PURPOSE
Chronic graft-versus-host disease (cGVHD) remains the major cause of late morbidity after allogeneic hematopoietic cell transplantation. Colony-stimulating factor 1 receptor (CSF-1R)–dependent macrophages promote cGVHD fibrosis, and their elimination in preclinical studies ameliorated cGVHD. Axatilimab is a humanized monoclonal antibody that inhibits CSF-1R signaling and restrains macrophage development.
PATIENTS AND METHODS
This phase I (phI)/phase II (phII) open-label study (ClinicalTrials.gov identifier: NCT03604692) evaluated safety, tolerability, and efficacy of axatilimab in patients age ≥ 6 years with active cGVHD after ≥ 2 prior systemic therapy lines. Primary objectives in phI were to identify the optimal biologic and recommended phII dose and in phII to evaluate the overall (complete and partial) response rate (ORR) at the start of treatment cycle 7.
RESULTS
Forty enrolled patients (17 phI; 23 phII) received at least one axatilimab dose. In phI, a dose of 3 mg/kg given once every 4 weeks met the optimal biologic dose definition. Two dose-limiting toxicities occurred at the 3 mg/kg dose given once every 2 weeks. At least one treatment-related adverse event (TRAE) was observed in 30 patients with grade ≥ 3 TRAEs in eight patients, the majority known on-target effects of CSF-1R inhibition. No cytomegalovirus reactivations occurred. With the 50% ORR at cycle 7 day 1, the phII cohort met the primary efficacy end point. Furthermore, the ORR in the first six cycles, an end point supporting regulatory approvals, was 82%. Responses were seen in all affected organs regardless of prior therapy. Fifty-eight percent of patients reported significant improvement in cGVHD-related symptoms using the Lee Symptom Scale. On-target activity of axatilimab was suggested by the decrease in skin CSF-1R–expressing macrophages.
CONCLUSION
Targeting profibrotic macrophages with axatilimab is a therapeutically promising novel strategy with a favorable safety profile for refractory cGVHD.
BACKGROUND
Chronic graft-versus-host disease (cGVHD) is the most common late complication after allogeneic hematopoietic cell transplantation affecting 30%-70% of recipients.1,2 Multiorgan involvement, irreversible fibrotic manifestations, and systemic toxicities related to immunosuppression use make cGVHD a major cause of late morbidity2,3 and nonrelapse mortality.2,4,5 Fibrotic cGVHD manifestations, including skin sclerosis, joint and fascial involvement, and bronchiolitis obliterans syndrome, affect up to 40% of all patients and impart the greatest morbidity,6-9 with a significant impact on decline in patient functioning and quality of life.10 Systemic glucocorticoids remain the frontline therapy for moderate and severe cGVHD, but the majority of patients with cGVHD require additional treatments, which demonstrate progressively decreasing response rates and increasing cumulative toxicities.11,12 This is particularly true for patients with fibrotic disease in whom clinical responses on the basis of the 2014 NIH consensus criteria are difficult to achieve. Despite the recent approvals of ibrutinib,13 belumosudil,14 and ruxolitinib,15 cGVHD remains an area of unmet need as therapy failures remain common.16
CONTEXT
Key Objective
Chronic graft-versus-host disease (cGVHD) is a major and difficult-to-treat cause of late complications after allogeneic hematopoietic cell transplantation. Colony-stimulating factor 1 receptor–dependent macrophages are important for cGVHD development and worsening. This study examined safety and preliminary efficacy of colony-stimulating factor 1 receptor blockade with the monoclonal antibody axatilimab in patients with advanced cGVHD.
Knowledge Generated
This phase I/II study provided the proof of concept that blocking pathologic macrophage development in cGVHD is safe and can lead to therapeutic benefits in heavily pretreated patients.
Relevance (C.F. Craddock)
-
Axatilimab shows promising efficacy in patients with advanced chronic GVHD, supporting its current evaluation in a randomized prospective trial.*
*Relevance section written by JCO Associate Editor Charles F. Craddock, MD.
Dysregulated inflammation, chronic tissue injury, and impaired remodeling are hallmarks of cGVHD.17-19 During this process, colony-stimulating factor 1 receptor (CSF-1R)–dependent monocytes instruct key aspects of profibrotic (M2) macrophage differentiation, polarization, and function and promote sustained inflammation and tissue injury and accelerated maladaptive tissue repair and fibrosis.20-22 On the basis of the key role for CSF-1R–driven signaling in macrophage biology and preclinical results documenting benefit of CSF-1R targeting in cGVHD models,20 we initiated a phase I (phI)/phase II (phII) study of axatilimab in patients with cGVHD after the failure of at least two prior systemic therapy lines.
Axatilimab is a high-affinity (KD 4-8 pM) humanized IgG4 monoclonal antibody recognizing the ligand-binding domain on CSF-1R, with binding demonstrated to known CSF-1R variants (V32G, A245S, P247H, and V279M). Axatilimab blocks binding of both colony-stimulating factor 1 (CSF-1) and interleukin-34 ligands and potently inhibits ligand-induced monocyte activation (IC50 100-400 pM), without antibody-mediated receptor internalization or activation. In early-phase clinical trials, axatilimab demonstrated preferential elimination of nonclassical monocytes from peripheral blood and a safety profile consistent with its mechanism of action.23
Here, we present the primary analysis of a phI/II study of axatilimab and describe its safety and efficacy in a heavily pretreated patient cohort with recurrent or refractory cGVHD, highlighting the first evidence of promising clinical activity of CSF-1R–targeting in a human fibroproliferative disease.
PATIENTS AND METHODS
Eligible patients were at least 6 years of age, had undergone allogeneic hematopoietic cell transplantation, and had evidence of active cGVHD per the 2014 National Institutes of Health (NIH) consensus criteria.24 Patients must have received at least two prior lines of systemic therapy for cGVHD. Concomitant use of systemic glucocorticoids and/or a calcineurin inhibitor was allowed. Patients with evidence of underlying malignancy relapse or post-transplant lymphoproliferative disease and/or active uncontrolled infection at the time of screening were excluded.
The study sponsor (Syndax Pharmaceuticals, Waltham, MA), in collaboration with subject matter experts, designed the SNDX-6352-0503 (ClinicalTrials.gov identifier: NCT03604692) phI/II open-label, multicenter trial and analyzed the data. The trial was conducted in accordance with the guidelines for Good Clinical Practice of the International Council for Harmonisation, principles of the Declaration of Helsinki, and applicable local regulations. The Protocol (online only) was approved at each participating site by the institutional review board. All patients (or their guardians) provided informed consent. Eligible patients were assigned doses sequentially on the basis of the time at which they enrolled.
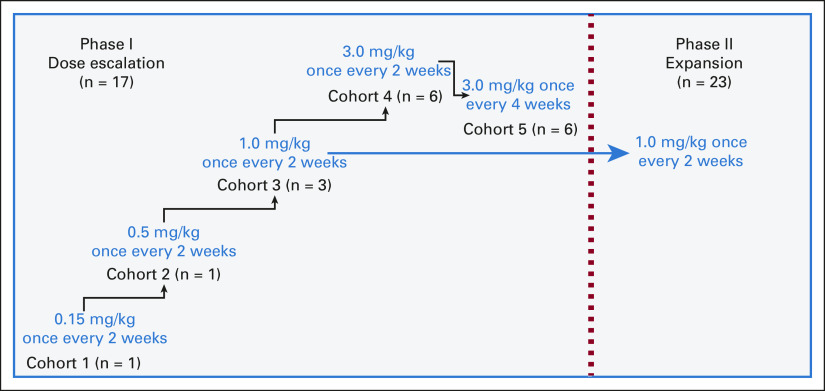
The phI portion followed a dose-escalation design, enrolling patients at doses of 0.15 mg/kg, 0.5 mg/kg, and 1 mg/kg once every two weeks and 3 mg/kg once every two weeks or once every four weeks (Fig 1). Toxicities were graded by investigators according to the common terminology criteria for adverse events version 5.0. The dose-limiting toxicity (DLT) assessment window covered the first 28 days from axatilimab treatment initiation or administration of third dose for once every 2 weeks dosing regimens (cycle 2 day 1), whichever was later. DLT definitions are outlined in the Data Supplement. On the basis of the prior clinical experience with axatilimab in healthy volunteers25 and relapsed/refractory solid tumors,26 if the first patient enrolled into the 0.15 mg/kg or 0.5 mg/kg once every two weeks cohort did not experience a ≥ grade 2 toxicity (non-DLT) during the toxicity assessment window, the next patient would be treated at the subsequent dose level. A 3 + 3 design was used to determine the maximum tolerated dose in cohorts 3, 4, and 5 (1 mg/kg once every 2 weeks, 3 mg/kg once every 2 weeks, and 3 mg/kg once every 4 weeks). Cohort 5 enrolled six patients per the safety review committee (consisting of investigators and the sponsor) recommendation.
FIG 1.
Study schema.
The key objectives for the phI portion were characterization of an optimal biologic dose (OBD), identification of a recommended phII dose (RP2D), evaluation of safety and tolerability, and description of the pharmacokinetic profile of axatilimab in patients with cGVHD. OBD was defined as the lowest safe dose with the highest rate of biologic activity (100% reduction of nonclassical monocytes at the time of dose interval and plateaued increase of circulating CSF-1 levels that persist for an entire dosing level). OBD and RP2D(s) for further evaluation were defined by the safety review committee.
A phII dose-expansion portion was added to the design with version 7 of the protocol, with the decision to expand at the 1 mg/kg once every 2 weeks dose level on the basis of observations from the phI portion to date (clinical benefit in cGVHD and conserved pharmacokinetic/pharmacodynamic effects comparable with those observed in previous studies using this dose25,26). The primary objective for the phII portion was evaluation of the cGVHD overall response rate (ORR) at cycle 7 day 1 (complete and partial response, per the 2014 NIH consensus criteria27) with axatilimab. Secondary objectives included assessment of patient-reported outcomes using the Lee Symptom Scale28 and further characterization of efficacy and safety/tolerability. In both phases, treatment could continue if there was ongoing evidence of benefit without progressive disease requiring additional therapy and/or unacceptable toxicity.
For the phI portion, statistical analysis was descriptive without formal hypothesis testing. In the phII, the sample size was calculated by hypothesizing a true ORR of 60%. With a 1-sided α = .05, 22 patients provided > 90% power to test the null hypothesis of 30% ORR. The data were summarized using descriptive statistics, including mean, standard deviation, median, and range for continuous variables and frequency and percentage for discrete variables. The Kaplan-Meier method was used to analyze the failure-free survival (FFS).
Detailed descriptions of correlative analysis methods are outlined in the Data Supplement. All investigators contributed to the development of this manuscript and approved its submission.
RESULTS
Patients
From February 2019 to April 2021, 40 patients were enrolled at 10 study sites and had received at least one dose of axatilimab by the time of the data cutoff (October 22, 2021). Seventeen patients were enrolled in the phI portion (once every 2 weeks: 0.15 mg/kg [n = 1]; 0.5 mg/kg [n = 1]; 1 mg/kg [n = 3]; 3 mg/kg [n = 6]; and once every 4 weeks: 3 mg/kg [n = 6]). All 23 patients in the phII received 1 mg/kg of axatilimab once every 2 weeks. No significant differences in baseline characteristics were noted when comparing phI and phII patients (Table 1). Before study entry, patients had received a median of four prior lines of treatment, including ibrutinib (n = 26), ruxolitinib (n = 21), and belumosudil (n = 8). At baseline, patients had a median of four organ systems affected by cGVHD (range, 1-9). At the time of the data cutoff, 17 patients (5 phI; 12 phII) remain on study treatment (Data Supplement).
TABLE 1.
Baseline Patient Demographics and Clinical Characteristics
Safety
In the phI cohort, two DLTs were reported, both at the 3 mg/kg once every 2 weeks dose level. One patient with pre-existing myositis and grade 2 creatinine phosphokinase (CPK) elevation at baseline developed a grade 4 CPK increase with evidence of inflammatory myopathy. Another patient required a 2-week treatment delay because of a grade 3 lipase elevation without evidence of pancreatitis arising during the DLT assessment period. Overall, 39 patients (98%) experienced at least one treatment-emergent adverse event across phI and phII (Table 2) with most events related either to cGVHD or the on-target effect of CSF-1R inhibition (Table 3). Treatment-related adverse events (TRAEs) occurred in 75% (n = 30 of 40) of patients, with 20% (n = 8 of 40) of patients experiencing grade ≥ 3 TRAEs. Serious adverse events were noted in 40% (n = 16) of patients, with seven patients discontinuing the study intervention because of adverse events, four of which were deemed treatment-related (Table 2). The only death that occurred on study was unrelated to the study intervention and was the result of a fall.
TABLE 2.
Safety End Point Results
TABLE 3.
Adverse Events Related to On-Target Activity of the CSF-1R Blockade
Observed transient elevations of serum enzymes (AST, ALT, CPK, amylase, and lipase) were consistent with the described effect of CSF-1R inhibition of Kupffer cell–mediated enzymatic clearance29,30 and were not accompanied by other evidence of end-organ damage except in a single patient with a history of myositis described above. Reversible periorbital edema, a class effect of CSF-1R targeting related to macrophage depletion,31 was largely mild and infrequent although more common in patients receiving axatilimab at 3 mg/kg, regardless of dosing interval. No ≥ grade 3 on-target toxicities of CSF-1R blockade were seen in the phII cohort (Table 3). Serial neurologic examination monitoring showed no clinically significant changes from baseline in all patients. Finally, axatilimab had a negligible impact on hematopoietic reserve, with neutropenia (grade 2) observed in only one and thrombocytopenia in two patients (grades 1 and 3), respectively.
No relapse of primary hematologic malignancy was seen on the study. Infections were reported in 19 patients across all dose cohorts (48%; Data Supplement). While on study, none of the patients developed an invasive fungal infection and no CMV reactivations or other systemic viral infections were observed. The observed infection rates and their profiles are similar to or lower than those reported in the cGVHD patient population treated on recent clinical trials.13-15 Thus, these findings further support a favorable safety profile of axatilimab.
Efficacy
Thirty-nine patients were evaluable for response across phI and phII (one patient in phII withdrew from study because of reasons unrelated to axatilimab tolerance and before a postbaseline assessment). In phI, the dose of 3 mg/kg given once every 4 weeks demonstrated the highest rate of biologic activity and was considered the optimal biologic dose per protocol.
The primary efficacy end point in the phII cohort, ORR at cycle 7 day 1, was 50% (n = 11 of 22; 90% CI, 31 to 69; Fig 2A). The ORR by cycle 7 day 1, an end point consistent with the contemporary end points supporting regulatory approvals in cGVHD,14,15 was 82% (n = 18 of 22; 95% CI, 60 to 95) in the phII cohort and 67% (n = 26 of 39; 95% CI, 50 to 81) among all evaluable patients on study (Fig 2B). The best ORR observed at any point during the study was 69% (n = 27 of 39; 95% CI, 52 to 83), was similar in patients previously treated with ibrutinib (58%; n = 15 of 26), ruxolitinib (65%; n = 13 of 20), and belumosudil (50%; n = 4 of 8; Data Supplement), and did not differ between patients with moderate (83%; n = 5 of 6) and severe cGVHD (67%; n = 22 of 33; Data Supplement). Chronic GVHD progression led to discontinuation of the study medication in seven patients (Data supplement), six of whom discontinued axatilimab within the first six cycles.
FIG 2.
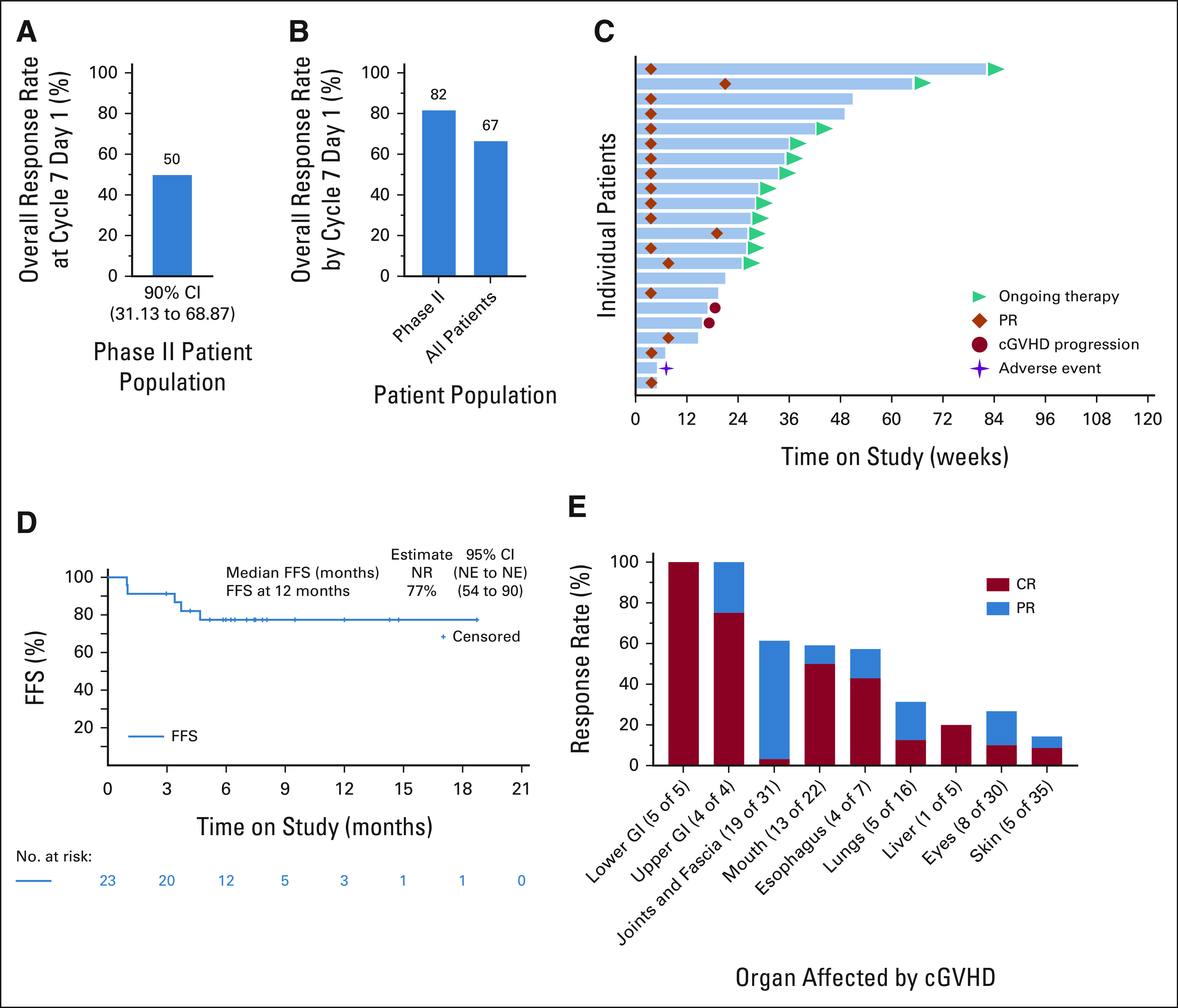
Clinical efficacy of axatilimab. (A and B) Axatilimab treatment leads to responses in a heavily pretreated patient population. Bar graphs show (A) overall response rate at cycle 7 day 1 (primary end point of the phII study portion) in the phII patient cohort and (B) overall response rate by cycle 7 day 1 (regulatory approval end point) for both phII patients and all patients combined. (C) The swimmer lane plot bars show individual patients treated in the phII cohort and temporal relationship of their outcomes to the treatment initiation and response durability. Teal triangles indicate patients who continue on the study, orange diamonds indicate PR, red circles indicate cGVHD progression, and purple stars show adverse events leading to treatment discontinuation. (D) FFS (defined as time from first dose of axatilimab to unequivocal progression of cGVHD, addition of another systemic immunosuppression, discontinuation of axatilimab because of toxicity, relapse of underlying malignancy, or death for any reason) in patients with recurrent or refractory active cGVHD. (E) Axatilimab induces responses in all cGVHD-involved organs. Bars show cumulative response rate on the basis of observations at any point on study and highlight PR and CRs. Lung responses are based on %FEV1 if test results were available (pulmonary function testing was not mandated by the study protocol; 1 of 5 responders) and/or symptom score improvement. cGVHD, chronic graft-versus-host disease; CR, complete response; FFS, failure-free survival; NE, not evaluable; NR, not reached; PR, partial response.
Median times to response of 4 weeks (range, 4-20 weeks; phII cohort, Fig 2C) and 5 weeks (range, 4-48 weeks, phI cohort; Data Supplement) were noted. In all responding patients, the median duration of axatilimab exposure was 38 weeks (range, 6-116 weeks, 16 patients ongoing). In all study participants, the median duration of axatilimab use was 29 weeks (range, 2-116 weeks, 17 patients ongoing). The overall FFS rate (using a broadened failure definition that incorporates toxicity-related discontinuation and cGVHD progression not included in the standard cGVHD FFS reporting11,32) at 12 months was 77% (95% CI, 54 to 90; Fig 2D) for the phII cohort and 68% (95% CI, 51 to 81; data not shown) for all patients. Median duration of response for phII responding patients was not reached (95% CI, 2.79 months to not evaluable; Data Supplement), with 33% of patients experiencing sustained response lasting ≥ 20 weeks. Among all treated patients, responses were noted in all involved organs with the best response commonly the initial partial response. The joints and fascia response rate was 61% (n = 19 of 31; 53% in the phII cohort assessed per the refined NIH algorithm33; Data Supplement), lung 31% (n = 5 of 16) and skin response was seen in 14% of all patients (n = 5 of 35; Fig 2E), including four patients with sclerosis improvement (90% of patients had sclerotic disease; n = 31 of 35). Improvement in the investigator-reported severity of skin tightening and/or improvement in skin-tightening symptoms recorded in the Lee Symptom Scale was recorded in 84% (n = 26 of 31) of patients with sclerotic skin cGVHD, suggesting a measurable benefit in this difficult-to-treat manifestation.
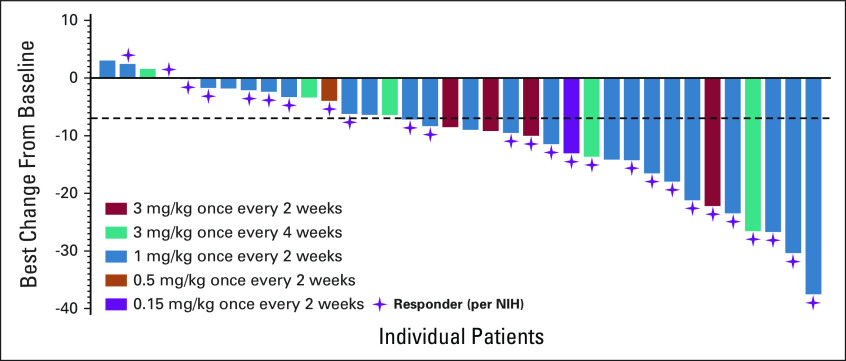
Among responders, the dose decrease in glucocorticoids was observed in 52% of patients (11 of 21 patients with glucocorticoid use at baseline), with a mean dose reduction of 22% (from 0.23 mg/kg once daily to 0.18 mg/kg once daily of prednisone dose equivalent). Finally, a clinically meaningful improvement in the summary Lee Symptom Scale of least 7 points was seen in 58% (n = 21 of 36) of all evaluable patients (four patients did not complete either baseline or at least one postbaseline assessment; Fig 3).
FIG 3.
Improvement in cGVHD symptoms with axatilimab treatment. Clinical axatilimab responses are accompanied by a reduction in cGVHD symptom burden. The waterfall plot shows the best change in cGVHD symptoms from baseline in all evaluable patients treated on study, as measured using the summary score of the Lee Symptom Scale. Bars depict individual patients and purple stars indicate patients who achieved clinical response per the NIH 2014 consensus criteria. The dashed line indicates a 7-point decrease threshold. cGVHD, chronic graft-versus-host disease; NIH, National Institutes of Health.
Correlative Studies
To assess the impact of axatilimab on biomarkers of the CSF-1R blockade, correlative studies were conducted on collected samples. Patients were included in the analyses based solely on the sample availability. The on-target effect of the CSF-1R blockade with axatilimab was seen in reduction of nonclassical, but not in classical and intermediate monocyte levels in peripheral blood (Fig 4A), with a parallel increase in plasma CSF-1 and interleukin-34 levels (not shown). Reduction in the skin density of CSF-1R+ macrophages was observed in analysis of four paired skin biopsies (Fig 4B). Three of the four patients analyzed had baseline cGVHD skin involvement, and all showed improvements in both total and skin-specific patient-reported outcomes. Analysis of plasma biomarkers centered on concentrations of cytokines, chemokines, and growth factors associated with profibrotic macrophage homeostasis. Among analyzed patients, we observed a rapid and significant decrease in transforming growth factor-β in the responding patients, with no change noted in tumor necrosis factor-α or interleukin-6, commonly associated with classical macrophages (Fig 4C).
FIG 4.
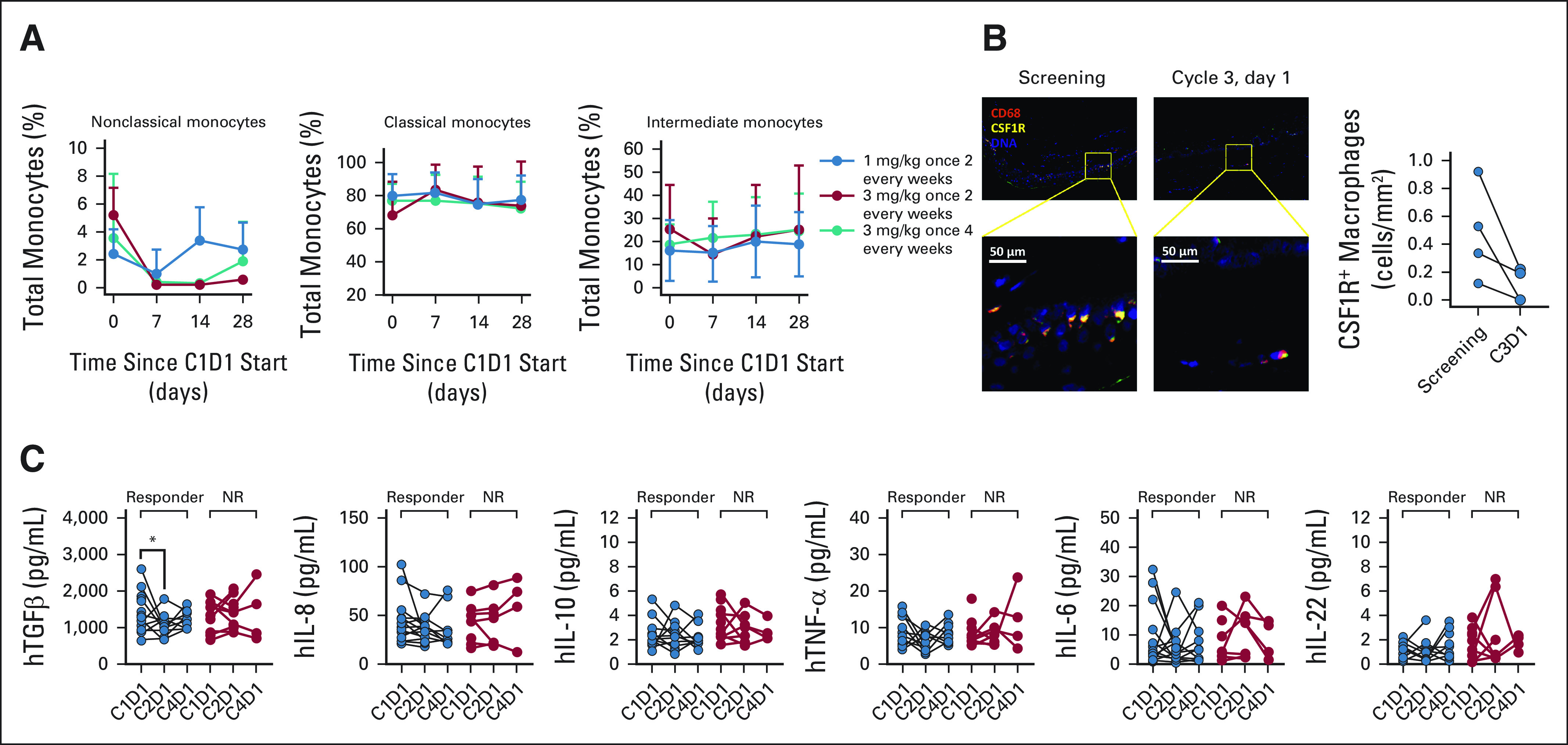
Correlative studies highlight potential response and pharmacodynamic biomarkers of axatilimab. (A) Axatilimab therapy modulates the monocyte profile in peripheral blood by reducing prevalence of nonclassical CD14+CD16++ monocytes. Cumulative data show changes in monocyte subsets in peripheral blood of patients treated with axatilimab doses of 1 mg/kg (blue circles) and 3 mg/kg (red circles) once every 2 weeks and 3 mg/kg once every 4 weeks (teal circles) during the first cycle of therapy. (B) Axatilimab reduces skin macrophage density in patients with cGVHD. Multiplex immunohistochemistry analysis of skin macrophage density documents a decrease in skin CSF-1R+ macrophage infiltration after two cycles of therapy. Representative pseudocolored images and cumulative data from analysis of four paired samples are shown. (C) Response to axatilimab is associated with a rapid decrease in key profibrotic macrophage cytokines. Plasma collected at baseline and day 1 of cycles 2 and 4 was analyzed for levels of cytokines involved in monocyte and macrophage homeostasis (TGF-β, IL-8, IL-10, TNF-α, IL-6, and IL-22), and paired samples are shown. *P = .03 (CI, 60 to 803 pg/mL). C1D1, cycle 1 day 1; C2D1, cycle 2 day 1; C3D1, cycle 3 day 1; C4D1, cycle 4 day 1; cGVHD, chronic graft-versus-host disease; CSF-1R, colony-stimulating factor 1 receptor; IL, interleukin; NR, nonresponder; TGF, transforming growth factor; TNF, tumor necrosis factor.
DISCUSSION
In this phI/II study, we document safety and promising efficacy of axatilimab in a heavily pretreated patient population and highlight CSF-1R targeting as a novel approach for cGVHD control. Forty patients were enrolled and received at least one dose of axatilimab. Their baseline characteristics were reflective of advanced cGVHD, including multiorgan fibrotic disease. Patients received a median of four prior treatment lines, including the use of at least one of the Food and Drug Administration recently approved agents in the majority of the study population. Aside from the two DLTs noted in the 3 mg/kg once every 2 weeks dose cohort, axatilimab was well-tolerated across all other dose levels. Importantly, likely owing to the negligible impact on classical monocytes and lack of myelosuppression, observed infection rates were lower than those seen in contemporary reports.13-15 In this population prone to toxicities, TRAEs led to discontinuation in only three (8%) patients. TRAEs were largely predictable on the basis of the on-target effects of the CSF-1R blockade. Serum elevations of AST, ALT, and CPK reflected decreased clearance because of Kupffer cell impact29,30 and were not accompanied by symptomatic organ dysfunction except in a single patient with pre-existing myositis.
In the phII cohort, axatilimab demonstrated a high response rate (50% ORR at cycle 7 day 1), meeting the primary efficacy end point. Furthermore, the observed ORR was even higher (82%) when assessed by the ORR by cycle 7 day 1, the regulatory end point used in recent approvals.14,15 The observed efficacy rates may be even more noteworthy considering that the only allowed concomitant agents were glucocorticoids and one calcineurin inhibitor, and the cohort included refractory patients after failure of a median of four prior lines of systemic therapy. The activity seen in fibrotic manifestations, short median time to response, durability of response, and the observation of clinically significant improvement in patient-reported outcomes in 58% of patients further support the meaningful clinical benefits of axatilimab and the potential for CSF-1R targeting in this disease. The small size of our study is the major limitation to the broader inference on axatilimab efficacy, which will be addressed in the ongoing AGAVE-201 study. Furthermore, study design with follow-up and data collection ending at 90 days after the end of treatment per protocol and a relatively short on-treatment follow-up duration may limit accurate appraisal of long-term axatilimab effects, including duration of sustained cGVHD response, potential for its deepening, and impact on FFS.
In correlative studies, documentation of an early decrease in plasma transforming growth factor-β concentrations and tissue CSF-1R+ macrophages in responding patients highlights possible response and pharmacodynamic biomarkers in cGVHD consistent with the CSF-1R blockade. However, limited numbers of analyzed patients warrant further studies in the ongoing AGAVE-201 clinical trial.
Despite significant changes in the cGVHD therapeutic landscape in recent years with the approval of ibrutinib, belumosudil, and ruxolitinib, challenges to successful cGVHD management persist. Nowhere is that more pronounced than in the cGVHD phenotypes characterized by significant fibrosis, such as joints and fascia and bronchiolitis obliterans, in which complete responses are rare (< 15%14,15) and the need for prolonged treatment commonly adds to the disease burden. CSF-1R targeting was initially proposed as a strategy to enhance anticancer benefits of chemoimmunotherapy through elimination of CSF-1R–dependent and immunosuppressive tumor-associated macrophages.29 However, outside of tenosynovial giant cell tumor, which is uniquely accompanied by a genetic alteration in CSF1 expression, studies have largely failed to demonstrate a measurable clinical benefit for oncology indications. CSF-1R–driven macrophage signaling, however, plays a critical role in a host of human diseases and is essential in fibroproliferative conditions, which may contribute to as many as 45% of all deaths in the United States.34 In cGVHD, CSF-1R–dependent donor-derived macrophages are essential disease mediators, with an increasing amount of evidence suggesting their bidirectional role in enhancing extracellular matrix responses and collagen deposition, while sustaining dysregulated adaptive alloimmunity.20,22,35 The latter may partly explain the observed short time to response seen in this study, where inflammatory modulation may herald antifibrotic effects seen in the improvements in joints and fascia scores and reports of skin-tightening benefits by patients and clinicians.
Our study provides the proof of concept of CSF-1R targeting in cGVHD, and observation of tolerability and clinical benefit lends further credence to development in this disease. Furthermore, because axatilimab is an antibody, minimal drug-drug interactions may facilitate the potential for future combinatorial approaches to target nonoverlapping pathways and provide therapeutic synergy with limited toxicity. The ongoing registrational clinical trial AGAVE-201 (ClinicalTrials.gov identifier: NCT04710576) will further test axatilimab efficacy in advanced cGVHD in which the need for more efficacious approaches persists despite recent therapeutic progress.
ACKNOWLEDGMENT
The authors acknowledge all the patients who participated in the study, Dr Briggs Morrison for contributions to the conceptualization of the study, and technical support from Ms Nell Kirchenberger, Ms Konjit Betre, and Ms Shamilene Sivagnanam for the correlative analyses. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Carrie L. Kitko
Consulting or Advisory Role: Horizon Therapeutics
Travel, Accommodations, Expenses: Mallinckrodt
Mukta Arora
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Research Funding: Syndax (Inst), Kadmon (Inst), Pharmacyclics (Inst)
Zachariah DeFilipp
Consulting or Advisory Role: Kadmon, Omeros, Incyte, MorphoSys
Research Funding: Incyte, REGiMMUNE, Taiho Oncology
Mohammad Abu Zaid
Stock and Other Ownership Interests: Pieris Pharmaceuticals
Consulting or Advisory Role: Syndax, Ossium Health
Research Funding: Syndax (Inst), Pharmacyclics (Inst), Janssen (Inst), Incyte (Inst), AlloVir (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1350343
Vedran Radojcic
Employment: Syndax Pharmaceuticals, Inc
Stock and Other Ownership Interests: Syndax Pharmaceuticals, Inc
Consulting or Advisory Role: Regeneron, Allakos
Open Payments Link: https://openpaymentsdata.cms.gov/physician/114735
Courtney B. Betts
Other Relationship: Akoya Biosciences
Lisa M. Coussens
Employment: Oregon Health & Science University (OHSU)
Honoraria: Lustgarten Foundation for Pancreatic Cancer Research, Syndax, Carisma Therapeutics, Verseau Therapeutics, Inc, Scientific Advisory Board, Zymeworks, CytomX Therapeutics, Inc, Kineta Inc, Starr Cancer Consortium, Therapeutics Working Group:, Susan G Komen Foundation, Komen Scholar, AACR: Cancer Immunology Research
Consulting or Advisory Role: Cell Signaling Technologies, Pharmacyclics, CytomX Therapeutics, Carisma Therapeutics, Verseau Therapeutics, Zymeworks, Kineta, Inc, AbbVie, Shasqi Inc, HiberCell, Alkermes, AstraZeneca Partner of Choice Network, OHSU site leader:, Genenta Science, Susan G Komen Foundation, Komen Scholar, Syndax, PDX Pharmaceuticals, Inc, Pio Therapeutics Pty Ltd
Research Funding: Syndax, Pharmacyclics, Cell Signaling Technologies, Innate Pharma, Acerta Pharma (Inst), Susan G. Komen for the Cure (Inst), ZellBio, Inc, HiberCell
Other Relationship: Prospect Creek Foundation, Lustgarten Foundation for Pancreatic Cancer Research, (P30) Koch Institute for Integrated Cancer Research, Massachusetts Institute of Technology (2012-present; honorarium), (P30) Salk Institute Cancer Center (2016-2020; honorarium), Bloomberg-Kimmel Institute for Cancer Immunotherapy, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (2016-present; honorarium), Dana Farber Cancer Center Breast SPORE (2017-present; honorarium), (P30) Dana Farber/Harvard Cancer Center (2019-present; honorarium), (P30) University of California, San Diego Moores Cancer Center (2019-present; honorarium), Cancer Research Institute (CRI; 2013-present; unpaid), The V Foundation for Cancer Research (2013-present; unpaid), Starr Cancer Consortium (2011-present, honorarium), Therapeutics Working Group (2019-present; paid), NIH/NCI-Frederick National Laboratory Advisory Committee (FNLAC; 2016-present; daily honorarium), Susan G Komen Foundation, Komen Scholar (2020-2023; honorarium), (P50) Dana Farber Cancer Center Breast SPORE, (P30) The Jackson Laboratory Cancer Center, (P01) Columbia University Medical Center, Prostate P01, (P50) MDACC GI SPORE, American Association for Cancer Research, AACR: Cancer Discovery, Cancer Cell, National Foundation for Cancer Research
Michael L. Meyers
Employment: Syndax
Leadership: Syndax
Stock and Other Ownership Interests: Syndax, Nuvalent, Inc
Consulting or Advisory Role: Syndax, Nuvalent, Inc
Patents, Royalties, Other Intellectual Property: Syndax, Nuvalent, Inc
Peter Ordentlich
Employment: Syndax
Leadership: Syndax
Stock and Other Ownership Interests: Syndax
Consulting or Advisory Role: Patrys, Twentyeight-Seven Therapeutics
Patents, Royalties, Other Intellectual Property: Issued patents and patents pending
Christine Quaranto
Employment: Syndax, Aerovate Therapeutics
Stock and Other Ownership Interests: Syndax Pharmaceuticals, Aerovate Therapeutics
Aaron Schmitt
Employment: Syndax Pharmaceuticals Inc
Stock and Other Ownership Interests: Syndax
Yu Gu
Employment: Syndax, AstraZeneca
Stock and Other Ownership Interests: Syndax, AstraZeneca
Bruce R. Blazar
Stock and Other Ownership Interests: BlueRock Therapeutics, Tmunity Therapeutics, Inc, Magenta Therapeutics
Consulting or Advisory Role: BlueRock Therapeutics, Magenta Therapeutics, Obsidian Therapeutics, Editas Medicine
Research Funding: BlueRock Therapeutics, Rheos Medicines, Équilibre Biopharmaceuticals Corp, Carisma Therapeutics
Patents, Royalties, Other Intellectual Property: Inducible regulatory T-cell generation for hematopoietic cell transplants (UMN Z09026), US 9,228,172, TALEN-based gene correction, Patent No. 9,393,257, Generation of natural killer cells and lymphoid tissue inducer–like (LTI-like) NK-22 cells, 9,862,928, Method for correcting a genetic sequence, 10,648,002
Travel, Accommodations, Expenses: Incyte, Magenta Therapeutics, Rheos Medicines
Amandeep Salhotra
Consulting or Advisory Role: Kadmon, Syros Pharmaceuticals, Sobi
Research Funding: Bristol Myers Squibb
Iskra Pusic
Consulting or Advisory Role: Kadmon, Incyte, Syndax
Madan Jagasia
Employment: Iovance Biotherapeutics
Stock and Other Ownership Interests: Iovance Biotherapeutics
Consulting or Advisory Role: Kadmon
Stephanie J. Lee
Honoraria: Wolters Kluwer, PER
Consulting or Advisory Role: EMD Serono, Pfizer, 4SC, Mallinckrodt/Therakos, Almirall Hermal GmbH, Rain Therapeutics, Kadmon, Equillium
Research Funding: Kadmon, Amgen, Bristol Myers Squibb, EMD Serono, Incyte, Syndax, Pfizer, AstraZeneca
Patents, Royalties, Other Intellectual Property: Patent pending for high-affinity T-cell receptors that target the Merkel polyomavirus
Other Relationship: National Marrow Donor Program, Society for Investigative Dermatology (SID)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the American Society of Hematology 2020 (virtual, December 6, 2020) and 2021 (Atlanta, GA, December 11, 2021) Annual Meetings; and at the 2021 Transplantation & Cellular Therapy Meeting, Salt Lake City, UT, April 23, 2022.
SUPPORT
Supported by Syndax Pharmaceuticals, Inc. Individual investigators were also supported by the funding from the National Institutes of Health (B.R.B.: P01CA142106, P01AI056299, and R37AI34495; V.R.: K08HL145116; L.M.C.: U01CA224012 and U2CCA233280), the Knight Cancer Institute, and the Brenden-Colson Center for Pancreatic Care at Oregon Health & Science University (L.M.C.). T.P.W. is a K12 Scholar supported by the National Cancer Institute of the National Institutes of Health under Award No. K12CA226330.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Provision of study materials or patients: Carrie L. Kitko, Mukta Arora, Zachariah DeFilipp, Mohammad Abu Zaid, Antonio Di Stasi, Vedran Radojcic, Trent P. Wang, Amandeep Salhotra, Iskra Pusic, Madan Jagasia, Stephanie J. Lee
Collection and assembly of data: Carrie L. Kitko, Mukta Arora, Zachariah DeFilipp, Mohammad Abu Zaid, Antonio Di Stasi, Vedran Radojcic, Courtney B. Betts, Lisa M. Coussens, Michael L. Meyers, Hope Qamoos, Peter Ordentlich, Vinit Kumar, Christine Quaranto, Aaron Schmitt, Trent P. Wang, Amandeep Salhotra, Iskra Pusic, Madan Jagasia, Stephanie J. Lee
Data analysis and interpretation: Carrie L. Kitko, Mukta Arora, Zachariah DeFilipp, Mohammad Abu Zaid, Antonio Di Stasi, Vedran Radojcic, Courtney B. Betts, Lisa M. Coussens, Michael L. Meyers, Hope Qamoos, Peter Ordentlich, Vinit Kumar, Christine Quaranto, Yu Gu, Trent P. Wang, Amandeep Salhotra, Iskra Pusic, Madan Jagasia, Stephanie J. Lee
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Axatilimab for Chronic Graft-Versus-Host Disease After Failure of at Least Two Prior Systemic Therapies: Results of a Phase I/II Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Carrie L. Kitko
Consulting or Advisory Role: Horizon Therapeutics
Travel, Accommodations, Expenses: Mallinckrodt
Mukta Arora
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Research Funding: Syndax (Inst), Kadmon (Inst), Pharmacyclics (Inst)
Zachariah DeFilipp
Consulting or Advisory Role: Kadmon, Omeros, Incyte, MorphoSys
Research Funding: Incyte, REGiMMUNE, Taiho Oncology
Mohammad Abu Zaid
Stock and Other Ownership Interests: Pieris Pharmaceuticals
Consulting or Advisory Role: Syndax, Ossium Health
Research Funding: Syndax (Inst), Pharmacyclics (Inst), Janssen (Inst), Incyte (Inst), AlloVir (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1350343
Vedran Radojcic
Employment: Syndax Pharmaceuticals, Inc
Stock and Other Ownership Interests: Syndax Pharmaceuticals, Inc
Consulting or Advisory Role: Regeneron, Allakos
Open Payments Link: https://openpaymentsdata.cms.gov/physician/114735
Courtney B. Betts
Other Relationship: Akoya Biosciences
Lisa M. Coussens
Employment: Oregon Health & Science University (OHSU)
Honoraria: Lustgarten Foundation for Pancreatic Cancer Research, Syndax, Carisma Therapeutics, Verseau Therapeutics, Inc, Scientific Advisory Board, Zymeworks, CytomX Therapeutics, Inc, Kineta Inc, Starr Cancer Consortium, Therapeutics Working Group:, Susan G Komen Foundation, Komen Scholar, AACR: Cancer Immunology Research
Consulting or Advisory Role: Cell Signaling Technologies, Pharmacyclics, CytomX Therapeutics, Carisma Therapeutics, Verseau Therapeutics, Zymeworks, Kineta, Inc, AbbVie, Shasqi Inc, HiberCell, Alkermes, AstraZeneca Partner of Choice Network, OHSU site leader:, Genenta Science, Susan G Komen Foundation, Komen Scholar, Syndax, PDX Pharmaceuticals, Inc, Pio Therapeutics Pty Ltd
Research Funding: Syndax, Pharmacyclics, Cell Signaling Technologies, Innate Pharma, Acerta Pharma (Inst), Susan G. Komen for the Cure (Inst), ZellBio, Inc, HiberCell
Other Relationship: Prospect Creek Foundation, Lustgarten Foundation for Pancreatic Cancer Research, (P30) Koch Institute for Integrated Cancer Research, Massachusetts Institute of Technology (2012-present; honorarium), (P30) Salk Institute Cancer Center (2016-2020; honorarium), Bloomberg-Kimmel Institute for Cancer Immunotherapy, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (2016-present; honorarium), Dana Farber Cancer Center Breast SPORE (2017-present; honorarium), (P30) Dana Farber/Harvard Cancer Center (2019-present; honorarium), (P30) University of California, San Diego Moores Cancer Center (2019-present; honorarium), Cancer Research Institute (CRI; 2013-present; unpaid), The V Foundation for Cancer Research (2013-present; unpaid), Starr Cancer Consortium (2011-present, honorarium), Therapeutics Working Group (2019-present; paid), NIH/NCI-Frederick National Laboratory Advisory Committee (FNLAC; 2016-present; daily honorarium), Susan G Komen Foundation, Komen Scholar (2020-2023; honorarium), (P50) Dana Farber Cancer Center Breast SPORE, (P30) The Jackson Laboratory Cancer Center, (P01) Columbia University Medical Center, Prostate P01, (P50) MDACC GI SPORE, American Association for Cancer Research, AACR: Cancer Discovery, Cancer Cell, National Foundation for Cancer Research
Michael L. Meyers
Employment: Syndax
Leadership: Syndax
Stock and Other Ownership Interests: Syndax, Nuvalent, Inc
Consulting or Advisory Role: Syndax, Nuvalent, Inc
Patents, Royalties, Other Intellectual Property: Syndax, Nuvalent, Inc
Peter Ordentlich
Employment: Syndax
Leadership: Syndax
Stock and Other Ownership Interests: Syndax
Consulting or Advisory Role: Patrys, Twentyeight-Seven Therapeutics
Patents, Royalties, Other Intellectual Property: Issued patents and patents pending
Christine Quaranto
Employment: Syndax, Aerovate Therapeutics
Stock and Other Ownership Interests: Syndax Pharmaceuticals, Aerovate Therapeutics
Aaron Schmitt
Employment: Syndax Pharmaceuticals Inc
Stock and Other Ownership Interests: Syndax
Yu Gu
Employment: Syndax, AstraZeneca
Stock and Other Ownership Interests: Syndax, AstraZeneca
Bruce R. Blazar
Stock and Other Ownership Interests: BlueRock Therapeutics, Tmunity Therapeutics, Inc, Magenta Therapeutics
Consulting or Advisory Role: BlueRock Therapeutics, Magenta Therapeutics, Obsidian Therapeutics, Editas Medicine
Research Funding: BlueRock Therapeutics, Rheos Medicines, Équilibre Biopharmaceuticals Corp, Carisma Therapeutics
Patents, Royalties, Other Intellectual Property: Inducible regulatory T-cell generation for hematopoietic cell transplants (UMN Z09026), US 9,228,172, TALEN-based gene correction, Patent No. 9,393,257, Generation of natural killer cells and lymphoid tissue inducer–like (LTI-like) NK-22 cells, 9,862,928, Method for correcting a genetic sequence, 10,648,002
Travel, Accommodations, Expenses: Incyte, Magenta Therapeutics, Rheos Medicines
Amandeep Salhotra
Consulting or Advisory Role: Kadmon, Syros Pharmaceuticals, Sobi
Research Funding: Bristol Myers Squibb
Iskra Pusic
Consulting or Advisory Role: Kadmon, Incyte, Syndax
Madan Jagasia
Employment: Iovance Biotherapeutics
Stock and Other Ownership Interests: Iovance Biotherapeutics
Consulting or Advisory Role: Kadmon
Stephanie J. Lee
Honoraria: Wolters Kluwer, PER
Consulting or Advisory Role: EMD Serono, Pfizer, 4SC, Mallinckrodt/Therakos, Almirall Hermal GmbH, Rain Therapeutics, Kadmon, Equillium
Research Funding: Kadmon, Amgen, Bristol Myers Squibb, EMD Serono, Incyte, Syndax, Pfizer, AstraZeneca
Patents, Royalties, Other Intellectual Property: Patent pending for high-affinity T-cell receptors that target the Merkel polyomavirus
Other Relationship: National Marrow Donor Program, Society for Investigative Dermatology (SID)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Arai S, Arora M, Wang T, et al. : Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: A report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant 21:266-274, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora M, Cutler CS, Jagasia MH, et al. : Late acute and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 22:449-455, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood WA, Chai X, Weisdorf D, et al. : Comorbidity burden in patients with chronic GVHD. Bone Marrow Transplant 48:1429-1436, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wingard JR, Majhail NS, Brazauskas R, et al. : Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol 29:2230-2239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Defilipp Z, Alousi AM, Pidala JA, et al. : Nonrelapse mortality among patients diagnosed with chronic GVHD: An updated analysis from the chronic GVHD Consortium. Blood Adv 5:4278-4284, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martires KJ, Baird K, Steinberg SM, et al. : Sclerotic-type chronic GVHD of the skin: Clinical risk factors, laboratory markers, and burden of disease. Blood 118:4250-4257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Au BKC, Au MA, Chien JW: Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 17:1072-1078, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inamoto Y, Storer BE, Petersdorf EW, et al. : Incidence, risk factors, and outcomes of sclerosis in patients with chronic graft-versus-host disease. Blood 121:5098-5103, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhm J, Hamad N, Shin EM, et al. : Incidence, risk factors, and long-term outcomes of sclerotic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 20:1751-1757, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Pidala J, Kurland B, Chai X, et al. : Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: Report on baseline data from the Chronic GVHD Consortium. Blood 117:4651-4657, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin PJ, Storer BE, Inamoto Y, et al. : An endpoint associated with clinical benefit after initial treatment of chronic graft-versus-host disease. Blood 130:360-367, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velickovic VM, McIlwaine E, Zhang R, et al. : Adverse events in second- and third-line treatments for acute and chronic graft-versus-host disease: Systematic review. Ther Adv Hematol 11:204062072097703, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miklos D, Cutler CS, Arora M, et al. : Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 130:2243-2250, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutler CS, Lee SJ, Arai S, et al. : Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: The ROCKstar study. Blood, 2021;138:2278-2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeiser R, Polverelli N, Ram R, et al. : Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med 385:228-238, 2021 [DOI] [PubMed] [Google Scholar]
- 16.Chin K-K, Kim HT, Inyang E-A, et al. : Ibrutinib in steroid-refractory chronic graft-versus-host disease, a single-center experience. Transplant Cell Ther 27:990.e1-990.e7, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Cooke KR, Luznik L, Sarantopoulos S, et al. : The biology of chronic graft-versus-host disease: A Task Force Report from the National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. Biol Blood Marrow Transplant 23:211-234, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeiser R, Blazar BR: Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med 377:2565-2579, 2017 [DOI] [PubMed] [Google Scholar]
- 19.MacDonald KPA, Hill GR, Blazar BR: Chronic graft-versus-host disease: Biological insights from preclinical and clinical studies. Blood 129:13-21, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander KA, Flynn R, Lineburg KE, et al. : CSF-1-dependant donor-derived macrophages mediate chronic graft-versus-host disease. J Clin Invest 124:4266-4280, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du J, Paz K, Flynn R, et al. : Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-beta production. Blood 129:2570-2580, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono R, Watanabe T, Kawakami E, et al. : Co-activation of macrophages and T cells contribute to chronic GVHD in human IL-6 transgenic humanised mouse model. EBioMedicine 41:584-596, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ordentlich P, Wolfreys A, Da Costa A, et al. : Targeting colony stimulating factor- 1 receptor (CSF-1R) with SNDX-6352, a novel anti-CSF-1R targeted antibody. J Immunother Cancer 4:P402, 2016 [Google Scholar]
- 24.Jagasia MH, Greinix HT, Arora M, et al. : National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 21:389-401 e1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiessen R, Visser A, Tadema H, et al. : First in human, single ascending dose study in healthy volunteers of SNDX-6352, a humanized IgG4 monoclonal antibody targeting colony stimulating factor-1 receptor (CSF-1R). J Immunother Cancer 5:P505, 2016 [Google Scholar]
- 26.Azad N, Rasco D, Sharma S, et al. : SNDX-6352-0502 - a phase 1, open-label, dose escalation trial to investigate the safety, tolerability, pharmacokinetics and pharmacodynamic activity of SNDX-6352 monotherapy in patients with unresectable, recurrent, locally-advanced, or metastatic solid tumors. Cancer Res 80, 2020. (suppl 16; abstr CT149) [Google Scholar]
- 27.Lee SJ, Wolff D, Kitko C, et al. : Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant 21:984-999, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SJ, Cook EF, Soiffer R, et al. : Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant 8:444-452, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Ries C, Cannarile MichaelA, Hoves S, et al. : Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25:846-859, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Cannarile MA, Weisser M, Jacob W, et al. : Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer 5:53, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bissinger S, Hage C, Wagner V, et al. : Macrophage depletion induces edema through release of matrix-degrading proteases and proteoglycan deposition. Sci Transl Med 13:eabd4550, 2021 [DOI] [PubMed] [Google Scholar]
- 32.Inamoto Y, Flowers MED, Sandmaier BM, et al. : Failure-free survival after initial systemic treatment of chronic graft-versus-host disease. Blood 124:1363-1371, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inamoto Y, Lee SJ, Onstad LE, et al. : Refined National Institutes of Health response algorithm for chronic graft-versus-host disease in joints and fascia. Blood Adv 4:40-46, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wynn TA: Fibrotic disease and the TH1/TH2 paradigm. Nat Rev Immunol 4:583-594, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jardine L, Cytlak U, Gunawan M, et al. : Donor monocyte–derived macrophages promote human acute graft-versus-host disease. J Clin Invest 130:4574-4586, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]