Abstract
Introduction: Implant surface disinfection is the most difficult phases in treatment of peri-implantitis. This study sought to assess the efficacy of antimicrobial photodynamic therapy (aPDT) with phycocyanin and diode laser for the reduction of Porphyromonas gingivalis.
Methods: In this in vitro, experimental study, first the minimum inhibitory concentration (MIC) of phycocyanin, the sub-lethal exposure time of the diode laser, and the sub-lethal dose of aPDT were determined. The wells containing P. gingivalis suspension were randomly divided into three experimental groups for exposure to ½ MIC phycocyanin for 5 minutes, minimum lethal dose of diode laser (635 nm, 4 minutes), and aPDT with phycocyanin and diode laser. The positive control group was exposed to 0.2% chlorhexidine (CHX) for 5 minutes, and the negative control group received no treatment. The colony count was calculated in the five groups and compared using one-way ANOVA and t test.
Results: aPDT with a diode laser (635 nm, 4 minutes) and 125 µg/mL phycocyanin caused a significantly greater reduction in P. gingivalis count (mean reduction of 44.24%) compared with other groups (P<0.0001). Minimum and maximum colony counts were noted in 0.2% CHX and negative control groups respectively. The reduction in the colony count was significant in all experimental groups, compared with the control group (P<0.0001).
Conclusion: aPDT with 635-nm diode laser and phycocyanin can significantly decrease P. gingivalis count in vitro. Considering the conservative nature of this modality, it may be used for the decontamination of peri-implant.
Keywords: Antimicrobial photodynamic therapy, Porphyromonas gingivalis, Phycocyanin, Diode laser, Decontamination
Introduction
With an increase in the use of dental implants for the replacement of lost teeth, the prevalence of peri-implant diseases is also on the rise. According to the existing studies, peri-implant mucositis has an incidence of 80% while the incidence of peri-implantitis ranges from 28% to 56%.1,2 Peri-implantitis is described by the inflammation of peri-implant tissues, which is often associated with infection, formation of deep pockets, and marginal bone loss.2 The microorganisms causing peri-implantitis are the same as those causing periodontitis and include Staphylococcus aureus, Capnocytophagasp, Spirochetes sp, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, Prevotella intermedia, and Porphyromonas gingivalis.3,4
Disinfection of implant surfaces is among the most difficult phases in the treatment of peri-implantitis. Mechanical methods by the application of curettes, ultrasonic scalers, and air power abrasion, and chemical methods by the use of citric acid, hydrogen peroxide, EDTA, chlorhexidine (CHX), and topical and systemic antibiotic therapy, as well as different laser types such as erbium, CO2 and diode lasers have been proposed for this purpose. Bacterial biofilm removal with minimal damage to the implant surface, removal of bacterial toxins, and management of inflammatory reactions are the main goals in the treatment of peri-implantitis. If not properly managed, peri-implantitis can lead to pain, bone loss, and implant mobility, requiring implant extraction.5,6 The reported complications in the process of implant surface decontamination include damage to the implant surface, an increase in microbial resistance, limitations in the complete elimination of microbial plaque, and heat damage following the use of high-power lasers.7,8
Antimicrobial photodynamic therapy (aPDT) with a laser has been proposed for the treatment of peri-implantitis and the elimination of periopathogens. aPDT is characterized by photochemical reactions between three components including photosensitizer, oxygen, and light source.8 The mechanism of action of aPDT is related to the attachment of the photosensitizer to the target cells. Irradiation of light at a certain wavelength in the presence of oxygen results in the formation of high-energy singlet oxygen and oxygen radicals, which are lethal to the target cells.9 Such reactions occur in a small area, which makes aPDT suitable for local and selective destruction at the target site.10 aPDT is a safe, non-invasive procedure and can be repeated without concerning about the development of bacterial resistance.11
Evidence shows that light absorption by the bacteria, laser characteristics (wavelength, power, etc), duration of radiation, nature of the bacterial matrix, and type of photosensitizer can all affect the efficacy of aPDT.12 Phycocyanin is a new natural photosensitizer with several bioactive, anti-inflammatory, anti-cancer, and anti-microbial properties, which is commonly used in food and pharmaceutical industries.13
Chiniforush et al in assessing different light sources for the activation of chlorophyllin–phycocyanin mixture in the aPDT procedure against Enterococcus faecalis concluded that red lasers (635 and 660 nm) are more effective for the activation of the photosensitizer in the reduction of E. faecalis.13
Etemadi et al in assessing aPDT with phycocyanin on A. actinomycetemcomitansbiofilm on titanium discs concluded that aPDT with phycocyanin at a concentration of 125 μg/mL accompanied by 635-nm diode laser irradiation for 4 min. decreased A. actinomycetemcomitans biofilm and can be considered a safe and non-invasive method for the decontamination of the implant surface.14
To our knowledge, no study has evaluated the effects of phycocyanin as a photosensitizer in the aPDT procedure on P. gingivalis; thus, this study sought to assess the efficacy of aPDT with phycocyanin and diode laser for reduction of P. gingivalis. The null hypothesis was that aPDT with phycocyanin can significantly decrease the P. gingivalis count.
Materials and Methods
In this experimental study, the minimum sample size was calculated to be 6 in each group according to a study by Saffarpour et al,15 using one-way ANOVA power analysis of PASS 11 software and assuming alpha = 0.05, beta = 0.2, standard deviation of the mean colony count = 77 × 104, and effect size of 0.75.
Preparation of Bacterial Suspension
Porphyromonas gingivalis (ATCC33277) was cultured on brain heart infusion (BHI) agar (Merck, Germany) supplemented with 5 g/L yeast extract, 5 mg/L hemin, 1 mg/L vitamin K (Sigma, Germany), and 5% defibrinated sheep blood. The plates were incubated in a glove box in microaerophilic conditions at 37°C, 5% CO2, 10% H2, and 85% N2 for 48 hours. To prepare a microbial suspension, fresh P. gingivalis colonies were transferred from the BHI agar to BHI broth supplemented with 1 mg/mL hemin and 5% horse serum. To assess the bacteria in the planktonic phase, the sub-minimum inhibitory concentration (MIC), sub-lethal exposure time of the diode laser, and sub-lethal dosage of aPDT had to be determined first.
Preparation of Photosensitizers and Light Source
Phycocyanin (Photoactive + , Weber medical, Germany) was used as a photosensitizer in this study. After preparation, it was kept under dark conditions at room temperature prior to use. A 635-nm diode laser (Konftec, Taiwan) with an output of 220 mW in continuous mode was used. The power density of the device was 0.34 W/cm2. The laser was used for 1, 2, 3, 4 and 5 minutes with energy densities of 20.4, 40.8, 61.2, 81.6 and 102 J/cm2 respectively. Laser power was checked with a power meter (Coherent, USA) before experiments.
Determination of MIC of Phycocyanin, Minimum Lethal Exposure Time of Diode Laser, and Minimum Lethal Dose of aPDT
To determine the MIC of phycocyanin (Photoactive + ; Weber Medical, Germany) against P. gingivalis, first 100 µL of 2X BHI broth was added to each well of a round-end 96-well plate. Next, 100 µL of phycocyanin with a final concentration of 2 mg/mL was added to the wells in the first column and diluted by 1:2. Afterwards, 100 µL of P. gingivalis suspension with a concentration of 1.5 × 106 colony-forming units (CFUs)/mL was added to each well.
In this study, wells containing P. gingivalis suspension alone and wells containing BHI broth without bacterial suspension or phycocyanin were considered the positive and negative controls respectively. Next, the microplates were incubated in the dark at room temperature (25 ± 2°C) under microaerophilic conditions for 5 minutes. Next, 100 µL of the contents of each well was spread-cultured on enriched agar plates. The plates were incubated at 37°C for 48 hours. under microaerophilic conditions. Finally, the colony count (CFUs/mL) in each plate was determined according to the method by Miles et al.16
To determine the minimum lethal exposure time of the laser against P. gingivalis, 200 µL of P. gingivalis suspension with a final concentration of 1.5 × 106 CFUs/mL in a flat-end 96-well plate was irradiated with the diode laser (Konftec, Taiwan) with a 635-nm wavelength, 220 mW power, power density of 0.34 w/cm2 and tip diameter of 7 mm for 1, 2, 3, 4 and 5 minutes at room temperature. After irradiation, the plate was incubated at 37°C for 48 hours under microaerophilic conditions, and the minimum lethal dose of the laser was determined according to the colony count, as described earlier.17
To determine the minimum lethal dose of aPDT against P. gingivalis, 100 µL of 2X BHI broth was added to each well of a flat-end 96-well plate, and then 100 µL of phycocyanin with a concentration 4 times that of MIC was added to the first well and then serially diluted to ½ MIC. Next, 100 µL of fresh P. gingivalis suspension with a final concentration of 1.5 × 106 CFUs/mL was added to each well, and the microplate was finally incubated in the dark at room temperature under microaerophilic conditions for 5 minutes. The bacterial suspension was then laser-irradiated and a minimum lethal dose of aPDT was determined based on the colony count, as described earlier.17
Antimicrobial Photodynamic Therapy
The wells were randomly allocated into three experimental and two control (positive and negative) groups.
In the negative control group, the bacterial suspension did not undergo any treatment.
In the positive control group, bacterial suspension was exposed to 0.2% CHX at 37°C for 5 minutes.
In the first experimental group, bacterial suspension was exposed to phycocyanin in ½ MIC concentration at 37 °C for 5 minutes.
In the second experimental group, bacterial suspension was exposed to a minimum lethal dose of the laser.
In the third experimental group, bacterial suspension was exposed to aPDT with phycocyanin in such a way that bacterial suspension was first exposed to ½ MIC phycocyanin in the dark at 37°C for 5 minutes and was then irradiated with a minimum lethal dose of laser at 635-nm laser with 220 mW power and 7-mm tip diameter for 4 minutes. The laser was irradiated vertically at a 1-mm distance from the surface of the wells.
Next, 10 µL of the suspension of each microtube was transferred into one well of a 96-well microplate containing 100 µL BHI broth, and after 5 serial dilutions, 10 µL of the contents of each well was spread-cultured on a BHI agar plate supplemented with defibrinated sheep blood, 5 g/L yeast extract, 5 mg/L hemin, and 1 mg/L vitamin K. Next, the plate was incubated at 37°C under microaerophilic conditions for 48 hours and then the colony count (CFUs/mL) was calculated according to Miles et al.16
Statistical Analysis
Each intervention was repeated three times. The collected data were analyzed by SPSS version 23 (SPSS Inc., IL, USA). One-way ANOVA and t test were first applied to compare the groups. Pairwise comparisons were then performed by a post-hoc test. P < 0.05 was considered statistically significant.
Results
According to the analysis of colony counts by the t test to determine the MIC of phycocyanin, phycocyanin at a concentration of 250 µg/mL showed a significant difference in the colony count with the control group compared with lower concentrations. Thus, 250 µg/mL concentration was considered the lethal dose of phycocyanin, and its previous concentration (125 µg/mL) was considered the sublethal dose (Figure 1, P < 0.05).
Figure 1.

Colony Count Following Exposure to Different Concentrations of Phycocyanin
For the determination of the minimum lethal exposure time of the diode laser according to the colony count, the t test showed a significant difference in the 5-minute laser irradiation group with the control group, compared with other groups. Thus, the 5-minute irradiation time was considered the lethal exposure time, and its previous exposure time (4 minutes) was considered the sublethal exposure time (Figure 2, P < 0.05).
Figure 2.
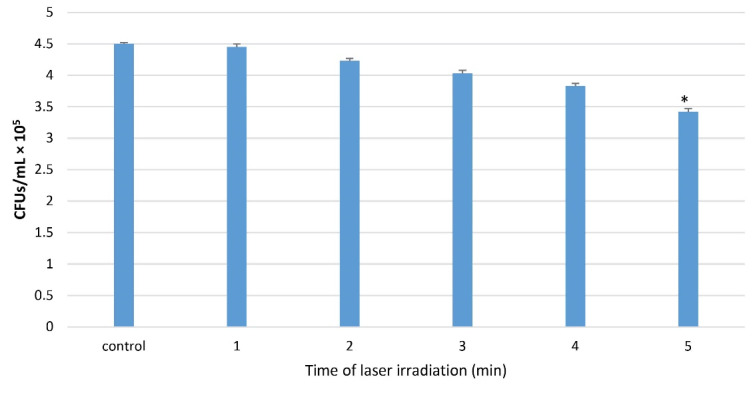
Colony Count Following Different Exposure Times of Laser at a Wavelength of 635 nm
For the determination of the minimum lethal dose of aPDT according to the colony count, the t test showed a significant difference in 125 µg/mL phycocyanin + 4-min laser irradiation time with the control group, compared with other groups. Thus, aPDT with 125 µg/mL phycocyanin + 4 minutes laser irradiation time was considered the minimum lethal dose of aPDT (Figure 3, P < 0.05).
Figure 3.

Colony Count Following aPDT With Different Protocols
Finally, the colony count (CFUs/mL) in the five groups was compared. The maximum colony count was noted in the negative control group followed by the phycocyanin, laser, and aPDT groups. No bacterial colony was noted in the 0.2% CHX group (Table 1).
Table 1. Mean and Standard Deviation of Porphyromonas gingivalis Colony Count (CFUs/mL) After the Interventions .
| Group | Mean±SD×105 | Range×105 | |
| Minimum | Maximum | ||
| Control | 4.5 ± 0.03 | 4.47 | 4.53 |
| CHX | 0.0 ± 0.0 | 0.0 | 0.0 |
| Phycocyanin | 3.93 ± 0.08 | 3.86 | 4.02 |
| Laser | 3.83 ± 0.02 | 3.78 | 3.88 |
| Phycocyanin + Laser | 2.51 ± 0.08 | 2.44 | 2.6 |
SD, Standard deviation; CHX, chlorhexidine.
Next, one-way ANOVA was applied to compare the groups and calculate the percentage of reduction in the colony count compared with the control group. The percentage of reduction in the colony count was 100% in the 0.2% CHX group, 44.24% in the aPDT group, 15.02% in the laser group, and 12.8% in the phycocyanin group; the difference in this respect was significant between the afore-mentioned four groups (Table 2, P < 0.0001).
Table 2. Mean Percentage of Reduction in the Colony Count of Porphyromonas gingivalis in the Experimental and Positive Control Groups Compared With the Negative Control Group .
| Groups | Percentage of Reduction in the Colony Count Compared With the Control Group | P Value |
| CHX (0.2%) | 100% | ˂0.0001 |
| Phycocyanin (125µg/mL) | 12.8% | |
| Laser (4 min) | 15.02% | |
| Phycocyanin + Laser (125 µg/mL + 4 min) | 44.24% |
Discussion
This study evaluated the effectiveness of aPDT with phycocyanin and diode laser for the reduction of P. gingivalis. The results indicated that aPDT with a 635-nm diode laser irradiated for 4 minutes and phycocyanin with a final concentration of 125 µg/mL caused a significantly greater reduction in the P. gingivalis count (mean reduction of 44.24%) compared with other groups (P < 0.0001). Minimum and maximum colony counts were noted in the 0.2% CHX and negative control groups respectively. The reduction in the colony count was significant in all experimental groups compared with the control group (P < 0.0001).
Moslemi et al18 confirmed the optimal efficacy of aPDT with Radachlorine and toluidine blue photosensitizers against P. gingivalis, which was in line with our results although the photosensitizers were different. According to Braham et al,19 aPDT with methylene blue and 670-nm diode laser can decrease the P. gingivalis count by the direct elimination of bacteria and also by the inactivation of proteases that are responsible for the pathogenesis of P. gingivalis. It also inactivates the destructive host cytokines such as tumor necrosis factor-alpha and interleukin 1β. Furthermore, de Oliveira et al20 evaluated the changes in gingival crevicular fluid cytokines after aPDT and reported that aPDT decreased the level of tumor necrosis factor-alpha and RANKL in gingival crevicular fluid. It should be noted that RANKL is responsible for bone loss and has osteoclastogenic properties.
In the present study, phycocyanin was used as a photosensitizer, which has blue pigments and is obtained from Spirulina, which is a type of blue-green algae with anti-oxidative, anti-inflammatory, antibacterial and anti-cancer properties. It also boosts the immune system and has pharmacologic effects on the liver and kidneys.21,22 Afrasiabi et al23 assessed the effects of aPDT with phycocyanin and diode laser on Streptococcus mutans biofilm and reported that diode laser irradiation accompanied by the use of phycocyanin and chlorophyll photosensitizers caused a significant reduction in bacterial biofilm (36.93%) and also in the metabolic activity of bacteria (77%). Also, in low concentrations (156 µg/mL), phycocyanin did not cause tooth discoloration. Moreover, it did not reduce significantly the viability of human gingival fibroblasts in any concentration; these advantages point to the benefits of aPDT with phycocyanin in comparison with decontamination with CHX. Pourhajibagher et al24 evaluated the efficacy of aPDT with phycocyanin and diode laser against E. faecalis biofilm. They reported that aPDT with phycocyanin and diode laser caused an 89.45% reduction in the viability of E. faecalis and its biofilm, and it down-regulated the expression of fsrB gene, which is an important virulence factor of E. faecalis and leads to a significant increase in the production of intracellular reactive oxygen species.
Conversely, Minnock et al25 stated that the cell wall of gram-negative bacteria includes an internal cytoplasmic membrane and an external membrane that serves as a barrier and decreases the bond and penetration of photosensitizer. However, phycocyanin can bond to the external membrane of Gram-negative bacteria due to its positive charge and serve as a suitable photosensitizer for Gram-negative bacteria. Considering the favorable properties of phycocyanin and limited studies on its efficacy in aPDT procedures, phycocyanin was used in this study as a photosensitizer.23,24
Chan and Lai26 evaluated the efficacy of different light sources in aPDT for the elimination of P. gingivalis and found that the diode laser with a 665-nm wavelength caused a maximum significant reduction in the P. gingivalis count. They explained the reason to be the presence of protohaemin and protoporphyrin in P. gingivalis, which can highly absorb red light, increasing the antimicrobial efficacy of aPDT. Furthermore, Safaei et al27 reported that maximum light absorption by phycocyanin occurred in wavelengths between 620 nm and 650 nm. Minkova et al28 stated that peak light absorption by phycocyanin ranged between 620 nm and 642 nm; it had maximum light absorption at 620 nm and maximum fluorescence at 642 nm. According to the aforementioned studies and calculation of sub-lethal exposure time of laser, the 635-nm diode laser with 220-mW power was used for 4 min. in the current study.
In the present study, aPDT with phycocyanin and 635-nm diode laser resulted in a 44% reduction in the P. gingivalis count, which was the highest after CHX. CHX caused a 100% reduction in the bacterial count; however, its cytotoxic effects cannot be overlooked. Giannelli et al29 showed that although CHX was washed off the surface of titanium discs before the culture, it still damaged the adjacent macrophages. High cytotoxicity of CHX can be due to the fact that it increases oxidative stress, changes the balance of intracellular calcium ions, and impairs mitochondrial function, leading to eventual tissue damage. In a study by Giannelli et al,29 macrophages next to the discs subjected to aPDT showed a normal pattern of cytoplasm and nucleus and only experienced slight mitochondrial swelling.
Laser irradiation alone caused reductions in the count of P. gingivalis compared with the control group that can be related to the phenomenon that bacterial cells have endogenous porphyrins which act as photosensitizers.
The use of one type of laser with a specific power was a limitation of this study. Future studies are required to use different laser powers and assess the efficacy of aPDT with phycocyanin in other periopathogenic microorganisms.
Conclusion
The use of phycocyanin alone and aPDT with a 635-nm diode laser and phycocyanin can significantly decrease the P. gingivalis count in vitro. Considering the conservative nature of this modality, it may be used as an adjunctive treatment for the decontamination of implant surfaces and peri-implant areas.
Acknowledgements
We thank Tehran Medical Sciences, Islamic Azad University, Tehran, Iran.
Author Contributions
All of the authors contributed to the investigation, supervision, writing, review, and editing of the study. The study was conceptualized by AE and NCH. Data curation, data visualization, and formal analysis were carried out by MP and AA.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of Interests
The authors declare that they have no competing interests.
Ethics Considerations
Not applicable. This study was in vitro.
Funding
No funding was received for this research.
Please cite this article as follows: Etemadi A, Azizi A, Pourhajibagher M, Chiniforush N. In vitro efficacy of antimicrobial photodynamic therapy with phycocyanin and diode laser for the reduction of porphyromonas gingivalis. J Lasers Med Sci. 2022;13:e55. doi:10.34172/ jlms.2022.55.
References
- 1.Lindhe J, Meyle J. Peri-implant diseases: consensus report of the sixth European workshop on periodontology. J Clin Periodontol. 2008;35(8 Suppl):282–5. doi: 10.1111/j.1600-051X.2008.01283.x. [DOI] [PubMed] [Google Scholar]
- 2.Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35(8 Suppl):286–91. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 3.Becker W, Becker BE, Newman MG, Nyman S. Clinical and microbiologic findings that may contribute to dental implant failure. Int J Oral Maxillofac Implants. 1990;5(1):31–8. [PubMed] [Google Scholar]
- 4.Mombelli A, Marxer M, Gaberthüel T, Grunder U, Lang NP. The microbiota of osseointegrated implants in patients with a history of periodontal disease. J Clin Periodontol. 1995;22(2):124–30. doi: 10.1111/j.1600-051x.1995.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 5.Renvert S, Roos-Jansåker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol. 2008;35(8 Suppl):305–15. doi: 10.1111/j.1600-051X.2008.01276.x. [DOI] [PubMed] [Google Scholar]
- 6.Smeets R, Henningsen A, Jung O, Heiland M, Hammächer C, Stein JM. Definition, etiology, prevention and treatment of peri-implantitis--a review. Head Face Med. 2014;10:34. doi: 10.1186/1746-160x-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louropoulou A, Slot DE, Van der Weijden FA. Titanium surface alterations following the use of different mechanical instruments: a systematic review. Clin Oral Implants Res. 2012;23(6):643–58. doi: 10.1111/j.1600-0501.2011.02208.x. [DOI] [PubMed] [Google Scholar]
- 8.Sayar F, Chiniforush N, Bahador A, Etemadi A, Akhondi N, Azimi C. Efficacy of antimicrobial photodynamic therapy for elimination of Aggregatibacteractinomycetemcomitans biofilm on Laser-Lok titanium discs. Photodiagnosis Photodyn Ther. 2019;27:462–6. doi: 10.1016/j.pdpdt.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Takasaki AA, Aoki A, Mizutani K, Schwarz F, Sculean A, Wang CY, et al. Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontol 2000. 2009;51:109–40. doi: 10.1111/j.1600-0757.2009.00302.x. [DOI] [PubMed] [Google Scholar]
- 10.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections--state of the art. Photodiagnosis Photodyn Ther. 2009;6(3-4):170–88. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiniforush N, Pourhajibagher M, Parker S, Shahabi S, Bahador A. The in vitro effect of antimicrobial photodynamic therapy with indocyanine green on Enterococcus faecalis: influence of a washing vs non-washing procedure. Photodiagnosis Photodyn Ther. 2016;16:119–23. doi: 10.1016/j.pdpdt.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Schär D, Ramseier CA, Eick S, Arweiler NB, Sculean A, Salvi GE. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: six-month outcomes of a prospective randomized clinical trial. Clin Oral Implants Res. 2013;24(1):104–10. doi: 10.1111/j.1600-0501.2012.02494.x. [DOI] [PubMed] [Google Scholar]
- 13.Chiniforush N, Pourhajibagher M, Parker S, Benedicenti S, Bahador A, Sălăgean T, et al. The effect of antimicrobial photodynamic therapy using chlorophyllin–phycocyanin mixture on Enterococcus faecalis: the influence of different light sources. Appl Sci. 2020;10(12):4290. doi: 10.3390/app10124290. [DOI] [Google Scholar]
- 14.Etemadi A, Eftekhari Bayati S, Pourhajibagher M, Chiniforush N. In vitro effect of antimicrobial photodynamic therapy with phycocyanin on Aggregatibacteractinomycetemcomitans biofilm on SLA titanium discs. Photodiagnosis Photodyn Ther. 2020;32:102062. doi: 10.1016/j.pdpdt.2020.102062. [DOI] [PubMed] [Google Scholar]
- 15.Saffarpour A, Fekrazad R, Heibati MN, Bahador A, Saffarpour A, Rokn AR, et al. Bactericidal effect of erbium-doped yttrium aluminum garnet laser and photodynamic therapy on Aggregatibacteractinomycetemcomitans biofilm on implant surface. Int J Oral Maxillofac Implants. 2016;31(3):e71–8. doi: 10.11607/jomi.4224. [DOI] [PubMed] [Google Scholar]
- 16.Miles AA, Misra SS, Irwin JO. The estimation of the bactericidal power of the blood. J Hyg (Lond) 1938;38(6):732–49. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pourhajibagher M, Chiniforush N, Shahabi S, Ghorbanzadeh R, Bahador A. Sub-lethal doses of photodynamic therapy affect biofilm formation ability and metabolic activity of Enterococcus faecalis. Photodiagnosis Photodyn Ther. 2016;15:159–66. doi: 10.1016/j.pdpdt.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Moslemi N, Rouzmeh N, Shakerinia F, Bahador A, Soleimanzadeh Azar P, Kharazifard MJ, et al. Photodynamic inactivation of Porphyromonasgingivalis utilizing radachlorin and toluidine blue O as photosensitizers: an in vitro study. J Lasers Med Sci. 2018;9(2):107–12. doi: 10.15171/jlms.2018.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braham P, Herron C, Street C, Darveau R. Antimicrobial photodynamic therapy may promote periodontal healing through multiple mechanisms. J Periodontol. 2009;80(11):1790–8. doi: 10.1902/jop.2009.090214. [DOI] [PubMed] [Google Scholar]
- 20.de Oliveira RR, Schwartz-Filho HO, Novaes AB, Garlet GP, de Souza RF, Taba M, et al. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: cytokine profile in gingival crevicular fluid, preliminary results. J Periodontol. 2009;80(1):98–105. doi: 10.1902/jop.2009.070465. [DOI] [PubMed] [Google Scholar]
- 21.Hfaiedh M, Brahmi D, Zourgui L. Protective role of cactus cladodes extract on sodium dichromate-induced testicular injury and oxidative stress in rats. Biol Trace Elem Res. 2014;159(1-3):304–11. doi: 10.1007/s12011-014-9969-8. [DOI] [PubMed] [Google Scholar]
- 22.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118–26. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afrasiabi S, Pourhajibagher M, Chiniforush N, Aminian M, Bahador A. Anti-biofilm and anti-metabolic effects of antimicrobial photodynamic therapy using chlorophyllin-phycocyanin mixture against Streptococcus mutans in experimental biofilm caries model on enamel slabs. Photodiagnosis Photodyn Ther. 2020;29:101620. doi: 10.1016/j.pdpdt.2019.101620. [DOI] [PubMed] [Google Scholar]
- 24.Pourhajibagher M, Chiniforush N, Bahador A. Antimicrobial action of photoactivated C-phycocyanin against Enterococcus faecalis biofilms: attenuation of quorum-sensing system. Photodiagnosis Photodyn Ther. 2019;28:286–91. doi: 10.1016/j.pdpdt.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 2000;44(3):522–7. doi: 10.1128/aac.44.3.522-527.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan Y, Lai CH. Bactericidal effects of different laser wavelengths on periodontopathic germs in photodynamic therapy. Lasers Med Sci. 2003;18(1):51–5. doi: 10.1007/s10103-002-0243-5. [DOI] [PubMed] [Google Scholar]
- 27.Safaei M, Maleki H, Soleimanpour H, Norouzy A, Shahbani Zahiri H, Vali H, et al. Development of a novel method for the purification of C-phycocyanin pigment from a local cyanobacterial strain Limnothrix sp. NS01 and evaluation of its anticancer properties. Sci Rep. 2019;9(1):9474. doi: 10.1038/s41598-019-45905-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minkova KM, Tchernov AA, Tchorbadjieva MI, Fournadjieva ST, Antova RE, Busheva M. Purification of C-phycocyanin from Spirulina (Arthrospira) fusiformis. J Biotechnol. 2003;102(1):55–9. doi: 10.1016/s0168-1656(03)00004-x. [DOI] [PubMed] [Google Scholar]
- 29.Giannelli M, Pini A, Formigli L, Bani D. Comparative in vitro study among the effects of different laser and LED irradiation protocols and conventional chlorhexidine treatment for deactivation of bacterial lipopolysaccharide adherent to titanium surface. Photomed Laser Surg. 2011;29(8):573–80. doi: 10.1089/pho.2010.2958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


