Abstract
Osteogenesis imperfecta (OI) is a rare disorder of bone fragility. Gravid patients with OI usually need cesarean and may present multiple challenges. We present a case of an OI patient with severe scoliosis and an anticipated difficult airway undergoing cesarean section, with details on perioperative assessment and strategy of anesthesia.
Keywords: cesarean section, Fibrobronchoscope‐guided conscious endotracheal intubation, general anesthesia, osteogenesis imperfecta
1. INTRODUCTION
Osteogenesis imperfecta (OI), also as known “brittle bone disease,” is a genetically heterogeneous connective tissue disorder that encodes gene mutations affecting type I collagen. More than 80% of cases are caused by COL1A1 or COL1A2, and genomic advances have identified 18 additional genes as causes for OI. 1 The primary manifestations include low bone mass and bone fragility that generate recurrent skeletal fractures and deformities. Additional complications include short stature, blue sclera, hearing loss, dentinogenesis imperfecta, pulmonary dysfunction, and cardiac valvular abnormalities. 2 According to the genetic pattern and clinical manifestations, OI is classified into types I, II, III, and IV, corresponding with the phenotypic range of mild, lethal, severe progressive deforming, and moderate, respectively. 3 OI is common in women, with a population prevalence of approximately 1/10,000, with a maternal prevalence of approximately 1/25,000–1/30,000. 4 With therapeutic advances, more women with OI are reaching reproductive age and have a higher rate of cesarean section. The reports on the anesthetic modality for cesarean deliveries in patients with severe OI (type III or IV) indicate the multiple challenges to anesthesiologists (Table 1).
TABLE 1.
Anesthetic challenges expected and the management.
| Anesthetic concern | Challenges expected | Management in our case |
|---|---|---|
| Limited neck mobility, a Mallampati IV classification, a short thyromental distance and dysgenic teeth | Potential difficult airway | Awake fibrobronchoscope‐guided endotracheal intubation |
| Brittle bones and limb deformities |
|
|
| Severe scoliosis |
|
|
| Risk of malignant hyperthermia | Increased risk of GA | Inhalation anesthetics and succinylcholine avoided |
We present a case of a 36‐year‐old woman with OI type IV with severe scoliosis and an anticipated difficult airway undergoing cesarean section. We describe details on perioperative assessment and strategy of anesthesia, particularly on awake fibrobronchoscope‐guided endotracheal intubation.
Written informed consent was obtained from the patient for the collection of samples and the publication of medical data.
2. CASE PRESENTATION
A 36‐year‐old gravida 2 para 0 woman was admitted at 27 + 4 weeks to wait for delivery because of OI. During her pregnancy, she had sporadic chest tightness and shortness of breath. She had been frequently hospitalized due to repeated limb fractures in childhood and was definitively diagnosed with OI by genetic testing. She entered in a wheelchair and had a short stature of 100 cm height and 34 kg weight. Her sclera was white. She presented with a barrel‐shaped chest and severe scoliosis. She had bilateral hip dysplasia and short limbs with significant deformities. X‐ray images of the patient obtained after surgery showed the deformities of spine and limbs (Figure 1). The muscle strength of both lower extremities was abnormally low, and movement was limited. The left lower extremity had grade III muscle strength, and the patient could lift it off the bed for a few seconds; the right lower extremity had grade II–III muscle strength, and the patient could lift the knee joint, but not the ankle joint, off the bed.
FIGURE 1.
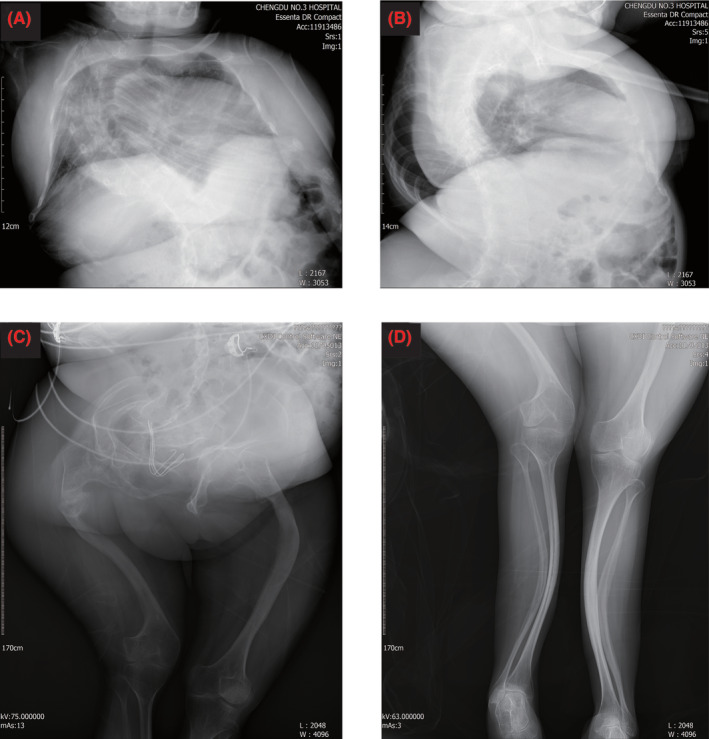
X‐ray images of the patient taken after surgery. (A, B) X‐ray of chest, severe scoliosis was observed. (C, D) X‐ray of lower extremity, dysplasia of both hips and short limbs with significant deformities were showed.
Cardiac ultrasound suggested mild enlargement of the left atrium, and the electrocardiogram was normal. Pulmonary function suggested minor airway dysfunction. Laboratory results including hematology, coagulation studies, and renal function tests were within the normal range. The patient received liver‐protective treatment because of her liver function tests (ALT 106 U/L, AST 68.2 U/L).
The preoperative airway examination revealed a mouth opening of two finger breadths, a Mallampati class IV classification, a short and restricted neck, missing lower teeth, and irregular upper teeth (Figure 2). This was clearly a case at risk of having a difficult airway as expected. At a multidisciplinary consultation, general anesthesia with an awake fibrobronchoscope‐guided endotracheal intubation was planned. The anesthetic management in such a patient was challenging.
FIGURE 2.
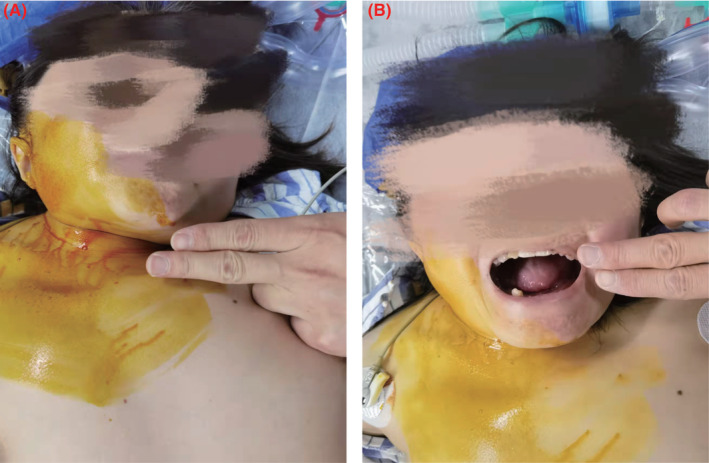
Preoperative airway examination. (A) Short and restricted neck. (B) Restricted mouth opening, a Mallampati class IV classification, missing lower teeth, and irregular upper teeth.
Persistent elevation of liver enzymes prompted cesarean section at 30 + 2 weeks of gestation. Once the patient was admitted to the operating room, standard monitoring including HR and SPO2 was performed. An arterial cannula was placed in the right radial artery in addition to standard monitoring, and the arterial blood gas results were pH 7.43, pCO2 26.9 mmHg, pO2 90 mmHg, HCO3 18 mEq/L, and base excess −5 mEq/L. Ultrasound‐guided placement of the right internal jugular vein was performed.
The anesthesiologist provided topical local anesthesia to the airway by applying lidocaine mucilage (1%) to the patient's throat. However, the patient had a sensitive throat and could not tolerate this and vomited after a few seconds. The anesthesiologist then decided to spray the local anesthetic onto her tongue and epiglottal root with 1% tetracaine in small, repeated doses via a laryngeal anesthesia tube. The patient still felt discomfort but did not vomit. After approximately 30 min, supraglottic airway anesthesia was achieved. The anesthesiologist immediately performed a cricothyroid puncture with 4 mL of 2% lidocaine to achieve subglottic airway anesthesia. After 5 min, a 6.0 mm tracheal tube was successfully placed with guidance from the fibroscope. The intubation depth was 19 cm measured at the lips. During the procedure, the patient did not choke, cough, or experience significant discomfort. Considering the possibility of airway edema, which could become an emergent difficult airway, the otolaryngologist remained at the bedside with the tracheostomy tray ready for emergency airway access.
After inflation of the cuff and confirmation of positioning by the fibroscope and end‐tidal carbon dioxide, general anesthesia was induced with remifentanil 40 μg, etomidate 16 mg, and cisatracurium 10 mg. Anesthesia was maintained with propofol (plasma target controlled infusion, 2.5 μg/mL) and remifentanil (plasma target controlled infusion, 2.5 ng/mL). Midazolam (2 mg), sufentanil (15 μg), and dexmedetomidine (20 μg/h) were added after the birth. An oxygen–air mixture was delivered through closed‐circuit pressure‐controlled ventilation. Lung protective ventilation strategies were performed: tidal volume (VT) of 6 mL/kg, respiratory rate (RR) of 16 times/min, inspiration: expiration (I:E) ratio of 1:2, and 5 cmH2O PEEP. The end‐expiratory carbon dioxide pressure (PetCO2) fluctuated between 35 and 40 mmHg during the operation.
A female infant 980 g in weight and 35 cm in length with normal morphology was delivered. The baby had mild neonatal asphyxia with Apgar scores of 6, 8, and 8 after 1, 5, and 10 min, respectively, and was transferred to the neonatal intensive care unit with tracheal intubation. Oxytocin (10 μ) and hemabate (250 μg) were administered to the myometrium to reduce the risk of uterine atony. The operation lasted 45 min, and estimated blood loss was 500 mL. Crystalloid (700 mL) and colloidal solutions (200 mL) were applied, and urine volume was 250 mL. At the end of the surgery, the arterial blood gas results were pH 7.35, pCO2 39.4 mmHg, pO2 253 mmHg, HCO3 21.8 mEq/L, and base excess −3 mEq/L. The patient was transferred to the intensive care unit for ventilatory support. Patient‐controlled intravenous analgesia with butorphanol was used for pain control until postoperative Day 2. She returned to the obstetrics department after 2 days and was discharged home 8 days later.
3. DISCUSSION
OI is an inherited disorder of connective tissue with multiple complications including bone fragility, short stature, and scoliosis. Cesarean delivery is often performed for other maternal or fetal indications. 5 Management of the complications of OI in a pregnant patient necessitates significant anesthetic planning and multidisciplinary communication. In this case, we describe the general anesthesia management for a cesarean section in a patient with severe OI, and particularly the strategies for managing an anticipated difficult airway.
Both general anesthesia and neuraxial anesthesia have been reported for management of cesarean delivery of a parturient with OI. 6 , 7 However, severe scoliosis precluded the use of neuraxial anesthesia in our case.
The Canadian Airway Focus Group provided definitions for a “difficult airway” and its individual components, encompassing problems encountered with facemask ventilation, insertion of supraglottic airway devices, laryngoscopy, tracheal intubation, and front‐of‐neck airway access. 8 In the general obstetric population, the predictors associated with difficult intubation appear to be the same as for non‐pregnant patients, including high Mallampati score, short neck, receding mandible, protruding maxillary incisors, and increased neck circumference. 9 Airway examination of this patient revealed a potentially difficult airway as difficult tracheal intubation due to a reduced cervical spine range of motion, a Mallampati class IV classification, dysgenic teeth, and a short thyromental distance. Tracheal intubation using glidescope can be difficult and may increase the risk of atlantoaxial dislocation, mandibular fracture, or cervical fracture during airway manipulation. 10 The use of a supraglottic airway has been reported in patients with OI, and the safety of a laryngeal mask airway in cesarean sections has also been reported. 6 , 11 However, a laryngeal mask airway is not the preferred technique for a patient with OI undergoing cesarean delivery. Mushambi, et al. advised that, if general anesthesia for pregnant women with anticipated airway difficulty is required, a risk assessment must be made as to the probability of safe airway management after the induction of anesthesia, and awake tracheal intubation should be used if this cannot be assured. 12 Therefore, we finally opted for a fiberoptic‐guided awake tracheal intubation.
However, it was difficult to topicalize the airway on account of the patient's sensitive pharynx. To avoid an emergent difficult airway and to ensure the patient's safety, we chose to administer local anesthetic slowly and repeatedly in small amounts. An ultrasound‐guided bilateral superior laryngeal nerve block may reduce the patient discomfort. 13 Since we were not familiar with this technology, we gave up. In this case, we had prepared strategies for both an anticipated difficult airway and an emergent difficult airway (the otolaryngologist remained at the bedside).
A parturient with OI may pose other challenges for anesthesiologists. Given the nature of OI, extreme care should be taken to avoid fractures. In severe cases, invasive blood pressure management may be advisable to reduce the risk of fracture from noninvasive blood pressure cuffs. 14 Considering the increased risk of uterine atony, inhaled anesthetic was avoided, a central vein was prepared, and blood products were available. Although genetic and muscle contracture testing did not confirm a diagnosis of malignant hyperthermia, a higher risk of perioperative hyperthermia has been reported; therefore, inhalation anesthetics and succinylcholine were avoided in favor of total intravenous anesthesia. 15
In summary, the management of a pregnant patient with OI demands multidisciplinary collaboration. Anesthesiologists may meet many challenges, especially an anticipated difficult airway. We need to pay particular attention, such as taking advantage of the most familiar tools and preparing emergency airway plans.
AUTHOR CONTRIBUTIONS
Tao Hu: Data curation; writing – original draft. Tao Chen: Writing – original draft. Lin Luo: Data curation. Ying Zhou: Data curation. Yu Zhang: Supervision. Qiang Fu: Conceptualization; writing – review and editing.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST STATEMENT
No potential conflict of interest relevant to this article was reported.
ETHICAL APPROVAL
This study was approved by the Medical Ethics Committee of The Third People's Hospital of Chengdu and was conducted in accordance with the Declaration of Helsinki.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Hu T, Chen T, Luo L, Zhou Y, Zhang Y, Fu Q. The anesthetic consideration of a gravid patient with osteogenesis imperfecta undergoing cesarean section: A case report.. Clin Case Rep. 2023;11:e7179. doi: 10.1002/ccr3.7179
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Rossi V, Lee B, Marom R. Osteogenesis imperfecta: advancements in genetics and treatment. Curr Opin Pediatr. 2019;31:708‐715. doi: 10.1097/mop.0000000000000813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marom R, Rabenhorst BM, Morello R. Osteogenesis imperfecta: an update on clinical features and therapies. Eur J Endocrinol. 2020;183:R95‐R106. doi: 10.1530/eje-20-0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Dijk FS, Cobben JM, Maugeri A, et al. Osteogenesis imperfecta: clinical and genetic heterogeneity. Ned Tijdschr Geneeskund. 2012;156:A4585. [PubMed] [Google Scholar]
- 4. Ruiter‐Ligeti J, Czuzoj‐Shulman N, Spence AR, et al. Pregnancy outcomes in women with osteogenesis imperfecta: a retrospective cohort study. J Perinatol. 2016;36:828‐831. doi: 10.1038/jp.2016.111 [DOI] [PubMed] [Google Scholar]
- 5. Rao R, Cuthbertson D, Nagamani SCS, et al. Pregnancy in women with osteogenesis imperfecta: pregnancy characteristics, maternal, and neonatal outcomes. Am J Obstet Gynecol MFM. 2021;3:100362. doi: 10.1016/j.ajogmf.2021.100362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sutton CD, Carvalho B. Supraglottic airway rescue after failed fiberoptic intubation in a patient with osteogenesis imperfecta: a case report. A&A Practice. 2019;13:7‐9. doi: 10.1213/xaa.0000000000000968 [DOI] [PubMed] [Google Scholar]
- 7. Dinges E, Ortner C, Bollag L, et al. Osteogenesis imperfecta: cesarean deliveries in identical twins. Int J Obstet Anesth. 2015;24:64‐68. doi: 10.1016/j.ijoa.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 8. Law JA, Duggan LV, Asselin M, et al. Canadian airway focus group updated consensus‐based recommendations for management of the difficult airway: part 2. Planning and implementing safe management of the patient with an anticipated difficult airway. Can J Anaesth. 2021;68:1405‐1436. doi: 10.1007/s12630-021-02008-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rocke DA, Murray WB, Rout CC, et al. Relative risk analysis of factors associated with difficult intubation in obstetric anesthesia. Anesthesiology. 1992;77:67‐73. doi: 10.1097/00000542-199207000-00010 [DOI] [PubMed] [Google Scholar]
- 10. Gupta A, Kamal G, Gupta N, et al. Combined spinal‐epidural anesthesia with dexmedetomidine‐based sedation for multiple corrective osteotomies in a child with osteogenesis imperfecta type III: a case report. A & A Case Rep. 2017;9:60‐63. doi: 10.1213/xaa.0000000000000527 [DOI] [PubMed] [Google Scholar]
- 11. Kostopanagiotou G, Coussi T, Tsaroucha N, et al. Anaesthesia using a laryngeal mask airway in a patient with osteogenesis imperfecta. Anaesthesia. 2000;55:506. doi: 10.1046/j.1365-2044.2000.01425-28.x [DOI] [PubMed] [Google Scholar]
- 12. Mushambi MC, Athanassoglou V, Kinsella SM. Anticipated difficult airway during obstetric general anaesthesia: narrative literature review and management recommendations. Anaesthesia. 2020;75:945‐961. doi: 10.1111/anae.15007 [DOI] [PubMed] [Google Scholar]
- 13. Wang H, Huang X, Wu A, et al. Management of anesthesia in a patient with osteogenesis imperfecta and multiple fractures: a case report and review of the literature. J Int Med Res. 2021;49:3000605211028420. doi: 10.1177/03000605211028420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oakley I, Reece LP. Anesthetic implications for the patient with osteogenesis imperfecta. AANA J. 2010;78:47‐53. [PubMed] [Google Scholar]
- 15. Fürderer S, Stanek A, Karbowski A, et al. Intraoperative hyperpyrexia in patients with osteogenesis imperfecta. Z Orthop Ihre Grenzgeb. 2000;138:136‐139. doi: 10.1055/s-2000-10128 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


