Abstract
The free and bound phenolic constituents in Dendrocalamus hamiltonii shoots were evaluated and compared to processed bamboo candy. Preliminary proximate analysis revealed a percent reduction in moisture and protein with a less significant change in fibre content. The fresh free phenolic extract (FFPE) exhibited a total phenolics of 131.22 mg GAE/g and recovered 48.29 mg GAE/g phenolic content in bound fraction (FBPE). Results demonstrated higher loss of free phenolics after processing compared to bound fraction (CBPE). Although similar results were observed in total flavonoid content. Antioxidant activity was reduced after candy processing, with fresh shoots having the lowest percent inhibition (IC50) against DPPH· and ABTS· radicals. Although both free and bound fractions of candy demonstrated effective antioxidant activity. HPLC analysis revealed that FFPE contained more chlorogenic acid (0.14 mg/10 g) and cinnamic acid (0.75 mg/10 g) than CFPE. Quercetin was undetected in all free fractions but was found in bound form.
Keywords: Bamboo shoots, Free phenolics, Bound phenolics, Antioxidant activity, High performance liquid chromatography, Scanning electron microscope
Introduction
Bamboo is referring as the most valuable edible plant distributed throughout the world about 280 species within 10 genera. There is a number of species like Dendrocalamus hamiltonii, Bambusa tulda, Bambusa vulgaris and Phyllostachys edulis which are harvested and consumed as a vegetable in the form of fresh, sliced, fermented and canned food (Nirmala et al., 2018). They are employed in a large number of Asian dishes and also available in markets. Even in India, different bamboo food products have been made like candy, chutney, chukh, and crackers (Bajwa et al., 2016). Because of the diversified nature of bamboo shoots, it is referred to as ‘the top-grade vegetable (Zhang et al., 2011). The edible portion of bamboo shoot is covered by protective non-edible leaf sheaths comprised of meristematic tissue which is the regions of rapid cell multiplication (Luo et al., 2012). Bamboo shoots also gained popularity for its health benefits and its potential ingredient for modern functional foods. For improving the living standard and demand of natural foods, especially organic and natural food has been greatly increased. So, it is important to establish a link between natural diet and health benefits, a wide range of research was made in all sorts of food products. These products mainly include functional foods and nutraceuticals that confer health benefits to consumer (Malla et al., 2014).
Polyphenols play a vital role in the production of nutraceuticals. Hence, phenolics widely present in the fruits, vegetables and cereals grains work foremost for health benefits as bioactive compounds that prevent many chronic diseases including antiinflammatory, antithrombotic, antibacterial, antiallergic, antiviral and vasodilation (Rockenbach et al., 2011; Sharma et al. 2012; Inglett et al., 2011; Dadwal and Gupta, 2021). Similarly, bamboo phenolics are well known for its antioxidants, antiaging, cardiovascular disease, and anticancer activity (Kaliora and Dedoussis, 2007) and it could be act as a medicinal food. Free and bound phenolics are most discussed topic in present scientific community. In plant matrix, bound phenolic compounds are covalently attached which makes its extraction difficult with any organic solvents (Pérez-Jiménez and Torres, 2011). Through ester bonds these phenolic compounds covalently combine to pectin, cellulose and polysaccharides and become hard to hydrolyze (Cuevas Montilla et al., 2011). As such work is already performed on crops like wheat (Chandrasekara and Shahidi, 2011) and fruits like litchi (Su et al., 2014). These free and bound phenolics are generally deteriorate and reduced during food processing and a significant loss has previously been observed. Processing involves higher temperatures, dissolution during washing, pH, and processing environments. As a result, extensive research is required to determine the potential losses of health-promoting phenolics for the enhancement of future product development procedures. Considering the health benefits of edible bamboo shoots, a nutritional and phenolic profiling was performed in their free and bound forms while compared with processed sweet bamboo candy. Previously, the antioxidant activities, phenolics, flavonoids, and ascorbic acid content has been determined in D. hamiltonii shoots (Dadwal et al., 2022). However, there hasn’t been much progress in terms of free and bound phenolics in D. hamiltonii shoots, followed by the candy processing effect. As a result, present study compares free and bound phenolics in D. hamiltonii shoots in fresh and residual fractions and determining nutritional and phenolic content changes after developing commercially consumed bamboo candy.
Materials and methods
Procurement of raw material
D. hamiltonii bamboo shoots were obtained from the plantations raised at CSIR-Himalayan Bio-resource Technology, Palampur fields in the month of July–August 2020.
Pre-processing of bamboo shoots
The fresh bamboo shoots were washed properly under tap water to clean the impurities, unwanted hairs and dust particles adhere to it. Thereafter, shoots were boiled for 20 min to eliminate bitter compounds containing cyanogenic glycosides like taxiphyllin (Sonar et al., 2015). Fresh bamboo shoots and its processed bamboo candy were dried in hot air oven at 40 °C (Macro Scientific Work, Pvt, Ltd., India) and for further analysis dried shoots were ground into fine powder using a grinder (Philips Electronics, India).
Chemicals and reagents
All the chemical compounds including methanol, ethanol, diethyl ether, hexane, ethyl acetate, sodium hydroxide, and hydrochloric acid were acquired from Himedia Laboratories, Mumbai. The chemicals like Folin-Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl, 2,2′-azino-bis 3-ethylbenzothiazoline-6-sulphonic acid, HPLC grade acetonitrile, trifloroacetic acid and phenolic standards like cinnamic, gallic, chlorogenic and p-coumaric acid, catechin, pro-catechuic acid was purchased from Sigma-Aldrich, Bangalore.
Proximate analysis
The moisture content, ash, fat (Soxhlet extraction method) and crude fibre of fresh bamboo shoot and candy were determined by following the protocols given by Godswill (2019). The amount of protein was recorded by using modified Lowry method (Mæhre et al. 2018) and for the estimation of sugar content anthrone, reagent test procedure was followed as described by Pandey and Ojha (2014).
Free phenolic extraction
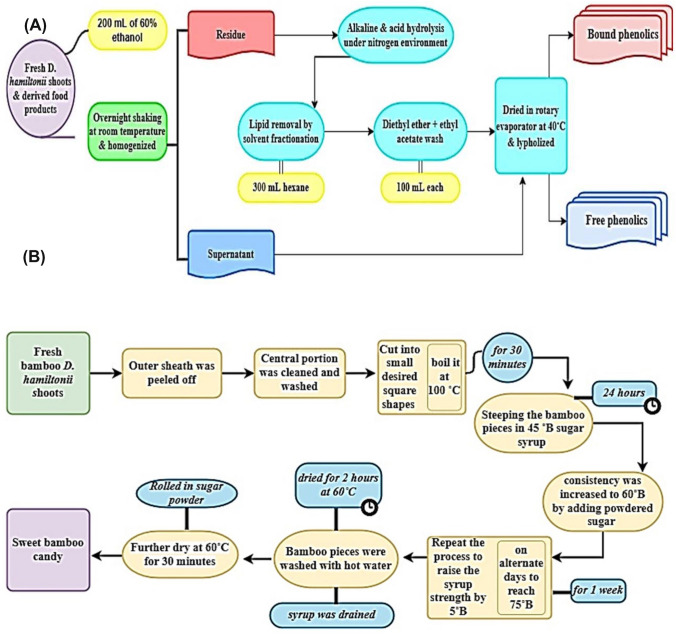
Isolation of free phenolic compounds was conducted by the procedure described in Verardo et al. (2011) with slight modifications. The dried powder (20 g) of fresh bamboo shoots and processed bamboo candy was treated with 60% ethanol (200 mL) and homogenized for 10 min. Then it was kept for overnight shaking (500 rpm) at room temperature. After that the supernatant containing free phenols was filtered and dried in rotary-evaporator at 40 °C. Thereafter, each sample was lyophilized at − 80 °C. Residue part containing bound fraction was carried out for further hydrolysis (Fig. 1A).
Fig. 1.
Free and bound phenolics extraction method and processing of bamboo candy
Bound phenolic extraction
The residual portion of free phenolic extraction was hydrolyzed with 5 M sodium hydroxide (300 mL) with continuous shaking under nitrogen gas for 4 h at room temperature. Alkaline sample was further treated with 1 N hydrochloric acid till at pH, 2–3 under ice cold condition. For the removal of lipids, extraction was made under hexane (300 mL). Finally, the solution was treated with ethyl acetate: diethyl ether (1:1 v/v, 100 mL). Then the organic fractions were separated and dried at 40 °C at rotary evaporator followed by lyophilization at − 80 °C (Fig. 1A) (Verardo et al., 2011).
Total phenolic content
The amount of total phenolic content of fresh free phenolic extract (FFPE), fresh bound phenolic extract (FBPE), candy free phenolic extract (CFPE) and candy bound phenolic extract (CBPE) was calculated by using Folin–Ciocalteu method (Dadwal et al., 2021) with slight modifications. Folin-ciocalteu reagent (500 µL, 1 N) was added to the prepared extracts followed by 7.5% saturated sodium bicarbonate (100 µL). For half an hour the reaction mixtures were incubated at ambient temperature. Thereafter in Shimadzu UV–Vis spectrophotometer the absorbance was noted at 730 nm against blank. All determinations were replicated thrice and gallic acid was used as standard. The results were denoted in milligram equivalents of gallic acid per gram of extract (mg/g GAE of extracts).
Total flavonoid content
Aluminum chloride method (Dadwal et al., 2021) was followed for the evaluation of total flavonoid content. In a fraction of diluted ethanolic extract (1 mL), sodium nitrite (5%) was added (incubated for 5 min.) followed by 10% aluminum chloride (incubation for 6 min). After adding 1 M sodium hydroxide the reaction mixture was left for incubation for 10 min at ambient temperature. At 510 nm absorbance was recorded against blank and using quercetin as positive control. The amount of total flavonoid can be indicated as µg RE/mg Rutin equivalent (RE) of extracted compound. All determinations were carried out in triplicates.
DPPH· free radical scavenging assay
The antioxidant capacity of FFPE, FBPE, CFPE and CBPE was measured according to the procedure given by Joshi et al., 2015, however some modifications were also made. Bamboo shoot extract (50–500 µL) was mixed with 0.1 mM DPPH· (50 µL) in methanol (95%). At ambient temperature the mix was kept for incubation in dark for 30 min. The amount of DPPH· was determined using kinetic bio spectrophotometer (Eppendorf) at 517 nm. The % inhibition was calculated by using the given equation:
A0 is the absorbance of control, A1: absorbance of sample.
ABTS· free radical scavenging assay
For the determination of ABTS· free radical scavenging activity previously described method by Joshi et al. (2015) was used for FFPE, FBPE, CFPE and CBPE extracts. Radical cation ABTS· was prepared through oxidation of 2, 2′-azinobis-(3-ethyl-benzothiazoline-6-sulphonate) by potassium persulfate. A mixture of 7 mM ABTS· (5 mL) and 140 mM potassium persulphate (88 µL) was kept overnight in dark at ambient temperature. Afterwards this working solution was diluted with ethanol till it reached absorbance of 0.70 ± 0.05 at 734 nm. Aliquots of 20 µL of ethanolic extract with different concentrations was then mixed with ABTS· working solution (980 µL), followed by incubation in the dark for 10 min at ambient temperature. Further, at a wavelength of 734 nm absorbance was noted in kinetic Bio-spectrophotometer (Eppendorf). The ABTS· free radical-scavenging activity of the samples was calculated by using following equation:
A0 is the absorbance of control, A1 is the absorbance of sample.
Hydroxyl radical scavenging (OH·) Assay
The scavenging ability of hydroxyl radical was evaluated by the method described by Ajibola et al., 2011 with modest changes. A part of FFPE, FBPE, CFPE and CBPE (25 µL) was mixed with 3 mM of ferrous sulphate (25 µL) and 3 mM 1,10-phenanthroline (25 µL) dissolved in 0.1 M phosphate buffer (pH 7.4). To initiate the reaction 0.01% (v/v) hydrogen peroxide (25 µL) was added to it. The reaction mixture was incubated for 1 h at 37 °C and the absorbance was recorded at 536 nm using a UV/VIS spectrophotometer. Hydroxyl radical-scavenging capacity was found according to the given equation:
A0: absorbance of control, A1: absorbance of sample.
HPLC quantification for ascorbic acid and phenolic compounds
Ascorbic acid and phenolic compounds were observed in FFPE, FBPE, CFPE and CBPE were analyzed using a Shimadzu analytical HPLC with column oven (C40-10ASVP), auto-sampler (SIL-10AF), vacuum solvent degas module model-DGU-20A5 and diode-array detector model-CBM-20A, auto-sampler model-SIL-20AC. The mobile phases were (A) 0.1% TFA (trifloro acetic acid) in water and (B) acetonitrile in gradient elution. Ascorbic acid was identified using isocratic solvent system with 0.1% trifloroacetic acid as mobile phase. Injection volume was 20 µL. Method used on this system was prescribed by Tanaka et al. (2014) with some modifications.
Morphological examination using scanning electron microscopy (SEM)
SEM was used to study the microstructural variations in the fresh bamboo shoots and bamboo candy using a modified Nunes et al. (2008) technique. The samples were first dried at room temperature, then fixed on a steel surface and coated with gold using a sputter coating equipment (at 10 Pa vacuum for 10 s) and double-sided carbon tape (E1010 ion sputter Hitachi, Japan). The final photos were acquired on a SEM (Hitachi S3400N) and preserved for future reference.
Statistical analysis
The mean total phenol content and antioxidant activity of all the four samples including FFPE, FBPE, CFPE and CBPE were compared using one-way ANOVA process. Statistically significant difference was performed at p < 0.01. All the results were statistically analyzed using GraphPad prism software and Microsoft excel.
Result and discussion
Polyphenols are the secondary metabolites distributed throughout the plant kingdom at different concentration. Currently, based on their degree of solubility and binding covalent nature they are classified into intact and non-intact form. Some phenolics being soluble in nature could easily be extracted by the simple solvent method of extraction but the bound phenolic compounds occur in covalently intact structure. These compounds can only be extracted by alkaline hydrolysis method or other enzyme and acid hydrolysis techniques (Verardo et al., 2011). The composition of free and bound phenolics and their activity were analyzed in the both the cases of fresh and candy of bamboo shoots.
Proximate composition
Food processing has a significant impact on primary metabolites (Arias-Rico et al., 2020). As a result, free and bound phenolics in fresh bamboo shoots were analyzed and compared to bamboo candy for proximate composition. There was no significant difference in fat, although a slight increase in ash content was observed in the bamboo candy (p < 0.05), which could be due to the addition of sugar bound while preparing the candy (Table 1). The fibre content of fresh bamboo shoots and candy was also reduced slightly, from 6.67 to 6.17%, respectively. Which could be the result of washing and boiling bamboo shoots prior to processing. However, moisture content was found to be significantly decreased in bamboo candy (24.81%) as compared to fresh bamboo shoots containing 78.41% moisture (p < 0.05). Conversely, protein content was also significantly decreased in candy with an amount of 3.87% whereas 7.87% of protein was recorded in fresh shoots (p < 0.05). Likewise in case of sugar content, very less amount (2.28%) was found in fresh bamboo shoots but increased to a significant amount in bamboo candy containing 31.76% sugar (p < 0.05) as it was dipped in sugar syrup during processing. Overall, the proximate parameters were very supported by previous studies (Dadwal et al., 2022).
Table 1.
Proximate analysis of fresh bamboo shoots and bamboo candy
| Proximate parameters | Fresh bamboo shoots | Bamboo candy |
|---|---|---|
| Moisture (%) | 78.41 ± 2.10a | 24.81 ± 0.10b |
| Ash (%) | 1.28 ± 0.05b | 2.04 ± 0.05a |
| Protein (%) | 4.87 ± 0.24a | 3.87 ± 0.02b |
| Fat (%) | 0.29 ± 0.002a | 0.27 ± 0.001b |
| Fibre (%) | 6.67 ± 0.08a | 6.17 ± 0.05b |
| Sugar (%) | 2.28 ± 0.05b | 31.76 ± 1.22a |
Results are presented as the mean ± standard deviation (n = 3). Superscripts letter (a and b) represents the significance difference at p < 0.05 with in the row
Total phenolic and flavonoid content in free/bound fractions
The data for total phenolic content of free and bound fractions in fresh bamboo shoots and bamboo candy are clearly tabulated in Table 2. The phenolic content of free or soluble (131.22 ± 1.6 mg GAE/mg) and bound fraction (48.29 ± 0.8 mg GAE/mg) were found maximum in fresh D. hamiltonii bamboo shoots. Likewise, total phenolic content of free fraction (98.42 ± 1.2 mg GAE/mg) and bound fraction (46.96 ± 1.2 mg GAE/mg) was also observed in the D. hamiltonii bamboo candy. Total flavonoids were quantified in free fraction (189.66 ± 0.2 mg RE/mg) and bound fraction (110.00 ± 0.1 mg RE/mg) of fresh D. hamiltonii bamboo shoots. Similarly, total flavonoids in free fraction (106.97 ± 0.6 mg RE/mg) and bound fraction (97.67 ± 0.5 mg RE/mg) were also reported in the D. hamiltonii bamboo candy. The yield of phenolics was less encountered by Sonar et al. (2015) because of the different mode of extraction process, but still noticeable changes were observed in this study. Results illustrated that phenolics and flavonoid content was much higher in free forms as compared to bound phenolics. But it reduced to a large extent when it was compared to candy. During the food processing, D. hamiltonii bamboo shoots were treated with multiple washing. Hence, a large fraction of free or soluble phenolics were washed out during the removal of volatile cyanogenic compounds named taxiphyllin (Haque and Bradbury, 2002). Further candy processing, hot water boiling, dipped in hot syrup (60 °C–70 °C) and dried at 60 °C were applied for healthier acceptability of the final product (Fig. 1B). Such temperature variations result the significant declination of phenolic constituents with a greater extend. Earlier reports also been demonstrated that a continuous variation in phenolic content when the temperature rises from 50 to 100 °C (Réblová, 2012; Chen and Lin, 2007; Kong et al., 2007). The results showed that there was no significant loss of bound phenolics and flavonoids, but a slight change could be due to heating, which penetrates deeply inside the solid matrices and causes structural deformation. Dipping in hot sugar syrup causes sugar to acquire the spaces and generate stress on natural structural arrangements, which may result in loss of bound phenolics. Previously, Yeo, and Shahidi (2017) discussed how boiling causes the loss of insoluble phenolics by loosening the cellular matrices. Similar noticeable changes in free and bound phenolics were detected in this research.
Table 2.
Phenolic profile of free and bound fractions of fresh D. hamiltonii shoots and processed bamboo candy and antioxidant activity and IC50 values of bamboo extracts using DPPH and ABTS assays
| Assays | Fresh bamboo shoots | Bamboo candy | ||
|---|---|---|---|---|
| FFPE | FBPE | CFPE | CBPE | |
| Total phenolic content (mg GAE/mg) | 131.22 ± 1.6* | 48.29 ± 0.8 | 98.42 ± 1.2 | 46.96 ± 1.2 |
| Total flavonoid content (mg RE/mg) | 189.66 ± 0.2 | 110.00 ± 0.1 | 106.97 ± 0.6 | 97.67 ± 0.5 |
| Hydroxyl reducing assay (% inhibition) | 89.78 | 34.09 | 61.71 | 41.46 |
| IC50 of radical scavenging activity (µg/mL) | ||||
| DPPH | 62.8 ± 1.22 | 210.5 ± 1.87 | 131.2 ± 1.05 | 283.2 ± 0.85 |
| ABTS | 195.1 ± 1.61 | 334.5 ± 2.18 | 117.6 ± 1.05 | 239.2 ± 2.05 |
FFPE fresh free phenolic extract, FBPE fresh bound phenolic extract, CFPE candy free phenolic extract, CBPE candy bound phenolic extract
*Values are the mean of three replicates ± standard deviation, values with common letters in each column do not differ statistically according to Duccans’ Multiple Range Test at p ≤ 0.01
Hydroxyl radical scavenging (HO·) activity of extracts
The highly reactive HO· radical retaliate moieties of the cell membrane and can react with almost any molecule in its neighbor. It is responsible for most oxidative damage to DNA, proteins and lipids. These radicals are strongly reactive species in the biological system and in the human body there is no specific enzyme to defend against them (Lobo et al., 2010). Therefore, it is requisite to discover a good scavenger molecule.
The HO· radical scavenging activity of the various free and bound extracts was observed (Table 2). In all the extracts, with increase in concentration strong hydroxyl radical scavenging potential was reflected. Maximum scavenging ability of free phenolic compounds was found in fresh D. hamiltonii bamboo shoots. At concentration of 1 mg/mL an inhibition of 89.78 and 34.09% was noticed in its free and bound form, respectively. Free phenolics in D. hamiltonii bamboo candy showed an inhibition of 61.71% and in bound phenolics 41.46% inhibition at 1 mg/mL. Results illustrated the scavenging ability of hydroxyl radical was decreased when shoots were applied for the simple candy processing. But a large proportion has remained in the bound form that could be assimilated by the body when consumed. Such results can be compared with experiment performed by Sandhiya et al. (2013) on the leaf extracts of Bambusa arundinacea for similar activity. Scavenging activity of free and bound phenols was compared in candy which results the loss of activity.
Effect on antioxidative properties
DPPH· and ABTS· have extensively been used as in vitro free radical scavenging assays to evaluate the reducing substances of compounds. The antioxidant activity was recorded as decline in the absorbance at 517 and 734 nm for DPPH· and ABTS·, respectively. The phenolic compounds as hydrogen donating antioxidant emerge in samples to scavenge the free radicals by making a non-radical form DPPH-H and ABTS· to form ABTS-H. The IC50 is defined as concentration of extract that led to decrease in the initial concentration of DPPH· and ABTS· free radicals by 50%. Minimum IC50 employs maximum antioxidant activity of an extract. IC50 for ascorbic acid by using DPPH· (3.25 µg/mL ± 0.03) and ABTS· (3.45 µg/mL ± 0.06) assays were used as a standard. From the results of DPPH·, lowest IC50 was noted in FFPE (62.8 µg/mL ± 1.2) compared to FBPE (210.5 µg/mL ± 1.87). In the other hand, higher IC50 was noticed in CFPE (131.2 µg/mL ± 1.05) compared to CBPE (283.2 µg/mL ± 0.85). As per ABTS· assay, the value for IC50 in FFPE and FBPE was found as 195.1 µg/mL ± 1.61 and 334.5 µg/mL ± 2.18, respectively. Whereas in CFPE IC50 was observed with a concentration of 117.6 µg/mL ± 1.05 and in CBPE 239.2 µg/mL ± 2.05 IC50 was recorded (Table 2). Maximal radical-scavenging potential was observed by DPPH· as opposed to ABTS·. These antioxidant activities were strongly related to the content of phenolics and flavonoids, which tend to decrease after bamboo processing for candy preparation. The antioxidant activities of extracts against both DPPH· and ABTS· radicals were reduced, indicating that fewer phenolic constituents participated in the antioxidant reaction. Processing results loss of antioxidant activity was previously demonstrated by Yeo, and Shahidi (2017) also supporting present results. Similar study was also performed on wheat and porcino flour regarding changes in the free and bound free radical scavenging activity which supports current investigation in bamboo shoots and bamboo candy (Stojanović et al., 2014). It was concluded that free radical scavenging activity declined while moving from free to bound phenolics followed by bamboo processing for food formulations.
Correlation among antioxidant activities
Extracted fresh bamboo shoots and derived bamboo candy were taken at the rate of 50 µg/mL concentration. Free phenolic compounds showed highest antioxidant activities than bound phenolic compounds in both fresh bamboo shoots and candy. The correlation allying in these two antioxidant activities was noted as r2 = 0.447. Similar correlation was noted by Li et al. (2013) demonstrated in buckwheat brans.
Correlation among phenolic content and antioxidant activity
The antioxidant potential of fresh bamboo extracts and developed bamboo candy acquired from DPPH· and ABTS· assays equated well with total phenolic content. The linear correlations attained among total phenolic content and antioxidant capacity of given methods were recorded as r2DPPH = 0.180 and r2ABTS = 0.777 (Fig. 2).
Fig. 2.
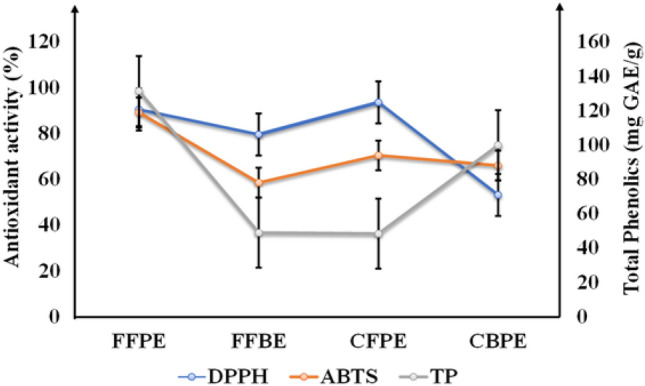
Antioxidant activity and correlation between of fresh bamboo shoots and processed bamboo candy phenolics versus antioxidant
Estimation of ascorbic acid and phenolic compounds using HPLC
Ascorbic acid is an essential constituent required for the functioning of immune system also count in the making of collagen, tissue repair, production of neurotransmitters and structural material for bones, skin, and blood vessels. Level of ascorbic acid in the fresh bamboo shoots was detected as 0.35 µg/mg, which was higher compared to bound phenolic extract (0.23 µg/mg). Similarly, in bamboo candy 0.18 µg/mg amount of ascorbic acid has been detected in free form and 0.17 µg/mg in its bound fraction. It is evident from given data that the amount of ascorbic acid remained in the bound form. However, when it was employed for candy processing the ascorbic acid amount was declined to a large extend which was about half of the total amount detected in fresh D. hamiltonii shoots. Again, food processing reduces the total ascorbic acid. This could be the result of numerous temperature treatments and multiple washing treatments while removing the toxic taxiphyllin (Haque and Bradbury, 2002). A temperature dependent ascorbic acid loss has previously been determined in fruit juices showed the decrease in level of ascorbic acid with the increase in temperature (El-Ishaq and Obirinakem, 2015).
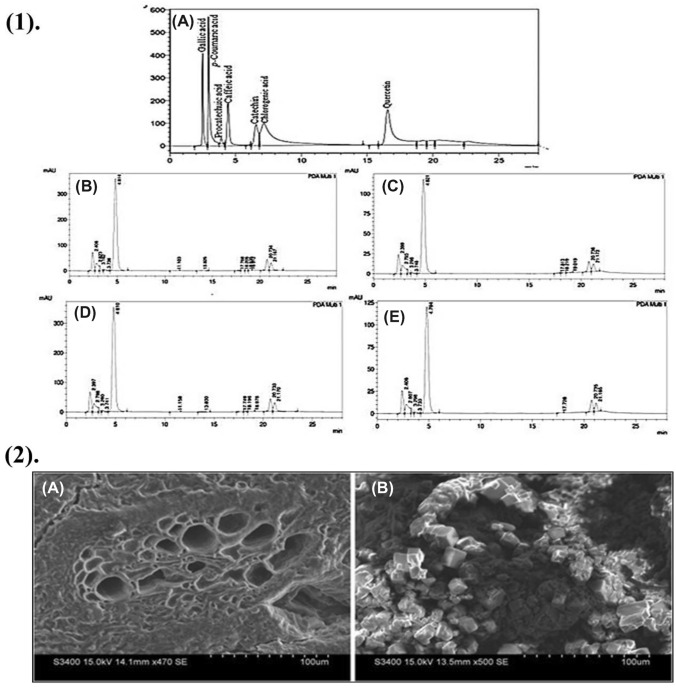
Multifunctional nature including free radical scavenger, singlet oxygen quencher and metal chelators action of polyphenols makes it a quality bioactive compound (Kris-Etherton et al., 2004). Its presence in bamboo shoots makes it an excellent edible healthy item that makes it a medicinal food. Phenolics loss during the different cooking methods has previously been reported (Zhang et al., 2011). Bamboo polyphenols were already reported previously (Chongtham et al., 2011). Although present study demonstrated cinnamic acid content in FFPE and FBPE as 0.75 ± 0.002 mg/10 g and 0.47 ± 0.005 mg/10 g, respectively. Whereas CFPE shown slight reduction after processing as 0.52 ± 0.001 mg/10 g and increased in bound fraction as 0.60 ± 0.001 mg/10 g. In case of chlorogenic acid in FFPE was recorded as 1.44 ± 0.01 mg/10 g and 1.09 ± 0.006 mg/10 g in FBPE. On the other hand, the amount of chlorogenic acid was lesser in CFPE (0.62 ± 0.002 mg/10 g) and CBPE (0.57 ± 0.01 mg/10 g). p-Coumaric acid was only reported in FFPE (10.2 ± 0.01 mg/10 g) and FBPE (10.13 ± 0.05 mg/10 g). Similarly, gallic acid was only present in FFPE (0.14 ± 0.02 mg/10 g) and FBPE (0.09 ± 0.01 mg/10 g) form but absent in processed bamboo candy. Quercetin was detected only in the bound forms as 0.69 ± 0.02 mg/10 g and 0.65 ± 0.008 mg/10 g in FBPE and CBPE, respectively (Table 3; Fig. 3 (1)). The antioxidant property showed that bound phenols had decreased antioxidant capacities in fresh D. hamiltonii bamboo shoots and its processed bamboo candy. A wide range of changes cleared the concept of phenolic loss in a different prospective. It was examined from the results that chlorogenic acid and cinnamic acid were present in both free and bound forms of fresh and food candy of D. hamiltonii bamboo shoots. p-Coumaric and gallic acid were present in all free and bound form of fresh D. hamiltonii (Table 3). Quercetin was detected only in the bound forms and some phenolic compounds such as protocatechuic acid and catechin were completely undetected in all extracts.
Table 3.
HPLC quantification of free and bound phenolics and ascorbic acid content in fresh bamboo shoots and bamboo candy
| Extracts | p-Coumaric acid | Gallic acid | Cinnamic acid | Chlorogenic acid | Quercetin | Ascorbic acid |
|---|---|---|---|---|---|---|
| (mg/10 gextract basis) | ||||||
| FFPE | 10.2 ± 0.01* | 0.14 ± 0.02 | 0.75 ± 0.002 | 1.44 ± 0.01 | ND | 3.5 ± 0.02 |
| FBPE | 10.13 ± 0.05 | 0.09 ± 0.01 | 0.47 ± 0.005 | 1.09 ± 0.006 | 0.69 ± 0.02 | 2.3 ± 0.07 |
| CFPE | ND | ND | 0.52 ± 0.001 | 0.62 ± 0.002 | ND | 1.8 ± 0.05 |
| CBPE | ND | ND | 0.60 ± 0.001 | 0.57 ± 0.01 | 0.65 ± 0.008 | 1.7 ± 0.02 |
FFPE fresh free phenolic extract, FBPE fresh bound phenolic extract, CFPE candy free phenolic extract, CBPE candy bound phenolic extract
*Values are the mean of three replicates ± standard deviation, values with common letters in each column do not differ statistically according to Duccans’ Multiple Range Test at p ≤ 0.01
ND not detected
Fig. 3.
HPLC profile of phenolics (A phenolics standard mix; B FFPE; C CFPE; D FBPE; E CBPE) and scanning electron microscopy of fresh D. hamiltonii shoots and bamboo candy
Morphological examination of fresh D. hamiltonii shoots and bamboo candy
Structural morphology of fresh D. hamiltonii shoots and derived food product is well studied by Habibi and Lu (2014). Present study explored the morphological changes by investigating the images of the transversal section of fresh D. hamiltonii shoots and its processed bamboo candy. The vascular bundles and regions nearly parenchyma cells in fresh D. hamiltonii shoots [Fig. 3 (2) A] were visualized clearly but there is a structural deformation in the candy. Sugar coating as crystal granules can be easily visualized in the image of bamboo candy [Fig. 3 (2) B]. Temperature changes during food processing caused changes in the parenchyma cells and cell wall of D. hamiltonii bamboo candy, resulting in the degradation of solid matrices. These morphological examinations revealed the physical changes in the tissues at the microscale level, which has been linked to structural integrity and loss of phenolic constituents. Dadwal et al. (2022) previously demonstrated the morphological variation and effect of preservation on D. hamiltonii shoots using SEM. It has been assumed that bamboo shoots in their fresh state have a strong structural makeup, whereas processing causes significant structural changes that result in the loss of nutritional and phenolic constituents. Overall, these results quite bring a useful information for bamboo-based food products and health promotion.
Bamboo shoots are an important part of Asian cuisine and are consumed in a variety of edible forms. Due to the health benefits of this traditional crop, free and bound phenolics were monitored in fresh D. hamiltonii shoots and sugar-dipped sweet candy. Free phenolics were found to be more abundant than insoluble or bound fractions, whereas after candy processing, a significant loss in free phenolics was observed, but no major shift has been found in bamboo candy. These losses of phenolic constituents were found to be co-related with the antioxidant activities. All extracts with lesser phenolic content demonstrated lower free radical scavenging potential. Although an effective antioxidant activity was recovered in bamboo candy, indicating its potential health benefits. Further HPLC analysis revealed a similar pattern, with higher levels of p-coumaric and chlorogenic acid in fresh bamboo shoots but no p-coumaric acid was detected in bamboo candy. Gallic acid was also washed out after preprocessing and was found to be undetected. After bamboo candy processing, a loss of nutritional and phenolic constituents was observed. This was assumed to be due to a loss of structural integrity, which was supported by SEM results that showed deformation in vascular tissues in bamboo candy. Finally, it was determined that the pre-processing of commercially prepared sugar-dipped bamboo candy results in the loss of free and bound phenolics, followed by nutritional components, but an effective amount is retained, which might also contribute to health benefits.
Acknowledgements
We express our gratitude to the Director, Institute of Himalayan Bioresource Technology (CSIR), for valuable suggestions and encouragement. Authors acknowledge financial assistance received from DST project “Promoting socio-economic development in Shivalik hills through alternate farming, bamboo and novel nutraceutical products” (GAP 0176).
Declarations
Conflict of interest
The authors report no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ajibola CF, Fashakin JB, Fagbemi TN, Aluko RE. Effect of peptide size on antioxidant properties of African yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fractions. International Journal of Molecular Sciences. 2011;12(10):6685–6702. doi: 10.3390/ijms12106685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Rico J, Macías-León FJ, Alanís-García E, Cruz-Cansino NDS, Jaramillo-Morales OA, Barrera-Gálvez R, Ramírez-Moreno E. Study of edible plants: Effects of boiling on nutritional, antioxidant, and physicochemical properties. Foods. 2020;9(5):599. doi: 10.3390/foods9050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajwa HK, Nirmala C, Koul A, Bisht MS. Changes in organoleptic, physicochemical and nutritional qualities of shoots of an edible bamboo Dendrocalamus hamiltonii Nees. and Arn. Ex Munro during processing. Journal of Food Processing and Preservation. 2016;40(6):1309–1317. doi: 10.1111/jfpp.12716. [DOI] [Google Scholar]
- Chandrasekara A, Shahidi F. Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MSN. Journal of Functional Foods. 2011;3(3):144–158. doi: 10.1016/j.jff.2011.03.007. [DOI] [Google Scholar]
- Chen YT, Lin KW. Effects of heating temperature on the total phenolic compound, antioxidative ability and the stability of dioscorin of various yam cultivars. Food Chemistry. 2007;101(3):955–963. doi: 10.1016/j.foodchem.2006.02.045. [DOI] [Google Scholar]
- Chongtham N, Bisht MS, Haorongbam S. Nutritional properties of bamboo shoots: potential and prospects for utilization as a health food. Comprehensive Reviews in Food Science and Food Safety. 2011;10(3):153–168. doi: 10.1111/j.1541-4337.2011.00147.x. [DOI] [Google Scholar]
- Cuevas Montilla E, Hillebrand S, Antezana A, Winterhalter P. Soluble and bound phenolic compounds in different Bolivian purple corn (Zea mays L.) cultivars. Journal of Agricultural and Food Chemistry. 2011;59(13):7068–7074. doi: 10.1021/jf201061x. [DOI] [PubMed] [Google Scholar]
- Dadwal, V. Gupta, M. Recent developments in citrus bioflavonoid encapsulation to reinforce controlled antioxidant delivery and generate therapeutic uses. Critical Reviews in Food Science and Nutrition. (2021). 10.1080/10408398.2021.1961676 [DOI] [PubMed]
- Dadwal, V., Joshi, R. Gupta, M. Effect of physical and chemical preservation techniques on nutritional, morphological, phenolic and antioxidant profile of Dendrocalamus hamiltonii sprouts. Vegetos. 35: 969-977 (2022). 10.1007/s42535-022-00377-4
- Dadwal V, Joshi R, Gupta M. Formulation, characterization and in vitro digestion of polysaccharide reinforced Ca-alginate microbeads encapsulating Citrus medica L. phenolics. LWT. 2021;152:112290. doi: 10.1016/j.lwt.2021.112290. [DOI] [Google Scholar]
- El-Ishaq A, Obirinakem S. Effect of temperature and storage on vitamin C content in fruits juice. International Journal of Chemical and Biomolecular science. 2015;1(2):17–21. [Google Scholar]
- Godswill AC. Proximate composition and functional properties of different grain flour composites for industrial applications. International Journal of Food Sciences. 2019;2(1):43–64. doi: 10.47604/ijf.1010. [DOI] [Google Scholar]
- Habibi MK, Lu Y. Crack propagation in bamboo’s hierarchical cellular structure. Scientific Reports. 2014;4(1):1–7. doi: 10.1038/srep05598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque MR, Bradbury JH. Total cyanide determination of plants and foods using the picrate and acid hydrolysis methods. Food Chemistry. 2002;77(1):107–114. doi: 10.1016/S0308-8146(01)00313-2. [DOI] [Google Scholar]
- Inglett GE, Chen D, Berhow M, Lee S. Antioxidant activity of commercial buckwheat flours and their free and bound phenolic compositions. Food Chemistry. 2011;125(3):923–929. doi: 10.1016/j.foodchem.2010.09.076. [DOI] [Google Scholar]
- Joshi R, Rana A, Gulati A. Studies on quality of orthodox teas made from anthocyanin-rich tea clones growing in Kangra valley, India. Food Chemistry. 2015;176:357–366. doi: 10.1016/j.foodchem.2014.12.067. [DOI] [PubMed] [Google Scholar]
- Kaliora AC, Dedoussis GV. Natural antioxidant compounds in risk factors for CVD. Pharmacological Research. 2007;56(2):99–109. doi: 10.1016/j.phrs.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Kong F, Tang J, Rasco B, Crapo C, Smiley S. Quality changes of salmon (Oncorhynchus gorbuscha) muscle during thermal processing. Journal of Food Science. 2007;72(2):S103-S111. doi: 10.1111/j.1750-3841.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Lefevre M, Beecher GR, Gross MD, Keen CL, Etherton TD. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: the antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annual Review of Nutrition. 2004;24:511–538. doi: 10.1146/annurev.nutr.23.011702.073237. [DOI] [PubMed] [Google Scholar]
- Li FH, Ya YU AN, Yang XL, Tao SY, Jian MING. Phenolic profiles and antioxidant activity of buckwheat (Fagopyrum esculentum Möench and Fagopyrum tartaricum L. Gaerth) hulls, brans and flours. Journal of Integrative Agriculture. 2013;12(9):1684–1693. doi: 10.1016/S2095-3119(13)60371-8. [DOI] [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy reviews. 2010;4(8):118. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Feng S, Pang J, Mao L, Shou H, Xie J. Effect of heat treatment on lignification of postharvest bamboo shoots (Phyllostachys praecox f. prevernalis.) Food Chemistry. 2012;135(4):2182–2187. doi: 10.1016/j.foodchem.2012.07.087. [DOI] [PubMed] [Google Scholar]
- Mæhre HK, Dalheim L, Edvinsen GK, Elvevoll EO, Jensen IJ. Protein determination-method matters. Foods. 2018;7(1):5. doi: 10.3390/foods7010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malla S, Hobbs JE, Sogah EK. Functional foods, health benefits and health claims. Athens Journal of Health. 2014;1(1):37–46. doi: 10.30958/ajh.1-1-3. [DOI] [Google Scholar]
- Nirmala C, Bisht MS, Bajwa HK, Bamboo Santosh O. A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends in Food Science & Technology. 2018;77:91–99. doi: 10.1016/j.tifs.2018.05.003. [DOI] [Google Scholar]
- Nunes C, Santos C, Pinto G, Lopes-da-Silva JA, Saraiva JA, Coimbra MA. Effect of candying on microstructure and texture of plums (Prunus domestica L.) LWT-Food Science and Technology. 2008;41(10):1776–1783. doi: 10.1016/j.lwt.2008.02.006. [DOI] [Google Scholar]
- Pandey AK, Ojha V. Precooking processing of bamboo shoots for removal of anti-nutrients. Journal of Food Science and Technology. 2014;51(1):43–50. doi: 10.1007/s13197-011-0463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Jiménez J, Torres JL. Analysis of nonextractable phenolic compounds in foods: the current state of the art. Journal of Agricultural and Food Chemistry. 2011;59(24):12713–12724. doi: 10.1021/jf203372w. [DOI] [PubMed] [Google Scholar]
- Réblová Z. Effect of temperature on the antioxidant activity of phenolic acids. Czech Journal of Food Sciences. 2012;30(2):171–175. doi: 10.17221/57/2011-CJFS. [DOI] [Google Scholar]
- Rockenbach II, Rodrigues E, Gonzaga LV, Caliari V, Genovese MI, Gonçalves AEDSS, Fett R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chemistry. 2011;127(1):174–179. doi: 10.1016/j.foodchem.2010.12.137. [DOI] [Google Scholar]
- Sandhiya S, Subhasree N, Shivapriya S, Agrawal A, Dubey GP. In vitro antioxidant and antimicrobial potential of Bambusa arundinacea (Retz.) Willd. International Journal of Pharmacy and Pharmaceutical Sciences. 2013;5(2):359–362. [Google Scholar]
- Sharma P, Ghimeray AK, Gurung A, Jin CW, Rho HS, Cho DH. Phenolic contents, antioxidant and α-glucosidase inhibition properties of Nepalese strain buckwheat vegetables. African Journal of Biotechnology. 2012;11(1):184–190. [Google Scholar]
- Sonar NR, Vijayendra SVN, Prakash M, Saikia M, Tamang JP, Halami PM. Nutritional and functional profile of traditional fermented bamboo shoot based products from Arunachal Pradesh and Manipur states of India. International Food Research Journal. 22(2): 788-797 (2015)
- Stojanović JS, Nikolić NČ, Lazić ML, Karabegović IT, Mastilović JS, Stojanović GS. DPPH radical scavenging capacity and the reducing power of free and bound phenolics from wheat and porcino (Boletus edulis) flours. Savremene Tehnologije. 2014;3(2):52–57. doi: 10.5937/savteh1402052S. [DOI] [Google Scholar]
- Su D, Zhang R, Hou F, Zhang M, Guo J, Huang F, Wei Z. Comparison of the free and bound phenolic profiles and cellular antioxidant activities of litchi pulp extracts from different solvents. BMC Complementary and Alternative Medicine. 2014;14(1):1–10. doi: 10.1186/1472-6882-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Zhu Q, Tan H, Horiba H, Ohnuki K, Mori Y, Shimizu K. Biological activities and phytochemical profiles of extracts from different parts of bamboo (Phyllostachys pubescens) Molecules. 2014;19(6):8238–8260. doi: 10.3390/molecules19068238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verardo V, Arraez-Roman D, Segura-Carretero A, Marconi E, Fernandez-Gutierrez A, Caboni MF. Determination of free and bound phenolic compounds in buckwheat spaghetti by RP-HPLC-ESI-TOF-MS: Effect of thermal processing from farm to fork. Journal of Agricultural and Food Chemistry. 2011;59(14):7700–7707. doi: 10.1021/jf201069k. [DOI] [PubMed] [Google Scholar]
- Yeo J, Shahidi F. Effect of hydrothermal processing on changes of insoluble-bound phenolics of lentils. Journal of Functional Foods. 2017;38:716–722. doi: 10.1016/j.jff.2016.12.010. [DOI] [Google Scholar]
- Zhang JJ, Ji R, Hu YQ, Chen JC, Ye XQ. Effect of three cooking methods on nutrient components and antioxidant capacities of bamboo shoot (Phyllostachys praecox CD Chuhet CS Chao) Journal of Zhejiang University Science B. 2011;12(9):752–759. doi: 10.1631/jzus.B1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]