Abstract
Poultry is one of the most consumed sources of animal protein around the world. To meet the global demands for poultry meat and eggs, it is necessary to improve their nutrition to sustain the poultry industry. However, the poultry industry faces several challenges, including feedstuff availability, the banning of antibiotics as growth promoters, and several environmental stressors. Therefore, there is a critical need to include available nutraceuticals in the diet to sustain the poultry industry. Nutraceuticals are natural chemical substances that positively influence animal physiological and productive traits. Botanical products (such as fenugreek seeds, ginger roots, and olive leaves) are among the most commonly used nutraceuticals and are gradually gaining popularity in the poultry industry due to their immense benefits in nutrition and therapeutic properties. They can be added to the diet separately or in combination (as a natural antioxidant and immunostimulant) to improve poultry health and production. Botanical products are rich in essential oils and essential fatty acids, which have multiple benefits on the animal’s digestive system, such as activating the digestive enzymes and restoring microbiota balance, enhancing poultry health, and production. These nutraceuticals have been shown to stimulate the expression of several genes related to growth, metabolism, and immunity. In addition, the essential oil supplementation in poultry diets up-regulated the expression of some crucial genes associated with nutrient transportation (such asglucose transporter-2 and sodium-glucose cotransporter-1). Previous studies have suggested that supplementation of botanical compounds increased broiler body weight and hen egg production by approximately 7% and 15%, respectively. Furthermore, the supplementation of botanical compounds enhanced the reproductive efficiency of hens and the semen quality of roosters by 13%. This review article discusses the significant effects of some botanical products in the poultry industry and how they can benefit poultry, especially in light of the ban on antibiotics as growth promoters.
Keywords: antioxidant, egg production, essential fatty acids, fertility rate, immunological response, meat quality, nutraceuticals, welfare
Introduction
The global poultry industries, particularly in developing countries, face feed shortages year after year. The continued use of conventional feed additives from plant or animal sources in poultry diets has become a critical issue due to the high competition among livestock species and industrial purposes. This leads to an increase in the price of these feedstuff and livestock products. There is a global trend toward finding healthier natural alternatives to synthetic pharmaceuticals and therapeutic drugs in poultry farms [1–3]. Therefore, discovering alternative and readily available feed additives is essential to protecting the poultry industry, particularly in developing countries.
The use of alternatives for feed additives in the poultry diet has recently been considered. The main purpose is that these feed additives should produce safe and high-quality food [4–6]. Nutraceuticals offer these advantages and should be considered as alternative feed additives. Nutraceuticals, also known as bioceuticals, are natural chemical compounds (natural pharmaceuticals/therapeutic) that modify and maintain normal physiological functions that support the healthy host [7]. Nutraceuticals are divided into plant essential oils [8], and established nutraceuticals, such as omega-3, 6, and 9 fatty acids [9]. They are frequently used in poultry diets as alternative feed supplements to enhance animal productivity and welfare due to their numerous growth and health benefits [2, 3, 10]. These compounds exert several biological functions in poultry and may enhance their production (obtaining high-quality products) and welfare. They also can potentially improve the balance of the intestinal microbiota, which is important in regulating metabolism, intestinal epithelial proliferation, and vitamin synthesis [10, 11]. Nutraceuticals are developed as nano-substances to treat several diseases, including nutritional deficiency (malnutrition), and coccidiosis. Furthermore, nutraceuticals in poultry diets can stimulate various gene expressions related to growth, metabolism, and immunity [12].
Botanical products (such as medicinal seeds and essential oils) are one of these nutraceuticals that are gradually gaining popularity in poultry farms due to their nutritional value and therapeutic properties (Figure-1), as well as leaving no residue in products [13]. Essential oils from plants have been routinely used in chicken farms to keep birds healthy and improve their productive performance. These essential oils include active components that positively impact physiological functions and medicinal features, such as anti-inflammatory and antibacterial effects [8, 14]. Therefore, this review discusses the impacts of using commercially available botanical products in the Middle East and beyond on poultry health and production. This article also emphasizes their properties, doses, biological functions, and welfare impact for future use in the poultry industry.
Figure-1.
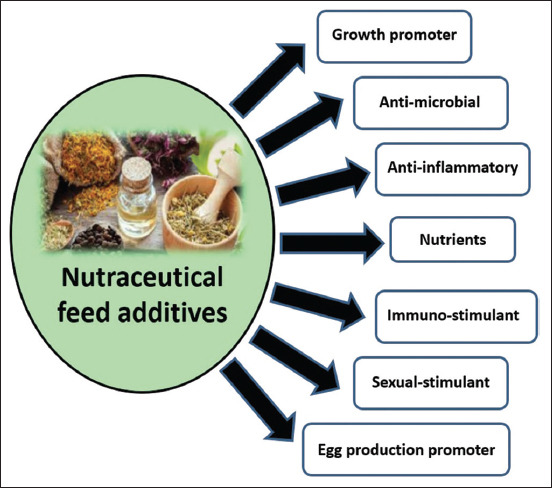
Schematic overview of the biological functions of botanical products as nutraceuticals added to poultry diets.
Effect of Botanical Products on Poultry Production and Health Status
Botanical products are natural and vital substances widely used as dietary additives in the poultry industry. Their nutritional and medicinal properties positively influence performance (Table-1) [8, 15–35]. They contain phytobiotics, phenols, flavonoids, tannins, and essential oils, which have many roles in the birds. They can benefit a broad range of poultry species by improving digestion and promoting health [3, 10, 36, 37]. As a result, they perform a variety of vital roles in enhancing poultry productivity and immune functions. In addition, botanical or herbal products are considered phytogenic feed additives for poultry birds to improve egg weight and ovary characteristics and decrease yolk trimethylamine levels in laying hens [38]. Dietary supplementation of different botanical feed additives, such as camphor (50 mg/kg of feed), can enhance seminal characteristics and the reproductive performance of roosters [39].
Table-1.
The key health and production benefits of nutraceutical/botanical product supplementation in poultry diets.
| Botanical additives | Studied traits | Species | Key benefits | References |
|---|---|---|---|---|
| Rosemary and Cinnamon essential oils | Egg production, meat quality, blood biochemical, immunity, and antioxidative status | Laying chickens Broiler chickens | Improved hen performance, liver and kidney functions, immunity, antioxidative capacity, and egg production quality. Cinnamon oil has hypocholesterolemic and antioxidant properties, improving broiler meat quality. | [8, 16] |
| Pumpkin (Cucurbita maxima) | Egg production and quality | Laying chickens | Pumpkin can be added to the laying’s diet (10%) to improve egg production and quality (by reducing bad cholesterol levels). | [31] |
| Garden cress (Lepidium sativum) | Birds’ health and production | Broiler chickens | Garden cress can be added to the broiler’s diet (up to 1%) to improve the broiler’s body weight, biological performance, and health status. | [32, 33] |
| Fenugreek (Trigonella foenum-gracum) | Growth performance and live body weight | Broiler chickens | Fenugreek addition (1–1.5%) in the broiler diet has some growth-promoting effects on broiler chicken, improving the feed conversion ratio and body weight of birds. | [23] |
| Carcass characteristics and meat quality | Broiler chickens | Better product quality was observed when fenugreek seed was added up to 3% in the commercial broiler diet. | [24] | |
| Black cumin (Nigella sativa) | Egg production and quality | Laying chickens | Improve egg production, weight, eggshell thickness, and haugh unit. Reduction in yolk cholesterol content. | [25] |
| Poultry health and production | Broiler and laying chickens | Promotes bords’ health and production performance. Plays a significant role as a natural antioxidant and immunostimulant. | [27] | |
| Productive performance, oxidative status, and meat quality | Broiler chickens | Improving broiler performance and meat quality by enhancing antioxidant activities and suppressing lipid peroxidation in meat. | [26] | |
| Ginger (Zingiber officinale) | Growth performance and antioxidative status | Broiler chickens | The supplementation of ginger at the level of 5 g/kg improved the antioxidant status and bodyweight of broilers | [28] |
| Feed conversion ratio, body weight, and humoral immunity | Broiler quails | Improving birds’ feed conversion ratio, body weight, and humoral immunity. | [29] | |
| Egg production and quality | Laying quails | Oral administration of 100–150 μL/kg body weight of ginger essential oil to quails positively affects egg weight and reduces cholesterol. | [30] | |
| Thyme (Thymus vulgaris L.) | Growth, body weight, egg production, and quality | Broiler and laying chickens | Improvement in broiler’s growth and body weight. Improvement in egg production and egg nutritive value of laying hens and reduction in yolk bad cholesterol and triglyceride levels. | [15, 17-19] |
| Turmeric (Curcuma longa) | Antioxidant enzymes’ activity and body weight | Broiler chickens | Turmeric powder (0.5 g/kg diets) improved final body weight, feed conversion ratio, digestibility, and lipid status in broiler chicks under heat stress conditions. | [20, 21] |
| Egg production and quality | Laying chickens | Improvement in egg production. Eggs’ cholesterol was reduced by 25% when layers were fed 4% turmeric in the diet. | [22] | |
| Chamomile (Matricaria spp.) | Birds’ health and production | Broiler chickens | Adding 200–400 mg/kg of chamomile oils to broiler diets improves weight gain and final body weight and decreases microbe activities. | [34, 35] |
To feed the growing human population, current poultry breeds are developed for higher production of meat and eggs. However, these breeds are metabolically more active, resulting in the production of cellular free radicals. These free radicals alter cellular functions by accelerating oxidative stress, inflammation, and immunosuppression. Recently, researchers have tested several botanical compounds and their bioactive molecules, which scavenge free radicals and restore the cellular functions required for poultry health and production.
Thyme (vulgaris L.)
Thyme (T. vulgaris L.) is one of the most common herbal medicinal plants worldwide that belong to the Lamiaceae family. It is widely used in human food to provide a distinct flavor. Due to its antioxidant, antibacterial, and therapeutic characteristics, it is usually used in livestock farms (as an alternative to antibiotics) for enhancing animal productivity and health [40–42]. The essential oil in dry thyme (active extract) is a combination of monoterpenes, mainly thymol (2-isopropyl-5-methylphenol) and phenol isomer carvacrol (2-methyl-5-(propan-2-yl) phenol) [43]. Thyme also contains phenolics, some biphenylics, and flavonoids which have been shown to have antioxidative properties and other benefits for birds [41–46]. Essential oil phenolic compounds increased catalase activity, detoxifying hydrogen peroxide and converting lipid hydroperoxides to nontoxic substances [47]. Thyme extracts are advised in laying farms to improve egg quality, especially the fatty acid profile in the yolk [48]. Lipid profiles of the egg can be modified by feeding hens plant seed oils to reduce the content of n-6 fatty acids while increasing the content of n-3 fatty acids [49, 50]. Layer’s diet enriched with dry thyme leaves at 2% has several positive impacts on laying hens’ physiological and productive performance and their antioxidant activity [15]. Thyme leaves significantly influence egg production quality and nutritional value by decreasing bad yolk cholesterol (low-density lipoprotein [LDL]) and total saturated fatty acid concentrations and increasing omega-3 fatty acid concentrations. It also raises the a-linolenic acid level of eggs while significantly reducing palmitic acid content in the yolk. Furthermore, dietary thyme at 2% can reduce yolk malondialdehyde, unhealthy blood cholesterol, and triglyceride levels. Blood bad cholesterols (such as LDL) reduction may be related to 3-hydroxy-3-methylglutaryl- coenzyme A (HMG-CoA) reductase activity inhibition, which is one of the enzymes involved in regulating cholesterol metabolism; thus, cholesterol synthesis reduction [16]. The previous studies [15, 17, 18, 48, 51] reported that dietary thyme supplementation could enhance poultry growth performance and egg production and the humoral immune response without harming the birds. In addition, dietary supplementation of thyme (1–5 g/kg) in broiler diets as a growth promoter increased feed intake, feed conversion efficiency, and final body weight. It can also increase birds’ meat dressing percentage and quality [17, 19, 52]. Thyme extracts also have the potential to improve broiler meat oxidative stability by interrupting free radicalchains, resulting in a product with higher oxidative stability [53]. Its mode of action is related to intramuscular fat stimulation and flavor amino deposition. Phytogenic products containing thymol and carvacrol enhanced broilers’ growth performance, digestive enzyme activities, antioxidant enzyme activities, and retarded lipid oxidation [54]. In the poultry diet, phytogenic feed additives functioned for overall broiler gut antioxidant capacity. A 5% reduction in dietary energy and protein intake primed important antioxidant responses, particularly on phytogenic addition [55]. Diet-type interactions with phytogenic feed additives were determined for critical antioxidant and cytoprotective genes (such as nuclear factor erythroid 2-like 2 pathway) and the total antioxidant capacity in the proximal gut. The overall antioxidant response along the broiler gut was increased by reduced dietary energy and protein intake, and the addition of phytogenic feed additives consistently increased it. On the other hand, thyme contains flavonoids, which can increase the activity of Vitamin C, which acts as an antioxidant and thus improves immune function [54, 56]. It also plays an important role in reducing stress on birds, especially during the hot seasons (such as summer), and increasing feed intake, metabolism, and body weight in broiler chickens [57]. Thyme essential oils can improve birds’ productivity by increasing the secretion of digestive enzymes and improving nutrient utilization through improved liver function [19, 58, 59]. Thyme’s antibacterial activity was associated with increased broiler productivity [40]. Broilers fed a diet supplemented with thyme had higher productivity, which they attributed to increasing apparent fecal and crude protein digestibility [60]. It has been reported that essential oils (25% thymol and 25% carvacrol) supplementation on growth performance, gut lesions, intestinal morphology, and immune responses of broiler chickens infected with Clostridium perfringens [45]. The supplementation induced gut lesions and increased crypt depth in the ileum. It also downregulated the claudin-1 and occludin mRNA expression, up-regulated the mRNA expression of interleukin-1β, and tended to increase toll-like receptor 2 mRNA expression in the ileum, and improved mucosal secretory immunoglobulin A production. These studies concluded that dietary essential oil supplementation alleviated gut lesions and improved the ratio of villus height to crypt depth in the treated birds. Furthermore, they revealed that essential oils supplementation (120 and 240 mg/kg) increased the serum antibody titers against the Newcastle disease virus and improved birds’ immune responses. Essential oil supplementation quadratically and linearly up-regulated the expression levels of critical genes associated with nutrient transportation (such as glucose transporter-2 [GLUT2], sodium-glucose cotransporter-1 [SGLT1], and Sodium-coupled neutral amino acid transporter 1), and barrier function of tight junction protein-1 [61]. These findings indicate that essential oil positively affects growth, immunity, and intestinal health in broilers, and 200 mg/kg of essential oil is recommended in the broiler diet.
Turmeric (Curcuma longa L.)
Turmeric (C. longa L.), a member of the Zingiberaceae family, is a rhizomatous herbaceous plant belonging to the ginger family. Curcumin is a major component of turmeric and is commonly used as a spice and food-coloring agent [62]. It has been considered a natural polyphenol nutraceutical substance and is well known for reducing oxidative stress and repairing the damage caused by oxidative stress. According to Sharma et al. [63], curcumin has been identified for various biological activities, including antioxidant and anti-cancer effects. Furthermore, several studies have shown that curcumin has anti-inflammatory properties and lipid and cholesterol-reducing effects [64, 65]. It can also be used in animal diets, such as broiler diets, to mitigate heat stress [20, 21]. Furthermore, it can reduce free radicals; reactive oxygen species (ROS), and reactive nitrogen species (RNS), by activating glutathione, catalase, and superoxide dismutase, and limiting ROS-producing enzymes [66, 67]. Thus, it becomes a vital medical and antioxidant substance in chicken feeds, especially in tropical areas where high temperatures throughout the year can delay growth, decrease egg production, and increase disease outbreaks and mortality of birds [68]. These issues worsen when the birds are subjected to heat stress [69, 70]. Sadeghi and Moghaddam [20] observed that adding turmeric (0.5%) to broiler diets during heat stress raised blood concentrations of triiodothyronine (T3) and thyroxine (T4), feed consumption, body weight, and decreased bird feed conversion ratio. Moreover, El-Maaty et al. [71] revealed that the addition of turmeric to broiler diets (0.5%) increased final body weight (11%), feed conversion ratio (17%), crude protein digestibility (5%), and high-density lipoprotein (HDL) (28%) while lowering creatinine, triglycerides, cholesterol (>15%), LDL (39%), and very-LDL (VLDL) (>13%) during thermal stress conditions. Triglycerides are secreted from the liver into the blood by triglyceride-rich lipoproteins; thus, reduced hepatic lipogenesis lowers triglyceride concentrations in plasma [72]. The reduction of bad cholesterols (LDL and VLDL) may be related to the inhibition of HMG-CoA reductase (one of the enzymes involved in regulating cholesterol metabolism in animals) activity [16].
Turmeric and other natural plant extracts can be added to poultry diets in small amounts to enhance several productive and health benefits [22, 73]. Supplementing turmeric in the poultry diet at a 1%–5% concentration improves feed intake and significantly lowers cholesterol in poultry products. In laying hens, dietary supplementation of turmeric at 1% and 4% reduced eggs’ cholesterol by 16% and 25%, respectively [22]. Compared to a control group, birds fed a high carbohydrate content supplemented with turmeric for a month before sexual maturity produced higher eggs (20%). The active turmeric extract (curcumin) influences lipid metabolism and inhibits peroxidation [74]. It stimulates bile production, which is necessary for lipid emulsification [75]. Turmeric supplementation can improve liver function and vitellogenin production [22]. Turmeric enhances liver function by reducing serum levels of glutamic pyruvic transaminase/alanine aminotransferase and glutamic oxaloacetic transaminase/aspartate aminotransferase [22, 76].
Fenugreek (Trigonella foenum-graecum)
Fenugreek seeds (T. foenum-graecum) are known for their medicinal properties, including antibacterial and anti-inflammatory effects [77, 78]. They are also high in protein, fat, total carbohydrates, minerals, biotin, and trimethylamine, stimulating the animal’s appetite [77, 79]. Galactomannan is the main polysaccharide in fenugreek seeds, representing approximately 50% of the seed weight [80]. It has been recognized as an anti-diabetic nutraceutical due to its capacity to decrease blood glucose levels. Adding fenugreek seed powder (1%–1.5%) to the broiler diet improved the feed conversion ratio and increased final body weight in birds [23]. While adding up to 3% fenugreek in broiler diets can improve birds’ body weight gain and carcass quality [24]. Adding 1% fenugreek to laying hens’ diet improved feed intake and egg yolk color, particularly in the second production cycle [81]. Fenugreek supplementation at 0.4% in laying diets improved egg production and quality [82]. These studies have suggested that supplementation of fenugreek can potentially improve meat and egg production and their quality.
Black Cumin (Nigella sativa)
Black cumin seeds (N. sativa) are well known for their medicinal and therapeutic characteristics. They contain antioxidants, volatile oils, alkaloids, and several pharmacologically active substances, such as thymols [25, 83–85]. Dietary supplementation of black cumin enhanced broilers’ production and meat quality by increasing antioxidant activities and inhibiting meat lipid peroxidation [26]. Black cumin seeds are also rich in essential oils and essential fatty acids, and have multiple benefits on the animal’s digestive system, such as activating the digestive enzymes, restoring microbiota balance, and increasing nutrient absorption [86, 87], and this may be one of the reasons behind birds’ production and health improvements. Azeem et al. [27] found that black cumin enhances poultry productivity and immunological resistance. They reported that black cumin seeds play bioactive roles in the avian body as a natural antioxidant and immunostimulant. Black cumin seeds added to the chicken diet at the dose rate of 10,000–30,000 mg/kg increased nutrient utilization, body weight gain, immunological response, and lower fatty acid levels in the products [88]. Furthermore, N. sativa meal could be added up to 15% in growing quail diets with no negative effects on productive performance. Compared to the control, the inclusion of 10% N. sativa meal in the quail diet resulted in the best values of live body weight and daily weight gain [89]. The up-regulated expression of some nutrient transporters, such as SGLT1 and GLUT2, may be another reason for birds’ production and health improvements [61].
Olive (Olea europaea L.)
Olive by-products (leaves, cake or pulp meal, and oil) are among the most important nutraceutical substances because of their nutritional and healthful effects. Polyunsaturated fatty acids (PUFA), polyphenols, and minor phytochemical compounds in olive products improved productivity and the immune system by influencing metabolism processes and the synthesis of white blood cells and cytokine [45, 90–93]. Olive leaves, cake meal (a by-product of olive oil extraction), and oil are used in poultry diets as a source of energy and antioxidant compounds [94]. Olive leaves have high antioxidant activity, originating from phenolics, thus exhibiting strong preventive effects against oxidation [95, 96]. Olive cake meal has a high nutritional value (crude fat, 13%–18%; crude proteins, 9%–10%) [97, 98]. It is rich in lignin and includes a high content of non-starch polysaccharides [94, 99]. Furthermore, it contains Vitamin E, calcium, iron, potassium, magnesium, sodium, and phosphorus [100]. Olive cake meals are found worldwide and used as a plant source for broiler diets at a fair price in several countries [101]. Olive oil is distinguished from other vegetable oils by its high oleic acid content (the most common monounsaturated fatty acid in the diet) [102]. The importance of olive oil stems from its high levels of monounsaturated fatty acids and the presence of low-represented components such as alpha-tocopherol, phenolics, chlorophyll, and carotenoids [103]. Adding 5% olive oil to broiler diets can decrease serum triglyceride levels while increasing HDL-cholesterol levels in treated birds [104]. This addition reduced saturated fatty acid contents in breast and drumstick meat and increased the total unsaturated fatty acid contents. Moreover, supplementing 2% or 5% olive oil to layer diets boosted yolk color, increased total yolk unsaturated fatty acids, and decreased serum cholesterol concentration in laying hens [102]. Laying hens’ diet supplemented with dried olive pulp at rates of 5% and 6% improved egg nutrition quality by increasing the PUFA percentage in eggs, decreasing that of saturated fatty acids, and improving the PUFA to saturated fatty acids ratio [105]. Olive by-products beneficial effects could be due to their high content of vital and biological compounds, such as monounsaturated fatty acids, PUFA, and polyphenols, as well as their high antioxidant capacity [106]. Furthermore, olive oil improves the absorption of Vitamin A, another fat-soluble vitamin, which might increase HDL-cholesterol levels in the sera of laying hens [107]. Olive oil supplementation also increased calcium concentration in non-ruminant animal livers, such as poultry, and made it more available for absorption [108]. Hens can absorb calcium through their digestive systems and mobilize calcium from the medullar bones [109]. On the other hand, olive oil is a good solvent of Vitamin D and thus can improve calcium concentrations in eggshells [110]. These studies demonstrated that supplementing olive products and by-products has beneficial effects on poultry meat and egg production.
Ginger (Zingiber officinale)
Ginger roots (Z. officinale) are rich in volatile oils, gingerols, and zingerone and are widely used as medicinal plants worldwide [111]. Ginger roots enhance digestive enzymes and antioxidative activities in birds [112]. Dietary supplementation of 5000 mg/kg ginger powder as a nutritional addition in broiler diets increased antioxidant capacity and serum metabolites [28]. Quails fed a ginger-supplemented diet (125 mg/kg) had the best feed conversion ratio, body weight, and humoral immunity [29]. Furthermore, the addition of ginger aided in the optimization of the lipid profile in blood serum and enhances the bird’s antioxidative status. Bee propolis (500 mg/kg) mixed with ginger powder (125 mg/kg) displayed better growth performance and poultry health [29, 113]. Dietary supplementation of ginger powder (10–15 g/kg) in laying diets improved the laying performance and serum antioxidant status of hens [114]. In addition, oral administration of 100–150 μL/kg body weight of ginger essential oil to laying Japanese quails positively influenced egg weight and decreased egg cholesterol [30]. On the other hand, adding 2.5 and 5 g/kg ginger to the broiler breeder’s diet increased the reproductive performance of males, including semen ejaculate volume and sperm concentration per ejaculate, live sperms, and viability [115].
Pumpkin (Cucurbita maxima) and Garden Cress (Lepidium sativum)
Pumpkin (C. maxima) and garden cress (L. sativum) seeds are important medicinal plant seeds that have recently gained attention on a global scale due to their nutritional and pharmaceutical properties, leading to the development of innovative sources of feed additives. They are rich in protein (25%–30%) and an almost equal amount of healthy fats. They also have a good amount of vitamins and minerals, essential for physiological and biological functions in poultry [116–122]. Moreover, they contain phenolic components (phytochemicals) that are responsible for their high antioxidant capacity [116–124]. Pumpkin and garden cress seeds are also rich in polyphenols, flavonoids, antioxidants, and PUFA [125]. These compounds have various biological activities in bird’s body, including growth gene stimulation, metabolic process promotion, and final product quality improvement by increasing PUFA and decreasing bad cholesterol levels [12, 45, 126, 127].
Pumpkin and garden cress seeds can be added combined or separately to poultry diets to improve birds’ development and production, as well as the health and immune response [13, 116, 122, 128–130]. They contain essential aromatic oils with various nutritional and medicinal properties for birds. They are excellent sources of unsaturated fatty acids, such as linoleic acid, and saturated fatty acids, such as arachidic acid [131, 132]. Furthermore, they contain a-linolenic acid, which can be transformed into eicosapentaenoic acid and docosahexaenoic acid in the body [133].
Pumpkin seed oil is high in Vitamin E and β-carotene, which have powerful antioxidant and anti-cancer properties [120, 121, 123, 134]. It is also rich in omega-3 and omega-6 fatty acids, L-tryptophan, and some trace minerals such as zinc [135, 136]. It is recommended that laying hen diets contain 10% pumpkin to improve ether extract and healthy fatty acid levels while decreasing harmful cholesterol and fatty acids [31]. Moreover, pumpkin is a good source of crude protein and can potentially substitute soybean meal in broiler diets up to 20% without causing any adverse effect on the bird’s internal organs [122].
Garden cress oil contains tocopherol (a natural antioxidant), carotenoid, oleic acid, and a-linolenic acid, which reduce various types of radicals [131, 137]. Garden cress can be added, as a potential feed additive, to the broiler’s diet at the level of 0.75% for better biological performance and health status [32]. In addition, garden cress supplementation (1%) increased broiler feed consumption, body weight, blood globulin, behavior, and economic efficiency [33].
Azolla (Azolla pinnata)
Azolla (A. pinnata) is a small aquatic fern plant that lives afloat on the water’s surface and can resist hot temperatures. It provides a carbon source and a favorable environment for algae growth and development. It is rich in proteins (25%–35%), minerals (10%–15%), and vitamins (20%–30%) and has a low fiber content (11%–13%) [138–141]. Furthermore, it contains a good amount of carotenoids, which play a useful biological role that contributes to therapeutic effects, such as immunomodulators, antibacterial, and anti-inflammatory effects, and improves poultry productive performance and product quality [142]. However, due to its high nutrient content, Azolla is used as a feedstuff in poultry and livestock diets. It can be used as a low-cost animal feed and a partial replacement for high-priced traditional proteins in poultry diets. According to Mishra et al. [138] and Azolla can be used in poultry diets at 25%–30% for chickens and a higher rate of 40%–45% for ducks and geese [138, 141]. The high enzyme concentration in the Azolla plant may be due to active components in this plant, which can inhibit free radical activity within the animal’s body as one of the most important natural antioxidants [138].
Chamomile (Matricaria spp.)
Chamomile flower (Matricaria spp.) is one of the most common herbs used for medicinal purposes worldwide. Egyptian chamomile (Matricaria recutita) is famous for its high-quality; therefore, large quantities of this plant are exported to European countries. It contains flavones (such as apigenin), coumarins, and essential volatile oils (1%–2%) (such as bisabolol oxide A and B (25%–50%), and chamazulene (10%) [143]. Besides its medicinal properties, it has antioxidant, anti-inflammatory, antimicrobial, and cholesterol-lowering activities [143–147].
It was reported that feed conversion, weight gain, and market body weight were significantly improved with 200–400 mg chamomile oils for each kilogram broiler diet [34]. Chamomile also has a beneficial effect on broiler plasma cholesterol and glucose. It was found that increasing doses of chamomile in the feeding ration decreased the numbers of coliform microbes in the digestive tract of chicks and reduced the population of Clostridium perfringens [35]. In laying Japanese quails, supplementing chamomile at 0.50 g/kg of the diet improved productivity, reproductive performance, and economic efficiency [144]. Furthermore, including 2.5–5 g chamomile/kg in a Japanese quail diet reduces the behavior of aggressive pecking and improves birds’ welfare [147].
Conclusion
Nutraceuticals are bioactive chemical substances found in several botanical products. They displayed promises as effective feed additives for sustaining poultry productivity while improving their health. Botanical products have been widely used as dietary supplementation in poultry diets due to their nutritional and therapeutic properties. Therefore, botanical products are recommended as natural feed additives and alternatives to any artificial chemical material. These natural substances can be added to the poultry diet individually or in combination to improve the physiological, productive, reproductive, and immunological parameters which impact poultry welfare and their quantitative and qualitative productivity. This review summarized the botanical products’ health and production benefits as nutraceuticals. These nutraceuticals can potentially be used in broiler and layer farms as natural growth and egg production promoters and aid in the sustainability of the poultry.
Authors’ Contributions
KE: Conceptualization, supervision, visualization, and writing–original draft. KE and BM: Data curation and writing–review and editing. KE, BM, and AK: Formal analysis, investigation, and resources. All authors have read, reviewed, and approved the final manuscript.
Acknowledgments
The authors of this manuscript are grateful to their respective universities and institutes for their technical assistance and valuable support in completing this review. The authors did not receive any funds for this study.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.El-Hanoun A, Elkomy A.E, El-Sabrout K, Abdella M. Effect of bee venom on reproductive performance and immune response of male rabbits. Physiol. Behav. 2020;223:112987. doi: 10.1016/j.physbeh.2020.112987. [DOI] [PubMed] [Google Scholar]
- 2.Alagawany M, Elnesr S.S, Farag M.R, Tiwari R, Yatoo M.I, Karthik K, Michalak I, Dhama K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health-a comprehensive review. Vet. Q. 2020;41(1):1–29. doi: 10.1080/01652176.2020.1857887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alagawany M, Elnesr S.S, Farag M.R, Abd El-Hack M.E, Barkat R.A, Gabr A.A, Foda M.A, Noreldin A.E, Khafaga A.F, El-Sabrout K, Elwan H.A.M, Tiwari R, Yatoo M.I, Michalak I, Di Cerbo A, Dhama K. Potential role of important nutraceuticals in poultry performance and health-a comprehensive review. Res. Vet. Sci. 2021;137:9–29. doi: 10.1016/j.rvsc.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Açıkgöz Z, Yücel B, Altan Ö. The effects of propolis supplementation on broiler performance and feed digestibility. Arch. Fur. Geflugelkunde. 2005;69(3):117–122. [Google Scholar]
- 5.El-Sabrout K, El-Deek A, Ahmad S, Usman M, Dantas M.R.T, Souza-Junior J.B.F. Lighting, density, and dietary strategies to improve poultry behavior, health, and production. J. Anim. Behav. Biometeorol. 2022;10(1):2212. [Google Scholar]
- 6.Arain M.A, Nabi F, Marghazani I.B, Ul-Hassan F, Soomro H, Kalhoro H, Soomro F, Buzdar J.A. In ovo delivery of nutraceuticals improves health status and production performance of poultry birds:A review. Worlds Poult. Sci. J. 2022;78(3):765–788. [Google Scholar]
- 7.Das L, Bhaumik E, Raychaudhuri U, Chakraborty R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012;49((2)):173–183. doi: 10.1007/s13197-011-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abo Ghanima M.M, Elsadek M.F, Taha A.E, Abd El-Hack M.E, Alagawany M, Ahmed B.M, Elshafie M.M, El-Sabrout K. Effect of housing system and rosemary and cinnamon essential oils on layers performance, egg quality, haematological traits, blood chemistry, immunity, and antioxidant. Animals. 2020;10(2):245. doi: 10.3390/ani10020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alagawany M, Elnesr S.S, Farag M.R, El-Sabrout K, Alqaisi O, Dawood M.A.O, Soomro H, Abdelnour S.A. Nutritional significance and health benefits of omega-3,-6 and -9 fatty acids in animals. Anim. Biotechnol. (2022a);33((7)):1678–e1690. doi: 10.1080/10495398.2020.1869562. [DOI] [PubMed] [Google Scholar]
- 10.Dhama K, Latheef S.K, Mani S, Samad H.A, Karthik K, Tiwari R, Khan R.U, Alagawany M, Farag M.R, Alam G.M, Laudadio V, Tufarelli V. Multiple beneficial applications and modes of action of herbs in poultry health and production-a review. Int. J. Pharm. 2015;11(3):152–176. [Google Scholar]
- 11.Adil S, Magray S.N. Impact and manipulation of gut microflora in poultry:A review. J. Anim. Vet. Adv. 11((6)):873–877. [Google Scholar]
- 12.Alagawany M, Elnesr S.S, Farag M.R, El-Naggar K, Madkour M. Nutrigenomics and nutrigenetics in poultry nutrition:An updated review. Worlds Poult. Sci. J (2012) (2022b);78((2)):377–396. [Google Scholar]
- 13.Mathewos Z, Girma M, Ameha N, Zeryehun T. Effects of Neem (Azadirachta indica) and pumpkin (Cucurbita maxima) seeds and their combination as feed additive on growth and carcass characteristics of broilers. Livest. Res. Rural. Dev. 2019;31(6):83. [Google Scholar]
- 14.El-Husseiny O.M, Abd-Elsamee M.O, Hassane M.I, Omara I.I. Response of egg production and eggshell quality to dietary vegetable oils. Int. J. Poult. Sci. 2008;7(10):1022–1032. [Google Scholar]
- 15.Yalçın S, Eser H, Onbasilar I, Yalçın S. Effects of dried thyme (Thymus vulgaris L.) leaves on performance, some egg quality traits and immunity in laying hens. Ank. Univ. Vet. Fak. Derg. 2020;67:303–311. [Google Scholar]
- 16.Ciftci M, Simsek U.G, Yuce A, Yilmaz O, Dalkilic B. Effects of dietary antibiotic and cinnamon oil supplementation on antioxidant enzyme activities, cholesterol levels and fatty acid compositions of serum and meat in broiler chickens. Acta Vet. Brno. 2010;79:33–40. [Google Scholar]
- 17.Mansoub N.H. Comparison of effects of using thyme and probiotic on performance and serum composition of broiler chickens. Adv. Environ. Biol. (2011a);5((7)):2012–2015. [Google Scholar]
- 18.Mansoub N.H. Assessment on effect of thyme on egg quality and blood parameters of laying hens. Ann. Biol. Res. (2011b);2((4)):417–422. [Google Scholar]
- 19.Toghyani M, Tohidi M, Gheisari A.I, Tabeidian S.A. Performance, immunity, serum biochemical and hematological parameters in broiler chicks fed dietary thyme as an alternative for antibiotic growth promoter. Afr. J. Biotechnol. 2010;9(40):6819–6825. [Google Scholar]
- 20.Sadeghi A.A, Moghaddam M. The effects of turmeric, cinnamon, ginger and garlic powder nutrition on antioxidant enzymes'status and hormones involved in energy metabolism of broilers during heat stress. Iran. J. Appl. Anim. Sci. 2018;8(1):125–130. [Google Scholar]
- 21.Sugiharto S. Alleviation of heat stress in broiler chicken using turmeric (Curcuma longa)-a short review. J. Anim. Behav. Biometeorol. 2020;8(3):215–222. [Google Scholar]
- 22.Kosti D, Dahiya D.S, Dalal R.P, Tewatia B.S, Vijayalakshmy K. Role of turmeric supplementation on production, physical and biochemical parameters in laying hens. Worlds Poult. Sci. J. 2020;76(3):625–637. [Google Scholar]
- 23.Gaikwad B.S, Patil R.A, Padghan P.V, Shinde S.S. Effect of fenugreek (Trigonella foenum-gracum L.) seed powder as natural feed additive on growth performance of broilers. Int. J. Curr. Microbiol. Appl. Sci. 2019;8(10):1137–1146. [Google Scholar]
- 24.Yassin M, Nurfeta A, Banerjee S. The effect of supplementing Fenugreek (Trigonella foenum-graecum L.) seed powder on growth performance, carcass characteristics and meat quality of Cobb 500 broilers reared on conventional ration. Ethiopian J. Agric. Sci. 2020;30(3):129–142. [Google Scholar]
- 25.Nasir Z, Abid A.R, Hayat Z, Shakoor H.I. Effect of Kalongi (Nigella sativa) seeds on egg production and quality in white Leghorn layers. J. Anim. Plant Sci. 2005;15(1–2):22. [Google Scholar]
- 26.Rahman M, Kim S.J. Effects of dietary Nigella sativa seed supplementation on broiler productive performance, oxidative status and qualitative characteristics of thighs meat. Italian. J. Anim. Sci. 2016;15(2):241–247. [Google Scholar]
- 27.Azeem T, Rehman Z.U, Umar S, Asif M, Arif M, Rahman A. Effect of Nigella sativa on poultry health and production:A review. Sci. Lett. 2014;2(2):76–82. [Google Scholar]
- 28.Zhang G.F, Yang Z.B, Wang Y, Yang W.R, Jiang S.Z, Gai G.S. Effects of ginger root (Zingiber officinale) processed to different particle sizes on growth performance antioxidant status, and serum metabolites of broiler chickens. Poult. Sci. 2009;88(10):2159–2166. doi: 10.3382/ps.2009-00165. [DOI] [PubMed] [Google Scholar]
- 29.Zeweil H.S, El-Rahman M.H.A, Dosoky M, Salma W, Hafsa H.A, Abdulhamid A.B. Effects of ginger and bee propolis on the performance, carcass characteristics and blood constituents of growing Japanese quail. Egypt. Poult. Sci. J. (2016a);36((1)):143–159. [Google Scholar]
- 30.Herve T, Raphaël K.J, Ferdinand N, Victor Herman N, Marvel N.M.W, D'Alex T.C, Vitrice F.T.L. Effects of ginger (Zingiber officinale, Roscoe) essential oil on growth and laying performances, serum metabolites, and egg yolk antioxidant and cholesterol status in laying Japanese quail. J. Vet. Med. 2019;2019:7857504. doi: 10.1155/2019/7857504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez Y, Valdivié M, Solano G, Estarrón-Espinosa M, Martínez O, Córdova J. Effect of pumpkin (Cucurbita maxima) seed meal on total cholesterol and fatty acids of laying hen eggs. Cuban J. Agric. Sci. 2012;46(1):73–78. [Google Scholar]
- 32.Shawle K, Urge M, Animut G. Effect of different levels of Lepidium sativum Lon growth performance, carcass characteristics, hematology and serum biochemical parameters of broilers. SpringerPlus, 2016;5((1)):1441. doi: 10.1186/s40064-016-3118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassan R.I.M, El Shoukary R.D. Impact of dietary supplementation with cress seeds (Lepidium Sativum L.) on growth performance, carcass characteristics and behavior of broilers. Alex. J. Vet. Sci. (2019);61((2)):38–44. [Google Scholar]
- 34.Al-Mashhadani E.H, Al-Mashhadani H, Al-Shamire J.S. Effect of supplementing different levels of chamomile oil on broiler performance and some physiological traits. Int. J. Poult. Sci. 2013;12(7):426–429. [Google Scholar]
- 35.Jakubcova Z, Zeman L, Mares P, Mlcek J, Jurikova T, Dostalova L, Mrazkova E, Mrkvicova E, Balla S, Sochor J. Effect of chamomile supplements to feeding doses on antimicrobial parameters in poultry. Potrav. Slovak. J. Food Sci. 2014;8(1):228–232. [Google Scholar]
- 36.Liu Y.F, Zhang K.Y, Zhang Y, Bai S.P, Ding X.M, Wang J.P, Peng H.W, Xuan Y, Su Z.W, Zeng Q.F. Effects of graded levels of phytase supplementation on growth performance, serum biochemistry, tibia mineralization, and nutrient utilization in Pekin ducks. Poult. Sci. 2020;99(10):4845–4852. doi: 10.1016/j.psj.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yadav A.S, Kolluri G, Gopi M, Karthik K, Malik Y, Dhama K. Exploring alternatives to antibiotics as health promoting agents in poultry-a review. J. Exp. Biol. Agric. Sci. 2016;4(3):368–383. [Google Scholar]
- 38.Saki A.A, Aliarabi H, Siyar S.A.H, Salari J, Hashemi M. Effect of a phytogenic feed additive on performance, ovarian morphology, serum lipid parameters and egg sensory quality in laying hen. Vet. Res. Forum. 2014;5(4):287–293. [PMC free article] [PubMed] [Google Scholar]
- 39.Raei H, Torshizi M.A.K, Sharafi M, Ahmadi H. Improving seminal quality and reproductive performance in male broiler breeder by supplementation of camphor. Theriogenology. 2021;166:1–8. doi: 10.1016/j.theriogenology.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 40.El-Ghousein S.S, Al-Beitawi N.A. The effect of feeding of crushed thyme (Thymus valgaris L.) on growth, blood constituents, gastrointestinal tract and carcass characteristics of broiler chickens. J. Poult. Sci. 2009;46(2):100–104. [Google Scholar]
- 41.Fachini-Queiroz F.C, Kummer R, Estevão-Silva C.F, Carvalho M.D, Cunha J.M, Grespan R, Bersani-Amado C.A, Cuman R.K. Effects of thymol and carvacrol, constituents of Thymus vulgaris Lessential oil, on the inflammatory response. Evid Based Complement. Altern. Med. 2012;2012:657026. doi: 10.1155/2012/657026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosseinzadeh S, Jafarikukhdan A, Hosseini A, Armand R. The application of medicinal plants in traditional and modern medicine:A review of Thymus vulgaris. Int. J. Clin. Med. 2015;6(9):635–642. [Google Scholar]
- 43.Kowalczyk A, Przychodna M, Sopata S, Bodalska A, Fecka I. Thymol and thyme essential oil-new insights into selected therapeutic applications. Molecules. 2020;25(18):4125. doi: 10.3390/molecules25184125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding X, Yu Y, Su Z, Zhang K. Effects of essential oils on performance, egg quality, nutrient digestibility and yolk fatty acid profile in laying hens. Anim. Nutr. 2017;3(2):127–131. doi: 10.1016/j.aninu.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du E, Wang W, Gan L, Li Z, Guo S, Guo Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016;7:19. doi: 10.1186/s40104-016-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, Chen H, Li W, Miao J, Chen N, Shao X, Cao Y. Polyphenols in Eucalyptus leaves improved the egg and meat qualities and protected against ethanol-induced oxidative damage in laying hens. J. Anim. Physiol. Anim. Nutr. (Berl) 2018;102(1):214–223. doi: 10.1111/jpn.12680. [DOI] [PubMed] [Google Scholar]
- 47.Fki I, Bouaziz M, Sahnoun Z, Sayadi S. Hypocholesterolemic effects of phenolic-rich extracts of Chemlali olive cultivar in rats fed a cholesterol-rich diet. Bioorg. Med. Chem. 2005;13(18):5362–5370. doi: 10.1016/j.bmc.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 48.Vakili R, Heravi R.M. Performance and egg quality of laying hens fed diets supplemented with herbal extracts and flaxseed. Poult. Sci. 2016;4(2):107–116. [Google Scholar]
- 49.Leskanich C.O, Noble R.C. Manipulation of the n-3 polyunsaturated fatty acid composition of avian eggs and meat. Worlds Poult. Sci. J. 53((2)):155–183. [Google Scholar]
- 50.Grobas S, Mendez J, Lazaro R, De Blas C, Mateo G.G. Influence of source and percentage of fat added to diet on performance and fatty acid composition of egg yolks of two strains of laying hens. Poult. Sci (1997) 2001;80(8):1171–1179. doi: 10.1093/ps/80.8.1171. [DOI] [PubMed] [Google Scholar]
- 51.Ghasemi R, Zarei M, Torki M. Adding medicinal herbs including garlic (Allium sativum) and thyme (Thymus vulgaris) to diet of laying hens and evaluating productive performance and egg quality characteristics. Am. J. Anim. Vet. Sci. 2010;5(2):151–154. [Google Scholar]
- 52.Al-Kassie G.A.M. Influence of two plant extracts derived from thyme and cinnamon on broiler performance. Pak. Vet. J. 2009;29(4):169–173. [Google Scholar]
- 53.Franz C, Baser K.H, Windisch W.M. Essential oils and aromatic plants in animal feeding-a European perspective. A review. Flav. Fragr. J. 2010;25(5):327–340. [Google Scholar]
- 54.Hashemipour H, Kermanshahi H, Golian A, Veldkamp T. Metabolism and nutrition:Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013;92(8):2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- 55.Griela E, Paraskeuas V, Mountzouris K.C. Effects of diet and phytogenic inclusion on the antioxidant capacity of the broiler chicken gut. Animals. 2021;11((3)):739. doi: 10.3390/ani11030739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Acamovic T, Brooker J.D. Biochemistry of plant secondary metabolites and their effects in animals. Proc. Nutr. Soc. 2005;64(3):403–412. doi: 10.1079/pns2005449. [DOI] [PubMed] [Google Scholar]
- 57.Lin H, Jiao H.C, Buyse J, Decuypere E. Strategies for preventing heat stress in poultry. Worlds Poult. Sci. J. 2006;62(1):71–86. [Google Scholar]
- 58.Langhout P. New additives for broiler chickens. World Poult. 2000;16(3):22–27. [Google Scholar]
- 59.Williams P, Losa R. The use of essential oils and their compounds in poultry nutrition. World Poult. 2001;17(4):14–15. [Google Scholar]
- 60.Hernandez F, Madrid J, García V, Orengo J, Megías M.D. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 2004;83(2):169–174. doi: 10.1093/ps/83.2.169. [DOI] [PubMed] [Google Scholar]
- 61.Su G, Zhou X, Wang Y, Chen D, Chen G, Li Y, He J. Effects of plant essential oil supplementation on growth performance, immune function and antioxidant activities in weaned pigs. Lipids Health Dis. 2018;17((1)):139. doi: 10.1186/s12944-018-0788-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Govindarajan V.S. Turmeric--chemistry, technology, and quality. Crit. Rev. Food Sci. Nutr. 1980;12(3):199–301. doi: 10.1080/10408398009527278. [DOI] [PubMed] [Google Scholar]
- 63.Sharma R.A, Gescher A.J, Steward W.P. Curcumin:The story so far. Eur. J. Cancer. 2005;41(13):1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Lantz R.C, Chen G.J, Solyom A.M, Jolad S.D, Timmermann B.N. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine. 2005;12(6–7):445. doi: 10.1016/j.phymed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Zava D.T, Dollbaum C.M, Blen M. Estrogen and progestin bioactivity of foods, herbs, and spices. Exp. Biol. Med. 1998;217(3):369–378. doi: 10.3181/00379727-217-44247. [DOI] [PubMed] [Google Scholar]
- 66.Hewlings S.J, Kalman D.S. Curcumin:A review of its'effects on human health. Foods. 2017;6(10):92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mishra B, Jha R. Oxidative stress in the poultry gut:Potential challenges and interventions. Front. Vet. Sci. 2019;6:60. doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sugiharto S, Yudiarti T, Isroli I, Widiastuti E, Kusumanti E. Dietary supplementation of probiotics in poultry exposed to heat stress-a review. Ann. Anim. Sci. (2017a);17((3)):591–604. [Google Scholar]
- 69.Sugiharto S, Yudiarti T, Isroli I, Widiastuti E, Putra F.D. Effect of dietary supplementation with Rhizopus oryzae or Chrysoniliacrassa on growth performance, blood profile, intestinal microbial population, and carcass traits in broilers exposed to heat stress. Arch. Anim. Breed. (2017β);60:347–356. [Google Scholar]
- 70.Mohamed A.S.A, Lozovskiy A.R, Ali A.M.A. Nutritional strategies to alleviate heat stress effects through feed restrictions and feed additives (vitamins and minerals) in broilers under summer conditions. J. Anim. Behav. Biometeorol. 2019;7(3):123–131. [Google Scholar]
- 71.El-Maaty A, Hayam M.A, Rabie M.H, El-Khateeb A.Y. Response of heat-stressed broiler chicks to dietary supplementation with some commercial herbs. Asian J. Anim. Vet. Adv. 2014;9(12):743–755. [Google Scholar]
- 72.Zhou T.X, Chen Y.J, Yoo J.S, Huang Y, Jee J.H, Jang H.D, Shin S.O, Kim H.J, Cho J.H, Kim I.H. Effects of chitooligosaccharide supplementation on performance, blood characteristics, relative organ weight, and meat quality in broiler chickens. Poult. Sci. 2009;88((3)):593–600. doi: 10.3382/ps.2008-00285. [DOI] [PubMed] [Google Scholar]
- 73.Mahesh M.G, Bhandary Y.P. Natural antibiotic effect of turmeric in poultry management. Int. J. Poult. Fish Sci. 2018;2(2):1–2. [Google Scholar]
- 74.Kohli K, Ali J, Ansari M.J, Raheman Z. Curcumin:A natural anti-inflammatory agent. Indian J. Pharm. 2005;37(3):141. [Google Scholar]
- 75.Seo K.I, Choi M.S, Jung U.J, Kim H.J, Yeo J, Jeon S.M, Lee M.K. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol. Nutr. Food Res. 2008;52(9):995–1004. doi: 10.1002/mnfr.200700184. [DOI] [PubMed] [Google Scholar]
- 76.Rao D.C, Sekhara N.C, Satyanarayana M.N, Srinivasan M. Effect of curcumin on serum and liver cholesterol levels in the rat. J. Nutr. 1970;100(11):1307–1316. doi: 10.1093/jn/100.11.1307. [DOI] [PubMed] [Google Scholar]
- 77.Adil S, Qureshi S, Pattoo R.A. A review on positive effects of fenugreek as feed additive in poultry production. Int. J. Poult. Sci. 2015;14(12):664–669. [Google Scholar]
- 78.Bash E, Ulbricht C, Kuo G, Szapary P, Smith M. Therapeutic Applications of fenugreek. Altern. Med. Rev. 2003;8(1):20–27. [PubMed] [Google Scholar]
- 79.Al-Habori M, Roman A. Pharmacological properties in fenugreek. In: Petropoulos G.A, editor. The Genus Trigonella. London and New York: Taylor and Francis; (2002). pp. 163–182. [Google Scholar]
- 80.Raghuram T.C, Sharma R.D, Sivakumar B, Sahay B.K. Effect of fenugreek seeds on intravenous glucose disposition in non-insulin dependent diabetic patients. Phytother. Res. 1994;8(2):83–86. [Google Scholar]
- 81.Samani S.K, Ghorbani M.R, Fayazi J, Salari S. The effect of different levels of Fenugreek (Trigonella foenum-graecum L.) powder and extract on performance, egg quality, blood parameters and immune responses of laying hens in second production cycle. Vet. Res. Forum, 2020;11(1):53–57. doi: 10.30466/vrf.2019.80961.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Aqil A.A. Effects of adding dietary fenugreek (Trigonella foenum graecum L.) powder on productive performance and egg quality of laying hens. Int. J. Poult. Sci. 2016;15(7):259–263. [Google Scholar]
- 83.Ghosheh O.A, Houdi A.A, Crooks P.A. High-performance liquid chromatography analysis of the pharmacologically active quinines and related compounds in the oil of the black seed (Nigella sativa) J. Pharm. Biomed. Anal. 1999;19(5):757–762. doi: 10.1016/s0731-7085(98)00300-8. [DOI] [PubMed] [Google Scholar]
- 84.Abd El-Hack M.E.A, Alagawany M, Farag M.R, Tiwari R, Karthik K, Dhama K. Nutritional, healthical and therapeutic efficacy of black cumin (Nigella sativa) in animals, poultry and humans. Int. J. Pharm. 2016;12(3):232–248. [Google Scholar]
- 85.Al-Homidan A, Al-Qarawi A.A, Al-Waily S.A, Adam S.E. Response of broiler chicks to dietary Rhazya stricta and Nigella sativa. Br. Poult. Sci. 2002;43(2):291–296. doi: 10.1080/00071660120121526. [DOI] [PubMed] [Google Scholar]
- 86.Mountzouris K.C, Paraskevas V, Tsirtsikos P, Palamidi I, Steiner T, Schatzmayr G, Fegeros K. Assessment of a phytogenic feed additive effect on broiler growth performance, nutrient digestibility and caecal microflora composition. Anim. Feed Sci. Techol. 2011;168(3–4):223. [Google Scholar]
- 87.Zhang R, Wang X.W, Liu L.L, Cao Y.C, Zhu H. Dietary oregano essential oil improved the immune response, activity of digestive enzymes, and intestinal microbiota of the koi carp, Cyprinus carpio. Aquaculture. 2020;518:734781. [Google Scholar]
- 88.Kumar P, Patra A.K. Beneficial use of black cumin (Nigella sativa L.) seeds as a feed additive in poultry nutrition. Worlds Poult. Sci. J. 2017;73(4):872–885. [Google Scholar]
- 89.Abd El-Hack M.E, Attia A.I, Arif M, Soomro R.N, Arain M.A. The impacts of dietary Nigella sativa meal and Avizyme on growth, nutrient digestibility and blood metabolites of meat-type quail. Anim. Prod. Sci. 2018;58(2):291–298. [Google Scholar]
- 90.De la Lastra C, Barranco M, Motilva V, Herrerías J. Mediterranean diet and health biological importance of olive oil. Curr. Pharm. Des. 2001;7(10):933–950. doi: 10.2174/1381612013397654. [DOI] [PubMed] [Google Scholar]
- 91.Harwood J.L, Yaqoob P. Nutritional and health aspects of olive oil. Eur. J. Lipid Sci. Technol. 2002;104(9-10):685. [Google Scholar]
- 92.Silva S, Gomes L, Leitão F, Coelho A.V, Boas L.V. Phenolic compounds and antioxidant activity of Olea europaea L. fruits and leaves. Food Sci. Technol. Int. 2006;12(5):385–396. [Google Scholar]
- 93.Paiva-Martins F, Ribeirinha T, Silva A, Gonçalves R, Pinheiro V, Mourão J.L, Outor-Monteiro D. Effects of the dietary incorporation of olive leaves on growth performance, digestibility, blood parameters and meat quality of growing pigs. J. Sci. Food Agric. 2014;94(14):3023–3029. doi: 10.1002/jsfa.6650. [DOI] [PubMed] [Google Scholar]
- 94.Saleh A, Alzawqari M. Effects of replacing yellow corn with olive cake meal on growth performance, plasma lipid profile, and muscle fatty acid content in broilers. Animals. 2021;11(8):2240. doi: 10.3390/ani11082240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bouaziz M, Fki I, Jemai H, Ayadi M, Sayadi S. Effect of storage on refined and husk olive oils composition:Stabilization by addition of natural antioxidants from Chemlali olive leaves. Food Chem. 2008;108(1):253–262. [Google Scholar]
- 96.Kiritsakis K, Kontominas M.G, Kontogiorgis C, Litina D.H, Moustakas A, Kiritsakis A. Composition and antioxidant activity of olive leaf extracts from Greek olive cultivars. J. Am. Oil Chem. Soc. 2010;87(4):369–376. [Google Scholar]
- 97.Al-Harthi M. The effect of different dietary contents of olive cake with or without Saccharomyces cerevisiae on egg production and quality, inner organs and blood constituents of commercial layers. Eur. Poult. Sci. 2015;79:83–87. [Google Scholar]
- 98.Al-Harthi M.A. The efficacy of using olive cake as a by-product in broiler feeding with or without yeast. Italian J. Anim. Sci. 2016;15(3):512–520. [Google Scholar]
- 99.Abd El-Moneim A.E, Sabic E.M. Beneficial effect of feeding olive pulp and Aspergillus awamori on productive performance, egg quality, serum/yolk cholesterol and oxidative status in laying Japanese quails. J. Anim. Feed Sci. 2019;28(1):52–61. [Google Scholar]
- 100.Ozcan C, Cimrin T, Yakar Y, Alasahan S. Effects of olive cake meal on serum constituents and fatty acid levels in breast muscle of Japanese quail. S. Afr. J. Anim. Sci. 2020;50(6):874–880. [Google Scholar]
- 101.Al-Harthi M. The effect of olive cake, with or without enzymes supplementation, on growth performance, carcass characteristics, lymphoid organs and lipid metabolism of broiler chickens. Braz. J. Poult. Sci. 2017;19:83–90. [Google Scholar]
- 102.Zhang Z.F, Kim I.H. Effects of dietary olive oil on egg quality, serum cholesterol characteristics, and yolk fatty acid concentrations in laying hens. J. Appl. Anim. Res. 2014;42(2):233–237. [Google Scholar]
- 103.Tarchoune I, Sgherri C, Eddouzi J, Zinnai A, Quartacci M.F, Zarrouk M. Olive leaf addition increases olive oil nutraceutical properties. Molecules. 2019;24(3):545. doi: 10.3390/molecules24030545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Z.F, Zhou T.X, Kim I.H. Effects of dietary olive oil on growth performance, carcass parameters, serum characteristics, and fatty acid composition of breast and drumstick meat in broilers. Asian Australas. J. Anim. Sci. 2013;26(3):416–422. doi: 10.5713/ajas.2012.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dedousi A, Kritsa M.Z, Stojčić M.D, Sfetsas T, Sentas A, Sossidou E. Production performance, egg quality characteristics, fatty acid profile and health lipid indices of produced eggs, blood biochemical parameters and welfare indicators of laying hens fed dried olive pulp. Sustainability. 2022;14(6):3157. [Google Scholar]
- 106.Turner R, Etiene N, Garcia-Alonso M, de Pascual-Teresa S, Minihane A.M, Weinberg P.D, Rimbach G. Antioxidant and anti-atherogenic activities of olive oil phenolics. Int. J. Vitam. Nutr. Res. 2010;75(1):61–70. doi: 10.1024/0300-9831.75.1.61. [DOI] [PubMed] [Google Scholar]
- 107.Kaya S, Kececi T, Haliloglu S. Effects of zinc and vitamin A supplements on plasma levels of thyroid hormones, cholesterol, glucose and egg yolk cholesterol of laying hens. Res. Vet. Sci. 2001;71(2):135–139. doi: 10.1053/rvsc.2001.0500. [DOI] [PubMed] [Google Scholar]
- 108.Chetty K.N, Calahan L, Oliver R, Chetty S.N. Garlic induced alteration in liver mineral concentrations in corn oil and olive oil fed rats. Pathophysiology. 2004;11(2):129–131. doi: 10.1016/j.pathophys.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Keshavarz K, Nakajima S. Re-evaluation of calcium and phosphorus requirements of laying hens for optimum performance and eggshell quality. Poult. Sci. 1993;72(1):144–153. [Google Scholar]
- 110.Bar A, Vax E, Striem S. Relationships among age, eggshell thickness and vitamin D metabolism and its expression in the laying hen. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1999;123(2):147–154. doi: 10.1016/s1095-6433(99)00039-2. [DOI] [PubMed] [Google Scholar]
- 111.Chrubasik S, Pittler M.H, Roufogalis B.D. Zingiber is rhizoma:A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine. 2005;12(9):684–701. doi: 10.1016/j.phymed.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 112.Dieumou F.E, Teguia A, Kuiate J.R, Tamokou J.D, Fonge N.B, Dongmo M.C. Effects of ginger (Zingiber officinale) and garlic (Allium sativum) essential oils on growth performance and gut microbial population of broiler chickens. Livest. Res. Rural. Dev. 2009;21(8):25–34. [Google Scholar]
- 113.Zeweil H.S, Zahran S.M, El-Rahman M.H.A. Effect of using bee propolis as a natural supplement on productive and physiological performance of Japanese quail. Egypt. Poult. Sci. J. (2016b);36((1)):161–175. [Google Scholar]
- 114.Zhao X, Yang Z.B, Yang W.R, Wang Y, Jiang S.Z, Zhang G.G. Effects of ginger root (Zingiber officinale) on laying performance and antioxidant status of laying hens and on dietary oxidation stability. Poult. Sci. 2011;90(8):1720–1727. doi: 10.3382/ps.2010-01280. [DOI] [PubMed] [Google Scholar]
- 115.Saed Z.G.M, Mohammed T, Farhan S.M. Effect of ginger and celery seeds as feed additives on reproductive performance of broiler breeder males. Plant Arch. 2018;18(2):1823–1829. [Google Scholar]
- 116.Al-Sayed H.M.A, Zidan N.S, Abdelaleem M.A. Utilization of garden cress seeds (Lepidium sativum L.) as a natural source of protein and dietary fiber in noodles. Int. J. Pharm. Res. Allied Sci. 2019;8(3):17–28. [Google Scholar]
- 117.Bryan R.M, Shailesh N.S, Jill K.W, Steven F.V, Roque L.E. Composition and physical properties of cress (Lepidium sativum L.) and field pennycress (Thlaspi arvense L.) oils. Ind. Crop. Prod. 2009;30(2):199–205. [Google Scholar]
- 118.Deshmukh Y.R, Thorat S.S, Mhalaskar S.R. Influence of garden cress seed (Lepidium sativum L.) bran on quality characteristics of cookies. Int. J. Curr. Microbiol. Appl. Sci. 2017;6(9):586–593. [Google Scholar]
- 119.Lotfi S, Fakhraei J, Yarahmadi H.M. Dietary supplementation of pumpkin seed oil and sunflower oil along with vitamin E improves sperm characteristics and reproductive hormones in roosters. Poult. Sci. 2021;100(9):101289. doi: 10.1016/j.psj.2021.101289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mitra P, Ramaswamy S.H, Chang S.K. Pumpkin (Cucurbita maximus) seed oil extraction using supercritical carbon dioxide and physicochemical properties of the oil. J. Food Eng. 2009;95(1):208–213. [Google Scholar]
- 121.Srbinoska M, Hrabovski N, Rafajlovska V, Sinadinovic-Fiser S. Characterization of the seed and seed extracts of Pumpkins Cucurbita maxima D and Cucurbita pepo L from Macedonia. Macedonian J. Chem. Chem. Eng. 2012;31(1):65–78. [Google Scholar]
- 122.Wafar R.J, Hannison M, Abdullahi U.U, Makinta A. Effect of pumpkin (Cucurbita pepo L.) seed meal on the performance and carcass characteristics of broiler chickens. Asian J. Adv. Agric. Res. 2017;2(3):1–7. [Google Scholar]
- 123.Stevenson D.G, Eller F.J, Wang L, Jane J.L, Wang T, Inglett G.E. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J. Agric. Food Chem. 2007;55(10):4005–4013. doi: 10.1021/jf0706979. [DOI] [PubMed] [Google Scholar]
- 124.Vlaicu P.A, Panaite T.D. Effect of dietary pumpkin (Cucurbita moschata) seed meal on layer performance and egg quality characteristics. Anim. Biosci. 2022;35(2):236–246. doi: 10.5713/ab.21.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.El-Saadany A.S, El-Barbary A.M, Shreif E.Y, Elkomy A, Khalifah A.M, El-Sabrout K. Pumpkin and garden cress seed oils as feed additives to improve the physiological and productive traits of laying hens. Italian J. Anim. Sci. 2022;21(1):1047–1057. [Google Scholar]
- 126.Parham S, Kharazi A.Z, Bakhsheshi-Rad H.R, Nur H, Ismail A.F, Sharif S, RamaKrishna S, Berto F. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants. 2020;9((12)):1309. doi: 10.3390/antiox9121309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bilal R.M, Liu C, Zhao H, Wang Y, Farag M.R, Alagawany M, Hassan F.U, Elnesr S.S, Elwan H, Qiu H, Lin Q. Olive oil:Nutritional applications, beneficial health aspects and its prospective application in poultry production. Front Pharmacol. 2021;12:723040. doi: 10.3389/fphar.2021.723040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Adamu M, Boonkaewwan C. Effect of Lepidium sativum L(Garden Cress) seed and its extract on experimental Eimeria tenella infection in broiler chickens . Kasetsart J. Nat. Sci. 2014;48(1):28–37. [Google Scholar]
- 129.Singh C.S, Paswan V.K. Ch. 14. Advances in Seed Biology. London: IntechOpen Publisher; (2017). The potential of garden cress (Lepidium sativum L.) seeds for the development of functional foods; pp. 280–290. [Google Scholar]
- 130.Achilonu M.C, Nwafor I.C, Umesiobi D.O, Sedibe M.M. Biochemical proximates of pumpkin (Cucurbitaeae spp) and their beneficial effects on the general well-being of poultry species. J. Anim. Physiol. Anim. Nutr (Berl) 2018;102((1)):5–16. doi: 10.1111/jpn.12654. [DOI] [PubMed] [Google Scholar]
- 131.Diwakar B.T, Dutta P.K, Lokesh B.R, Naidu K.A. Physicochemical properties of garden cress (Lepidium sativum L.) seed oil. J. Am. Oil. Chem. Soc. 2010;87(5):539–548. [Google Scholar]
- 132.Bardaa S, Ben Halima N, Aloui F, Ben Mansour R, Jabeur H, Bouaziz M, Sahnoun Z. Oil from pumpkin (Cucurbita pepo L.) seeds:Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016:15–73. doi: 10.1186/s12944-016-0237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Richardson A.J. Omega-3 fatty acids in ADHD and related neuro-developmental disorders. Int. Rev. Psychiatry, (2006);18(2):155–172. doi: 10.1080/09540260600583031. [DOI] [PubMed] [Google Scholar]
- 134.Abbas R.J, Al-Shaheen S.A, Majeed T.I. Association of Genetic and Environmental Resources Conservation (AGERC). In:Proceeding of the 4th. Cairo, Egypt: International Conference of Genetic and Environment; 2016. Effect of Supplementing Different Levels of Pumpkin Seed Oil in the Diets of Spent Laying Japanese Quail (Coturnix coturnix japonica) [Google Scholar]
- 135.Hashemi J.M. Pumpkin seed oil and Vitamin E improve reproductive function of male rats inflicted by testicular injury. World Appl. Sci. J. (2013);23(10):1351–1359. [Google Scholar]
- 136.Kóňa J, Ďurovka M, Tancík J. Pumpkin vegetables. Garmond, Nitra. 2007:148. [Google Scholar]
- 137.Zia-Ul-Haq M, Ahmad S, Calani L, Mazzeo T, Rio D.D, Pellegrini N, De Feo V. Compositional study and antioxidant potential of Ipomoea hederacea Jacq and Lepidium sativum L. seeds. Molecules, 2012;17(9):10306–10321. doi: 10.3390/molecules170910306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mishra D.B, Roy D, Kumar V, Bhattacharyya A, Kumar M, Kushwaha R, Vaswani S. Effect of feeding different levels of Azolla pinnata on blood biochemicals, hematology and immunocompetence traits of Chabro chicken. Vet. World, 2016;9(2):192–198. doi: 10.14202/vetworld.2015.192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Naghshi H, Khojasteh S, Jafari M. Investigation of the effect of different levels of Azolla (Azolla pinnata) on performance and characteristics of Cobb broiler chicks. Int. J. Farming Allied Sci. 2014;3(1):45–49. [Google Scholar]
- 140.Alalade O.A, Iyayi E.A. Chemical composition and the feeding value of Azolla pinnata meal for egg-type chicks. Int. J. Poult. Sci. 2006;5(2):137–141. [Google Scholar]
- 141.Al-Rekabi M.M, Ali N.A, Abbas F.R. Effect of partial and total substitution for Azolla plant (Azolla pinnata) powder instead of soybean meal in broiler chickens diets on blood biochemical traits. Plant Arch. 2020;20(1):1344–1348. [Google Scholar]
- 142.Nabi F, Arain M.A, Rajput N, Alagawany M, Soomro J, Umer M, Soomro F, Wang Z, Ye R, Liu J. Health benefits of carotenoids and potential application in poultry industry:A review. J. Anim. Physiol. Anim. Nutr. (Berl) 2020;104(6):1809–1818. doi: 10.1111/jpn.13375. [DOI] [PubMed] [Google Scholar]
- 143.McKay D.L, Blumberg J.B.A. Review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) Phytother. Res. 2006;20(7):519–530. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- 144.El-Galil K.A, Mahmoud H.A, Hassan A.M, Morsy A.A. Effect of chamomile flowers meal as feed additives in laying Japanese quail diets on productive and reproductive performance. J. Anim. Poult. Prod. 2010;1((10)):517–533. [Google Scholar]
- 145.Isaac O. Pharmacological investigations with compounds of chamomile 1On the pharmacology of (-)-c--bisabolol and bisabolol oxides (review) Planta Med. (1979);35(2):118–124. doi: 10.1055/s-0028-1097193. [DOI] [PubMed] [Google Scholar]
- 146.Mann C, Staba E.J. The chemistry, pharmacology and formulations of Chamomile. In: Graker L.E, Simon J.E, editors. Herbs, Spices and Medicinal Plants:Recent Advances in Botany, Horticulture and Pharmacology, Phoenix. Arizona: Oryx Press; (1986). pp. 235–280. [Google Scholar]
- 147.Tenório K.I, Sgavioli S, Roriz B.C, Ayala C.M, Santos W, Rodrigues P.H.M, Almeida V.R, Garcia R.G. Effect of chamomile extract on the welfare of laying Japanese quail. Rev. Bras. Zootec. 2017;46(9):760–765. [Google Scholar]


