Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically on the based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
We present 5-year outcomes from the phase 3 KEYNOTE-189 study (ClinicalTrials.gov identifier: NCT02578680). Eligible patients with previously untreated metastatic nonsquamous non–small-cell lung cancer without EGFR/ALK alterations were randomly assigned 2:1 to pembrolizumab 200 mg or placebo once every 3 weeks for up to 35 cycles with pemetrexed and investigator's choice of carboplatin/cisplatin for four cycles, followed by maintenance pemetrexed until disease progression or unacceptable toxicity. Primary end points were overall survival (OS) and progression-free survival (PFS). Among 616 randomly assigned patients (n = 410, pembrolizumab plus pemetrexed-platinum; n = 206, placebo plus pemetrexed-platinum), median time from random assignment to data cutoff (March 8, 2022) was 64.6 (range, 60.1-72.4) months. Hazard ratio (95% CI) for OS was 0.60 (0.50 to 0.72) and PFS was 0.50 (0.42 to 0.60) for pembrolizumab plus platinum-pemetrexed versus placebo plus platinum-pemetrexed. 5-year OS rates were 19.4% versus 11.3%. Toxicity was manageable. Among 57 patients who completed 35 cycles of pembrolizumab, objective response rate was 86.0% and 3-year OS rate after completing 35 cycles (approximately 5 years after random assignment) was 71.9%. Pembrolizumab plus pemetrexed-platinum maintained OS and PFS benefits versus placebo plus pemetrexed-platinum, regardless of programmed cell death ligand-1 expression. These data continue to support pembrolizumab plus pemetrexed-platinum as a standard of care in previously untreated metastatic non–small-cell lung cancer without EGFR/ALK alterations.
INTRODUCTION
In the phase 3 KEYNOTE-189 study, pembrolizumab (an anti–programmed cell death protein-1 monoclonal antibody) plus pemetrexed and carboplatin/cisplatin significantly prolonged overall survival (OS; hazard ratio [HR], 0.49; 95% CI, 0.38 to 0.64; P < .001) and progression-free survival (PFS; 0.52; 0.43 to 0.64; P < .001) versus placebo plus pemetrexed-platinum in previously untreated metastatic nonsquamous non–small-cell lung cancer (NSCLC) without EGFR/ALK alterations.1 In the protocol-specified final analysis, OS and PFS continued to improve with HRs (95% CI) of 0.56 (0.46 to 0.69) and 0.49 (0.41 to 0.59), respectively.2 We present a 5-year exploratory analysis from KEYNOTE-189.
CONTEXT
Key Objective
We examined whether patients with previously untreated metastatic nonsquamous non–small-cell lung cancer without EGFR/ALK alterations treated with pembrolizumab plus pemetrexed and platinum chemotherapy continue to experience improved survival outcomes versus placebo plus pemetrexed and platinum chemotherapy after 5 years of follow-up.
Knowledge Generated
After 5 years, pembrolizumab plus pemetrexed-platinum was associated with improved overall survival (OS) and progression-free survival compared with placebo plus pemetrexed-platinum in patients with metastatic nonsquamous non–small-cell lung cancer, regardless of programmed cell death ligand-1 expression. Five-year OS rates were 19.4% versus 11.3% in the intention-to-treat population.
Relevance (T.E. Stinchcombe)
-
The 5-year follow-up demonstrates durable benefit for the combination of platinum-pemetrexed and pembrolizumab and the benefit in the subsets of patients on the basis of tumor programmed cell death ligand-1 expression. In the future, the landmark analyses of progression-free survival or OS at 3 or 5 years may be used to assess the long-term benefit of novel immunotherapies.*
*Relevance section written by JCO Associate Editor Thomas E. Stinchcombe, MD.
METHODS
Study Design and Patients
The study design for KEYNOTE-189 (ClinicalTrials.gov identifier: NCT02578680) has been previously described.1-3 The Protocol (online only) and its amendments were approved by the appropriate institutional review boards and ethics committees. Patients provided written informed consent before enrollment.
Patients were randomly assigned 2:1 to pembrolizumab 200 mg or placebo once every 3 weeks for up to 35 cycles (approximately 2 years). Patients also received pemetrexed 500 mg/m2 plus cisplatin 75 mg/m2 or carboplatin area under the curve 5 mg/mL/min once every 3 weeks for four cycles followed by pemetrexed maintenance therapy. Treatment continued until the maximum number of cycles or until radiographic progression, unacceptable toxicity, investigator's decision, or patient withdrawal. Patients in the placebo plus pemetrexed-platinum group could cross over to receive pembrolizumab monotherapy upon documented progressive disease (PD) per RECIST v1.1 by blinded independent central review (BICR) if eligibility criteria were met. Patients could receive a second course of pembrolizumab monotherapy for up to 17 cycles (approximately 1 year) upon PD after either completing 35 cycles of pembrolizumab with a best overall response of stable disease or better or having achieved confirmed investigator-assessed complete response (CR) after receiving ≥ 8 cycles of pembrolizumab and ≥ 2 cycles beyond the initial CR assessment.
End Points and Statistical Analysis
Primary end points were PFS per RECIST v1.1 by BICR and OS. Secondary end points were objective response rate (ORR) and duration of response (DOR) per RECIST v1.1 by BICR and safety. Exploratory end points included PFS2 (time from random assignment to second/subsequent PD on next-line treatment or death from any cause). No alpha was assigned to this analysis.
RESULTS
Patients
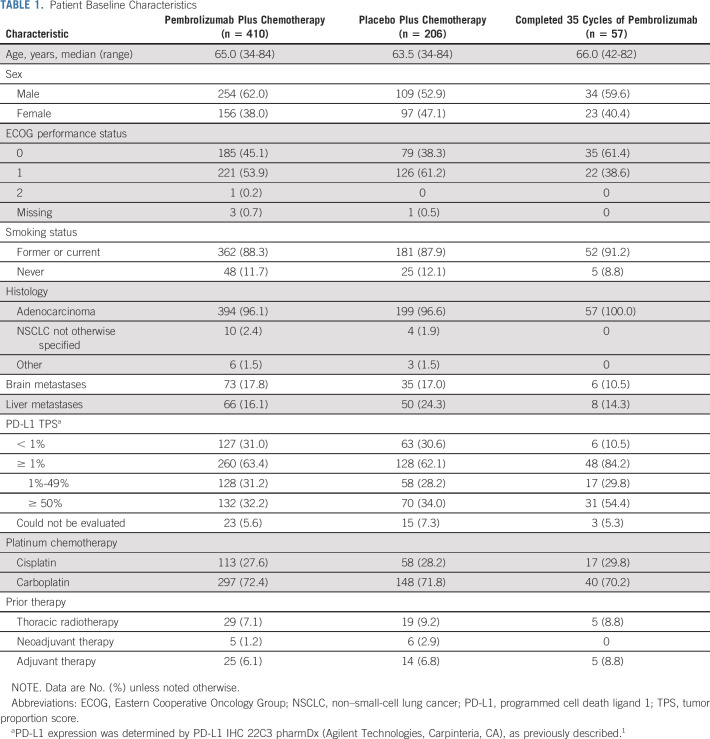
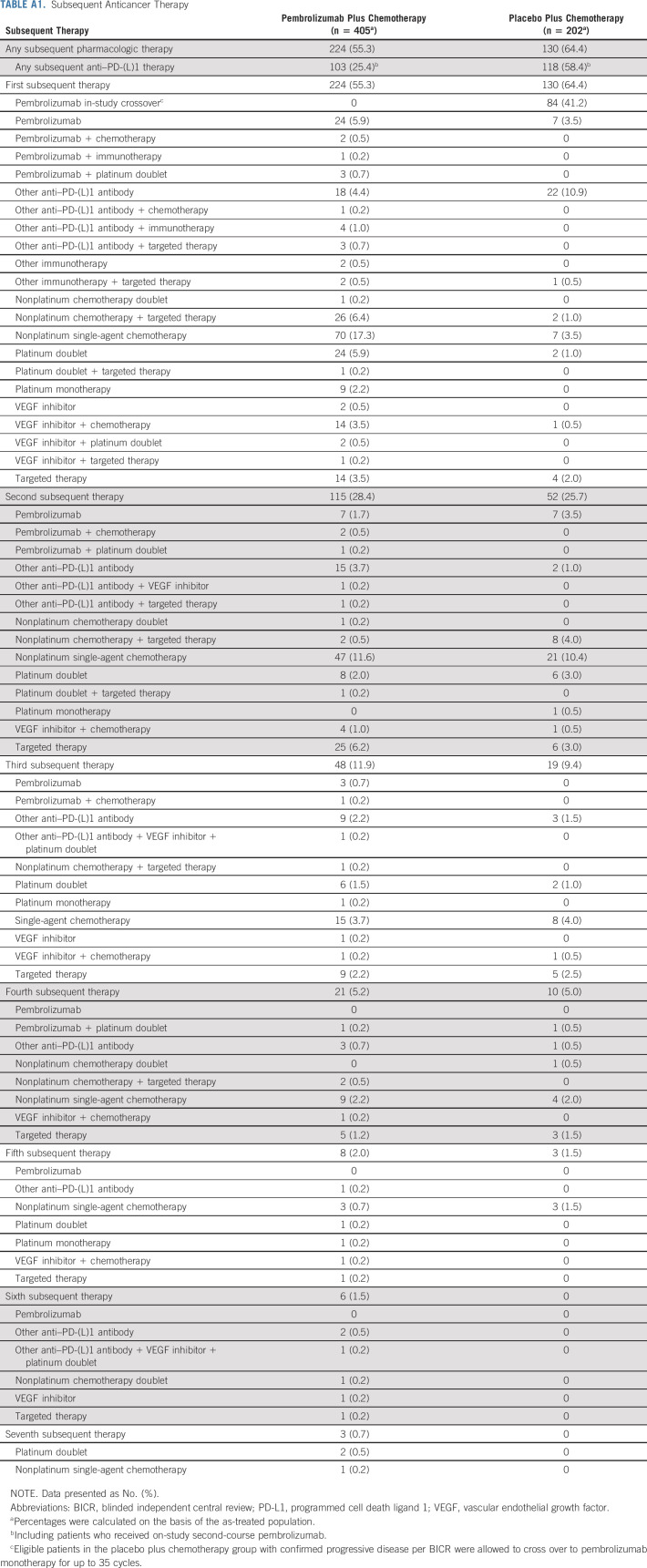
Overall, 616 patients were randomly assigned to pembrolizumab plus pemetrexed-platinum (n = 410) or placebo plus pemetrexed-platinum (n = 206; Table 1). At data cutoff (March 8, 2022), seven patients (all in the pembrolizumab plus pemetrexed-platinum group) were continuing to receive pemetrexed. Among patients randomly assigned to pembrolizumab plus pemetrexed-platinum, 224 (54.6%) received subsequent anticancer therapy (103 received anti–PD-1 or anti–programmed cell death ligand 1 [PD-L1] therapy, including nine who began on-study second-course pembrolizumab; Appendix Table A1, online only). In the placebo plus pemetrexed-platinum group, 84 patients crossed over to pembrolizumab monotherapy on-study; an additional 34 patients received subsequent anti–PD-(L)1 therapy outside the study for an effective crossover rate of 57.3%.
TABLE 1.
Patient Baseline Characteristics
Efficacy Outcomes
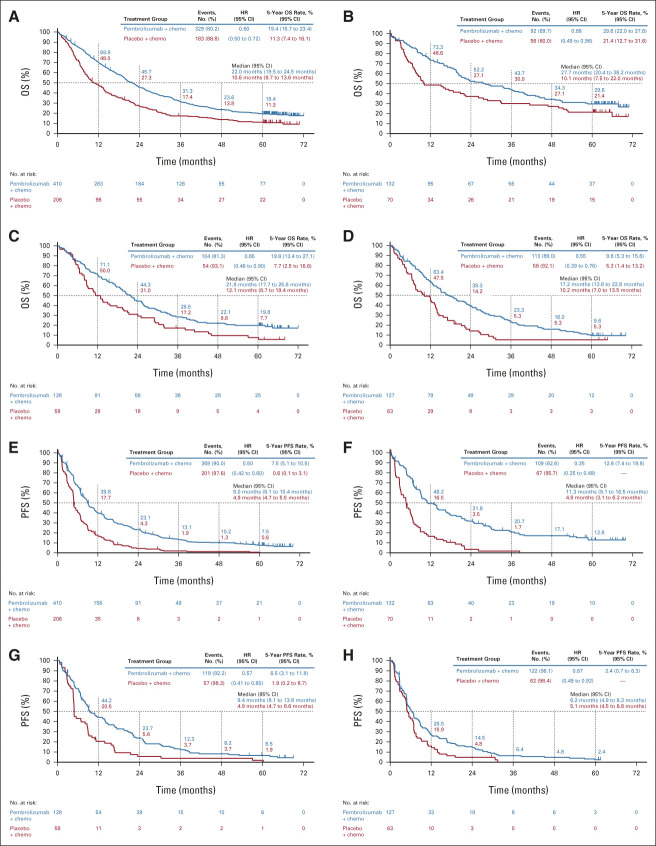
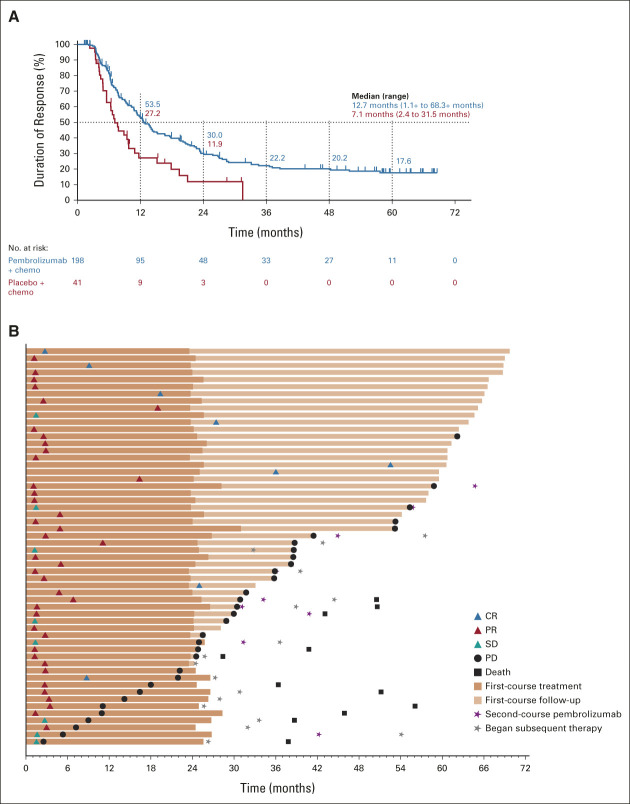
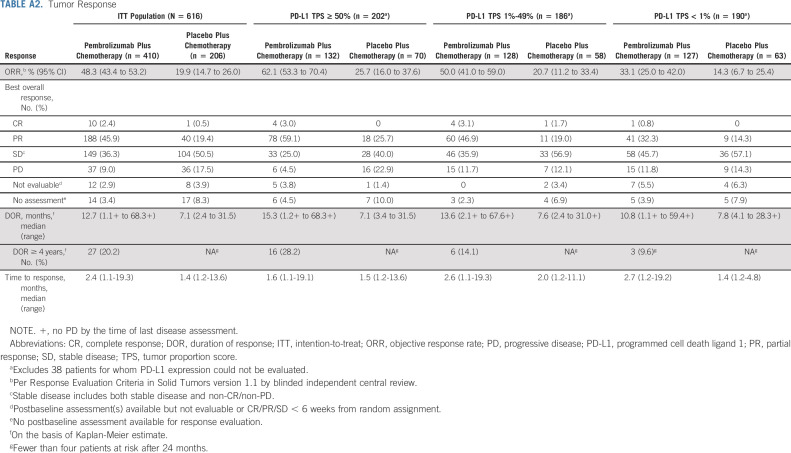
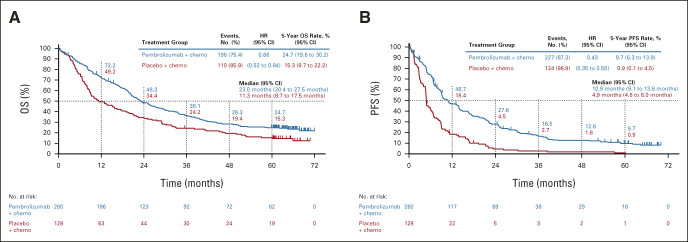
Median time from random assignment to data cutoff was 64.6 (range, 60.1-72.4) months. In the intention-to-treat (ITT) population, HRs (95% CI) for pembrolizumab plus pemetrexed-platinum versus placebo plus pemetrexed-platinum were 0.60 (0.50 to 0.72) for OS and 0.50 (0.42 to 0.60) for PFS (Figs 1A and 1E). Five-year OS rates were 19.4% versus 11.3%, and 5-year PFS rates were 7.5% versus 0.6%. ORR (95% CI) was 48.3% (43.4 to 53.2) and 19.9% (14.7 to 26.0), respectively. Median (range) DOR was 12.7 (1.1+ to 68.3+) and 7.1 (2.4 to 31.5) months, respectively (Fig 2A). Similar trends were observed across the PD-L1 subgroups analyzed (Figs 1B‐1D, 1F‐1H; Appendix Table A2 [online only]; and Appendix Fig A1 [online only]).
FIG 1.
Kaplan-Meier estimates of OS in the (A) ITT, (B) PD-L1 TPS ≥ 50%, (C) PD-L1 TPS 1%-49%, and (D) PD-L1 TPS < 1% populations and PFS by RECIST version 1.1 by blinded independent central review in the (E) ITT, (F) PD-L1 TPS ≥ 50%, (G) PD-L1 TPS 1%-49%, and (H) PD-L1 TPS < 1% populations. Chemo, chemotherapy; ITT, intention-to-treat; OS, overall survival; PD-L1, programmed cell death ligand 1; PFS, progression-free survival; TPS, tumor proportion score.
FIG 2.
(A) Duration of response in the ITT population and (B) treatment duration and time to response in patients who completed 35 cycles of pembrolizumab. Response assessments are shown per RECIST version 1.1 by BICR. Median PFS was not reached (95% CI, 14.7 months to not reached). PFS rate 3 years after completion of 35 cycles was 56.2% (95% CI, 39.7 to 69.8). BICR, blinded independent central review; chemo, chemotherapy; CR, complete response; ITT, intention-to-treat; PD, progressive disease; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
Median (95% CI) PFS2 was 17.0 (15.0 to 19.2) months in the pembrolizumab plus pemetrexed-platinum group versus 9.1 (7.6 to 10.8) months in the placebo plus pemetrexed-platinum group (HR, 0.54; 95% CI, 0.45 to 0.65). Five-year PFS2 rates (95% CI) were 16.7% (13.2 to 20.5) versus 7.8% (4.7 to 12.1).
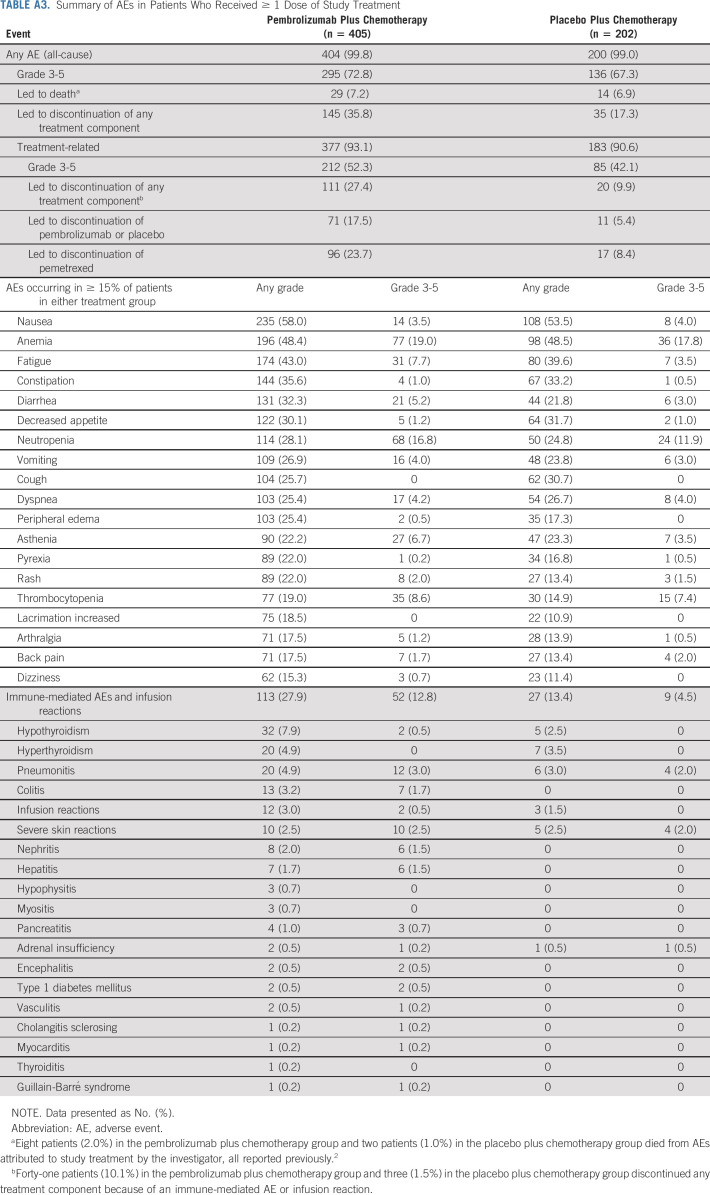
Safety
Overall, 404 patients (99.8%) in the pembrolizumab plus pemetrexed-platinum group and 200 (99.0%) in the placebo plus pemetrexed-platinum group experienced adverse events (AEs). All fatal AEs were previously reported.2,3 Immune-mediated AEs and infusion reactions occurred in 113 (27.9%) and 27 (13.4%) patients, respectively (Appendix Table A3, online only).
Patients Who Completed 35 Cycles of Pembrolizumab
Of the patients randomly assigned to pembrolizumab plus pemetrexed-platinum, 57 (13.9%) completed 35 cycles of pembrolizumab (Table 1) and received a median of 35 (range, 2-98) cycles of pemetrexed. ORR was 86.0% (8 CRs, 41 partial responses) and eight patients had best response of stable disease (Fig 2B). Median DOR was 57.7 (range, 4.2 to 68.3+) months. Estimated OS rate 3 years after completion of 35 cycles (ie, approximately 5 years from random assignment) was 71.9% (95% CI, 58.3 to 81.8). At data cutoff, 23 of 57 patients (40.4%) were alive without PD or subsequent therapy.
All patients experienced ≥ 1 AE (grade 3/4 AEs, 38 [66.7%]); none were grade 5. Immune-mediated AEs and infusion reactions occurred in 23 patients (40.4%; grade 3/4 in 7 [12.3%]).
DISCUSSION
In this 5-year update from KEYNOTE-189, pembrolizumab plus pemetrexed-platinum continued to prolong OS and PFS regardless of PD-L1 expression versus placebo plus pemetrexed-platinum with manageable toxicity (consistent with previous reports)2,3 in patients with previously untreated metastatic nonsquamous NSCLC without EGFR/ALK alterations. Five-year OS rate was approximately 20% with pembrolizumab plus pemetrexed-platinum in the ITT population (v 11% with placebo plus pemetrexed-platinum) and was higher in patients with higher PD-L1 tumor proportion score (TPS), especially in the TPS ≥ 50% subgroup (29.6% v 21.4%; similar to the 5-year OS rates in the KEYNOTE-024 study of pembrolizumab monotherapy v chemotherapy [31.9% v 16.3%]4). However, there were a limited number of patients at risk at 5 years in some subgroups. Importantly, benefits were also observed in the PD-L1 TPS < 1% subgroup, for whom pembrolizumab monotherapy is not indicated.
Sustained improvements in OS were observed with pembrolizumab plus pemetrexed-platinum versus placebo plus pemetrexed-platinum despite an effective crossover rate of 57% from placebo plus pemetrexed-platinum to subsequent anti–PD-(L)1 therapy, which is reflected in the plateauing of the placebo plus pemetrexed-platinum Kaplan-Meier curve and was not previously observed in studies of chemotherapy alone.5 These factors likely attenuated the differences in 5-year OS rates in the ITT population and in OS HR observed in later analyses compared with the first interim analysis.1 The improvement in PFS2 with pembrolizumab plus pemetrexed-platinum versus placebo plus pemetrexed-platinum also suggests the benefit of pembrolizumab plus pemetrexed-platinum is maintained after initial disease progression, further supporting its use as first-line treatment.
Pembrolizumab demonstrated robust and durable antitumor activity in patients who completed 35 cycles of pembrolizumab, with a majority of patients (72%) alive 3 years after completion (approximately 5 years after random assignment). These data support the feasibility of a 2-year treatment duration with pembrolizumab plus pemetrexed-platinum and are consistent with the outcomes reported in patients who completed 35 cycles of pembrolizumab monotherapy6,7 and in the KEYNOTE-407 study of pembrolizumab plus carboplatin and paclitaxel/nab-paclitaxel versus placebo plus carboplatin and paclitaxel/nab-paclitaxel in previously untreated metastatic squamous NSCLC.8 Although this is the first report of 5-year outcomes for anti–PD-(L)1 therapy plus chemotherapy from a phase 3 study, improved survival outcomes at 5 years were reported with nivolumab (anti–PD-1) plus ipilimumab (anti–CTLA-4) versus chemotherapy in the CheckMate 227 study.9
In conclusion, pembrolizumab plus pemetrexed-platinum continued to demonstrate prolonged survival and durable antitumor activity versus placebo plus pemetrexed-platinum after 5 years of follow-up, with manageable toxicity,2 in patients with previously untreated metastatic nonsquamous NSCLC without EGFR/ALK alterations. These results continue to support the combination of first-line pembrolizumab plus a platinum and pemetrexed as a standard of care for these patients.
ACKNOWLEDGMENT
The authors thank the patients and their families and caregivers for participating in this study, along with all investigators and site personnel. Additional study support was provided by Gregory M. Lubiniecki, MD, and Mark Shamoun, MD, of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ. Eli Lilly provided pemetrexed but had no additional role in trial conduct. Medical writing assistance was provided by Kathleen Estes, PhD, and Shilpa Kamboj, PhD, of ICON plc (Blue Bell, PA). This assistance was funded by MSD.
APPENDIX
TABLE A1.
Subsequent Anticancer Therapy
TABLE A2.
Tumor Response
TABLE A3.
Summary of AEs in Patients Who Received ≥ 1 Dose of Study Treatment
FIG A1.
Kaplan-Meier estimates of (A) OS and (B) PFS per Response Evaluation Criteria in Solid Tumors version 1.1 by blinded independent central review in patients with PD-L1 TPS ≥ 1%. Chemo, chemotherapy; OS, overall survival; PD-L1, programmed cell death ligand 1; pembro, pembrolizumab; PFS, progression-free survival; TPS, tumor proportion score.
Marina C. Garassino
Honoraria: MSD Oncology, AstraZeneca/MedImmune, GlaxoSmithKline, Takeda, Roche, Bristol Myers Squibb
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Novartis, Takeda, Roche, Tiziana Life Sciences, Sanofi, Celgene, Daiiki Sankyo, Inivata, Incyte, Pfizer, Seattle Genetics, Lilly, GlaxoSmithKline, Bayer, Blueprint Medicines, Janssen, Regeneron
Speakers' Bureau: AstraZeneca, Takeda, MSD Oncology, Celgene, Incyte, Roche, Bristol Myers Squibb, Otsuka, Lilly
Research Funding: Bristol Myers Squibb (Inst), MSD (Inst), Roche/Genentech (Inst), AstraZeneca/MedImmune (Inst), AstraZeneca (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Merck (Inst), Incyte (Inst), Takeda (Inst), Spectrum Pharmaceuticals (Inst), Blueprint Medicines (Inst), Lilly (Inst), Ipsen (Inst), Janssen (Inst), Exelixis (Inst), MedImmune (Inst), Sanofi (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca
Shirish Gadgeel
Honoraria: Merck
Consulting or Advisory Role: Genentech/Roche, AstraZeneca, Bristol Myers Squibb, Takeda, Daichii-Sanyko, Novartis, Blueprint Medicines, Lilly, Pfizer, Janssen Oncology, Mirati Therapeutics, Merck, Esai Pharma, Gilead Sciences, GlaxoSmithKline
Research Funding: Genentech/Roche (Inst), Merck (Inst), Blueprint Medicines (Inst), Astellas Pharma (Inst), Daiichi Sankyo (Inst), I-Mab (Inst), Nektar (Inst), AstraZeneca (Inst), Pfizer (Inst), Amgen (Inst), Turning Point Therapeutics (Inst), Regeneron (Inst), Mirati Therapeutics (Inst), Janssen Oncology (Inst), BioMed Valley Discoveries (Inst), Ymabs Therapeutics Inc (Inst), Calithera Biosciences (Inst), InventisBio (Inst), Daichii Sanyko (Inst), Dragonfly Therapeutics (Inst), eFFECTOR Therapeutics (Inst), Elevation Oncology (Inst), Erasca, Inc (Inst), Helsinn Therapeutics (Inst), Incyte (Inst), Numab (Inst), Verastem (Inst)
Travel, Accommodations, Expenses: Mirati Therapeutics
Other Relationship: AstraZeneca
Giovanna Speranza
Consulting or Advisory Role: Bayer
Enriqueta Felip
Consulting or Advisory Role: Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Lilly, GlaxoSmithKline, Janssen, Merck Serono, Novartis, Pfizer, Sanofi, Takeda, Peptomyc, Daiichi Sankyo Europe GmbH, F. Hoffmann LaRoche, Merck Sharp & Dohme
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Lilly, Medscape, Merck Sharp & Dohme, PeerVoice, Pfizer, Takeda, Amgen, F. Hoffmann LaRoche, Janssen, Medical Trends, Merck Serono, Sanofi, TouchONCOLOGY
Research Funding: Merck (Inst), Merck KGaA (Inst)
Other Relationship: GRIFOLS
Emilio Esteban
Travel, Accommodations, Expenses: Pfizer/EMD Serono
Manuel Dómine
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, MSD Oncology, Pfizer, Roche, Takeda
Maximilian J. Hochmair
Consulting or Advisory Role: Takeda, Roche, Lilly, AstraZeneca/Daiichi Sankyo
Speakers' Bureau: Lilly, MSD Oncology, Novartis, Roche
Steven F. Powell
Consulting or Advisory Role: Bristol Myers Squibb (Inst)
Speakers' Bureau: Alkermes (Inst)
Research Funding: Merck Sharp & Dohme (Inst), Novartis (Inst), Genentech/Roche (Inst), Incyte (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Vyriad (Inst), Actuate Therapeutics (Inst), AstraZeneca/MedImmune (Inst), Seattle Genetics (Inst), Sorrento (Inst)
Francesco Grossi
Consulting or Advisory Role: MSD Oncology, Bristol Myers Squibb, AstraZeneca, Roche, Pfizer, Bayer, Lilly, Novartis Italy, Sanofi
Speakers' Bureau: MSD Oncology, Bristol Myers Squibb, AstraZeneca, Roche, Pierre Fabre, Amgen, Celgene, Lilly, Pfizer, Sanofi
Research Funding: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb, MSD, Roche, AstraZeneca, Pierre Fabre, Celgene, Amgen, Lilly, Novartis
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, AbbVie, Amgen, Mirati Therapeutics, Samsung Bioepis, Sanofi/Regeneron, Daiichi Sankyo Europe GmbH
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics, Sanofi/Aventis
Rina Hui
Honoraria: Merck Sharp & Dohme, Novartis, Roche, AstraZeneca, Bristol Myers Squibb, Lilly, Pfizer, Seattle Genetics, Oncosec, Merck, Eisai
Consulting or Advisory Role: Merck Sharp & Dohme, AstraZeneca, Roche, Bristol Myers Squibb, Novartis, Lilly, Pfizer, Seattle Genetics, Oncosec, Merck, Eisai
Research Funding: AstraZeneca (Inst), Lilly (Inst), MSD (Inst), Roche (Inst), Seattle Genetics (Inst), OncoSec (Inst), Novartis (Inst), BMSi (Inst), Corvus Pharmaceuticals (Inst), Olema Pharmaceuticals (Inst), Janssen (Inst)
Edward B. Garon
Consulting or Advisory Role: Novartis, GlaxoSmithKline, Merck, Boehringer Ingelheim, Shionogi, Eisai, Bristol Myers Squibb, ABL Bio, Xilio Therapeutics, Natera, Sanofi/Regeneron, Lilly, Personalis, Gilead Sciences, AstraZeneca, AbbVie/ABBOTT, Ipsen
Research Funding: Merck (Inst), Genentech (Inst), AstraZeneca (Inst), Novartis (Inst), Lilly (Inst), Bristol Myers Squibb (Inst), Mirati Therapeutics (Inst), Dynavax Technologies (Inst), Iovance Biotherapeutics (Inst), Neon Therapeutics (Inst), EMD Serono (Inst), ABL Bio (Inst)
Patents, Royalties, Other Intellectual Property: Diagnosistic and therapeutic use of "Motif Neoepitopes" as defined by Cummings et al in Nature Cancer (Inst)
Takayasu Kurata
Honoraria: AstraZeneca, Ono Pharmaceutical, Bristol Myers Squibb, Chugai Pharma, Lilly, Boehringer Ingelheim, MSD Oncology, Pfizer, Nippon Kayaku
Research Funding: MSD Oncology (Inst), AstraZeneca (Inst), Lilly (Inst), Ono Pharmaceutical (Inst), Novartis (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), Amgen
Jhanelle E. Gray
Honoraria: Merck Sharp & Dohme, Axiom Healthcare Strategies, Inivata, Jazz Pharmaceuticals, Merck, OncoCyte
Consulting or Advisory Role: AstraZeneca, Blueprint Medicines, Bristol Myers Squibb, EMD Serono, Lilly, Sanofi, Merck Sharp & Dohme, Loxo, Jazz Pharmaceuticals, Novartis, AstraZeneca/MedImmune, Janssen Scientific Affairs, National Comprehensive Cancer Network
Research Funding: Merck (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Genentech/Roche (Inst), G1 Therapeutics (Inst), Novartis (Inst), Pfizer (Inst), Ludwig Institute for Cancer Research (Inst), SWOG (Inst), Array BioPharma (Inst), ECOG-ACRIN (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Inivata, Merck, EMD Serono, Novartis, OncoCyte
Paul Schwarzenberger
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck & Co, Inc
Travel, Accommodations, Expenses: AstraZeneca
Erin Jensen
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck & Co, Inc
M. Catherine Pietanza
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck & Co, Inc
Delvys Rodríguez-Abreu
Consulting or Advisory Role: Roche, Bristol Myers Squibb, MSD, AstraZeneca Spain, Novartis
Speakers' Bureau: Roche, Bristol Myers Squibb, MSD
Travel, Accommodations, Expenses: Roche, Bristol Myers Squibb, MSD
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the European Society for Medical Oncology Congress, Paris, France, September 9‐13, 2022.
SUPPORT
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
AUTHOR CONTRIBUTIONS
Conception and design: Maximilian J. Hochmair, Helge G. Bischoff, Martin Reck, Jhanelle E. Gray, Paul Schwarzenberger, M. Catherine Pietanza, Delvys Rodríguez-Abreu
Administrative support: Enriqueta Felip, Maximilian J. Hochmair, Helge G. Bischoff
Provision of study materials or patients: Shirish Gadgeel, Giovanna Speranza, Enriqueta Felip, Manuel Dómine, Steven F. Powell, Helge G. Bischoff, Nir Peled, Francesco Grossi, Ross R. Jennens, Martin Reck, Rina Hui, Edward B. Garon, Takayasu Kurata, Jhanelle E. Gray, Delvys Rodríguez-Abreu
Collection and assembly of data: Marina C. Garassino, Shirish Gadgeel, Giovanna Speranza, Enriqueta Felip, Maximilian J. Hochmair, Steven F. Powell, Helge G. Bischoff, Nir Peled, Francesco Grossi, Ross R. Jennens, Martin Reck, Rina Hui, Edward B. Garon, Takayasu Kurata, Paul Schwarzenberger, M. Catherine Pietanza, Delvys Rodríguez-Abreu
Data analysis and interpretation: Marina C. Garassino, Shirish Gadgeel, Enriqueta Felip, Manuel Dómine, Maximilian J. Hochmair, Steven F. Powell, Helge G. Bischoff, Nir Peled, Ross R. Jennens, Martin Reck, Rina Hui, Jhanelle E. Gray, Paul Schwarzenberger, Erin Jensen, M. Catherine Pietanza, Delvys Rodríguez-Abreu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non–Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Marina C. Garassino
Honoraria: MSD Oncology, AstraZeneca/MedImmune, GlaxoSmithKline, Takeda, Roche, Bristol Myers Squibb
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Novartis, Takeda, Roche, Tiziana Life Sciences, Sanofi, Celgene, Daiiki Sankyo, Inivata, Incyte, Pfizer, Seattle Genetics, Lilly, GlaxoSmithKline, Bayer, Blueprint Medicines, Janssen, Regeneron
Speakers' Bureau: AstraZeneca, Takeda, MSD Oncology, Celgene, Incyte, Roche, Bristol Myers Squibb, Otsuka, Lilly
Research Funding: Bristol Myers Squibb (Inst), MSD (Inst), Roche/Genentech (Inst), AstraZeneca/MedImmune (Inst), AstraZeneca (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Merck (Inst), Incyte (Inst), Takeda (Inst), Spectrum Pharmaceuticals (Inst), Blueprint Medicines (Inst), Lilly (Inst), Ipsen (Inst), Janssen (Inst), Exelixis (Inst), MedImmune (Inst), Sanofi (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca
Shirish Gadgeel
Honoraria: Merck
Consulting or Advisory Role: Genentech/Roche, AstraZeneca, Bristol Myers Squibb, Takeda, Daichii-Sanyko, Novartis, Blueprint Medicines, Lilly, Pfizer, Janssen Oncology, Mirati Therapeutics, Merck, Esai Pharma, Gilead Sciences, GlaxoSmithKline
Research Funding: Genentech/Roche (Inst), Merck (Inst), Blueprint Medicines (Inst), Astellas Pharma (Inst), Daiichi Sankyo (Inst), I-Mab (Inst), Nektar (Inst), AstraZeneca (Inst), Pfizer (Inst), Amgen (Inst), Turning Point Therapeutics (Inst), Regeneron (Inst), Mirati Therapeutics (Inst), Janssen Oncology (Inst), BioMed Valley Discoveries (Inst), Ymabs Therapeutics Inc (Inst), Calithera Biosciences (Inst), InventisBio (Inst), Daichii Sanyko (Inst), Dragonfly Therapeutics (Inst), eFFECTOR Therapeutics (Inst), Elevation Oncology (Inst), Erasca, Inc (Inst), Helsinn Therapeutics (Inst), Incyte (Inst), Numab (Inst), Verastem (Inst)
Travel, Accommodations, Expenses: Mirati Therapeutics
Other Relationship: AstraZeneca
Giovanna Speranza
Consulting or Advisory Role: Bayer
Enriqueta Felip
Consulting or Advisory Role: Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Lilly, GlaxoSmithKline, Janssen, Merck Serono, Novartis, Pfizer, Sanofi, Takeda, Peptomyc, Daiichi Sankyo Europe GmbH, F. Hoffmann LaRoche, Merck Sharp & Dohme
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Lilly, Medscape, Merck Sharp & Dohme, PeerVoice, Pfizer, Takeda, Amgen, F. Hoffmann LaRoche, Janssen, Medical Trends, Merck Serono, Sanofi, TouchONCOLOGY
Research Funding: Merck (Inst), Merck KGaA (Inst)
Other Relationship: GRIFOLS
Emilio Esteban
Travel, Accommodations, Expenses: Pfizer/EMD Serono
Manuel Dómine
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, MSD Oncology, Pfizer, Roche, Takeda
Maximilian J. Hochmair
Consulting or Advisory Role: Takeda, Roche, Lilly, AstraZeneca/Daiichi Sankyo
Speakers' Bureau: Lilly, MSD Oncology, Novartis, Roche
Steven F. Powell
Consulting or Advisory Role: Bristol Myers Squibb (Inst)
Speakers' Bureau: Alkermes (Inst)
Research Funding: Merck Sharp & Dohme (Inst), Novartis (Inst), Genentech/Roche (Inst), Incyte (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Vyriad (Inst), Actuate Therapeutics (Inst), AstraZeneca/MedImmune (Inst), Seattle Genetics (Inst), Sorrento (Inst)
Francesco Grossi
Consulting or Advisory Role: MSD Oncology, Bristol Myers Squibb, AstraZeneca, Roche, Pfizer, Bayer, Lilly, Novartis Italy, Sanofi
Speakers' Bureau: MSD Oncology, Bristol Myers Squibb, AstraZeneca, Roche, Pierre Fabre, Amgen, Celgene, Lilly, Pfizer, Sanofi
Research Funding: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb, MSD, Roche, AstraZeneca, Pierre Fabre, Celgene, Amgen, Lilly, Novartis
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, AbbVie, Amgen, Mirati Therapeutics, Samsung Bioepis, Sanofi/Regeneron, Daiichi Sankyo Europe GmbH
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics, Sanofi/Aventis
Rina Hui
Honoraria: Merck Sharp & Dohme, Novartis, Roche, AstraZeneca, Bristol Myers Squibb, Lilly, Pfizer, Seattle Genetics, Oncosec, Merck, Eisai
Consulting or Advisory Role: Merck Sharp & Dohme, AstraZeneca, Roche, Bristol Myers Squibb, Novartis, Lilly, Pfizer, Seattle Genetics, Oncosec, Merck, Eisai
Research Funding: AstraZeneca (Inst), Lilly (Inst), MSD (Inst), Roche (Inst), Seattle Genetics (Inst), OncoSec (Inst), Novartis (Inst), BMSi (Inst), Corvus Pharmaceuticals (Inst), Olema Pharmaceuticals (Inst), Janssen (Inst)
Edward B. Garon
Consulting or Advisory Role: Novartis, GlaxoSmithKline, Merck, Boehringer Ingelheim, Shionogi, Eisai, Bristol Myers Squibb, ABL Bio, Xilio Therapeutics, Natera, Sanofi/Regeneron, Lilly, Personalis, Gilead Sciences, AstraZeneca, AbbVie/ABBOTT, Ipsen
Research Funding: Merck (Inst), Genentech (Inst), AstraZeneca (Inst), Novartis (Inst), Lilly (Inst), Bristol Myers Squibb (Inst), Mirati Therapeutics (Inst), Dynavax Technologies (Inst), Iovance Biotherapeutics (Inst), Neon Therapeutics (Inst), EMD Serono (Inst), ABL Bio (Inst)
Patents, Royalties, Other Intellectual Property: Diagnosistic and therapeutic use of "Motif Neoepitopes" as defined by Cummings et al in Nature Cancer (Inst)
Takayasu Kurata
Honoraria: AstraZeneca, Ono Pharmaceutical, Bristol Myers Squibb, Chugai Pharma, Lilly, Boehringer Ingelheim, MSD Oncology, Pfizer, Nippon Kayaku
Research Funding: MSD Oncology (Inst), AstraZeneca (Inst), Lilly (Inst), Ono Pharmaceutical (Inst), Novartis (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), Amgen
Jhanelle E. Gray
Honoraria: Merck Sharp & Dohme, Axiom Healthcare Strategies, Inivata, Jazz Pharmaceuticals, Merck, OncoCyte
Consulting or Advisory Role: AstraZeneca, Blueprint Medicines, Bristol Myers Squibb, EMD Serono, Lilly, Sanofi, Merck Sharp & Dohme, Loxo, Jazz Pharmaceuticals, Novartis, AstraZeneca/MedImmune, Janssen Scientific Affairs, National Comprehensive Cancer Network
Research Funding: Merck (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Genentech/Roche (Inst), G1 Therapeutics (Inst), Novartis (Inst), Pfizer (Inst), Ludwig Institute for Cancer Research (Inst), SWOG (Inst), Array BioPharma (Inst), ECOG-ACRIN (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Inivata, Merck, EMD Serono, Novartis, OncoCyte
Paul Schwarzenberger
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck & Co, Inc
Travel, Accommodations, Expenses: AstraZeneca
Erin Jensen
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck & Co, Inc
M. Catherine Pietanza
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck & Co, Inc
Delvys Rodríguez-Abreu
Consulting or Advisory Role: Roche, Bristol Myers Squibb, MSD, AstraZeneca Spain, Novartis
Speakers' Bureau: Roche, Bristol Myers Squibb, MSD
Travel, Accommodations, Expenses: Roche, Bristol Myers Squibb, MSD
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. : Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078-2092, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Abreu D, Powell SF, Hochmair MJ, et al. : Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: Protocol-specified final analysis from KEYNOTE-189. Ann Oncol 32:881-895, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Gadgeel S, Rodriguez-Abreu D, Speranza G, et al. : Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 38:1505-1517, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Reck M, Rodriguez-Abreu D, Robinson AG, et al. : Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol 39:2339-2349, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiller JH, Harrington D, Belani CP, et al. : Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92-98, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Reck M, Rodriguez-Abreu D, Robinson AG, et al. : Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol 39:2339-2349, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Castro G, Kudaba I, Wu Y-L, et al. : Five-year outcomes with pembrolizumab versus chemotherapy as first-line therapy in patients with non–small-cell lung cancer and programmed death ligand-1 tumor proportion score ≥1% in the KEYNOTE-042 study. J Clin Oncol 41:1986-1991, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novello S, Kowalski DM, Luft A, et al. : Pembrolizumab plus chemotherapy in squamous non–small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol 41:1999-2006, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer JR, Lee J-S, Ciuleanu T-E, et al. : Five-year survival outcomes with nivolumab (NIVO) plus ipilimumab (IPI) versus chemotherapy (chemo) as first-line (1L) treatment for metastatic non–small cell lung cancer (NSCLC): Results from CheckMate 227. J Clin Oncol 40, 2022. (suppl abstr LBA9025) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.