Abstract
Introduction: In everyday life, electrical devices are the primary sources of extremely low-frequency electromagnetic fields (ELF-EMF), and the human body may be a great conductor of these fields. We chose alpha band power, especially at 10 Hz frequency, due to its prior beneficial role in memory. The purpose was to clarify whether there is a relationship between ELF-EMF exposure and cognitive deficits in rats, clinical signs, behavioral analysis, and the impact of ELF-EMF during different times of exposure on neuroplasticity via the expression of BDNF.
Methods: Forty adult male rats were selected randomly. The rats were exposed to ELF-EMF (10 Hz, 4 mT) for 7 days and 30 days, one hour daily. The expression of BDNF proteins in the hippocampus was evaluated after sacrificing animals to assess learning and memory function. The body weight of rats in the long-term exposed group differed significantly (P<0.05). The level of BDNF mRNA in the hippocampus was found by the RT-PCR method.
Results: Our findings indicate that exposure to ELF-EMF affects spatial learning and memory and can improve memory, especially with long-term exposure. In addition, we discovered a significant difference in the long-term exposed group (P<0.05), where radiation for 30 days resulted in a substantial rise in BDNF levels.
Conclusion: After prolonged exposure, male rats spent more time and traveled a greater percentage of their distance in the target quadrant, demonstrating that long-term exposure improves spatial memory and that 10 Hz might be safe.
Keywords: ELF-EMF, Spatial memory, Morris water maze test, Learning and memory
Introduction
Electrical gadgets and infrastructure, as well as wireless communication, are all defining characteristics of contemporary living.1 When electrical equipment is turned on or power lines transmit, an electromagnetic field (EMF) is generated.2-4 There are several debates about the safety of extremely low-frequency electromagnetic fields (ELF-EMF) on human health and cognitive function.2 A variety of studies have been conducted to determine how EMF affects people and animal models biologically, physiologically, and behaviorally.5 Due to the generation of EMF and the resultant electromagnetic radiation, modern technology has become a source of ubiquitous electromagnetic pollution.6 However, the current is considered harmless to the human body since it cannot break bonds or heat biological tissues.3 For example, it does not cause direct damage to DNA.7 Even though ELF-EMF is non-ionizing and has no thermal impact on cells and tissues, increasing scientific interest in this topic has resulted in the World Health Organization (WHO) Report (2007) and the WHO Environmental Health Criteria (EHC) Report. According to the WHO’s 10-year follow-up research, ELF-EMF had no detrimental effect on the human body.8 There is, however, substantial controversy about the safety of ELF-EMF due to varied exposure circumstances, such as length and intensity of exposure.3 Inconsistent and incomparable results of ELF-EMF biological effect tests are the most obvious problems caused by various experimental parameters (frequency, flow density, and duration of ELF-EMF exposure) and individuals (cell lines, species, strains, sex, and age),9 and they also have a variable impact on the behavior of experimental animals.2 However, a few consider research that suggests ELF-EMF may influence nerve cells.3 ELF-EMF has a significant impact on fundamental brain processes and synaptic plasticity.10 It would be suggested that the impact may heavily depend on exposure factors, including frequency and intensity.11 Others showed that a 28-day high-dose ELF-EMF (50 Hz, 8 mT) might impair the memory of hippocampal rats by elevating neurotransmitters such as glutamate and gamma-aminobutyric acid.12
The ELF-EMF is available from several sources, such as power lines, transmission systems, and household appliances, in a frequency range of 1 to 300 Hz.10,13 In this context, the effects of EMF on critical biological mechanisms, including cell proliferation, ion exchanges across the biodiversity membranes, bone repair, nerve repair, free radical production, hormonal changes, modulation of enzyme activity, and changes in membrane and intracellular proteins, were investigated.5 Hence, ELFs have been shown in extensive studies on human and animal models to influence the activity of brain neurons and therefore interfere with brain waves.5 Preclinical studies, especially in rodent models, have played and will continue to play an essential role in clarifying the genetic and environmental parameters that affect normal and pathological activities related to neurodevelopmental failures.14 The primary oscillations in the human brain are alpha-band oscillations, which have a mean frequency of around 10 Hz in the ELF range.15 The frequency of alpha waves can range between 8 and 13 Hz.16 Event-related desynchronization (ERD) is defined as a power decline in the signal power in the EEG.17 Several studies have found an association between ERD of alpha oscillations in this range and memory performance.18,19 As part of molecular studies, short-term synaptic capacities and activity-dependent synaptic plasticity include long-term potentiation control by the brain-derived neurotrophic factor (BDNF)20 that plays an essential part in memory and learning.21 However, neuroplasticity and synaptic transmission are maintained by BDNF in the mature brain. It entails enhancing long-term potentiation as well as encouraging and regulating neuronal development. Tropomyosin receptor kinase B (TrkB) and its shortened isoform, p75 NTR, have an extremely high affinity for BDNF. The neurotrophin activates TrkB, initiating a cascade of signaling pathways that culminate in neurogenesis, neuroplasticity, cell survival, and stress tolerance.22 In this study, we hypothesized that exposure to pulsed ELF-EMF at 10 Hz would increase the BDNF mRNA expression. On the other hand, we selected the alpha band, particularly at 10 Hz, due to its involvement in focus modulation, sensory inhibition and working memory.23
Hence, the endogenous expression of BDNF can be associated with learning and increases in function.24 The up-regulation of BDNF, which is involved in controlling high-frequency synaptic transmission and long-term potentiation in the hippocampus, may help alleviate cognitive impairments and learning problems in Alzheimer’s disease (AD).25 The hippocampus is an important part of the brain when it comes to learning and memory, especially spatial memory and flexible recollection of earlier experiences.26 Some benefits of ELF-EMF are illustrated in Figure 1.
Figure 1.
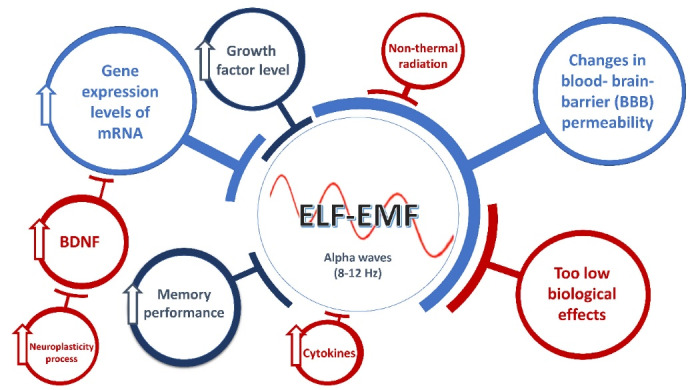
Some alterations exposed by ELF-EMF (adapted from papers by27-31)
The Morris water maze (MWM) is a frequently used paradigm in behavioral cognitive function to investigate the cognitive functions and neural mechanisms underlying spatial learning and memory,32,33 and it is broadly utilized for the investigation of hippocampal-dependent memory.33 The MWM is composed of multiple components; the hidden platform test and probing trial are the most specific tests of spatial learning and memory respectively.32 Rats are needed to identify a submerged platform in an opaque circular pool in this behavioral experiment. The benefit of this test is that it is rapid without pre-learning or confinement of nourishment and water.14 The MWM plays a critical part in the approval of rat models for neurocognitive illnesses such as AD.34 AD is characterized by persistent and progressive neurodegeneration results in progressive cognitive impairment and patient mortality.35 García et al conducted a review of epidemiological research, suggesting a relationship between ELF-EMF and AD occupational exposures, but certain restrictions affect the accuracy of the study.36 Spatial memory is considered a type of short-term memory. Hence, MWM is suggested as an optimal model for studying rodent spatial memory, leading to forming learning and memory. On the other hand, some modern techniques use zebrafish to detect cognitive function instead of rodents.37 The purpose of the current study was to assess the effects of an extremely low-frequency magnetic field on learning and memory, utilizing a Morris water maze. Also, we tried to examine the effect of short-term and long-term ELFs on BDNF levels in the hippocampus of male rats.
Materials and Methods
Animals and Groups
A total of 40 Wistar rats, aged 5–6 weeks with an average weight of 180 –250 g, were randomly assigned to the ELF-EMF exposure groups or the sham exposure group at the Experimental Animal Center of Iran University of Medical Sciences. We performed animal experiments following the UK Animals (Scientific Procedures) Act 1986 and its associated guidelines and the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). For the experiment, four groups were considered for the MWM using the ELF-EMF exposure: the long-term sham group, the long-term exposure group, the short-term sham group, and the short-term exposure group, with ten rats for each group. Both sham groups were affected by the waves in similar conditions. The only difference was that they did not affect the waves. All the equipment was switched on for this reason, and the rats could hear the fan and machine activities. The animals were housed in a 12:12 hour dark/light cycle at room temperature (24°C ± 2°C) and provided with sufficient food and water. Every attempt has been made to reduce the pain.
ELF Exposure Procedure
The computer software attached to the power amplifier that converted the audio to the electric current on the antenna to produce the ELF-EMF generated an audio signal. The microwave output of a horn antenna with a power range of 1-200 W was used. The electromagnetic radiation device includes a power amplifier (ZHL-16W-43 + , USA) to amplify the signal generated and a signal generator (the Agilent HP 83732B, USA), which can generate signals in the frequency bandwidth range of 10 MHz to 20 GHz. A horn antenna (model LB-OH-320-10-C-NF) with dimensions of 257 × 124 × 164 mm, which can operate at a frequency of 10 Hz, a power meter, a radiation box (20 × 30 cm), and a power density measuring device were used. Electromagnetic waves (pulsed modulation) with a frequency of 10 Hz, a density of 4 mW/cm3, and a pulse width of 5 ms were irradiated from above on the surface of the radiation cage. The spacing between the horn antenna and the tested animals was 25 cm. As a result, the parameters of the ELF-EMF exposure in our investigation were 10 Hz and 4 mT, and it was set for 7 and 30 days as short-term and long-term exposures respectively. The sham (control) group was subjected to the same circumstances as the experimental group but without applying ELF-EMF.
Morris Water Maze
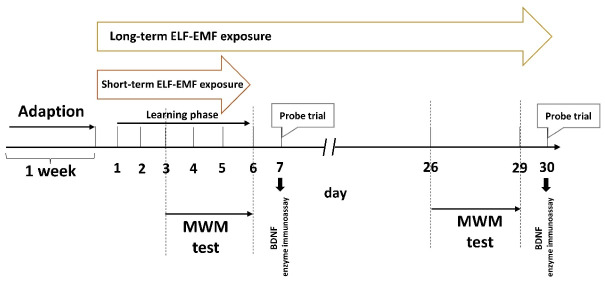
According to the selected procedure (four days of training started on day 3 in the water tank followed by the seventh day of the experiment as the last day), the MWM started on the third day for the short-term sham group. The maze comprises a circular pool (width of 140 cm, height of 50 cm) filled to a depth of 30 cm with warm water (22–25°C) and set in a gloomy room with minimal lighting. The pool was filled with water, and the wall of the pool was covered with dark glass. For the long-term group, the tests started on days 26 and 30, and a probe trial was conducted. The tank was separated into four quadrants, and an invisible platform (diameter 10 cm) was placed in the center of one of the quadrants of the tank that extended 1 cm below the water surface. The rats learned to identify the fixed platform using spatial indicators. Each rat was free to swim for 90 seconds during training days and 60 seconds during the probe trial until it came across and climbed onto the platform, where it remained for fifteen seconds to recall the cue signs on the walls. Data on latency (the time the rat was placed on the platform when the platform was identified) and swimming distance were extracted from the video using a behavioral analysis system (Panlab, Barcelona, Spain). A recall or probe trial was conducted after the last training session on days 7 and 30 to measure the time spent in the target quadrant where the platform had been located during training. The number of times the animal crossed the platform area and the amount of time spent in the target quadrant were recorded and evaluated in order to determine the animal’s spatial memory. The rats were taken out of the water, patted dry with a terrycloth towel, and then placed in a warming cage (containing a heating pad set to a low setting beneath a standard shoebox cage) for at least 5 minutes before returning to their home cage. Twenty-four hours following the previous training session, a probe trial was carried out to assess spatial reference memory by removing the platform. Then the rats were permitted to swim without restriction in the pool for 60 seconds.
During the retention test (60 seconds), the platform was removed, and the animal was permitted to look for it. In case rats might not discover the platform inside 60 seconds (for training days only 90 seconds), they were guided to the platform by hand and permitted to stay there for 15 seconds to learn cue signs on the walls to remember the situation of the platform, and then their escape latency was acknowledged as 60 seconds. In the retention tests, we assessed four different measures of platform memory: time spent (%) in the platform quadrant, mean distance (cm) to the previous position of the platform, latency (s) to the primary crossing over the prior platform situation, and the number of crossings over the former platform position. As a result, escape latency was utilized to examine the learning and memory abilities of the rats.
BDNF Enzyme Immunoassay
BDNF mRNA levels in brain tissues were measured using RT-PCR. The levels of BDNF were determined using an enzyme immunoassay (EIA) as previously described by Nawa et al.38 The internal control was β-actin mRNA, which was co-amplified with BDNF mRNA. The following primers were used as mentioned in Table 1.
Table 1. The Primary Sequences of the Selected Genes .
| Gene | Sequence |
| β-actin | Forward: 5′-TCCTCCTGAGCGCAAGTAC-3′ |
| Reverse: 5′-CCTGCTTGCTGATCCACATCT-3′ | |
| BDNF | Forward: 5′-CGTGATCGAGGAGCTGTTGG-3′ |
| Reverse: 5′-CTGCTTCAGTTGGCCTTTCG-3′ |
In an early experiment, the number of PCR cycles and denaturation temperature were tested to identify a linear working range for all PCR products. According to the protocol by Mizuno et al,20 the experimental amplification protocol is comprised of the first round at 94°C for 5 minutes, followed by 27 cycles of denaturation for 1 minute at 94°C, annealing for 1 minute at 58°C, and extension for 2 minutes at 72°C (PCR Thermal Cycler; Takara, Shiga, Japan).
Statistical Analysis
All results are presented as the mean ± standard deviation (SD). All statistical analysis was performed using GraphPad Prism® 8.1 software for Windows (GraphPad Software, San Diego, CA, USA). The data were analyzed using a two-way repeated measures ANOVA with sham groups and exposed groups as two between-subject factors, followed by Sidak’s test to compare the groups and time exposure (short-term and long-term). A one-way ANOVA was performed using Dunnett’s test to compare groups in the learning phase with the first day of the MWM test. To compare groups and time, as well as their interactions, a two-way ANOVA was used, followed by the Bonferroni post hoc test. Also, a student’s t test was used when necessary. We used a one-way ANOVA using Dunnett’s test to compare exposed groups to their sham groups in the training days in the MWM test. The swim paths were assessed utilizing a computerized video-tracking system (EthoVision 3.1; Noldus Information Technology, Wageningen, the Netherlands). It was considered statistically significant only when the P value was less than 0.05, 0.01, 0.001, and 0.0001. The test rejects the hypothesis of normality when the P value is less than or equal to 0.05.
Results
The MWM test was created to evaluate spatial learning and memory. The MWM task required the animal to locate a concealed platform to escape from floating in a pool of water. The animal used visual input from extra-maze cues in the testing room to create a “spatial orientation map” in the brain to perform this task. Figure 2 depicts the experimental design and schedule for the MWM test.
Figure 2.
Experimental Design and Schedule for the Morris Water Maze Test
Evaluation of Clinical Signs
In all the one-week and four-week studies, all groups showed no signs of death.
Weight, Water and Food Intake Profiles
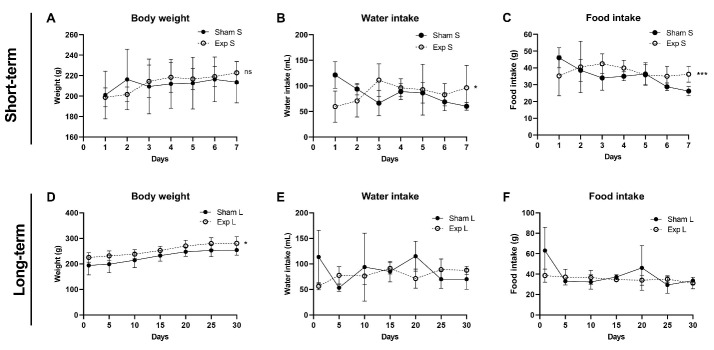
Figure 3 depicts several patterns of body weight, water, and food intake. The body weight in exposed groups, including short-term and long-term groups, was comparable with that of their sham groups at the end of the study. Only the long-term group’s body weight rate increased significantly from week 0 to week 4 (P= 0.0364), as shown in Figures 3A and D. In contrast, the short-term exposure group showed no significant difference. In Figure 3D, both the long-term exposed group and its sham group rose slightly from day 0 to day 20 and reached the plateau. For analysis in Figures 3B and E, animals in the sham groups consumed high water intakes on the first day, and then all four groups showed a fluctuating trend. Only the short-term exposed group exhibited a significant difference at the end of the study (P < 0.05). Similarly, there was a significant difference compared with the short-term sham group in terms of food consumption (P < 0.001, Figure 3C). We noticed that the long-term exposed group exhibited a straight line (horizontal) from day 0 to day 30 (Figure 3F). The food intake did not differ between the two groups (P > 0.05).
Figure 3.
Effects of ELF-EMF on Body Weight, Food Intake and Water Intake. (A, D) body weight, (B, E) water intake, and (C, F) water intake. Sham S: short-term sham group, Exp S: short-term exposed group, Sham L: long-term sham group, Exp L: long-term exposed group; all data are presented as mean ± SD (n = 10 per group); the statistical difference in data obtained from the tested groups was performed using a t test. ns = no significant, *P < 0.05, ***P < 0.001, compared with its sham group at the end of the study.
Behavioral analysis
The behavioral study of the sham short-term and long-term groups showed the same passive mode (25% and 20% respectively), which was lower than the exposed groups. In this study, however, the sham groups followed the same trend. However, we noticed increased activity in the rats in both exposed groups (Figure 4).
Figure 4.

Effects of ELF-EMF on Temperament in Rats
Effects of ELF-EMF on Rats in MWM Test
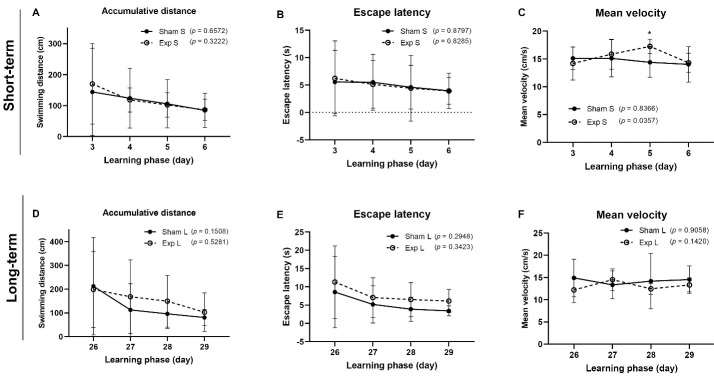
Following training, the amount of time that passes until the animal steps onto the platform to leave the water (escape latency) and the proportion of time or path length spent in the quadrant, including the platform, are used to determine whether to learn (the target quadrant). The daily data comparison graphs revealed that each group had successfully completed the four-day training. The height of the chart would be reduced if the training was done correctly, as was plainly shown in each group. Figures 4A and 4B show that the accumulative distance traveled and the escape latency decreased in all groups from the third to the sixth day of training. Our research showed that long-term exposure to ELF-EMF was more beneficial than short-term exposure. However, no significant difference was found (P > 0.05). For the short-term groups, the mean swimming velocity fluctuated during the training days (Figure 5C), except on the 5th day, when it showed a significant difference compared to the 3rd day (P < 0.05). Rests remained constant during training. The decline in latency in Figures 4B and 4E, in the learning phase in the short-term and long-term groups, was similar (P > 0.05) based on a one-way ANOVA using Dunnett’s test compared to day 3. In MWM, all groups showed decreased mean latency, particularly in the long-term exposed group. These results showed that the long-term exposed group discovered the hidden platform faster than the long-term sham group. The mean escape latency was 3.4 s versus 3.9 s respectively (Figure 5E): at the end of the learning phase, their latency period stopped, indicating that it had reached its minimum. Although no significant improvements in learning were seen throughout the four days, the escape latency plot indicates that the rats could still recall the hidden platform by decreasing the time between days 3 and 6. However, during training sessions, ELF-EMF did not affect rats’ spatial memory in the short-term or long-term groups (Figure 5).
Figure 5.
Effects of ELF Field Exposure on Spatial Learning and Memory During the Training Days in the Morris Water Maze in Different Groups. (A, D) accumulated distance during the learning phase (days) in short-term and long-term groups respectively. In long-term exposure (D), day 5 had a shorter swimming distance prior to escaping onto the hidden platform; (B, E) escape latency during the training phase in short-term and long-term groups respectively. The escape latency was calculated from the data collected from day 2 through day 5 of the learning phase. Both groups showed similar latency to escape onto the platform (P > 0.05) using one-way ANOVA and Dunnett’s test compared with the 3rd day; (C, F) mean velocity in training days in short-term and long-term groups respectively. The decreasing trend in latency (P > 0.05) could not be explained by changes in swim speed; Sham S: short-term sham group, Exp S: short-term exposed group, Sham L: long-term sham group, Exp L: long-term exposed group; all data are presented as mean ± SD (n = 10 per group), (*P < 0.05).
The results of the probe trial are illustrated in Figure 6. In Figure 6A, the time required to cross the platform area was determined. The results showed that the rats in the long-term exposed group remembered and swam across the platform area seventeen times on average, which was comparable to the sham ELF-EMF exposed rats. However, no significant difference was found between the long-term exposure and the long-term sham groups (P > 0.05). A two-way ANOVA revealed that the duration of exposure (time exposure) showed a significant difference (F(1, 28) = 6.95; P = 0.0135). Also, there was a significant difference between the groups (sham and exposed groups) (F(1, 28) = 9.759; P = 0.0041). Hence, the interaction between time exposure and groups was considered extremely significant (F(1, 28) = 26.77; P < 0.0001; Figure 6A). Moreover, the accumulative distance traveled in the probe trial revealed that the short-term and long-term ELF-EMF exposure groups were not significantly different from their sham groups (P > 0.05). Based on a one-way ANOVA using Tukey’s post hoc test, the short-term exposed group was significantly different (P < 0.05) from the long-term sham group. A two-way ANOVA revealed a significant difference between the sham and exposed groups (F(1, 14) = 6.963; P = 0.0194); however, different time exposures and interactions between groups and time exposure showed no significant difference (P > 0.05, Figure 6B). Additionally, the impacts of ELF-EMF on spatial memory were assessed using the percentage of residence time spent in the target quadrant, which indicated a significant difference (P < 0.05) between the long-term group and its sham group. Also, there was a significant difference between the two groups, the short-term exposed group and the long-term exposed group (P < 0.05). We performed a two-way ANOVA using Bonferroni’s test for velocity measurement. However, although there was a significant difference in the interaction between groups and time exposure (F (1, 14) = 16.69; P = 0.0011), the ELF-EMF alone at various times and groups did not show a significant difference (P> 0.05, Figure 6C).
Figure 6.
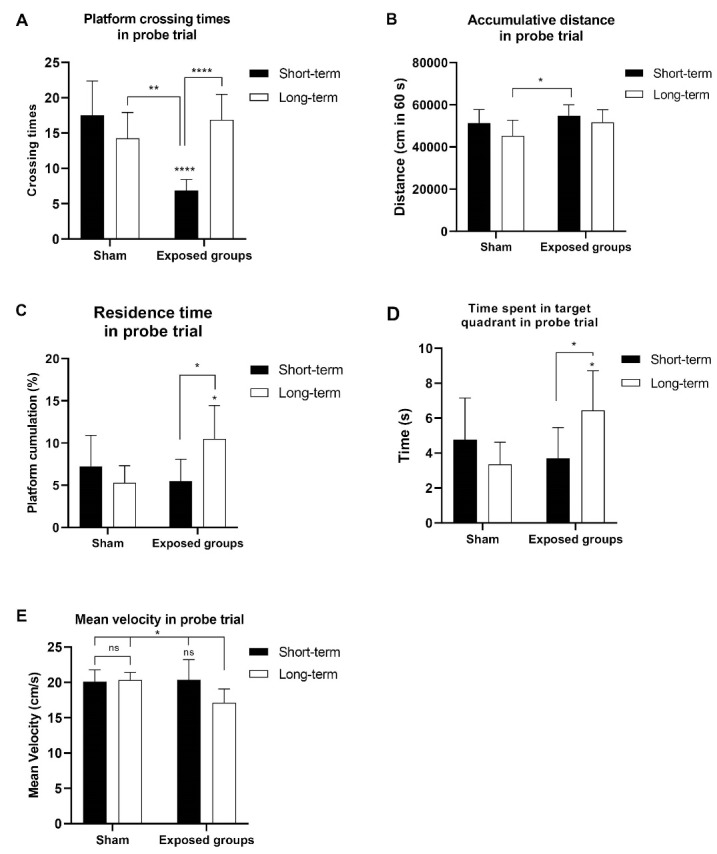
On training day 6, a probe trial was performed to test spatial memory in relation to the following: (A) the number of crossing the platform area in the probe trial; (B) the accumulative distance travelled in the probe trial; (C) the percentage ofresidence time in the probe trial; (D) time spent in the target quadrant in the probe trial; (E) mean velocity in the probe trial. On day 6, the probe trial was conducted without the platform, and the total distance travelled, time spent traversing the platform area and time spent in the target quadrant, percentage of platform cumulation, and mean velocity were all recorded, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The long-term exposed groups in terms of time spent (s) in the target quadrant showed a significant difference compared with their sham group (P= 0.0197). Only the long-term exposed group differed significantly from the sham-exposed group at P < 0.05. According to the two-way ANOVA, there was a significant difference in the interaction between groups and time exposure (F(1, 14) = 16.09; P= 0.0013), and no significant difference in groups and time exposure was found (P > 0.05, Figure 6D). The mean swimming velocity in the probe trial changed significantly in the long-term exposed group compared with its sham group and exposed short-term group (P < 0.05), suggesting overt problems with swimming ability may have happened in this group (Figure 6E). Also, we found a significant difference in interaction (groups vs. time exposure) (F(1, 14) = 6.405; P = 0.0240) and time exposure alone (F(1, 14) = 4.717; P= 0.0475). Heat maps showing the swim paths (during the 60s swims) of both short-term and long-term groups exposed to ELF-EMF are depicted in Figure 7A. During the probe trial day, the rats from the four groups showed representative swimming traces (Figure 7B). Taken together, these findings indicate that exposure to ELF-EMF has a positive effect on spatial learning and memory, especially after long-term radiation.
Figure 7.
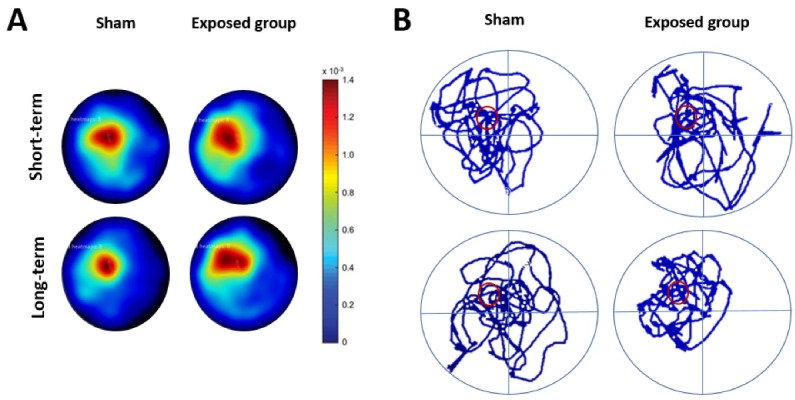
Effect of ELF-EMF on Locomotor Activity in Rats During the Morris Water Maze Test. (A) Swim paths density plots (heat maps) during the probe trial for ELF-EMF exposure during short-term and long-term studies. The heat maps are based on the tracking data from EthoVision where the rat’s position is given by an x, y coordinate for each time point. The heat maps were generated as described in the “Materials and Methods” section. Red color indicates that more time was spent in that area while the blue color indicates less time; (B) Representative swimming traces of the four groups of rats were observed on the probe trial day.
Effects of ELF-EMF in Alterations in BDNF mRNA Expression
BDNF mRNA in the entire hippocampus was measured by RT-PCR (data not shown). However, Figure 8 represents the alterations in BDNF mRNA expression in the hippocampus of the rats linked to spatial memory formation.
Figure 8.

Alterations in BDNF mRNA Expression in the hippocampus of rats are linked to the development of spatial memory. The amount of BDNF mRNA was compared to the level of β-action mRNA. Each value represents the mean ± SD. ns = not significant, *P < 0.05, ****P < 0.0001.
A one-way ANOVA using Tukey’s test revealed no significant difference in BDNF mRNA in the short-term exposed group compared to its sham group (P > 0.05). However, we found a significant difference in the long-term exposed group (P< 0.05) in which radiation for 30 days showed an increase in BDNF level significantly. There was a significant difference in BDNF mRNA levels between the long-term exposed group and the short-term exposed group, and also between the long-term exposed group and the short-term sham group, at a significant P < 0.0001, respectively. A two-way ANOVA with Bonferroni’s test was performed on the results of exposed groups and sham groups versus time and showed a significant effect of various groups (F(1,14) = 5.971; P = 0.0284), exposure (F(1,14) = 71.43; P < 0.0001), and groups by exposure time interaction (F(1,14) = 6.179; P = 0.0262). Based on our results, short-term exposure to 10 Hz did not change the BDNF level in the hippocampus at this time.
Discussion
Morris initially described the MWM in 1984,39 and it quickly became one of the most commonly used laboratory techniques in cognitive neuroscience.3 In order to reach a hidden escape platform,7 rats were required to navigate the perimeter of an open swimming arena utilizing cues. This test was used to evaluate the impact of this task on the rats’ spatial learning and memory.32,40 In general, our study showed that the male rats during long-term exposure had an increased number of targets in each quadrant and the percentage of their distance traveled in the target quadrant, which indicates a long-term improvement in spatial memory latency, one of the first dependent measures of performance on learning trials in the MWM. However, cumulative distance has been recommended as a superior measure of spatial learning capacity.7 The selected EMF microwave radiation for the long-term study has helped form better spatial memory in the hippocampus. However, the short-term exposure group did not improve the residence time or distance traveled in the target quadrant. Hence, the number of crossing platforms was reduced to enter the target quadrant. Gao et al showed that ELF-EMF significantly enhanced the learning capacity and memory of cerebral ischemia rats. In hippocampus rats, the average latency and distance traveled to the concealed platform were considerably reduced by ELF-EMF (50 Hz, 1 mT), and the entry to the target quadrant was enhanced.13 However, Lai et al mentioned that exposure to ELF-EMF did not impact brain morphology and histology during 24 weeks.3 In the current study, we discovered a reduction in escape latency in the exposed long-term group; however, the mean swimming speed of the groups did not change significantly. This result is similar to that reported by He et al32 as we concluded that this difference was not attributed to sensory-motor development. During long-term ELF-EMF exposure, the probe trial revealed longer duration spent in the target quadrant and a greater frequency of annulus crossings. These results show that exposure to ELF-EMF considerably improves the long-term retention of spatial memory while having no effect on short-term memory.
In vivo research on the effects of ELF-EMF on AD is currently lacking, and only a few scientists have conducted relevant experiments on neuroblastoma cell lines, demonstrating that such AD-related cells are not exposed to ELF-EMF.7 Liu et al discovered that prolonged exposure to ELF-EMF (50-Hz magnetic field of 2 mT) decreased the latency required to locate the hidden platform and enhanced long-term memory for the previous position of the platform without impairing short-term memory or motor activity. For the first time, their data shows that prolonged exposure to ELF-EMF is beneficial for developing and preserving spatial memory.41 Another study discovered that exposure to ELF-EMFs slowed weight gain in rats and reduced cognitive and clinicopathologic symptoms in animals with AD.9 Furthermore, Szemerszky et al demonstrated that exposure to 500 mT ELF-EMF for 4–6 weeks had no impact on anxiety-like behavior. They found that the hormonal stress response was comparable in control and short-term exposed rats, whereas long-term ELF-EMF exposure resulted in a substantial increase in proopiomelanocortin and depressive-like behavior.42 Rezaei-Tavirani et al previously discovered that increased ELF-EMF severity resulted in behavioral changes such as movement deformities and significantly reduced memory. Further, adjustment timing at the molecular scale was associated with the down-regulation of vital hippocampus protein expression, the majority of which is involved in cytoskeletal mechanisms in brain injury.43 Both hippocampal atrophy and low BNDF levels were associated with memory loss.44 Cirulli et al. discovered that intrahippocampal infusions of BDNF improved spatial memory performance and had long-term impacts on emotional behavior.45 Pelleymounter et al discovered that rats lost weight following central BDNF infusions.46 We observed decreased food consumption in the long-term exposed group, which was confirmed by our findings of increased BDNF gene expression. On the basis of our data and those in Pelleymounter and collegues’ study, BDNF could reduce appetite and induce weight loss. In another study by Li et al, their findings imply that BDNF/TrkB signal transduction pathways may play a role in improving learning and memory after multiple chronic stressors47; the present study confirmed their results. Therefore, reduced levels of BDNF could lead to neurite atrophy and synaptic loss seen in AD patients’ brains, while the up-regulation of BDNF could slow the progression of AD and cognitive impairment.25 Our findings show that the development of spatial memory is linked to an increase in BDNF mRNA in the hippocampus, a brain region implicated in spatial learning and memory in the Morris Water Maze test. This result is similar to that of Olton et al48 working on the radial arm maze test.
The average swimming velocity was between 10 and 20 cm per second, which was similar to other studies in mice.49,50 For example, we found similar results by Woods et al49 for the mean swimming velocity in the range of 10–20 cm/s. They gave no explanation in detail of the meaning of the swimming velocity and latency results. Our results confirmed Yuan’s findings regarding that the training video revealed that the movement speed was lower than the typical speed din the training film and the animals spent more time floating on occasion, but the direction of the movement virtually matched the ideal path.33 These findings suggest that the effects of the magnetic field should be linked to the timing of the exposure. From the findings of this study, it can be concluded that non-ionizing magnetic fields in the range of ELF-EMF can increase the development of spatial memory formation in long-term exposure and help improve memory. Therefore, more research is required to fully understand the impact of ELF-EMF on human and animal behavior in the future.
Conclusion
In conclusion, our research found that the male rats spent more time in the target quadrant and traveled a more significant proportion of their distance in the target quadrant after long-term exposure, suggesting a long-term exposure gain in spatial memory, which was previously stated by other researchers that exposure to an ELF-EMF range of 8-12 Hz could enhance memory due to its nonthermal effects on the permeability of the membrane and channels. These effects could interfere with the interaction between neurons and cause neuroplasticity. However, this frequency did not increase the time or distance traveled in the target quadrant in the short-term exposure group, although it did decrease the number of platforms crossed to reach the target quadrant. On the basis of our molecular research and findings, we conclude that long-term exposure to ELF-EMF at 10 Hz may induce neuroplasticity and the process of memory function.
Author Contributions
SMSdesigned and supervised the study; FZ collected data; FZ and AMC analyzed the data; AMC wrote and edited the manuscript.
Conflict of Interest
The authors report no conflicts of interest.
Ethical Considerations
All tests were carried out following the guidelines established by the Medical Ethics Committee of Malek Ashtar University.
Please cite this article as follows: Zarrin F, Mahdavi SM, Modarresi Chahardehi A, Razzaghi Z, Ahmadi N. The Effect of Time-Dependence of 10 Hz Electromagnetic Field on Spatial Learning and Memory in Rats. J Lasers Med Sci. 2022;13:e64. doi:10.34172/jlms.2022.64.
References
- 1.PaK Aeen M, Mahdavi SM, Maghami P, Modarresi Chahardehi A. The effect of non-ionizing electromagnetic fields in the range of 2.4 GHz on memory, thermal sensitivity and serum protein in male rats. Act Nerv Super Rediviva. 2022;64(2-3):77–85. [Google Scholar]
- 2.Karimi SA, Salehi I, Shykhi T, Zare S, Komaki A. Effects of exposure to extremely low-frequency electromagnetic fields on spatial and passive avoidance learning and memory, anxiety-like behavior and oxidative stress in male rats. Behav Brain Res. 2019;359:630–8. doi: 10.1016/j.bbr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Lai J, Zhang Y, Liu X, Zhang J, Ruan G, Chaugai S, et al. Effects of extremely low frequency electromagnetic fields (100μT) on behaviors in rats. Neurotoxicology. 2016;52:104–13. doi: 10.1016/j.neuro.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbar Shemirani S, Nasiri Khalili MA, Mahdavi SM, Modarresi Chahardehi A. The effect of mirtazapine on reducing chronic stress in male rats. Int Clin Neurosci J. 2022;9:e21. doi: 10.34172/icnj.2022.21. [DOI] [Google Scholar]
- 5.Kazemi M, Sahraei H, Aliyari H, Tekieh E, Saberi M, Tavacoli H, et al. Effects of the extremely low frequency electromagnetic fields on NMDA-receptor gene expression and visual working memory in male rhesus macaques. Basic Clin Neurosci. 2018;9(3):167–76. doi: 10.29252/nirp.bcn.9.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redlarski G, Lewczuk B, Żak A, Koncicki A, Krawczuk M, Piechocki J, et al. The influence of electromagnetic pollution on living organisms: historical trends and forecasting changes. Biomed Res Int. 2015;2015:234098. doi: 10.1155/2015/234098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Liu X, Zhang J, Li N. Short-term effects of extremely low frequency electromagnetic fields exposure on Alzheimer’s disease in rats. Int J Radiat Biol. 2015;91(1):28–34. doi: 10.3109/09553002.2014.954058. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization (WHO). Extremely Low Frequency Fields. WHO; 2007.
- 9.Liu X, Zuo H, Wang D, Peng R, Song T, Wang S, et al. Improvement of spatial memory disorder and hippocampal damage by exposure to electromagnetic fields in an Alzheimer’s disease rat model. PLoS One. 2015;10(5):e0126963. doi: 10.1371/journal.pone.0126963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khaki AA, Khaki A, Ahmadi SS. The effect of Non-ionizing electromagnetic field with a frequency of 50 Hz in rat ovary: a transmission electron microscopy study. Int J Reprod Biomed. 2016;14(2):125–32. [PMC free article] [PubMed] [Google Scholar]
- 11.Mahdavi SM, Sahraei H, Yaghmaei P, Tavakoli H. Effects of electromagnetic radiation exposure on stress-related behaviors and stress hormones in male Wistar rats. Biomol Ther (Seoul) 2014;22(6):570–6. doi: 10.4062/biomolther.2014.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan Y, Wang Z, Zhang H, He Y, Fan R, Cheng Y, et al. Extremely low frequency electromagnetic field exposure causes cognitive impairment associated with alteration of the glutamate level, MAPK pathway activation and decreased CREB phosphorylation in mice hippocampus: reversal by procyanidins extracted from the lotus seedpod. Food Funct. 2014;5(9):2289–97. doi: 10.1039/c4fo00250d. [DOI] [PubMed] [Google Scholar]
- 13.Gao Q, Leung A, Yang YH, Lau BW, Wang Q, Liao LY, et al. Extremely low frequency electromagnetic fields promote cognitive function and hippocampal neurogenesis of rats with cerebral ischemia. Neural Regen Res. 2021;16(7):1252–7. doi: 10.4103/1673-5374.301020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnhart CD, Yang D, Lein PJ. Using the Morris water maze to assess spatial learning and memory in weanling mice. PLoS One. 2015;10(4):e0124521. doi: 10.1371/journal.pone.0124521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012;16(12):606–17. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moini J, Piran P. Cerebral cortex. In: Moini J, Piran P, eds. Functional and Clinical Neuroanatomy. Academic Press; 2020. p. 177-240. 10.1016/b978-0-12-817424-1.00006-9. [DOI]
- 17.Wianda E, Ross B. The roles of alpha oscillation in working memory retention. Brain Behav. 2019;9(4):e01263. doi: 10.1002/brb3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnefond M, Jensen O. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr Biol. 2012;22(20):1969–74. doi: 10.1016/j.cub.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Hanslmayr S, Spitzer B, Bäuml KH. Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cereb Cortex. 2009;19(7):1631–40. doi: 10.1093/cercor/bhn197. [DOI] [PubMed] [Google Scholar]
- 20.Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20(18):7116–21. doi: 10.1523/jneurosci.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etemad A, Sheikhzadeh F, Ahmadi Asl N. Evaluation of brain-derived neurotrophic factor in diabetic rats. Neurol Res. 2015;37(3):217–22. doi: 10.1179/1743132814y.0000000428. [DOI] [PubMed] [Google Scholar]
- 22.Klimek A, Rogalska J. Extremely low-frequency magnetic field as a stress factor-really detrimental?-Insight into literature from the last decade. Brain Sci. 2021;11(2):174. doi: 10.3390/brainsci11020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rana KD, Vaina LM. Functional roles of 10 Hz alpha-band power modulating engagement and disengagement of cortical networks in a complex visual motion task. PLoS One. 2014;9(10):e107715. doi: 10.1371/journal.pone.0107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellweg R, Lohmann P, Huber R, Kühl A, Riepe MW. Spatial navigation in complex and radial mazes in APP23 animals and neurotrophin signaling as a biological marker of early impairment. Learn Mem. 2006;13(1):63–71. doi: 10.1101/lm.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Fang Y, Lian Y, Chen Y, Wu T, Zheng Y, et al. Brain-derived neurotrophic factor ameliorates learning deficits in a rat model of Alzheimer’s disease induced by aβ1-42. PLoS One. 2015;10(4):e0122415. doi: 10.1371/journal.pone.0122415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reshetnikov VV, Kisaretova PE, Ershov NI, Shulyupova AS, Oshchepkov DY, Klimova NV, et al. Genes associated with cognitive performance in the Morris water maze: an RNA-seq study. Sci Rep. 2020;10(1):22078. doi: 10.1038/s41598-020-78997-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klimesch W, Doppelmayr M, Hanslmayr S. Upper alpha ERD and absolute power: their meaning for memory performance. Prog Brain Res. 2006;159:151–65. doi: 10.1016/s0079-6123(06)59010-7. [DOI] [PubMed] [Google Scholar]
- 28. Markov MS. Electromagnetic Fields in Biology and Medicine. CRC Press; 2015.
- 29.Cichoń N, Bijak M, Czarny P, Miller E, Synowiec E, Sliwinski T, et al. Increase in blood levels of growth factors involved in the neuroplasticity process by using an extremely low frequency electromagnetic field in post-stroke patients. Front Aging Neurosci. 2018;10:294. doi: 10.3389/fnagi.2018.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao HJ, Li ZZ, Wang LM, Sun W, Yu JC, Wang B. Association of lower serum Brain-derived neurotrophic factor levels with larger infarct volumes in acute ischemic stroke. J Neuroimmunol. 2017;307:69–73. doi: 10.1016/j.jneuroim.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29(41):12764–7. doi: 10.1523/jneurosci.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He LH, Shi HM, Liu TT, Xu YC, Ye KP, Wang S. Effects of extremely low frequency magnetic field on anxiety level and spatial memory of adult rats. Chin Med J (Engl) 2011;124(20):3362–6. doi: 10.3760/cma.j.issn.0366-6999.2011.20.028. [DOI] [PubMed] [Google Scholar]
- 33.Yuan Z, Zhou H, Zhou N, Dong D, Chu Y, Shen J, et al. Dynamic evaluation indices in spatial learning and memory of rat vascular dementia in the Morris water maze. Sci Rep. 2019;9(1):7224. doi: 10.1038/s41598-019-43738-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bromley-Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J Vis Exp. 2011(53):2930. 10.3791/2920. [DOI] [PMC free article] [PubMed]
- 35.Zhao Y, Gu JH, Dai CL, Liu Q, Iqbal K, Liu F, et al. Chronic cerebral hypoperfusion causes decrease of O-GlcNAcylation, hyperphosphorylation of tau and behavioral deficits in mice. Front Aging Neurosci. 2014;6:10. doi: 10.3389/fnagi.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García AM, Sisternas A, Hoyos SP. Occupational exposure to extremely low frequency electric and magnetic fields and Alzheimer disease: a meta-analysis. Int J Epidemiol. 2008;37(2):329–40. doi: 10.1093/ije/dym295. [DOI] [PubMed] [Google Scholar]
- 37. Modarresi Chahardehi A, Arsad H, Lim V, Seeni A. Zebrafish a new development in the pharmaceutical industry for the treatment of anxiety. In: The 3rd National Conference on Knowledge and Technology of Psychology, Educational Sciences and Sociology of Iran. 2019. https://civilica.com/doc/929667/.
- 38.Nawa H, Carnahan J, Gall C. BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: partial disagreement with mRNA levels. Eur J Neurosci. 1995;7(7):1527–35. doi: 10.1111/j.1460-9568.1995.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 39.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 40.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 41.Liu T, Wang S, He L, Ye K. Chronic exposure to low-intensity magnetic field improves acquisition and maintenance of memory. Neuroreport. 2008;19(5):549–52. doi: 10.1097/WNR.0b013e3282f8b1a0. [DOI] [PubMed] [Google Scholar]
- 42.Szemerszky R, Zelena D, Barna I, Bárdos G. Stress-related endocrinological and psychopathological effects of short- and long-term 50Hz electromagnetic field exposure in rats. Brain Res Bull. 2010;81(1):92–9. doi: 10.1016/j.brainresbull.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Rezaei-Tavirani M, Hasanzadeh H, Seyyedi S, Ghoujeghi F, Semnani V, Zali H. Proteomic analysis of extremely low-frequency electromagnetic field (ELF-EMF) with different intensities in rats hippocampus. Arch Neurosci. 2018;5(1):e62954. doi: 10.5812/archneurosci.62954. [DOI] [Google Scholar]
- 44.Mizoguchi Y, Yao H, Imamura Y, Hashimoto M, Monji A. Lower brain-derived neurotrophic factor levels are associated with age-related memory impairment in community-dwelling older adults: the Sefuri study. Sci Rep. 2020;10(1):16442. doi: 10.1038/s41598-020-73576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cirulli F, Berry A, Chiarotti F, Alleva E. Intrahippocampal administration of BDNF in adult rats affects short-term behavioral plasticity in the Morris water maze and performance in the elevated plus-maze. Hippocampus. 2004;14(7):802–7. doi: 10.1002/hipo.10220. [DOI] [PubMed] [Google Scholar]
- 46.Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131(2):229–38. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 47.Li XH, Liu NB, Zhang MH, Zhou YL, Liao JW, Liu XQ, et al. Effects of chronic multiple stress on learning and memory and the expression of Fyn, BDNF, TrkB in the hippocampus of rats. Chin Med J (Engl) 2007;120(8):669–74. [PubMed] [Google Scholar]
- 48.Olton DS, Becker JT, Handelmann GE. Hippocampus, space, and memory. Behav Brain Sci. 1979;2(3):313–22. doi: 10.1017/s0140525x00062713. [DOI] [Google Scholar]
- 49.Woods R, Vallero RO, Golub MS, Suarez JK, Ta TA, Yasui DH, et al. Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation. Hum Mol Genet. 2012;21(11):2399–411. doi: 10.1093/hmg/dds046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang D, Kim KH, Phimister A, Bachstetter AD, Ward TR, Stackman RW, et al. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ Health Perspect. 2009;117(3):426–35. doi: 10.1289/ehp.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]