Abstract
Aims:
We analyzed the impact of frailty on readmission rates for ST-elevated myocardial infarctions (STEMIs) and the utilization of percutaneous coronary intervention (PCI) in STEMI admissions.
Methods and Results:
The 2016–2019 Nationwide Readmission Database was analyzed for patients admitted with an acute STEMI. Patients were categorized by frailty risk and analyzed for 30-day readmission risk after acute STEMIs, PCI utilization and outcomes, and healthcare resource utilization.
Qualifying index admissions were found in 584,918 visits. Low risk frailty was noted in 78.20%, intermediate risk in 20.67%, and high risk in 1.14% of admissions. Thirtyday readmissions occurred in 7.74% of index admissions, increasing with frailty (p < 0.001). Readmission risk increased with frailty, 1.37 times with intermediate and 1.21 times with high-risk frailty.
PCI was performed in 86.40% of low-risk, 66.03% of intermediate-risk, and 58.90% of high-risk patients (p < 0.001). Intermediate patients were 55.02% less likely and high-risk patients were 61.26% less likely to undergo PCI (p < 0.001). Length of stay means for index admissions were 2.96, 7.83, and 16.32 days for low, intermediate, and high-risk groups. Intermediate and high-risk frailty had longer length of stay, higher total cost, and were more likely to be discharged to a skilled facility (p < 0.001).
Conclusion:
Among adult, all-payer inpatient visits, frailty discerned by the hospital frailty risk score was associated with increased readmissions, increased healthcare resource utilization, and lower PCI administration.
Keywords: acute myocardial infarction, frailty, percutaneous intervention, readmission, STEMI
1 |. INTRODUCTION
Frailty is a dynamic clinical syndrome marked by limitation of physiological reserve leading to impairment in physical and mental performance.1–3 Rising life expectancy and an aging population have front lined the burden of comorbid cardiovascular conditions and their implications on healthcare’s resources and outcomes.4,5 Many studies have shown frailty to be independently associated with cardiovascular hospitalization, falls, morbidity, and long-term mortality, independent of age, comorbidity, or disability.1,6–9 Consequently, interest has risen in outcomes associated with frailty.
Frailty is increasingly recognized as an essential assessment tool in perioperative evaluation for patients undergoing invasive procedures and determining cardiovascular outcomes.10,11 Frailty is associated with single and multivessel coronary artery disease12,13 and has been identified as an independent predictor of mortality and morbidity in this population.14,15 Compared with non-frail counterparts, frailty is associated with extended hospital stays and increased hospitalization index costs, especially after percutaneous coronary intervention (PCI).12,16,17 However, the impact of frailty in healthcare utilization and outcomes may be underestimated, as frail patients are more likely to be excluded from clinical trials.18
Previously, a visually subjective Canadian study of health and aging clinical frailty scale (CFS) has shown to predict outcomes in ST-elevated myocardial infarctions (STEMI) patients undergoing PCI.19,20 However, given the subjective nature of the CFS, it is prone to observer variability bias. Additionally, lower sample size in these prior studies limits generalizability of results to the national population. We used the hospital frailty risk score (HFRS), an advantageous method, developed and validated by Gilbert et al.,19 using administrative data to predict 30-day outcomes,20 that can be easily implemented into electronic medical record systems. The HFRS has been validated against the Fried Phenotype and the Rockwood frailty index,21,22 despite requiring fewer resources for implementation, and has shown significant predictive capabilities in the United States of America, Canada, the United Kingdom, and Australia.19,23,24 Frailty has been identified as a strong independent factor in reinfarction, in-hospital mortality, and 30-day mortality for patients experiencing myocardial infarctions25 and has also shown implications in pneumonia, heart failure, acute coronary syndrome, valvular replacement, and noncardiac etiologies.26–28 The Healthcare Cost and Utilization Project’s (HCUP) National Inpatient Sample (NIS) and Nationwide Readmission Databases (NRD)29 have been analyzed to determine the effect of frailty on inflammatory bowel diseases,26 heart failure,17 transcatheter aortic valve implantations,27 and hepatocellular carcinoma.30 Recent literature has also highlighted the benefit of PCI in frail population using the NIS.21 Our study adds to this important finding by analyzing the NRD to determine the impact of frailty on readmissions in acute STEMI patients undergoing PCI. We aimed to determine the influence of frailty on readmission rates for acute STEMIs, PCI implementation, and healthcare utilization.
2 |. METHODS
We performed a retrospective cohort study utilizing the NRD from the Agency for Healthcare Research and Quality’s HCUP database from 2016 to 2019. The NRD is a database of inpatient admissions and readmissions representing about 60% of all-payer hospitalizations in the United States population.31 International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify diagnoses and procedures. Patients were included in the study if they were at least 18 years of age with a nonelective admission for an acute STEMI between January and November. Acute STEMI was defined as admissions with the primary diagnosis ICD-10-CM code I21.0x, I21.1x, I21.2x, or I21.3. Patients were excluded if admitted in December to track 30-day readmission rates.
Frailty was identified by employing methodology described by Gilbert et al.19 ICD-10 codes and weights used in calculating the frailty score can be found in Supporting Information: Table 1. Frailty scores were categorized as low (<5), intermediate (5–15), and high (>15), based on the methods in Gilbert’s hospital frailty score.19
Readmissions were identified using the unique identifier “nrd_visitlink.” Readmissions were included if they occurred after an index admission, met the inclusion criteria, and were within 30 days. Readmissions were excluded if related to a traumatic injury. Inherently, patients who died during the index hospitalization were excluded. Patients with multiple readmissions had their primary and subsequent readmissions identified and separated for analysis. Time to readmission was calculated from the day of index admission discharge to the day of readmission.
Patient demographics present on admission were obtained from reported data. The Elixhauser and Charlson comorbidity software by Quan et al.32,33 is a user-available program utilized to classify comorbidities. Additional comorbidities and admission-related procedures were identified using unique ICD-10 codes, listed in Supporting Information: Table 2.
Missing data were examined quantitatively and plotted for visualization. Little’s test was used to determine if data were missing completely at random (MCAR) with significance at p < 0.05. Data were also analyzed using the covariate-dependent missingness (CDM) assumption, an extension of Little’s test, accounting for covariates and unequal variances.34 Variables with more than 2% missing data that failed Little’s MCAR and CDM testing underwent multiple imputations (25 data sets) for sensitivity analysis.35
The effect of frailty was analyzed for the following endpoints: 30-day readmissions after acute STEMIs, PCI utilization and outcomes, and healthcare resource utilization. Descriptive trends of admissions were also described. Statistical analysis was performed using Stata 17. National estimates were obtained using discharge weights supplied by HCUP. In concordance with the inferential nature of complex survey statistics, categorical variables are presented as mean percentages with 95% confidence intervals (CI), and continuous variables are presented as means with accompanying standard errors. Pearson’s χ2 tests were used to compare categorical variables. Kaplan–Meier estimates compared survival rates between the three cohorts. Cox proportion regression analyses determined predictors of inpatient mortality. Multivariate regression analyses evaluated the effect of frailty and confounding independent variables on outcomes. Annual hospital procedural volumes were determined using the weighted quantity of PCIs by unique hospital identifier; hospital volume status was divided into quintiles based on relative procedural volumes per analyzed year. The incidence of a primary or prior PCI could not be determined due to the inherent nature of the NRD. Correlation of variables and marginal prediction was assessed for frailty score, age, and Elixhauser comorbidities. The study was determined to be exempt from Institutional Review Board review. Study design with cohort separation and outcome identification is presented in Figure 1. The data underlying this article were provided by HCUP under license.
FIGURE 1.
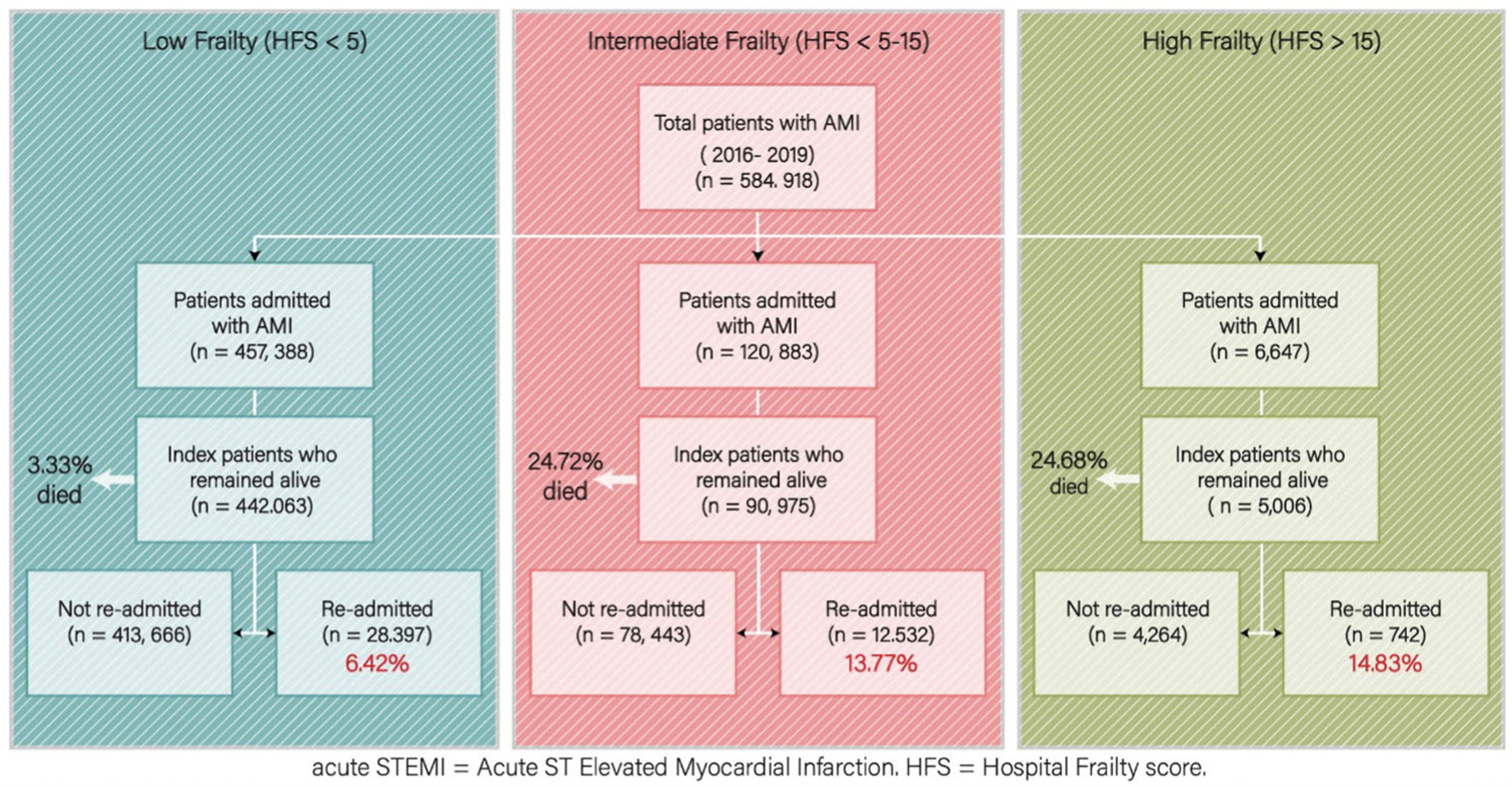
Methodology. Criteria and methods of analysis. [Color figure can be viewed at wileyonlinelibrary.com]
3 |. RESULTS
Baseline characteristics for 584,918 index admissions are included in Table 1. The proportions of patients in the low-risk group were 78.20% (n = 457,388), intermediate risk 20.67% (n = 120,883), and high risk 1.14% (n = 6647). Mean age was 63.58 ± 13.08 years and 30.63% were female.
TABLE 1.
Index admission characteristics.
| Frailty risk |
Obs | p Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (n = 457,388) |
Intermediate (n = 120,883) |
High (n = 6647) |
Total |
||||||||
| % | CI | % | CI | % | CI | % | CI | ||||
| Age, mean (SE) | 61.84 (0.03) | 69.61 (0.06) | 73.61 (0.22) | 63.58 (0.03) | 584,918 | <0.001 | |||||
| Elixhauser comorbidity sum, mean (SE) | 2.63 (0.00) | 5.26 (0.01) | 6.59 (0.04) | 3.22 (0.00) | 584,918 | <0.001 | |||||
| Charlson index, mean (SE) | 1.26 (0.00) | 2.97 (0.01) | 4.28 (0.04) | 1.65 (0.00) | 584,918 | <0.001 | |||||
| CHA2DS2-VASc score, mean (SE) | 2.38 (0.00) | 3.62 (0.01) | 4.34 (0.03) | 2.66 (0.00) | 584,918 | <0.001 | |||||
| Age 75 and over | |||||||||||
| Under 75 years old | 83.61 | [83.41–83.80] | 61.48 | [60.98–61.99] | 49.03 | [47.12–50.93] | 78.64 | [78.43–78.85] | 459,984 | <0.001 | |
| 75 or older | 16.39 | [16.20–16.59] | 38.52 | [38.01–39.02] | 50.97 | [49.07–52.88] | 21.36 | [21.15–21.57] | 124,934 | ||
| Indicator of sex | |||||||||||
| Male | 71.73 | [71.53–71.94] | 61.13 | [60.70–61.56] | 55.82 | [53.98–57.64] | 69.36 | [69.17–69.55] | 405,711 | <0.001 | |
| Female | 28.27 | [28.06–28.47] | 38.87 | [38.44–39.30] | 44.18 | [42.36–46.02] | 30.64 | [30.45–30.83] | 179,206 | ||
| History of myocardial infarction | 11.46 | [11.24–11.69] | 13.17 | [12.83–13.52] | 12.98 | [11.79–14.28] | 11.83 | [11.62–12.05] | 69,220 | <0.001 | |
| Diabetes | 28.61 | [28.35–28.86] | 40.62 | [40.14–41.11] | 43.79 | [41.99–45.59] | 31.26 | [31.01–31.52] | 182,863 | <0.001 | |
| Hypertension | 70.66 | [70.32–71.00] | 78.34 | [77.91–78.77] | 82.55 | [81.18–83.83] | 72.39 | [72.07–72.69] | 423,395 | <0.001 | |
| Dyslipidemia | 66.19 | [65.68–66.69] | 59.08 | [58.48–59.68] | 60.29 | [58.52–62.03] | 64.65 | [64.19–65.12] | 378,168 | <0.001 | |
| History of smoking | 54.28 | [53.90–54.67] | 47.50 | [46.94–48.07] | 42.13 | [40.30–43.98] | 52.74 | [52.39–53.10] | 308,515 | <0.001 | |
| History of stroke | 5.01 | [4.91–5.12] | 16.94 | [16.62–17.27] | 41.26 | [39.55–42.99] | 7.89 | [7.77–8.01] | 46,159 | <0.001 | |
| Congestive heart failure | 29.66 | [29.27–30.05] | 58.46 | [57.96–58.97] | 68.60 | [66.84–70.31] | 36.05 | [35.67–36.44] | 210,883 | <0.001 | |
| Cardiac arrhythmias | 32.89 | [32.59–33.20] | 59.72 | [59.24–60.19] | 67.82 | [66.05–69.55] | 38.83 | [38.53–39.14] | 227,151 | <0.001 | |
| Valvular disease | 7.86 | [7.66–8.07] | 15.36 | [14.96–15.77] | 19.66 | [18.29–21.10] | 9.55 | [9.33–9.76] | 55,841 | <0.001 | |
| Pulmonary circulation disorders | 1.91 | [1.83–1.99] | 6.82 | [6.59–7.05] | 8.77 | [7.73–9.93] | 3.00 | [2.91–3.09] | 17,541 | <0.001 | |
| Peripheral vascular disorders | 6.36 | [6.24–6.48] | 13.41 | [13.08–13.75] | 16.37 | [15.04–17.79] | 7.93 | [7.79–8.07] | 46,381 | <0.001 | |
| Chronic pulmonary disease | 13.34 | [13.14–13.54] | 22.26 | [21.85–22.68] | 23.14 | [21.59–24.76] | 15.29 | [15.09–15.50] | 89,450 | <0.001 | |
| Renal failure | 6.94 | [6.80–7.08] | 34.83 | [34.36–35.30] | 48.11 | [46.31–49.92] | 13.17 | [12.98–13.37] | 77,043 | <0.001 | |
| Obesity | 17.64 | [17.30–17.98] | 16.93 | [16.53–17.33] | 15.85 | [14.50–17.29] | 17.47 | [17.16–17.79] | 102,195 | <0.001 | |
| Alcohol abuse | 3.14 | [3.06–3.23] | 5.25 | [5.06–5.45] | 5.38 | [4.65–6.21] | 3.61 | [3.52–3.69] | 21,089 | <0.001 | |
| History of PCI | 1.17 | [1.11–1.24] | 1.45 | [1.34–1.57] | 1.26 | [0.94–1.70] | 1.23 | [1.17–1.30] | 7210 | <0.001 | |
| History of coronary artery bypass graft | 3.96 | [3.87–4.06] | 6.17 | [5.95–6.39] | 6.09 | [5.30–7.00] | 4.44 | [4.35–4.54] | 25,982 | <0.001 | |
| History of carotid artery occlusion | 0.80 | [0.76–0.84] | 2.13 | [2.01–2.26] | 3.91 | [3.27–4.67] | 1.11 | [1.06–1.16] | 6490 | <0.001 | |
| History of chronic ischemic heart disease | 85.94 | [85.58–86.30] | 82.79 | [82.36–83.21] | 82.26 | [80.88–83.56] | 85.25 | [84.93–85.56] | 498,626 | <0.001 | |
| History of cardiac arrest | 3.66 | [3.57–3.75] | 14.92 | [14.55–15.29] | 16.90 | [15.56–18.32] | 6.14 | [6.02–6.26] | 35,892 | <0.001 | |
| History of cardiogenic shock | 6.89 | [6.75–7.03] | 34.95 | [34.45–35.46] | 40.71 | [38.81–42.62] | 13.07 | [12.88–13.27] | 76,464 | <0.001 | |
| History of cardiac pacemaker | 0.81 | [0.77–0.85] | 1.87 | [1.76–1.98] | 2.80 | [2.24–3.50] | 1.05 | [1.01–1.09] | 6128 | <0.001 | |
| HxCard implants grafts | 0.84 | [0.80–0.88] | 1.60 | [1.49–1.72] | 1.60 | [1.18–2.15] | 1.00 | [0.96–1.05] | 5872 | <0.001 | |
| History of dialysis | 0.46 | [0.43–0.49] | 3.31 | [3.16–3.47] | 3.03 | [2.49–3.68] | 1.08 | [1.03–1.12] | 6296 | <0.001 | |
| Location and hospital characteristics | |||||||||||
| Calendar year | |||||||||||
| 2016 | 25.43 | [24.42–26.46] | 24.32 | [23.33–25.34] | 19.30 | [17.26–21.51] | 25.13 | [24.16–26.12] | 146,986 | <0.001 | |
| 2017 | 25.14 | [24.13–26.17] | 25.02 | [24.02–26.04] | 25.18 | [23.25–27.22] | 25.11 | [24.15–26.10] | 146,892 | ||
| 2018 | 24.54 | [23.56–25.55] | 24.61 | [23.62–25.63] | 26.18 | [24.18–28.29] | 24.57 | [23.63–25.54] | 143,743 | ||
| 2019 | 24.89 | [23.92–25.89] | 26.05 | [25.03–27.10] | 29.34 | [27.19–31.58] | 25.18 | [24.24–26.15] | 147,297 | ||
| Insurance carrier, cleaned | |||||||||||
| Medicare | 42.78 | [42.47–43.10] | 67.55 | [67.05–68.04] | 76.48 | [74.91–77.97] | 48.34 | [48.03–48.64] | 270,540 | <0.001 | |
| Medicaid | 10.78 | [10.53–11.04] | 9.19 | [8.88–9.51] | 7.63 | [6.71–8.66] | 10.41 | [10.18–10.65] | 58,290 | ||
| Private insurance | 39.21 | [38.87–39.55] | 19.55 | [19.15–19.96] | 14.13 | [12.88–15.48] | 34.82 | [34.51–35.13] | 194,880 | ||
| Self-pay | 7.23 | [7.01–7.45] | 3.72 | [3.51–3.93] | 1.76 | [1.33–2.33] | 6.43 | [6.24–6.63] | 36,001 | ||
| Median household income national quartile for patient ZIP code | |||||||||||
| 0–25th percentile | 28.00 | [27.16–28.86] | 28.79 | [27.89–29.70] | 29.59 | [27.72–31.53] | 28.18 | [27.36–29.03] | 162,310 | <0.001 | |
| 26th–50th percentile (median) | 28.74 | [28.11–29.38] | 28.24 | [27.57–28.93] | 26.40 | [24.66–28.22] | 28.61 | [28.00–29.23] | 164,778 | ||
| 51st–75th percentile | 25.34 | [24.76–25.93] | 24.41 | [23.80–25.03] | 24.63 | [23.00–26.34] | 25.14 | [24.58–25.71] | 144,785 | ||
| 76th–100th percentile | 17.92 | [17.19–18.66] | 18.55 | [17.74–19.40] | 19.38 | [17.84–21.02] | 18.06 | [17.34–18.81] | 104,034 | ||
| Bed size of hospital | |||||||||||
| Small | 13.21 | [12.34–14.14] | 12.77 | [11.88–13.71] | 13.69 | [11.95–15.63] | 13.12 | [12.27–14.03] | 76,769 | <0.001 | |
| Medium | 28.20 | [27.22–29.20] | 26.44 | [25.49–27.41] | 24.76 | [22.88–26.73] | 27.80 | [26.86–28.75] | 162,591 | ||
| Large | 58.59 | [57.44–59.73] | 60.79 | [59.64–61.93] | 61.56 | [59.21–63.86] | 59.08 | [57.97–60.17] | 345,559 | ||
| Hospital urban-rural designation | |||||||||||
| Large metropolitan areas with at least 1 million residents | 47.20 | [45.74–48.68] | 53.74 | [52.19–55.28] | 61.20 | [58.67–63.68] | 48.71 | [47.26–50.17] | 284,931 | <0.001 | |
| Small metropolitan areas with less than 1 million residents | 46.42 | [44.94–47.90] | 40.84 | [39.32–42.37] | 34.16 | [31.79–36.62] | 45.13 | [43.67–46.59] | 263,959 | ||
| Micropolitan areas | 5.98 | [5.37–6.64] | 4.82 | [4.35–5.35] | 4.07 | [3.27–5.05] | 5.72 | [5.15–6.33] | 33,428 | ||
| Not metropolitan or micropolitan (nonurban residual) | 0.40 | [0.29–0.56] | 0.61 | [0.50–0.73] | 0.56 | [0.33–0.97] | 0.44 | [0.34–0.58] | 2599 | ||
| Teaching status of urban hospitals | |||||||||||
| Metropolitan nonteaching | 23.23 | [22.33–24.15] | 20.42 | [19.61–21.26] | 18.57 | [17.01–20.23] | 22.59 | [21.74–23.47] | 132,158 | <0.001 | |
| Metropolitan teaching | 70.40 | [69.35–71.42] | 74.15 | [73.21–75.07] | 76.80 | [74.94–78.56] | 71.25 | [70.26–72.21] | 416,733 | ||
| Nonmetropolitan hospital | 6.38 | [5.76–7.05] | 5.43 | [4.94–5.96] | 4.63 | [3.79–5.66] | 6.16 | [5.59–6.79] | 36,028 | ||
| Patient location: NCHS urban-rural code | |||||||||||
| Central counties of metro areas of ≥1 million population | 20.29 | [19.30–21.31] | 24.24 | [23.11–25.40] | 28.08 | [25.86–30.42] | 21.19 | [20.19–22.23] | 123,565 | <0.001 | |
| Fringe counties of metro areas of ≥1 million population | 24.47 | [23.36–25.62] | 25.68 | [24.44–26.96] | 28.95 | [26.72–31.28] | 24.77 | [23.65–25.92] | 144,437 | ||
| Counties in metro areas of 250,000–999,999 population | 23.67 | [22.55–24.83] | 22.28 | [21.16–23.44] | 17.73 | [16.06–19.54] | 23.32 | [22.22–24.45] | 135,952 | ||
| Counties in metro areas of 50,000–249,999 population | 11.46 | [10.75–12.21] | 10.01 | [9.36–10.70] | 9.99 | [8.78–11.35] | 11.14 | [10.46–11.86] | 64,972 | ||
| Micropolitan counties | 11.12 | [10.54–11.72] | 9.88 | [9.36–10.43] | 8.01 | [6.88–9.30] | 10.83 | [10.28–11.40] | 63,129 | ||
| Not metropolitan or micropolitan counties | 9.00 | [8.51–9.51] | 7.92 | [7.44–8.42] | 7.24 | [6.25–8.38] | 8.75 | [8.28–9.25] | 51,033 | ||
| Admission day is a weekend | 28.84 | [28.64–29.03] | 28.18 | [27.80–28.56] | 28.10 | [26.55–29.70] | 28.69 | [28.52–28.87] | 167,830 | 0.008 | |
Note: Baseline characteristics of patients admitted for STEMI.
Abbreviations: CI, confidence intervals; PCI, percutaneous coronary intervention; SE, standard error; STEMI, ST-elevated myocardial infarctions.
3.1 |. PCI use and outcomes
PCI was performed in 86.40% of low-risk, 66.03% of intermediate-risk, and 58.90% of high-risk patients (p < 0.001). Intermediate patients were 55.02% less likely to undergo PCI (95% CI: 53.71%–56.31%; p < 0.001), while high-risk patients were 61.26% less likely to undergo PCI (95% CI: 57.74%–64.48%; p < 0.001). Cox regression analysis revealed an inpatient mortality hazard ratio 1.70 times higher (95% CI: 1.56–1.685; p < 0.001) for intermediate risk, and 21.82% lower risk (95% CI: 8.28%–33.36%; p = 0.003) for high-risk, compared to the low-risk frailty group. The Cox regression analysis is presented in Table 2.
TABLE 2.
Cox regression for inpatient mortality.
| Cox regression | |||||
|---|---|---|---|---|---|
|
|
|||||
| Hazard ratio | p Value | 95% CI | |||
| Frailty risk | |||||
| Low | 1.00 | ||||
| Intermediate | 4.10 | 0.000 | [3.91–4.31] | ||
| High | 2.56 | 0.000 | [2.33–2.82] | ||
| PCI | |||||
| Medical management | 1.00 | ||||
| Percutaneous coronary intervention | 0.48 | 0.000 | [0.45–0.52] | ||
| Intervention within 24 h | 1.25 | 0.000 | [1.16–1.35] | ||
| Hospital rank by procedure volume (quintile) | |||||
| 1st | 1.00 | ||||
| 2nd | 1.08 | 0.320 | [0.93–1.25] | ||
| 3rd | 1.05 | 0.488 | [0.91–1.22] | ||
| 4th | 1.00 | 0.947 | [0.86–1.15] | ||
| 5th | 1.01 | 0.850 | [0.87–1.18] | ||
| Age over 75 years | 1.54 | 0.000 | [1.48–1.60] | ||
| Female (sex) | 1.08 | 0.000 | [1.05–1.12] | ||
| Calendar year | |||||
| 2016 | 1.00 | ||||
| 2017 | 0.96 | 0.132 | [0.91–1.01] | ||
| 2018 | 0.97 | 0.274 | [0.92–1.02] | ||
| 2019 | 0.94 | 0.027 | [0.89–0.99] | ||
| Insurance carrier, cleaned | |||||
| Medicare | 1.00 | ||||
| Medicaid | 0.73 | 0.000 | [0.68–0.77] | ||
| Private insurance | 0.73 | 0.000 | [0.70–0.77] | ||
| Self-pay | 1.07 | 0.119 | [0.98–1.16] | ||
| Median household income national quartile for patient ZIP code | |||||
| 0–25th percentile | 1.00 | ||||
| 26th–50th percentile (median) | 1.02 | 0.386 | [0.98–1.07] | ||
| 51st–75th percentile | 0.95 | 0.050 | [0.91–1.00] | ||
| 76th–100th percentile | 0.98 | 0.415 | [0.93–1.03] | ||
| Bed size of hospital | |||||
| Small | 1.00 | ||||
| Medium | 1.04 | 0.244 | [0.97–1.11] | ||
| Large | 1.03 | 0.394 | [0.96–1.10] | ||
| Hospital urban-rural designation | |||||
| Large metropolitan areas with at least 1 million residents | 1.00 | ||||
| Small metropolitan areas with less than 1 million residents | 1.10 | 0.013 | [1.02–1.18] | ||
| Micropolitan areas | 1.07 | 0.250 | [0.95–1.20] | ||
| Not metropolitan or micropolitan (nonurban residual) | 1.01 | 0.953 | [0.69–1.49] | ||
| Teaching status of urban hospitals | |||||
| Metropolitan nonteaching | 1.00 | ||||
| Metropolitan teaching | 1.00 | 0.932 | [0.95–1.05] | ||
| Nonmetropolitan hospital | 1.00 | ||||
| Patient location: NCHS urban-rural code | |||||
| Central counties of metro areas of ≥1 million population | 1.00 | ||||
| Fringe counties of metro areas of ≥1 million population | 0.93 | 0.002 | [0.88–0.97] | ||
| Counties in metro areas of 250,000–999,999 population | 0.96 | 0.319 | [0.89–1.04] | ||
| Counties in metro areas of 50,000–249,999 population | 1.01 | 0.766 | [0.93–1.11] | ||
| Micropolitan counties | 1.01 | 0.763 | [0.93–1.10] | ||
| Not metropolitan or micropolitan counties | 0.99 | 0.860 | [0.90–1.10] | ||
| Admission day is a weekend | 1.03 | 0.080 | [1.00–1.07] | ||
| Congestive heart failure | 0.86 | 0.000 | [0.83–0.89] | ||
| Cardiac arrhythmias | 1.63 | 0.000 | [1.57–1.69] | ||
| Valvular disease | 0.97 | 0.242 | [0.93–1.02] | ||
| Renal failure | 0.96 | 0.041 | [0.92–1.00] | ||
| Diabetes | 1.17 | 0.000 | [1.13–1.21] | ||
| Hypertension | 0.83 | 0.000 | [0.80–0.86] | ||
| Dyslipidemia | 0.64 | 0.000 | [0.62–0.66] | ||
| History of smoking | 0.85 | 0.000 | [0.82–0.88] | ||
| History of PCI | 0.95 | 0.543 | [0.81–1.11] | ||
| History of CABG | 1.14 | 0.000 | [1.06–1.22] | ||
| History of carotid artery occlusion | 0.66 | 0.000 | [0.58–0.76] | ||
| History of chronic ischemic heart disease | 0.65 | 0.000 | [0.62–0.68] | ||
| History of myocardial infarction | 1.00 | 0.904 | [0.95–1.06] | ||
Note: Cox proportional hazard regression analysis for inpatient mortality.
Abbreviations: CI, confidence intervals; PCI, percutaneous coronary intervention.
PCI was associated with reduced all-cause in-hospital mortality in each frailty group (p < 0.001, all) and significantly associated with reduced early readmissions in the low and intermediate frailty groups. Supporting Information: Tables 3a, 3b, and 3c provide the multivariate regression odds ratio for inpatient mortality and early readmission for each frailty group; separate analyses are noted for any PCI, PCI by stent type and placement, and coronary artery bypass grafting. Inpatient survival after PCI is compared to overall survival by the Kaplan–Meier survival graph in Figure 2.
FIGURE 2.
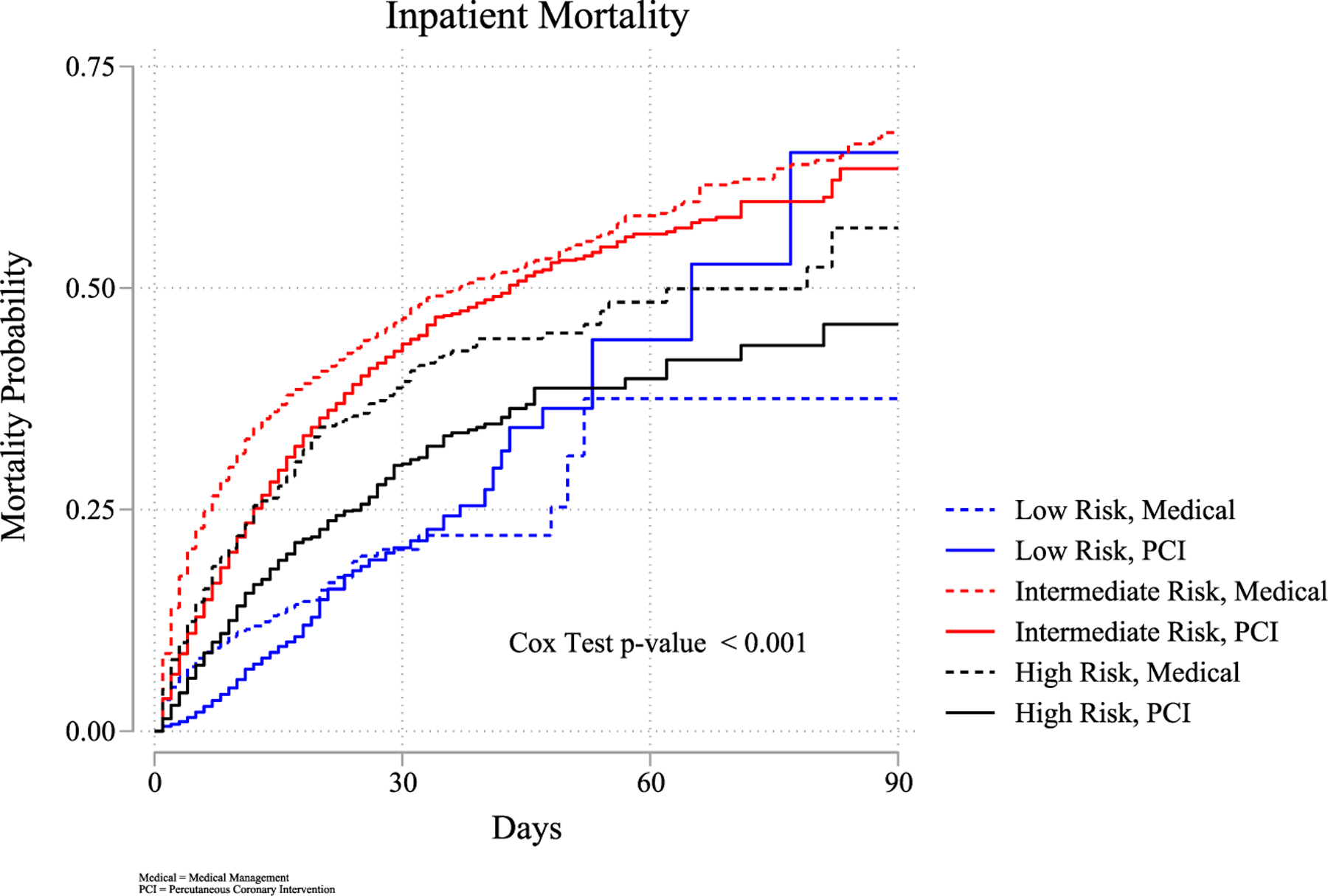
Inpatient mortality risk during index admission. Risk of inpatient mortality by frailty and PCI utilization. PCI, percutaneous coronary intervention.
3.2 |. Acute STEMI 30-day readmission
Thirty-day readmissions occurred in 7.74% of total index admissions, increasing with frailty risk (p < 0.001), at 6.42%, 13.77%, and 14.83% for low, intermediate, and high-risk groups, respectively. Multivariate regression showed that when compared to the low-risk frailty patients, intermediate-risk frail patients were 1.37 times higher risk of readmission (95% CI: 1.32–1.43; p < 0.001), and high-risk frail patients were 1.21 times higher risk of readmission (95% CI: 1.06–1.40; p = 0.005). The Kaplan–Meier readmission curve representing readmission risk by frailty group is presented in Figure 3. After discharge to a skilled facility, the adjusted odds of readmission were 1.20 (95% CI: 1.13–1.26; p < 0.001). Early readmissions accounted for 41,670 visits, totaling 185,383 days at a total cost of $584.26 million. The top 10 cause of readmissions are presented in Table 3.
FIGURE 3.
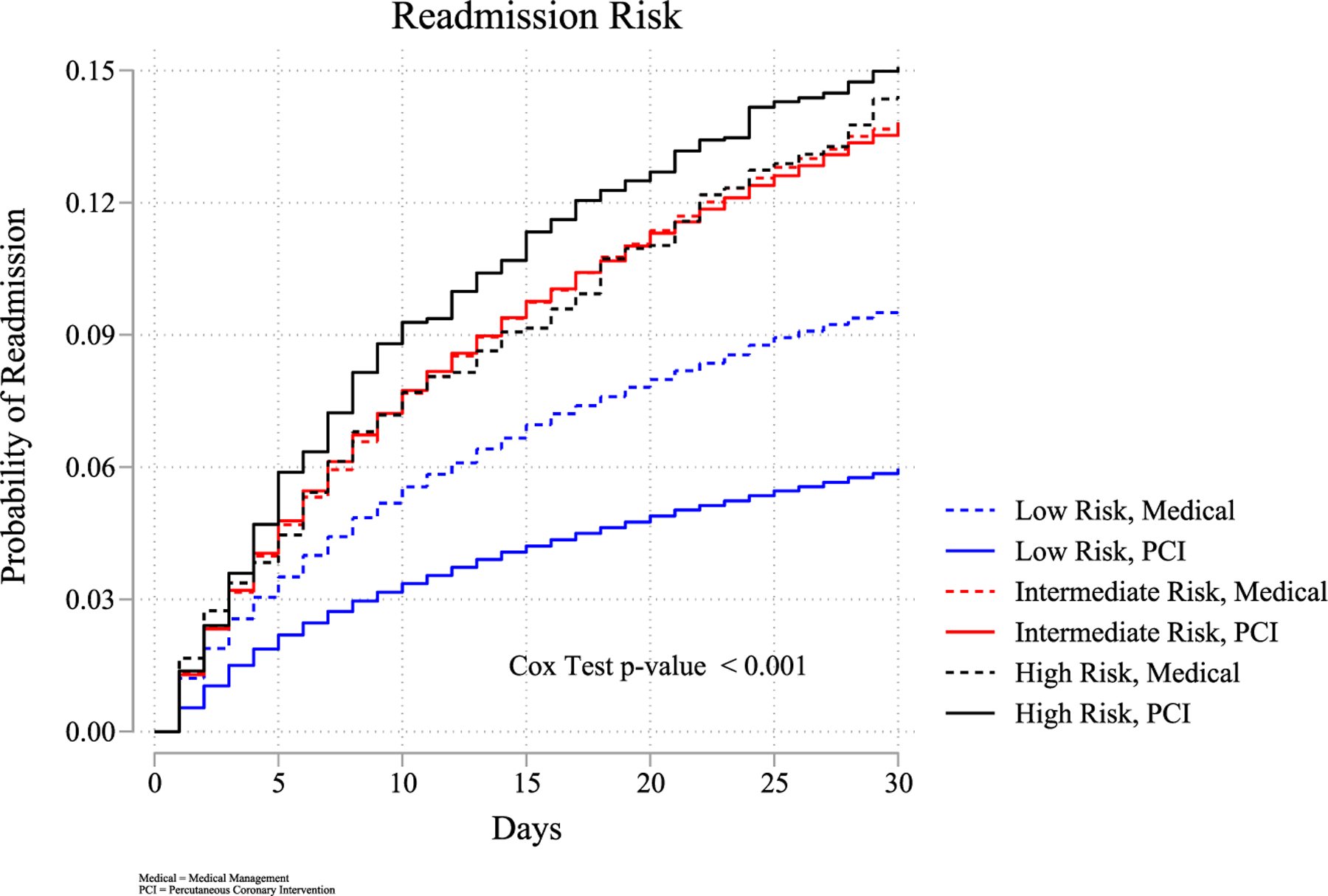
Readmission by frailty risk. Readmission risk by frailty and PCI utilization. PCI, percutaneous coronary intervention. [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 3.
Top 10 causes of readmission, overall (cardiovascular and non-cardiovascular).
| Count | Diagnosis | |
|---|---|---|
| 1. | 2883 | Hypertensive heart disease with heart failure |
| 2. | 1861 | Non-ST-elevation (NSTEMI) myocardial infarction |
| 3. | 1856 | Hypertensive heart and chronic kidney disease with heart failure |
| 4. | 1852 | Unstable angina |
| 5. | 1621 | Atherosclerotic heart disease of native coronary artery |
| 6. | 1501 | Sepsis |
| 7. | 1266 | Chest pain |
| 8. | 1115 | Acute exacerbation of chronic heart failure with reduced ejection fraction |
| 9. | 852 | Subsequent non-ST-elevation (NSTEMI) myocardial infarction |
| 10. | 820 | ST-elevation (NSTEMI) myocardial infarction |
Note: Most common diagnosis on readmission.
Abbreviation: STEMI, ST-elevated myocardial infarctions.
3.3 |. Healthcare resource utilization
Length of stay means for index admissions were 2.96, 7.83, and 16.32 days for low, intermediate, and high-risk groups (p < 0.001). Multivariate linear regression indicated 2.95 days longer stay for intermediate-risk (95% CI: 2.88–3.03; p < 0.001), and 10.43 days longer for high risk (95% CI: 9.86–11.01; p < 0.001). Length of stay distribution by frailty group is represented in Figure 4.
FIGURE 4.
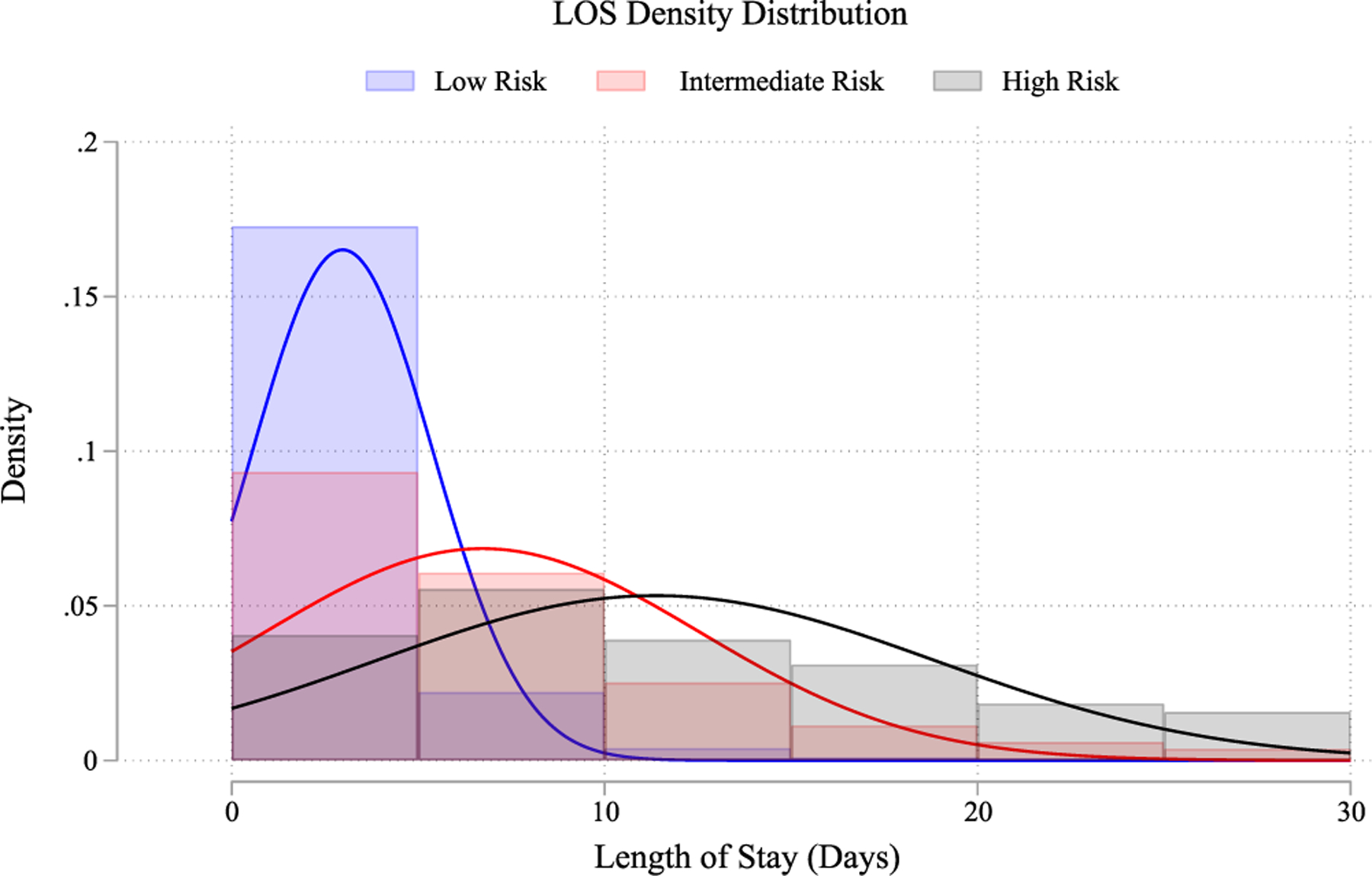
Length of stay distribution. Mean length of stay by frailty risk status. [Color figure can be viewed at wileyonlinelibrary.com]
Total costs increased significantly as frailty risk increased (p < 0.001) and are listed in Table 4. Discharge to a skilled facility also increased significantly (p < 0.001) with increasing frailty risk, 2.73% for low, 26.15% for intermediate, and 53.45% for high-risk. Multivariate logistic regression showed intermediate-risk patients were 4.31 times more likely (95% CI: 4.11–4.51; p < 0.001) and high risk 8.66 times more likely (95% CI: 7.68–9.77; p < 0.001) to be discharged to a skilled facility. Additional inpatient trends and outcome characteristics are listed in Table 4.
TABLE 4.
In hospital and outcome characteristics.
| Procedures and in hospital interventions, index admission | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low (n = 457,388) | Intermediate (n = 120,883) | High (n = 6647) | Total | Obs | p Value | |||||
| % | CI | % | CI | % | CI | % | CI | |||
| Hemodialysis, recieved inpatient | 0.19 | [0.17–0.21] | 3.66 | [3.46–3.87] | 5.94 | [5.16–6.84] | 0.97 | [0.92–1.02] | 5667 | <0.001 |
| Cardiac intervention | ||||||||||
| No intervention | 10.83 | [10.60–11.07] | 27.33 | [26.85–27.82] | 34.04 | [32.30–35.82] | 14.51 | [14.26–14.76] | 84,854 | <0.001 |
| Balloon angioplasty | 3.17 | [3.08–3.25] | 4.11 | [3.95–4.29] | 4.24 | [3.58–5.01] | 3.38 | [3.30–3.46] | 19,741 | |
| Bare metal stent | 6.08 | [5.85–6.32] | 6.95 | [6.64–7.28] | 6.44 | [5.55–7.45] | 6.27 | [6.04–6.50] | 36,645 | |
| Drug eluting stent | 75.84 | [75.46–76.22] | 52.24 | [51.66–52.82] | 45.64 | [43.88–47.41] | 70.62 | [70.24–71.00] | 413,075 | |
| Coronary artery bypass graft | 4.08 | [3.93–4.22] | 9.36 | [9.02–9.73] | 9.65 | [8.54–10.88] | 5.23 | [5.07–5.40] | 30,603 | |
| Underwent PCI within 24 h | 82.46 | [82.15–82.76] | 59.98 | [59.40–60.57] | 50.03 | [48.05–52.01] | 77.45 | [77.12–77.77] | 452,998 | <0.001 |
| Time to PCI, days (SE) | 0.14 (0.00) | 0.43 (0.01) | 0.86 (0.04) | 0.19 (0.00) | 452,998 | <0.001 | ||||
| PCI by hospital procedure volume (quintile) | ||||||||||
| 1st | 0.78 | [0.67–0.90] | 1.34 | [1.18–1.53] | 1.85 | [1.43–2.38] | 0.91 | [0.79–1.04] | 5254 | <0.001 |
| 2nd | 8.82 | [8.22–9.45] | 7.82 | [7.25–8.43] | 7.06 | [6.08–8.17] | 8.59 | [8.02–9.20] | 49,797 | |
| 3rd | 16.50 | [15.49–17.58] | 16.00 | [14.95–17.10] | 15.06 | [13.46–16.80] | 16.38 | [15.38–17.44] | 94,948 | |
| 4th | 24.54 | [23.12–26.01] | 23.77 | [22.33–25.27] | 25.21 | [22.95–27.60] | 24.39 | [22.99–25.84] | 141,330 | |
| 5th | 49.36 | [47.66–51.06] | 51.07 | [49.35–52.80] | 50.84 | [48.06–53.61] | 49.73 | [48.06–51.40] | 288,180 | |
| Encounter for palliative care | 1.23 | [1.18–1.29] | 13.50 | [13.13–13.88] | 19.95 | [18.52–21.46] | 3.98 | [3.87–4.09] | 23,280 | <0.001 |
| Do not resuscitate order | 2.79 | [2.70–2.88] | 21.47 | [21.02–21.92] | 31.63 | [29.96–33.35] | 6.97 | [6.83–7.12] | 40,792 | <0.001 |
| Use of extracorporeal membrane oxygenation | 0.11 | [0.09–0.12] | 1.78 | [1.60–1.98] | 1.44 | [1.03–2.00] | 0.47 | [0.42–0.52] | 2733 | <0.001 |
| Implantation of cardiac device | 0.97 | [0.90–1.03] | 6.45 | [6.12–6.80] | 7.12 | [6.24–8.11] | 2.17 | [2.06–2.28] | 12,696 | <0.001 |
| Implantation of cardiac ouput assitance device | 5.93 | [5.78–6.10] | 23.61 | [23.12–24.12] | 25.68 | [23.88–27.58] | 9.81 | [9.60–10.03] | 57,398 | <0.001 |
| Use of mechanical ventilator | 4.52 | [4.41–4.63] | 37.15 | [36.61–37.70] | 51.68 | [49.84–53.52] | 11.80 | [11.61–12.00] | 69,026 | <0.001 |
| Use of vasopressors | 1.03 | [0.95–1.11] | 7.27 | [6.74–7.83] | 10.53 | [9.18–12.06] | 2.42 | [2.25–2.61] | 14,179 | <0.001 |
| Transfusion of blood products | 1.08 | [1.02–1.15] | 7.46 | [7.07–7.86] | 14.07 | [12.69–15.56] | 2.55 | [2.42–2.68] | 14,901 | <0.001 |
| CPR performed in hospital | 2.08 | [2.01–2.16] | 9.81 | [9.48–10.15] | 11.33 | [10.20–12.57] | 3.78 | [3.68–3.89] | 22,134 | <0.001 |
| In hospital defibrillation or cardiovsersion | 3.46 | [3.36–3.56] | 7.79 | [7.51–8.07] | 8.86 | [7.86–9.98] | 4.42 | [4.31–4.53] | 25,825 | <0.001 |
| Postprocedural anemia | 2.68 | [2.57–2.81] | 13.25 | [12.82–13.69] | 18.29 | [16.86–19.82] | 5.05 | [4.88–5.22] | 29,512 | <0.001 |
| Acute kidney injury | 5.63 | [5.48–5.79] | 52.44 | [51.90–52.97] | 73.56 | [71.97–75.09] | 16.08 | [15.82–16.34] | 94,041 | <0.001 |
| Outcomes | ||||||||||
| Index admissions, overall | ||||||||||
| Died during hospitalization | 3.33 | [3.24–3.41] | 24.72 | [24.31–25.13] | 24.68 | [23.12–26.31] | 7.99 | [7.87–8.12] | 46,718 | <0.001 |
| Transfer to skilled facility | 2.64 | [2.56–2.73] | 19.68 | [19.30–20.07] | 40.25 | [38.47–42.05] | 6.59 | [6.47–6.72] | 38,551 | <0.001 |
| Length of stay, mean (SE) | 2.96 (0.01) | 7.83 (0.04) | 16.33 (0.33) | 4.12 (0.01) | 584,912 | <0.001 | ||||
| Total cost, mean (SE) | $22,637.53 (31.70) | $42,726.1 (201.65) | $65,517.63 (1311.23) | $27,272.66 (53.56) | 582,622 | <0.001 | ||||
| Index admissions with PCI utilization | ||||||||||
| Died during hospitalization | 2.06 | [1.99–2.13] | 20.50 | [20.04–20.96] | 21.50 | [19.59–23.55] | 5.29 | [5.18–5.40] | 25,318 | <0.001 |
| Transfer to skilled facility | 1.84 | [1.77–1.91] | 17.60 | [17.16–18.04] | 39.89 | [37.55–42.28] | 4.78 | [4.67–4.89] | 22,870 | <0.001 |
| Length of stay, mean (SE) | 2.79 (0.00) | 7.78 (0.04) | 17.56 (0.44) | 3.74 (0.01) | 584,912 | <0.001 | ||||
| Total cost, mean (SE) | $22,970.68 (30.19) | $45,876.52 (229.34) | $75,847.13 (1802.81) | $27,218.49 (52.00) | 584,912 | <0.001 | ||||
| Readmissions within 30 days, mean (SE) | 6.42 (0.00) | 13.77 (0.00) | 14.83 (0.01) | 7.74 (0.00) | 41,670 | <0.001 | ||||
| Died during hospitalization | 1.55 | [1.34–1.78] | 8.50 | [7.83–9.22] | 13.04 | [9.51–17.64] | 4.11 | [3.83–4.40] | 1720 | <0.001 |
| Transfer to skilled facility | 7.29 | [6.84–7.78] | 25.64 | [24.60–26.70] | 47.69 | [42.77–52.66] | 14.25 | [13.76–14.76] | 5967 | <0.001 |
| Length of stay, mean (SE) | 3.20 (0.03) | 6.32 (0.09) | 12.24 (0.56) | 4.43 (0.04) | 584,912 | <0.001 | ||||
| Total cost, mean (SE) | $11,723.94 (120.24) | $17,627.89 (305.88) | $30,042.94 (1882.48) | $14,062.72 (136.59) | 584,912 | <0.001 | ||||
Note: Outcomes by frailty risk status.
Abbreviations: PCI, percutaneous coronary intervention; SE, standard error.
4 |. DISCUSSION
Our study used a validated risk score model to examine frailty in acute myocardial infarctions and has significant findings. Frailty is common amongst patients admitted for AMI, with more than 1 in 5 patients being classified as intermediate or high risk. Mortality was higher in intermediate and high-risk groups than in low risk. Frailty is also associated with greater length of stays, total costs, and 30-day readmissions. Finally, frailty is associated with lower utilization of PCI, which may be associated with the observed increased in-hospital mortality.
PCI has been increasingly utilized in elderly patients admitted for an AMI from 2000 to 2016 (6% vs. 12%) with a concomitant 41% mortality reduction, despite frail patients being less likely to undergo PCI (15% vs. 33%).26 Our work builds upon the important findings by Borovac et al. depicting that frail patients, although less likely to undergo PCI, derived significant benefit from it.21 Additionally, we included discharge disposition to a skilled care facility in our study with an aim to minimize physician selection bias, that impacts decision to perform PCI in frail patients. In our analysis, frail patients were also less likely to undergo PCI yet still benefited from the intervention; in-hospital mortality was significantly reduced for each frailty level, while readmission risk was reduced in low and intermediate groups. It is unclear why our study revealed a higher in-patient mortality in the intermediate-risk group compared with the highest-risk frailty group. A plausible explanation could be an increased number of interventions for comorbidities, both cardiovascular and non-cardiovascular, in the intermediate-risk frailty group, compared with the highest frailty group that could have skewed this data. Additionally, underutilization of PCI in the highest frailty group may have contributed to this finding. Noteworthily, all frailty groups benefitted from coronary intervention, compared with medical management.
A substantial finding of our analysis is the impact of frailty on healthcare utilization and costs. The cost of index admissions was significantly higher in increasingly frail groups, which may be difficult to control due to the increasing complexity of care required. Nevertheless, extraordinary additional costs are incurred due to early readmissions, which are more prominent in intermediate and high-risk frailty groups. This may unduly penalize hospitals that care for higher populations of frail patients.36 In combination with a postdischarge visiting service, an in-hospital multidisciplinary team may aid in identifying those at risk of readmission and optimize care. Together, these services will assist in reducing readmissions, healthcare costs, and provide better patient care.37,38
The utilization of frailty risk is recommended by international guidelines,39,40 which makes the accessibility of routine data a significant advantage to determine the HFR score. It is important to note that age is not well correlated with frailty (correlation coefficient 0.301, p < 0.001). The interaction between age and comorbidities’ correlation to frailty score is visualized in Figure 5. Frailty’s relationship with age may be better correlated with frailty subdomains, including physical, social, and cognitive, as described by Matsue et al. in the FRAGILE-HF study40; however, further studies will be needed to discriminate these subdomains using a model based on administrative data.
FIGURE 5.
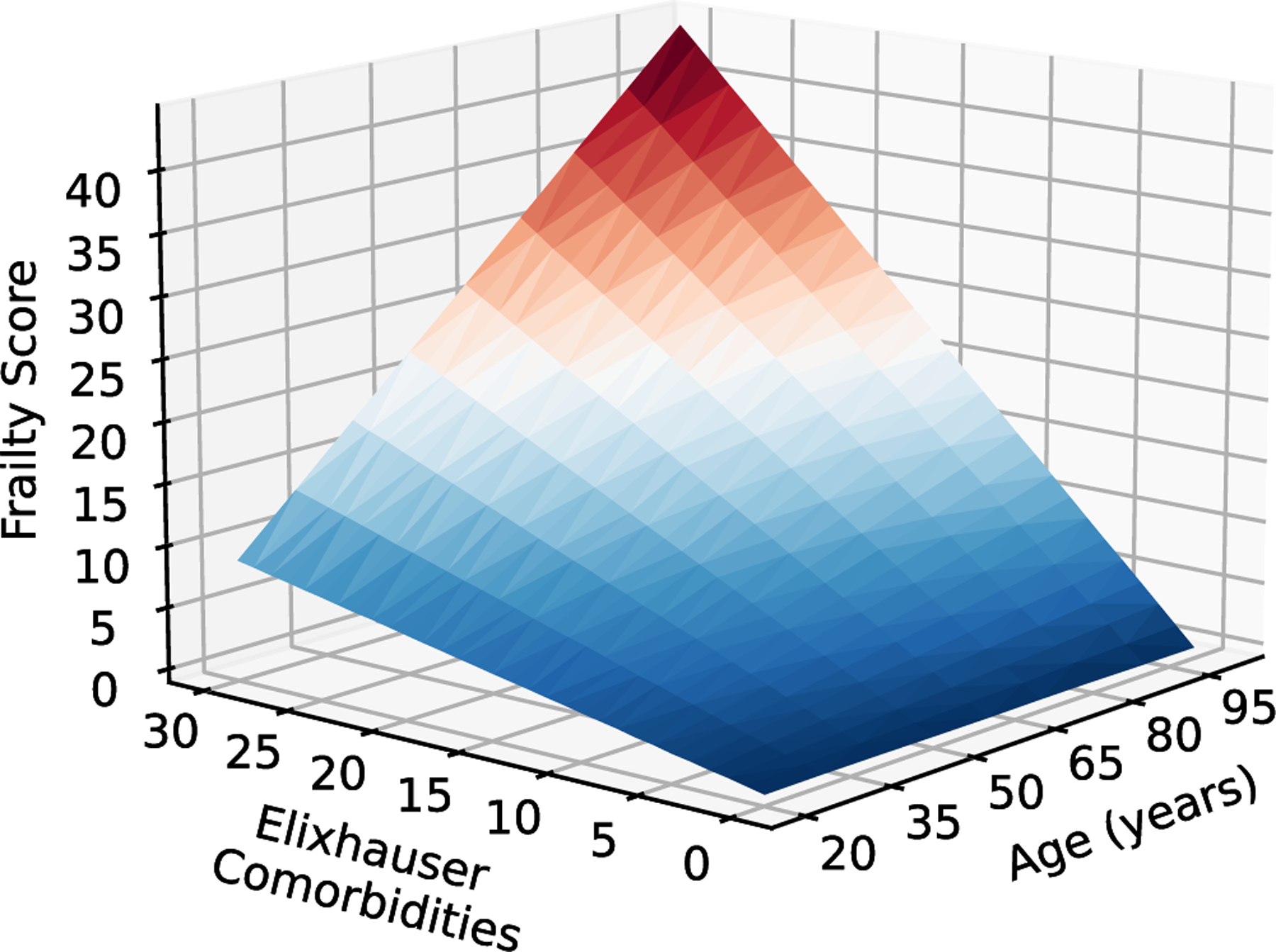
Relationship of age and comorbidities to frailty. Frailty risk in relation to age and Elixhauser comorbidities. [Color figure can be viewed at wileyonlinelibrary.com]
The findings identified in this study contribute significantly to understanding AMI outcomes. Our paper builds upon the work by Borovac et al. to utilize an all-payer nationwide database, the NRD, by including patients less than 65 years of age admitted for acute ST-elevated myocardial infarctions. Furthermore, we analyzed procedural outcomes while accounting for contemporary factors. Since STEMIs are commonly managed with the use of PCI, this finding is paramount. Given that the elderly frail population is less likely to undergo an intervention, we aimed to investigate whether revascularization prevented readmission rates in frail populations.
Further reviews are required to finalize a consensus on a broader acceptable definition of frailty and its assessment. There is an unmet need to include frailty as a routine risk assessment tool, especially in cardiovascular care and preoperative evaluation, given wide-ranging implications on short- and long-term clinical outcomes.12,40,41 Primary care physicians should consider increased vigilance toward recognizing frailty as it is an independent predictor of ED visits and hospitalizations.17 We suggest the involvement of Geriatricians in multidisciplinary teams, as this may portend better outcomes for the frail population.5,42 Finally, more studies are needed to assess the feasibility and best practices for treating this vulnerable population after discharge.43–46
4.1 |. Limitations
Our study has several limitations. First, admissions were identified using only the primary diagnosis, consistent with best use methodologies provided by HCUP. This accepted protocol ensures that only acute episodes of myocardial infarctions are identified. Nonetheless, patients may be missed if their AMI occurred leading to admission but was not listed as the first diagnosis. Second, due to the retrospective observational nature of this study, residual confounders may be missed, and causality cannot be established. Third, the NRD does not provide granular information, including medications, laboratory, or imaging results. Fourth, albeit previously validated, the HFRS could not be compared to other validated frailty measures in our study due to these missing data elements. Fifth, as comorbidities are identified using the presence of specific ICD-10 codes, the potential for coding errors or missed coding may skew influence. Additionally, the inclusion of palliative care consults, which may holistically affect the decision to perform PCI, was not a part our study. The NRD also does not account for competing risk of mortality, that varies among different frailty groups. This may underestimate the risk for readmission in patients who are more likely to die postdischarge, Finally, the lack of discharge prescriptions and therapies prevents identifying factors for readmission. This is particularly important in identifying evidence-based therapies and their influence on readmission rates, though evidence suggests no difference in the use of medication between frail and non-frail patients.47
5 |. CONCLUSION
Among adult, all-payer inpatient visits, frailty discerned by the HFRS was associated with increased readmissions, increased healthcare resource utilization, and lower PCI administration. Future studies are needed to assess the impact of postdischarge compliance on reducing risk and adverse outcomes. As population age continually increases, the burden of frailty will become a significant priority. Utilizing the hospital frailty score provides an improved prediction model for adverse outcomes, risk-benefit assessments of interventions, and will allow for goal-oriented care of patients experiencing acute ST-elevated myocardial infarctions.
Supplementary Material
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data underlying this article were provided by the Agency for Healthcare Research and Quality’s HCUP under license. Data can be requested from the Agency for Healthcare Research and Quality.
REFERENCES
- 1.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 2005;173(5): 489–495. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381(9868):752–762. doi: 10.1016/s0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet 1999;353(9148):205–206. doi: 10.1016/s0140-6736(98)04402-x [DOI] [PubMed] [Google Scholar]
- 4.Damluji AA, Ramireddy A, Otalvaro L, Forman DE. Secondary cardiovascular prevention in older adults: an evidence based review. J Geriatric Cardiol: JGC 2015;12(5):459–464. doi: 10.11909/j.issn.1671-5411.2015.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell SP, Orr NM, Dodson JA, et al. What to expect from the evolving field of geriatric cardiology. JACC 2015;66(11):1286–1299. doi: 10.1016/j.jacc.2015.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56(3): M146–M157. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 7.Cacciatore F, Abete P, Mazzella F, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest 2005;35(12):723–730. doi: 10.1111/j.1365-2362.2005.01572.x [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Mañas L, Féart C, Mann G, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol: Series A 2013;68(1):62–67. doi: 10.1093/gerona/gls119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JSM, Olde Rikkert MGM, Nijhuis-van der Sanden MWG. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev 2011;10(1):104–114. doi: 10.1016/j.arr.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 10.Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. JACC 2014;63(8):747–762. doi: 10.1016/j.jacc.2013.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr 2016;16(1):157. doi: 10.1186/s12877-016-0329-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh M, Rihal CS, Lennon RJ, Spertus JA, Nair KS, Roger VL. Influence of frailty and health status on outcomes in patients with coronary disease undergoing percutaneous revascularization. Circulation: Cardiovasc Quality Outcomes 2011;4(5):496–502. doi: 10.1161/circoutcomes.111.961375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001;56(3):M158–M166. doi: 10.1093/gerona/56.3.m158 [DOI] [PubMed] [Google Scholar]
- 14.Wahl TS, Graham LA, Hawn MT, et al. Association of the modified frailty index with 30-day surgical readmission. JAMA Surg 2017;152(8):749–757. doi: 10.1001/jamasurg.2017.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIsaac DI, Taljaard M, Bryson GL, et al. Frailty as a predictor of death or new disability after surgery: a prospective cohort study. Ann Surg 2020;271(2):283–289. doi: 10.1097/sla.0000000000002967 [DOI] [PubMed] [Google Scholar]
- 16.Madrigal J, Emami S, Christian-Miller N, Cale M, Benharash P, Ebrahimi R. TCT-644 the effects of frailty on mortality and complications following percutaneous coronary interventions. JACC 2019;74(13):B632. doi: 10.1016/j.jacc.2019.08.763 [DOI] [Google Scholar]
- 17.McNallan SM, Singh M, Chamberlain AM, et al. Frailty and healthcare utilization among patients with heart failure in the community. JACC: Heart Failure 2013;1(2):135–141. doi: 10.1016/j.jchf.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanenawa K, Yamaji K, Tashiro H, et al. Patient selection and clinical outcomes in the STOPDAPT-2 trial: an all-comer single-center registry during the enrollment period of the STOPDAPT-2 randomized controlled trial. Circulation: Cardiovasc Interven 2021;14(2):e010007. doi: 10.1161/circinterventions.120.010007 [DOI] [PubMed] [Google Scholar]
- 19.Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018;391(10132):1775–1782. doi: 10.1016/s0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwok CS, Zieroth S, Van Spall HGC, et al. The hospital frailty risk score and its association with in-hospital mortality, cost, length of stay and discharge location in patients with heart failure short running title: frailty and outcomes in heart failure. Int J Cardiol 2020;300:184–190. doi: 10.1016/j.ijcard.2019.09.064 [DOI] [PubMed] [Google Scholar]
- 21.Borovac JA, Mohamed MO, Kontopantelis E, et al. Frailty among patients with acute ST-elevation myocardial infarction in the United States: the impact of the primary percutaneous coronary intervention on in-hospital outcomes. J Invasive Cardiol 2022;34(1):55. [DOI] [PubMed] [Google Scholar]
- 22.Aguayo GA, Donneau AF, Vaillant MT, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol 2017;186(4):420–434. doi: 10.1093/aje/kwx061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAlister F, van Walraven C. External validation of the hospital frailty risk score and comparison with the hospital-patient one-year mortality risk score to predict outcomes in elderly hospitalised patients: a retrospective cohort study. BMJ Quality Safety 2019;28(4):284–288. doi: 10.1136/bmjqs-2018-008661 [DOI] [PubMed] [Google Scholar]
- 24.Smith RJ, Reid DA, Santamaria JD. Frailty is associated with reduced prospect of discharge home after in-hospital cardiac arrest. Intern Med J 2019;49(8):978–985. doi: 10.1111/imj.14159 [DOI] [PubMed] [Google Scholar]
- 25.Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011;124(22):2397–2404. doi: 10.1161/circulationaha.111.025452 [DOI] [PubMed] [Google Scholar]
- 26.Qian AS, Nguyen NH, Elia J, Ohno-Machado L, Sandborn WJ, Singh S. Frailty is independently associated with mortality and readmission in hospitalized patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2021;19(10):2054–2063. doi: 10.1016/j.cgh.2020.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malik AH, Yandrapalli S, Zaid S, et al. Impact of frailty on mortality, readmissions, and resource utilization after TAVI. Am J Cardiol 2020;127:120–127. doi: 10.1016/j.amjcard.2020.03.047 [DOI] [PubMed] [Google Scholar]
- 28.Kundi H, Wadhera RK, Strom JB, et al. Association of frailty with 30-day outcomes for acute myocardial infarction, heart failure, and pneumonia among elderly adults. JAMA Cardiol 2019;4(11): 1084–1091. doi: 10.1001/jamacardio.2019.3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malik AH, Yandrapalli S, Zaid S, et al. Impact of frailty on mortality, readmissions, and resource utilization after TAVI. Am J Cardiol 2020;127:120–127. doi: 10.1016/j.amjcard.2020.03.047 [DOI] [PubMed] [Google Scholar]
- 30.Ramai D, Dang-Ho KP, Kewalramani A, et al. Hospital frailty risk score is independently associated with mortality and encephalopathy in hospitalized patients with hepatocellular carcinoma. Biomedicines 2021;9(11):1693. doi: 10.3390/biomedicines9111693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.HCUP Nationwide Readmissions Database (NRD). Healthcare Cost and Utilization Project (HCUP). Agency for healthcare research and quality R, MD 2019. https://www.hcup-us.ahrq.gov/nrdoverview.jsp.
- 32.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 33.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 2009;47(6):626–633. doi: 10.1097/MLR.0b013e31819432e5 [DOI] [PubMed] [Google Scholar]
- 34.Li Cheng. Little’s test of missing completely at random. Stata J 2013;13(4):795–809. [Google Scholar]
- 35.Royston P Multiple imputation of missing values. Stata J 2004; 4(3):227–241. [Google Scholar]
- 36.McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation 2015;131(20):1796–1803. doi: 10.1161/circulationaha.114.010270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kripalani S, Theobald CN, Anctil B, Vasilevskis EE. Reducing hospital readmission rates: current strategies and future directions. Annu Rev Med 2014;65:471–485. doi: 10.1146/annurev-med-022613-090415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradley EH, Curry L, Horwitz LI, et al. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circulation: Cardiovas Quality and Outcomes 2013;6(4):444–450. doi: 10.1161/circoutcomes.111.000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dent E, Morley JE, Cruz-Jentoft AJ, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging 2019;23(9):771–787. doi: 10.1007/s12603-019-1273-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner G, Clegg A. Best practice guidelines for the management of frailty: a British Geriatrics Society, age UK and Royal College of general practitioners report. Age Ageing 2014;43(6):744–747. doi: 10.1093/ageing/afu138 [DOI] [PubMed] [Google Scholar]
- 41.Matsue Y, Kamiya K, Saito H, et al. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILE-HF cohort study. Eur J Heart Fail 2020;22(11):2112–2119. doi: 10.1002/ejhf.1926 [DOI] [PubMed] [Google Scholar]
- 42.Singh M, Alexander K, Roger VL, et al. Frailty and its potential relevance to cardiovascular care. Mayo Clin Proc 2008;83(10): 1146–1153. doi: 10.4065/83.10.1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric comanagement of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc 2008;56(7):1349–1356. doi: 10.1111/j.1532-5415.2008.01770.x [DOI] [PubMed] [Google Scholar]
- 44.Walters K, Frost R, Kharicha K, et al. Home-based health promotion for older people with mild frailty: the HomeHealth intervention development and feasibility RCT. Health Technol Assess (Rockv) 2017;21(73):1–128. doi: 10.3310/hta21730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kennedy MA, Hatchell KE, DiMilia PR, et al. Community health worker interventions for older adults with complex health needs: a systematic review. J Am Geriatr Soc 2021;69(6):1670–1682. doi: 10.1111/jgs.17078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adja KYC, Lenzi J, Sezgin D, et al. The importance of taking a patient-centered, community-based approach to preventing and managing frailty: a public health perspective. Front Public Health 2020;8:599170. doi: 10.3389/fpubh.2020.599170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martín-Sánchez FJ, Rodríguez-Adrada E, Mueller C, et al. The effect of frailty on 30-day mortality risk in older patients with acute heart failure attended in the emergency department. Acad Emerg Med 2017;24(3):298–307. doi: 10.1111/acem.13124 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by the Agency for Healthcare Research and Quality’s HCUP under license. Data can be requested from the Agency for Healthcare Research and Quality.


