Abstract
Background:
Among various visual functions, stereoacuity, or the ability to perceive depth, is the most sophisticated binocular function. Many publications discuss the influence of retinal image formation by multifocal intraocular lenses on glare and contrast sensitivity, but only a few present results of testing binocular vision in patients with multifocal intraocular lenses.
Objective:
This article is designed to review the results of testing binocular vision in patients with multifocal intraocular lenses implanted in cataract surgery.
Methods:
This article was performed based on a literature review and Internet search through scientific databases such as PubMed, Scopus, Web of Science, and Google Scholar.
Results:
Some reports found that patients implanted with the monofocal lens, when measured with a near addition, presented statistically significant better stereoacuity scores than those implanted with any of the multifocal intraocular lens types. When the TNO test was used for measurement, statistically significant better stereoacuity was disclosed with the refractive multifocal intraocular lens than with the diffractive-based multifocal intraocular lens design. Stereoacuity scores, even within the same types of lenses, were significantly better with the Titmus test than with the TNO test.
Conclusion:
Stereoacuity is not affected by multifocality-induced retinal blur as it is by other causes of image degradation such as small residual refractive error very early opacification of ocular media or dry eye. Multifocal intraocular lenses do not cause more functional aniseikonia than would be expected with a monofocal intraocular lens. Since stereoacuity is compromised with unilateral multifocal intraocular lens implantation bilateral implantation should be attempted.
Keywords: Stereoacuity, multifocal lens, Titmus test, TNO test
1. BACKGROUND
Among various visual functions, stereoacuity, or the ability to perceive depth, is the most sophisticated binocular function. The visual system requires binocularity to achieve stereoacuity and retains a single representation of a world viewed through two eyes. Good stereoacuity is required for accurate hand-eye coordination when using tools, threading a needle, performing surgery, or even using a computer (1). Reduced stereoacuity may cause symptoms of discomfort such as eyestrain, headaches, and diplopia (2).
Factors related to stereoacuity in phakic patients are uncorrected refractive error (3, 4) (contrast reduced by optical blur), decreased visual acuity, sensory and motor fusion problems (5-7), and age (8-11) (loss of light transmission through ocular media). In aphakic and pseudophakic patients stereoacuity is additionally affected by the difference in visual acuity (12, 13), spherical equivalent (14), astigmatism (14), the axial length between fellow eyes, aniseikonia (15-17), pupil diameter, and IOL decentration and tilt (18).
Stereoacuity tests
Stereoacuity testing is important in clinical practice because many of the adverse conditions that affect the visual system’s function will also affect stereoacuity. Various tests have been proposed for stereoacuity testing. Howard-Dolman device is considered the most sensitive and accurate procedure to determine stereoacuity (19). However, the Howard-Dolman apparatus is rarely used in clinical practice, with clinicians opting for either vectographic tests, such as the Titmus Wirth test, Randot test, or anaglyphic tests such as TNO, which is a highly dissociative stereotest that does not present monocular cues.
Picture 1. TNO test.
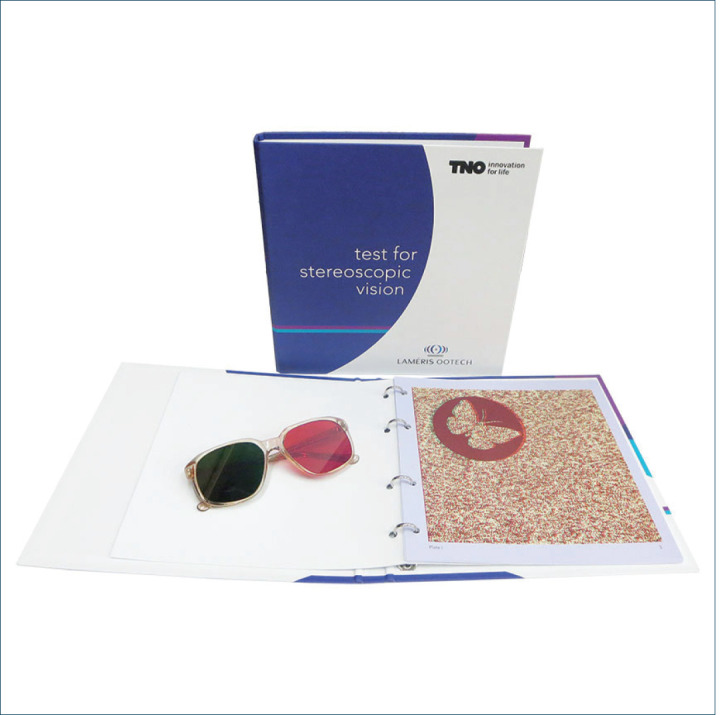
Stereoacuity and cataract
Age-related cataract is the most common eye disorder in the western world (20). Cataract (opaque lens) causes visual disability in terms of decreased visual acuity, decreased contrast sensitivity, anisometropia, and aniseikonia, leading to impaired binocular function. Impaired visual function is associated with an increased number of falls, decreased driving performances, and decreased quality of life (ie psychological effects such as depression and anxiety) (21, 22).
In quality of life visual acuity is required for functional tasks, while stereopsis and contrast were more important determinants of quality of life (23). However, stereoacuity was not found to be indicative of overall subjective visual difficulty attributable to the lens opacity. In other words, we can say that stereoacuity is a psychophysical test that is affected by lens opacities but may not be a good reflection of the subjective visual difficulty in the presence of good CDVA in the fellow eye (23, 24).
Multifocal intraocular lenses
With great advances in cataract surgery, as well as intraocular lens design, and preoperative diagnostics much attention has been paid to improving postoperative optical quality. Due to those advancements postoperative anisometropia, astigmatism and aniseikonia are almost eliminated. To this day, the most frequently implanted intraocular lens (IOL) is a monofocal lens designed to provide excellent outcomes for distance, but usually, patients require refractive compensation for intermediate and near activities. In comparison to monofocal intraocular lenses, multifocal intraocular lenses (MIOLs) are designed to provide patients with an expanded range of vision and consequently reduce spectacle dependence after cataract surgery, also improving some quality of life aspects (25). The basic principle underlying the multifocal lenses is the simultaneous creation of more than one image point for a single object point. The corollary of this principle is that multiple object points (e.g. distance and near) simultaneously can be brought into the same image point (26). Multifocal intraocular lenses present a variety of designs: from purely refractive to full aperture diffractive, and hybrid diffractive-refractive. Whereas refractive multifocal intraocular lenses provide two or more foci by varying the surface curvature in the sectors defined within the lens aperture, diffractive multifocal intraocular lenses rely on diffractive principles to split the incoming light energy into two or more foci. Hybrid designs combine refractive and diffractive zones on the same surface, commonly a central diffractive zone and a refractive periphery (27). Currently, the majority of multifocal intraocular lens models are based on a diffractive platform that sends light to the retina with a predefined light distribution to two different foci, far and near, with a near addition of + 3.00 D or below (28).
Picture 2. Titmus Wirt test.

All multifocal intraocular lens designs lead to simultaneous vision, which has been documented to result in a variety of photic phenomena described as halos and/or glare (29), with their negative impact on vision being modulated by several factors, such as pupil size and illumination conditions, lens power and addition (30), lens design, and sensitivity and possible neuroadaptation of the patient to the phenomena, among others. Besides, the simultaneous imaging of near and distant objects, in addition to the possible presence of post-operative residual defocus or astigmatism, as well as higher-order aberrations (such as spherical aberration and coma), have been found to lead to a reduction in contrast sensitivity (31) and may also influence the natural capability of human binocular vision for three-dimensional perception. Although, there have been reports that a new generation of multifocal intraocular lenses present with improvement in contrast sensitivity (32, 33).
Preoperative planning for multifocal intraocular lens implantation
Although new generations of MIOLs went through several modifications to improve distance, intermediate, and near vision, reduce undesired photic phenomena (ie, glare and halos), and offer good contrast sensitivity in comparison with their predecessors, they are still far from perfect (34). Potential patients should undoubtedly be informed about potential adverse events because they constitute, apart from reduced visual acuity, the main reason for dissatisfaction after the implantation of such a lens (35-37). Therefore, meticulous preoperative examination, careful patient selection for each of these technologies, and thorough preoperative information are key to postoperative patient satisfaction (35-37). Careful patient selection should not only focus on biometry, ophthalmologic findings, and preoperative astigmatism but also on personality characteristics (35-37).
Main ophthalmic inclusion criteria should be similar visual acuity on both eyes with binocular function within normal limits, clear cornea without topographic irregularities (ie asymmetrical astigmatism, irregular astigmatism), no macular pathology or PNO damage (ie uncontrolled glaucoma), and systemic diseases that might affect the postoperative vision (i.e. Uncontrolled diabetes mellitus). Biometry and corneal topography are mandatory and appropriate lens design should be chosen according to the amount of corneal astigmatism (toric lenses in astigmatism > 0.75D) (34, 38).
2. OBJECTIVE
The aim of this article is to review the results of testing binocular vision in patients with multifocal intraocular lenses implanted in cataract surgery.
3. MATERIAL AND METHODS
This article was performed based on a literature review and Internet search through scientific databases such as PubMed, Scopus, Web of Science, and Google Scholar.
4. RESULTS
Influence of multifocality on stereoacuity
Many publications discuss the influence of retinal image formation by multifocal intraocular lenses on glare and contrast sensitivity (29-33, 39), but only a few present results of testing binocular vision in patients with multifocal intraocular lenses (38-47).
There is some variability among results in published literature (Table 1). There are reports which found that patients implanted with the monofocal lens, when measured with a near addition, presented statistically significant better stereoacuity scores than those implanted with any of the multifocal intraocular lens types (40, 42, 45, 46). In addition, when the TNO test was used for measurement, statistically significant better stereoacuity was disclosed with the refractive multifocal intraocular lens than with the diffractive based multifocal intraocular lens design (42, 44). The differences may originate from the different clinical tests used (eg Titmus, Lang, Randot test, anaglyphic TNO test, Howard–Dolman test) and the different optical principles to design the lens, refraction, diffraction, or hybrid, and hence the image created on the retina. Stereoacuity scores, even within the same types of lenses, were significantly better with the Titmus test than with the TNO test. With the Titmus test, a higher percentage of patients implanted with multifocal intraocular lenses reached the best possible stereoacuity value that can be measured with this test (40 arc seconds). Patients implanted with diffractive multifocal intraocular lenses presented the worst stereoacuity with the TNO test and the largest between test differences when compared with the values of the Titmus test. To explain these findings, it is necessary to understand the mechanisms involved in distance and near image focusing of refractive and diffractive-based multifocal intraocular lenses. With refractive-based multifocal intraocular lenses, the only relevant factor is the chromatic dispersion of the material. While in diffractive-based multifocal intraocular lenses there is a combination of chromatic dispersion of the material and dependence on the wavelength of both add power and diffraction efficiency. However, the majority of the reports agree that stereoacuty is preserved in the normal range after binocular implantation of multifocal intraocular lenses and that multifocality does not cause stereoacuity to deteriorate (43).
Table 1. Outcomes of several studies evaluating stereopsis after multifocal IOL implantation.
| First author | Patients | Mean age±SD | IOL | Test | Stereoacuity | Binocular CDVA | Binocular CNVA |
|---|---|---|---|---|---|---|---|
| Häring | 29 | 70.0±11.3 | Array SA-40 N | Titmus | 4.25±0.91 cm* | 0.01±0.17 | 0.31±0.30 |
| Shoji | 26 | 63.7±9.0 | UV360M4-071optex | Titmus | 84.6% ≥ 60” | 100% ≥ 20/20 |
92.3% ≥ J2 100% ≥ J3 |
| Souza | 24 | 68.3±9.2 | AcrySof ReSTOR SA60D3 | Titmus | 92% ≥ 50” | 0.02±0.17 logMAR | 0.02±0.10 logMAR** |
| Chang | 14 13 |
66.5±8.0 64.9±6.1 |
AcrySof ReSTOR SN60D3 ReZoom |
Titmus Titmus |
74.1% ≥ 80” 100% ≥ 80” |
0.06 logMAR -0.04 logMAR |
0.07 logMAR** 0.24 logMAR** |
| Cionni | 15 | N/A | AcrySof ReSTOR SN60D3 | Titmus | 60 ± 30” | N/A | N/A |
| Ferrer-Blasco | 15 | 55.2±2.7 | AcrySof ReSTOR SN60D3 | Titmus | 48.67 ± 1.13 | 0.02± 0.04 logMAR | 0.03±0.04 logMAR |
| Chen | 15 | 71.0±7.0 | ReZoom NXG1/Tecnis ZM900 | Titmus | 62 ± 34 | -0.04±0.09 logMAR | N/A |
| Varon | 24 23 25 28 |
68.1±7.3 69.3±10.7 67.7±7.9 68.2±7.6 |
ReZoom NXG1 AcrySof ReSTOR SN6ADI AcrySof ReSTOR SN60D3 Tecnis ZMA00 |
Titmus/TNO Titmus/TNO Titmus/TNO Titmus/TNO |
240” / 55” Figures / 60” Figures a / 50” 480-Figures/ 50” |
0.00 logMAR 0.02 logMAR 0.01 logMAR 0.00 logMAR |
0.17 logMAR 0.12 logMAR 0.09 logMAR 0.12 logMAR |
| Ferrer-Blasco | 30 | 55.43.8 | AcrySof ReSTOR SN6AD3 | Howard-Dolman method/Titmus/Random dot | 18.42±6.10”/ 44.55±1.08”/ 41.25±1.12” | 0.04±0.05 logMAR | 0.04±0.04 logMAR |
| SD–standard deviation, CDVA–corrected distance visual acuity, CNVA–corrected near visual acuity, N/A–not available, “–arc seconds, *–subjective height quantitatively evaluated, **–at a patient-preferred distance, a–the Figures of the TNO test have stereo-acuity values > 480” | |||||||
Picture 3. Randot test.
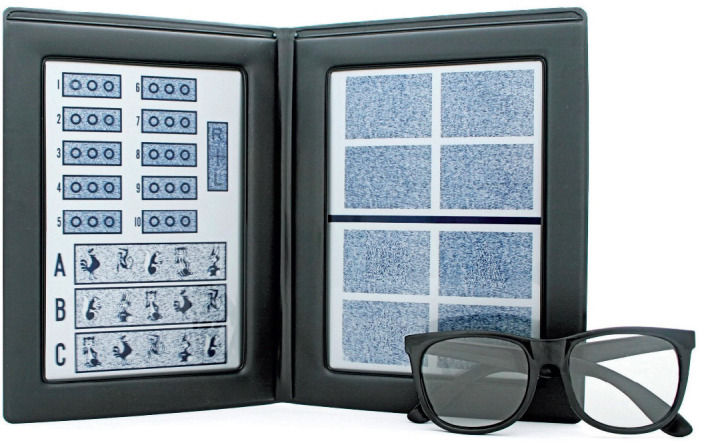
Picture 4. Lang test.

One possible explanation for the favorable results in stereoacuity, besides the improvement in optical properties (eg decrease in photic phenomena and increase in contrast sensitivity, could be the Stiles–Crawford effect (48, 49). The refractive qualities of the multifocal intraocular lens, which lead to the formation of multiple retinal images, “wrap” the retinal receptor cells within “scattered” light. One could hypothesize that in this way, the multifocal intraocular lens comes closer to the optical performance of the natural human lens than unnaturally clear monofocal intraocular lenses with their physically perfect image formation at one focus point. Concerning the physiology of other vertebrates, the following attempt to explain this seemingly paradoxical mechanism can be made: birds and reptiles do not have a microtremor of the eyes, which in mammals prevents local adaption to the viewed retinal image. In mammals, the microtremor limits the quality of the retinal image. In contrast, the retinal cones of birds and reptiles contain tiny drops with an oily consistency, which focus the light within the receptor cells and hence improve the quality of the retinal image. Oily drops of this kind would not be an advantage in mammal eyes because the microtremor inevitably causes the cones to be “wrapped” by light. A cortical program recognizes the limiting factors (microtremor, lateral illumination of the retinal cones) and needs them for the brain to actuate complex random-dot binocular vision. It might be assumed that, as with other visual processes, a “phylogenetic flaw” is part of the physiologic “program” and cannot be eliminated for the brain to recognize the “program” (43).
In a quest to enhance visual quality and reduce unwanted side effects the idea of combining different types of multifocal intraocular lenses, the so-called “Mix and Match”, came into focus. The procedure usually involved implantation or refractive multifocal intraocular lens in the dominant eye and a diffractive multifocal intraocular lens in the non-dominant eye, other options included two bifocal diffractive lenses in which lens of lower addition is implanted in the dominant eye or a combination of the extended range of vision intraocular lens (EDOF) in the dominant eye and diffractive bifocal lens in the nondominant eye. It was reported that those combinations could enhance reading acuity, reading speed and near stereoacuity without obvious photic phenomena consequently decreasing the need for spectacle correction (38). However, combinations of the multifocal intraocular lens in one eye and monofocal lens in the other could compromise stereoacuity (41, 43). The possible reason for those findings could be aniseikonia (43). Although, the amount of aniseikonia tolerated is individually variable and there is no linear relationship between aniseikonia and stereoacuity (17,43,49). The same principle can be applied in monocular multifocal intraocular lens implantation where the other eye is left phakic and stereopsis would depend on the accommodation of the phakic eye (41).
Postoperative follow-up and managing patients’ satisfaction
Due to the visual performances of multifocal intraocular lenses, that is unaided vision at all distances, they came as a solution for presbyopia treatment with crystalline lens exchange. That is called refractive lens exchange (50, 51). Patients who undergo refractive lens exchange generally are younger and have higher expectations regarding refractive and visual outcomes than those with cataracts (51, 52). To enable patients to be spectacle-free while engaging in daily activities, the multifocal intraocular lenses must provide adequate functional vision at various distances (51). Spectacle independence is a useful measure of the performance of a multifocal intraocular lens, however good postoperative visual acuity cannot always guarantee patient satisfaction (35, 56, 57). Postoperative residual refraction and astigmatism, spectacle independence, visual performances under different lighting conditions, postoperative photic phenomena, large pupil size, posterior capsule opacification, and dry eye are correlated to postoperative patient satisfaction (35). Good binocular function does not always correlate with independence from spectacles for near vision which indicates that disturbances of the binocular vision or a high level of aniseikonia do not necessarily correspond to the dependence on spectacles for near vision (35, 41). In addition, patient psychometric characteristics can have a significant impact on patient satisfaction: compulsive checking, orderliness, competence, and dutifulness are significantly correlated to the perception of halos and glare and postoperative dissatisfaction (35). But even with careful selection and perfect surgery, some patients are not as satisfied as could be expected by their clinical findings (35, 53, 54). Most unsatisfied patients reported blurred vision, photic phenomena, or both (35). Studies showed that patient motivation for spectacle independence may be the deciding factor if improvement outweighs the side effects of multifocal intraocular lenses (35, 53, 54). Studies showed that bilateral implantation of MIOL significantly improved distance, intermediate, and near vision as a result of neural summation (55). Nevertheless, overall satisfaction is still high (up to 96%) (51).
5. CONCLUSION(S)
Stereoacuity is not affected by multifocality-induced retinal blur as it is by other causes of image degradation such as small residual refractive error (3, 4) very early opacification of ocular media (8-11) or dry eye. Multifocal intraocular lenses do not cause more functional aniseikonia than would be expected with a monofocal intraocular lens. Since stereoacuity is compromised with unilateral multifocal intraocular lens implantation bilateral implantation should be attempted.
Author’s contribution:
All authors were involved in all steps of preparation this article. Final proofreading was made by the first author.
Conflict of interest:
None of the authors have any conflict to disclose.
Financial support and sponsorship:
Nil.
REFERENCES
- 1.Edwards K, Llewellyn R. 1st. London: Butterworths; 1988. Optometry. [Google Scholar]
- 2.Lehmann OJ, Verity DH, Coombes AGA, Ah-Fat FG, Francis PJ, Ionides ACW. 1st. Oxford: Butterworth-Heinemann; 1988. Clinical optics and refraction 1998. [Google Scholar]
- 3.Wood ILC. Stereopsis with spatially-degrade images. Ophthalmic Physiol Opt. 1983;3:337–340. [PubMed] [Google Scholar]
- 4.Legge GE, Gu Y. Stereopsis and contrast. Vis Res. 1989;29:989–1004. doi: 10.1016/0042-6989(89)90114-4. [DOI] [PubMed] [Google Scholar]
- 5.Saladin JJ. Effects of heterophoria on stereopsis. Optom Vis Sci. 1995;72:487–492. [PubMed] [Google Scholar]
- 6.Reading RE, Tanlamai T. The threshold of stereopsis in the presence of differences in magnification of the ocular images. J Am Optom Assoc. 1908;51:593–595. [PubMed] [Google Scholar]
- 7.Lovasik JV, Szymkiw M. Effects of aniseikonia, anisometropia, accommodation, retinal illuminance, and pupil size on stereopsis. Invest Ophthalmol Vis Sci. 1985;26:741–750. [PubMed] [Google Scholar]
- 8.Jani SN. The age factor in stereopsis screening. Am J Optom Arch Am Acad Optom. 1966;43:653–657. doi: 10.1097/00006324-196610000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Heckmann T, Schor CM. Is edge information for stereoacuity spatially channeled? Vis Res. 1989;29:593–607. doi: 10.1016/0042-6989(89)90045-x. [DOI] [PubMed] [Google Scholar]
- 10.Greene HA, Madden DJ. Adult age difference in visual acuity, stereopsis, and contrast sensitivity. Am J Optom Physiol Opt. 1987;64:749–753. doi: 10.1097/00006324-198710000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Adams AJ, Wong LS, Wong L, et al. Visual acuity changes with age: some new perspectives. Am J Optom Physiol Opt. 1988;65:403–406. doi: 10.1097/00006324-198805000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Kwapiszeski BR, Gallagher CC, Holmes JM. Improved stereoacuity: an indication for unilateral cataract surgery. J Cataract Refract Surg. 1996;22:441–445. doi: 10.1016/s0886-3350(96)80039-3. [DOI] [PubMed] [Google Scholar]
- 13.Laidlaw A, Harrad R. Can second eye cataract extraction be justified? Eye. 1993;7:680–686. doi: 10.1038/eye.1993.155. [DOI] [PubMed] [Google Scholar]
- 14.Lightholder PA, Phillips LJ. Evaluation of the binocularity of 147 unilateral and bilateral pseudophakic patients. Am J Optom Physiol Opt. 1979;56:451–459. doi: 10.1097/00006324-197907000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Highman VN. Stereopsis and aniseikonia in uniocular aphakia. Br J Ophthalmol. 1977;61:30–33. doi: 10.1136/bjo.61.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsumi O, Tanino T, Hirose T. Effect of aniseikonia on binocular function. Invest Ophthalmol Vis Sci. 1986;27(4):601–604. [PubMed] [Google Scholar]
- 17.Katsumi O, Miyajima H, Ogawa T, et al. Aniseikonia and stereoacuity in pseudophakic patients; unilateral and bilateral cases. Ophthalmology. 1992;99:1270–1277. doi: 10.1016/s0161-6420(92)31813-5. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi K, Hayashi H. Stereopsis in bilaterally pseudophakic patients. J Cataract Refract Surg. 2004;30:1466–1470. doi: 10.1016/j.jcrs.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Madrid-Costa D, Cervinõ A, Ferrer-Blasco T, et al. Visual and optical performance with hybrid multifocal intraocular lenses. Clin Exp Optom. 2010;93:426–440. doi: 10.1111/j.1444-0938.2010.00518.x. [DOI] [PubMed] [Google Scholar]
- 20.Klein BE, Klein R, Lee KE. Incidence of age-related cataract over a 10-year interval: the Beaver Dam Eye Study. Ophthalmology. 2002;109(11):2052–2057. doi: 10.1016/s0161-6420(02)01249-6. [DOI] [PubMed] [Google Scholar]
- 21.Elliot DB, Patla A, Furniss M, et al. Improvements in clinical and functional vision and quality of life after second eye cataract surgery. Optom Vis Sci. 2000;77:13–24. doi: 10.1097/00006324-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa T, Desapriya E, Puri M, et al. Evaluating the benefits of second eye cataract surgery among the elderly. J Cataract Refract Surg. 2013;39:1593–1603. doi: 10.1016/j.jcrs.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 23.Datta S, Foss AJE, Grainge MJ, et al. The importance of acuity, stereopsis, and contrast sensitivity for health-related quality of life in elderly women with cataracts. Invest Ophthalmol Vis Sci. 2008;49:1–6. doi: 10.1167/iovs.06-1073. [DOI] [PubMed] [Google Scholar]
- 24.Charalampidou S, Nolan J, Loughman J, et al. Psychophysical impact and optical and morphological characteristics of symptomatic non-advanced cataract. Eye (Lond) 2011;25:1147–1154. doi: 10.1038/eye.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alió JL, Plaza-Puche AB, Piñero DP, et al. Quality of life evaluation after implantation of 2 multifocal intraocular lens models and a monofocal model. J Cataract Refract Surg. 2011;37:638–648. doi: 10.1016/j.jcrs.2010.10.056. [DOI] [PubMed] [Google Scholar]
- 26.Holladay JT, van Dijk H, Lang A, et al. Optical performance of multifocal intraocular lenses. J Cataract Refract Surg. 1990;16:413–422. doi: 10.1016/s0886-3350(13)80793-6. [DOI] [PubMed] [Google Scholar]
- 27.Davison JA, Simpson MJ. History and development of the apodized diffractive intraocular lens. J Cataract Refract Surg. 2006;32:849–858. doi: 10.1016/j.jcrs.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Percival SP. Prospective study of the new diffractive bifocal intraocular lens. Eye (Lond) 1989;3:571–575. doi: 10.1038/eye.1989.89. [DOI] [PubMed] [Google Scholar]
- 29.Vingolo EM, Grenga P, Iacobelli L, et al. Visual acuity and contrast sensitivity: AcrySof ReSTOR apodized diffractive versus AcrySof SA60AT monofocal intraocular lenses. J Cataract Refract Surg. 2007;33:1244–1247. doi: 10.1016/j.jcrs.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 30.Pieh S, Lackner B, Hanselmayer G, et al. Halo size under distance and near conditions in refractive multifocal intraocular lenses. Br J Ophthalmol. 2001;85:816–821. doi: 10.1136/bjo.85.7.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gil MA, Varon C, Rosello N, et al. Visual acuity, contrast sensitivity, subjective quality of vision, and quality of life with 4 different multifocal IOLs. Eur J Ophthalmol. 2012;22:175–187. doi: 10.5301/EJO.2011.8371. [DOI] [PubMed] [Google Scholar]
- 32.Mencucci R, Favuzza E, Caporossi O, et al. Comparative analysis of visual outcomes, reading skills, contrast sensitivity, and patient satisfaction with two models of trifocal diffractive intraocular lenses and an extended range of vision intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2018 Jul 6; doi: 10.1007/s00417-018-4052-3. [DOI] [PubMed] [Google Scholar]
- 33.Pedrotti E, Carones F, Aiello F, et al. Comparative analysis of visual outcomes with 4 intraocular lenses: monofocal, multifocal, and extended range of vision. J Cataract Refract Surg. 2018 Feb;44(2):156–167. doi: 10.1016/j.jcrs.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Cillino G, Casuccio A, Pasti M, et al. Working-age cataract patients: visual results, reading performance, and quality of life with three diffractive multifocal intraocular lenses. Ophthalmology. 2014;121:34–44. doi: 10.1016/j.ophtha.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 35.Mester U, Vaterrodt T, Goes F, et al. Impact of personality characteristics on patient satisfaction after multifocal intraocular lens implantation: results from the “Happy Patient Study”. J Refract Surg. 2014;30(10):674–678. doi: 10.3928/1081597X-20140903-05. [DOI] [PubMed] [Google Scholar]
- 36.de Vries NE, Webers CA, Touwslager WR, et al. Dissatisfaction after implantation of multifocal intraocular lenses. J Cataract Refract Surg. 2009;35:859–997. doi: 10.1016/j.jcrs.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 37.Woodward MA, Randleman JB, Stulting RD. Dissatisfaction after multifocal intraocular lens implantation. J Cataract Refract Surg. 2009;35:992–997. doi: 10.1016/j.jcrs.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen W, Meng Q, Ye H, et al. Reading ability and stereoacuity with combined implantation of refractive and diffractive multifocal intraocular lenses. Acta Ophthalmol. 2011;89:376–381. doi: 10.1111/j.1755-3768.2009.01702.x. [DOI] [PubMed] [Google Scholar]
- 39.Alfonso JF, Fernández-Vega L, Valcárcel B. Visual performance after AcrySof ReSTOR aspheric intraocular lens implantation. J Optom. 2008;1:30–35. doi: 10.3921/joptom.2008.30. [DOI] [Google Scholar]
- 40.Ferrer-Blasco T, Madrid Costa D, García-Lázaro S, et al. Stereopsis in bilaterally multifocal pseudophakic patients. Graefes Arch Clin Exp Ophthalmol. 2011;249:245–251. doi: 10.1007/s00417-010-1558-8. [DOI] [PubMed] [Google Scholar]
- 41.Shoji N, Shimizu K. Binocular function of the patient with the refractive multifocal intraocular lens. J Cataract Refract Surg. 2002;28:1012–1017. doi: 10.1007/s00417-010-1558-8. [DOI] [PubMed] [Google Scholar]
- 42.Varón C, Gil MA, Alba-Bueno F, et al. Stereo-acuity in patients implanted with multifocal intraocular lenses: is the choice of stereo test relevant? Current Eye Research. 2014;39(7):711–719. doi: 10.3109/02713683.2013.865758. [DOI] [PubMed] [Google Scholar]
- 43.Häring G, Gronemeyer A, Hedderich J, et al. Stereoacuity and aniseikonia after unilateral and bilateral implantation of the Array refractive multifocal intraocular lens. J Cataract Refract Surg. 1999;25:1151–1156. doi: 10.1016/s0886-3350(99)00136-4. [DOI] [PubMed] [Google Scholar]
- 44.Chang DF. Prospective functional and clinical comparison of bilateral ReZoom and ReSTOR intraocular lenses in patients 70 years or younger. J Cataract Refract Surg. 2008;34:934–941. doi: 10.1016/j.jcrs.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 45.Cionni RJ, Chang DF, Donnenfeld ED, et al. Clinical outcomes and functional visual performance comparison of the ReSTOR apodized diffractive intraocular lens to a monofocal control. Br J Ophthalmol. 2009;93:1215–1219. doi: 10.1136/bjo.2008.146647. [DOI] [PubMed] [Google Scholar]
- 46.Souza CE, Muccioli C, Soriano ES, et al. Visual performance of AcrySof Restor apodized diffractive IOL: A prospective comparative trial. Am J Ophthalmol. 2006;141:827–832. doi: 10.1016/j.ajo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 47.Ferrer-Blasco T, Montés-Micó R, Cerviño A, et al. Stereoacuity after refractive lens exchange with AcrySof ReSTOR intraocular lens implantation. J Refract Surg. 2009;25:1000–1004. doi: 10.3928/1081597X-20091016-05. [DOI] [PubMed] [Google Scholar]
- 48.Stiles WS, Crawford BH. The luminous efficiency of rays entering the eye pupil at different points. Proc Roy Soc B. 1993;112:428–450. [Google Scholar]
- 49.Lakshminarayann V, Bailey JE, Enoch JM. The optics of phakic, pseudophakic, and aphakic eyes: effect of the Stiles-Crawford (SCE I) function. Optom Vision Sci. 1993;70:404–408. [PubMed] [Google Scholar]
- 50.Dick HB, Gross S, Tehrani M, et al. Refractive lens exchange with an array multifocal intraocular lens. J Refract Surg. 2002;5:505–518. doi: 10.3928/1081-597X-20020901-04. [DOI] [PubMed] [Google Scholar]
- 51.Chang JS, Ng JC, Chan VK, et al. Visual Outcomes, and patient satisfaction after refractive lens exchange with a single piece diffractive multifocal intraocular lens. J Ophthalmol. 2014;2014:458296. doi: 10.1155/2014/458296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westin O, Koskela T, Behndig A. Epidemiology and outcomes in refractive lens exchange surgery. Acta Ophthalmol. 2015;93(1):41–45. doi: 10.1111/aos.12460. [DOI] [PubMed] [Google Scholar]
- 53.Hofmann T, Zuberbuhler B, Cervino A, et al. Retinal straylight and complaint scores 18 months after implantation of the AcrySof monofocal reSTOR diffractive intraocular lenses. J Refract Surg. 2009;25:485–492. doi: 10.3928/1081597X-20090512-02. [DOI] [PubMed] [Google Scholar]
- 54.Calladine D, Evans JR, Shah S, et al. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst Rev. 2012 Sep 12;(9):CD003169. doi: 10.1002/14651858.CD003169.pub3. [DOI] [PubMed] [Google Scholar]
- 55.Tsaousis KT, Plainis S, Dimitrakos SA, et al. Binocularity enhances visual acuity of eyes implanted with multifocal intraocular lenses. J Refract Surg. 2013;29(4):246–250. doi: 10.3928/1081597X-20130318-03. [DOI] [PubMed] [Google Scholar]


