Advances in genomics have ushered in promising therapies tailored to the individual. Personalized medicine is promoted and has begun to positively influence care. For example, medications such as trastuzumab for the 30% of breast cancers that overexpress ERBB2 and vemurafenib for patients with late-stage melanoma who carry the V600E variant have been beneficial.1 Despite these advances, for many sectors of the population—children, older adults, pregnant and lactating women, and individuals with physical and intellectual disabilities—limited evidence-based therapies optimized to their specific medical needs exist. Combined, these groups comprise as much as 58% of the US population (eTable in the Supplement). Research focusing onorat the very least includes members of these groups is critically needed.
Until the initial passage of the Best Pharmaceuticals for Children Act in 2002, pediatric drug doses were based on extrapolation from adults. Importantly, body composition and metabolic processes change as children develop, resulting in different safety and efficacy profiles.2 Similarly, medication needs change with age and with life events. Older patients often have a range of comorbidities and declining organ function that affect drug dosing and effectiveness. Physiological changes during pregnancy not only alter metabolism but include slowing of intestinal transport, doubling of blood volume, increasing renal excretion, and changing of circulating binding proteins. These processes alter pharmacodynamics and effectiveness. Without optimal levels, pregnant women and their fetuses may be exposed to a medication at a nontherapeutic or subtherapeutic dose. For example, a pharmacokinetic study of amoxicillin treatment for anthrax exposure during pregnancy found that the required concentrations were not achievable using the recommended dosing.3
For individuals with intellectual disabilities, pharmacokinetic studies rarely address alternative delivery routes, such as gastrostomy tubes or rectal suppositories. Children with Down syndrome who develop acute leukemia have a higher incidence of treatment-related toxic effects from certain chemotherapeutic drugs. Populations affected by physical disabilities have little data available to inform pharmacological care. Inasystematic review, individuals with spinal cord injuries demonstrated significant variation in drug metabolism, half-life, and clearance.4 In addition, people with intellectual or physical disabilities often require additional time needed for consent and follow-up, and the uncertainty regarding their comprehension. This likely affects their inclusion in clinical trials. In one analysis, only 2% of 300 clinical trials included people with intellectual disabilities, yet with only minor accommodations, at least 70% of these trials could have included them.5 In the same populations, however, medications are often prescribed with minimal evidence to support their use, especially psychotropic drugs with significant adverse effects.
Recently, discussions have arisen about the need for inclusion in research and elimination these gaps. In 2017, the National Institutes of Health (NIH) held a workshop, “Inclusion Across the Lifespan,” that highlighted current federal regulations that include protections for “vulnerable populations” (pregnant women, fetuses, neonates, prisoners, and children). Although these regulations were originally designed to protect these individuals, many investigators have called for reconsideration, opting to protect them through research, rather than from research. Inclusion will likely yield data that will benefit more people.
Many underrepresented populations encounter barriers to participation in research. In a review of 338 phase 3 and 4 NIH-funded actively recruiting studies in Clinicaltrials.gov, explicit exclusion was found in 68% for pregnant women, 47.3% for lactating women, 75.7% for children, 27.8% for older people, 12.4% for those with intellectual or developmental disabilities, and 1.8% for those with physical disabilities (Figure). Additionally, the results of most of these trials did not mention individuals with disabilities in either the inclusion or exclusion criteria. Participation by those with cognitive impairment may be limited by lack of ability to provide informed consent or comply with the study protocol or procedures. Rather than explicit exclusion, individuals with physical disabilities are often excluded because of limited access to the study facility or the challenges associated with obtaining physiological measurements.
Figure. Open NIH-Funded Phase 3 and 4 Studies as of October 19, 2017.
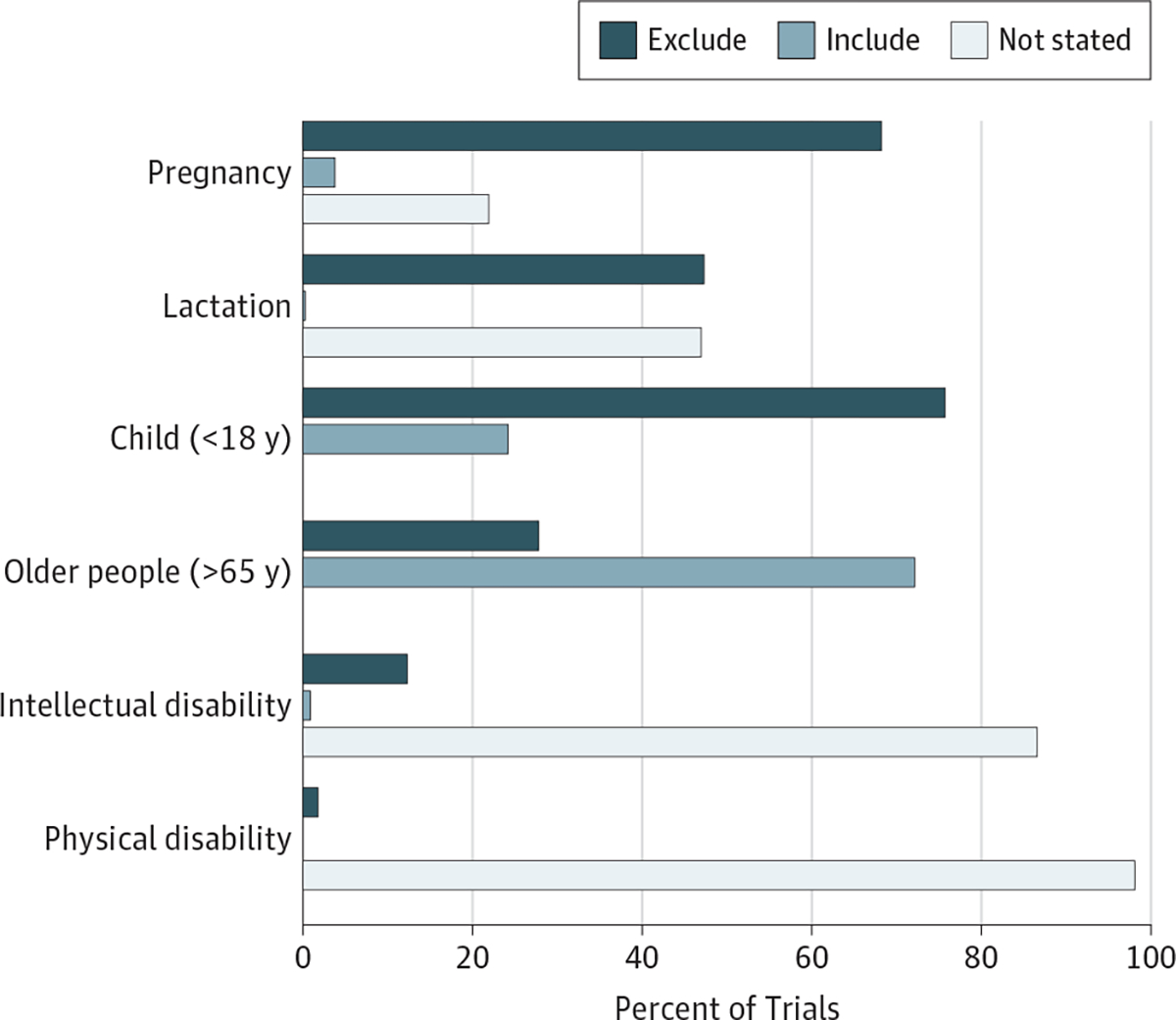
Clinicaltrials.gov records (N=338) were reviewed. Exclusion for intellectual disabilities was based on IQ and defined intellectual disability or cognitive impairment; physical disabilities: exclusions for physical disabilities were inability to ambulate, extreme immobility, and paraplegia.
An assumption that research studies should include rather than exclude a range of populations would represent a seminal shift. Investigators would still, however, have important decisions to make when designing a study. They would need to determine if the study would have potential benefits and whether the diverse physiological changes would dilute or eliminate an effect. To design an appropriately powered study to test for an effect in all subgroups, a large sample size would be needed. This would result in higher costs and diminished feasibility.
Clear justifications exist for exclusion in specific research studies. One is biological, when a condition does not exist in a population. Excluding men from a study on preeclampsia is justified because men do not get pregnant. A second is when there is an unacceptable risk that outweighs the knowledge to be gained, such as testing a known teratogen in pregnant women. However, exclusion of populations simply because they may take more time to include or are considered vulnerable is unacceptable and stands in contrast to the ethical principles of equity and justice. If a therapy is potentially useful to a specific population, that population should be included in the research.
A less frequently discussed issue is that some groups are not identified as a subpopulation, even though their physiological variations may affect the research results. For example, lactation status may not be considered when enrolling women; this prevents the analysis of the potential impact of lactation on the participant’s altered metabolism.
National Institutes of Health policies require plans for the inclusion of subgroups, particularly children and women, in funded clinical trials. The Pediatric Research Equity Act authorizes the US Food and Drug Administration to require pharmaceutical companies to study their products in children. Using these as examples, policies need to be developed for pregnant women, older adults, and people with intellectual and physical disabilities. One opportunity is to use alternative study designs for these groups, especially when placebo use is of concern. A study design involving individuals who do not respond to standard therapy, that uses a surrogate end point, or includes a provision of an early escape or early advance may provide needed data. The use of convenience samples (ie, studying those who are already exposed to the medication) is another method to provide information. This allows the collection of data from individuals using a medication off-label, which is the case for most of the populations described herein.
Although alternative study designs provide an opportunity for data acquisition when traditional models are not feasible, they should not replace appropriate randomized clinical trials. For example, placebo-controlled trials of therapies for depression in pregnancy have raised concerns because of the use of a placebo in the setting of depression. The authors of a study specifically evaluated this issue and concluded that placebo-controlled trials were ethically justified, emphasizing the importance of using the best research designs to improve the quality of care for pregnant women.6 In considering drug trials for children, especially those with intellectual disabilities, special issues exist when trying to assess whether an intervention has an effect, due to the lack of robust, reproducible outcome assessments.7 Recent high-profile drug trials to improve cognition, behavior, or both in individuals with Down syndrome or fragile X syndrome in part failed because of lack of sensitive outcome measures.8 Also, safety concerns leading to the recruitment of older persons for participation in a clinical trial may have missed a window of cognitive plasticity that would have been present in younger research participants. The placebo effect has been described as a real phenomenon for families and individuals with intellectual disabilities who are desperate for a cure.9People with physical disabilities have a range of challenges that can affect their participation in clinical trials, including different etiologies of their impairments, difficulties in measuring impairment due to limits in physical mobility, heterogeneity within specific conditions, and illnesses or injuries that pose a challenge to stratification. For many of these populations, off-label use of drugs is common, with few methodologically sound studies to inform evidence-based practices.
Although personalized medicine offers the opportunity to tailor therapies to the individual, given the large gaps in data for certain populations, in actuality it is “exclusive medicine.” Now, more than ever, it is imperative not to lose sight of the critical need to obtain evidence for medical therapies for major underrepresented populations. Without these data, more than half of the US population will be unable to benefit from personalized care.
Supplementary Material
Footnotes
Conflict of Interest Disclosures: Both authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Drs Spong and Bianchi are employees of the US federal government and performed this work as part of their official duties.
Contributor Information
Catherine Y. Spong, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland..
Diana W. Bianchi, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland..
REFERENCES
- 1.Cutter GR, Liu Y. Personalized medicine. Neurol Clin Pract. 2012;2(4):343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brussee JM, Calvier EA, Krekels EH, et al. Children in clinical trials. Expert Rev Clin Pharmacol. 2016;9(9):1235–1244. [DOI] [PubMed] [Google Scholar]
- 3.Andrew MA, Easterling TR, Carr DB, et al. Amoxicillin pharmacokinetics in pregnant women. Clin Pharmacol Ther. 2007;81(4):547–556. [DOI] [PubMed] [Google Scholar]
- 4.Feldman MA, Bosett J, Collet C, Burnham-Riosa P. Where are persons with intellectual disabilities in medical research. J Intellect Disabil Res. 2014;58(9): 800–809. [DOI] [PubMed] [Google Scholar]
- 5.Mestre H, Alkon T, Salazar S, Ibarra A. Spinal cord injury sequelae alter drug pharmacokinetics. Spinal Cord. 2011;49(9):955–960. [DOI] [PubMed] [Google Scholar]
- 6.Coverdale JH, McCullough LB, Chervenak FA. The ethics of randomized placebo-controlled trials of antidepressants with pregnant women. Obstet Gynecol. 2008;112(6):1361–1368. [DOI] [PubMed] [Google Scholar]
- 7.Berry-Kravis E, Hessl D, Abbeduto L, Reiss AL, Beckel-Mitchener A, Urv TK; Outcome Measures Working Groups. Outcome measures for clinical trials in fragile X syndrome. J Dev Behav Pediatr. 2013;34(7):508–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart SJ, Visootsak J, Tamburri P, et al. Pharmacological interventions to improve cognition and adaptive functioning in Down syndrome. Am J Med Genet A. 2017;173(11):3029–3041. [DOI] [PubMed] [Google Scholar]
- 9.Jensen KB, Kirsch I, Pontén M, et al. Certainty of genuine treatment increases drug responses among intellectually disabled patients. Neurology. 2017;88(20):1912–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


