Abstract
Background
Data on the effectiveness of SARS-CoV-2 vaccines and the durability of protection against the prevalent Omicron variant are scarce, especially in patients with autoimmune rheumatic diseases (AIRDs). Hence, we prospectively studied Omicron breakthrough infections in patients with AIRDs and attempted to isolate associated risk factors.
Methods
Patients with AIRDs who had completed primary vaccination with either AZD1222 or BBV152 vaccines were included and prospectively followed up from January 2022 onwards for the development of breakthrough Omicron infections. The time interval from the last event [2nd dose of vaccination (V) or past COVID-19 infection (I) whichever was later] to Omicron infection was recorded. Patients were divided based on the events and their order of occurrence into V + V, V + I, I + V, V + I + V, and V + V + I groups. The incidence of breakthrough infections and their predictors were studied with a focus on the vaccine type and hybrid (H) immunity (vaccinated individuals with a history of COVID-19 infection).
Results
We included 907 patients with AIRDs (53.5 ± 11.7 years and a male-to-female ratio of 1:5.1), and the majority of patients had received AZD1222 (755, 83.2%). Breakthrough infections were observed in 158 of 907(17.4%) of which 97 (10.4%) were confirmed by RT-PCR.
Breakthrough infections were significantly greater in the V versus the H group (15.7% and 3.5%, log-rank test, p = < 0.01). Among the hybrid group, the order of infection and vaccination had no bearing on the risk of breakthrough infections. On multivariate analysis, breakthrough infections were significantly lesser in the H versus the V group [HR: 0.2(0.1–0.4); p = 0.01].
Conclusion
The risk of breakthrough Omicron infections in fully vaccinated patients with AIRDs was 17.4% with a significantly lower risk in patients with hybrid immunity.
Keywords: COVID-19 vaccines, COVID-19, SARS-CoV-2 variants, SARS-CoV-2, Omicron
Introduction
Omicron (B.1.1.529) variant was labeled as a variant of concern by the World Health Organization in November 2021 and it spread like wildfire throughout 2022. It has multiple mutations in the receptor-binding domain (RBD) of the spike protein with much higher infectivity than the previous strains [1]. Infections with Omicron were observed across India from January 2022 onwards [2]. Previous studies had shown a modest decline in vaccine effectiveness against the delta variant from the original alpha strain in terms of breakthrough infections [3–6]. Patients with autoimmune rheumatic diseases are at increased risk for severe COVID-19 due to their medications and prevalent comorbidities [7].
More than 60 mutations were identified in the Omicron variant with several of them in the spike protein involved in transmissibility, severity, and immune escape [8]. This raised doubts regarding the effectiveness of SARS-CoV-2 vaccine-induced and past COVID infection-induced immunity against Omicron. In vitro neutralization studies demonstrated a reduced response to Omicron in previously vaccinated individuals [9, 10]. A study from the United Kingdom compared symptomatic Omicron infections compared to Delta infections and found limited protection by primary immunization with AZD1222 (ChAdOx1 nCoV-19, ‘Covishield’) or BNT162b2 which improved following a booster mRNA vaccine [11]. A study from Qatar found limited protection by mRNA boosters against symptomatic infection, but strong protection against hospitalization and death was observed [12]. Similar results were observed from a study in the USA [13].
A previous prospective cohort study in patients with AIRDs from our center, conducted at the time of the second wave dominated by the delta variant in India, showed that hybrid immunity provides better protection against breakthrough infection [14]. Breakthrough infections were observed in 7.4% of the cohort and were associated with poor humoral response to vaccines. Data regarding the effectiveness of primary immunization, boosters, and hybrid immunity against the Omicron variant in patients with AIRDs are scarce with in vitro studies suggesting poor vaccine-induced immune response against the Omicron variant [15, 16]. The duration of protection offered by vaccination or past infection in this subgroup of patients is also questionable.
Hence, we aimed to study the incidence of breakthrough infections with the Omicron variant in patients with AIRDs overall as well as across various subgroups of vaccination and hybrid immunity. We also studied the influence of other factors such as the type of rheumatic disease and treatment of breakthrough infections.
Methodology
Aim: Our objectives were to assess the incidence of breakthrough COVID-19 infection (Omicron variant) in a vaccinated cohort of patients with AIRDs and compare it across those who had received primary immunization without previous SARS-CoV-2 infection (V), and those with hybrid immunity (H). Additionally, we also aimed to assess other factors associated with breakthrough infections like the type of vaccine, underlying AIRD, drugs used, etc.
Inclusion criteria: Patients with AIRD who had completed both the dose of SARS-CoV2 vaccines (AZD1222/ChAdOx1 and BBV152/ Bharat Biotech’s Covaxin’) or single vaccine dose with SARS-CoV-2 infection who had been included from March 2021 onwards were actively followed up till March 2022 at the Centre for Arthritis and Rheumatism Excellence (CARE) in Southern India.
Ethics approval: The study was approved by the Ethics Committee of Sree Sudheendra Medical Mission and written informed consent from all the participants was taken.
Type of study: a prospective cohort study.
All patients enrolled in the cohort were followed on each follow-up visit for clinical details, exposure to COVID-19, symptoms suggestive of COVID-19, or any documented COVID-19. If any participant missed a visit, they were contacted over the telephone and these details were updated and they were provided another appointment date.
Clinical details: Demographic details, type of AIRD, immunosuppressive drugs, comorbidities, details of vaccination, breakthrough infection, and type of contact were recorded. The time interval from the last event [2nd dose of vaccination (V) or past COVID-19 infection (I) whichever was later] to Omicron infection was recorded. Patients were divided based on the events and their order of occurrence into V + V, V + I, I + V, V + I + V, and V + V + I groups (eg. V + I means, one dose of vaccine and one episode of COVID-19).
Definitions: Breakthrough infections: Vaccine breakthrough cases are defined as instances in which an individual tested positive for COVID-19 after at least 2 weeks of being fully vaccinated with the primary series (both doses in the case of AZD1222 and BBV152) [17, 18].
Confirmed COVID-19: COVID-19 infection was defined as a positive reverse transcriptase-polymerase chain reaction (RT-PCR). Positivity before January 1st, 2022 was assumed to be due to the Delta variant and those after due to the Omicron variant.
Probable COVID-19: A probable case was defined as those with symptoms of COVID-19 with a negative or unavailable RTPCR or positive family history of COVID-19.
A COVID-19 contact was defined as per WHO (World Health Organization) recommendations [19].
Hybrid Immunity was defined as those with past COVID-19 infection with one or two doses of vaccination.
Statistical analysis: Data are expressed as mean and standard deviation. Baseline characteristics were compared across the three groups (V, H groups). A p-value of < 0.05 was deemed as statistically significant, and all reported values were two sided. SPSS v.24 was used for statistical analysis.
Univariate analysis comparing V and H groups was done using unpaired t-test for continuous and Chi-square or Fisher’s exact test for categorical variables.
For the survival analysis, Kaplan–Meir survival curves were used to illustrate the proportions of survival across the two groups (V, H) and comparisons were done using the log-rank test. Confirmed COVID-19 and probable COVID-19 were taken as events in two separate analyses.
To determine predictors for breakthrough Omicron infection, a log-rank test was used for univariate analysis using Kaplan–Meir survival analysis. Drugs that were used in less than 20 individuals were not analyzed. All clinically relevant parameters were included in the multivariate analysis. Multivariate analysis was carried out using cox regression with breakthrough infection as the dependent variable, duration of follow-up till the event as the time factor, and using forward likelihood ratio test.
Results
We studied 907 patients of AIRDs with an average age of 53.5 ± 11.7 years and a male-to-female ratio of 1:5.1. Table 1 contains details of the cohort including the background rheumatic disease, vaccine received, and immunosuppressants used. The majority of patients had received AZD1222 (755, 83.2%) while the remaining received the BBV152 vaccine. The most common AIRD was RA (661, 72.8%) and methotrexate (465, 51.3%) was the most commonly used disease-modifying anti-rheumatic drug (DMARD). When V (n = 535) and H (n = 373) were compared, those in the H group were significantly younger, had a higher proportion of spondyloarthritis patients and AZD1222 use, and had lower proportions on methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, tofacitinib, and rituximab.
Table 1.
Baseline characteristics
| N = 907 | Vaccine (V) only, n = 535 | Hybrid (H) Immunity, n = 372 | P value, across V and H groups | |
|---|---|---|---|---|
| Age | 53.5 ± 11.7 | 55.5 ± 11.6 | 50.5 ± 11.3 | 0.0001 |
| Female: Male | 5.1:1 | 5.5:1 | 4.7:1 | 0.4 |
| Diagnosis | ||||
| RA | 661 (72.8) | 400 (74.8) | 261 (70.2) | 0.2 |
| Spondyloarthritis | 103 (11.3) | 50 (9.3) | 53 (14.2) | 0.02, 0.6 (0.4–0.9) |
| SLE | 35 (3.8) | 16 (2.9) | 19 (5.1) | 0.1 |
| Vasculitis | 38 (4.1) | 25 (4.6) | 13 (3.5) | 0.3 |
| Other CTDs | 70(7.6) | 44 (8.2) | 26 (6.9) | 0.2 |
| Vaccine Group | ||||
| V + V | 535 (58.9) | 535 (100) | ||
| I + V | 17 (18.7) | 17 (4.6) | ||
| V + I | 25 (2.7) | 25 (6.7) | ||
| I + V + V | 121 (13.3) | 121 (32.5) | ||
| V + I + V | 97 (10.7) | 97 (26.1) | ||
| V + V + I | 112 (12.3) | 112 (30.1) | ||
| Past COVID-19 | 372 (41) | |||
| Time since last event to COVID infection in days | ||||
| Confirmed | 191.4 ± 97.2 | 205 ± 109.5 | 171.9 ± 71.6 | 0.0001 |
| Confirmed + Probable | 186.8 ± 95.8 | 200.2 ± 104.9 | 167.4 ± 77 | 0.0001 |
| Primary Immunization | 0.0001, 1.9 (1.3–2.9) | |||
| AZD1222 | 755 (83.2) | 426 (79.6) | 329 (88.4) | |
| BBV152 | 152 (16.8) | 109 (20.4) | 43 (11.6) | |
| Drugs | ||||
| Methotrexate | 465 (51.3) | 310 (57.9) | 155 (41.7) | 0.0001, 1.9 (1.5–2.5) |
| Sulfasalazine | 164 (18.1) | 124 (23.2) | 40 (10.8) | 0.0001, 2.5 (1.7–3.7) |
| Hydroxychloroquine | 454 (50) | 338 (63.2) | 116 (31.2) | 0.0001, 3.7 (2.9–5.1) |
| Leflunomide | 46 (5) | 38 (7.1) | 8 (2.2) | 0.01, 3.5 (1.6–7.5) |
| Tofacitinib | 56 (6.2) | 41 (7.7) | 15 (4) | 0.03, 1.9 (1.1–3.6) |
| Rituximab | 38 (4.2) | 29 (5.4) | 9 (2.4) | 0.03, 2.3 (1.1–4.9) |
| Tacrolimus | 10 (1.1) | 7 (1.3) | 3 (0.8) | 0.4 |
| Mycophenolate Mofetil | 49 (5.4) | 33 (6.2) | 16 (4.3) | 0.2 |
| Azathioprine | 6 (0.6) | 3 (0.6) | 3 (0.8) | 0.6 |
| TNF inhibitors | 9 (0.9) | 4 (0.6) | 6 (1.6) | 0.1 |
| Glucocorticoids | 105 (11.6) | 71 (13.3) | 34 (9.1) | 0.06 |
V Vaccine, I COVID-19 infection, TNF Tumor Necrosis Factor, RA Rheumatoid Arthritis, SLE Systemic Lupus Erythematosus, CTD Connective Tissue Disease, AZD1222 ChAdOx1 nCoV-19 or AztraZeneca, BNT162b2 Pfizer BioNTech, BBV152 Bharat Biotech
Breakthrough infections: Frequency and severity
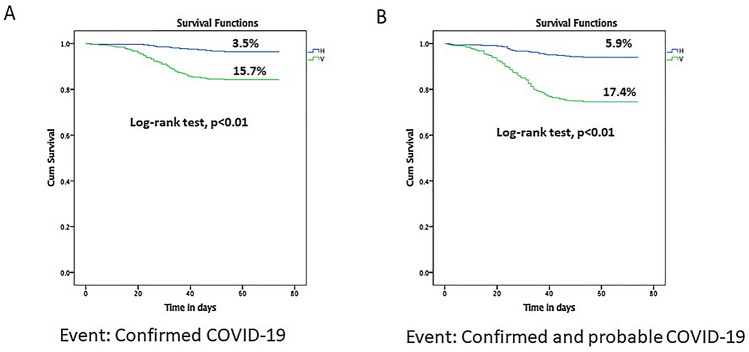
Confirmed COVID-19: Breakthrough Omicron infections were seen in 97 of 907, 10.4% of the cohort — 84 of 535, 15.7% in the V group, 13 of 372, 3.5% in the H group (log-rank test, p = < 0.01) (Fig. 1).
Fig. 1.
Survival analysis with A confirmed COVID-19 B confirmed and probable COVID-19 as events assessed from 1st January 2022 till 15th March 2022 across Vaccine (V) and Hybrid (H) groups
Confirmed and probable COVID-19: Breakthrough Omicron infections were seen in 158 of 907, 17.4% — 136 of 535, 24.4% in the V group and 22 of 372, 5.9% in the H group (log-rank test, p = < 0.01) (Fig. 1).
The majority of breakthrough infections were asymptomatic (80 of 158, 50.6%) and mild (73 of 158, 46.2%). Only 5 (3.1%) had moderate COVID-19 and none had a severe infection.
Predictors of breakthrough infections for confirmed COVID-19
Univariate
Using the log-rank test, V versus H group (15.7% and 3.5%, p = < 0.01) was significantly associated with breakthrough infections (Table 2).
Table 2.
Univariate analysis (log-rank test) for factors associated with breakthrough infections (confirmed COVID-19 as the event)
| Parameter | Patients | Events (Confirmed COVID-19) | Log rank |
|---|---|---|---|
| Gender | Male, 148 | 14 | 0.6 |
| Female, 759 | 83 | ||
| Rheumatoid arthritis | Yes, 661 | 63 | 0.05 |
| No, 246 | 34 | ||
| Spondyloarthritis | Yes, 103 | 13 | 0.4 |
| No, 804 | 84 | ||
| Systemic lupus erythematosus | Yes, 35 | 4 | 0.8 |
| No, 872 | 93 | ||
| Other connective tissue diseases | Yes, 67 | 11 | 0.1 |
| No, 840 | 86 | ||
| Vasculitis | Yes, 38 | 6 | |
| No, 869 | 91 | ||
| Type of vaccine | AZD1222, 755 | 78 | 0.4 |
| BBV152, 152 | 19 | ||
| Subgroup | V, 535 | 84 | 0.0001 |
| H, 372 | 13 | ||
| Methotrexate | Yes, 465 | 49 | 0.8 |
| No, 442 | 48 | ||
| Sulfasalazine | Yes, 164 | 20 | 0.4 |
| No, 743 | 77 | ||
| Hydroxychloroquine | Yes, 454 | 51 | 0.6 |
| No, 453 | 46 | ||
| Leflunomide | Yes, 46 | 7 | 0.7 |
| No, 861 | 90 | ||
| Tofacitinib | Yes, 56 | 7 | 0.7 |
| No, 851 | 90 | ||
| Rituximab | Yes, 38 | 5 | 0.6 |
| No, 869 | 92 | ||
| Mycophenolate Mofetil | Yes, 49 | 4 | 0.5 |
| No, 858 | 93 | ||
| Glucocorticoids | Yes, 105 | 11 | 0.9 |
| No, 802 | 86 |
Age (52.2 ± 11.6 vs 53.7 ± 11.7, p = 0.2) and time since the last event in days (191.6 ± 107.2 vs 191.4 ± 96, p = 0.9) were not significantly associated with breakthrough infections.
Among the hybrid group, subgroups based on the order of infection and vaccination had no significant effect on the risk of breakthrough infections[I + V, 0 of 17; I + V + V, 4 of 121(3.3%); V + I, 0 of 25; V + I + V, 6 of 97(6.2%); V + V + I, and 3 of 106(2.8%). P = 0.4] (Fig. 2).
Fig. 2.
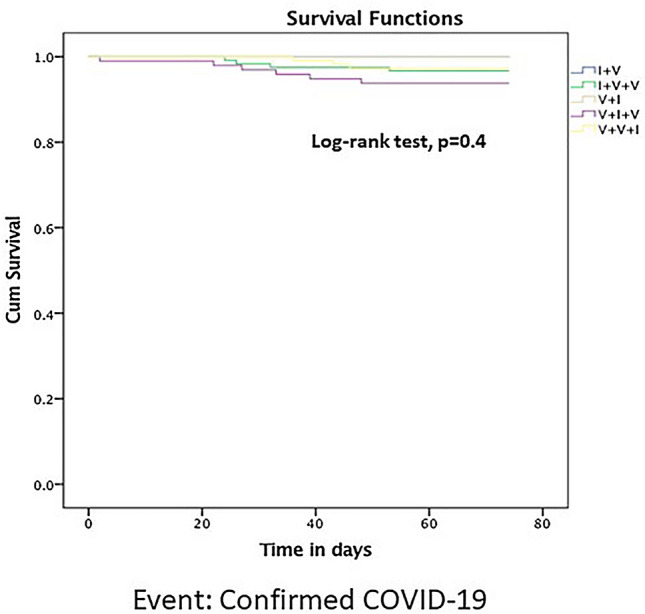
Survival analysis across subgroups of hybrid immunity with confirmed COVID-19 as an event assessed from 1st January 2022 till 15th March 2022. (V- Vaccine, I- Infection)
Multivariate
On cox regression analysis using clinically relevant variables(age, type of AIRD, type of vaccine, V or H group, time since last event, drugs used), only age [(p = 0.01, 0.98 (0.96–0.99)] and the subgroup of V and H were significantly associated with the risk of breakthrough Omicron infections (p = 0.001, 0.2 (0.1–0.4).
Discussion
We studied 907 patients with AIRDs and breakthrough Omicron infections were observed in 158 of 907 (17.4%) of which 97 (10.4%) were confirmed by RT PCR. Breakthrough infections were significantly greater in the V versus the H group age and time since the last event in days was not significantly associated with breakthrough infections on univariate analysis. Among the hybrid group, order of infection and vaccination had no bearing on the risk of breakthrough infections. On multivariate analysis, breakthrough infections were significantly lesser in the H versus the V group.
We had previously shown that AIRD patients with hybrid immunity have higher antibody titers with greater SARS-CoV-2 neutralizing capacity in vitro [14]. We had also shown that post-vaccination antibody titers were a correlate of protection for breakthrough infection [20]. The current study provides one more layer of confirmation with real-world data that hybrid immunity leads to lower breakthrough infections. This was protective even during the Omicron wave that had shown higher breakthrough infections in fully vaccinated persons [21, 22].
Our recent study had shown that the neutralization potential of serum against Omicron in vitro was reduced in those having hybrid immunity after receiving the second dose of the vaccine [16]. However, this did not translate to reduce protection against the Omicron variant in vivo in a very large cohort followed up through the Omicron wave in India.
A nationwide cohort study from the Netherlands reported the frequency of breakthrough Omicron infections confirmed by RT-PCR to be 29.6% in 1593 patients with immune-mediated inflammatory diseases (IMIDs) compared to 31.3% in controls. We found a lower rate of confirmed breakthrough infections of 10.4%. This could be because of a higher proportion of patients with hybrid immunity (41% versus 21.6%) in our cohort as well as a lesser use of biologic drugs like rituximab (4.2% versus 12%) [23, 24]. Another Dutch study also reported a similar rate of breakthrough Omicron infections of 23% [25]. The risk of breakthrough Omicron infection was higher than delta breakthrough infections as previously reported by us and others [20, 26]. This could be multifactorial, due to the long interval between primary immunization and Omicron infection, higher infectivity of Omicron strain, and less stringent masking towards the latter part of the pandemic.
On univariate and multivariate analyses, we found that the single most important factor responsible for enhanced protection against breakthrough infections was a history of past COVID-19. This is similar to other studies from the Netherlands for the Omicron variant as well as older studies from our group on delta breakthrough infections [14, 23, 27, 28]. Additional vaccine doses were also found to be protective in both the Dutch cohorts [23, 25]. We could not assess the effects of boosters as booster vaccination began in January 2022 as per the national guidelines [29]. Similar findings were observed from the United Kingdom and Israel with > 80% protection against the Omicron variant observed with mRNA booster doses, especially in the initial three months [30–32].
Previously, the waning of protection with time since the last event was reported in a study from Israel with the risk of breakthrough delta infection significantly increased if the last event was more than a year ago compared to 4 to 6 months [27]. We did not find an association with the last event most likely as the majority were of 4–6 months after the last event. An assessment of booster vaccination and breakthrough infections over an extended time would be able to confirm this observation for the Omicron variant which is still the predominant variant of SARS-CoV-2[1].
The majority of our patients had an asymptomatic or mild Omicron infection. A study from Israel reported a significantly lower risk of hospitalization and mortality of Omicron breakthrough infections compared to delta infections [33]. Similar trends were observed in the USA [34].
Strengths of the study include prospective data collection, the inclusion of patients with symptoms, and family history of COVID-19 as probable COVID-19 as well as factoring the time interval between infection or vaccination and the Omicron infection. The significance of this study is that it provides a valuable explanation that the majority of Indian patients with AIRDs did not suffer greatly during the Omicron wave since they already possessed hybrid immunity.
Limitations include the lack of data on the severity of infection as well as an assumption of the variant as Omicron after January 1st, 2022. Additional factors such as neutralizing activity against Omicron and T cell assays were not carried out. A larger sample size for the BBV152 vaccine group is needed to assess the difference in the effectiveness of the two vaccine types used.
In conclusion, the risk of breakthrough Omicron infections in fully vaccinated patients with RDs is still 17.4%. Those in the group having hybrid immunity had better protection during the Omicron wave.
Author contributions
All authors were involved in the data collection, refinement, statistical analysis, manuscript writing, and proofing. All authors take full responsibility for the integrity and accuracy of all aspects of the work.
Funding
None.
Data Availability
Data are available on request due to privacy restrictions.
Declarations
Conflict of interest
SA has received an honorarium as a speaker from Pfizer, Dr. Reddy’s, Cipla, and Novartis (unrelated to the current work). The other authors declare no conflicts of interest.
Open data sharing
The corresponding author can be contacted for details regarding data sharing.
Ethics approval
The study was approved by the Ethics Committee of Sree Sudheendra Medical Mission (IEC/2021/35) and written informed consent from all the participants was taken.
Patient consent for publication
Consent obtained directly from patient(s).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tracking SARS-CoV-2 variants [Internet]. [cited 2022 May 17]. Available from: https://www.who.int/activities/tracking-SARS-CoV-2-variants
- 2.IndiaFightsCorona COVID-19 in India, Vaccination, Dashboard , Corona Virus Tracker | mygov.in [Internet]. [cited 2022 May 17]. Available from: https://www.mygov.in/covid-19
- 3.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Risk M, Shen C, Hayek SS, Holevinski L, Schiopu E, Freed G, et al. Comparative effectiveness of COVID-19 vaccines against the delta variant. Clin Infect Dis. 2022;75(1):e623–e629. doi: 10.1093/cid/ciac106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruxvoort KJ, Sy LS, Qian L, Ackerson BK, Luo Y, Lee GS, et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ. 2021;375:e068848. doi: 10.1136/bmj-2021-068848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoff LS, Ravichandran N, Shinjo SK, Day J, Sen P, Junior JG, et al. COVID-19 severity and vaccine breakthrough infections in idiopathic inflammatory myopathies, other systemic autoimmune and inflammatory diseases, and healthy controls: a multicenter cross-sectional study from the COVID-19 Vaccination in Autoimmune Diseases (COVAD) survey. Rheumatol Int. 2023;43(1):47–58. doi: 10.1007/s00296-022-05229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed S, Gasparyan AY, Zimba O. Comorbidities in rheumatic diseases need special consideration during the COVID-19 pandemic. Rheumatol Int. 2021;41(2):243–256. doi: 10.1007/s00296-020-04764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CoVariants: 21K (Omicron) [Internet]. [cited 2022 May 17]. Available from: https://covariants.org/variants/21K.Omicron
- 9.Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2023;602(7898):654–656. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mileto D, Micheli V, Fenizia C, Cutrera M, Gagliardi G, Mancon A, et al. Reduced neutralization of SARS-CoV-2 Omicron variant by BNT162b2 vaccinees' sera: a preliminary evaluation. Emerg Microbes Infect. 2022;11(1):790–792. doi: 10.1080/22221751.2022.2045878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, AlMukdad S, Yassine HM, Al-Khatib HA, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N Engl J Med. 2022;386(19):1804–1816. doi: 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and delta variants. JAMA. 2022;327(7):639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shenoy P, Ahmed S, Paul A, Cherian S, Umesh R, Shenoy V, et al. Hybrid immunity versus vaccine-induced immunity against SARS-CoV-2 in patients with autoimmune rheumatic diseases. Lancet Rheumatol. 2022;4(2):e80–e82. doi: 10.1016/S2665-9913(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mageau A, Ferré VM, Goulenok T, Charpentier C, Delory N, Francois C, et al. Severely impaired humoral response against SARS-CoV-2 variants of concern following two doses of BNT162b2 vaccine in patients with systemic lupus erythematosus (SLE) Ann Rheum Dis. 2022;81(8):1194–1196. doi: 10.1136/annrheumdis-2022-222498. [DOI] [PubMed] [Google Scholar]
- 16.Menon AR, Cherian S, Paul A, Kumar K, Ahmed S, Mehta P, et al. Effects of the second dose of COVID-19 vaccines in patients with autoimmune rheumatic diseases with hybrid immunity. Rheumatol Int. 2022;43(3):449–457. doi: 10.1007/s00296-022-05265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.COVID-19 Breakthrough Data [Internet]. Department of Health. [cited 2023 Mar 2]. Available from: https://coronavirus.health.ny.gov/covid-19-breakthrough-data
- 18.CDC. COVID-19 Vaccination [Internet]. Centers for Disease Control and Prevention. 2020 [cited 2022 Oct 6]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html
- 19.Contact tracing in the context of COVID-19 [Internet]. [cited 2021 Nov 2]. Available from: https://www.who.int/publications-detail-redirect/contact-tracing-in-the-context-of-covid-19
- 20.Ahmed S, Mehta P, Paul A, Anu S, Cherian S, Shenoy V, et al. Postvaccination antibody titres predict protection against COVID-19 in patients with autoimmune diseases: survival analysis in a prospective cohort. Ann Rheum Dis. 2022;81(6):868–874. doi: 10.1136/annrheumdis-2021-221922. [DOI] [PubMed] [Google Scholar]
- 21.Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.152.9) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;99(10332):1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q, Guo Y, Iketani S, Nair MS, Li Z, Mohri H, et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608(7923):603–608. doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stalman EW, Wieske L, van Dam KPJ, Kummer LY, van Kempen ZLE, Killestein J, et al. Breakthrough infections with the SARS-CoV-2 omicron (B.1.1.529) variant in patients with immune-mediated inflammatory diseases. Ann Rheum Dis. 2022;81(12):1757–1766. doi: 10.1136/ard-2022-222904. [DOI] [PubMed] [Google Scholar]
- 24.Moor MB, Suter-Riniker F, Horn MP, Aeberli D, Amsler J, Möller B, et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2022;3(11):e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boekel L, Besten YR, Hooijberg F, Wartena R, Steenhuis M, Vogelzang E, et al. SARS-CoV-2 breakthrough infections in patients with immune-mediated inflammatory diseases during the omicron dominant period. Lancet Rheumatol. 2022;4(11):e747–e750. doi: 10.1016/S2665-9913(22)00221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calabrese CM, Kirchner E, Husni EM, Moss BP, Fernandez AP, Jin Y, et al. Breakthrough SARS-CoV-2 infections in immune mediated disease patients undergoing B cell depleting therapy: a retrospective cohort analysis. Arthritis Rheumatol. 2022;74(12):1906–1915. doi: 10.1002/art.42287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman LS, Ash N, et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med. 2022;386(23):2201–2212. doi: 10.1056/NEJMoa2118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crotty S. Hybrid immunity. Science. 2021;372:1392–1393. doi: 10.1126/science.abj2258. [DOI] [Google Scholar]
- 29.MoHFW | Home [Internet]. [cited 2022 Mar 2]. Available from: https://www.mohfw.gov.in/
- 30.Ferdinands JM, Rao S, Dixon BE, Mitchell PK, DeSilva MB, Irving SA et al (2022) Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance - VISION Network, 10 States, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 71(7):255–263. 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed]
- 31.COVID-19 vaccine surveillance report - week 6. [Internet].[cited 2022 Mar 2]. Available from: https://www.gov.uk/government/publications/covid-19-vaccine-surveillance-report
- 32.Amanatidou E, Gkiouliava A, Pella E, Serafidi M, Tsilingiris D, Vallianou NG, et al. Breakthrough infections after COVID-19 vaccination: Insights, perspectives and challenges. Metabol Open. 2022;14:100180. doi: 10.1016/j.metop.2022.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bieber A, Brikman S, Novack L, Ayalon S, Abu-Shakra M, Zeller L, et al. SARS-CoV-2 infection among patients with autoimmune rheumatic diseases; comparison between the Delta and Omicron waves in Israel. Semin Arthritis Rheum. 2023;58:152129. doi: 10.1016/j.semarthrit.2022.152129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawano Y, Patel NJ, Wang X, Cook CE, Vanni KM, Kowalski EN, et al. Temporal trends in COVID-19 outcomes among patients with systemic autoimmune rheumatic diseases: from the first wave through the initial Omicron wave. Ann Rheum Dis. 2022;81(12):1742–1749. doi: 10.1136/ard-2022-222954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request due to privacy restrictions.