Abstract
Background
The impact of body composition (BC) abnormalities on COVID-19 outcomes remains to be determined.
Objectives
We summarized the evidence on BC abnormalities and their relationship with adverse clinical outcomes in patients with COVID-19.
Methods
A systematic search was conducted up until 26 September, 2022 for observational studies using BC techniques to quantify skeletal muscle mass (or related compartments), muscle radiodensity or echo intensity, adipose tissue (AT; or related compartments), and phase angle (PhA) in adults with COVID-19. Methodological quality of studies was assessed using the Newcastle-Ottawa Scale. A synthesis without meta-analysis was conducted to summarize the prevalence of BC abnormalities and their significant associations with clinical outcomes.
Results
We included 62 studies (69.4% low risk of bias) with 12–1138 participants, except 3 studies with ≤490,301 participants. Using CT and different cutoff values, prevalence ranged approximately from 22% to 90% for low muscle mass, 12% to 85% for low muscle radiodensity, and 16% to 70% for high visceral AT. Using BIA, prevalence of high FM was 51%, and low PhA was 22% to 88%. Mortality was inversely related to PhA (3/4 studies) and positively related to intra- and intermuscular AT (4/5 studies), muscle echo intensity (2/2 studies), and BIA-estimated FM (2/2 studies). Intensive care unit (ICU) admission was positively related to visceral AT (6/7 studies) and total AT (2/3 studies). Disease severity and hospitalization outcomes were positively related to intra- and intermuscular AT (2/2 studies). Inconsistent associations were found for the rest of the BC measures and hospitalization outcomes.
Conclusions
Abnormalities in BC were prevalent in patients with COVID-19. Although conflicting associations were observed among certain BC abnormalities and clinical outcomes, higher muscle echo intensity (reflective of myosteatosis) and lower PhA were more consistently associated with greater mortality risk. Likewise, high intra- and intermuscular AT and visceral AT were associated with mortality and ICU admission, respectively.
This trial was registered at PROSPERO as CRD42021283031.
Keywords: COVID-19, body composition, muscle, adipose tissue, phase angle, hospitalization, mortality, computed tomography, ultrasound, bioelectrical impedance analysis
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- ALST
appendicular lean soft tissue
- AT
adipose tissue
- BC
body composition
- BIA
bioelectrical impedance analysis
- BMI
body mass index
- COVID-19
coronavirus disease 2019
- CSA
cross-sectional area
- CT
computed tomography
- DXA
dual-energy X-ray absorptiometry
- HU
Hounsfield unit
- ICU
intensive care unit
- IMAT
intra- and intermuscular adipose tissue
- L3
third lumbar vertebra
- LOS
length of stay
- LST
lean soft tissue
- MV
mechanical ventilation
- NOS
Newcastle-Ottawa Scale
- PhA
phase angle
- RT-PCR
real-time reverse transcriptase-polymerase chain reaction
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SAT
subcutaneous adipose tissue
- SMI
skeletal muscle index
- SPhA
standardized phase angle
- T4
fourth thoracic vertebra
- TAT
total adipose tissue
- US
ultrasound
- VAT
visceral adipose tissue.
Introduction
COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has a wide spectrum of clinical manifestations, ranging from asymptomatic to severely symptomatic. Advanced age and comorbid conditions, such as obesity, diabetes mellitus, hypertension, and cardiovascular or respiratory system diseases, have been associated with severe or lethal COVID-19 [1]. Body composition (BC) may also play a role in COVID-19 severity. Evidence suggests that individuals with abnormal BC phenotypes—including low muscle mass, low muscle radiodensity (reflective of myosteatosis), and/or excess adiposity—may be at higher risk for greater disease severity and death [[2], [3], [4]]. However, although BC is a better predictor of health outcomes than BMI [5,6], its assessment during and after COVID-19 hospitalization has been particularly difficult in such an overburdened clinical scenario [7].
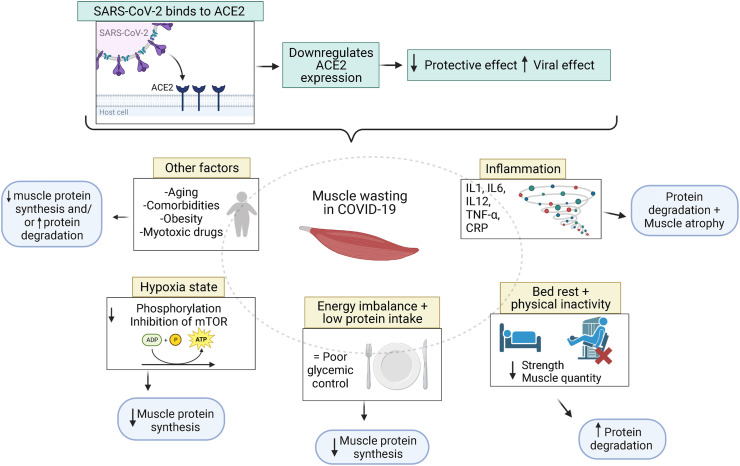
SARS-CoV-2 infection directly contributes to muscle loss and myosteatosis through increased production of proinflammatory cytokines, leading to muscle protein breakdown and/or inhibition of protein synthesis [[8], [9], [10]] (Figure 1 ). Prolonged bed rest and hospital stay (>2 wk) are also associated with reduced muscle mass in critical care [11,12]. This can be extrapolated to COVID-19 and might contribute to worse clinical course and long-term sequelae [13,14]. Patients with COVID-19 discharged from the intensive care unit (ICU) may experience an especially challenging post-acute functional recovery, requiring specific rehabilitation strategies [15].
FIGURE 1.
Potential muscle wasting mechanisms of COVID-19. SARS-CoV-2 induces ACE2 downregulation and increases the viral effect on the body. Inflammation, physical inactivity, energy imbalance, and hypoxia may also downregulate muscle protein synthesis and cause muscle wasting. Figure created with BioRender.com. ACE2: angiotensin-converting enzyme 2; mTOR, mammalian/mechanistic target of rapamycin; P, phosphate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Obesity [using BMI as a proxy of high adipose tissue (AT)] is a risk factor for developing severe COVID-19 [16]. Thus, AT content and distribution may also predict COVID-19 prognosis [3,17]. Excess visceral adipose tissue (VAT), which is highly metabolically active, is detrimental to several health aspects. VAT secretes inflammatory mediators that may amplify the cytokine storm triggered by SARS-CoV-2, thereby possibly contributing to disease severity [[18], [19], [20]].
In view of the above-mentioned literature, this systematic review aimed to summarize the evidence on the prevalence of BC abnormalities in COVID-19. We also investigated the relationship between BC phenotypes and adverse outcomes, including mortality, ICU admission, mechanical ventilation (MV), disease severity, hospitalization, and length of stay (LOS), among others.
Methods
Study overview and eligibility criteria
We conducted this systematic review in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses [21] and the Synthesis Without Meta-Analysis [22] reporting guidelines. The study was registered in PROSPERO (CRD42021283031), and no deviations from the original protocol were made.
We used the PECOS criteria (population, exposure, comparison, outcome, study design) to formulate the eligibility criteria of studies for our review. Our population was defined as adults (aged ≥18 y) diagnosed with COVID-19 using positive RT-PCR, with or without specific morbidities. For exposure, studies had to assess ≥1 of the following body composition measures: 1) quantity of skeletal muscle or its related compartments [that is, FFM, lean soft tissue (LST), and appendicular lean soft tissue (ALST)]; 2) muscle radiodensity or muscle echo intensity as markers of skeletal muscle composition; 3) quantity of AT or its related compartments [that is, VAT, subcutaneous adipose tissue (SAT), intra- and intermuscular adipose tissue (IMAT), and FM]; and 4) phase angle (PhA), which is a composite value derived from raw BIA measures (that is, resistance and reactance) and is considered a marker of muscle mass and composition [23]. We also included studies where conditions of sarcopenia and sarcopenic obesity were diagnosed using acknowledged BC techniques. BC measures had to be assessed using BC techniques compared with anthropometric approaches [for example, CT, BIA, DXA, and ultrasound (US)] at any time point. Comparator was defined as patients with COVID-19 but with normal BC (that is, absence of abnormalities in BC measures using different approaches, such as pre-established or data-driven cutoff values). Due to the challenges in defining cutoff values for abnormal BC, we also included studies that did not categorize participants into groups (for example, “normal” compared with “abnormal”) but instead assessed relationships between BC and outcomes on a continuous scale. Additional outcomes included prevalence of BC abnormalities and/or clinical outcomes, such as mortality, ICU admission, disease severity, MV, hospitalization rate, LOS or ICU stay, comorbidities, hospital discharge, physical function (for example, grip strength, physical performance, and mobility) or reduced muscle function (for example, grip strength), and inflammatory markers. We included observational studies [for example, cross-sectional, longitudinal cohort (retrospective or prospective), and case-controls]; data from control groups of randomized controlled trials were also eligible for this review.
Outcomes with incomplete data were not included. We excluded studies combining children or adolescent data with adult data or reports on post–COVID-19 assessments. Case reports, case series, narrative reviews, systematic reviews, meta-analyses, conference abstracts, and non–peer-reviewed articles were also excluded.
Search strategy
A comprehensive search of electronic databases including MEDLINE (Ovid), Embase (Ovid), CINAHL, and SCOPUS was developed by a professional librarian in collaboration with the team. Searches included articles published between 1 December, 2019 and 26 September, 2022 (last search update). The search combined keywords related to COVID-19 and BC [compartments (for example, muscle mass and FFM) and clinical condition (for example, sarcopenia)] (Supplemental Table 1). Searches were restricted to English language and human studies. Retrieved articles were screened for eligibility using Covidence online software (Vertitas Health Innovation Ltd). Records from each database were automatically merged, and duplicates were removed. Three independent reviewers (MMI, CEO, and ATLM) screened titles and abstracts for inclusion criteria in duplicate, and full text of relevant articles were retrieved and examined by these independent reviewers. Disagreements were resolved by consensus between reviewers. During the last search update, it was noticed that Covidence automatically excluded as “duplicate” any non–peer-reviewed articles that had become peer-reviewed between the first and last searches. As such, electronic databases were manually searched (RIS files) for non–peer-reviewed articles to identify potential eligible articles missed during screening (see details in Supplemental Table 2).
Data extraction and synthesis
Relevant data were extracted by one reviewer (MMI, CEO, or ATLM) using an Excel spreadsheet. Data extracted included general study information, demographic and sample characteristics, study design, study methods, exposure, and outcomes. An independent reviewer (MMI, CEO, or ATLM) checked data for accuracy. Discrepancies were corrected by consensus among the 3 reviewers (MMI, CEO, ATLM). When necessary, an open source software (Digitizer V.2.6.8; http://plotdigitizer.sourceforge.net) was used to convert data plots into numerical values [24].
Body composition was classified into the following categories based on measures and techniques used for assessment: muscle quantity [that is, mass, area, or thickness of skeletal muscle or its related compartments (FFM, LST, and ALST)], muscle composition [that is, the degree of IMAT infiltration (or myosteatosis) depicted by surrogate measures including muscle radiodensity or echo intensity], PhA, and adipose tissue quantity (that is, mass, area, or thickness of adipose tissue and its related compartments). For simplicity, CT-assessed IMAT was considered under the category of AT because it is usually identified using the same Hounsfield unit (HU) range of AT (−190 to −30 HU) compared with that of skeletal muscle (−29 to +150 HU) [25]. However, it is noteworthy that IMAT also reflects muscle composition and is strongly associated with muscle radiodensity and echo intensity [26,27]. Notably, high muscle echo intensity and low muscle radiodensity are reflective of myoesteatosis and are therefore markers of BC abnormality.
The physical principle of BIA involves estimating total body water and related compartments (that is, FFM, ALST, LST, or skeletal muscle mass depending on the reference tool used to develop the BIA equation). As a result, BIA-estimated visceral fat data was not included because it is more closely related to anthropometric measures (that is, waist circumference) than to body composition per se and is also poorly related to CT-assessed VAT [28,29]. Studies exclusively assessing diaphragm muscle were also excluded, as this is currently a poorly explored and accepted approach to define low muscle mass [30]. The variation in prevalence of BC abnormalities among studies was summarized using range (that is, minimum to maximum value reported).
The association between BC and outcomes [correlation or association (beta, OR, and HR) coefficients] and/or differences in BC or outcomes between analyzed groups were reported. Some studies evaluated >1 BC compartment, and therefore, data presentation is reflected by that (that is, total number of studies reporting the relationship is higher than the absolute number of studies included in the review). The direction of the association was described as positive (for example, higher AT related to greater mortality rate) or inverse (for example, lower muscle mass related to greater mortality rate) for studies where the association was significant (P < 0.05). Studies evaluating different indices of the same BC compartment [for example, skeletal muscle cross-sectional area (CSA) and skeletal muscle index (SMI)] or outcomes within the same domain (for example, hospitalization rate and LOS) were labeled as “mixed” if <70% of the relationships between exposures and outcomes had a consistent direction [31]. The term “unrelated” was used to report a nonsignificant relationship. Data from studies not showing P values were not abstracted. Study characteristics and findings were reported in summary tables and graphs.
Study quality assessment
The methodological quality of the included studies was assessed independently by 3 reviewers (MMI, CEO, or ATLM) in duplicate using the Newcastle-Ottawa Scale (NOS) [32], and disagreements were resolved by consensus. NOS uses 2 slightly different rating scales for case-control and cohort studies. In the absence of an official and validated rating scale for cross-sectional studies [33], we adapted the case-control scale using signaling questions specific to our research question and evaluated cross-sectional studies (that is, those collecting outcome data on the same day or close to the BC assessment day) included in this review [34]. Cohort studies were defined as those that assessed outcomes over time (for example, mortality and LOS). Research-specific adaptations to these scales have been observed in the literature [35,36]. Both forms can have a maximum of 9 points across the following 3 domains: 1) selection of study groups (4 points); 2) comparability of groups (2 points); and 3) ascertainment of exposure and outcomes (3 points) (Supplemental Table 3). For quality assessment, each point was collected and summed to obtain a total score. Studies with ≥6 points were considered high quality [36].
Results
Study selection and patients’ characteristics
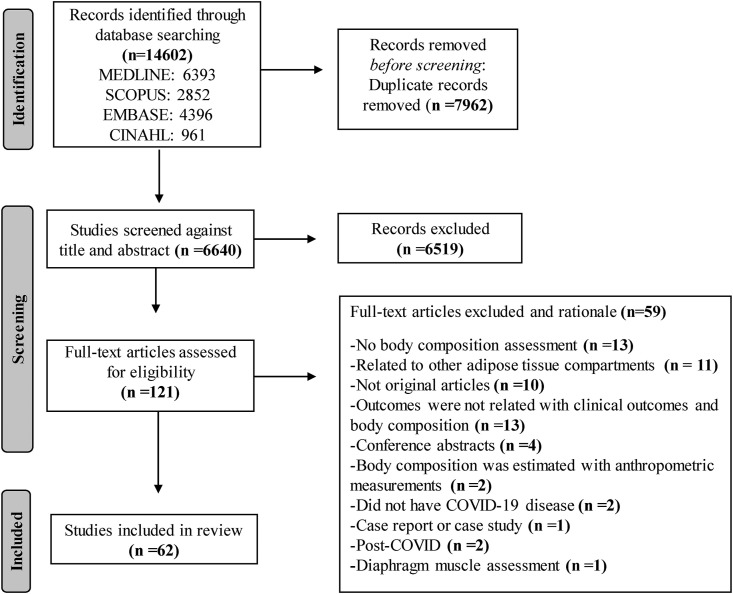
The search identified 14,602 records (Figure 2 ). After screening for duplicates and study characteristics, we reviewed 121 full-text articles, 62 of which met the inclusion criteria. Included articles were cohort (n = 56) or cross-sectional (n = 6) studies, with most having a retrospective (n = 41) design. Sample sizes ranged from 12 to 1138 participants, except for 3 studies that included participants from the UK Biobank—one with 435,504 participants, another with 461,460 participants, and the largest with 490,301 participants [[37], [38], [39]]. One of these population-based studies performed Mendelian randomization analysis and included participants from diverse datasets. In this case, BC data (that is, exposure) were obtained from UK Biobank participants, whereas outcome data (COVID-19 severity and hospitalization) were obtained from 19,444 individuals from the COVID-19 Host Genetic Initiative [39].
FIGURE 2.
PRISMA flow diagram for systematic reviews of databases and registers.
Participant age ranged from 18 to 93 y (where range was available). Studies assessed body composition in hospitalized patients using CT imaging (n = 45), BIA (n = 12), and US (n = 4). One study used 2 different techniques (CT and BIA) [40]. Most studies were from Italy (n = 15), followed by Germany (n = 7), United States (n = 6), Mexico (n = 4), and Brazil (n = 4). Included articles were published between 2020 and 2022. Two studies reported data from both the first and second COVID-19 wave [41,42], and 4 studies investigated changes over time in BC [[43], [44], [45], [46]].
Study quality
A total of 43 articles (69.4%) were rated as having a low risk of bias (≥6 points) (Table 1 ). The number of studies scoring the maximum number of points within each domain were as follows: 10 studies (16.1%) for selection of study group; 38 studies (61.3%) for comparability of groups; and 29 studies (46.8%) for ascertainment of exposure and outcomes. Under the “comparability of groups” domain, 16 studies (25.8%) received 1 point for controlling for covariables (that is, age, sex), and 38 (61.3%) were assigned 2 points as they controlled for additional covariables (for example, disease severity, BMI). As noted under this category, multivariate analyses were conducted in 54 studies (87.1%) and univariate analyses in 8 studies (12.9%).
TABLE 1.
Quality assessment of included studies (N = 62) using the Newcastle-Ottawa Scale.
| Study | Cohort or cross-sectional1 | Selection |
Comparability |
Exposure/outcome |
Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 1 | 2 | 3 | |||
| Antonarelli [81] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Attaway [43] | Cohort | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 7 | ||
| Aykin [97] | Cohort | ∗ | ∗∗ | ∗ | ∗ | ∗ | 6 | |||
| Battisti [90] | Cohort | ∗ | ∗ | ∗∗ | ∗ | ∗ | 6 | |||
| Beltrao [47] | Cohort | ∗ | ∗ | ∗∗ | ∗ | ∗ | 6 | |||
| Besutti [74] | Cohort | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 7 | ||
| Beypinar [77] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | 5† | |||
| Bunnell [95] | Cohort | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 8 | |
| Chandarana [96] | Cross-sectional | ∗ | ∗ | ∗ | 3† | |||||
| Chandarana [99] | Cross-sectional | ∗ | ∗∗ | ∗ | ∗ | ∗ | 6 | |||
| Cornejo-Pareja [48] | Cohort | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | 7 | ||
| Damanti [4] | Cohort | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 7 | ||
| Da Porto [82] | Cohort | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | 7 | ||
| De Andrade-Junior [44] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| De Lorenzo [72] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | 5† | |||
| Del Giorno [49] | Cohort | ∗ | ∗ | ∗∗ | 4† | |||||
| Do Amaral E Castro [98] | Cross-sectional | ∗ | ∗∗ | ∗ | ∗ | 5† | ||||
| Erdöl [50] | Cohort | ∗ | ∗∗ | ∗ | ∗ | ∗ | 6 | |||
| Favre [20] | Cohort | ∗ | ∗∗ | ∗ | ∗ | 5† | ||||
| Feng [51] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Formenti [75] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | 5† | |||
| Gao [37] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | |
| Giraudo [52] | Cross-sectional | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | |
| Goehler [70] | Cohort | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 7 | ||
| Hocaoglu [76] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Kang [53] | Cohort | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | 7 | ||
| Kardas [83] | Cohort | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 8 | |
| Kellnar [45] | Cohort | ∗ | ∗ | ∗ | ∗ | 4† | ||||
| Kim [54] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Koehler [55] | Cohort | ∗ | ∗ | ∗∗ | 4† | |||||
| Kottlors [91] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 8 |
| Kremer [42] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| McGovern [56] | Cohort | ∗ | ∗ | ∗ | 3† | |||||
| Menozzi [41] | Cohort | ∗ | ∗ | ∗∗ | ∗ | 5† | ||||
| Moctezuma-Velázquez [57] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 8 |
| Molwitz [58] | Cohort | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | 7 | ||
| Moonen [89] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | 5† | |||
| Moonen [93] | Cohort | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 8 | |
| Nobel [86] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | 5† | |||
| Ogata [6] | Cohort | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 8 | |
| Osuna-Padilla [59] | Cohort | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 7 | ||
| Osuna-Padilla [40] | Cohort | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 8 | |
| Padiha [60] | Cohort | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 8 | |
| Pediconi [71] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Petersen [94] | Cross-sectional | ∗ | ∗∗ | ∗ | 4† | |||||
| Polat [84] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | 5† | |||
| Poros [78] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | 5† | |||
| Reyes-Torres [100] | Cohort | ∗ | ∗ | ∗ | ∗∗ | ∗ | 6 | |||
| Rossi [79] | Cohort | ∗ | ∗ | ∗ | ∗ | 4† | ||||
| Sahin [87] | Cohort | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | 7 | ||
| Scheffler [85] | Cohort | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 7 | ||
| Schiaffino [80] | Cohort | ∗ | ∗ | ∗ | ∗∗ | ∗ | 6 | |||
| Stevanovic [73] | Cohort | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | 7 | ||
| Surov [88] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Ufuk[61] | Cohort | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 7 | ||
| Umbrello [46] | Cohort | ∗ | ∗ | ∗ | ∗ | 4† | ||||
| Watanabe [92] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | 8 | |
| Wilkinson [38] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | 8 | |
| Yang [62] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 7 | |
| Yi [63] | Cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 6 | ||
| Ying-Hao [64] | Cohort | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 7 | ||
| Yoshiji [39] | Cross-sectional | ∗ | ∗ | ∗ | ∗ | 4† | ||||
Each asterisk (∗) denotes 1 point. The maximum number of points for each study is 9, and studies with ≥6 total points were considered to be of high quality. Numbers with † in the “Total” column refer to studies with a low rating.
Cross-sectional studies were evaluated using an adapted version of the Newcastle-Ottawa Scale for case-control studies (see details on the criteria in Supplemental Material). The following domains were used: selection: 1: definition adequate; 2: representative of the cases; 3: selection of controls; 4: definition of controls. Comparability: 1: study controls; exposure: 1: ascertainment of exposure; 2: same method of ascertainment for cases and controls; 3: non-response rate. For the cohort scale the following domains were used: selection: 1: representativeness of the exposed cohort; 2: selection of the nonexposed cohort; 3: ascertainment of exposure; 4: demonstration that outcome of interest was not present at the start of study. Comparability: 1: study controls; outcomes: 1: assessment of outcome; 2: sufficient length of follow-up for outcomes to occur 3: adequacy of follow-up.
Prevalence of BC abnormalities
Thirty of 62 studies (48.4%) reported data on the prevalence of BC abnormalities in patients with COVID-19 using CT (n = 25), US (n = 1), or BIA (n = 4) (Table 2 ). Of those studies, 23 identified patients with low muscle mass, low FFM, low radiodensity, or low PhA [4,38,[40], [41], [42],[47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64]]. The prevalence of low muscle mass using different cutoff values for CT-based SMI [54,[65], [66], [67], [68], [69]] ranged from 22.2% to 90.0% [4,[40], [41], [42],47,50,51,[53], [54], [55], [56], [57], [58],61,63,64]. Additionally, 2 studies estimated low muscle mass based on FFM measures and reported a prevalence ranging between 1.7% and 84.4% [38,49]. Low muscle radiodensity (based on HU by CT imaging) was reported in 9 studies, with a prevalence ranging from 11.8% to 85.2% [4,[51], [52], [53],55,56,60,62,63]. Low PhA was reported in 3 studies, with a prevalence ranging from 22.0% to 87.8% [48,49,59]. Furthermore, 8 studies identified patients with abnormal AT using CT scans [6,20,53,56,62,[70], [71], [72]] and 2 with BIA [49,73]. Prevalence of high VAT ranged from 16.1% to 69.6% [20,56,70,71], 75.0% of COVID-19 patients had high SAT [56], 31.5% to 50.3% had high VAT:SAT ratio [53,62,72], 50.9% had high percent FM (by BIA) [73], and 84.4% had FM adjusted by height below the 15th data-driven percentile [49].
TABLE 2.
Body composition abnormalities in patients with COVID-19
| Study | Body compartment analyzed | Technique | Prevalence of body composition abnormalities | Cutoff values used to define abnormalities |
|---|---|---|---|---|
| Beltrao [47] | Muscle mass | CT | Low SMA: 111 of 200 (55.5%) | SMA: <92 cm2 |
| Cornejo-Pareja [48] | Muscle mass | BIA | SPhA quartiles (lowest to highest) | Categorized SPhA into quartiles |
| Q1: 34 of 127 (26.8%) | Q1: lower 25th percentile (<−2) | |||
| Q2: 31 of 127 (24.4%) | Q2: 25th–50th percentile (−1.9 to −0.8) | |||
| Q3: 34 of 127 (26.8%) | Q3: 50th to 75th percentile (−0.7 to 0.2) | |||
| Q4: 28 of 127 (22%) | Q4: >75th percentile (≥0.3) | |||
| Damanti [4] | Muscle mass and radiodensity | CT | Low SMI: 53 of 81 (65.4%) | SMI: specific to each sex and vertebra level according to Derstine et al. [66] |
| Low SMD: 69 of 81 (85.2%) | Low SMD: specific to each vertebra level according to Derstine et al. [66] | |||
| De Lorenzo [72] | Adipose tissue | CT (estimated %BF) | Obese: 12 of 22 (54.5%) | Defined using age- and sex-specific %BF cutoffs [128] |
| Del Giorno [49] | FFM FM Body cell mass |
BIA | At nutritional risk | Defined malnourished when BIA parameters were lower than the 15th percentile |
| Low PhA: 79 of 90 (87.8%) | Low PhA: 4.3° | |||
| Low FFM: 76 of 90 (84.4%) | Low FFM: 27.9 kg/m | |||
| Low FM: 76 of 90 (84.4%) | Low FM: 6.2 kg/m | |||
| Erdöl [50] | Muscle mass | CT | Low SMI: 77 of 232 (33.2%) | Low SMI: data-driven tertiles (specific cutoff values not reported) |
| Favre [20] | Adipose tissue | CT | High VAT: 64 of 165 (38.8%) | High VAT: ≥128.5 cm2 |
| Feng [51] | Muscle mass and radiodensity | CT | Low paraspinal muscle index: Males: 32 of 63 (50.8%) Females: 26 of 53 (49.1%) |
Low paraspinal muscle index: defined using gender-specific medians (values not reported) |
| Low paraspinal SMD: Males: 32 of 63 (50.8%) Females: 27 of 53 (50.9%) |
Low paraspinal SMD: defined using gender-specific medians (values not reported) | |||
| Giraudo [52] | Muscle radiodensity | CT | Low SMD: 43 of 150 (28.7%) | Low SMD: <30 HU |
| Goehlr [70] | Adipose tissue | CT | High VAT: 263 of 378 (69.6%) | High VAT: ≥100 cm2 |
| Kang [53] | Muscle mass and adipose tissue | CT | Low SMI: 103 of 127 (81.1%) | Low SMI at L2 <50 cm2/m2 for males; <39 cm2/m2 for females |
| High VAT:SAT ratio: 40 of 127 (31.5%) | High VAT:SAT ratio: as a proxy of high visceral adiposity >1.33 for males; >0.71 for females |
|||
| Low SMD: 15 of 127 (11.8%) | Low SMD: as a proxy of myosteatosis <32.7 HU for males; <28.9 for females |
|||
| Kim [54] | Muscle mass | CT | Low SMI: 29 of 121 (24%) | SMI: ≤24 cm2/m2 for males; ≤20 cm2/m2 for females |
| Koehler [55] | Muscle mass and radiodensity | CT | Low SMI at L3: 83 of 162 (51.2%) | Low SMI at L3: <52.3 cm2/m2 for males; <38.6 cm2/m2 for females (both BMI < 30 kg/m2) 54.3 cm2/m2 for males 46.6 cm2/m2 for females (both BMI > 30 kg/m2) |
| Low SMD at L3: 105 of 162 (64.8%) | Low SMD: <32.5 HU for females; <35.5 HU for males |
|||
| Kremer [42] | Muscle mass | US | Low psoas muscle area index: Males: 33 of 69 (47.8%) Females: 20 of 44 (45.5%) |
Low psoas muscle area index: Defined using gender-specific median muscle indices from reference cohort I (≤ median) |
| Males: 246.9 mm2/m2 | ||||
| Females: 224.1 mm2/m2 | ||||
| McGovern [56] | Muscle mass and radiodensity | CT | Low SMI: 39 of 63 (62%) | Low SMI: <43 cm2/m2 for males; <41 cm2/m2 for females (both BMI < 25 kg/m2) <53 cm2/m2 for males; SMI <41 cm2/m2 for females (both BMI >25 kg/m2) |
| Low SMD: 51 of 63 (81%) | Low SMD: <41 HU for BMI < 25 kg/m2; <33 HU for BMI ≥ 25 kg/m2) |
|||
| Adipose tissue | High VAT: 42 of 63 (67%) | VAT: males >160 cm2; females >80 cm2 | ||
| High SAT: 47 of out 63 (75%) | SAT: males >50.0 cm2/m2; females >42.0 cm2/m2) | |||
| Menozzi [41] | Muscle mass | CT | Low SMA: 41.5% (overall) First wave: 88 of 155 (57.9%) Second wave: 25 of 117 (21.6%) |
Low SMA: <92.3 cm2 for males; <56.1 cm2 in females |
| Moctezuma-Velázquez [57] | Muscle mass | CT | Low SMI: 115 of 519 (22.2%) | Low SMI: <42.6 cm2/m2 for males; <30.6 cm2/m2 for females |
| Molwitz [58] | Muscle mass | CT | Low SMI: Prado et al. [68]: Male: 18 of 20 (90%) Female: 6 of 12 (50%) Martin et al. [67]: Male: 17 of 20 (85%) Female: 7 of 12 (58.3%) Werf et al. [69]: Male: 11 of 20 (55%) Female: 3 of 12 (25%) |
Low SMI: specific to each sex and vertebra level according to Prado et al. [68], Martin et al. [67], Werf et al. [69] cutoffs |
| Ogata [6] | Adipose tissue | CT | VAT/TAT: | Defined using data-driven tertiles |
| Lowest: 17 of 53 (32.1%) | Lowest VAT/TAT: ≤48.9% | |||
| Middle: 18 of 53 (34.0%) | Middle VAT/TAT: 49.0% to 66.1% | |||
| Highest: 18 of 53 (34.0%) | Highest VAT/TAT: ≥66.2% | |||
| Osuna-Padilla [59] | Muscle mass | CT | Low SPhA: Nonsurvivors: 24 of 25 (96%) Survivors: 29 of 42 (69%) |
Low SPhA: < −1.65 |
| Osuna-Padilla [40] | Muscle mass | CT | Low SMI: 41 of 86 (48%) | Low SMI: ≤52.3 cm2/m2 for males; ≤38.6 cm2/m2 for females (both BMI < 30 kg/m2) ≤54.3 cm2/m2 for males; ≤46.6 cm2/m2 for females (both BMI > 30 kg/m2) |
| Padilha [60] | Muscle radiodensity | CT | Low SMD: 71 of 200 (35.5%) | Low SMD: <35.5 HU for males; <27.7 HU for females |
| Pediconi [71] | Adipose tissue | CT | VAT-defined obesity: 40 of 62 (64.5%) | VAT-defined obesity: >130 cm2 |
| VAT-defined overweight: 10 of 62 (16.1%) | VAT-defined overweight: 100–129 cm2 | |||
| Stevanovic [73] | FM | BIA | Very high %BF:50.9% | Very high %BF: Males: >25% for ages 20–39; >27.5% for ages 40–59; >30% for ages ≥60 Females: >39.5% for ages 20–39; >40% for ages 40–59; >41.5% for ages ≥60 |
| Ufuk [61] | Muscle mass | CT | Low pectoralis muscle index: Males: 25 of 76 (32.9%) Females: 19 of 54 (35.2%) |
Low pectoralis muscle index: smallest tertile of height-square adjusted pectoralis area values for each sex |
| Watanabe [92] | Adipose tissue | CT | N/R | High VAT: defined using data-driven quartiles |
| Wilkinson [38] | Muscle mass | BIA | Low ALST: ALST index: 8321 of 490,301 (1.7%) ALST/BMI: 8293 of 490,301 (1.7%) Either index: 9342 of 490,301 (1.9%) |
Low ALST (defined using 2 distinct indices): ALST index (ALST/height2): <7.26 kg/m2 for males and <5.45 kg/m2 for females [129] ALST/BMI: <0.789 in males and <0.512 in females [130] High % BF: >25% in men and >35% in women [131]. Sarcopenic obesity: defined as the presence of obesity and low muscle mass (using both ALST index and ALST/BMI definitions) |
| Yang [62] | Muscle radiodensity | CT | Low SMD: 71 of 143 (49.7%) | Low SMD: 32.7 HU in males and 28.9 HU in females |
| Adipose tissue | High VAT/SAT: 72 of 143 (50.3%) | High VAT/SAT: median values of 1.33 for males and 0.71 in females | ||
| Yi [63] | Muscle mass | CT | Low SMI: 78 of 234 (33.3%) | Low SMI: data-driven tertiles (specific cutoff values not reported) |
| Low SDM: 77 of 234 (32.9%) | Low SMD: data-driven tertiles (specific cutoff values not reported) | |||
| Ying-Hao [64] | Muscle mass | CT | Low pectoralis muscle index: 39 of 116 (33.6%) | Low pectoralis muscle index: 16.4 cm2/m2 for males and 13.8 cm2/m2 for females |
ALST, appendicular lean soft tissue; HU, Hounsfield unit; L2, second lumbar vertebra; L3, third lumbar vertebra; N/R, not reported; PhA, phase angle; SAT, subcutaneous adipose tissue; SMA, skeletal muscle area; SMD, skeletal muscle radiodensity; SMI, skeletal muscle index; SPhA, standardized phase angle; TAT, total adipose tissue; VAT, visceral adipose tissue. Note: The study by Watanabe et al. [92] categorized patients into data-driven quartiles of VAT; however, the authors did not specify the final number of patients in each group, so data could not be abstracted here.
Body composition abnormalities and clinical outcomes
Mortality
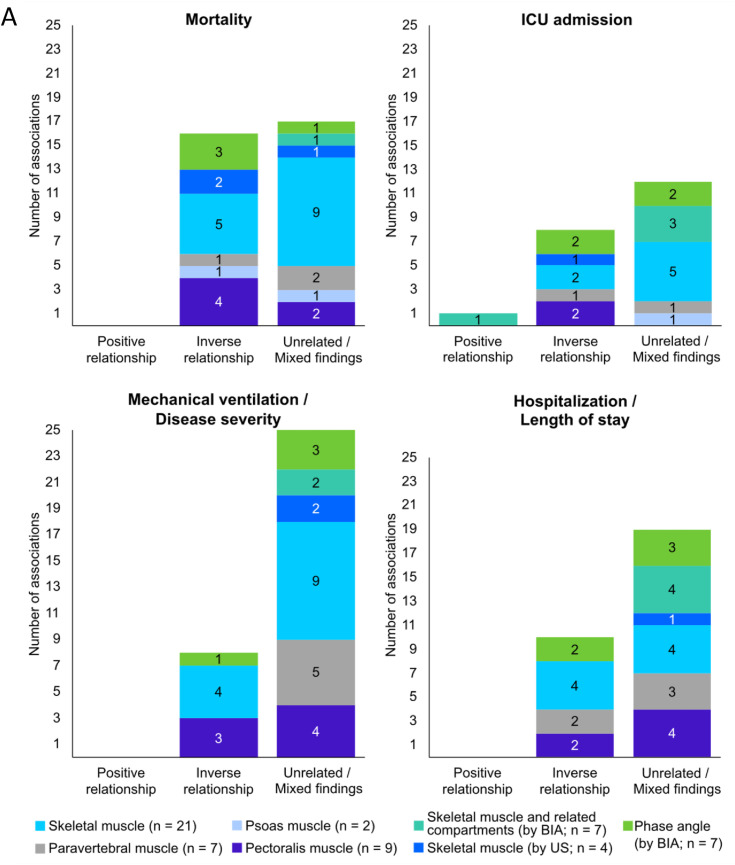
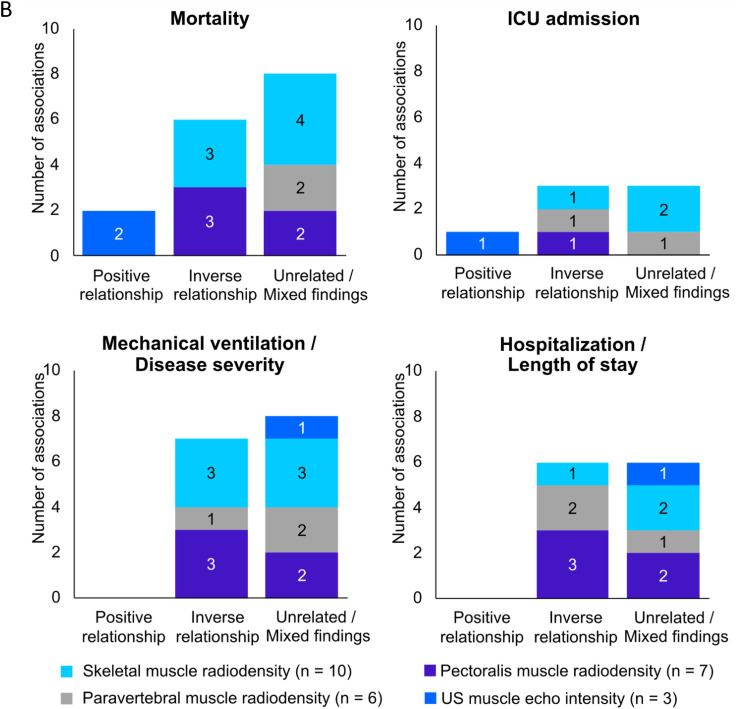
The relationship between mortality and body composition abnormalities was investigated in 36 studies [4,37,40,42,43,[46], [47], [48],50,53,54,[56], [57], [58], [59], [60], [61], [62],70,[73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89]] (Figure 3 , Supplemental Table 4). Out of 24 of these studies, 12 showed that skeletal muscle mass assessed by either CT [43,47,50,56,61,77,78,80,86,88] or US [42,46] was negatively associated with in-hospital mortality and 30-d mortality [4,40,42,43,46,47,50,53,54,[56], [57], [58],60,61,75,77,78,80,81,83,84,[86], [87], [88]]. Lower muscle radiodensity was also related to higher mortality rate in 6 [53,60,74,76,87,88] of 13 studies [4,53,56,58,60,74,76,[79], [80], [81],83,87,88]. Two studies reporting muscle echo intensity using US found a positive association with mortality [46,75]. Low PhA predicted mortality in 3 [48,59,89] of 4 studies [48,59,82,89].
FIGURE 3.
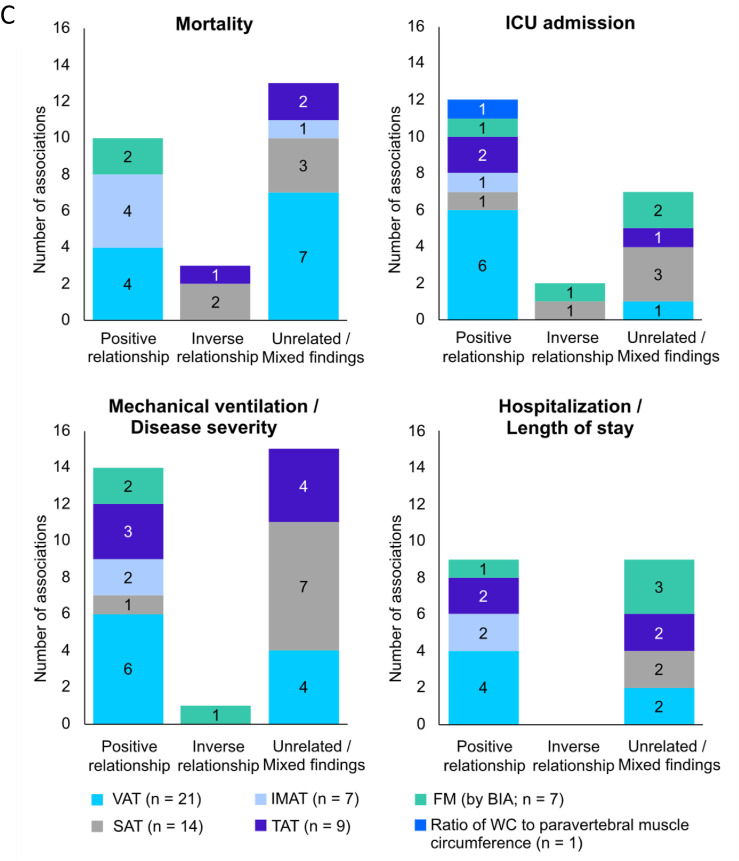
Associations between clinical outcomes and body composition abnormalities related to: A) muscle mass; B) muscle composition; C) adipose tissue, in acute COVID-19 patients. Number of studies per variables is shown in parenthesis. Most variables were obtained from computed tomography images studies. Ten studies used bioelectrical impedance analysis and only 4 used ultrasound. Some studies evaluated >1 body composition compartment, and therefore, data presentation is reflected by that (that is, total number of studies reporting the relationship is higher than the absolute number of studies included in the review). ICU, intensive care unit; IMAT, intra- and intermuscular adipose tissue; SAT, subcutaneous adipose tissue; TAT, total adipose tissue; US, ultrasound; VAT, visceral adipose tissue; WC, waist circumference.
Fifteen studies evaluated the relationships between AT depots and mortality [37,47,53,56,58,62,70,73,74,[76], [77], [78], [79],85,86]. Higher VAT was related to greater mortality rates in 4 [47,56,70,78] of 11 studies [47,53,56,58,62,70,74,77,78,85,86]. Positive relationships were also found between IMAT and mortality risk in 4 [62,76,79,86] of 5 studies [62,74,76,79,86]. Nevertheless, 1 study found that patients with lower SAT had higher mortality rates [53]. Hospitalized older adults who survived COVID-19 also had greater SAT and total AT (TAT) than nonsurvivors, suggesting a protective effect of higher SAT and TAT [85]. Two studies [37,73] found that BIA-estimated FM was positively related to mortality. Findings for each adiposity compartment can be found in Supplemental Figure 1.
Overall, a small number of studies showed that mortality measures were positively related to muscle echo intensity assessed by US and inversely with PhA; however, skeletal muscle mass and radiodensity were inconsistently related to mortality. Greater mortality risk was also found among patients with high IMAT in most studies, but the relationships between VAT or SAT with mortality were mixed (Table 3 ).
TABLE 3.
Number and direction of associations between body composition abnormalities and clinical outcomes in acute COVID-19 (N = 62 studies).
| Main outcomes | Assessment | Direction of associations |
Summary of findings | ||
|---|---|---|---|---|---|
| Positive | Inverse | Unrelated/mixed | |||
| Mortality | Skeletal muscle (by CT) | — | 11 | 14 | Muscle mass was inversely associated with mortality in 13 of 29 (44.8%) instances |
| Skeletal muscle (by US) | — | 2 | 1 | ||
| Compartments related to muscle mass (by BIA) | — | — | 1 | ||
| Muscle radiodensity (by CT) | — | 6 | 8 | Muscle radiodensity was inversely associated with mortality in 6 of 14 (42.9 %) instances | |
| Muscle echo intensity (by US) | 2 | — | — | Muscle eco intensity was positively associated with mortality in 2 of 2 (100%) instances | |
| Phase angle (by BIA) | — | 3 | 1 | Phase angle was inversely associated with mortality in 3 of 4 (75.0%) instances | |
| FM (by BIA) | 2 | — | — | Adipose tissue and FM were positively associated with mortality in 10 of 26 (38.5%) instances | |
| Adipose tissue (that is, VAT, SAT, TAT, IMAT by CT) | 8 | 3 | 13 | ||
| ICU admission | Skeletal muscle (by CT) | — | 5 | 7 | Muscle mass was inversely associated with ICU admission in 6 of 17 (35.3%) instances |
| Skeletal muscle (by US) | — | 1 | — | ||
| Compartments related to muscle mass (by BIA) | 1 | — | 3 | ||
| Muscle radiodensity (by CT) | — | 3 | 3 | Muscle radiodensity was inversely associated with ICU admission in 3 of 6 (50.0%) instances | |
| Muscle echo intensity (by US) | 1 | — | — | Muscle echo intensity positively associated with mortality in 1 of 1 (100%) instance | |
| Phase angle (by BIA) | — | 2 | 2 | Phase angle was inversely associated with ICU admission in 2 of 4 (50.0%) instances | |
| FM (by BIA) | 1 | 1 | 2 | Adipose tissue and FM were positively associated with ICU admission in 11 of 20 (55.0%) instances | |
| Adipose tissue (that is, VAT, SAT, TAT, IMAT by CT) | 10 | 1 | 5 | ||
| MV/degree of severity | Skeletal muscle (by CT) | — | 7 | 18 | Muscle mass was inversely associated with MV/severity in 7 of 29 (24.1%) instances |
| Skeletal muscle (by US) | — | — | 2 | ||
| Compartments related to muscle mass (by BIA) | — | — | 2 | ||
| Muscle radiodensity (by CT) | — | 7 | 7 | Muscle radiodensity was inversely associated with MV/severity in 7 of 14 (50.0%) instances | |
| Muscle echo intensity (by US) | — | — | 1 | Muscle eco intensity was unrelated/mixed associated with MV/severity in 1 of 1 (100%) instance | |
| Phase angle (by BIA) | — | 1 | 3 | Phase angle was inversely associated with MV/severity in 1 of 4 (25.0%) instances | |
| FM (by BIA) | 2 | 1 | — | Adipose tissue and FM were positively associated with MV/severity in 14 of 30 (46.7%) instances | |
| Adipose tissue (that is, VAT, SAT, TAT, IMAT by CT) | 12 | — | 15 | ||
| Hospitalization/LOS | Skeletal muscle (by CT) | — | 8 | 11 | Muscle mass was inversely associated with hospitalization/LOS in 8 of 24 (33.3%) instances |
| Skeletal muscle (by US) | — | — | 1 | ||
| Compartments related to muscle mass (by BIA) | — | — | 4 | ||
| Muscle radiodensity (by CT) | — | 6 | 5 | Muscle radiodensity was inversely associated with hospitalization/LOS in 6 of 11 (54.5%) instances | |
| Muscle echo intensity (by US) | — | — | 1 | Muscle echo intensity was unrelated/mixed associated with hospitalization/LOS in 1 of 1 (100%) instance | |
| Phase angle (by BIA) | — | 2 | 3 | Phase angle was inversely associated with hospitalization/LOS in 2 of 5 (40.0%) instances | |
| FM (by BIA) | 1 | — | 3 | Adipose tissue and FM were positively associated with hospitalization/LOS in 9 of 18 (50.0%) instances | |
| Adipose tissue (that is, VAT, SAT, TAT, IMAT by CT) | 8 | — | 6 | ||
ICU, intensive care unit; IMAT, intra- and intermuscular adipose tissue; LOS, length of hospital stay; MV, mechanical ventilation; SAT, subcutaneous adipose tissue; TAT, total adipose tissue; US, ultrasound; VAT, visceral adipose tissue. The ratio of waist circumference to paravertebral muscle circumference was positively associated with ICU admission in one study [91], which was not included in the summary above due to this measurement significantly differing from other included body composition measures.
ICU admission
The relationship between BC abnormalities and ICU admission in patients with COVID-19 was evaluated in 24 studies [37,43,46,[48], [49], [50],52,[55], [56], [57],71,73,78,80,84,[87], [88], [89], [90], [91], [92], [93], [94], [95]] (Figure 3, Supplemental Table 4). Among 16 studies assessing muscle mass [37,43,46,49,50,[55], [56], [57],78,80,84,[87], [88], [89],93,95], 4 using CT [43,50,55,88] and 1 using US [46] found that low muscle mass was related to a greater likelihood of being admitted to the ICU. Using BIA, FFM [49,89], ALST [37], LST, and SMI [89] were unrelated to ICU admission in 3 of 4 studies [37,49,89,93]. Lower muscle radiodensity was associated with greater ICU admission in 3 [52,87,88] of 6 studies [52,55,56,80,87,88]. Only 1 study assessed muscle echo intensity and reported increases in this BC measure during the first 7 d of ICU stay [46]. PhA was lower in the ICU group, or inversely correlated with ICU admission, in 2 [48,93] of 4 studies [48,49,89,93].
VAT was positively related to ICU admission in 6 studies [55,56,71,90,92,94], whereas mixed results were found in 1 study [95]. SAT was positively related [71], inversely related [90], or unrelated to ICU admission [55,56,92]. Also using CT, positive associations were found between ICU admission and IMAT [95], TAT [92,94], and pectoralis muscle adjusted by waist circumference [91]. Three [37,49,89] of 4 studies [37,49,73,89] reported unrelated/mixed findings between BIA-assessed FM and ICU admission. (Supplemental Figure 1).
Altogether, patients with COVID-19 who had high VAT were more likely to be admitted to the ICU. However, inconsistent associations were found between ICU admission and muscle mass or radiodensity, PhA, SAT, TAT, and FM by BIA (Table 3).
Mechanical ventilation and disease severity
Thirty-seven studies investigated the relationship between BC abnormalities and MV or severe COVID-19 [4,6,20,[38], [39], [40], [41], [42], [43], [44],47,50,51,54,[57], [58], [59], [60], [61], [62], [63], [64],74,78,[81], [82], [83],85,[87], [88], [89],[92], [93], [94],[96], [97], [98]] (Figure 3, Supplemental Table 4). Twenty-one of these studies assessed muscle mass using CT [4,40,41,43,47,50,51,54,57,58,[61], [62], [63], [64],78,81,83,87,88,97,98] and 2 by US [42,44]. Low muscle mass by CT was inversely related to greater disease severity and/or need for intubation in 6 studies [47,50,61,63,64,88], and unrelated/mixed findings were found in 15 studies [4,40,41,43,51,54,57,58,62,78,81,83,87,97,98]. US muscle measurements showed conflicting or no relationships with these same outcomes [42,44]. Using BIA, FFM [39] and ALST [38] were unrelated to severe COVID-19 in 2 studies. Lower muscle radiodensity was related to a greater likelihood of severe illness, including the need for MV and/or greater disease severity in 5 studies [51,62,63,87,88], and unrelated/mixed findings were found in 6 studies [4,58,60,81,83,98]. In addition, 1 study showed that increased muscle radiodensity was protective against MV or death (as a composite score) [74]. Low PhA showed unrelated/mixed findings in relation to COVID-19 severity in 3 [59,82,89] of 4 studies [59,82,89,93]. In another study, patients with low muscle mass had poor clinical outcome (greater disease severity) during the first wave but not the second wave of COVID-19 [41].
The relationships between adiposity measures and MV or disease severity was evaluated in 14 studies [6,20,38,39,47,58,62,74,85,[92], [93], [94],96,98]. Seven studies found a positive association between these clinical outcomes and VAT [20,47,74,85,92,94], IMAT [62,74], SAT [85], and TAT [74,85,94]. Conflicting findings were reported between AT (VAT, TAT, and SAT) and COVID-19 severity [6,20,58,62,92,96,98]. FM by BIA was positively related to COVID-19 severity [38,39] but inversely with complications [93] (Supplemental Figure 1).
Inconsistent findings were found for all reported body compartments, with the exception of a small number of studies (2 studies) that found positive associations between IMAT and MV or disease severity (Table 3).
Additional outcomes
Hospitalization and LOS
The relationships between BC abnormalities and hospitalization outcomes in patients with COVID-19 was explored in 28 studies [4,37,39,40,[42], [43], [44], [45],[47], [48], [49], [50], [51],54,[58], [59], [60], [61],64,74,76,78,81,83,93,96,97,99] (Figure 3, Supplemental Table 4). Seven studies found that muscle mass assessed by CT [40,47,51,54,61,81,97] was inversely related to hospitalization or LOS (hospital or ICU), from a total of 19 studies [4,37,39,40,42,43,47,[49], [50], [51],54,58,61,64,81,83,93,97,99]. Using BIA, unrelated/mixed findings were shown for hospitalization in relation to FFM [39,49,93], LST [93], ALST [37], or SMI [93] in 4 studies. Low muscle radiodensity was evaluated in 9 studies [4,50,51,58,60,74,76,81,83] and was associated with a greater likelihood of hospitalization [74] and longer hospital or ICU LOS [50,51,60,81]; however, unrelated/mixed relationships were also found between muscle radiodensity and LOS [4,58,76,83]. Moreover, a study assessing echo intensity found no relationship with ICU stay [44]. PhA was inversely related to LOS in 2 [45,48] of 5 studies [45,48,49,59,93].
The relationship between hospitalization outcomes and adipose tissue was explored in 10 studies [37,39,47,49,58,74,78,93,96,99]. Higher hospitalization risk was associated with greater VAT in 4 studies [47,74,96,99] and TAT and IMAT in 2 studies [74,99]. However, unrelated/mixed findings (by CT) were found in 3 studies [58,78,96]. FM by BIA showed unrelated/mixed findings in relation to hospitalization outcomes in 3 [39,49,93] of 4 studies [37,39,49,93] (Supplemental Figure 1).
Except for a small number of studies showing positive associations between IMAT and hospitalization outcomes, inconsistent associations were found between these outcomes and all BC measures.
Comorbidities/conditions
As a surrogate of obesity, BMI was positively associated with AT measures (VAT, SAT, TAT, and IMAT) in 5 [56,70,74,90,94] of 6 studies [56,70,74,90,94,96] (Supplemental Table 4). Muscle mass was positively related to BMI in 2 studies [4,57], inversely related in 1 study [56], and unrelated in 4 studies [40,42,64,97]. Low muscle radiodensity was related to hypertension, diabetes, and 2 or more comorbidities in 1 study [60] but unrelated to the development of pulmonary fibrosis in another study [51] or the presence of comorbidities (that is, hypertension, diabetes, cardiovascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, chronic liver and kidney disease, and cancer) in a third study [62]. Most studies reported mixed/unrelated findings for the relationship between muscle mass and comorbidities [40,42,51,54,56,57,61,64]. Patients with greater VAT and/or SAT had higher rates of diabetes [70], active cancer [56], and renal failure and asthma [56]; 2 studies found mixed/unrelated associations with these or other comorbidities [62,70]. Overall, high BMI was associated with higher AT, but mixed findings were observed for the relationship between BC measures and other comorbidities.
Hospital discharge and physical function
Low muscle mass was a predictor of delayed hospital discharge in 1 of 1 study [54] (Supplemental Table 4). Lower PhA was associated with a lower swallowing recovery rate at hospital discharge in 1 of 1 study [100]. Furthermore, patients with low muscle radiodensity had lower Barthel index scores, indicating increased disability in 1 of 1 study [52]. Regarding measures of physical function, only 1 study was found; the study used handgrip strength and reported a 22.3% decrease in strength between baseline and day 10 [44].
Inflammatory biomarkers
The relationship between inflammation and BC measures was evaluated in 16 studies [41,47,48,[50], [51], [52],54,56,57,60,70,[72], [73], [74],79,92] (Supplemental Table 4). An inverse correlation was observed between muscle mass and white blood cell or lymphocyte count in 2 of 2 studies [54,57]. In contrast, 3 [51,56,57] of 5 studies [50,51,54,56,57] found nonsignificant correlations between muscle mass and CRP concentrations. Moreover, 3 [51,56,57] of 4 studies [50,51,56,57] found nonsignificant correlations between albumin and muscle mass. Muscle radiodensity was inversely correlated with CRP concentrations in 3 [50,52,74] of 6 studies [[50], [51], [52],56,60,74], and positively with creatine kinase [79], creatinine [60], and albumin [50,51]. CRP was positively related to VAT in 3 studies [70,74,92] and TAT in 2 studies [74,92] but unrelated to these or other AT/fat measurements (that is, IMAT, SAT, and percentage of FM) in 6 studies [47,56,[72], [73], [74],92]. Furthermore, 1 study compared COVID-19 patients with low muscle mass across the 2 pandemic waves [41]. Patients with low muscle mass in the first wave had higher concentrations of CRP and lower albumin concentrations than those in the second wave [41]. Overall mixed findings were observed between diverse inflammatory biomarkers and measurements of muscle mass, muscle radiodensity, and AT/FM.
Discussion
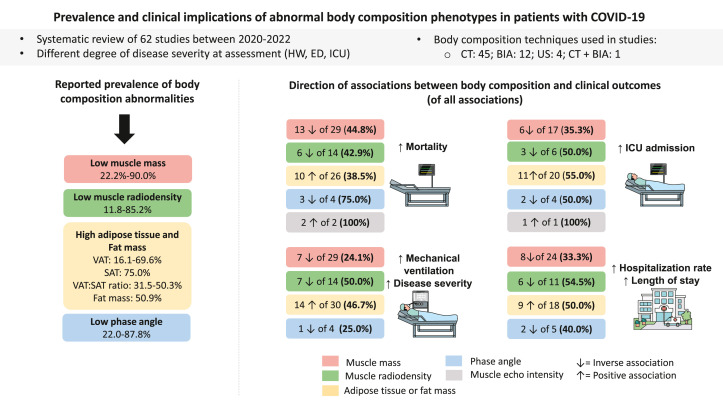
This systematic review integrated findings from 62 published studies examining the prevalence and relationship between BC abnormalities and clinical outcomes among hospitalized patients with COVID-19 between 2020 and 2022. Studies hereby included showed that the prevalence of low muscle mass in individuals with COVID-19 was high (≤90%). This exceeds the 69.1% prevalence of low muscle mass in patients with metastatic cancer, for example [101]. The prevalence of high AT, particularly stored as VAT or SAT, was also high (≤75%). Regarding clinical outcomes, a small number of studies showed that higher mortality risk was more consistently associated with lower PhA, higher muscle echo intensity (reflective of myoesteatosis), and higher IMAT. Patients admitted to the ICU often presented with higher VAT. Furthermore, higher disease severity was associated with higher IMAT in a few studies (Supplemental Figure 1). Hospitalization outcomes showed inconsistent findings across all body composition measurements in patients with COVID-19 (Figure 4 ).
FIGURE 4.
Summary of findings of the associations related to abnormal body composition and clinical outcomes in patients with COVID-19. Image represents different body compartments (muscle and adipose tissue). Prevalence of body composition abnormalities in each compartment is represented with a color box. The same colors are used to show the positive and inverse associations between body composition abnormalities and clinical outcomes across the 4 main outcomes (that is, mortality, length of hospital stay, mechanical ventilation/disease severity, and ICU admission). Either positive or inverse associations are shown for low muscle mass and its related compartments (for example, FFM, lean soft tissue; red boxes), low muscle radiodensity (green boxes), high adipose tissue (yellow boxes), low phase angle (blue boxes), and high muscle echo intensity. Please refer to Supplemental Figure 1 for specific findings by each adipose tissue compartment. Remaining percentages in each box represent unrelated/mixed findings or associations in the opposite direction. Some associations evaluated >1 body composition compartment, and therefore, data presentation is reflected by that (that is, total number of studies reporting the relationship is higher than the absolute number of studies included in the review). ED, emergency department; HW, hospital ward; ICU, intensive care unit; PhA, phase angle; SAT, subcutaneous adipose tissue; US, ultrasound; VAT, visceral adipose tissue.
BC abnormalities including low muscle mass or radiodensity or low PhA were also associated with higher circulating concentrations of inflammatory markers [41,47,48,[50], [51], [52],54,56,57,60,72,79], in line with previous literature [102,103]. Patients with COVID-19 and elevated inflammatory biomarkers on admission presented with greater disease severity [104]. Previous studies have shown that a long-lasting high AT accumulation [105] and low muscle mass [106] may be related to disease severity (Figure 1). Although our systematic review cannot confirm this association, we showed a positive association between VAT and ICU admission. This is consistent with prior research showing that individuals with high VAT are more susceptible to severe SARS-CoV-2 infection due to greater ACE2 enzyme expression in VAT [70].
Acute respiratory distress syndrome is a serious and relatively common complication of COVID-19, and CT scans play a key role in its diagnosis and follow-up [107]. Given the availability of these scans in the medical records of patients with COVID-19, CT imaging has been opportunistically used for BC assessment. Although the third lumbar vertebra (L3) is the reference site for this purpose [108], this landmark is not readily available in COVID-19 patients [109]. In this review, only 13 of 45 studies evaluated BC abnormalities using a single image at the L3 level. In the absence of abdominal CT scans, other measurement sites (that is, fourth thoracic vertebra [T4], twelfth thoracic vertebra, and second lumbar vertebra) have been explored in other clinical conditions [110,111]. Similarly, our review shows that different landmarks have also been used for body composition assessment in COVID-19, ranging from the T4 to the fourth lumbar vertebra. Notably, heterogeneous associations between measurement sites and clinical outcomes were observed, with some studies reporting significant associations at one vertebra level but not at another for the same body compartment (Figure 3). For example, skeletal muscle showed associations with mortality when assessed at L3 [56] but not at T4 [81]. Interestingly, most studies using images at T4 only included the pectoralis muscle, which has been previously shown to have a weak correlation with total muscle CSA [112]. As such, the use of single muscle approaches is not recommended [113].
Despite the inconsistencies across measurement sites, BC assessed by CT images was more frequently associated with outcomes compared with BIA (that is, more unrelated/mixed findings). This is not surprising, as BIA provides indirect/estimated measures of BC using predictive equations that are population-, equation-, and device-specific [114,115]. Nonetheless, PhA is derived from the raw BIA value and is considered a marker of muscle mass and composition. PhA was more consistently associated with mortality and ICU admission. As a marker of cell membrane health and integrity, low PhA has been associated with increased inflammation and oxidative stress [116], which may impact COVID-19 pathogenesis and severity [117]. Thus, PhA may be the most reliable BIA value to be used as a marker of abnormal BC and predictor of adverse outcomes in clinical practice. This measurement can also be adjusted for age and sex, known as standardized PhA [48,59]. The clinical value of BIA for tracking longitudinal changes in muscle mass (or its related compartments such as FFM, ALST, LST, and SMI) in hospitalized patients with COVID-19 remains to be established. No longitudinal studies were included in our review. Interestingly, US-assessed muscle mass and echo intensity were also associated with clinical outcomes in longitudinal studies [42,46,75]. US is a convenient and emerging bedside technique, especially suitable for BC assessment of critically ill patients, such as those with severe COVID-19 [118].
Additional findings revealed that VAT adjusted for TAT was a stronger predictor of COVID-19 severity than BMI [6]. This supports the notion that AT distribution is a more accurate predictor of health outcomes than BMI [5,119].
Although we included cohort studies with large sample sizes [[37], [38], [39],88], large longitudinal studies are needed to address the effects of confounding factors (for example, age, sex, ethnicity, physical activity, comorbidities, COVID-19 waves) on the relationship between abnormal BC and COVID-19 outcomes. Notably, we did not stratify our results based on the presence or absence of multivariate analyses, and we were also unable to evaluate whether studies were appropriately powered. Although the “comparability” criteria domain within our scoring system showed that most studies (87.1%) controlled for covariables, the number or type of variables was not explored. For instance, age could be an important confounder because older adults are more likely to develop severe COVID-19 and hence worse clinical outcomes due to the high prevalence of multimorbidity [120] and sarcopenia [106,121,122]. Studies that found significant associations with a specific measure or within a group (for example, SMI, males) but not with other measures or groups (for example, muscle CSA, females) were classified as mixed. For practical reasons, this category was counted as an unrelated finding category; however, this could bias the analysis.
Methodological variability across studies precluded a formal meta-analysis. A previous publication examined the pooled prevalence of CT-assessed low muscle mass and the association between BC abnormalities with in-hospital mortality (n = 6 studies) [36]. Although we were unable to conduct a meta-analysis, our systematic approach allowed the inclusion of a greater number of studies (n = 62) and the ability to explore relationships between BC assessed by different techniques and several clinical outcomes. This is particularly relevant as CT scans are not widely available for BC assessment, and BIA and US have been increasingly used in the context of COVID-19 and in clinical practice in general [123,124]. Another limitation is that the prevalence of low muscle mass at baseline (that is, at the time of COVID-19 diagnosis) could be related to comorbidities, poor nutrition/low physical activity, and/or aging [[125], [126], [127]]. These variables may per se impact disease severity or other outcomes. Additionally, we were unable to explore potential relationships between longitudinal changes in BC and clinical outcomes, as most studies reported only baseline measurements (for example, at hospital admission). Finally, we did not explore the prevalence and potential implications of abnormal BC in COVID-19 long-term health outcomes, such as the post-acute COVID-19 syndrome. Despite these inherent limitations, our review is the first to integrate a large number of studies over different COVID-19 waves as well as longitudinal changes in body composition during hospitalization using different techniques.
In conclusion, our findings showed that abnormalities in BC are prevalent in patients with acute COVID-19. Despite the limited number of selected studies, evidence suggests that high muscle echo intensity (reflective of myoesteatosis) and low PhA were associated with mortality. High IMAT and VAT were consistently associated with higher mortality and ICU admission, respectively. Inconsistent associations were found between other AT compartments and clinical outcomes. In contrast, low muscle mass and low radiodensity showed unrelated/mixed findings across all clinical outcomes. Heterogeneity of included studies and conflicting findings preclude definitive conclusions regarding the clinical significance of abnormal BC of these patients. BC assessment should be further explored as a potential prognostic tool with COVID-19, regardless of the patient’s BMI, disease severity, and age.
Acknowledgments
The authors’ responsibilities were as follows—MMI: searched the literature; MMI, CEO, ATLM: screened for relevant articles, evaluated the quality of the data and contribute to compose the tables; and all authors: conceived of the study, contributed to the interpretation of the findings, contributed to writing the final article, and read and approved the final manuscript.
CEO has received honoraria from Abbott Nutrition. CMP has previously received honoraria and/or paid consultancy from Abbott Nutrition, Nutricia, Nestlé Health Science, Fresenius Kabi, AMRA Medical, and Pfizer.
MCG has received honoraria and/or paid consultancy from Abbott Nutrition, Nutricia, and Nestlé Brazil. FL has previously received grant funding and paid consultancy from Abbott Nutrition. EM has previously received honoraria and/or paid consultancy from Abbott Nutrition, Nutricia, Nestlé Health Science, and Pfizer. SBH is on the Tanita Medical Advisory Board. MMI, ATLM, and RB report no conflicts of interest.
Funding
This work was supported by the Campus Alberta Innovation Program (to CMP) and the National Council of Science and Technology of Mexico (Consejo Nacional de Ciencia y Tecnología, CONACYT; to MMI).
Data availability
The data described in the manuscript, code book, and analytic code will be made available upon request pending approval from the corresponding author.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.04.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lian J., Jin X., Hao S., Cai H., Zhang S., Zheng L., et al. Analysis of epidemiological and clinical features in older patients with coronavirus disease 2019 (COVID-19) outside Wuhan. Clin. Infect. Dis. 2020;71(15):740–747. doi: 10.1093/cid/ciaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gil S., Filho W.J., Shinjo S.K., Ferriolli E., Busse A.L., Avelino-Silva T.J., et al. Muscle strength and muscle mass as predictors of hospital length of stay in patients with moderate to severe COVID-19: a prospective observational study. J. Cachexia Sarcopenia Muscle. 2021;12(6):1871–1878. doi: 10.1002/jcsm.12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pranata R., Lim M.A., Huang I., Yonas E., Henrina J., Vania R., et al. Visceral adiposity, subcutaneous adiposity, and severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. Clin. Nutr. ESPEN. 2021;43:163–168. doi: 10.1016/j.clnesp.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damanti S., Cristel G., Ramirez G.A., Bozzolo E.P., Da Prat V., Gobbi A., et al. Influence of reduced muscle mass and quality on ventilator weaning and complications during intensive care unit stay in COVID-19 patients. Clin. Nutr. 2022;41(12):2965–2972. doi: 10.1016/j.clnu.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothman K.J. BMI-related errors in the measurement of obesity. Int. J. Obes. (Lond). 2008;32(Suppl 3):S56–S59. doi: 10.1038/ijo.2008.87. [DOI] [PubMed] [Google Scholar]
- 6.Ogata H., Mori M., Jingushi Y., Matsuzaki H., Katahira K., Ishimatsu A., et al. Impact of visceral fat on the prognosis of coronavirus disease 2019: an observational cohort study. BMC Infect. Dis. 2021;21(1):1240. doi: 10.1186/s12879-021-06958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cava E., Carbone S. Coronavirus disease 2019 pandemic and alterations of body composition. Curr. Opin. Clin. Nutr. Metab. Care. 2021;24(3):229–235. doi: 10.1097/MCO.0000000000000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aschman T., Schneider J., Greuel S., Meinhardt J., Streit S., Goebel H.H., et al. Association between SARS-CoV-2 infection and immune-mediated myopathy in patients who have died. JAMA Neurol. 2021;78(8):948–960. doi: 10.1001/jamaneurol.2021.2004. [DOI] [PubMed] [Google Scholar]
- 9.Neufeldt C.J., Cerikan B., Cortese M., Frankish J., Lee J.Y., Plociennikowska A., et al. SARS-CoV-2 infection induces a pro-inflammatory cytokine response through cGAS-STING and NF-κB. Commun. Biol. 2022;5(1):45. doi: 10.1038/s42003-021-02983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pleguezuelos E., Del Carmen A., Llorensi G., Carcole J., Casarramona P., Moreno E., et al. Severe loss of mechanical efficiency in COVID-19 patients. J. Cachexia Sarcopenia Muscle. 2021;12(4):1056–1063. doi: 10.1002/jcsm.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wall B.T., Dirks M.L., van Loon L.J. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res. Rev. 2013;12(4):898–906. doi: 10.1016/j.arr.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Parry S.M., Puthucheary Z.A. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem. Physiol. Med. 2015;4(1):16. doi: 10.1186/s13728-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piotrowicz K., Gąsowski J., Michel J.P., Veronese N. Post-COVID-19 acute sarcopenia: physiopathology and management. Aging Clin. Exp. Res. 2021;33(10):2887–2898. doi: 10.1007/s40520-021-01942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirwan R., McCullough D., Butler T., Perez de Heredia F., Davies I.G., Stewart C. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. GeroScience. 2020;42(6):1547–1578. doi: 10.1007/s11357-020-00272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brugliera L., Spina A., Castellazzi P., Cimino P., Tettamanti A., Houdayer E., et al. Rehabilitation of COVID-19 patients. J. Rehabil. Med. 2020;52(4) doi: 10.2340/16501977-2678. [DOI] [PubMed] [Google Scholar]
- 16.Sawadogo W., Tsegaye M., Gizaw A., Adera T. Overweight and obesity as risk factors for COVID-19-associated hospitalisations and death: systematic review and meta-analysis. BMJ Nutr. Prev. Health. 2022;5(1):10–18. doi: 10.1136/bmjnph-2021-000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y., Lu Y., Huang Y.M., Wang M., Ling W., Sui Y., et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113 doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aslani M., Mortazavi-Jahromi S.S., Mirshafiey A. Cytokine storm in the pathophysiology of COVID-19: possible functional disturbances of miRNAs. Int. Immunopharmacol. 2021;101(A) doi: 10.1016/j.intimp.2021.108172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chait A., den Hartigh L.J. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front. Cardiovasc. Med. 2020;7:22. doi: 10.3389/fcvm.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favre G., Legueult K., Pradier C., Raffaelli C., Ichai C., Iannelli A., et al. Visceral fat is associated to the severity of COVID-19. Metabolism. 2021;115 doi: 10.1016/j.metabol.2020.154440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell M., McKenzie J.E., Sowden A., Katikireddi S.V., Brennan S.E., Ellis S., et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Souza N.C., Avesani C.M., Prado C.M., Martucci R.B., Rodrigues V.D., de Pinho N.B., et al. Phase angle as a marker for muscle abnormalities and function in patients with colorectal cancer. Clin. Nutr. 2021;40(7):4799–4806. doi: 10.1016/j.clnu.2021.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Jelicic Kadic A., Vucic K., Dosenovic S., Sapunar D., Puljak L. Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. J. Clin. Epidemiol. 2016;74:119–123. doi: 10.1016/j.jclinepi.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Aubrey J., Esfandiari N., Baracos V.E., Buteau F.A., Frenette J., Putman C.T., et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. (Oxf). 2014;210(3):489–497. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris-Love M.O., Avila N.A., Adams B., Zhou J., Seamon B., Ismail C., et al. The comparative associations of ultrasound and computed tomography estimates of muscle quality with physical performance and metabolic parameters in older men. J. Clin. Med. 2018;7(10):340. doi: 10.3390/jcm7100340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe Y., Ikenaga M., Yoshimura E., Yamada Y., Kimura M. Association between echo intensity and attenuation of skeletal muscle in young and older adults: a comparison between ultrasonography and computed tomography. Clin. Interv. Aging. 2018;13:1871–1878. doi: 10.2147/CIA.S173372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu H.K., Chen Y.Y., Yeh C., Chuang C.L., Chiang L.M., Lai C.L., et al. Discrepancies between leg-to-leg bioelectrical impedance analysis and computerized tomography in abdominal visceral fat measurement. Sci. Rep. 2017;7(1):9102. doi: 10.1038/s41598-017-08991-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Z., Liu Y., Yan C., Yang R., Xu L., Guo Z., et al. Measurement of visceral fat and abdominal obesity by single-frequency bioelectrical impedance and CT: a cross-sectional study. BMJ Open. 2021;11(10) doi: 10.1136/bmjopen-2020-048221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagano A., Wakabayashi H., Maeda K., Kokura Y., Miyazaki S., Mori T., et al. Respiratory sarcopenia and sarcopenic respiratory disability: concepts, diagnosis, and treatment. J. Nutr. Health Aging. 2021;25(4):507–515. doi: 10.1007/s12603-021-1587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boon M.H., Thomson H. The effect direction plot revisited: application of the 2019 Cochrane Handbook guidance on alternative synthesis methods. Res. Synth. Methods. 2021;12(1):29–33. doi: 10.1002/jrsm.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Internet]. The Ottawa Hospital Research Institute [cited March 2022]. Available from:
- 33.Moskalewicz A., Oremus M. No clear choice between Newcastle-Ottawa Scale and Appraisal Tool for cross-sectional studies to assess methodological quality in cross-sectional studies of health-related quality of life and breast cancer. J. Clin. Epidemiol. 2020;120:94–103. doi: 10.1016/j.jclinepi.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Luchini C., Stubbs B., Solmi M., Veronese N. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J. Meta Anal. 2017;5(4):80–84. doi: 10.13105/wjma.v5.i4.80. [DOI] [Google Scholar]
- 35.Gasbarrino K., Gorgui J., Nauche B., Côté R., Daskalopoulou S.S. Circulating adiponectin and carotid intima-media thickness: a systematic review and meta-analysis. Metabolism. 2016;65(7):968–986. doi: 10.1016/j.metabol.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Meyer H.J., Wienke A., Surov A. Computed tomography-defined body composition as prognostic markers for unfavourable outcomes and in-hospital mortality in coronavirus disease 2019. J. Cachexia Sarcopenia Muscle. 2022;13(1):159–168. doi: 10.1002/jcsm.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao M., Wang Q., Piernas C., Astbury N.M., Jebb S.A., Holmes M.V., et al. Associations between body composition, fat distribution and metabolic consequences of excess adiposity with severe COVID-19 outcomes: observational study and Mendelian randomisation analysis. Int. J. Obes. (Lond). 2022;46(5):943–950. doi: 10.1038/s41366-021-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson T.J., Yates T., Baker L.A., Zaccardi F., Smith A.C. Sarcopenic obesity and the risk of hospitalization or death from coronavirus disease 2019: findings from UK Biobank. JCSM Rapid Commun. 2022;5(1):3–9. doi: 10.1002/rco2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshiji S., Tanaka D., Minamino H., Lu T., Butler-Laporte G., Murakami T., et al. Causal associations between body fat accumulation and COVID-19 severity: a Mendelian randomization study. Front. Endocrinol. (Lausanne). 2022;13 doi: 10.3389/fendo.2022.899625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osuna-Padilla I.A., Rodríguez-Moguel N.C., Rodríguez-Llamazares S., Orsso C.E., Prado C.M., Ríos-Ayala M.A., et al. Low muscle mass in COVID-19 critically ill patients: prognostic significance and surrogate markers for assessment. Clin. Nutr. 2022;41(12):2910–2917. doi: 10.1016/j.clnu.2022.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menozzi R., Valoriani F., Prampolini F., Banchelli F., Boldrini E., Martelli F., et al. Impact of sarcopenia in SARS-CoV-2 patients during two different epidemic waves. Clin. Nutr. ESPEN. 2022;47:252–259. doi: 10.1016/j.clnesp.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kremer W.M., Labenz C., Kuchen R., Sagoschen I., Bodenstein M., Schreiner O., et al. Sonographic assessment of low muscle quantity identifies mortality risk during COVID-19: a prospective single-centre study. J. Cachexia Sarcopenia Muscle. 2022;13(1):169–179. doi: 10.1002/jcsm.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Attaway A., Welch N., Dasarathy D., Amaya-Hughley J., Bellar A., Biehl M., et al. Acute skeletal muscle loss in SARS-CoV-2 infection contributes to poor clinical outcomes in COVID-19 patients. J. Cachexia Sarcopenia Muscle. 2022;13(5):2436–2446. doi: 10.1002/jcsm.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Andrade-Junior M.C., de Salles I.C.D., de Brito C.M.M., Pastore-Junior L., Righetti R.F., Yamaguti W.P. Skeletal muscle wasting and function impairment in intensive care patients with severe COVID-19. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.640973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kellnar A., Hoppe J.M., Brunner S., Stremmel C. Hospitalization for COVID-19 is associated with significant changes in body composition. Clin. Nutr. ESPEN. 2021;45:499–502. doi: 10.1016/j.clnesp.2021.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Umbrello M., Guglielmetti L., Formenti P., Antonucci E., Cereghini S., Filardo C., et al. Qualitative and quantitative muscle ultrasound changes in patients with COVID-19-related ARDS. Nutrition. 2021;91–92 doi: 10.1016/j.nut.2021.111449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beltrão F.E.L., Beltrão D.C.A., Carvalhal G., Beltrão F.N.L., de Aquino I.M., Brito T.D.S., et al. Low muscle mass and high visceral fat mass predict mortality in patients hospitalized with moderate-to-severe COVID-19: a prospective study. Endocr. Connect. 2022;11(10) doi: 10.1530/EC-22-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cornejo-Pareja I., Vegas-Aguilar I.M., García-Almeida J.M., Bellido-Guerrero D., Talluri A., Lukaski H., et al. Phase angle and standardized phase angle from bioelectrical impedance measurements as a prognostic factor for mortality at 90 days in patients with COVID-19: a longitudinal cohort study. Clin. Nutr. 2022;41(12):3106–3114. doi: 10.1016/j.clnu.2021.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Del Giorno R., Quarenghi M., Stefanelli K., Capelli S., Giagulli A., Quarleri L., et al. Nutritional risk screening and body composition in COVID-19 patients hospitalized in an internal medicine ward. Int. J. Gen. Med. 2020;13:1643–1651. doi: 10.2147/IJGM.S286484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erdöl M.A., Kayaaslan B., Erdoğan M., Hasanoğlu İ., Yayla Ç., Civelek Eser F., et al. Sarcopenia and its prognostic role on hospitalization and in-hospital mortality in coronavirus disease 2019 patients with at least one cardiovascular risk factor. Turk. Kardiyol. Dern. Ars. 2022;50(2):103–111. doi: 10.5543/tkda.2022.21167. [DOI] [PubMed] [Google Scholar]
- 51.Feng Z., Zhao H., Kang W., Liu Q., Wu J., Bragazzi N.L., et al. Association of paraspinal muscle measurements on chest computed tomography with clinical outcomes in patients with severe coronavirus disease 2019. J. Gerontol. A Biol. Sci. Med. Sci. 2021;76(3):e78–e84. doi: 10.1093/gerona/glaa317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giraudo C., Librizzi G., Fichera G., Motta R., Balestro E., Calabrese F., et al. Reduced muscle mass as predictor of intensive care unit hospitalization in COVID-19 patients. PLOS ONE. 2021;16(6) doi: 10.1371/journal.pone.0253433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang M.K., Lee Y.R., Song J.E., Kweon Y.O., Tak W.Y., Jang S.Y., et al. Prognostic impact of myosteatosis on mortality in hospitalized patients with COVID-19. Diagnostics (Basel) 2022;12(9):2255. doi: 10.3390/diagnostics12092255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J.W., Yoon J.S., Kim E.J., Hong H.L., Kwon H.H., Jung C.Y., et al. Prognostic implication of baseline sarcopenia for length of hospital stay and survival in patients with coronavirus disease 2019. J. Gerontol. A Biol. Sci. Med. Sci. 2021;76(8):e110–e116. doi: 10.1093/gerona/glab085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koehler J., Boirie Y., Bensid L., Pereira B., Ghelis N., Dupuis C., et al. Thoracic sarcopenia as a predictive factor of SARS-COV2 evolution. Clin. Nutr. 2022;41(12):2918–2923. doi: 10.1016/j.clnu.2022.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGovern J., Dolan R., Richards C., Laird B.J., McMillan D.C., Maguire D. Relation between body composition, systemic inflammatory response, and clinical outcomes in patients admitted to an urban teaching hospital with COVID-19. J. Nutr. 2021;151(8):2236–2244. doi: 10.1093/jn/nxab142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moctezuma-Velázquez P., Miranda-Zazueta G., Ortiz-Brizuela E., González-Lara M.F., Tamez-Torres K.M., Román-Montes C.M., et al. Low thoracic skeletal muscle area is not associated with negative outcomes in patients with COVID-19. Am. J. Phys. Med. Rehabil. 2021;100(5):413–418. doi: 10.1097/PHM.0000000000001716. [DOI] [PubMed] [Google Scholar]
- 58.Molwitz I., Ozga A.K., Gerdes L., Ungerer A., Köhler D., Ristow I., et al. Prediction of abdominal CT body composition parameters by thoracic measurements as a new approach to detect sarcopenia in a COVID-19 cohort. Sci. Rep. 2022;12(1):6443. doi: 10.1038/s41598-022-10266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osuna-Padilla I.A., Rodríguez-Moguel N.C., Rodríguez-Llamazares S., Aguilar-Vargas A., Casas-Aparicio G.A., Ríos-Ayala M.A., et al. Low phase angle is associated with 60-day mortality in critically ill patients with COVID-19. JPEN J. Parenter. Enteral Nutr. 2022;46(4):828–835. doi: 10.1002/jpen.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Padilha D.M.H., Mendes M.C.S., Lascala F., Silveira M.N., Pozzuto L., Santos L.A.O., et al. Low skeletal muscle radiodensity and neutrophil-to-lymphocyte ratio as predictors of poor outcome in patients with COVID-19. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-20126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ufuk F., Demirci M., Sagtas E., Akbudak I.H., Ugurlu E., Sari T. The prognostic value of pneumonia severity score and pectoralis muscle area on chest CT in adult COVID-19 patients. Eur. J. Radiol. 2020;131 doi: 10.1016/j.ejrad.2020.109271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y., Ding L., Zou X., Shen Y., Hu D., Hu X., et al. Visceral adiposity and high intramuscular fat deposition independently predict critical illness in patients with SARS-CoV-2. Obesity (Silver Spring) 2020;28(11):2040–2048. doi: 10.1002/oby.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yi X., Liu H., Zhu L., Wang D., Xie F., Shi L., et al. Myosteatosis predicting risk of transition to severe COVID-19 infection. Clin. Nutr. 2022;41(12):3007–3015. doi: 10.1016/j.clnu.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ying-Hao P., Hai-Dong Z., Yuan F., Yong-kang L., Sen L., Wei-Long X., et al. Correlation of CT-derived pectoralis muscle status and COVID-19 induced lung injury in elderly patients. BMC Med. Imaging. 2022;22(1):144. doi: 10.1186/s12880-022-00872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caan B.J., Meyerhardt J.A., Kroenke C.H., Alexeeff S., Xiao J., Weltzien E., et al. Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS Study) Cancer Epidemiol. Biomarkers Prev. 2017;26(7):1008–1015. doi: 10.1158/1055-9965.EPI-17-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Derstine B.A., Holcombe S.A., Ross B.E., Wang N.C., Su G.L., Wang S.C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-29825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin L., Birdsell L., Macdonald N., Reiman T., Clandinin M.T., McCargar L.J., et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013;31(12):1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 68.Prado C.M., Lieffers J.R., McCargar L.J., Reiman T., Sawyer M.B., Martin L., et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 69.van der Werf A., Langius J.A.E., de van der Schueren M.A.E., Nurmohamed S.A., van der Pant K.A.M.I., Blauwhoff-Buskermolen S., et al. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur. J. Clin. Nutr. 2018;72(2):288–296. doi: 10.1038/s41430-017-0034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goehler A., Hsu T.H., Seiglie J.A., Siedner M.J., Lo J., Triant V., et al. Visceral adiposity and severe covid-19 disease: application of an artificial intelligence algorithm to improve clinical risk prediction. Open Forum Infect. Dis. 2021;8(7) doi: 10.1093/ofid/ofab275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pediconi F., Rizzo V., Schiaffino S., Cozzi A., Della Pepa G., Galati F., et al. Visceral adipose tissue area predicts intensive care unit admission in COVID-19 patients. Obes. Res. Clin. Pract. 2021;15(1):89–92. doi: 10.1016/j.orcp.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Lorenzo A., Tarsitano M.G., Falcone C., Di Renzo L., Romano L., Macheda S., et al. Fat mass affects nutritional status of ICU COVID-19 patients. J. Transl. Med. 2020;18(1):299. doi: 10.1186/s12967-020-02464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stevanovic D., Zdravkovic V., Poskurica M., Petrovic M., Cekerevac I., Zdravkovic N., et al. The role of bioelectrical impedance analysis in predicting COVID-19 outcome. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.906659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Besutti G., Pellegrini M., Ottone M., Cantini M., Milic J., Bonelli E., et al. The impact of chest CT body composition parameters on clinical outcomes in COVID-19 patients. PLOS ONE. 2021;16(5) doi: 10.1371/journal.pone.0251768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Formenti P., Umbrello M., Castagna V., Cenci S., Bichi F., Pozzi T., et al. Respiratory and peripheral muscular ultrasound characteristics in ICU COVID 19 ARDS patients. J. Crit. Care. 2022;67:14–20. doi: 10.1016/j.jcrc.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hocaoglu E., Ors S., Yildiz O., Inci E. Correlation of pectoralis muscle volume and density with severity of covid-19 pneumonia in adults. Acad. Radiol. 2021;28(2):166–172. doi: 10.1016/j.acra.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beypinar I., Bayav M., Ucan A., Efe S. The effect of body composition on prognosis in critically ill COVID-19 patients. Ann. Clin. Anal. Med. 2021;12(6):680–684. doi: 10.4328/ACAM.20383. [DOI] [Google Scholar]
- 78.Poros B., Becker-Pennrich A.S., Sabel B., Stemmler H.J., Wassilowsky D., Weig T., et al. Anthropometric analysis of body habitus and outcomes in critically ill COVID-19 patients. Obes. Med. 2021;25 doi: 10.1016/j.obmed.2021.100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rossi A.P., Gottin L., Donadello K., Schweiger V., Brandimarte P., Zamboni G.A., et al. Intermuscular adipose tissue as a risk factor for mortality and muscle injury in critically ill patients affected by COVID-19. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.651167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schiaffino S., Albano D., Cozzi A., Messina C., Arioli R., Bnà C., et al. CT-derived chest muscle metrics for outcome prediction in patients with COVID-19. Radiology. 2021;300(2):E328–E336. doi: 10.1148/radiol.2021204141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Antonarelli M., Fogante M. Chest CT-derived muscle analysis in COVID-19 patients. Tomography. 2022;8(1):414–422. doi: 10.3390/tomography8010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Da Porto A., Tascini C., Peghin M., Sozio E., Colussi G., Casarsa V., et al. Prognostic role of malnutrition diagnosed by bioelectrical impedance vector analysis in older adults hospitalized with COVID-19 pneumonia: a prospective study. Nutrients. 2021;13(11):4085. doi: 10.3390/nu13114085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kardas H., Thormann M., Bär C., Omari J., Wienke A., Pech M., et al. Impact of pectoral muscle values on clinical outcomes in patients with severe COVID-19 disease. Vivo. 2022;36(1):375–380. doi: 10.21873/invivo.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Polat M., Salbaş Ç.S., Sari S., Doğan M., Çam S., Karadağ A. The association between prognosis and sarcopenia assessed by psoas muscle measurement in elderly male patients with COVID-19, Turk. Geriatr. 2021;24(4):557–566. doi: 10.31086/tjgeri.2021.254. [DOI] [Google Scholar]
- 85.Scheffler M., Genton L., Graf C.E., Remuinan J., Gold G., Zekry D., et al. Prognostic role of subcutaneous and visceral adiposity in hospitalized octogenarians with COVID-19. J. Clin. Med. 2021;10(23):5500. doi: 10.3390/jcm10235500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nobel Y.R., Su S.H., Anderson M.R., Luk L., Small-Saunders J.L., Reyes-Soffer G., et al. Relationship between body composition and death in patients with COVID-19 differs based on the presence of gastrointestinal symptoms. Dig. Dis. Sci. 2022;67(9):4484–4491. doi: 10.1007/s10620-021-07324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sahin C., Yeniceri I.O., Oral Tapan O., Cakir T., Dirgen Caylak S., Togan T. Evaluation of skeletal muscle mass as a predictor of prognosis in patients treated in hospital for COVID-19 infection. Bratisl. Lek. Listy. 2022;123(3):197–204. doi: 10.4149/bll_2022_033. [DOI] [PubMed] [Google Scholar]
- 88.Surov A., Kardas H., Besutti G., Pellegrini M., Ottone M., Onur M.R., et al. Prognostic role of the pectoralis musculature in patients with COVID-19. A multicenter study. Acad. Radiol. 2023;30(1):77–82. doi: 10.1016/j.acra.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moonen H.P.F.X., van Zanten F.J.L., Driessen L., de Smet V., Slingerland-Boot R., Mensink M., et al. Association of bioelectric impedance analysis body composition and disease severity in COVID-19 hospital ward and ICU patients: the BIAC-19 study. Clin. Nutr. 2021;40(4):2328–2336. doi: 10.1016/j.clnu.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Battisti S., Pedone C., Napoli N., Russo E., Agnoletti V., Nigra S.G., et al. Computed tomography highlights increased visceral adiposity associated with critical illness in COVID-19. Diabetes Care. 2020;43(10):e129–e130. doi: 10.2337/dc20-1333. [DOI] [PubMed] [Google Scholar]
- 91.Kottlors J., Zopfs D., Fervers P., Bremm J., Abdullayev N., Maintz D., et al. Body composition on low dose chest CT is a significant predictor of poor clinical outcome in COVID-19 disease - a multicenter feasibility study. Eur. J. Radiol. 2020;132 doi: 10.1016/j.ejrad.2020.109274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Watanabe M., Caruso D., Tuccinardi D., Risi R., Zerunian M., Polici M., et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111 doi: 10.1016/j.metabol.2020.154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moonen H.P., Bos A.E., Hermans A.J., Stikkelman E., van Zanten F.J., van Zanten A.R. Bioelectric impedance body composition and phase angle in relation to 90-day adverse outcome in hospitalized COVID-19 ward and ICU patients: the prospective BIAC-19 study. Clin. Nutr. ESPEN. 2021;46:185–192. doi: 10.1016/j.clnesp.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petersen A., Bressem K., Albrecht J., Thieß H.M., Vahldiek J., Hamm B., et al. The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110 doi: 10.1016/j.metabol.2020.154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bunnell K.M., Thaweethai T., Buckless C., Shinnick D.J., Torriani M., Foulkes A.S., et al. Body composition predictors of outcome in patients with COVID-19. Int. J. Obes. (Lond). 2021;45(10):2238–2243. doi: 10.1038/s41366-021-00907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chandarana H., Dane B., Mikheev A., Taffel M.T., Feng Y., Rusinek H. Visceral adipose tissue in patients with COVID-19: risk stratification for severity. Abdom. Radiol. (NY) 2021;46(2):818–825. doi: 10.1007/s00261-020-02693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aykın Yığman Z., Karaca Umay E., Aktürk G., Ergün M.Ç. Is the thoracic and back muscle mass associated with disease severity in patients with COVID-19? Turk. Thorac. J. 2022;23(2):123–129. doi: 10.5152/TurkThoracJ.2022.21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.do Amaral E Castro A., Yokoo P., Fonseca E.K.U.N., Otoni J.C., Haiek S.L., Shoji H., et al. Prognostic factors of worse outcome for hospitalized COVID-19 patients, with emphasis on chest computed tomography data: a retrospective study. Einstein (São Paulo) 2022;20 doi: 10.31744/einstein_journal/2022AO6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chandarana H., Pisuchpen N., Krieger R., Dane B., Mikheev A., Feng Y., et al. Association of body composition parameters measured on CT with risk of hospitalization in patients with COVID-19. Eur. J. Radiol. 2021;145 doi: 10.1016/j.ejrad.2021.110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reyes-Torres C.A., Flores-López A., Osuna-Padilla I.A., Hernández-Cárdenas C.M., Serralde-Zúñiga A.E. Phase angle and overhydration are associated with post-extubating dysphagia in patients with COVID-19 discharged from the ICU. Nutr. Clin. Pract. 2022;37(1):110–116. doi: 10.1002/ncp.10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Raynard B., Pigneur F., Di Palma M., Deluche E., Goldwasser F. The prevalence of CT-defined low skeletal muscle mass in patients with metastatic cancer: a cross-sectional multicenter French study (the SCAN study) Support. Care Cancer. 2022;30(4):3119–3129. doi: 10.1007/s00520-021-06603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Makki K., Froguel P., Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013 doi: 10.1155/2013/139239. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dusseaux M.M., Antoun S., Grigioni S., Béduneau G., Carpentier D., Girault C., et al. Skeletal muscle mass and adipose tissue alteration in critically ill patients. PLOS ONE. 2019;14(6) doi: 10.1371/journal.pone.0216991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hachim I.Y., Hachim M.Y., Hannawi H., Naeem K.B., Salah A., Hannawi S. The inflammatory biomarkers profile of hospitalized patients with COVID-19 and its association with patient’s outcome: a single centered study. PLOS ONE. 2021;16(12) doi: 10.1371/journal.pone.0260537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sudhakar M., Winfred S.B., Meiyazhagan G., Venkatachalam D.P. Mechanisms contributing to adverse outcomes of COVID-19 in obesity. Mol. Cell. Biochem. 2022;477(4):1155–1193. doi: 10.1007/s11010-022-04356-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Montes-Ibarra M., Oliveira C.L.P., Orsso C.E., Landi F., Marzetti E., Prado C.M. The Impact of long COVID-19 on muscle health. Clin. Geriatr. Med. 2022;38(3):545–557. doi: 10.1016/j.cger.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zompatori M., Ciccarese F., Fasano L. Overview of current lung imaging in acute respiratory distress syndrome. Eur. Respir. Rev. 2014;23(134):519–530. doi: 10.1183/09059180.00001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shen W., Punyanitya M., Wang Z., Gallagher D., St-Onge M.P., Albu J., et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 1985;97(6):2333–2338. doi: 10.1152/japplphysiol.00744.2004. 2004. [DOI] [PubMed] [Google Scholar]
- 109.Barbas C.S.V. Thoracic computed tomography to assess ards and covid-19 lungs. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.829534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dabiri S., Popuri K., Cespedes Feliciano E.M., Caan B.J., Baracos V.E., Beg M.F. Muscle segmentation in axial computed tomography (CT) images at the lumbar (L3) and thoracic (T4) levels for body composition analysis. Comput. Med. Imaging Graph. 2019;75:47–55. doi: 10.1016/j.compmedimag.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rozenberg D., Orsso C.E., Chohan K., Orchanian-Cheff A., Nourouzpour S., Nicholson J.M., et al. Clinical outcomes associated with computed tomography-based body composition measures in lung transplantation: a systematic review. Transpl. Int. 2020;33(12):1610–1625. doi: 10.1111/tri.13749. [DOI] [PubMed] [Google Scholar]
- 112.Sanders K.J.C., Degens J.H.R.J., Dingemans A.C., Schols A.M.W.J. Cross-sectional and longitudinal assessment of muscle from regular chest computed tomography scans: L1 and pectoralis muscle compared to L3 as reference in non-small cell lung cancer. Int. J. Chron. Obstruct. Pulmon. Dis. 2019;14:781–789. doi: 10.2147/COPD.S194003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baracos V.E. Psoas as a sentinel muscle for sarcopenia: a flawed premise. J. Cachexia Sarcopenia Muscle. 2017;8(4):527–528. doi: 10.1002/jcsm.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gonzalez M.C., Heymsfield S.B. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: what are we really estimating? J. Cachexia Sarcopenia Muscle. 2017;8(2):187–189. doi: 10.1002/jcsm.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mulasi U., Kuchnia A.J., Cole A.J., Earthman C.P. Bioimpedance at the bedside: current applications, limitations, and opportunities. Nutr. Clin. Pract. 2015;30(2):180–193. doi: 10.1177/0884533614568155. [DOI] [PubMed] [Google Scholar]
- 116.da Silva B.R., Gonzalez M.C., Cereda E., Prado C.M. Exploring the potential role of phase angle as a marker of oxidative stress: a narrative review. Nutrition. 2022;93 doi: 10.1016/j.nut.2021.111493. [DOI] [PubMed] [Google Scholar]
- 117.Chernyak B.V., Popova E.N., Prikhodko A.S., Grebenchikov O.A., Zinovkina L.A., Zinovkin R.A. COVID-19 and oxidative stress. Biochemistry (Mosc) 2020;85(12):1543–1553. doi: 10.1134/S0006297920120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mundi M.S., Patel J.J., Martindale R. Body composition technology: implications for the ICU. Nutr. Clin. Pract. 2019;34(1):48–58. doi: 10.1002/ncp.10230. [DOI] [PubMed] [Google Scholar]
- 119.O’Rourke R.W. Adipose tissue and the physiologic underpinnings of metabolic disease. Surg. Obes. Relat. Dis. 2018;14(11):1755–1763. doi: 10.1016/j.soard.2018.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Statsenko Y., Al Zahmi F., Habuza T., Almansoori T.M., Smetanina D., Simiyu G.L., et al. Impact of age and sex on COVID-19 severity assessed from radiologic and clinical findings. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.777070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ali A.M., Kunugi H. Physical frailty/sarcopenia as a key predisposing factor to coronavirus disease 2019 (COVID-19) and its complications in older adults. BioMed. 2021;1(1):11–40. doi: 10.3390/biomed1010002. [DOI] [Google Scholar]
- 122.Martone A.M., Tosato M., Ciciarello F., Galluzzo V., Zazzara M.B., Pais C., et al. Sarcopenia as potential biological substrate of long COVID-19 syndrome: prevalence, clinical features, and risk factors. J. Cachexia Sarcopenia Muscle. 2022;13(4):1974–1982. doi: 10.1002/jcsm.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barazzoni R., Jensen G.L., Correia M.I.T.D., Gonzalez M.C., Higashiguchi T., Shi H.P., et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin. Nutr. 2022;41(6):1425–1433. doi: 10.1016/j.clnu.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 124.Compher C., Cederholm T., Correia M.I.T.D., Gonzalez M.C., Higashiguch T., Shi H.P., et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition diagnosis of malnutrition. JPEN J. Parenter. Enteral Nutr. 2022;46(6):1232–1242. doi: 10.1002/jpen.2366. [DOI] [PubMed] [Google Scholar]
- 125.Abate S.M., Chekole Y.A., Estifanos M.B., Abate K.H., Kabthymer R.H. Prevalence and outcomes of malnutrition among hospitalized COVID-19 patients: a systematic review and meta-analysis. Clin. Nutr. ESPEN. 2021;43:174–183. doi: 10.1016/j.clnesp.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oikawa S.Y., Holloway T.M., Phillips S.M. The impact of step reduction on muscle health in aging: protein and exercise as countermeasures. Front. Nutr. 2019;6:75. doi: 10.3389/fnut.2019.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pierik V.D., Meskers C.G.M., Van Ancum J.M., Numans S.T., Verlaan S., Scheerman K., et al. High risk of malnutrition is associated with low muscle mass in older hospitalized patients –a prospective cohort study. BMC Geriatr. 2017;17(1):118. doi: 10.1186/s12877-017-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.De Lorenzo A., Siclari M., Gratteri S., Romano L., Gualtieri P., Marchetti M., et al. Developing and cross-validation of new equations to estimate fat mass in Italian population. Eur. Rev. Med. Pharmacol. Sci. 2019;23(6):2513–2524. doi: 10.26355/eurrev_201903_17399. [DOI] [PubMed] [Google Scholar]
- 129.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Studenski S.A., Peters K.W., Alley D.E., Cawthon P.M., McLean R.R., Harris T.B., et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69(5):547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roh E., Choi K.M. Health consequences of sarcopenic obesity: a narrative review. Front. Endocrinol. (Lausanne). 2020;11:332. doi: 10.3389/fendo.2020.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data described in the manuscript, code book, and analytic code will be made available upon request pending approval from the corresponding author.