Abstract
The breadth and complexity of natural behaviors inspires awe. Understanding how our perceptions, actions, and internal thoughts arise from evolved circuits in the brain has motivated neuroscientists for generations. Researchers have traditionally approached this question by focusing on stereotyped behaviors, either natural or trained, in a limited number of model species. This approach has allowed for the isolation and systematic study of specific brain operations, which has greatly advanced our understanding of the circuits involved. At the same time, the emphasis on experimental reductionism has left most aspects of the natural behaviors that have shaped the evolution of the brain largely unexplored. However, emerging technologies and analytical tools make it possible to comprehensively link natural behaviors to neural activity across a broad range of ethological contexts and time scales, heralding new modes of neuroscience focused on natural behaviors. Here we describe a three-part roadmap that aims to leverage the wealth of behaviors in their naturally occurring distributions, linking their variance with that of underlying neural processes to understand how the brain is able to successfully navigate the everyday challenges of animals’ social and ecological landscapes. To achieve this aim, experimenters must harness one challenge faced by all neurobiological systems, namely variability, in order to gain new insights into the language of the brain.
In Brief:
Miller et al. argue that natural behavior can serve as a powerful lens into the full range of computations that brains perform by leveraging the dynamic covariance between behavior and neural activity.
Introduction.
Whereas the heart functions to pump blood through the circulatory system and the lungs to extract oxygen from the air around us, the nervous systems function to support an animal’s active participation in the world. Nervous systems have been shaped evolutionarily across species to successfully govern how individuals of a species navigate their unique ecological and social landscapes; a relationship elegantly articulated by Briscoe and Ragsdale1:“The evolution of vertebrate nervous systems is in large part the evolution of neural circuitry — whether through qualitative changes to circuit construction, increase or reduction of neuronal number at particular nodes, or through the reorganization of circuit components in space — with behavior as the target of selection, made manifest through neuronal activity”. Brains are not general-purpose machines: the cells, circuits, and areas of the brain have been collectively optimized to support behaviors in species’ repertoires.
Recent technological advances in the neurosciences have pushed the envelope in our understanding of the intricate relationship that exists between neural systems and behavior2,3. Technological development is necessary: without measuring specific dimensions of behavior or nervous system function, we are running blind. But an appreciation of these newly available data is tempered by the realization that tools alone will not illuminate the mysteries of the brain on their own4, no matter how ‘big’ the data. To meaningfully interpret such considerable datasets, integrating these diverse empirical observations into a higher-order understanding of complex systems mandates a solid theoretical foundation. If we are to understand brains as the dynamic biological systems they are, theory firmly rooted in behavior — the target of evolutionary selection — offers the most fertile ground for discovery of the brain’s computational principles, the generalizable neural dynamics that underlie if, when and how natural behaviors manifest across different contexts, including both the degree of plasticity and mechanistic constraints that shape the distributions of potential behavioral outcomes.
To address this considerable challenge, we propose a conceptual merger between two neuroscience sub-disciplines — systems neuroscience and neuroethology — that have at times been viewed as at odds but that are in fact complementary, each with its own theoretical advantages5,6. These disciplines are distinguished in large part by their distinct perspectives on the role of behavior to elucidate facets of brain function. Systems neuroscience has tended towards reductionistic paradigms designed to control behavior through conditioning7–9. From this perspective, behavior is viewed as one of many available lenses through which to view how brains work, with neural processes taking primacy. In contrast, neuroethology has focused on naturally occurring behaviors in a species’ repertoire, biasing towards highly specialized behaviors that result from intense evolutionary selection, such as survival and mate attraction10–13. For neuroethologists, natural behaviors are typically the anchor point for inquiry into brain function. While both frameworks have been powerful engines of discovery for how brains work, each has in its own way skewed our understanding of neural systems by focusing on stereotyped behaviors that have as little variability as possible. This focus follows to generate the large numbers of identical or nearly identical trials or behavioral events to gain repeatability and statistical power for traditional forms of data analysis. Whether isolated behaviors of the type studied in the laboratory are representative ‘models’ of all behaviors and brain function is debatable14. In fact, such stereotyped behaviors reflect only a small fraction of the behavioral repertoire (Figure 1). In other words, even for the most well studied species, we know embarrassingly little about how brains support what animals are doing most of their day.
Figure 1. Animals have remarkably diverse behavioral repertoires.
The stereotyped conditioned and specialized behaviors that are typically studied in systems neuroscience and neuroethology are a small part of the range of behaviors they produce, many of which are shared across species (social interaction, predator avoidance, prey capture, and so on) but for which we know very little about the underlying neural mechanisms. For example, with the exception of spatial representations in the medial temporal lobe first pioneered by O’Keefe15–19, relatively few data are available that detail the effects of navigation and exploration on perceptual and cognitive functions20–22 despite the fact that the ability to move through space has both been a foundational pressure on brain evolution and routinely accounts for large portions of daily activity in all animals. Similarly, the neuroscience of the song learning behavior in oscines has been studied extensively generating unique insights into sequence motor learning but has taught us very little about brain mechanisms involved in natural vocal exchanges23. Spatial exploration and natural communication behaviors, however, are highly variable, making them difficult to study in conventional frameworks.
How can neuroscientists interested in natural modes of brain function gain traction in understanding behaviors that are not isolated and stereotyped? How can we study the neural mechanisms underlying dynamic, variable, and infrequent behaviors? One important factor is to reconsider what constitutes a good or valid neuroscience experiment. The academic view of the scientific method emphasizes the virtue of high statistical repeatability and restricted outcomes for the pursuit of well-formed hypotheses. While this formulaic mode of scientific investigation has great value for many problems, it is not the only path to scientific inquiry, and it may not be well suited for studying many elements of natural behavior. In neurobiology, mechanistic hypotheses about brain circuits are often constrained by the narrow potential outcomes of a particular experimental paradigm. However, the long-term value of pursuing such hypotheses must ultimately be linked to an animal’s natural behavioral repertoire, including when that animal is a human. Thus, the distinct challenges posed by studying brain activity in animals undertaking natural behaviors are at least partially offset by the knowledge that the brain is operating within its normal range; the motivations for behaviors to manifest when they do are centered in the challenges the brain evolved to solve, rather than the experimentally compelled motivations like satiating thirst or avoiding pain that typify traditional neuroscientific approaches. Given the influence of an animal’s state on neural processes and behaviors24–28, such differences in behavioral motivation are likely to have profound effects on brain activity.
We consider that while natural behaviors include the range of observable actions that manifest in the daily lives of animals, behavior is not limited to motor actions. Instead, we endorse a more holistic view of behavior comprising events and processes that occur between actions that include — but are not limited to — sleep, decision-making (including the decision to suppress a behavior), planning, and related internal states of the individual. This view emerges from an appreciation of the fact that natural behaviors occur along overlapping short (millisecond, second) and long (circadian, lifecycle) time scales, each impacting neural computations and processes independently and collectively.
In this review, we present a three-part roadmap aimed at addressing the challenges of studying natural behaviors and brains. We champion using the full distribution of behavioral and neural data — rather than their central tendency — to evaluate the neuroscience of natural behaviors. The concept of distributions, or variance, as primary targets of experimental inquiry is a foundational tenet of our roadmap. We use the term variance broadly, to encompass different outcomes (behavioral output, neural activity, and so on) that occur within ostensibly similar conditions, such as repeated events, as well as across a range of contexts that often remain unexplored. We argue that keystone principles of brain function can only be discovered when animals execute dynamic behavioral sequences, whose details are subject to variation within a range of natural parameters. After all, variance is an inherent feature of all biological systems. Variability not only distinguishes biology from other sciences29, but it is one of the cornerstones of evolution by natural selection. Ultimately, the full mapping of covariance between the distributions of behaviors and brains is a key first step towards elucidating the core principles of neural computations that govern the breadth of behaviors in a repertoire.
Hence, rather than continue the tradition of neuroscience to curtail variability, our first challenge here is to embrace the distributions of the natural modes of behavior. Our second challenge addresses the experimental feasibility of an approach focused on dynamic, natural behaviors that we champion here. In this section, we describe how neural and behavioral recording advances have set the stage to optimize experimental designs that balance considerations of variance and control. The final challenge is one of interpretability. The recent step increase in the quality of data collection methods does not, by itself, guarantee a deeper understanding of brain function. New conceptual insights into the multi-dimensional relationship between complex brain signals and behaviors, and its bearing on core principles of brain function, require that scientists develop methods to harness the variance inherent to such rich datasets. For this, biological observations of behaviors and brains must be integrated into new theoretical frameworks that provide hitherto unseen perspectives on neural computations. Natural behaviors are not simply a useful source of variance. Natural behaviors encompass the map of possible neural computations that we seek to understand in neuroscience - how we interact with and navigate the world around us.
Importantly, here we distinguish between neural computations — processes in the brain itself, the representation and transformation of external and internal variables that govern behaviors — and computational methods — the analysis approaches that neuroscientists apply to characterize our observations and measurements of processes in the brain. Our ambition to develop theoretical principles from analysis approaches that fully capture neural computations within natural behavior remains the primary goal, but there are few, if any, convincing examples at present. A key motivation for the roadmap described here is to outline a path to that end.
Challenge 1: embrace the diversity of natural behaviors
Every action taken by an animal is unique. The Greek philosopher Heraclitus captured this principle in his famous adage, “No man ever steps in the same river twice, for it is not the same river and he is not the same man”. Thus, the brain is designed to achieve its goals amid an ever-changing set of circumstances. Nonetheless, neuroscience has traditionally sought to recreate, as precisely as possible, actions and behavioral conditions for the benefit of precise repeatability. This understandable pursuit has resulted in our view of the brain being filtered through a ‘mean/median’ framework, in which we record an event multiple times, averaging neural activity across those ‘trials/events’ and consider the mean/mode/median as ‘representative’. In fact, experimental approaches have often gone to great lengths to reduce the variance to focus the scope of inquiry to this central tendency outcome.
This traditional conceptual framework, which encapsulates many, though of course not all approaches to analyzing brain function, puts aside the important fact that variation in both the environmental context and the execution of a behavior is a core feature of brain function. Brains, however, must operate in a graded, analog fashion that continually adjusts actions and expectations with respect to the outside world. This natural variation should not be construed as noise against which the brain must contend, but rather the basic parameters of its operation. Harnessing the natural distribution of natural behaviors to study the brain in action — rather than focusing only on the mean/median — requires a tactical shift with new practical and theoretical considerations. This includes an appreciation of low-frequency neural or behavioral events as meaningful parts of the distribution, rather than as outliers that can be statistically ignored. Foremost is the capacity to measure and quantify the diverse modes of behavioral variance that mark natural experience across the full range that behaviors manifest.
Each instance of a given behavioral action is different because of changing environmental conditions30–32, ecological contexts (such as the presence or absence of particular conspecifics or predators12,33–36), internal cognitive states37–39, and a range of other factors40,41. One important source of variance stems from the observation that behaviors often occur within a continuous sequence, rather than as discrete events42. A lioness hunt is not only the attack, but a series of complementary actions including visually tracking, stalking, hiding and positioning, typically in coordination with group members43. Thus the significance of a given behavioral component, and the neural mechanisms supporting it, can depend on what came immediately before. This ubiquitous principle provides one avenue for dissecting contextual versus invariant neural contributions to a given behavior. It is exemplified in classic studies of human speech production, where the articulation of a word depends strongly on the preceding and subsequent syllables44,45(Figure 2).
Figure 2. Context-dependent variability of behavior, demonstrated by co-articulation of speech.
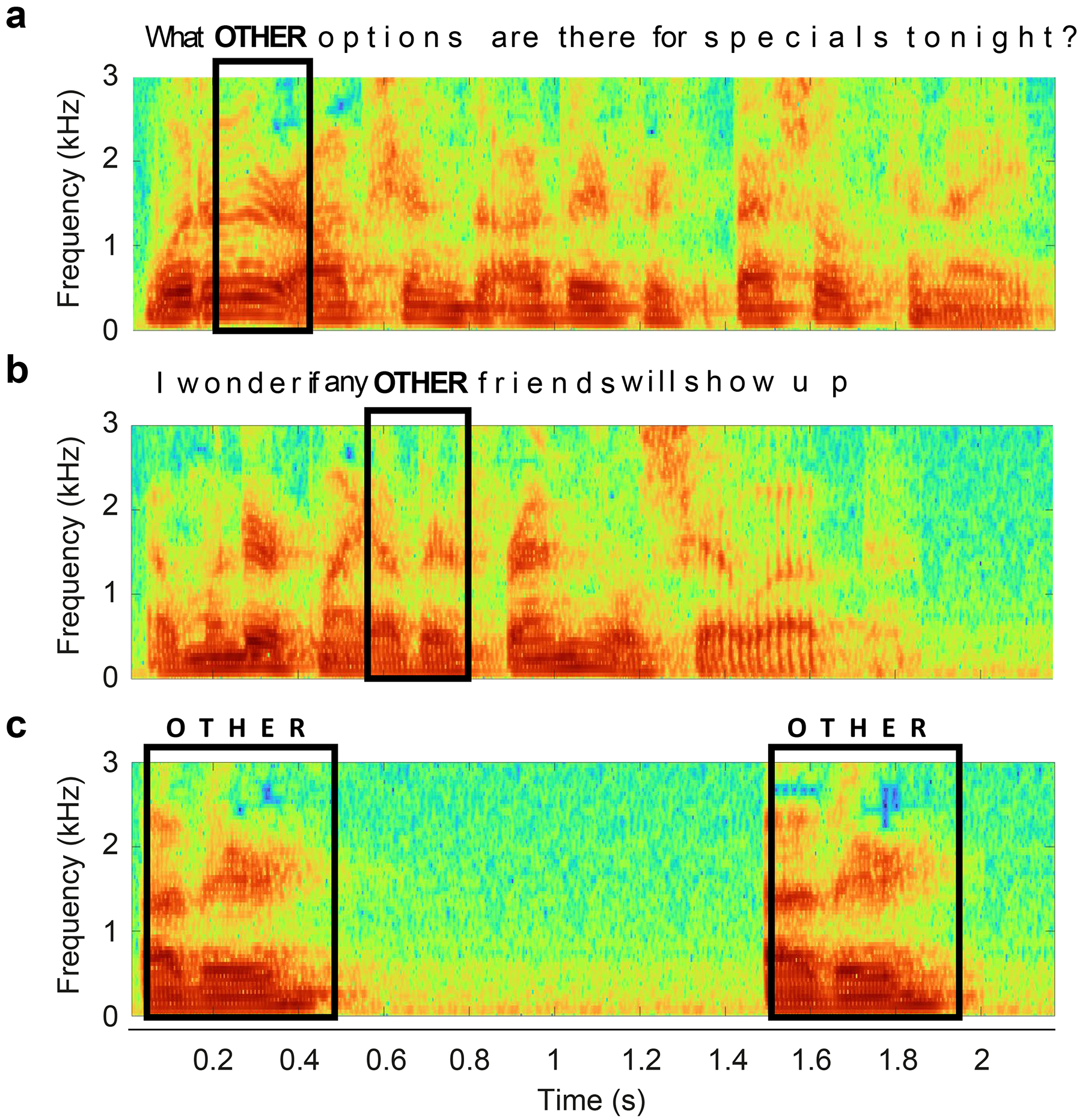
The formant frequency representation for single words (‘other’) can change dramatically in different sentences (A,B) and be distinct when spoken in isolation (C). This context dependence, and the contrast between natural context and repeated trials in isolation, is also mirrored in the sensory domain. For example, in vision an edge at a given orientation can appear in many different visual contexts, and such stimuli within natural scenes evoke different responses than the standard repeated presentation of a similar stimulus in isolation55,56.
The neural processes that generate natural behaviors must coordinate over both short and long time scales to accommodate sequential behavioral actions. Behavioral exchanges, and their underlying neural mechanisms, typically transpire over several seconds or longer. For example, the activity of frontal cortex neurons in marmosets prior to hearing a conspecific vocalization almost perfectly predicted whether the monkeys would engage in a conversational exchange, despite the fact that call response occurred up to 10 seconds after hearing a conspecific vocalization46. In other words, variance in the state of the neural population at the time an ‘event’ occurred (conspecific vocalization was heard) was a key source of variance in the probability of the subsequent social behavior several seconds later. Although experimental conventions focus analyses to select time periods at the time of a key event, designing tasks that involve extended time windows — including different sequences that comprise the same behavior — and the variance that emerges in these epochs can be harnessed to illuminate facets of brain activity that can at times have profound effects on behavior47. An important challenge will be determining how to define the windows of analysis, from the millisecond timescale of spiking activity, up to seconds for individual actions, and hours or days for state variability.
In addition to the retrospective dependency emphasized above, certain events in a sequence can have prospective consequences. The inclusion of a particular event, such as a goal-oriented decision, can be a key pivot point that marks a divergence of the subsequent sequence’s trajectory48. Decision-making illustrates this point as it is traditionally framed as the endpoint after weighing multiple options, a fact reflected in the various experimental paradigms conventionally used to address this issue9,49–52. Under natural conditions, however, decision-making more closely resembles a continuous, ongoing process. Decisions are made constantly, ranging from the choice to not walk into a wall to strategizing about climbing the social hierarchy. These types of decisions, and their effect on what follows, are not well captured in laboratory tests of decision-making that are most often discrete and dichotomous by nature53.
In natural behavior, choices are often expressed on a graded scale and at a chosen point in a continuous sequence. This principle is obvious in navigation, where local decisions in space and time often alter the course of what follows54. Moreover, each iteration of a certain kind of choice may have a different consequence. For example, a foraging bat, upon reaching a second feeding tree, may be more satiated and choose to socialize before consuming food. Thus, the way in which decision-making shapes the continuous flow of experience can provide natural experimental sources of variance for studying the neural principles underlying natural behavior.
Challenge 2: optimize experimental design for natural distributions.
From a practical perspective, addressing the points above requires significant changes in the acquisition, analysis, and conceptualization of data, particularly with respect to experimental design. Foremost is the continuous collection of behavioral and physiological data. Behavioral, neural, autonomic, and other signals must be acquired with sufficient temporal resolution to match the temporal dynamics of the behavioral motifs being investigated. At the same time, acquisition often needs to remain continuous over a period long enough to capture rare events and to study slower physiological processes. This contrast to trial-based paradigms reflects the desire to sample physiological activity during the broad distribution of natural behaviors. These requirements, combined with the desire to simultaneously sample a breadth of neural and other signals, place high demands on data recording, storage, and transmission. Fortunately, recent technological advances for data acquisition allow us to meet this challenge. For the quantification of behavior, machine learning approaches allow quick and continuous assessment of myriad behavioral variables. For physiological recordings, large-scale electrophysiological arrays and multiscale neuroimaging approaches allow for the simultaneous monitoring of hundreds, and sometimes thousands, of neurons in real time.
Here we discuss the bearing that these advances are beginning to have on experimental design. Importantly, this does not just simply mean acquiring larger quantities of data, but matching experimental design to the distributions that are naturally relevant: for example, sensory stimuli that represent the range an animal experiences in the real world, behavioral tasks that allow the broad repertoire of actions an animal can take, and recordings that capture the full diversity of neural cell types, response properties, and dynamics that underlie each of these.
New types of data — behavior
Tapping into the full richness of behavior will be facilitated by incorporating several key factors that have been traditionally eliminated in behavioral paradigms. First, studying natural behaviors that lack traditionally stereotyped characteristics, such as being innate and highly repeatable, or learned and over-trained, will allow exploration of the full space of possible mapping between neural activity and behavior. Second, task designs that have a continuous readout of behavior, rather than a single decision point, will both capture the richness of true behavior, which rarely has an isolated decision, and greatly expand the volume of data that can be acquired in a single session, which is necessary for many recent ‘data-hungry’ analysis methods. And last, natural behaviors take into account each species’ unique view of the world, its umwelt57, and what it is offered by that world, its affordances58 — with an ethological correspondence between the task variables and behavioral output, ensuring that meaningful computations are being studied.
Whenever constraints on the behavior are released, new phenomena are likely to be observed. Although the benefits may seem apparent, a combination of technical limitations and sociological issues have historically hampered researchers from taking such unconstrained approaches to behavior. Nonetheless, pioneering studies showed the richness of sensory processing during natural behavior59,60. More recently, it was found that simply allowing a mouse to run in place on a treadmill, rather than constraining it to sit, results in striking changes in visual and auditory processing that have expanded our view of cortical function20,61,62. These examples also emphasize the importance of active sensing63–65: in the real world animals determine how they acquire sensory information, as opposed to laboratory paradigms where the experimenter is in charge. Allowing naturalistic, active sensing therefore has the potential to introduce tremendous variability in both the sensory input and motor output, with concordant technical challenges. This also reflects the domain in which sensory systems are optimized to function22 which includes ongoing movements and time-varying sensory stimuli.
While one potential approach to incorporating natural behavior is to move on entirely from current reductionist paradigms, releasing all constraints and allowing an animal to behave freely, there are also more step-wise, transitional approaches available. For example, studies of active vision can begin with free viewing within a head-fixed paradigm66, or two-alternative forced choice paradigms can be modified to include richer stimuli and naturalistic response actions. Likewise, the study of natural scene statistics itself provides context for understanding the types of coding schemes the visual scene may employ67, although the utility of the natural scenes approach in probing vision experimentally has been debated68,69. As a further step, closed-loop experimental paradigms, which determine the timing and occurrence of stimuli and/or rewards based on a subject’s ongoing behavior, offer a path to balancing experimental control with the continuous nature of ethological behaviors70. One notable concern with such step-wise approaches, however, is whether each iterative step away from traditional reductionistic paradigms will eventually lead to truly natural behavior, or whether distinct challenges will emerge at each step limiting progress to this eventual goal. Thus, both the transitional approach of incorporating ethological components into current paradigms and the direct approach of pursuing purely natural behaviors are certain to be informative and challenging71.
New types of data — recording brain signals
Measurements of neural activity offer a notably narrow window into brain function. With very few exceptions, a nearly infinitesimal subset of the brain’s neurons is recorded over a brief period of time in the animal’s life, generally minutes to hours. From these small subsamples, we hope to infer a general population code for brain function. Traditionally, the solution to this has been to narrow down the subject of inquiry and corresponding experimental paradigm to match this window with the neural activity. In restricting visual processing to motion of moving dots, for example, neural recordings are often then limited to areas associated with motion processing (such as visual area MT) during a two-alternative forced choice motion discrimination task, and further restricted by matching the specific stimulus parameters to a subset of neurons in that particular recording session. As discussed above, however, brains rarely operate in such an isolated regime, and therefore as we increase the complexity of the behavioral context, we must broaden this window so that the dimensionality of our neural data matches the dimensionality of the behavior or computations under investigation.
Increasing the throughput of recordings, in terms of number of simultaneous neurons as well as the neural populations — for example, cell types, circuits and brain regions — from which these neurons are sampled, ensures that there is a large and diverse enough population to represent the full behavioral or sensory repertoire being presented. Increasing the duration of recordings, whether over hours or days, not only increases the total data, but also allows sampling across multiple behavioral contexts and examination of variability over time (Figure 3). Some of these methods, such as two-photon calcium imaging, also allow estimation of coverage — the fraction of cells within a population that are responding in a given context — as opposed to methods such as single tungsten electrodes that will miss inactive neurons, leading to a ‘dark matter’ conundrum74.
Figure 3. Behavioral and neural signatures of variability.
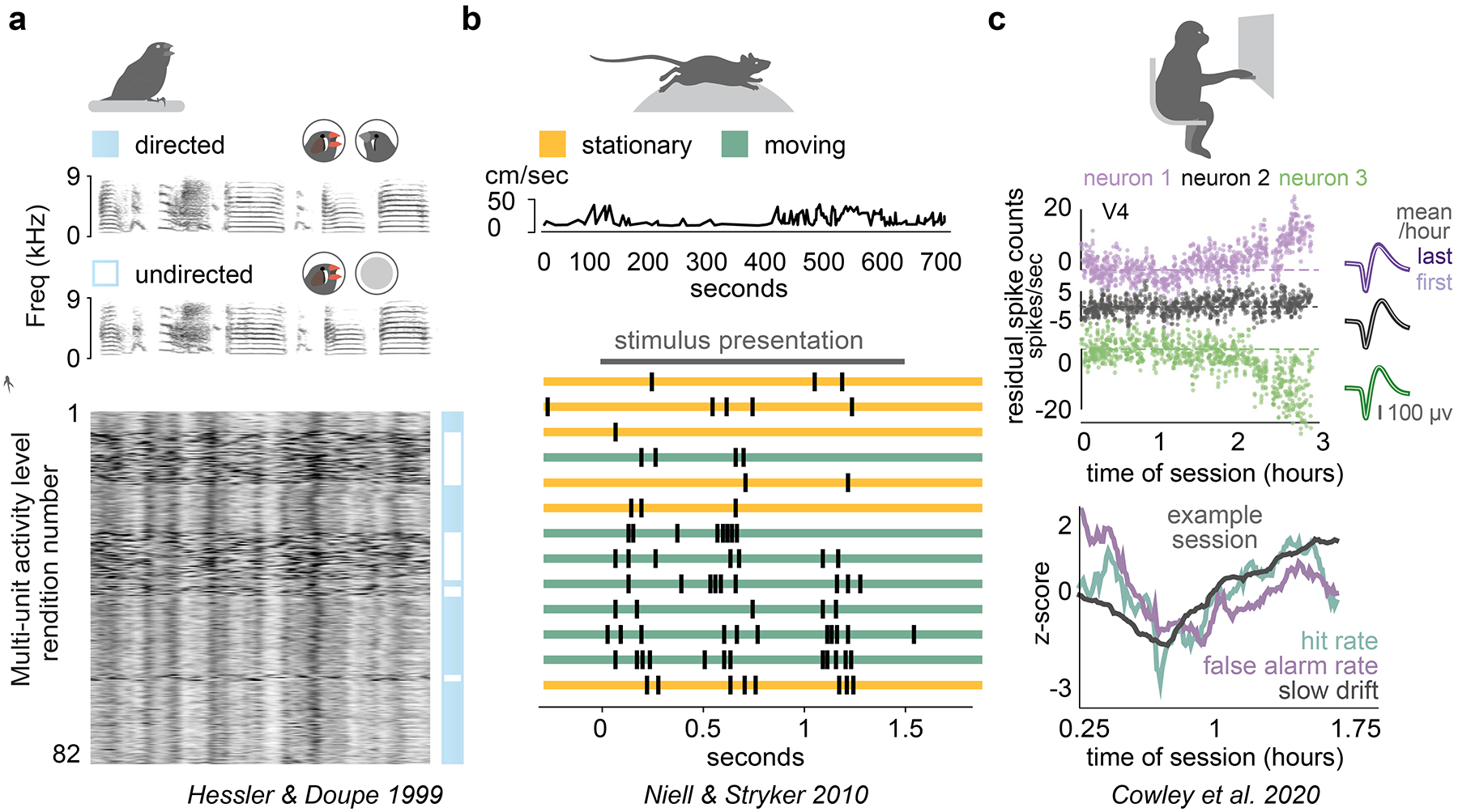
. (A) Zebrafinches sing to conspecifics of the opposite sex (directed song) as well as in isolation (non-directed song). While only small acoustic differences are evident between these contexts, the underlying neural activity shows striking variability72. Adapted with permission from Hessler and Doupe72. (B) Head-fixed mice on a spherical treadmill spontaneously alternate between a stationary state and locomotion. Responses to identical visual stimuli result in approximately two-times greater response when the mouse is running. Without quantification of behavioral state, this would appear as unexplained variance20,72. Adapted with permission from Niell and Stryker20. (C) In recordings of monkeys performing a visual task over several hours, task performance and arousal (as measured by pupil diameter) vary slowly. This is accompanied by a slow drift in the first principal component of neural activity, evident at the population level73. Adapted with permission from Cowley et al.73.
One example of the insights that can be gained from such an approach is the elucidation of a ‘slow drift’ signal in macaque V4 and PFC73, which found that activity distributed across populations of neurons in these areas was modulated over the course of minutes and hours during performance of visual attention task (Figure 3C). The amplitude of this spontaneous modulation was up to five-times larger than the effect of attention that was the basis of the task design, and co-varied with behavioral performance, thus revealing a large-scale determinant of brain activity and behavior that would not be detected if averaging over trials or recording a single neuron at a time. Furthermore, this study in primates parallels findings on the effects of behavioral state, particularly locomotion and arousal, across a range of model species from flies to mice20,75–78.
Challenge 3: analyses to harness the variance.
In the section above, we have proposed dramatically expanding the distribution of experimental data across many dimensions — but how then do we extract interpretable findings? In addition to enabling the acquisition of more diverse data, recent advances will hopefully yield a richer understanding of the data, with data analysis methods and models that consider more than tuning curves or choice probabilities to allow a full mapping between neural activity and behavior. These analysis techniques offer powerful approaches that both distill the complexity of natural brain function into cogent models while still capturing the richness of these dynamic datasets. Ultimately, this delicate balance is key to harnessing the variance inherent to the natural brain and elucidating the fundamental principles that underlie how countless species successfully navigate the world. Given the need to account for the many sources of variance that act on different levels of the brain–behavior interactions, answers to these questions in this framework need not resemble current descriptions of neural circuits that attempt to assign a specific computational role to individual neuron classes in highly constrained contexts.
Quantifying behavior
Over the past several years, machine-learning based approaches to quantifying behavior through video analysis, such as DeepLabCut, and LEAP79,80, and acoustic analysis81,82, have rapidly become pervasive in neuroscience. The tremendous strength of these approaches is that they allow easy quantification of many kinematic variables during an animal’s action. This contrasts with previous approaches, which required either reducing measurements down to one or a few task variables (binary choice, reaction time); laborious manual or semi-automated tracking of a small number of body points; or categorical, often qualitative, assessment into an ethogram. Hence, these new methods improve our ability to capture the true variance of natural behavior.
But this wealth of data raises the question of how to effectively make use of them for subsequent analysis. After all, although these behavioral quantification tools have dramatically increased our ability to annotate behavior, the path to translating these data into novel insight is not yet clear. One route might be to directly relate these measures to neural activity. However, raw kinematics are generally not the parameter space that is directly relevant for behavioral analysis. Instead, a number of methods have been developed that map raw kinematics into behavioral motifs or ethograms that are both more directly related to brain function and, ideally, more interpretable83–85 for researchers. In addition to providing labels for behaviors that can be correlated with neural activity, the transitions between these states may provide a way to divide continuous behavior into ‘decision points’ that allow current analysis methods to be applied.
Is this high-throughput method of annotation just a way of avoiding the labor of manual tracking / ethogram coding or will there be fundamental new insights emerge? Achieving this may depend on incorporating this rich ‘annotation’ into models that will allow fundamental principles of behavior to be derived that might not result from more limited datasets, such as those that may arise from more classical ethological treatments. For example, Calhoun et al.37 used a combined GLM–HMM (Generalized Linear Model–Hidden Markov Model) to determine latent states underlying shifts in Drosophila courtship song behavior. Depending on state, the same feedback cues from a conspecific elicit different distributions of behavioral output in terms of song production. Notably, this approach allowed identification of a specific cell type associated with one of the latent states rather than with the behavioral outputs. B-SOiD (Behavioral Segmentation of Open-field In DeepLabCut), likewise, provides a dimensionality reduction method for clustering behaviors and sequences of behaviors that might not be evident using more traditional ethological approaches86.
Importantly, these types of modeling are not the standard post hoc approaches often used to recapitulate experimental findings. Rather, in these approaches the model itself is an analysis tool that casts large-scale and complex data into interpretable terms, which can then be used to directly interrogate the system further through manipulation of the behavior or the brain. Translating these types of insight about specific behaviors into more broad theoretical frameworks that can provide a ‘first principles’ understanding of behavior is likely to remain a challenge for some time.
Analyzing neural activity
Current methods for recording neural activity during behavior generate a tremendous amount of data — countless individual spikes in many neurons across the duration of recording. As with behavioral data, the challenge is utilizing all these neural data87. What types of analyses are needed to extract the meaning from large-scale neural recordings? Traditional analyses often average across trials to obtain the mean response as a function of one or a few parameterized variables (stimulus orientation or frequency, task response). Similarly, in studying populations of neurons, traditional analyses often compute responses of neurons individually or after collapsing the data into pairwise interactions.
There are examples of classic studies that incorporate more sophisticated analyses, but a recent proliferation of advanced quantitative methods is now primed to make them more commonplace across systems neuroscience. Many of these approaches, such as PCA (Principal Components Analysis), non-linear manifold embedding and more generally data-driven statistical regularization techniques, involve dimensionality reduction: finding patterns in the raw high dimensional data that allows it to be represented by a much smaller number of variables87–90.
In contrast, other approaches, such as Canonical Component Analysis, directly interrogate the relationship between brain activity and behavior91. This has multiple potential advantages, including facilitating single trial analysis because multiple neurons provide more robust signal than individual neurons, incorporating data over groups of correlated neurons rather than examining individuals, and potentially leading to a set of interpretable factors that can be related to aspects of behavior and brain state.
The power of dimensionality reduction approaches in revealing principles of neural coding range from rotational dynamics in motor cortex92 to the temporal evolution of olfactory coding93, and across species from worms94 to primates95. These approaches can also reveal primary determinants of neural activity that may not be directly task related, such as the ‘slow drift’ described above and the impact of spontaneous movement on cortical dynamics96,97. Finally, these approaches are likely to be essential in determining how downstream regions use this information, as demonstrated by studies of the ability to decode visual stimulus properties from large populations of V1 neurons98,99.
Analysis at the level of single trials, rather than averaged responses across multiple trials, is particularly valuable in the context of natural behavioral and variability, as the exact same behavior is never repeated twice. A number of methods have been developed to facilitate this approach100, including analysis of second-order statistics (such as power spectra) that can be combined even when individual time series cannot be aligned101, point process models of individual neurons102, and GLMs (Generalized Linear Models) and related techniques that allow incorporation of multiple variables into the responses of populations of neurons103,104. Single trial analysis thus allows accounting for the unique combination of stimulus parameters, contextual and state variables, and motor output on a given trial (Figure 4). Furthermore, even in cases when the behavior itself is relatively reproducible, as in a two-alternative forced choice task, the underlying neural dynamics may have variability that is lost or even corrupted by focusing on the mean response. For example, while averaging activity of neurons across multiple trials of an information integration task shows gradual ramping activity, analysis at the level of single trials suggests that individual trials show discrete steps of activity at variable times, which then average out to a ramp over time105. Developing statistical approaches that allow rigorous assessment of rare or singular events that make up the long tail of variance in natural behavior remains a key challenge.
Figure 4. Pathways from natural behaviors to neural computations.
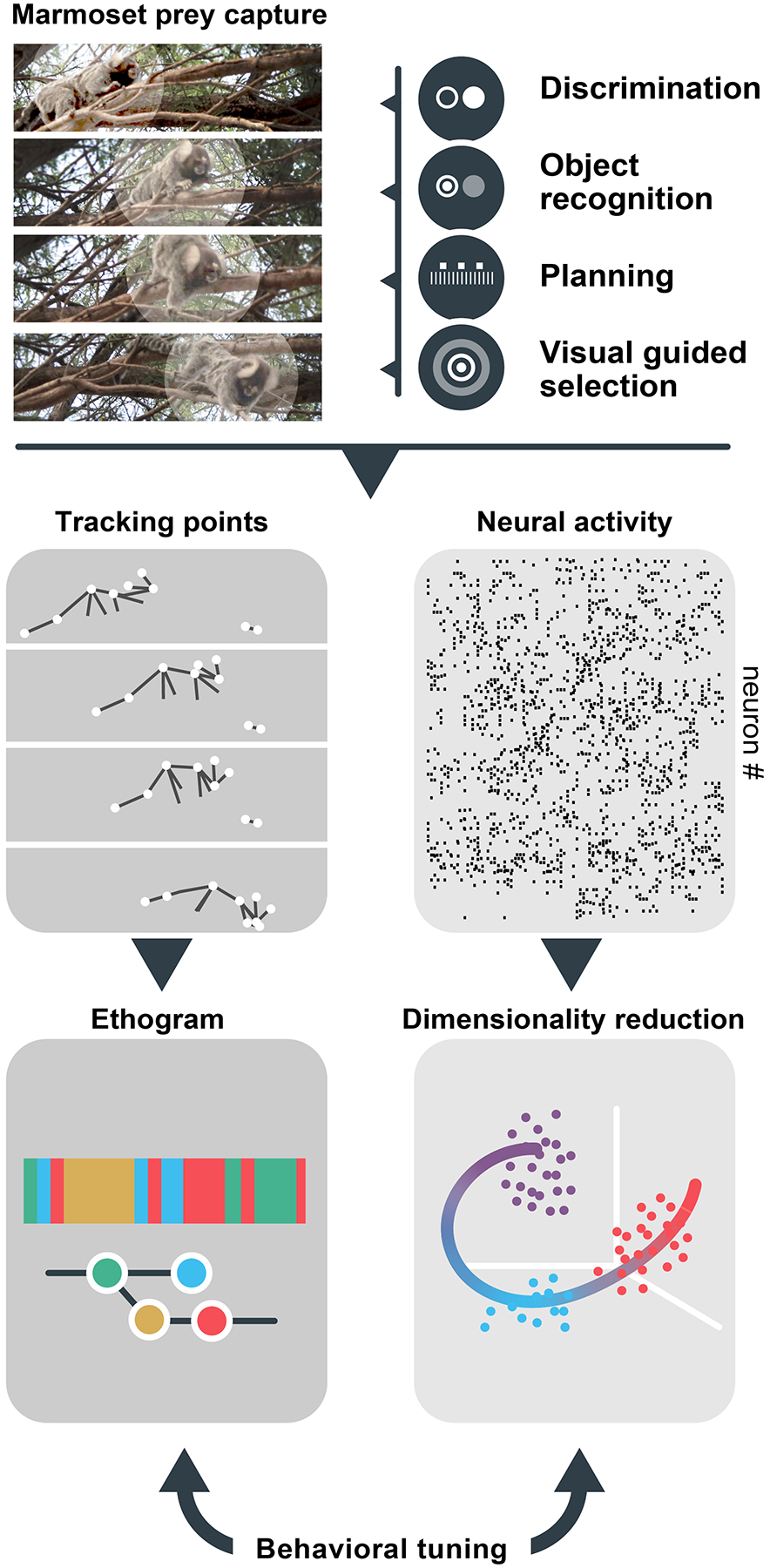
Marmoset prey capture comprises several visual processes that have heretofore been studied in isolation, rather than as different components of a single integrative, visuo-motor behavior. Employing complementary machine vision technologies to annotate the behavior of the animal and high-density neural recordings provides is a powerful strategy to identify behavioral components in different contexts through quantitative ethograms, while employing analytical approaches that reduce the dimensionality and identify critical covariance between brain and behavior. This approach is made possible by modern technologies for quantifying behaviors at time scales that mirror brain signals, longitudinal imaging and neural recordings and powerful modeling tools.
Conclusions
Ludwig Wittgenstein once wrote “If a lion could speak, we could not understand him”106. While this famous passage is often highlighted in discussions of communication and meaning in language, its connotations are directly relevant to our discussion here. Brains have many signals — spikes, oscillations, neuromodulators, and so on — that alone or together communicate an assemblage of information between cells, circuits and substrates across a range of different time scales and varying degrees of impact. Not unlike Willard Quine’s explorer who, upon hearing a local exclaim ‘Gavagai!’ in an unknown language, is left to ponder the meaning of the word — the indeterminacy of translation107–109 — we neuroscientists are tasked with both measuring various brain signals and interpreting their ‘meaning’. We are not, however, entirely without a Rosetta Stone. The computational principles that govern brains are routinely and elegantly illustrated in the natural behavioral repertoires of animals, amongst their dynamic, multidimensional distributions. In fact, they are so clearly evident because it is these very dynamics that have been sculpted and optimized over evolution for the myriad biological timescales across which animals operate. The language of the brain is natural behavior: the observable orchestral outcome of many brain signals working in concert. The impetus to combine natural, variable behaviors, large-scale neural recording and computational analysis to elucidate brain functions has been pursued by several neuroscience pioneers in previous decades101,110,111. What is different at this point in our field, however, is that we now have the tools to translate and leverage this prodigious diversity — both within single behaviors and across repertoires — as a powerful engine of discovery unto itself.
The significance of behavioral distributions for elucidating the principles governing brain functions is evident not only during natural behaviors, but also in more traditional paradigms. Consider first the highly reductionist studies of motion perception in the primate brain112,113. Subjects fixate a central spot, view an ambiguous dynamic moving dot display in their visual periphery, and then communicate their decision about the net direction of dot motion by making a discrete motor response to one of two response targets. While early generations of this task used a fixed duration of visual motion114,115, this behavioral parameter was iteratively extended to allow subjects to make their response as soon as they had made up their mind116. This modest change effectively grants volitional control of viewing duration to the experimental subject, increasing the natural variance of the behavior and yielding new insights into each single decision event117. Theoretical frameworks developed to account for these data therefore needed to graduate to more dynamic GLM approaches that revealed the complex combinations of sensory, decision and motor signals involved even in this highly stereotyped behavior104.
The need for brains to support behavioral diversity is further highlighted by the increasing awareness that mixed selectivity is likely the rule rather than the exception for neural coding, at least in vertebrate brains118–122. There is no reason to assume that the response of a single neuron should have a simple interpretation in terms of single sensory or behavioral variables that we impose upon them. There are simply too few cells in brains and too many actions they support for the vast majority of single neurons to be highly selective. The participation of individual neurons in many seemingly unrelated operations should not come as a surprise. At the same time, studies of mixed selectivity have typically relied on more conventional behavioral paradigms with correspondingly low-dimensional task parameters that do not fully reflect the breadth of operations that these cells, circuits and brains perform. These same limitations, however, do not extend to natural behaviors and repertoires. By examining the responses of neurons across multiple behavioral variables and/or contexts — thereby increasing the scope of behavioral distributions tested within individual neurons — these pioneering experiments have laid the groundwork for genuinely new perspectives on the neural computations governing flexible, variable behaviors.
Ethological repertoires offer perhaps the most powerful opportunity for insight into core neural computations that support brains. This suite of behaviors illustrates not only the particular challenges each species faces, but the distinct manner in which they are solved. Brains are analog systems, reflective of the very world in which they were selected to function over evolution. Information is not binned in nervous systems, but represented along covarying distributions in a multidimensional space.
The continued reliance on low-dimensional, stereotyped behaviors readily lends itself to testing binary hypotheses about cell types and circuits, but not necessarily to discovering the principles governing the full distribution of actions and processes that they support4. Ultimately, we do not record the activity of a single neuron in isolation, rather, we measure the perspective of a single neuron within a larger population. As the coding dynamics of that population change with respect to immediate behavioral and/or contextual challenges, it will be reflected in the distribution of a neuron’s activity covarying with behavioral distributions. As a result, neural responses to stimuli under more conventional conditions may not be representative of the putative natural analog because differences in behavioral context affect the animal’s perception of those signals in meaningful ways that are reflected in the patterns of neural activity123.
Similar to the challenge of indeterminacy in ascribing meaning to words in language108,109, one cannot identify the function of a brain cell, circuit, or substrate from limited contexts, but by embracing the myriad distributions of natural behaviors. For example, we have long measured sensory neuron responses to a battery of stimuli in order to determine the features that modulate activity: ‘sensory tuning’. Why not measure the covariance between neural activity and a large corpus of behaviors and/or sequences of behaviors that naturally occur within an animal’s repertoire? ‘Behavior tuning’ neurons in this way could yield unique insights into brains’ computational principles that generalize across events, as well as more idiosyncratic neural dynamics that emerge in response to distinct contextual challenges123 (Figure 4).
Developing a true theory of the brain requires experiments that take advantage of the breadth of distributions that only natural behaviors can offer. Such comparisons may be the only way to account for the indeterminacy between brain activity and function. As for the significance of cross-species comparisons for identifying generalizable principles of neural functions at the synaptic and cellular levels2,13, comparing neural processes across behaviors may reveal generalizable principles of neural function that resolve the indeterminacy of translation for brain signals and computations.
As neuroscientists we have traditionally sought to simplify brains and attribute single functions to single neurons. However, the single neuron focus fails to account for how animals survive and flourish. The path to discovery towards understanding how animals perform the impressive breadth of behaviors evident in natural repertoires is through an appreciation of the multidimensional relationships that exist between distributions of brains and behaviors. We anticipate that answers will include a broader set of principles than equating single neural cell types with responses to a specific stimulus feature or behavioral motif. Answers will associate the context-dependent dynamics of neural populations with generalizable neural computations essential for interacting with the natural social and ecological environments. Armed with the impressive modern tools to characterize behaviors and environmental factors at timescales on par with brains, data sciences approaches to quantify and model these multi-dimensional relationships, along with impressive molecular technologies to dissect these substrates, neuroscience is poised to transform our understanding of how brains actually function in the real world.
Acknowledgments.
We thank Gil Costa for assistance in generating figures. The corresponding authors (C.T.M, C.M.N.) were partially supported by NIH grant 1UF1NS116377-01.
Footnotes
Declaration of Interests.
The authors declare no competing interests.
References
- 1.Briscoe SD, and Ragsdale CW (2019). Evolution of the chordate telencephalon. Curr. Biol 29, R647–R662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller CT, Hale ME, Okano H, Okabe S, and Mitra P (2019). Comparative principles for next-generation neuroscience. Front. Behav. Neurosci 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereira TD, Shaevitz JW, and Murthy M (2020). Quantifying behavior to understand the brain. Nat. Neurosci 23, 1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krakauer JW, Ghazanfar AA, Gomez-Marin A, MacIver MA, and Poeppel D (2017). Neuroscience needs behavior: correcting a reductionist bias. Neuron 93, 480–490. [DOI] [PubMed] [Google Scholar]
- 5.Datta SR, Anderson DJ, Branson K, Perona P, and Leifer A (2019). Computational neuroethology: a call to action. Neuron 104, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennis EJ, El Hady A, Michaiel A, Clemens A, Tervo DRG, Voigts J, and Datta SR (2021). Systems neuroscience of natural behaviors in rodents. J. Neurosci 41, 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis RL (2005). Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu. Rev. Neurosci 28, 275–302. [DOI] [PubMed] [Google Scholar]
- 8.Lara AH, and Wallis JD (2015). The role of prefrontal cortex in working memory: a mini review. Front. Syst. Neurosci 9, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platt ML, and Glimcher PW (1999). Neural correlates of decision variables in parietal cortex. Nature 400, 233–238. [DOI] [PubMed] [Google Scholar]
- 10.Marler P (1991). Song learning behavior: the interface with neuroethology. Trends Neurosci. 14, 199–206. [DOI] [PubMed] [Google Scholar]
- 11.Emery NJ (2000). The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev 24, 581–604. [DOI] [PubMed] [Google Scholar]
- 12.Chang SWC, Brent LJN, Adams GK, Klein JT, Pearson JM, Watson KK, and Platt ML (2013). Neuroethology of primate social behavior. Proc. Natl. Acad. Sci. U. S. A 110 Suppl 2, 10387–10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yartsev MM (2017). The emperor’s new wardrobe: rebalancing diversity of animal models in neuroscience research. Science 358, 466. [DOI] [PubMed] [Google Scholar]
- 14.Striedter G, Belgard TG, Chen CC, Davis FP, Finlay BL, Gunturkun O, Hale ME, Harris JA, Hecht EI, Hof PR, et al. (2014). NSF workshop report: Discovering general principles of nervous system organization by comparing brain maps across species. J. Comp. Neurol 522, 1445–1453. [DOI] [PubMed] [Google Scholar]
- 15.O’Keefe J, and Dostrovsky J (1971). The hippocampus as a spatial map: preliminary evidence from unit activity in the freely moving rat. Brain Res. 34, 171–175. [DOI] [PubMed] [Google Scholar]
- 16.Moser E, Kropff E, and Moser M-B (2008). Place cells, grid cells, and the brain’s spatial representation system. Annu. Rev. Neurosci 31, 69–89. [DOI] [PubMed] [Google Scholar]
- 17.Courellis HS, Nummela SU, Metke M, Diehl GW, Bussell R, Cauwenberghs G, and Miller CT (2019). Spatial encoding in primate hippocampus during free navigation. PLoS Biol. 17, e3000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yartsev MM, and Ulanovsky N (2013). Representation of three-dimensional space in the hippocampus of flying bats. Science 340, 367. [DOI] [PubMed] [Google Scholar]
- 19.Buzsaki G, and Moser EI (2013). Memory, navigation and the theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci 16, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niell CM, and Stryker MP (2010). Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker PRL, Brown MA, Smear MC, and Niell CM (2020). Movement-related signals in sensory areas: roles in natural behavior. Trends Neurosci. 43, 581–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leopold DA, and Park SH (2020). Studying the visual brain in its natural rhythm. Neuroimage 216, 116790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elie JE, and Theunissen FE (2020). The Neuroethology of vocal communication in songbirds: production and perception of a call repertoire. In The Neuroethology of Birdsong, Sakata JT, Woolley SC, Fay RR, and Popper AN, eds. (Springer International Publishing; ), pp. 175–209. [Google Scholar]
- 24.McCormick DA, Nestvogel DB, and He BJ (2020). Neuromodulation of brain state and behavior. Annu. Rev. Neurosci 43, 391–415. [DOI] [PubMed] [Google Scholar]
- 25.Lovett-Barron M, Andalman AS, Allen WE, Vesuna S, Kauvar I, Burns VM, and Deisseroth K (2017). Ancestral circuits for the coordinated modulation of brain state. Cell 171, 1411–1423.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs EAK, Steinmetz NA, Peters AJ, Carandini M, and Harris KD (2020). Cortical state fluctuations during sensory decision making. Curr. Biol 30, 4944–4955.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell JF, Sundberg KA, and Reynolds JH (2009). Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron 63, 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beaman CB, Eagleman SL, and Dragoi V (2017). Sensory coding accuracy and perceptual performance are improved during the desynchronized cortical state. Nat. Commun 8, 1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez-Marin A, and Ghazanfar AA (2019). The life of behavior. Neuron 104, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells KD (1980). Behavioral ecology and social-organization of a dendrobatid frog (Colostethus-Inguinalis). Behav. Ecol. Sociobiol 6, 199–209. [Google Scholar]
- 31.Waser PM, and Brown CH (1986). Habitat acoustics and primate communication. Am. J. Primatol 10, 135–154. [DOI] [PubMed] [Google Scholar]
- 32.Gittleman JL, and Harvey PH (1982). Carnivore home-range size, metabolic needs and ecology. Behav. Ecol. Sociobiol 10, 57–63. [Google Scholar]
- 33.Rilling JK, and Sanfey AG (2011). The neuroscience of social decision-making. Annu. Rev. Psychol 62, 23–48. [DOI] [PubMed] [Google Scholar]
- 34.Herberholz J, and Marquart GD (2012). Decision making and behavioral choice during predator avoidance. Front. Neurosci 6, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connell LA, and Hofmann HA (2012). Evolution of a vertebrate social decision-making network. Science 336, 1154–1157. [DOI] [PubMed] [Google Scholar]
- 36.Tremblay S, Sharika KM, and Platt ML (2017). Social decision-making and the brain: a comparative perspective. Trends Cogn. Sci 21, 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calhoun AJ, Pillow JW, and Murthy M (2019). Unsupervised identification of the internal states that shape natural behavior. Nat. Neurosci 22, 2040–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nichols ALA, Eichler T, Latham R, and Zimmer M (2017). A global brain state underlies C. elegans sleep behavior. Science 356. [DOI] [PubMed] [Google Scholar]
- 39.Zagha E, and McCormick DA (2014). Neural control of brain state. Curr. Opin. Neurobiol 29, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naylor E (1996). Crab clockwork: the case for interactive circatidal and circadian oscillators controlling rhythmic locomotor activity of Carcinus maenas. Chronobiol. Int 13, 153–161. [DOI] [PubMed] [Google Scholar]
- 41.Morin LP, and Allen CN (2006). The circadian visual system, 2005. Brain Res. Rev 51, 1–60. [DOI] [PubMed] [Google Scholar]
- 42.Rose MC, Styr B, Schmid TA, Elie JE, and Yartsev MM (2021). Cortical representation of group social communication in bats. Science 374, eaba9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stander PE (1992). Cooperative hunting in lions: the role of the individual. Behav. Ecol. Sociobiol 29, 445–454. [Google Scholar]
- 44.Beddor PS, Harnsberger JD, and Lindemann S (2002). Language-specific patterns of vowel-to-vowel coarticulation: acoustic structures and their perceptual correlates. J. Phon 30, 591–627. [Google Scholar]
- 45.Daniloff RG, and Hammarberg RE (1973). On defining coarticulation. J. Phon 1, 239–248. [Google Scholar]
- 46.Nummela S, Jovanovic V, de la Mothe LA, and Miller CT (2017). Social context-dependent activity in marmoset frontal cortex populations during natural conversations. Journal of Neuroscience 37, 7036–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huk A, Bonnen K, and He BJ (2018). Beyond trial-based paradigms: continuous behavior, ongoing neural activity, and natural stimuli. J. Neurosci 38, 7551–7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neisser U (1976). Cognition and reality: principles and implications of cognitive psychology (W. H. Freeman; ). [Google Scholar]
- 49.Moore T, and Zirnsak M (2017). Neural mechanisms of selective visual attention. Annu. Rev. Psychol 68, 47–72. [DOI] [PubMed] [Google Scholar]
- 50.Hanks TD, and Summerfield C (2017). Perceptual decision making in rodents, monkeys, and humans. Neuron 93, 15–31. [DOI] [PubMed] [Google Scholar]
- 51.Gold JI, and Shadlen MN (2007). The neural basis of decision making. Annu. Rev. Neurosci 30, 535–574. [DOI] [PubMed] [Google Scholar]
- 52.Haroush K, and Williams ZM (2015). Neuronal prediction of opponent’s behavior during cooperative social interchange in primates. Cell 160, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pisupati S, Chartarifsky-Lynn L, Khanal A, and Churchland AK (2021). Lapses in perceptual decisions reflect exploration. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fajen BR, and Warren WH (2003). Behavioral dynamics of steering, obstable avoidance, and route selection. J. Exp. Psychol. Hum. Percept. Perform 29, 343–362. [DOI] [PubMed] [Google Scholar]
- 55.Felsen G, Touryan J, Han F, and Dan Y (2005). Cortical sensitivity to visual features in natural scenes. PLoS Biol. 3, e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker EY, Sinz FH, Cobos E, Muhammad T, Froudarakis E, Fahey PG, Ecker AS, Reimer J, Pitkow X, and Tolias AS (2019). Inception loops discover what excites neurons most using deep predictive models. Nat. Neurosci 22, 2060–2065. [DOI] [PubMed] [Google Scholar]
- 57.von Uexküll J (2013). A foray into the worlds of animals and humans: with a theory of meaning (U of Minnesota Press; ). [Google Scholar]
- 58.Gibson JJ (2013). The ecological approach to visual perception (Psychology Press; ). [Google Scholar]
- 59.Welker WI (1964). Analysis of sniffing of the albino rat 1). Behaviour 22, 223–244. [Google Scholar]
- 60.Somatic sensory transmission to the cortex during movement: Gating of single cell responses to touch (1982). Exp. Neurol 78, 654–669. [DOI] [PubMed] [Google Scholar]
- 61.Ayaz A, Saleem AB, Schölvinck ML, and Carandini M (2013). Locomotion controls spatial integration in mouse visual cortex. Curr. Biol 23, 890–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider DM, Nelson A, and Mooney R (2014). A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 513, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schroeder CE, Wilson DA, Radman T, Scharfman H, and Lakatos P (2010). Dynamics of Active Sensing and perceptual selection. Curr. Opin. Neurobiol 20, 172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang SC-H, Wolpert DM, and Lengyel M (2018). Theoretical perspectives on active sensing. Curr Opin Behav Sci 11, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kleinfeld D, Ahissar E, and Diamond ME (2006). Active sensation: insights from the rodent vibrissa sensorimotor system. Curr. Opin. Neurobiol 16, 435–444. [DOI] [PubMed] [Google Scholar]
- 66.Yates J, Coop S, Sarch G, Wu R-J, Butts D, Rucci M, and Mitchell J (2020). Beyond fixation: foveal receptive field estimation in freely viewing primates. Journal of Vision 20, 1470. [Google Scholar]
- 67.Simoncelli EP, and Olshausen BA (2001). Natural image statistics and neural representation. Annual Review of Neuroscience 24, 1193–1216. [DOI] [PubMed] [Google Scholar]
- 68.Rust NC, and Movshon JA (2005). In praise of artifice. Nat. Neurosci 8, 1647–1650. [DOI] [PubMed] [Google Scholar]
- 69.Felsen G, and Dan Y (2005). A natural approach to studying vision. Nat. Neurosci 8. [DOI] [PubMed] [Google Scholar]
- 70.Nourizonoz A, Zimmermann R, Ho CLA, Pellat S, Ormen Y, Prévost-Solié C, Reymond G, Pifferi F, Aujard F, Herrel A, et al. (2020). EthoLoop: automated closed-loop neuroethology in naturalistic environments. Nat. Methods 17, 1052–1059. [DOI] [PubMed] [Google Scholar]
- 71.Juavinett AL, Erlich JC, and Churchland AK (2018). Decision-making behaviors: weighing ethology, complexity, and sensorimotor compatibility. Curr. Opin. Neurobiol 49, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hessler NA, and Doupe AJ (1999). Social context modulates singing-related neural activity in the songbird forebrain. Nat. Neurosci 2, 209–211. [DOI] [PubMed] [Google Scholar]
- 73.Cowley BR, Snyder AC, Acar K, Williamson RC, Yu BM, and Smith MA (2020). slow drift of neural activity as a signature of impulsivity in macaque visual and prefrontal cortex. Neuron 108, 551–567.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olshausen BA, and Field DJ (2005). How close are we to understanding V1? Neural Comput. 17, 1665–1699. [DOI] [PubMed] [Google Scholar]
- 75.Maimon G, Straw AD, and Dickinson MH (2010). Active flight increases the gain of visual motion processing in Drosophila. Nat. Neurosci 13, 393–399. [DOI] [PubMed] [Google Scholar]
- 76.Chiappe ME, Seelig JD, Reiser MB, and Jayaraman V (2010). Walking modulates speed sensitivity in Drosophila motion vision. Curr. Biol 20, 1470–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vinck M, Batista-Brito R, Knoblich U, and Cardin JA (2015). Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86, 740–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reimer J, McGinley MJ, Liu Y, Rodenkirch C, Wang Q, McCormick DA, and Tolias AS (2016). Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun 7, 13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, and Bethge M (2018). DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci 21, 1281–1289. [DOI] [PubMed] [Google Scholar]
- 80.Pereira TD, Aldarondo DE, Willmore L, Kislin M, Wang SSH, Murthy M, and Shaevitz JW (2019). Fast animal pose estimation using deep neural networks. Nat. Methods 16, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sainburg T, Thielk M, and Gentner TQ (2020). Finding, visualizing, and quantifying latent structure across diverse animal vocal repertoires. PLoS Comput. Biol 16, e1008228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bianco MJ, Gerstoft P, Traer J, Ozanich E, Roch MA, Gannot S, and Deledalle C-A (2019). Machine learning in acoustics: theory and applications. J. Acoust. Soc. Am 146, 3590. [DOI] [PubMed] [Google Scholar]
- 83.Berman GJ, Choi DM, Bialek W, and Shaevitz JW (2014). Mapping the stereotyped behaviour of freely moving fruit flies. J. R. Soc. Interface 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, Abraira VE, Adams RP, and Datta SR (2015). Mapping sub-second structure in mouse behavior. Neuron 88, 1121–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kabra M, Robie AA, Rivera-Alba M, Branson S, and Branson K (2013). JAABA: interactive machine learning for automatic annotation of animal behavior. Nat. Methods 10, 64–67. [DOI] [PubMed] [Google Scholar]
- 86.Hsu AI, and Yttri EA (2021). B-SOiD, an open-source unsupervised algorithm for identification and fast prediction of behaviors. Nat. Commun 12, 5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whiteway MR, and Butts DA (2019). The quest for interpretable models of neural population activity. Curr. Opin. Neurobiol 58, 86–93. [DOI] [PubMed] [Google Scholar]
- 88.Cunningham JP, and Yu BM (2014). Dimensionality reduction for large-scale neural recordings. Nat. Neurosci 17, 1500–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Humphries MD (2020). Strong and weak principles of neural dimension reduction. arXiv, arXiv:2011.08088. [Google Scholar]
- 90.Hurwitz C, Kudryashova N, Onken A, and Hennig MH (2021). Building population models for large-scale neural recordings: opportunities and pitfalls. arXiv, arXiv:2102.01807. [DOI] [PubMed] [Google Scholar]
- 91.Li J, Kells PA, Osgood AC, Gautam SH, and Shew WL (2021). Collapse of complexity of brain and body activity due to excessive inhibition and MeCP2 disruption. Proc. Natl. Acad. Sci. U. S. A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Churchland MM, Cunningham JP, Kaufman MT, Foster JD, Nuyujukian P, Ryu SI, and Shenoy KV (2012). Neural population dynamics during reaching. Nature 487, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mazor O, and Laurent G (2005). Transient dynamics versus fixed points in odor representations by locust antennal lobe projection neurons. Neuron 48, 661–673. [DOI] [PubMed] [Google Scholar]
- 94.Kato S, Kaplan HS, Schrödel T, Skora S, Lindsay TH, Yemini E, Lockery S, and Zimmer M (2015). Global brain dynamics embed the motor command sequence of Caenorhabditis elegans. Cell 163, 656–669. [DOI] [PubMed] [Google Scholar]
- 95.Mante V, Sussillo D, Shenoy KV, and Newsome WT (2013). Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature 503, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Musall S, Kaufman MT, Juavinett AL, Gluf S, and Churchland AK (2019). Single-trial neural dynamics are dominated by richly varied movements. Nat. Neurosci 22, 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stringer C, Pachitariu M, Steinmetz N, Reddy CB, Carandini M, and Harris KD (2019). Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stringer C, Michaelos M, Tsyboulski D, Lindo SE, and Pachitariu M (2021). High-precision coding in visual cortex. Cell 184, 2767–2778.e15. [DOI] [PubMed] [Google Scholar]
- 99.Rumyantsev OI, Lecoq JA, Hernandez O, Zhang Y, Savall J, Chrapkiewicz R, Li J, Zeng H, Ganguli S, and Schnitzer MJ (2020). Fundamental bounds on the fidelity of sensory cortical coding. Nature 580, 100–105. [DOI] [PubMed] [Google Scholar]
- 100.Aljadeff J, Lansdell BJ, Fairhall AL, and Kleinfeld D (2016). Analysis of neuronal spike trains, deconstructed. Neuron 91, 221–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prechtl JC, Cohen LB, Pesaran B, Mitra PP, and Kleinfeld D (1997). Visual stimuli induce waves of electrical activity in turtle cortex. Proc. Natl. Acad. Sci. U. S. A 94, 7621–7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Truccolo W, Eden UT, Fellows MR, Donoghue JP, and Brown EN (2005). A point process framework for relating neural spiking activity to spiking history, neural ensemble, and extrinsic covariate effects. J. Neurophysiol 93, 1074–1089. [DOI] [PubMed] [Google Scholar]
- 103.Balzani E, Lakshminarasimhan K, Angelaki D, and Savin C (2020). Efficient estimation of neural tuning during naturalistic behavior. Adv. Neural Inf. Process. Syst 33, 12604–12614. [Google Scholar]
- 104.Park IM, Meister MLR, Huk AC, and Pillow JW (2014). Encoding and decoding in parietal cortex during sensorimotor decision-making. Nat. Neurosci 17, 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Latimer KW, Yates JL, Meister MLR, Huk AC, and Pillow JW (2015). Single-trial spike trains in parietal cortex reveal discrete steps during decision-making. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wittgenstein L (1958). Philosophical investigations (Blackwell; ). [Google Scholar]
- 107.Premack D (1986). Gavagai! or the future history of the animal language controversy (MIT Press; ). [DOI] [PubMed] [Google Scholar]
- 108.Quine WV (1973). On the reasons for the indeterminacy of translation. J. Philos 12, 178–183. [Google Scholar]
- 109.Van Orman Quine W (2013). Word and object, new edition (MIT Press; ). [Google Scholar]
- 110.Briggman KL, and Kristan WB Jr (2006). Imaging dedicated and multifunctional neural circuits generating distinct behaviors. J. Neurosci 26, 10925–10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abeles M, Bergman H, Gat I, Meilijson I, Seidemann E, Tishby N, and Vaadia E (1995). Cortical activity flips among quasi-stationary states. Proc. Natl. Acad. Sci. U. S. A 92, 8616–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Movshon JA, and Newsome WT (1992). Neural foundations of visual motion perception. Curr. Dir. Psychol. Sci 1, 35–39. [Google Scholar]
- 113.Andersen RA (1997). Neural mechanisms of visual motion perception in primates. Neuron 18, 865–872. [DOI] [PubMed] [Google Scholar]
- 114.Newsome WT, Britten KH, and Movshon JA (1989). Neuronal correlates of a perceptual decision. Nature 341, 52–54. [DOI] [PubMed] [Google Scholar]
- 115.Britten KH, Shadlen MN, Newsome WT, and Movshon JA (1992). The analysis of visual motion: a comparison of neuronal and psychophysical performance. J. Neurosci 12, 4745–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roitman JD, and Shadlen MN (2002). Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J. Neurosci 22, 9475–9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Churchland AK, Kiani R, Chaudhuri R, Wang X-J, Pouget A, and Shadlen MN (2011). Variance as a signature of neural computations during decision making. Neuron 69, 818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rigotti M, Barak O, Warden MR, Wang XJ, Daw ND, Miller EK, and Fusi S (2013). The importance of mixed selectivity in complex cognitive tasks. Nature 497, 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fusi S, Miller EK, and Rigotti M (2016). Why neurons mix: high dimensionality for higher cognition. Curr. Opin. Neurobiol 37, 66–74. [DOI] [PubMed] [Google Scholar]
- 120.Parthasarathy A, Herikstad R, Bong JH, Medina FS, Libedinsky C, and Yen S-C (2017). Mixed selectivity morphs population codes in prefrontal cortex. Nat. Neurosci 20, 1770–1779. [DOI] [PubMed] [Google Scholar]
- 121.Grunfeld IS, and Likhtik E (2018). Mixed selectivity encoding and action selection in the prefrontal cortex during threat assessment. Curr. Opin. Neurobiol 49, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bernardi S, Benna MK, Rigotti M, Munuera J, Fusi S, and Salzman CD (2020). The geometry of abstraction in the hippocampus and prefrontal cortex. Cell 183, 954–967.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jovanovic V, Fishbein AR, de la Mothe L, Lee K-F, and Miller CT (2022). Behavioral context affects social signal representations within single primate prefrontal cortex neurons. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]


