Highlights
-
•
The prevalence of antibiotic use in the outpatient setting in Sierra Leone is 31.8%.
-
•
Overall antibiotic consumption was 55.3 defined daily doses (DDDs) per 1000 outpatient-days.
-
•
Access antibiotics accounted for 38.6 DDDs per 1000 outpatient-days (69.8%).
-
•
Reserve antibiotics were not prescribed, probably because they were not available.
Keywords: Defined daily dose; AWaRe; Antibiotic consumption; Antibiotic use; Freetown, Sierra Leone; Antimicrobial resistance
Abstract
Objective
As there are no country-representative data on bacterial sensitivities to guide antimicrobial stewardship (AMS) interventions, an AMS programme was established in the outpatient clinics of three tertiary hospitals in Freetown, Sierra Leone.
Methods
The study employed a cross-sectional design to collect antibiotic prescribing data from 370 pregnant women and lactating mothers, 314 children and 229 regular patients in the outpatient clinics of the Princess Christian Maternity Hospital (PCMH), Ola During Children's Hospital and Connaught Hospital (CH), respectively, in April 2022. All data were analysed using Stata Version 16.
Results
Of 913 patients, most were female (n=635, 69.5%), treated at PCMH (n=370, 40.5%) and had a bacterial infection (n=661, 72.4%). The indication for prescribing antibiotics was inappropriate in 252 (27.6%) patients. Of the 1236 prescriptions, 393 (31.8%) were made at CH. The duration of antibiotic use was not stated in 230 (18.6%) prescriptions. Overall antibiotic consumption was 55.3 defined daily doses per 1000 outpatient-days.
Conclusion
Gaps in antibiotic prescriptions were identified in the outpatient clinics of three national referral hospitals in Sierra Leone. In order to combat antimicrobial resistance, AMS interventions are needed to reduce the prescription of antibiotics for inappropriate indications or without specified duration.
Introduction
Antimicrobial resistance (AMR) is a growing public health problem. AMR prolongs hospital stay, increases morbidity and mortality, diverts financial resources that could otherwise be used for improving health, and threatens global efforts to combat major infectious diseases [1,2]. Amongst the drivers of AMR, inappropriate antibiotic use and consumption in both healthcare and community settings is a major contributor to its growing burden [3,4]. Interventions to reduce inappropriate use and consumption of antibiotics are important tools in the fight against AMR [3]. Therefore, to reduce the burden of AMR, a functional antimicrobial stewardship programme (ASP) is required [4,5]. Antimicrobial stewardship (AMS) is a combination of interventions aimed at ensuring the appropriate use of anti-infective drugs to curb the emergence of resistance and preserve the potency of existing antimicrobials [5]. AMS is one of the three pillars of an integrated approach to strengthening health systems, and when its interventions are combined with robust antimicrobial surveillance systems, it can optimize antimicrobial use and reduce the development of resistance [6]. While AMS is widely implemented in high-income countries, reports suggest that the implementation of AMS in low- and middle-income countries can be challenging [5]. AMS interventions, while generic in principle, must be tailored to the specific environment in which they will be deployed [7]. Hence, these interventions must take into account locally generated data on the use of antimicrobials, antibiotic resistance, and infection prevention and control. Therefore, the synthesis of local normative data forms the basis for context-specific AMS interventions [4].
Sierra Leone is a low-income country with a per-capita income of $488 [8]. The country's health infrastructure was adversely affected by a decade-long civil war between 1991 and 2002 [9] and, more recently, by the 2014–2016 West African Ebola epidemic that claimed the lives of over 221 healthcare workers [10]. Both represented major challenges to the implementation of interventions in the prevention and control of diseases, including AMS, as they exacerbated an already-challenging human resource situation [11]. Currently, Sierra Leone has limited data on bacterial sensitivity to guide national AMS interventions. However, data from small studies have shown a high burden of antibiotic resistance, and identified gaps in hand hygiene and antibiotic prescribing practices [12], [13], [14], [15], [16], [17].
If this trend continues, it will seriously affect Sierra Leone's efforts to care for patients with both communicable and non-communicable diseases. In order to address this challenge, an ASP was established in 2022 in the outpatient clinics of three tertiary hospitals in the University of Sierra Leone Teaching Hospitals Complex (USLTHC), which encompasses several teaching hospitals in Freetown, the capital city, to carry out AMS activity. Amongst these activities, the outpatient ASP assesses antibiotic prescribing patterns, provides feedback through stakeholder engagement, reviews and updates antibiotic prescription protocols regularly, and tracks routine antibiotic consumption in the hospitals.
This paper reports the baseline findings of this ASP, and identifies areas of improvement in antibiotic prescribing practices in the outpatient clinics of three tertiary hospitals in Sierra Leone.
Methods
Study design, study population and timeline
This study employed a descriptive cross-sectional study design to collect data on antibiotic prescribing for patients in the outpatient settings of USLTHC in April 2022, including pregnant women, lactating mothers and children. Patients were recruited from Princess Christian Maternity Hospital (PCMH), Ola During Children's Hospital (ODCH) and Connaught Hospital (CH).
Study setting
General study settings
Sierra Leone is divided into five geographic regions, including the Western Area. The Western Area, which includes Freetown, is the most densely populated region of Sierra Leone. As of 2015, Sierra Leone reported a national population of approximately 7 million people, of which 22% (1.5 million people) reside in the Western Area [18]. Approximately 42% of the 10,350 healthcare workers employed by the Government reside in the Western Area [11].
Sierra Leone's health system is divided into three tiers of care: primary, secondary and tertiary. The national and regional hospitals provide tertiary care. Sierra Leone has 25 public hospitals, 10 of which provide tertiary services [11].
Specific study settings
Seven of the 10 tertiary hospitals in Sierra Leone are located in the Western Area, and three of them are national referral hospitals within USLTHC. These three tertiary hospitals – CH, PCMH and ODCH – are government-owned with capacities of 300, 199 and 121 beds, respectively. All hospitals provide inpatient and outpatient services for different populations. PCMH provides maternal and gynaecological services, while ODCH and CH provide services for children and regular patients, respectively.
Sample size and recruitment
Fisher's formula for single proportion studies was used to calculate the sample size by applying a coefficient interval of 95% (1.96); a degree of precision of 5%; and estimated prevalence rates of 81.8%, 68.4% and 26.0% among regular adult patients, pregnant women and lactating mothers, and children, respectively [19], [20], [21]: n = Z2 × p (1 − p)/d2. The minimum sample sizes calculated were 296 children, 332 pregnant women and lactating mothers, and 229 regular adult patients.
Data collection
Data for this study were collected by three nurses, with one nurse recruited from each of the three hospitals of interest. These nurses were designated as ‘AMS Focal Persons’ and trained on the study protocol and data collection procedures prior to the onset of patient enrolment. Convenience sampling was employed to enrol patients seen at the outpatient clinics of PCMH, ODCH and CH sequentially. Each day, the AMS Focal Person would review the case files of patients seen that day in order to assess prescription patterns and collect data on a study instrument which was encrypted into Epicollect (Epic, Verona, WI, USA). Patients who were admitted to the ward following an outpatient hospital visit were excluded from this study.
Diagnostic categories of patients prescribed antibiotics
The diagnoses of patients who were prescribed antibiotics were divided into:
-
•
Clinical bacterial infection: when a patient develops an infection that is clinically suspected to be of bacterial origin and thus requires empiric antibiotics.
-
•No clinical bacterial infection; these patients did not require antibiotics because the diagnosis they were being treated for was either unspecified or not a clinical bacterial infection. It is inappropriate to prescribe antibiotics to patients in these categories:
-
○‘No diagnosis’ when there was absence of a diagnosis.
-
○‘Non-bacterial process with HIV’ when a patient with human immunodeficiency virus had either a non-bacterial infective process or a non-infective process.
-
○‘Non-infective process’ when a patient had a non-communicable disease without evidence of infection.
-
○‘Non-bacterial infective process’ when a patient had an infection which was designated as non-bacterial in origin.
-
○
Definitions and categorization of antibiotic prescriptions
Antibiotics were categorized using the AWaRe framework and the Anatomic Therapeutic Classification (ATC) System based on the World Health Organization (WHO) Essential Model List [22], [23], [24]. Antibiotic consumption was estimated using WHO-assigned defined daily doses (DDDs) [23]. The DDDs were calculated by converting the total amount of antibiotic dispensed into grams. The resulting values were divided by the WHO-assigned DDDs based on the 2022 version of the ATC/DDD index [23]. The DDD per 1000 outpatient-days is calculated as follows: [consumption in DDDs/(number of outpatients visits during study x duration of study] x 1000. Total DDDs per 1000 outpatient-days and antibiotic consumption per 1000 outpatient-days were calculated for each hospital and by sex, using the number of outpatient visits for each hospital, and number of outpatient visits by sex during the study period, respectively.
Statistical analysis
After data collection was completed, data were exported into an Excel file (Microsoft Corp., Redmond, WA, USA), cleaned, coded and then transferred into Stata Version 16 (StataCorp LLC, College Station, TX, USA) for analysis. Frequencies and percentages were used to present demographic and clinical characteristics of study participants, as well as antibiotic consumption. Multi-variable regression models were built to estimate the association between inappropriate indications for antibiotic prescription and variables of interest. Poisson regression was used to model the number of antibiotics prescribed. For all models, the hospital, hospital unit, sex and age categories were entered sequentially. Variables were retained in the model if the estimate of hospital unit was >10%, or if the standard error of the estimate was reduced. Interactions between retention variables and hospital units were assessed, and a likelihood ratio test was conducted to determine if the model that included the interaction term was better. If this test was significant, the interaction term was retained in the model. All tests were two-tailed and significance was set at P=0.05.
Results
Demographic characteristics of the study participants
In total, 2830 patients were seen at the outpatient clinics of PCMH, ODCH and CH in April 2022; of these, 913 (32%) patients were included in the analysis (PCMH = 370, ODCH = 314, CH = 229). The majority of patients were women (n=635, 69.5%), and their median age was 22 years, with most being aged 18–44 years (n=499, 54.7%) (Table 1).
Table 1.
Demographic characteristics of study participants.
| Parameter | Total n (%) | CH n (%) | PCMH n (%) | ODCH n (%) |
|---|---|---|---|---|
| Overall total | 913 (100) | 229 (25.1) | 370 (40.5) | 314 (34.4) |
| Sex | ||||
| Female | 635 (69.5) | 117 (51.1) | 370 (100) | 148 (47.1) |
| Male | 278 (30.5) | 112 (48.9) | 0 (0) | 166 (52.9) |
| Age (years) | ||||
| <12 | 320 (35.1) | 11 (4.8) | 0 (0) | 309 (98.4) |
| 12–17 | 22 (2.4) | 8 (3.5) | 11 (3.0) | 3 (1.0) |
| 18–44 | 499 (54.7) | 139 (60.7) | 359 (97.0) | 1 (0.3) |
| ≥45 | 72 (7.9) | 71 (31.0) | 0 (0) | 1 (0.3) |
| Median (IQR) | ||||
| Marital status | ||||
| Single | 157 (17.2) | 39 (17.0) | 118 (31.9) | 0 (0) |
| Married | 285 (31.2) | 52 (22.7) | 232 (62.7) | 1 (0.3) |
| Separated/divorced/widowed | 8 (0.9) | 8 (3.5) | 0 (0) | 0 (0) |
| Not applicable | 130 (56.8) | 130 (56.8) | 20 (5.4) | 313 (99.7) |
| Occupation | ||||
| Formal | 71 (7.8) | 41 (17.9) | 30 (8.1) | 0 (0) |
| Informal | 356 (39.0) | 105 (45.9) | 250 (67.6) | 1 (0.3) |
| Retired | 7 (0.8) | 7 (3.1) | 0 (0) | 0 (0) |
| Student | 121 (13.3) | 46 (20.1) | 75 (20.3) | 0 (0) |
| Unemployed | 11 (1.2) | 0 (0) | 8 (2.2) | 3 (1.0) |
| Not applicable | 347 (38.0) | 30 (13.1) | 7 (1.9) | 310 (98.7) |
| Unit | ||||
| ANC | 281 (30.8) | 0 (0) | 281 (75.9) | 0 (0) |
| Medical | 204 (22.3) | 203 (88.7) | 0 (0) | 1 (0.3) |
| O&G | 89 (9.8) | 0 (0) | 89 (24.1) | 0 (0) |
| Paediatrics | 315 (34.5) | 2 (0.9) | 0 (0) | 313 (99.7) |
| Surgical | 24 (2.6) | 24 (10.5) | 0 (0) | 0 (0) |
| Patient category | ||||
| Child | 321 (35.2) | 8 (3.5) | 0 (0) | 313 (99.7) |
| Lactating mother | 6 (0.7) | 0 (0) | 6 (1.6) | 0 (0) |
| Pregnant woman | 364 (39.9) | 0 (0) | 364 (98.4) | 0 (0) |
| Regular | 222 (24.3) | 221 (96.5) | 0 (0) | 1 (0.3) |
CH, Connaught Hospital; PCMH, Princess Christian Maternity Hospital; ODCH, Ola During Children's Hospital; ANC, antenatal care; O&G, obstetrics & gynaecology; IQR, interquartile range.
Indications for prescribing antibiotics and antibiotic prescribing patterns
Most patients had a clinical bacterial infection (n=661, 72.4%), and 35 (3.8%) patients had no indications for prescribing antibiotics. Common clinical bacterial infections for prescribing antibiotics were pneumonia and other respiratory tract infections (n=324, 49.0%), and sexually transmitted and other genital infections (n=92, 14.9%) (Table 2). The majority of patients without an indication for antibiotic prescription were female (n=24, 68.6%), aged 18–44 years (n=24, 68.6%), received care at PMCH (n=20, 57.1%), and were pregnant (n=17, 48.6%) (Table 3). Nearly one-third of patients (n=252, 27.6%) had an inappropriate or no indication for antibiotic prescription (Table 3). The majority of patients with inappropriate or no indications for antibiotic prescription were seen for care at CH (n=121, 48.0%), and were either adult regular patients (n=117, 46.4%) or pregnant women (n=111, 44.4%) (Table 3).
Table 2.
Diagnosis of patients with suspected bacterial infections who received antibiotics.
| Parameter | Total n (%) | CH n (%) | PCMH n (%) | ODCH n (%) |
|---|---|---|---|---|
| Suspected bacterial infections | 661 (72.4) | 108 (47.2) | 254 (71.8) | 299 (94.6) |
| Pneumonia and other respiratory tract infections | 324 (49.0) | 47 (43.5) | 5 (2.0) | 272 (91.0%) |
| Sexually transmitted and other genital infections | 92 (13.9) | 21 (19.4) | 176 (69.2) | 0 (0.0) |
| Gastroenteritis and other gastrointestinal infections | 26 (3.9) | 12 (11.1) | 1 (0.4) | 13 (4.4%) |
| Skin and soft tissue infections | 23 (3.5) | 10 (9.3) | 1 (0.40 | 12 (4.0) |
| Urinary tract infections | 7 (1.5) | 12 (13.0) | 71 (28.0) | |
| Others: unspecified febrile illness, osteomyelitis and sepsis with undefined focus | 6 (0.9) | 5 (4.6) | 0 (0.0) | 1 (0.3) |
CH, Connaught Hospital; PCMH, Princess Christian Maternity Hospital; ODCH, Ola During Children's Hospital.
Table 3.
Different diagnostic categories displayed by variables of interest.
| Parameter | ND n (%) | BIP n (%) | nBIP +HIV n (%) | nIP n (%) | nBIP n (%) | InAdm n (%) |
|---|---|---|---|---|---|---|
| Overall total | 35 (3.8) | 661 (72.4) | 2 (0.2) | 169 (18.5) | 46 (5.0) | 252 (27.6) |
| Sex | ||||||
| Female | 24 (68.6) | 459 (69.4) | 2 (100) | 117 (69.2) | 33 (71.7) | 176 (68.7) |
| Male | 11 (31.4) | 202 (30.6) | 0 (0) | 52 (30.8) | 13 (28.3) | 79 (31.3) |
| Age (years) | ||||||
| <12 | 6 (17.1) | 297 (44.9) | 0 (0) | 11 (6.5) | 6 (13.0) | 23 (9.1) |
| 12–17 | 2 (5.7) | 12 (1.8) | 0 (0) | 5 (3.0) | 3 (6.5) | 10 (4.0) |
| 18–44 | 24 (68.6) | 324 (49.0) | 1 (50) | 121 (71.6) | 29 (63.0) | 174 (69.0) |
| ≥45 | 3 (8.6) | 28 (4.2) | 1 (50) | 32 (18.9) | 8 (17.4) | 45 (17.9) |
| Hospital | ||||||
| CH | 10 (28.6) | 108 (16.3) | 2 (100) | 84 (49.7) | 25 (54.4) | 121 (48.0) |
| PCMH | 20 (57.1) | 254 (38.4) | 0 (0) | 81 (47.9) | 15 (32.6) | 115 (45.6) |
| ODCH | 5 (14.3) | 299 (45.2) | 0 (0) | 4 (2.4) | 6 (13.0) | 16 (6.4) |
| Unit | ||||||
| ANC | 16 (45.7) | 181 (27.4) | 0 (0) | 77 (45.6) | 7 (15.2) | 100 (39.7) |
| Medical | 8 (22.9) | 102 (15.4) | 2 (100) | 68 (40.2) | 24 (52.2) | 100 (39.7) |
| O&G | 4 (11.4) | 73 (11.0) | 0 (0) | 4 (2.4) | 8 (17.4) | 16 (6.3) |
| Paediatric | 5 (14.3) | 299 (45.2) | 0 (0) | 5 (3.0) | 6 (13.0) | 16 (6.3) |
| Surgical | 2 (5.7) | 6 (0.9) | 0 (0) | 15 (8.9) | 1 (2.2) | 20 (8.0) |
| Patient category | ||||||
| Child | 5 (14.3) | 302 (45.7) | 0 (0) | 8 (4.7) | 6 (13.0) | 19 (7.5) |
| Lactating mother | 3 (8.6) | 1 (0.2) | 0 (0) | 0 (0) | 2 (4.4) | 5 (2.0) |
| Pregnant woman | 17 (48.6) | 253 (38.3) | 0 (0) | 81 (47.9) | 13 (28.3) | 111 (44.1) |
| Regular | 10 (28.6) | 105 (15.9) | 2 (100) | 80 (47.3) | 25 (54.3) | 117 (46.4) |
ND, no diagnosis; BIP, bacterial infective process; nBIP+HIV, non-bacterial infective process with human immunodeficiency virus; nIP, non-infective process; InAdmin, inappropriate indications for the administration of antibiotics; CH, Connaught Hospital; PCMH, Princess Christian Maternity Hospital; ODCH, Ola During Children's Hospital; ANC, antenatal care; O&G, obstetrics & gynaecology.
Whilst 638 (69.9%) patients were prescribed one antibiotic, 245 (26.8%) and 30 (3.2%) patients, respectively, received two or at least three antibiotics. The percentage of encounters with an antibiotic prescribed was 32.3%. An average of 84.1% of antibiotics were prescribed using their generic name. The mean number of drugs prescribed per encounter was 1.3, and the mean number of injectable antibiotics per encounter was 4.3 (Table 4).
Table 4.
World Health Organization (WHO) prescribing indicators.
| WHO prescribing indicator | Total | Documented practice | WHO standard reference |
|---|---|---|---|
| Percentage of encounters with an antibiotic prescribed | 2830 | 32.3% | 20.0–26.8% |
| Mean number of antibiotics prescribed per encounter | 913 | 1.3 | 1.6–1.8 |
| Percentage of antibiotics prescribed by generic name | 1236 | 84.1% | 100.0% |
| Mean number of injectable antibiotics per encounter | 1199 | 4.3 | 13.4–24.1 |
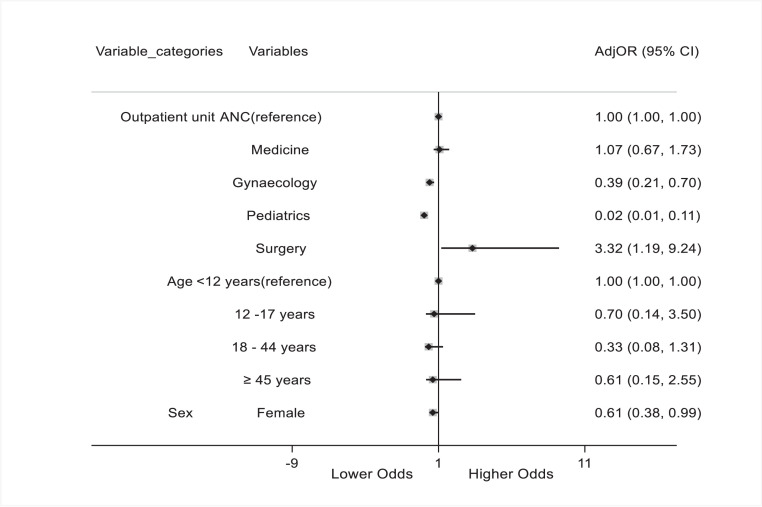
Following multi-variable logistic regression, patients in the paediatric and obstetrics outpatient units were significantly less likely to receive antibiotics for inappropriate indications compared with patients in the antenatal care unit [adjusted odds ratio (aOR) 0.39, 95% confidence interval (CI) 0.21–0.70; P=0.002 vs aOR 0.02, 95% CI 0.01–0.11; P<0.001]. Only patients in the specialist outpatient unit were significantly more likely to receive antibiotics for inappropriate indications compared with patients in the antenatal care unit. Overall, female patients were less likely to receive antibiotics for inappropriate indications compared with male patients (aOR 0.61, 95% CI 0.38–0.99; P=0.047] (Figure 1).
Figure 1.
Multi-variable logistic regression analysis of variables associated with inappropriate administration of antibiotics among outpatients in three hospitals in Freetown. ANC, antenatal care; AdjRR, adjusted risk ratio; CI, confidence interval.
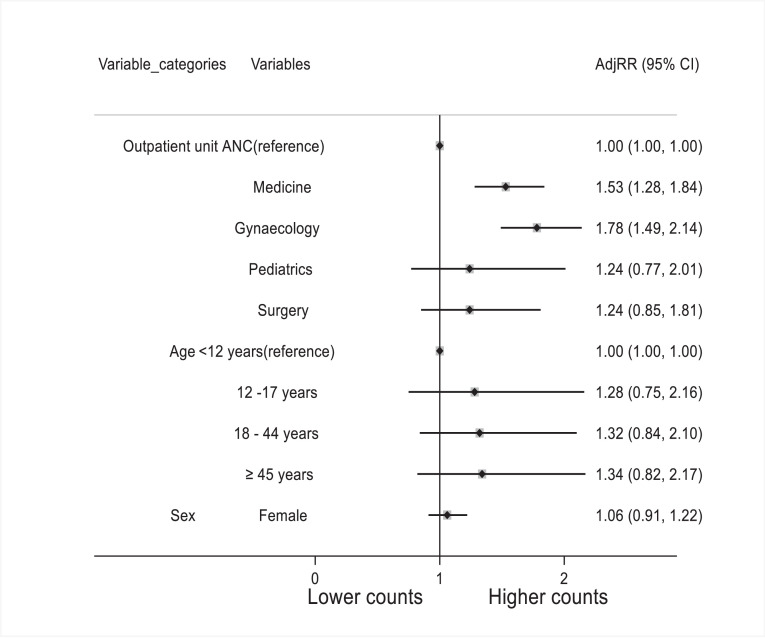
Modelling the number of prescribed antibiotics showed a 53% increase in the number of antibiotics prescribed to patients in the medical outpatient unit compared with patients in the antenatal unit [adjusted risk ratio (aRR) 1.53, 95% CI 1.28–1.84; P<0.001]. Likewise, the number of prescribed antibiotics in the obstetrics outpatient unit increased by 78% (aRR 1.78, 95% CI 1.49–2.14; P<0.001) (Figure 2).
Figure 2.
Poisson regression analysis of variables associated with number of antibiotics administered to outpatients in three hospitals in Freetown. ANC, antenatal care; AdjOR, adjusted odds ratio; CI, confidence interval.
Antibiotic consumption
In total, 1236 drug administrations were made, of which 393 (31.8%) were made at CH, 509 (41.2%) were made at PMCH and 334 (27.0%) were made at ODCH. Overall, the most commonly administered antibiotic in all of the outpatient visits to the three hospitals was amoxicillin (n=514, 51.1%). At ODCH and PCMH, amoxicillin was the most frequently administered antibiotic. The most frequently administered antibiotic at CH was amoxicillin-clavulanate (n=63, 21%). As the duration of antibiotic prescription was not stated, data for calculation of antibiotic consumption was incomplete for 230 (18.6%) administrations. These were eliminated, and only 1006 drug administrations were used in the calculation of consumption. Amoxicillin had the highest consumption at 21.9 DDDs per 1000 outpatient-days (Table 5).
Table 5.
Antibiotics and frequency of administration, AWaRe category and antibiotic consumption [in defined daily doses (DDDs) per 1000 outpatient-days].
| Variable | ATC code | AWaRe category | DDDs/1000 outpatient-days n (%) 55.3 (100) | Drug administration |
|||
|---|---|---|---|---|---|---|---|
| Total n (%) | CH n (%) | PCMH n (%) | ODCH n (%) | ||||
| Amoxicillin | J01CA04 | Access | 21.9 (36.6) | 514 (51.1) | 8 (2.7) | 246 (62.6) | 260 (83.1) |
| Amoxicillin-clavulanate | J01CR02 | Access | 4.67 (8.45) | 72 (7.2) | 63 (21.0) | - | 9 (2.9) |
| Amoxicillin-cloxacillin | J01CR51 | Access | 0.39 (0.71) | 26 (2.6) | 10 (3.3) | - | 16 (5.1) |
| Ampicillin | J01CA01 | Access | 0.04 (0.07) | 9 (0.9) | 1 (0.3) | 7 (1.8) | 1 (0.3) |
| Ampicillin-cloxacillin | J01CR50 | Access | 0.04 (0.07) | 2 (0.2) | 2 (0.7) | - | - |
| Azythromycin | J01DB01 | Watch | 0.87 (1.57) | 59 (5.9) | 49 (16.4) | 9 (2.3) | 1 (0.3) |
| Cefixime | J01DD08 | Watch | 2.36 (4.27) | 13 (1.3) | 13 (4.3) | - | - |
| Ceftriaxone | J01DD04 | Watch | 0.00 (0.00) | 19 (1.9) | 16 (5.3) | 2 (0.5) | 1 (0.3) |
| Cefuroxime | J01DC02 | Watch | 3.04 (5.50) | 12 (1.2) | 11 (3.7) | - | 1 (0.3) |
| Ciprofloxacin | J01MA02 | Watch | 4.70 (8.50) | 32 (3.2) | 20 (6.7) | - | 12 (3.8) |
| Clarithromycin | J01FA09 | Watch | 4.09 (7.40) | 12 (1.2) | 12 (4.0) | - | - |
| Clindamycin | J01FF01 | Access | 0.36 (0.65) | 5 (0.5) | 5 (1.7) | - | - |
| Doxycycline | J01AA02 | Access | 1.77 (3.20) | 8 (0.8) | 8 (2.6) | - | - |
| Erythromycin | J01FA01 | Watch | 0.20 (0.36) | 3 (0.3) | 1 (0.3) | - | 2 (0.6) |
| Flucloxacillin | J01CF05 | Access | 0.27 (0.49) | 5 (0.5) | 5 (1.7) | - | - |
| Gentamycin | J01GB03 | Access | 0.01 (0.02) | 2 (0.2) | - | 1 (0.3) | 1 (0.3) |
| Levofloxacin | J01MA12 | Watch | 1.51 (2.73) | 12 (1.2) | 12 (4.0) | - | - |
| Metronidazole | P01AB01 | Access | 7.94 (14.4) | 176 (17.5) | 39 (13.0) | 128 (32.6) | 9 (2.9) |
| Penicillin VK | J01CE02 | Access | 0.02 (0.04) | 1 (0.1) | 1 (0.3) | - | - |
| Tinidazole | P01AB02 | Access | 1.06 (1.92) | 22 (2.2) | 22 (7.3) | - | - |
| Tinifloxacin | J01RA13 | Access | 0.03 (0.05) | 2 (0.2) | 2 (0.7) | - | - |
CH, Connaught Hospital; PCMH, Princess Christian Maternity Hospital; ODCH, Ola During Children's Hospital.
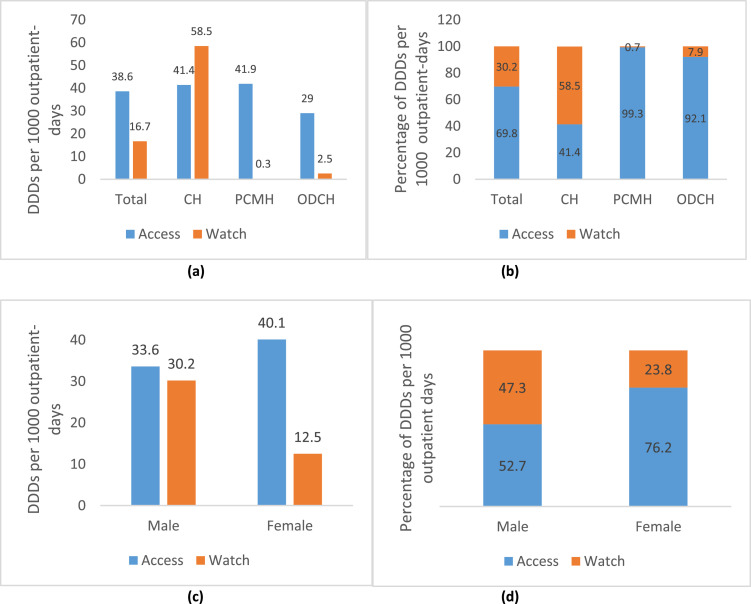
Overall antibiotic consumption was 55.3 DDDs per 1000 outpatient-days. Antibiotics in the Access class accounted for 38.6 DDDs per 1000 outpatient-days (69.8%), whereas antibiotics in the Watch class accounted for 16.7 DDDs per 1000 outpatient-days (30.2%). Total antibiotic consumption was 99.9 DDDs per 1000 outpatient-days at CH, 42.2 DDDs per 1000 outpatient-days at PCMH, and 31.5 DDDs per 1000 outpatient-days at ODCH. At PCMH and ODCH, Access antibiotics accounted for >90% of antibiotic consumption. Antibiotic consumption for males and females was 63.8 DDDs per 1000 outpatient-days and 52.6 DDDs per 1000 outpatient-days, respectively. Access antibiotics accounted for 76.2% of antibiotic consumption among females. No antibiotics in the Reserve class were utilized (Figure 3).
Figure 3.
(a) Total antibiotic consumption presented as defined daily doses (DDDs) per 1000 outpatient-days by World Health Organization (WHO) AWaRe category in three hospitals in Freetown. (b) Proportion of total antibiotic DDDs per 1000 outpatient-days by WHO AWaRe category in the hospitals. (c) Total antibiotic consumption presented as DDDs per 1000 outpatient-days by WHO AWaRe category according to sex. (d) Proportion of total antibiotic DDDs per 1000 outpatient-days by WHO AWaRe category according to sex. CH, Connaught Hospital; PCMH, Princess Christian Maternity Hospital; ODCH, Ola During Children's Hospital.
Discussion
This large-scale study on antibiotic prescribing patterns in the outpatient clinics of the three national referral hospitals of Sierra Leone reported that 32.2% of patients visiting the outpatient clinics were prescribed an antibiotic. This is lower than the figures reported previously for antibiotic prescriptions among hospitalized patients in the medical and surgical wards of a tertiary hospital in Sierra Leone (82%), and antibiotic prescriptions for surgical prophylaxis in four hospitals in two geographic regions of Sierra Leone (96%) [16,19]. Likewise, among suspected (47%) and confirmed (61%) cases of coronavirus disease 2019, the prevalence of antibiotic prescription was also higher [17]. These differences in antibiotic prescribing patterns were expected because this study was conducted in a different setting and applied different methods. This emphasizes the need to diversify AMS interventions in different hospital settings using context-specific data. Nonetheless, the prevalence rates of antibiotic prescribing in the outpatient clinics of these hospitals were higher than reported in outpatient facilities in Beijing, China (15%) [25] and Uganda (25%) [26]. This variation could be explained by the fact that there were no AMS structures to support the prescription of antibiotics in these hospitals in Sierra Leone before this study. The reported prevalence of antibiotic prescription (32.2%) is well above the WHO-recommended standard of 20–26.8% prevalence [27,28], reinforcing the need for more rigorous AMS activities and evidence-based recommendations to help guide antibiotic decision-making amongst clinicians at these hospitals. Thus, the authors used three ‘Rs’ to identify the gaps in prescribing antibiotics in the outpatient clinics of the three hospitals that, if addressed, will reduce inappropriate antibiotic prescription; right indication, right duration and right drug. Nearly one-third of patients prescribed an antibiotic had no indication for their use. These poor prescribing practices can be explained partly by the fact that two of the three hospitals provide free healthcare services for children aged <5 years, pregnant women and lactating mothers. Prescription of antibiotics can be influenced by the fact that the drugs are free and, in some cases, can be requested even when not needed. This fact further explains why the pregnant women in this study received significantly higher levels of antibiotics for inappropriate indications, even though nearly all of them were Access antibiotics. Another plausible reason for the inappropriate prescription of antibiotics may be related to the limited knowledge of healthcare workers about rational antibiotic prescribing, as clinicians tend to prescribe antibiotics as a safety net. In ongoing efforts to support AMS interventions in the outpatient clinics of these national referral hospitals, healthcare workers are being trained and mentored to optimize their antibiotic prescribing practices. Suspected bacterial infections were the reasons for prescribing antibiotics in >72% of patients. Whether the prescription of antibiotics for this empirical diagnosis reflects the treatment of a true bacterial infection is a question that can be answered during the implementation of AMS practices in these settings. Most importantly, antibiotic prescriptions should be guided by strong AMS structures to prevent inappropriate use and the development of resistance. Unfortunately, there are no AMS structures in most hospitals in Sierra Leone. One strategy employed to address these gaps was to initiate an outpatient ASP in the three national referral hospitals. For approximately 19% of the prescriptions, the duration of antibiotic prescription was not stated. The right duration is critical when implementing AMS interventions [29]. Clinicians are being educated to address this gap. Despite the prescription challenges described above, this ASP identified several positive antibiotic prescribing practices that can be built on and reinforced. First, the estimated antibiotic consumption of 55.3 DDDs per 1000 outpatient-days is lower than the 117.9 DDD per 100 bed-days of surgical antibiotic prophylaxis reported in a recent study [16]. Although the DDD of 55.3 per 1000 outpatient visits is higher than the reported national antibiotic consumption of 19 DDD per 1000 inhabitants per day [30], it can be reduced further by ongoing interventions to optimize the use of antimicrobial agents. Second, unlike studies conducted in China [31] and Ethiopia [32], the most commonly prescribed antibiotics in this study were in the Access class (J01CA04 amoxicillin and P01AB01 metronidazole).
Using the WHO/International Network for Rational Use of Drugs core prescribing indicators, the average number of antibiotics per encounter is lower than reported in Tanzania (1.30 vs 1.99), and is even below the recommended standard of 1.6–1.8 [28,33]. Although not optimal for CH (41.4%) and male patients (52.7%), narrow-spectrum Access antibiotics accounted for approximately 70% of prescriptions in this study, which is above the WHO-recommended threshold of 60%. These are good prescribing practices and will be reinforced in the ASP to reduce the selection pressure associated with the broad-spectrum Watch and Reserve antibiotics [34]. The lack of prescriptions for the Reserve antibiotics, although expected in these outpatient settings, may reflect unavailability as no Reserve antibiotics were reported in the 2017–2019 national antibiotic consumption estimate [30].
This study has strengths and limitations. While this is the first large-scale study to assess outpatient prescribing patterns in public hospitals and reflect on AMS interventions in Sierra Leone, it does not include primary and secondary health facilities that provide more outpatient services. An arbitrary method was used to categorize indications or diagnoses for outpatient antibiotic prescriptions in the study hospitals. Although it is an innovative approach, the authors did not verify diagnoses made by the clinicians at the bedside. Therefore, the findings may not represent the actual indications for which antibiotics were prescribed in these settings. Finally, antibiotic prescribing in the outpatient settings of any private facilities was not evaluated; this could be undertaken in a future project.
Conclusion
This study found some good AMS practices and identified gaps in antibiotic prescriptions in the outpatient clinics of three national referral hospitals in Sierra Leone. To combat the global health challenges of AMR, AMS interventions are needed at all levels to reduce the prescription of antibiotics for inappropriate indications or without specified duration.
Funding
This work was supported by Pfizer-ISID (International Society for Infectious Diseases) Global Medical Grant, Grant Number: 68687159.
Ethical approval
Ethical approval was obtained from the Sierra Leone Ethics and Scientific Review Committee of the Ministry of Health and Sanitation, Government of Sierra Leone. Written informed consent was not required for this study as the data on antibiotic prescribing were collected from patients’ case files.
Availability of data and materials
The data supporting this article are available in the repository of University of Sierra Leone, and will be made easily available on request to the corresponding author when required.
Author contributions
Conceptualization: S.L., I.F.K., R.D.C.L., O.A., D.F.J. and V.J.C.
Design: J.B.W.R., D.F.J., S.L., N.B. and B.D.F.
Data collection: U.B. and M.N.K.
Data analysis: E.F., U.B. and S.L.
Writing: S.L., E.F., T.D.M., S.K., U.B., G.F.D., G.A.Y., O.T.A., A.M., F.J.C, J.M.C and A.O.A.
All authors read and approved the final manuscript.
Conflict of interest statement
E.F. receives salary from the European Union's Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement (No 801076), through SSPH+ Global PhD Fellowship Programme in Public Health Sciences (GlobalP3HS). G.A.Y. reports salary support from the National Institutes of Health/AIDS Clinical Trials Group under Award Numbers 5UM1AI068636-15, 5UM1AI069501-09 and AI068636 (150GYD212), and consultancy fees from Pfizer. The other authors declare that they have no competing interests.
Acknowledgements
The authors appreciate the support provided by the administrations of the hospitals where the studies were conducted. In addition, the authors wish to thank the research team members, and acknowledge the cooperation of the ward staff, patients and patients’ relatives at the hospitals where the study was conducted.
References
- 1.World Health Organization . WHO; Geneva: 2015. Global action plan on antimicrobial resistance.https://www.who.int/publications/i/item/9789241509763 Available at: accessed 4 March 2022. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill J. Review on Antimicrobial Resistance; London: 2016. Tackling drug-resistant infections globally: final report and recommendations.https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf Available at: accessed 4 March 2022. [Google Scholar]
- 3.Collignon P, Beggs JJ, Walsh TR, Gandra S, Laxminarayan R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet Health. 2018;2:e398–e405. doi: 10.1016/S2542-5196(18)30186-4. [DOI] [PubMed] [Google Scholar]
- 4.Pierce J, Apisarnthanarak A, Schellack N, Cornistein W, Maani AA, Adnan S, et al. Global antimicrobial stewardship with a focus on low- and middle-income countries. Int J Infect Dis. 2020;96:621–629. doi: 10.1016/j.ijid.2020.05.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox JA, Vlieghe E, Mendelson M, Wertheim H, Ndegwa L, Villegas MV, et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect. 2017;23:812–818. doi: 10.1016/j.cmi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO; Geneva: 2019. Antimicrobial stewardship programmes in healthcare facilities in low-and-middle income countries.https://apps.who.int/iris/bitstream/handle/10665/329404/9789241515481-eng.pdf Available at: accessed 22 July 2022. [Google Scholar]
- 7.International Monetary Fund . IMF; Washington, DC: 2019. Sierra Leone: economic development documents. National Development Plan, 2019–23.https://www.imf.org/en/Publications/CR/Issues/2019/07/09/Sierra-Leone-Economic-Development-Documents-National-Development-Plan-2019-23-47099 Available at: accessed 14 January 2022. [Google Scholar]
- 8.Kaldor M, Vincent J. United Nations Development Programme Evaluation Office; New York: 2006. Evaluation of UNDP assistance to conflict-affected countries. Case study: Sierra Leone.https://cdn.sida.se/publications/files/sida48039en-sidas-support-to-undp-in-sierra-leone.pdf Available at: accessed 4 July 2022. [Google Scholar]
- 9.Ministry of Health and Sanitation. Government of Sierra Leone . Ministry of Health and Sanitation, Government of Sierra Leone; Freetown: 2015. Ebola viral disease situation report.https://reliefweb.int/sites/reliefweb.int/files/resources/Ebola-Situation-Report_Vol-260.pdf Available at: accessed 2 April 2022. [Google Scholar]
- 10.Ministry of Health and Sanitation, Government of Sierra Leone . Ministry of Health and Sanitation, Government of Sierra Leone; Freetown: 2021. Human resources for health strategy 2017–2021.https://www.afro.who.int/sites/default/files/2017-05/hrhstrategy2017.pdf Available at. accessed 1 March 2023. [Google Scholar]
- 11.Lakoh S, Li L, Sevalie S, Guo X, Adekanmbi O, Yang G, et al. Antibiotic resistance in patients with clinical features of healthcare-associated infections in an urban tertiary hospital in Sierra Leone: a cross-sectional study. Antimicrob Resist Infect Control. 2020;9:38. doi: 10.1186/s13756-020-0701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakoh S, Yi L, Sevalie S, Guo X, Adekanmbi O, Smalle IO, et al. Incidence and risk factors of surgical site infections and related antibiotic resistance in Freetown, Sierra Leone: a prospective cohort study. Antimicrob Resist Infect Control. 2022;11:39. doi: 10.1186/s13756-022-01078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakoh S, Maruta A, Kallon C, Deen GF, Russell JBW, Fofanah BD, et al. How well are hand hygiene practices and promotion implemented in Sierra Leone? A cross-sectional study in 13 public hospitals. Int J Environ Res Public Health. 2022;19:3787. doi: 10.3390/ijerph19073787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakoh S, Firima E, Williams CEE, Conteh SK, Jalloh MB, Sheku MG, et al. An intra-COVID-19 assessment of hand hygiene facility, policy and staff compliance in two hospitals in Sierra Leone: is there a difference between regional and capital city hospitals? Trop Med Infect Dis. 2021;6:204. doi: 10.3390/tropicalmed6040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakoh S, Yi L, Russell JBW, Zhang J, Sevalie S, Zhao Y, et al. The burden of surgical site infections and related antibiotic resistance in two geographic regions of Sierra Leone: a prospective study. Ther Adv Infect Dis. 2022;9 doi: 10.1177/20499361221135128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakoh S, Kanu JS, Conteh SK, Russell JBW, Sevalie S, Williams CEE, et al. High levels of surgical antibiotic prophylaxis: implications for hospital-based antibiotic stewardship in Sierra Leone. Antimicrob Steward Healthcare Epidemiol. 2022;2:e111. doi: 10.1017/ash.2022.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamara IF, Kumar AMV, Maruta A, Fofanah BD, Njuguna CK, Shongwe S, et al. Antibiotic use in suspected and confirmed COVID-19 patients admitted to health facilities in Sierra Leone in 2020–2021: practice does not follow policy. Int J Environ Res Public Health. 2022;19:4005. doi: 10.3390/ijerph19074005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Statistics Sierra Leone . SSL; Freetown: 2015. Sierra Leone 2015 population and housing census.https://www.statistics.sl/images/StatisticsSL/Documents/Census/2015/sl_2015_phc_thematic_report_on_pop_structure_and_pop_distribution.pdf Available at: accessed 14 January 2022. [Google Scholar]
- 19.Lakoh S, Adekanmbi O, Jiba DF, Deen GF, Gashau W, Sevalie S, et al. Antibiotic use among hospitalized adult patients in a setting with limited laboratory infrastructure in Freetown, Sierra Leone, 2017–2018. Int J Infect Dis. 2020;90:71–76. doi: 10.1016/j.ijid.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Kabba JA, James PB, Li Z, Hanson C, Chang J, Kitchen C, et al. Prescribing for patients seeking maternal and child healthcare in Sierra Leone: a multiregional retrospective cross-sectional assessments of prescribing pattern using WHO drug use indicators. Risk Manag Healthc Policy. 2020;13:2525–2534. doi: 10.2147/RMHP.S256648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole CP, Routledge P. An evaluation of rational prescribing in hospital outpatient practice in Sierra Leone and assessment of affordability of a prescription as an outcome. Pan Afr Med J. 2018;31:174. doi: 10.11604/pamj.2018.31.174.16729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization . WHO; Geneva: 2021. 2021 AWaRe classification.https://www.who.int/publications/i/item/2021-aware-classification Available at: accessed 3 December 2021. [Google Scholar]
- 23.WHO Collaborating Centre for Drug Statistics Methodology . WHO Collaborating Centre for Drug Statistics Methodology; Oslo: 2022. Guidelines for ATC classification and DDD assignment, 2023.https://www.whocc.no/atc_ddd_index_and_guidelines/guidelines/ Available at: accessed 2 December 2021. [Google Scholar]
- 24.World Health Organization . WHO; Geneva: 2021. Model list of essential medicines, 22nd list.https://apps.who.int/iris/bitstream/handle/10665/345533/WHO-MHP-HPS-EML-2021.02-eng.pdf Available at: accessed 2 December 2021. [Google Scholar]
- 25.Wushouer H, Du K, Chen S, Zhou Y, Zheng B, Guan X, et al. Outpatient antibiotic prescribing patterns and appropriateness for children in primary healthcare settings in Beijing City, China, 2017–2019. Antibiotics (Basel) 2021;10:1248. doi: 10.3390/antibiotics10101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonniface M, Nambatya W, Rajab K. An evaluation of antibiotic prescribing practices in a rural refugee settlement district in Uganda. Antibiotics (Basel) 2021;10:172. doi: 10.3390/antibiotics10020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desalegn AA. Assessment of drug use pattern using WHO prescribing indicators at Hawassa University Teaching and Referral Hospital, south Ethiopia: a cross-sectional study. BMC Health Serv Res. 2013;13:170. doi: 10.1186/1472-6963-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization . WHO; Geneva: 1993. How to investigate drug use in health facilities: selected drug use indicators.https://apps.who.int/iris/bitstream/handle/10665/60519/WHO_DAP_93.1.pdf Available at: accessed 1 March 2023. [Google Scholar]
- 29.Goebel MC, Trautner BW, Grigoryan L. The five Ds of outpatient antibiotic stewardship for urinary tract infections. Clin Microbiol Rev. 2021;34 doi: 10.1128/CMR.00003-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanu JS, Khogali M, Hann K, Tao W, Barlatt S, Komeh J, et al. National antibiotic consumption for human use in Sierra Leone (2017–2019): a cross-sectional study. Trop Med Infect Dis. 2021;6:77. doi: 10.3390/tropicalmed6020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White AT, Clark CM, Sellick JA, Mergenhagen KA. Antibiotic stewardship targets in the outpatient setting. Am J Infect Control. 2019;47:858–863. doi: 10.1016/j.ajic.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Fu M, Wushouer H, Hu L, Li N, Guan X, Shi L, et al. Outpatient prescribing pattern for acute bronchitis in primary healthcare settings in China. NPJ Prim Care Respir Med. 2021;31:24. doi: 10.1038/s41533-021-00234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilipamwambu A, Bwire GM, Myemba DT, Njiro BJ, Majigo MV. WHO/INRUD core prescribing indicators and antibiotic utilization patterns among primary health care facilities in Ilala district, Tanzania. JAC Antimicrob Resist. 2021;3 doi: 10.1093/jacamr/dlab049. dlab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharland M, Gandra S, Huttner B, Moja L, Pulcini C, Zeng M, et al. EML Expert Committee and Antibiotic Working Group Encouraging AWaRe-ness and discouraging inappropriate antibiotic use-the new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect Dis. 2019;19:1278–1280. doi: 10.1016/S1473-3099(19)30532-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this article are available in the repository of University of Sierra Leone, and will be made easily available on request to the corresponding author when required.