, AstraZeneca
Research Funding: MSD, Bristol Myers Squibb (Mexico), Roche, Celltrion, Lilly, BeiGene, AstraZeneca
Travel, Accommodations, Expenses: Roche, MSD Oncology, AstraZeneca, Boehringer Ingelheim
Ki Hyeong Lee
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Pfizer, Lilly
Research Funding: Merck
Shunichi Sugawara
Honoraria: AstraZeneca, Chugai Pharma, Nippon Boehringer Ingelheim, Taiho Pharmaceutical, Pfizer, Lilly, Novartis, Bristol Myers Squibb, Ono Pharmaceutical, MSD K.K, Yakult Honsha, Kyowa Kirin, Towa Pharmaceutical, Takeda, Nippon Kayaku, Otsuka, Merck, Amgen, Thermo Fisher Scientific, AbbVie
Andrew G. Robinson
Honoraria: Merck
Consulting or Advisory Role: AstraZeneca, Merck, Amgen
Research Funding: AstraZeneca (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Roche Canada (Inst)
Balazs Halmos
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Genentech/Roche, Pfizer, Takeda, Novartis, Merck, Bristol Myers Squibb, Turning Point Therapeutics, Apollomics, Janssen Oncology, Veracyte, BeiGene, Arcus Biosciences
Research Funding: Merck (Inst), AstraZeneca (Inst), Mirati Therapeutics (Inst), Boehringer Ingelheim (Inst), Roche/Genentech (Inst), Pfizer (Inst), Takeda (Inst), AbbVie (Inst), Bristol Myers Squibb (Inst), GlaxoSmithKline (Inst), Blueprint Medicines (Inst), Novartis (Inst), Advaxis (Inst), Janssen Oncology (Inst), Elevation Oncology (Inst), Amgen (Inst), BeiGene (Inst), Daiichi Sankyo/Astra Zeneca (Inst)
Erin Jensen
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck Sharp & Dohme LLC
Paul Schwarzenberger
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck Sharp & Dohme LLC
Travel, Accommodations, Expenses: AstraZeneca
M. Catherine Pietanza
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck Sharp & Dohme LLC
Luis Paz-Ares
Leadership: Genomica, ALTUM Sequencing
Honoraria: Roche/Genentech, Lilly, Pfizer, Bristol Myers Squibb, MSD, AstraZeneca, Merck Serono, PharmaMar, Novartis, Amgen, Sanofi, Bayer, Takeda, Mirati Therapeutics, Daiichi Sankyo, Hutchmed, BeiGene, GlaxoSmithKline, Janssen, Medscape, Regeneron
Speakers' Bureau: MSD Oncology, BMS, Roche/Genentech, Pfizer, Lilly, AstraZeneca, Merck Serono
Research Funding: BMS (Inst), AstraZeneca (Inst), PharmaMar (Inst), Kura Oncology (Inst), MSD (Inst), Pfizer (Inst)
Other Relationship: Novartis, Ipsen, Pfizer, SERVIER, Sanofi, Roche, Amgen, Merck, Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Paz-Ares L, Luft A, Vicente D, et al. : Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040-2051, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Paz-Ares L, Vicente D, Tafreshi A, et al. : A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: Protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol 15:1657-1669, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Garassino MC, Gadgeel S, Speranza G, et al. : Pembrolizumab plus pemetrexed and platinum in nonsquamous non–small‐cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol 41:1992-1998, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiller JH, Harrington D, Belani CP, et al. : Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92-98, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Reck M, Rodriguez-Abreu D, Robinson AG, et al. : Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol 39:2339-2349, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Castro G, Kudaba I, Wu Y-L, et al. : KEYNOTE-042 5-year survival update: Pembrolizumab versus chemotherapy in patients with previously untreated, PD-L1–positive, locally advanced or metastatic non–small-cell lung cancer. Paper presented at 36th Annual Meeting of the Society for Immunotherapy of Cancer, Washington, DC (virtual), November 10-14, 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and the European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.