Abstract
Sexual disturbances, and aggressivity are a major social problem. However, the molecular mechanisms involved in the control of these behaviors are largely unknown. FGF14, which is an intracellular protein controlling neuronal excitability and synaptic transmission, has been implied in neurologic and psychiatric disorders. Mice with Fgf14 deletion show blunted responses to drugs of abuse. By behavioral tests we show that male Fgf14 knockout mice have a marked reduction of several behaviors including aggressivity and sexual behavior. Other behaviors driven by spontaneous initiative like burying novel objects and spontaneous digging and climbing are also reduced in Fgf14 knockout mice. These deficits cannot be attributed to a generalized decrease of activity levels, because in the open field test Fgf14 knockout mice have the same spontaneous locomotion as wild types and increased rearing. Our results show that Fgf14 is important to preserve a set of behaviors and suggest that fine tuning of neuronal function by Fgf14 is an important mechanism of control for such behaviors.
Keywords: Fgf14, Aggressivity, Sexual behavior, Spontaneous behavior, Sociability, Mouse
1. Introduction
Disorders that include, alterations of sexual behavior and aggressivity are controlled by complex brain networks including the main olfactory system, basal ganglia (mainly ventral striatum and ventral pallidum), prefrontal cortex, ventral hippocampus, amygdala and hypothalamus [1], but little is known about the molecular determinants of such diseases.
Mutations of the FGF14 gene were initially reported in a spontaneously occurring human disease, the spino-cerebellar ataxia type 27 (SCA27), which, in addition to cerebellar symptoms, is associated to deficits in cognition, memory and behavior [2,3]. More recently, FGF14 has been implied in a series of psychiatric and neurologic disorders by several large-scale genome-wide association studies, which identified this gene as a potential molecular determinant of addictive behavior, schizophrenia, bipolar disorder, depression and epilepsy [4–11].
FGF14 is an intracellular protein that is related in sequence and structure to fibroblast growth factors, which are secreted, trophic molecules essential for proper development and function in a large variety of tissues [12]. In contrast to such fibroblast growth factors, some members of the FGF family (FGF11–14) are not secreted but act intracellularly, where they bind to target proteins including voltage-gated Na+ (Nav) channels [13–15]. More specifically, FGF14 binds to the C-terminal domain of Nav1.1, Nav1.2 and Nav1.6 [16,17]. The binding of FGF14 modulates the function of the channel, in an isoform specific manner [16,17]. Furthermore, the biophysical properties of Nav channels are regulated by phosphorylation of FGF14 by several kinases, like GSK3, which are implied in psychiatric disorders [11].
Fgf14 is expressed in the mouse central nervous system from embryonic day 12.5 [18] and in the adult brain the highest levels of Fgf14 expression have been found in cerebellum, basal ganglia, hippocampus, amygdala, cerebral cortex and thalamus [19,20], showing a large overlap with the regions critical for the control of food intake, sexual behavior and aggressivity.
Mice with a targeted deletion of the Fgf14 gene (Fgf14−/− mice) have a normal development of the central nervous system and a preserved overall brain anatomy [19]. In the adult brain, while the cerebellum and basal ganglia of Fgf14−/− mice are indistinguishable from wild types, in the hippocampus they show a disrupted inhibitory GABAergic circuit associated with altered action potential generation [21].
Fgf14−/− mice recapitulate several features of psychiatric disorders like working memory deficits and decreased gamma frequency oscillations of cortical networks, reminiscent of findings in patients with schizophrenia [21]. Moreover, Fgf14−/− mice display a strong suppression of locomotor responses normally elicited by the administration of drugs of abuse like cocaine and amphetamine, and to the administration of a D2 dopamine receptor agonist [19]. These results suggest that Fgf14−/− mice might have a deficit in behaviors that are related to decreased responsiveness to dopamine. However, the underlying mechanisms might be more complex, because the dopaminergic pathways were found to be intact in Fgf14−/− mice [19]. The presence of such morphological and behavioral alterations suggests the presence of aberrant neuronal activity in the critical regions for the control of food intake, sexual behavior and aggressivity. These regions include the striatum, hippocampus, neocortex (especially prefrontal cortex) and amygdala [1]. Therefore, we hypothesized that some of these behaviors might be affected by Fgf14 deletion.
2. Materials and methods
2.1. Animals
Two to four months old male Fgf14−/− mice and their wild type littermates were used for all experimental paradigms. All the animals used were male. The Fgf14−/− mice were obtained from Prof. Laezza Fernanda, University of Texas Medical Branch U.S.A. Adequate measures were taken to minimize pain and discomfort. All experimental procedures were carried out at NICO and approved by the Ethical Committee of the University of Torino and authorized by the Italian Ministry of Heath (authorization number: 822/2016-PR). Experiments have been carried out in accordance with the European Communities Parliament and Council Directives of 24 November 1986 (86/609/EEC) and 22 September 2010 (2010/63/EU).
2.2. Behavioral tests
Behavioral studies were performed in young adult male animals between 2 and 4 months, always during the light phase of the cycle. A general timeline of the behavioral tests is reported in Fig. 1. The same animals underwent all of the behavioral tests reported in the timeline. Where needed, all behavioral procedures were video-recorded and scored by an individual blind to the genotype of the mouse.
Fig. 1.
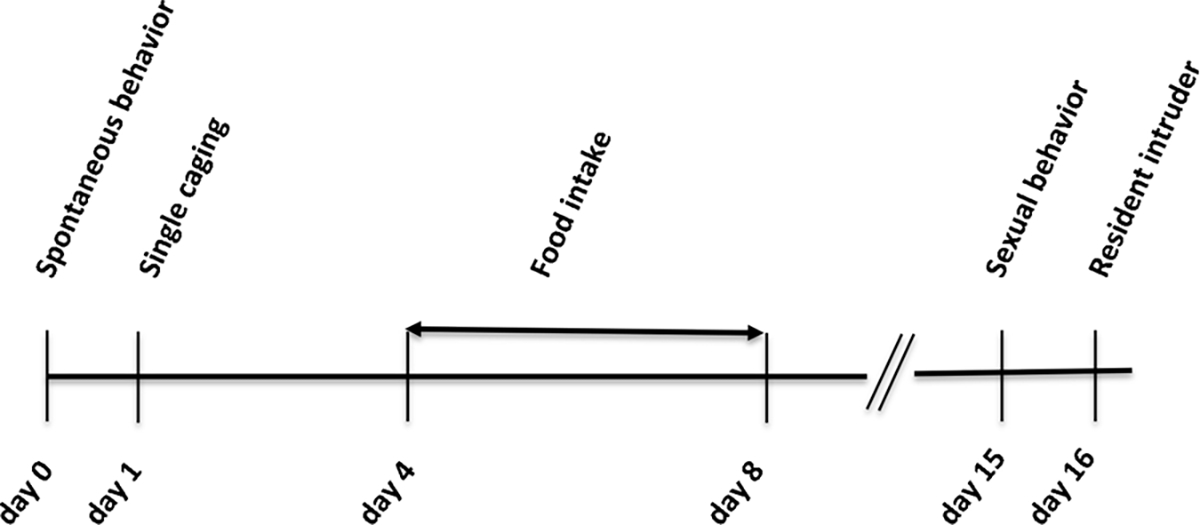
Experimental timeline showing the sequence of behavioral tests delivered to the animals.
2.2.1. Spontaneous behavior
For each mouse (n = 12 wild type; n = 12 Fgf14−/−) the evaluation of spontaneous behavior was performed as follows:
Mice were allowed to habituate to room for at least 45 min. Mice were removed from their home cages and individually placed in a clear cage (area 36 × 21, height 14 cm) with standard supply of food and water. The experiment was performed with constant lighting conditions. Each trial was video recorded for 20 min while the operator was out of the room. Each video was analyzed off-line for 10 min, between the 5th and 15th, by an individual blind to the genotype of the mouse. The ethologically-based behavioral checklist was similar to that described previously [22].
In particular, we measured the following parameters:
Rearing free (front paws reaching upwards away from any cage wall while standing on hind limbs).
Rearing towards a cage wall (front paws reaching upwards on a cage wall while standing on hind limbs).
Rearing towards central structure (front paws reaching upwards on that portion of grill where food and water are placed, while standing on hind limbs).
Digging.
Grooming (of any form).
Climbing (jumping onto cage top with climbing along grill in inverted or hanging position).
Stillness (motionless, with no behavior evident).
Jumping (every vertical movement, with detachment of both hind limbs from the cage floor, which is not included as total climbing).
We assessed, for each type of behavior, three different time parameters:
Total time (seconds) spent by the animal showing a single behavior.
Number of episodes of a specific behavior.
Mean time duration (seconds) of each episode for each specific behavior (this data was obtained by the division of total time spent for a specific behavior / number of episodes for the same behavior).
2.2.2. Sexual behavior
All mice (n = 14 wild type; n = 14 Fgf14−/−) were removed from their home cage and kept single caged (cage area 36 × 16, height 13 cm) for two weeks before performing the test. Only sexually naive male mice were used, because they are unable to discriminate between oestrus and non oestrus females [23]. Mice were transported in the test room and were allowed to habituate to room setting for at least 45 min. A sexually naive female (randomly chosen in a group of 2–4 months aged C57BL/6 female mice) was introduced in the home cage of the tested mouse. Each female was used only once. Each trial was carried out maintaining constant setting and lighting conditions in the same room. The tests were videotaped for 15 min. Each video was analyzed off-line by a trained single operator blind to the genotype of each animal. The male-female interactions were screened through a behavioral checklist similar to that described by [23,24].
The parameters analyzed were:
Mounting (pelvic thrusting with physical contact between male and female pelvic regions).
Latency to mount (seconds) (if no mount could be detected during the entire duration of the test, this latency was set to 900 s).
Intromission (mounting associated with a stable frequency continuously for at least 3 s and if the female’s anogenital area was elevated over the ground when finished).
Social approach (including sniffing on the body or head of the female, anogenital sniffing, social grooming, barbering and chasing).
Avoidance (leaving the female, both on the horizontal and vertical plane).
Passive behavior (the female exhibits one of the items included in the category “social approach” while the male stands in a submissive attitude).
The statistical comparison between the two genotypes was applied to three different time parameters for each behavioral item, excluding latency measures:
Total time (seconds) spent by the animal showing a single behavior.
Number of episodes of a specific behavior.
Mean time duration (seconds) of each episode of that specific behavior (these data were obtained with the division total time/number of episodes).
2.2.3. Resident intruder test
All mice (n = 14 wild type; n = 16 Fgf14−/−) used for this test were removed from their home cage and kept single caged (cage area 36 × 16, height 13 cm) for two weeks before being used as resident. This was in order to enhance their territorial attitude and to create a condition of mild social stress. Mice were transported in the test room and were allowed to habituate to room setting for at least 45 min. The test was carried out maintaining constant setting and lighting conditions. An intruder mouse of the same age and with a lower or same weight (intruders were group-housed C57BL/6 male mice) was placed in the home cage of the tested resident mouse. The intruders were used only once. The test was videotaped for 15 min. The analysis took place in a subsequent moment and was performed by a trained single operator blind to the genotype of each animal. The aggressive and territorial attitude of resident mice was assessed as previously described by [25].
Attacking (wrestling).
Latency to attack (seconds) (if no attack could be detected during the entire duration of the test, the attack latency was set to 900 s).
Threatening (including chasing, all offensive postures and aggressive grooming).
Social behavior (including sniffing on the body or head of the intruder, anogenital sniffing, social grooming and following).
Individual interaction (the sum of the time spent in attack, threat and social behavior).
Types of interaction (the behavioral pattern of the animals was studied by calculating the percentage of each type of behavior (i.e. social interaction, threatening and attacking) in relation to the total time of individual interaction).
The statistical comparison between the two genotypes was applied to three different time parameters for each behavioral item, excluding latency measures:
Total time (seconds) spent by the animal showing a single behavior.
Number of episodes of a specific behavior.
Mean time duration (seconds) of each episode of that specific behavior (these data were obtained with the division total time/number of episodes for each specific behavior).
2.2.4. Open field
Animals (n = 8 wild type; n = 11 Fgf14−/−) were tested in a 50 × 50 cm arena. The animal was placed in the corner of the open field and was allowed to explore the arena for 60 min. The distance traveled in the open field and the time spent in the center zone were measured every 10 min.
2.2.5. Three-chambered sociability test
The mice (n = 7 wild type; n = 7 Fgf14−/−) used for the experiment were transported to the test room and were allowed to habituate to room setting for at least 45 min. The apparatus, a custom-made clear Plexiglas box partitioned into three chambers of equal size (length 20 cm × width 40.5 cm × height 22 cm), was designed as previously described [26]. The test was divided in two phases, habituation and social behavior.
2.2.5.1. Habituation.
At the beginning of the test the mouse was confined in the middle chamber for 10 min. After this phase the doors were opened and the mouse was permitted to freely move in all chambers for other 10 min.
2.2.5.2. Social behavior.
The test mouse was confined again in the middle chamber. An inverted empty wire cup (wire pencil cup, Galaxy Cup, Kitchen Plus, http://www.kitchen-plus.com) and a wire cup containing a stranger mouse (stranger) were placed into left and right chambers. The doors were re-opened and the mouse was allowed to explore all chambers for other 10 min. The movements of the test mice were videotaped from the top and their approaches were analyzed using Ethovision XT video track system (Noldus Information Technology, Wageningen, The Netherlands). The comparison of the time spent with stranger vs. empty wire cup indicated the sociability of animals. Stranger mice were housed in the same facility but had no prior contact with test mice. They were trained for two sessions of 15 min a day before the test. The observer remained in the room and only mice that at the end of 15 min did not grip to the wire cup were chosen.
2.2.6. Marble burying test
The test was performed as in Hoeffer et al. [27]. Mice were placed individually in large clean cages (area 36 × 21, height 14 cm) containing five cm deep bedding. Twenty small black marbles were arranged in five spaced rows of four marbles. After a 30-min test period conducted under normal room lighting, mice were removed and the unburied marbles were counted. Marbles were considered buried if they were at least 2/3 covered with bedding.
2.3. Histological procedures
Fgf14−/− mice (n = 3) and their wild type littermates (n = 3) were anesthetized via intraperitoneal injection with ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight) and perfusion-fixed with 4% paraformaldehyde in 0.12 M phosphate buffer. The brains were removed and immersed in the same fixative at 4 °C for 18 h and then cryoprotected in 30% sucrose in 0.12 M phosphate buffer. The brains were frozen and serially cut by a freezing microtome in 50 μm-thick coronal sections and stained with cresyl violet. Volumetric analysis was performed on the basolateral amygdala complex by means of the StereoInvestigator software (MicroBrightField, Williston, VT, USA). The interval thickness between the serial sections analyzed was 300 μm.
2.4. Real time RT-PCR
mRNA expression levels of the dopamine D1 receptor (Drd1), D2 receptor (Drd2) and dopamine reuptake transporter DAT (Slc6a3) were evaluated by real time reverse transcription polymerase chain reaction (RT-PCR).
To obtain dissected brain regions, male mice (2–4 months old) were anesthetized by isoflurane inhalation and decapitated. Brains were removed immediately after decapitation and coronal sections were cut using a vibratome (Leica Microsystems GmbH, Wetzlar, Germany) following the coordinates of the mouse brain atlas [28]. From the slices, the prelimbic and infralimbic areas of the medial prefrontal cortex (mPFC), the nucleus accumbens (Acb) and the caudate-putamen (CPu) were dissected.
Total RNA from mPFC (mice: n = 8 wild type and n = 9 Fgf14−/−), Acb (mice: n = 4 wild type and n = 5 Fgf14−/−) and CPu (mice: n = 4 wild type and n = 5 Fgf14−/−), was extracted using the Pure link RNA Mini Kit (Life Tecnologies), according to manufacture’s instructions. Total RNA, for each region, was reversed transcripted at a final concentration of 20 ng/μl, using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). For primers and probes, Applied Biosystems’ TaqMan® Assay-on-demand-TM gene expression products were used. The catalogue number for each gene was Drd1 (Mm02620146_s1), Drd2 (Mm00438545_m1), Slc6a3 (Mm00438388_m1). Phosphoglycerate Kinase 1 (PGK1) (Mm00435617_m1) was used as the reference gene in the expression level analysis. Expression levels of target genes were calculated by the normalized comparative cycle threshold (Ct) method (2−ΔCt).
2.5. Statistical analysis
Statistical analyses were carried out by GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA) and included two-tailed unpaired Student’s t-test, Fisher’s test and Mann-Whitney U test In all instances, P < 0.05 was considered as statistically significant. Data were expressed as average ± standard error of the mean (SEM).
3. Results
3.1. Abnormal spontaneous behavior of Fgf14−/− mice
The observation of spontaneous behavior of mice in their housing cages represents a rich source of information not biased by experimental manipulation. For this reason, in order to gain insight in the behavioral alterations of Fgf14−/− mice we started with a thorough assessment of their spontaneous behavior, compared to their wild-type littermates. The parameters considered were rearing (defined as lifting the forepaws and keeping them either free or leaning to a vertical surface like the cage wall or the cage central structure), digging, grooming, climbing, jumping and remaining still. For each behavior we counted the total number of episodes, the duration of each episode and the total time spent in that behavior. The results are reported in Table 1. Fgf14−/− and wild type mice differed significantly in rearing, climbing and digging behaviors. Rearing wall and central structure behaviors differed between genotypes (t-test, t(22) = 2.465, p = 0.022 and t (22) = 3.025, p = 0.006; respectively, Fig. 2A, D and Table 1). Fgf14−/− mice reared significantly less often toward the central structure (t-test, t(22) = 2.374, p = 0.027, Fig. 2E and Table 1), but with a significantly increased duration of each episode (t-test, t(22) = 4.714, p = 0.0001, Fig. 2F and Table 1). Furthermore, an increased duration of each episode was observed for all types of rearing, also for free rearing and rearing wall behaviors (t-test, t(22) = 2.415, p = 0.025 and t (2) = 5.011, p = 0.0001; respectively, Fig. 2C, I and Table 1). Digging behavior was significantly reduced in Fgf14−/− mice compared to their wild type littermates (t-test, t(22) = 3.641, p = 0.001, Fig. 3A and Table 1). A reduction was also observed in the number of episodes for digging in Fgf14−/− mice (t-test, t(22) = 4.060, p = 0.0005, Fig. 3B and Table 1). The climbing behavior was reduced in Fgf14−/− mice both as total time and as number of episodes (t-test, t(22) = 2.624, p = 0.015 and t(22) = 3.034, p = 0.006; respectively, Fig. 4A, B and Table 1). Grooming, jumping and stillness behaviors did not differ between genotypes (t-test, p > 0.05). However, for stillness behavior there was a significant increase in the duration of each episode in Fgf14−/− mice (Table 1).
Table 1.
Analysis of 10 min of spontaneous behavior (n = 12 for wild type and Fgf14−/− mice). For each behavior, total time, number of episodes and episode mean time are reported.
| Stereotypic behaviors (sec) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Free rearing |
Rearing wall |
Rearing central structure |
Digging | Grooming | Climbing | Stillness | Jumping | |
|
| ||||||||
| Wild type | 11.4 ± 2.4 | 55.6 ± 3.8 | 68.0 ± 7.2 | 38.7 ± 6.7 | 28.4 ± 4 | 87.4 ± 22.4 | 20.6 ± 5.7 | 0.13 ± 0.09 |
| Fgf14 −/− | 21.1 ± 6.6 | 70.4 ± 4.7 | 116.7 ± 14.4 | 13.0 ± 2.2 | 33.1 ± 5.5 | 24.2 ± 8.8 | 22.2 ± 4.2 | 0.21 ± 0.16 |
| p value | 0.182 | * 0.022 | ** 0.006 | ** 0.001 | 0.499 | * 0.015 | 0.822 | 0.646 |
| Number of episodes for each behavior | ||||||||
| Wt | 12.5 ± 2.5 | 45.8 ± 3.8 | 43.4 ± 3.4 | 28.8 ± 3.6 | 12.1 ± 1.4 | 11.3 ± 2.9 | 9.4 ± 2.7 | 2 ± 1.5 |
| Fgf14 −/− | 12.8 ± 3.2 | 40.2 ± 2.2 | 33.8 ± 2.2 | 11.3 ± 2.3 | 10.4 ± 2.1 | 2.3 ± 0.8 | 7.1 ± 1.5 | 3.2 ± 2.3 |
| p value | 0.951 | 0.212 | * 0.027 | *** 0.0005 | 0.519 | ** 0.006 | 0.452 | 0.675 |
| Episode mean | time of each behavior (sec) | |||||||
| Wt | 0.9 ± 0.2 | 1.24 ± 0.05 | 1.5 ± 0.06 | 1.3 ± 0.1 | 2.6 ± 0.4 | 1.5 ± 0.8 | 2.2 ± 0.4 | 0.02 ± 0.01 |
| Fgf14 −/− | 1.5 ± 0.2 | 1.76 ± 0.09 | 3.4 ± 0.04 | 1.3 ± 0.2 | 3.8 ± 0.7 | 0.2 ± 0.16 | 3.6 ± 0.3 | 0.02 ± 0.01 |
| p value | * 0.025 | *** 0.0001 | *** 0.0001 | 0.814 | 0.198 | 0.122 | * 0.011 | 0.764 |
* p < 0.05.
** p < 0.01.
*** p < 0.001.
Fig. 2.
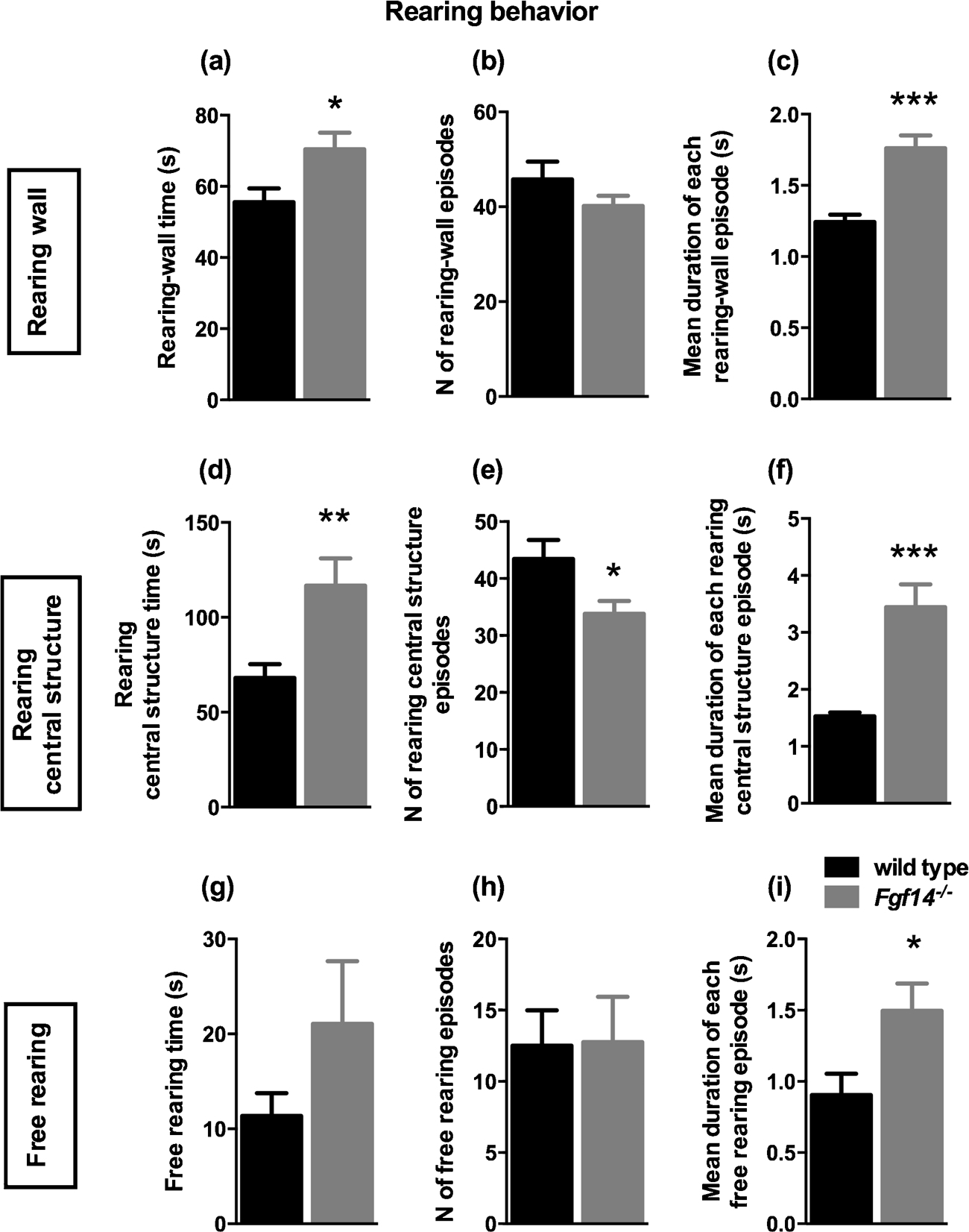
Rearing behavior observed in 10 min session. A–C. Rearing-wall behavior: Bars represent mean ± SEM of (A) rearing-wall time (p < 0.05), (B) number of episodes and (C) duration of each rearing-wall episode (p < 0.001). Rearing central structure behavior (D–F): (D) rearing-central structure time (p < 0.01), (E) number of episodes (p < 0.05) and (F) duration of each rearing-central structure episode (p < 0.001). Free rearing behavior (G–I): (G) free rearing time, (H) number of episodes and (I) duration of each free rearing episode of (p < 0.05).
Fig. 3.
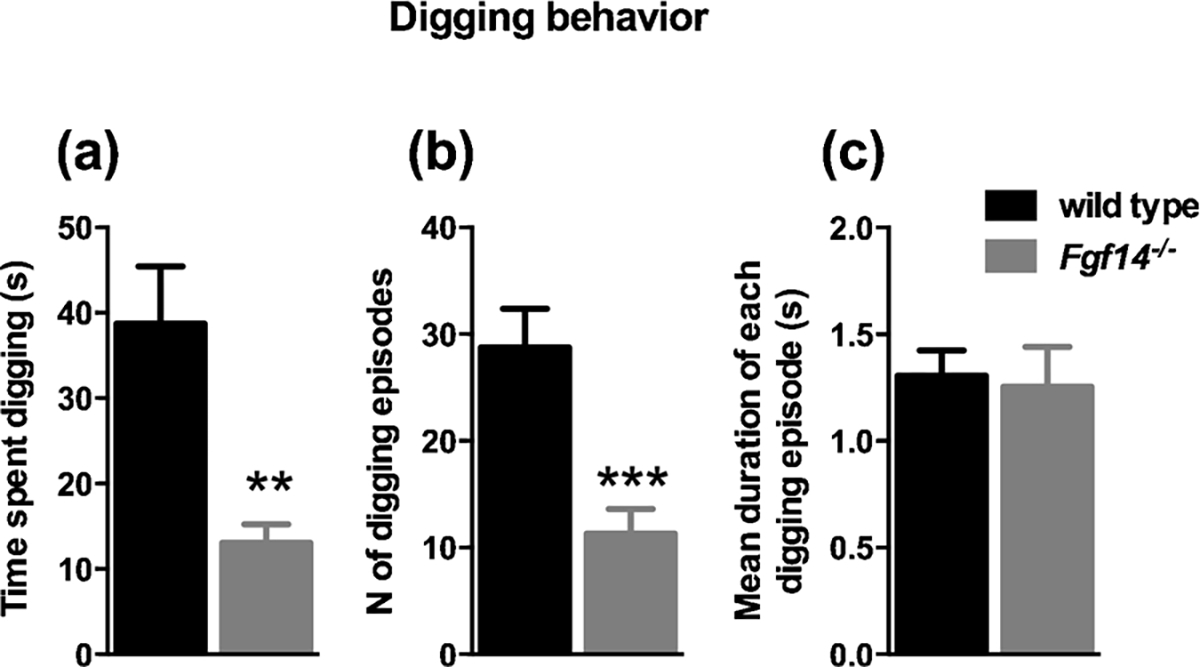
Fgf14−/− mice show a reduction in digging behavior. (A) Time spent in digging behavior (p < 0.01). (B) Number of digging episodes (p < 0.001). (C) Duration of each digging episode (p > 0.05).
Fig. 4.
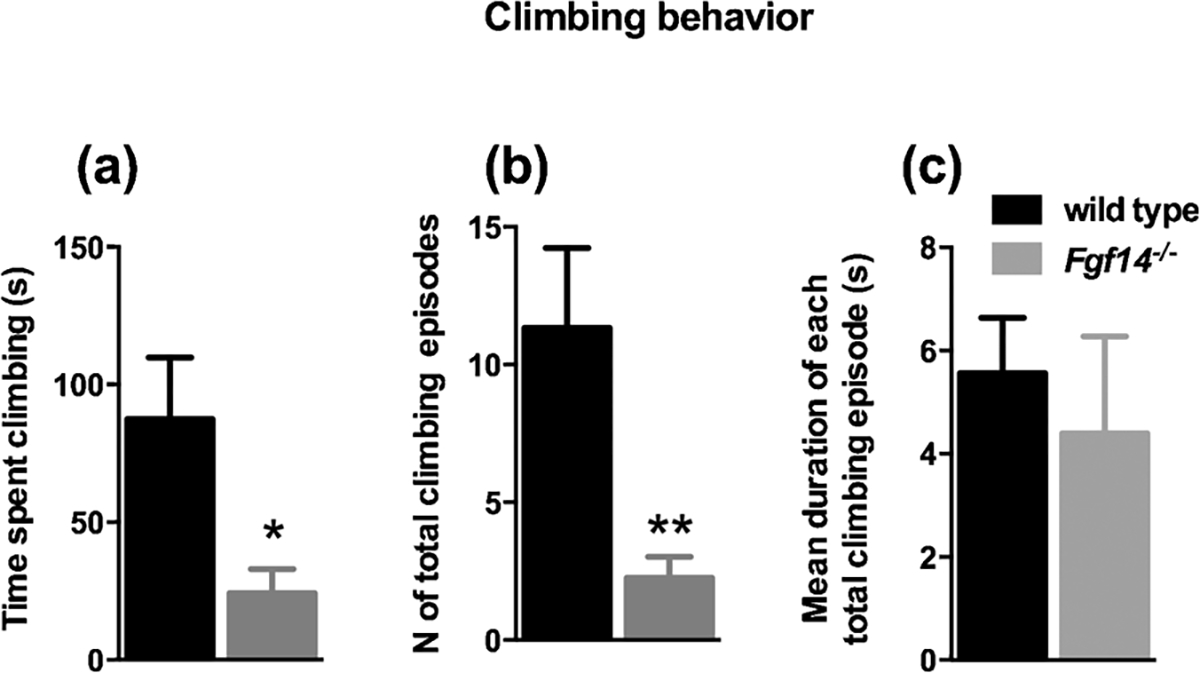
Fgf14−/− mice show a reduction in climbing behavior. (A) Bars represent mean ± SEM of total time spent climbing during the 10 min session. The total time spent climbing is significantly reduced compared to wild type mice * p < 0.05. (B) Fgf14−/− mice display a reduced number of climbing episodes ** p < 0.01. (C) Bars show no significant difference between genotypes in the mean duration of each climbing episode p > 0.05.
3.2. Reduced marble burying by Fgf14−/− mice
The marble burying test revealed a more than tenfold and significant difference between genotypes, with a reduced number of buried marble spheres for Fgf14−/− mice (0.8 ± 0.55) compared to wild types (10.75 ± 1.73) (t-test, t(16) = 6.007, p < 0.0001, Fig. 5).
Fig. 5.
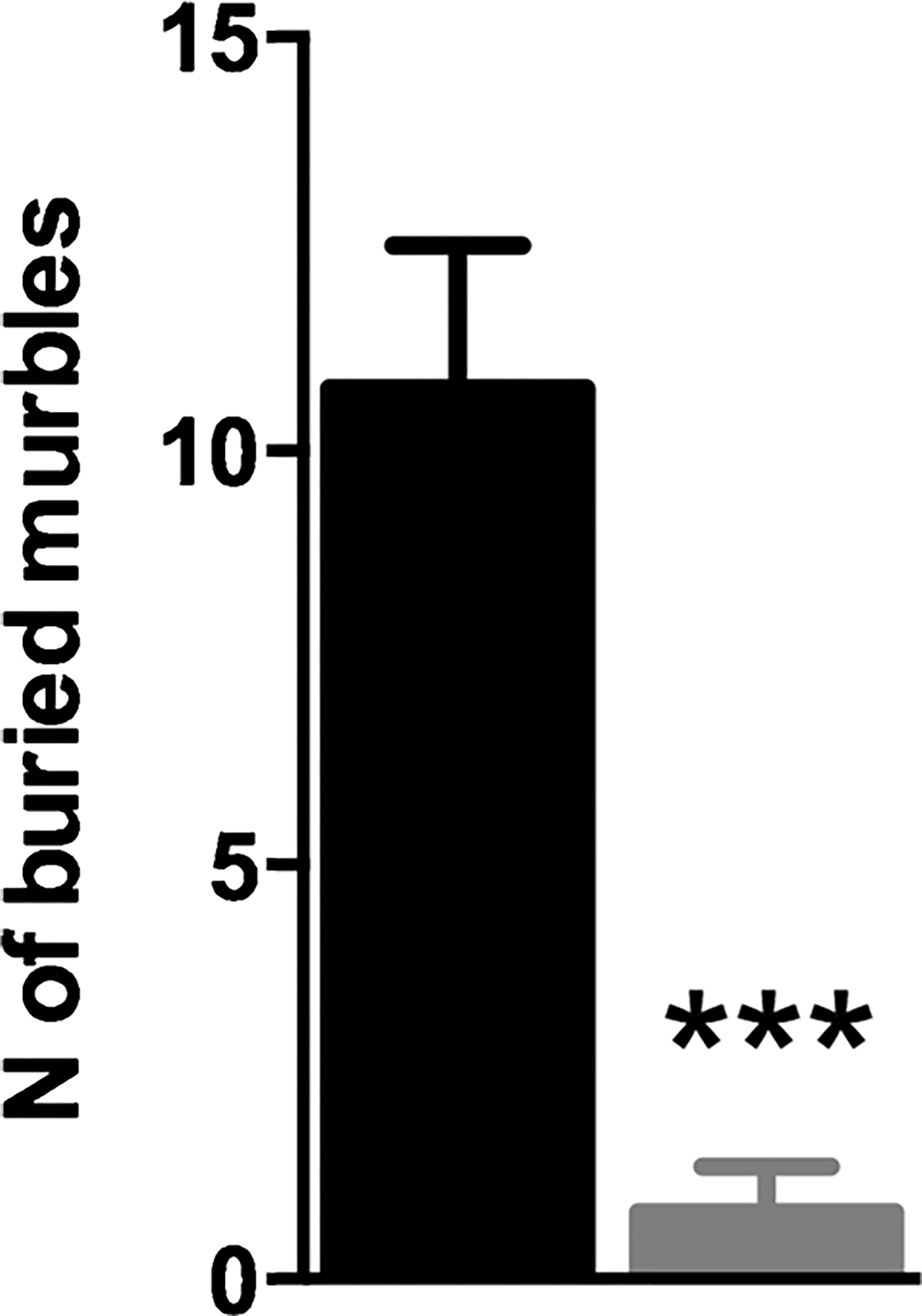
Fgf14−/− mice bury a smaller number of marbles in 30 min of test compared to their wild type littermates. The bars represent mean ± SEM. *** p < 0.001.
3.3. Reduced sexual behavior of Fgf14−/− mice
The sexual behavior of Fgf14−/− male mice resulted profoundly altered. The latency to the first mount was significantly prolonged in Fgf14−/− mice (t-test, t(26) = 5.331, p = 0.0001, Fig. 6A and Table 2). Both mean time of mounting and duration of each episode were significantly reduced in Fgf14−/− mice (t-test, t(26) = 2.484, p = 0.020 and t(26) = 3.449, p = 0.002 respectively, Fig. 6B, D and Table 2). The number of mounts was significantly reduced in Fgf14−/− mice (t-test, t (26) = 3.234, p = 0.003, Fig. 6C and Table 2). Furthermore, the number of Fgf14−/− males performing mounting was very low (1 of 14 mice) relative to their wild type littermates (12 of 14 mice) (Fisher’s test, p < 0.0001, Fig. 6E). Intromission, avoidance and affiliation approach were not significantly different between genotypes (p > 0.05, Table 2). An interesting data that arise from this test, in accord with a reduced sexual interaction, is the significant increase of passive behavior in Fgf14−/− mice (t-test, t(26) = 4.793, p < 0.0001, Fig. 6F and Table 2).
Fig. 6.
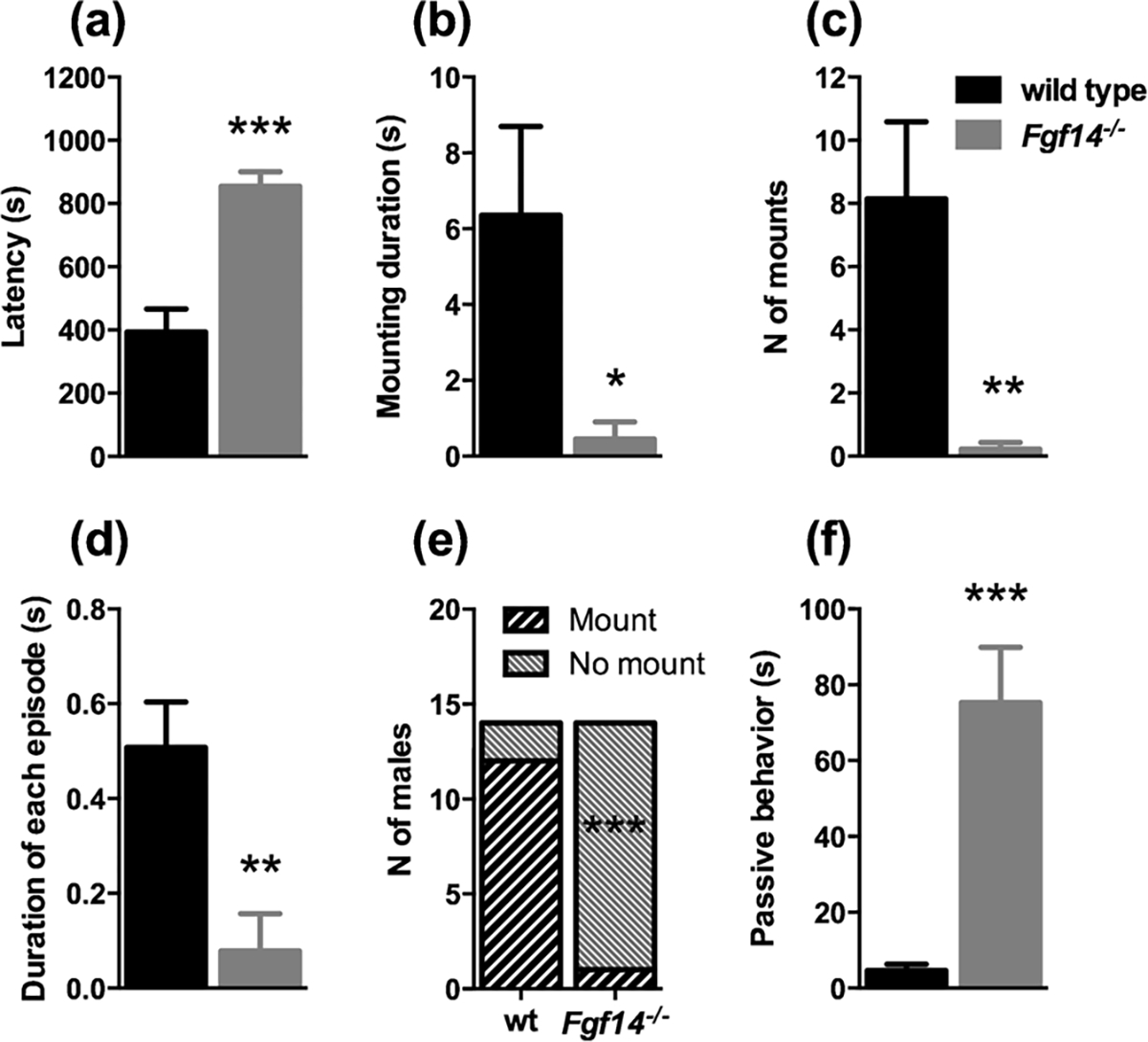
Fgf14−/− male mice show a reduced sexual interaction. (a) The latency for sexual interaction is increased while the mounting duration is decreased (b). The number of mounts (c), and the duration of each episode (d) are significantly fewer in Fgf14−/− mice compared to their wild type littermates. (e) The number of males that had a sexual approach was significantly lower in Fgf14−/− mice. Fgf14−/− mice during the test spent more time in passive behavior (f). The bars represent mean ± SEM. * p < 0.05; ** p < 0.01; *** p < 0.001.
Table 2.
Analysis of 15 min of sexual behavior (n = 14 for both genotypes).
| Sexual behavior | Wild type | Fgf14 −/− | P value |
|
| |||
| Latency to mount (sec) | 393.5 ± 73.36 | 854.3 ± 45.71 | *** 0.0001 |
| Time mounting (sec) | 6.36 ± 2.34 | 0.45 ± 0.45 | * 0.020 |
| Number of mounts (sec) | 8.14 ± 2.44 | 0.21 ± 0.21 | ** 0.003 |
| Duration of each episode (sec) | 0.51 ± 0.096 | 0.079 ± 0.079 | ** 0.002 |
| Social approach (sec) | 447.7 ± 26.84 | 406.1 ± 34.42 | 0.35 |
| Avoidance (sec) | 439.8 ± 26.48 | 417.5 ± 31.63 | 0.59 |
| Passive behavior (sec) | 4.61 ± 1.72 | 75.26 ± 14.64 | *** 0.0001 |
* p < 0.05.
** p < 0.01.
*** p < 0.001.
3.4. Reduced aggressive behavior of Fgf14−/− mice in the resident/intruder test
In the resident/intruder test, the latency of attacking the intruder mouse and the number of episodes were comparable between genotypes (p > 0.05, Table 3). On the contrary, the time spent attacking and the duration of each attack were significantly reduced in Fgf14−/− mice (u = 71.50, p = 0.045 and t(28) = 2.786, p = 0.0095, respectively, Fig. 7A, C and Table 3). The number of episodes for attack was comparable between genotypes (p > 0.05, Fig. 7C) Fgf14−/− mice showed a significant reduction in time threatening the intruder mouse, number of episodes and duration of each threat episode (t-test, t (28) = 4.007, p = 0.0004; t(28) = 4.076, p = 0.0003 and t (28) = 2.346, p = 0.026; respectively, Fig. 7D–F and Table 3). On the other hand, Fgf14−/− mice spent more time in social interaction than their wild type littermates (t(28) = 8.769, p < 0.0001, Fig. 7G and Table 3). Moreover, the percentage of time spent in social behavior relative to the total time of all types of inter-individual interaction was greater in Fgf14−/− mice than controls (97.59% for Fgf14−/− mice and 78.25% for wild type, Fig. 7H).
Table 3.
Analysis of 15 min of resident intruder test (n = 14 for wild type and n = 16 for Fgf14−/− mice.
| Resident intruder parameters | Wild type | Fgfl4 −/− | P value |
|---|---|---|---|
|
| |||
| Time threatening (sec) | 84.93 ± 21.4 | 4.08 ± 2.64 | *** 0.0004 |
| Number of threat episodes | 17.93 ± 4.39 | 1.0 ± 0.65 | *** 0.0003 |
| Mean time of each threat | 4.40 ± 0.68 | 1.45 ± 1.02 | * 0.026 |
| episode (sec) | |||
| Latency to first attack (sec) | 630.1 ± 82.4 | 769.3 ± 71.4 | 0.21 |
| Time attacking (sec) | 19.05 ± 8.8 | 2.51 ± 1.98 | * 0.045 |
| Number of attack episodes | 7.0 ± 3.03 | 1.8 ± 1.29 | 0.066 |
| Mean time of each attack | 1.32 ± 0.41 | 0.20 ± 0.12 | * 0.0095 |
| episode (sec) | |||
| Social behavior (sec) | 78.25 ± 5.05 | 445.2 ± 38.81 | *** < 0.0001 |
* p < 0.05.
*** p < 0.001.
Fig. 7.
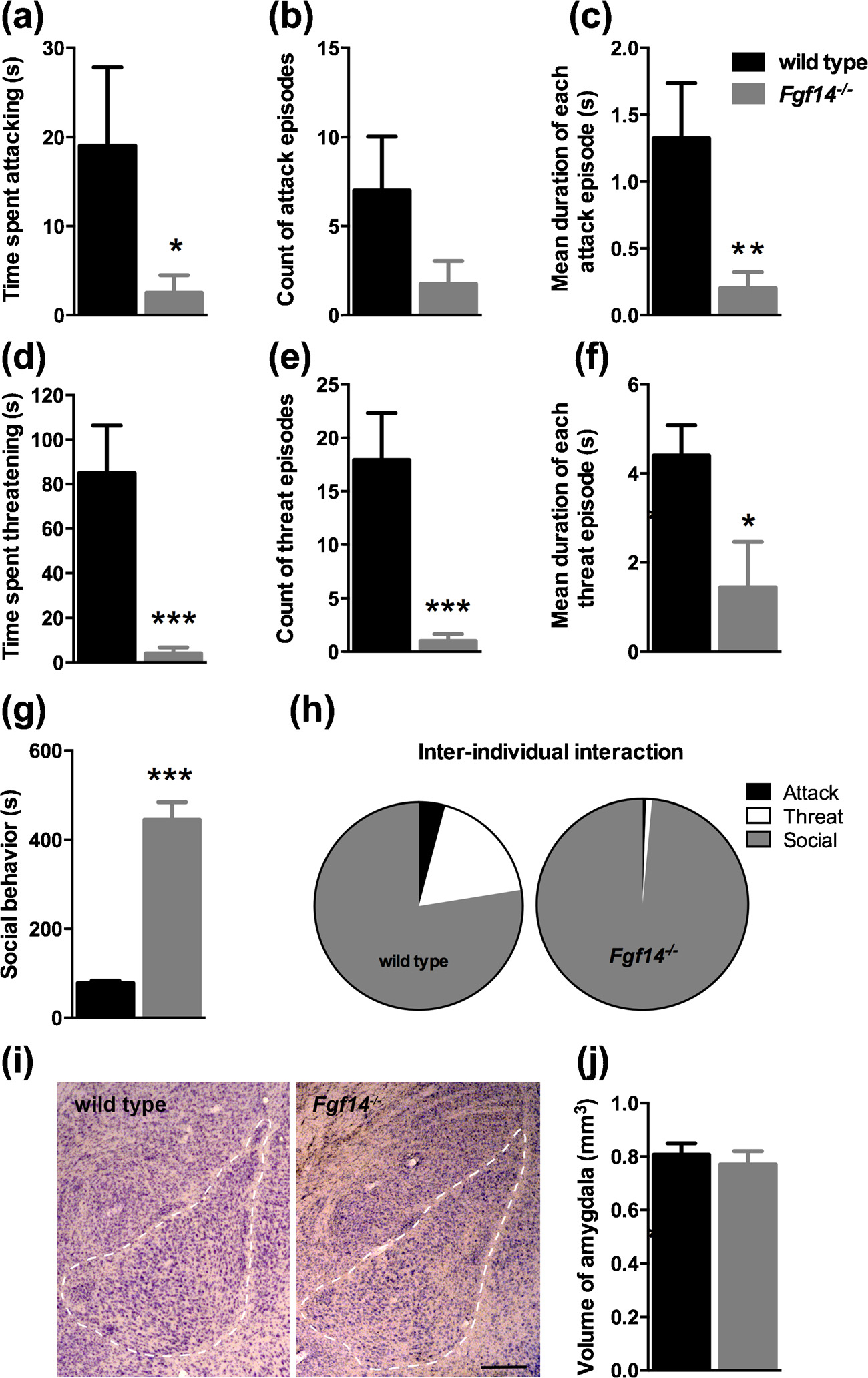
Fgf14−/− male mice show a reduced aggressivity in the resident intruder test. Bars represent means ± SEM of time (in seconds) spent in attack (a) and threat (d), number of episodes for attack (b) and threat (e), and mean duration of each episode for attack (c) and threat (f). (g) Duration of social behavior exhibited during the test. (h) Relative duration of inter-individual interaction types. (i) Representative Nissl-stained sections used to measure the size of the amygdala (calibration bar: 250 μm). (j) Volume of the amygdala in Fgf14−/− and wild type mice. The bars represent mean ± SEM. * p < 0.05; ** p < 0.01; *** p < 0.001.
The amygdala plays a pivotal role in the integration and expression of aggressivity and the volume of this structure has been reported to be altered in subjects with a history of aggressivity or violence [29–32]. We hypothesized that in Fgf14−/− mice the reduced levels of aggressivity could be related to an abnormal volume of the amygdala. With a stereological analysis of the amygdala, the volume in Fgf14−/− mice was 0.770 ± 0.050 mm3, which was not significantly different compared to wild type (0.807 ± 0.042 mm3; p > 0.05, Fig. 7I, J). This result suggests that the reduction in aggressivity is not due to an altered volume of the amygdala.
3.5. Normal sociability of Fgf14−/− mice in the three-chambered test
Modifications of social behavior might in some cases underlie alterations in sexual and aggressive behavior. To exclude the hypothesis of a reduced social interaction of Fgf14−/− mice with wild type mice of the same sex we performed the three-chambered test. The time spent in the chamber with the stranger mouse was significantly greater than the time spent in the chamber with the inanimate object for both wild type (t-test, t(12) = 4.21, p = 0.0012; Fig. 8A) and Fgf14−/− mice (t-test, t (12) = 3.21, p = 0.0074; Fig. 8A). These results demonstrate that the deficit in sexual and aggressive behavior of Fgf14−/− mice is not attributable to changes in social interaction.
Fig. 8.
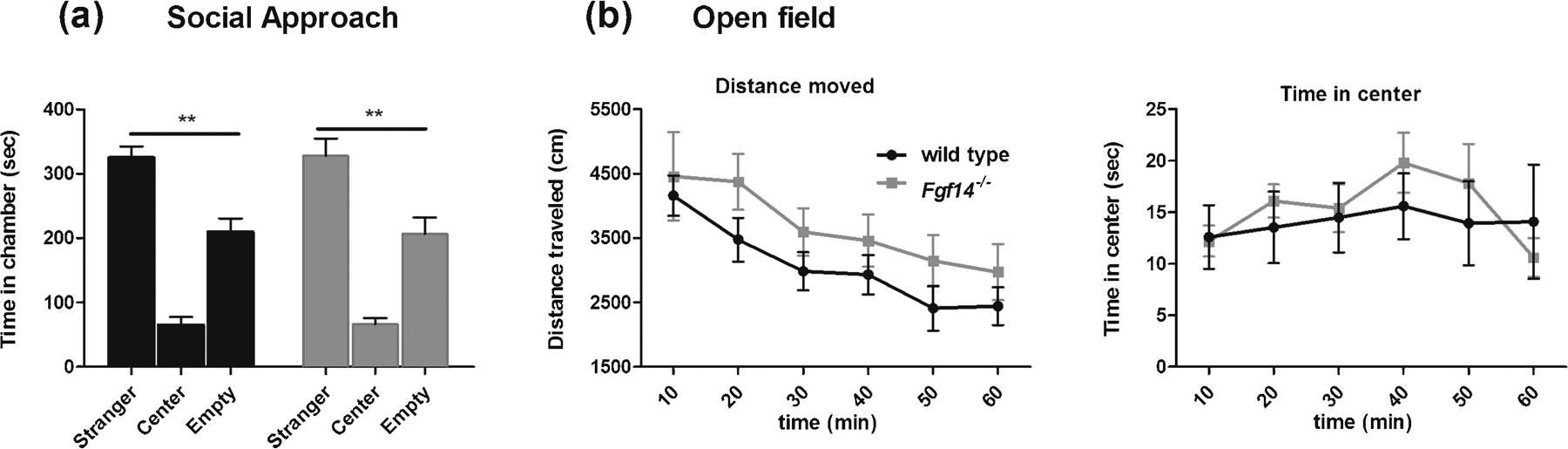
Fgf14−/− male mice show normal sociability, locomotor activity, exploratory behavior and time in center. (a) In the three chambers test both wild type and Fgf14−/− mice show a preference for a stranger mouse relative to an object (an empty wire cup identical to the one used to restrict the stranger mouse). (b) (left) In a novel open arena the locomotor activity related to the exploratory behavior and (right) the time in center are indistinguishable in Fgf14−/− relative to wild type mice. The bars represent mean ± SEM. ** p < 0.01.
3.6. Preserved locomotor activity and exploratory behavior of Fgf14−/− mice in the open field test
The reduction of some behaviors of Fgf14−/− mice, like aggressivity or sexual approach, might be due to a to a reduced locomotor activity or a lack of interest to explore a novel environment. To address this hypothesis, mice were subjected to the open field test. No significant differences were observed between genotypes for the distance traveled in the open field (two way ANOVA repeated measures, F(1, 15) = 0.2633, p = 0.62; Fig. 8B). Moreover, the distance traveled in the arena in the first 10 min was comparable between genotypes (Mann-Whitney test, u = 22, p = 0.20) indicating a normal exploratory behavior of Fgf14−/− mice in a new environment. The time spent on the center zone of the open field was comparable between genotypes (two way ANOVA repeated measures, F(1, 85) = 0.17, p = 0.68; Fig. 8B). These results demonstrate that the reduction of aggressive or sexual behavior is not linked to deficits in exploratory or locomotor activity.
3.7. Preserved dopamine receptors and transporter gene expression in Fgf14−/− mice
We quantified by real time RT-PCR the gene expression of the dopamine receptor D1 (Drd1), the dopamine receptor D2 (Drd2) and the dopamine reuptake transporter (Slc6a3) (Fig. 9) in the mPFC, Acb, and CPu, in wild type and Fgf14−/− mice. In the mPFC there was no significant difference between genotypes in mRNA levels for each gene analyzed (t-test, p > 0.05; Fig. 9A,D,G). Furthermore, the mRNA levels of Drd1, Drd2 and Slc6a3 did not change also for Acb and CPu in Fgf14−/− mice (t-test, p > 0.05; Fig. 9B,E,H and C,F,I respectively). These results demonstrate that the postsynaptic expression of receptors and transporters of the dopaminergic system is intact in Fgf14−/− mice, ruling out a possible major determinant of behavioral abnormality.
Fig. 9.
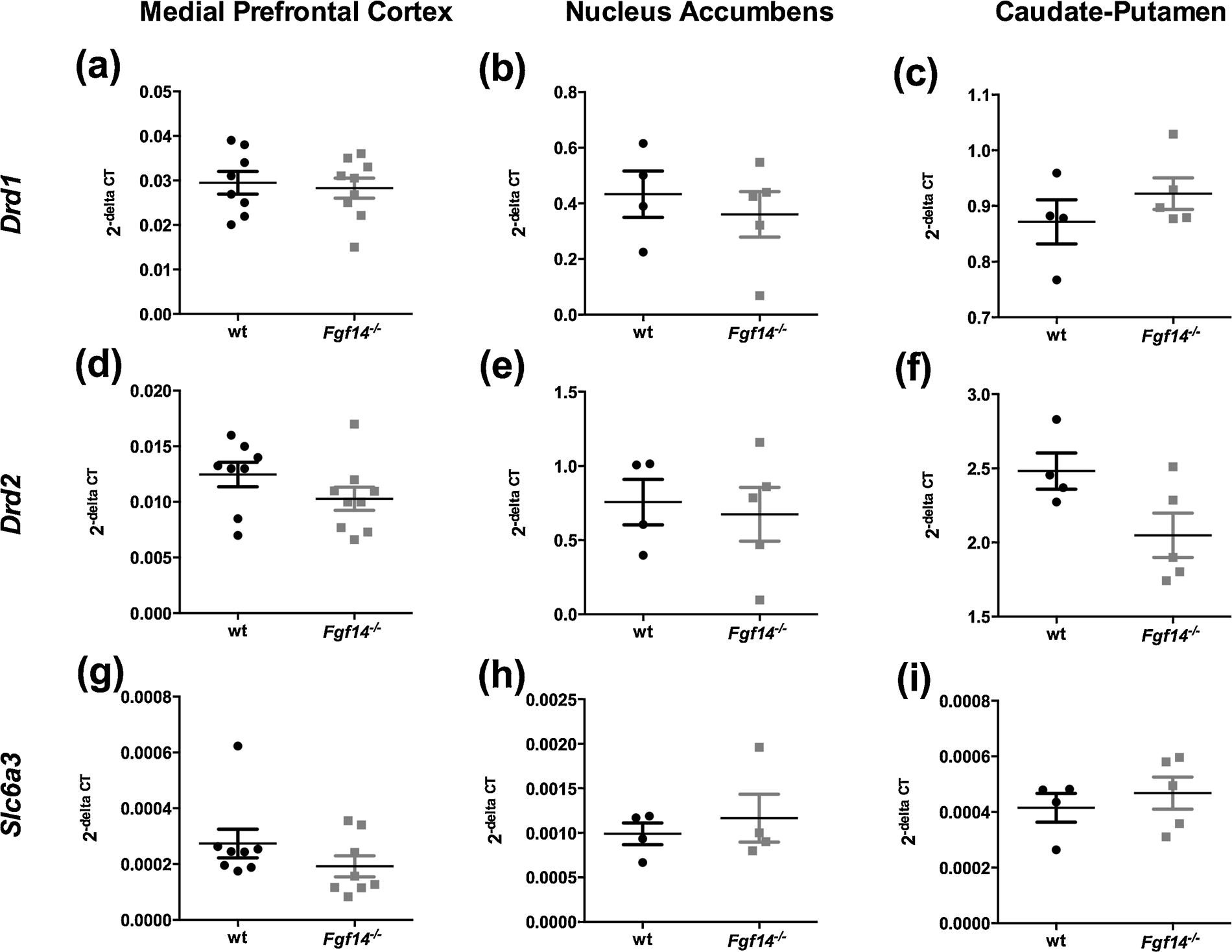
Drd1, Drd2 and Slc6a3 gene expression in the mPFC (a, d, g), Acb (b, e, h) and CPu (c, f, i) in wild type and Fgf14−/− mice. There were no significant changes for each gene expression in all the brain regions analyzed. Data are expressed as mean ± SEM.
4. Discussion
Previous reports on human mutations or variants of FGF14 indicate a correlation with aggressivity [33] and drug addiction [4,5,9]. Several studies on Fgf14−/− mice suggest that this gene might be involved in fine tuning of neuronal activity in circuits controlling spatial working memory and susceptibility to drug addiction [19–21]. The current study was performed to explore whether the lack of Fgf14 in mice is relevant for deficits in several behaviors ranging from aggressivity, to sexual behavior, sociability and spontaneous locomotion.
Our results confirm this hypothesis by showing that Fgf14−/− mice have a marked reduction of aggressivity and sexual behavior. The normal social interaction of Fgf14−/− with wild type mice suggests that the reduced level of aggressivity and sexual behavior is not due to deficits in social interaction between mice. Moreover, the normal levels of locomotor activity, observed in the open field test, also rule out the hypothesis that these deficits are caused by a reduced motor activity of mice in a novel environment. In addition, other behaviors driven by spontaneous initiative are also reduced in Fgf14−/− mice. These include burying novel objects and spontaneous digging and climbing. However, in the open field test, that evaluates the spontaneous locomotion in an arena, generally considered as a measure of the general level of activity and related to exploratory behavior [34], Fgf14−/− mice travel the same distance as control wild-type littermates. It is possible that the reduced number of marbles buried is due to a reduced propensity of Fgf14−/− mice to dig. Moreover, rearing, which is also related to exploratory activity [34], was more frequent in Fgf14−/− mice, indicating that the decrease was specific for behaviors like burying, digging and climbing. Rearing is also related to anxiety, because during this behavior the animal might be more vulnerable to predators [34]. However, Fgf14−/− male mice showed the same propensity to walk in the center as wild-type mice, suggesting that anxiety is not significantly affected. The different consequence of Fgf14 deletion on behaviors directed toward an object (burying, digging, climbing), relative to exploratory activity (locomotion, rearing), indicates that this gene specifically controls goal directed behaviors rather than unaimed, exploratory movements.
Our results show that Fgf14 is important for the control and modulation of sexual behavior, aggressivity and spontaneous behaviors toward targets like burying objects, digging and climbing. Regarding the mechanisms of these actions, it should be noted that Fgf14 controls neuronal excitability by modulating NaV channels by direct interaction [16,35,36] and neurotransmitter release by mechanisms, which are not yet completely clear [37,38]. Fgf14 increases neuronal excitability and action potential firing [35,36] with effects, which are NaV-subtype-specific [17]. Since each type of neuron expresses a specific repertoire of NaV channels, the modulation of membrane excitability by Fgf14 differs across neurons, with consequences, which are difficult to predict. For example, in the CA1 area of the hippocampus, Fgf14 deletion causes an alteration of inhibitory postsynaptic currents and a selective loss of some parvabumin-positive GABAergic interneurons. Similar alterations have been found in psychiatric disorders like schizophrenia [21]. Future studies are necessary to test whether similar alterations are also present in the brain regions involved in the behaviors, shown to be altered by this report. The modulation of neurotransmitter release by Fgf14 [37,38] adds further complexity to the control of signal processing in neuronal networks. Our finding that several types of behavior are impaired by the lack of Fgf14 opens a novel path in the field of investigation on the molecular mechanisms implied in sexual behavior, aggressivity and spontaneous behaviors such as burying, digging and climbing. This suggests that fine tuning of neuronal function by Fgf14 is an important mechanism of control for several behaviors in mice.
Funding
The research was funded by a grant of the University of Torino (Local Research Grant, FY 2015). There are no potential conflicts of interest to declare.
Abbreviations:
- Fgf14−/−mice
mice with a targeted deletion of the Fgf14 gene
- SEM
standard error of the mean
References
- [1].Hashikawa K, Hashikawa Y, Falkner A, Lin D, The neural circuits of mating and fighting in male mice, Curr. Opin. Neurobiol. 38 (2016) 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].van Swieten JC, Brusse E, de Graaf BM, Krieger E, van de Graaf R, de Koning I, Maat-Kievit A, Leegwater P, Dooijes D, Oostra BA, Heutink P, A mutation in the fibroblast growth factor 14 gene is associated with autosomal dominant cerebellar ataxia, Am. J. Hum. Genet. 72 (2003) 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brusse E, de Koning I, Maat-Kievit A, Oostra BA, Heutink P, van Swieten JC, Spinocerebellar ataxia associated with a mutation in the fibroblast growth factor 14 gene (SCA27): a new phenotype, Mov. Disord. 21 (2006) 396–401. [DOI] [PubMed] [Google Scholar]
- [4].Liu Q-R, Drgon T, Johnson C, Walther D, Hess J, Uhl GR, Addiction molecular genetics: 639,401 SNP whole genome association identifies many “cell adhesion” genes, Am. J. Med. Genet. Part B 141B (2006) 918–925. [DOI] [PubMed] [Google Scholar]
- [5].Drgon T, Johnson CA, Nino M, Drgonova J, Walther DM, Uhl GR, “Replicated” genome wide association for dependence on illegal substances: genomic regions identified by overlapping clusters of nominally positive SNPs, Am. J. Med. Genet. Part B 156 (2010) 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH, Modelling schizophrenia using human induced pluripotent stem cells, Nature 473 (2011) 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Verbeek EC, Bakker IM, Bevova MR, Bochdanovits Z, Rizzu P, Sondervan D, Willemsen G, de Geus EJ, Smit JH, Penninx BW, Boomsma DI, Hoogendijk WJ, Heutink P, A fine-mapping study of 7 top scoring genes from a GWAS for major depressive disorder, PLoS One 7 (2012) e37384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hunter AM, Leuchter AF, Power RA, Muthén B, McGrath PJ, Lewis CM, Cook IA, Garriock HA, McGuffin P, Uher R, Hamilton SP, A genome-wide association study of a sustained pattern of antidepressant response, J. Psychiatr. Res. 47 (2013) 1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Spencer JR, Darbyshire KM, Boucher AA, Kashem MA, Long LE, McGregor IS, Karl T, Arnold JC, Novel molecular changes induced by Nrg1 hypomorphism and Nrg1-cannabinoid interaction in adolescence: a hippocampal proteomic study in mice, Front. Cell. Neurosci. 7 (2013) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen HM, DeLong CJ, Bame M, Rajapakse I, Herron TJ, McInnis MG, O’Shea KS, Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients, Transl. Psychiatry 4 (2014) e375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Di Re J, Wadsworth PA, Laezza F, Intracellular fibroblast growth factor 14: emerging risk factor for brain disorders, Front. Cell. Neurosci. 11 (2017) 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ornitz DM, Itoh N, Fibroblast growth factors, Genome Biol. 2 (2001) REVIEWS3005.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu C, Dib-Hajj SD, Waxman SG, Fibroblast growth factor homologous factor 1B binds to the C terminus of the tetrodotoxin-resistant sodium channel rNav1.9a (NaN), J. Biol. Chem. 276 (2001) 18925–18933. [DOI] [PubMed] [Google Scholar]
- [14].Liu CJ, Dib-Hajj SD, Renganathan M, Cummins TR, Waxman SG, Modulation of the cardiac sodium channel Nav1.5 by fibroblast growth factor homologous factor 1B, J. Biol. Chem. 278 (2003) 1029–1036. [DOI] [PubMed] [Google Scholar]
- [15].Wittmack EK, Rush AM, Craner MJ, Goldfarb M, Waxman SG, Dib-Hajj SD, Fibroblast growth factor homologous factor 2B: association with Nav1.6 and selective colocalization at nodes of ranvier of dorsal root axons, J. Neurosci. 24 (2004) 6765–6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lou JY, Laezza F, Gerber BR, Xiao M, Yamada KA, Hartmann H, Craig AM, Nerbonne JM, Ornitz DM, Fibroblast growth factor 14 is an intracellular modulator of voltage-gated sodium channels, J. Physiol. 569 (2005) 179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Laezza F, Lampert A, Kozel MA, Gerber BR, Rush AM, Nerbonne JM, Waxman SG, Dib-Hajj SD, Ornitz DM, FGF14 N-terminal splice variants differentially modulate Nav1.2 and Nav1.6-encoded sodium channels, Mol. Cell. Neurosci. 42 (2009) 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang Q, McEwen DG, Ornitz DM, Subcellular and developmental expression of alternatively spliced forms of fibroblast growth factor 14, Mech. Dev. 90 (2000) 283–287. [DOI] [PubMed] [Google Scholar]
- [19].Wang Q, Bardgett ME, Wong M, Wozniak DF, Lou J, McNeil BD, Chen C, Nardi A, Reid DC, Yamada K, Ornitz DM, Ataxia and paroxysmal dyskinesia in mice lacking axonally transported FGF14, Neuron 35 (2002) 25–38. [DOI] [PubMed] [Google Scholar]
- [20].Wozniak DF, Xiao M, Xu L, Yamada KA, Ornitz DM, Impaired spatial learning and defective theta burst induced LTP in mice lacking fibroblast growth factor 14, Neurobiol. Dis. 26 (2007) 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alshammari TK, Alshammari MA, Nenov MN, Hoxha E, Cambiaghi M, Marcinno A, James TF, Singh P, Labate D, Li J, Meltzer HY, Sacchetti B, Tempia F, Laezza F, Genetic deletion of fibroblast growth factor 14 recapitulates phenotypic alterations underlying cognitive impairment associated with schizophrenia, Transl. Psychiatry 6 (2016) e806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Clifford JJ, Usiello A, Vallone D, Kinsella A, Borrelli E, Waddington JL, Topographical evaluation of behavioural phenotype in a line of mice with targeted gene deletion of the D2 dopamine receptor, Neuropharmacology 39 (2000) 382–390. [DOI] [PubMed] [Google Scholar]
- [23].D’Amato FR, Rizzi R, Moles A, A model of social stress in dominant mice: effects on sociosexual behaviour, Physiol. Behav. 73 (2001) 421–426. [DOI] [PubMed] [Google Scholar]
- [24].Egecioglu E, Prieto-Garcia L, Studer E, Westberg L, Jerlhag E, The role of ghrelin signalling for sexual behaviour in male mice, Addict. Biol. 21 (2016) 348–359. [DOI] [PubMed] [Google Scholar]
- [25].Studer E, Näslund J, Andersson E, Nilsson S, Westberg L, Eriksson E, Serotonin depletion-induced maladaptive aggression requires the presence of androgens, PLoS One 10 (2015) e0126462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yang M, Silverman JL, Crawley JN, Automated three-chambered social approach task for mice, Curr. Protoc. Neurosci. (2011), 10.1002/0471142301 Chapter 8, Unit 8.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hoeffer CA, Tang W, Wong H, Santillan A, Patterson RJ, Martinez LA, Tejada-Simon MV, Paylor R, Hamilton SL, Klann E, Removal of FKBP12 enhances mTOR-Raptor interactions, LTP, memory, and perseverative/repetitive behavior, Neuron 60 (2008) 832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Paxinos G, Franklin KBJ, The Mouse Brain in Stereotaxic Coordinates, Academic, New York, 2001. [Google Scholar]
- [29].Jacobs C, Van Den Broeck W, Simoens P, Increased volume and neuronal number of the basolateral nuclear group of the amygdaloid body in aggressive dogs, Behav. Brain Res. 170 (2006) 119–125. [DOI] [PubMed] [Google Scholar]
- [30].Pardini DA, Raine A, Erickson K, Loeber R, Lower amygdala volume in men is associated with childhood aggression, early psychopathic traits, and future violence, Biol. Psychiatry 75 (2014) 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Coccaro EF, Lee R, McCloskey M, Csernansky JG, Wang L, Morphometric analysis of amygdala and hippocampus shape in impulsively aggressive and healthy control subjects, J. Psychiatr. Res. 69 (2015) 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Thijssen S, Ringoot AP, Wildeboer A, Bakermans-Kranenburg MJ, El Marroun H, Hofman A, Jaddoe VW, Verhulst FC, Tiemeier H, van Ijzendoorn MH, White T, Brain morphology of childhood aggressive behavior: a multi-informant study in school-age children, Cogn. Affect. Behav. Neurosci. 15 (2015) 564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang-James Y, Faraone SV, Genetic architecture for human aggression: a study of gene-phenotype relationship in OMIM, Am. J. Med. Genet. Part B 171 (2016) 641–649. [DOI] [PubMed] [Google Scholar]
- [34].Crusio WE, Genetic dissection of mouse exploratory behaviour, Behav. Brain Res. 125 (2001) 127–132. [DOI] [PubMed] [Google Scholar]
- [35].Goldfarb M, Schoorlemmer J, Williams A, Diwakar S, Wang Q, Huang X, Giza J, Tchetchik D, Kelley K, Vega A, Matthews G, Rossi P, Ornitz DM, D’Angelo E, Fibroblast growth factor homologous factors control neuronal excitability through modulation of voltage-gated sodium channels, Neuron 55 (2007) 449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Laezza F, Gerber BR, Lou JY, Kozel MA, Hartman H, Craig AM, Ornitz DM, Nerbonne JM, The FGF14F145S mutation disrupts the interaction of FGF14 with voltage-gated Na+ channels and impairs neuronal excitability, J. Neurosci. 27 (2007) 12033–12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yan H, Pablo JL, Pitt GS, FGF14 regulates presynaptic Ca2+ channels and synaptic transmission, Cell Rep. 4 (2013) 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tempia F, Hoxha E, Negro G, Alshammari MA, Alshammari TK, Panova-Elektronova N, Laezza F, Parallel fiber to Purkinje cell synaptic impairment in a mouse model of spinocerebellar ataxia type 27, Front. Cell. Neurosci. 9 (2015) 205. [DOI] [PMC free article] [PubMed] [Google Scholar]


