Abstract
Introduction: Fear memories are influenced by psychological and environmental variables. We evaluated the effect of 2.45 GHz microwave radiation on rats’ fear learning and memory ability to determine the potential risks. The present study aimed to assess the impacts of corticosterone (CORT) levels on the consolidation and reconsolidation of fear conditioning memories.
Methods: The rats were evaluated in contextual fear conditioning using foot shocks in both short-term (7 days) exposure and long-term (30 days) exposure. Young male Wistar rats were continually exposed to radio frequency electromagnetic field radiation for 5-6 weeks (1 h/day) with a frequency, power density, and pulse width of 2.45 GHz, 6.0 mW/cm2, and 2 ms, respectively. Several animals housed in identical conditions without exposure to radiation were monitored.
Results: Based on the results, a significant increase and decrease in body weight and percentage of the freezing time were observed after the short-term group respectively. However, in the long term, we observed no significant difference in body weight, and the freezing time decreased substantially.
Conclusion: As CORT levels were analyzed, long-term radiation might increase stress, which was associated with significant weight loss in rats.
Keywords: Fear memory, Electromagnetic field, Corticosterone, Animal model
Introduction
Electromagnetic radiation (EMR) can be classified into many categories depending on its frequency and wavelength. The electromagnetic fields (EMFs) are broadly classified into two groups: EMFs (> 3 Hz–3 kHz) of extremely low frequency (ELF) and radiofrequency electromagnetic fields (RF-EMF) (3 kHz–300 GHz).1 EMF-generating devices, such as mobile phones, tablets, and other electrical gadgets, are now widely utilized in our everyday lives.2 EMF effects are a complicated phenomenon for biological beings.3,4 The detrimental effects of EMF on the cognitive and behavioral characteristics of humans and rodents have been widely debated, and there has been increasing concern regarding the adverse effects of EMF on general brain function.5 Some new laboratory studies, notably on the impacts of RF waves linked to mobile phone technology, have been published in recent years, and it is relevant to evaluate the results of all of these studies to further the argument.4,6 Hence, there are several factors to consider when assessing whether RF radiation may affect the human brain and its following outcomes in the type of cognition and behavior.7 Environmental disturbances are most evident in the brain, particularly in the hippocampus.8 Consequently, the purpose of this study was to investigate changes in some major parameters (body weight, levels of corticosterone [CORT], and contextual fear conditioning [CFC]) that are directly and indirectly related to the hippocampus following exposure to EMF radiation.9 Furthermore, stress hormones regulate neuropathological and physiological fear memories, and they may be involved in anxiety disorders.10
Fearful incidents are typically well remembered.11 Later in life, acute traumatic experiences may improve learning new connections.12 In other words, fear memory generalization is a learning mechanism that encourages adaptable answers to new situations.10 Fear is the most intensely studied emotion in brain anatomy and neural pathways.13 Stress improves fear learning, mediated through the basolateral amygdala, which has recently been demonstrated to regulate hippocampal plasticity and dendritic development.14 Fear memory is produced in three areas of the brain: the hippocampus, which includes inhibitory avoidance and contextual conditioning; the basolateral amygdala (inhibitory avoidance); and the lateral amygdala (tone conditioning).14 Repetitive stress promotes learning fear through brain action mediated by β-adrenoceptors and increases impulsiveness through an independent mechanism. The expression of ribonucleic acid (mRNA) of the zif268 messenger in the amygdala is increased.14 When stable memories are reactivated, they become labile and are subject to extinction or reconsolidation mechanisms.15 Several studies have shown that the release of stress hormones during and shortly after stress can impact the genesis and development of anxiety disorders both physiologically and pathologically.10 Compared to female rats, glucocorticoids control more inflammatory genes and induce a more robust anti-inflammatory response.16 Furthermore, dose-response experiments in the livers of male and female rats revealed that some genes in females are 10- to 100-fold less responsive to glucocorticoid regulation than those in males.17 Thus, the majority of studies on the effects of glucocorticoid hormones have been conducted with male participants.18 Stress activates the hypothalamic–pituitary–adrenal (HPA) axis and secretes these hormones,16 which cause the adrenal cortex to produce glucocorticoids (CORT in rats and cortisol in humans) that are linked to mineralocorticoid and glucocorticoid receptors in the brain.19
CFC is a Pavlovian conditioning model that instantly creates fear memories in a context like a room or a chamber. CFC is a procedure that involves placing an animal, typically a rat, in a context (conditioned stimulus) and exposing it to unpleasant stimuli (unconditioned stimulus). In conjunction with similar procedures for fear conditioning, CFC is used to simulate behavioral situations for studying the neurobiology of anxiety disorders in rats, such as post-traumatic stress disorder or phobias.20,21 Also, rats have been exposed to CFC to evaluate hippocampus-dependent memory.22 Experimental subjects learn to connect a neutral environment with an aversive stimulus and exhibit fear responses to a setting that predicts danger in CFC.23 In contextual fear conditions, rats get electrical foot shocks in the conditioned stimulus (CS).
In addition, they are aware that when the noise or light CS occurs, the foot will be shocked; thus, they fear it as well.24 When the tonic stimulus is heard during the testing phase, the typical reaction of fear-conditioned rats is to “freeze,” which means that they remain immobile for a variable amount of time.25 Freezing is a particular response to fear characterized as “lack of movement save for breathing.” This might take seconds to minutes, depending on the severity of aversive stimuli, the number of exposure, and the subject’s level of learning.26 On the other hand, animal research revealed that EMF exposure in various settings altered CORT plasma levels and increased stress-related behavior.2
The purpose of the present study was to discover the effects of EMF on some biological factors such as body weight, fear memory, and the relationship between stress-induced CORT levels in rodents at different times of exposure to EMF at 2.45 GHz. Hence, the effects of EMF at this frequency on animals’ fear memories remain unclear. However, in this study, we aimed to determine whether EMF at 2.45 GHz may impact the conditioning of fear memory in rats and could affect CORT levels immediately after CFC.
Materials and Methods
Animals
In this study, thirty-two Wistar male rats, 5–6 weeks old, with a bodyweight range of 180–250 g, were employed. The rats were housed on a 12-hour light-dark cycle and provided commercial rat chow. The rats were exposed to the laboratory setting for one week prior to handling.
Experimental Design
The animals were randomly divided into the following four groups (EMF groups and control groups):
Short-term exposed group: effects of 2.45 GHz EMF on rats for one week (60 minutes daily). The training phase of assessing fear memory was conducted on the sixth day of radiation. In the short-term groups, the sixth day was considered the training phase of the fear memory test. Also, on the seventh day of irradiation, animals were immediately subjected to the fear memory test.
Control short-term group: like the previous group (mentioned above), no radiation was performed, one hour per day for seven consecutive days.
Long-term exposed group: This group was subjected to the same frequency as that in the short-term groups, but for a period of 30 days and one-hour daily exposure. On the 29th day after radiation exposure, the training phase for evaluating fear memory was immediately performed using foot shock. In addition, on day 30, the fear memory test was carried out without foot shock.
Control long-term group: Similar to the last group (described above), no radiation was administrated (one hour per day for thirty days).
Every attempt was made to reduce the number and suffering of the animals utilized in the study.
EMF Application and Exposure System
The live animals’ 2.45 GHz EMR device incorporated a computer, microwave signal sources, and a two-dimensional moving loading platform. The microwave output of a horn antenna has a power range of 1–200 W. The EMR device includes a power amplifier (ZHL-16W-43 + , USA) to amplify the signal generated and a signal generator (the Agilent HP 83732B, USA), which can generate signals in the frequency bandwidth range from 10 MHz to 20 GHz. A horn antenna S-band (model LB-OH-320-10-C-NF) with dimensions of 257 × 124 × 164 mm, which can operate in the frequency range of 2 to 4 GHz, a power meter, a radiation box (20 × 30 cm), and a power density measuring device were used. On the surface of the radiation cage, electromagnetic waves (pulsed modulation) with a frequency of 2.45 GHz and a density of 6 mW/cm2, pulse width (2 ms), and pulse repetition frequency of 500 Hz were irradiated from above. The spacing between the horn antenna and the tested animals was 25 cm. For this study, we used pulse waves because, unlike continuous waves, they transmit a lower level of power to the subject, and they did not cause tissue heating or tissue destruction through heat.
Contextual Fear Conditioning
A CFC approach was used. Then the rats were subjected to CFC. Only one conditioning chamber was utilized. The conditioning chamber comprises a chamber (53 × 65 × 55 cm) with Plexiglas and aluminium and a grid floor with 0.3 cm steel bars separated at 1.8 cm. The complete behavioral technique is as follows:
The animals were transferred to the test chamber individually on the training day (6th in the short-term and 29th in the long-term groups). Rats were allowed two minutes to freely explore the area. The noise was then played for 30 seconds with a sound frequency of 4 MHz and an intensity of 90 dB. In the last two seconds, an electric shock using a current of 0.5 mA was delivered to the animal via the floor bars of the chamber. After 300 seconds, the animal was removed from the chamber. The following day, the animals were returned to the same chamber for 2 minutes for a fear memory test on day 7 for short-term study and on the 30th day for long-term exposure to radiation study without the shock but using the tone. Between the tests, the chamber was cleaned with 75% ethanol and dried. A researcher who was not aware of the procedure videotaped it and assessed behavioral reactions and the length of immobility as the freezing time was recorded. The initial 300 seconds of freezing was regarded as a general fear response to the chamber, similar to the previous day as a training day. The freezing behavior of the experiment (defined as no beam crossing over 1 s) was recorded in the database and the time spent freezing throughout the 300-second recording session measured fear memory retention. Throughout the duration of the study in the retrieval habitat, the percentage of time spent freezing (freezing time%) was computed (freezing time × 100/total recorded time) for each animal.
Measurement of Corticosterone
Chloroform was used to anesthetize rats, and blood samples were taken directly from their hearts. The blood was taken and kept immediately on ice in an EDTA-coated tube (Microvette, Sarstedt, Germany). The blood serum was centrifuged for 10 minutes at 1600 g, and the CORT level in plasma was measured (expressed in nmol/L) according to the manufacturer’s instructions using the Corticosterone ELISA Kit (Cat # KA0468). The absorbance of the samples was determined using an ELISA reader set to 450 nm.
Results
A one-month pulsed modulated RF exposure period did not result in any documented injuries or fatalities. The schematic design of the fear conditioning test is shown in Figure 1A. The body weight of the rats differed significantly between the exposed and control groups during the short-term study (P = 0.0016, Figure 1B).
Figure 1.
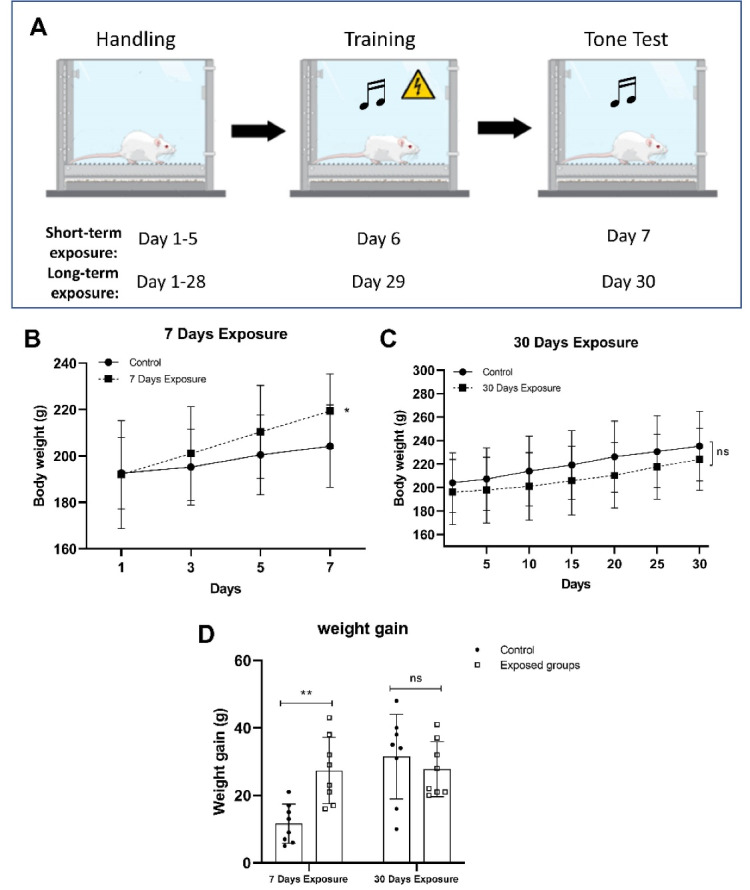
Schematic design for the fear conditioning test (A). Changes in body weight in rats exposed to 2.45 GHz electromagnetic fields during short-term exposure (7 days) (B), long-term (30 days) exposure (C), and weight gain (D). All data are presented as mean ± SD. Note a significant increase in the short-term exposed rats compared to their control group on day 1, P < 0.05, **P < 0.01
In the weight gain session in Figure 1D, the fear memory test itself, as a stress condition plus chronic exposure (1 hour daily for 30 days) to 2.45 GHz radiation, induced weight loss compared with its control group (P > 0.05). However, regardless of EMF exposure, all animals exposed to acute exposure (short-term) gained more weight significantly (P = 0.0016) during the experiment (Figure 1B). The short-term exposed group on day 7 showed a significant difference with day 1 (P = 0.0059), as shown in Figure 1B. A two-way ANOVA analysis revealed a significant interaction between groups and time exposure (F(1,24) = 10.72; P = 0.0032) and a significant difference in time exposure (F(1,24) = 6.203; P = 0.0201), but no significant difference was found in groups of animals (F(1,24) = 3.838; P = 0.0618). Also, the Student’s t-test showed that the loss in body weight in the long-term exposed group was not found significantly lower than the control long-term group (P > 0.05); however, a statistical difference was observed between the short-term exposed group and its control group (P = 0.0497) using student’s t test for 1 hour daily on day 7. Furthermore, the rats were subjected to fear conditioning to evaluate the ability of fear to learn.
Figure 2 indicates the freezing time during the conditioning session (tone test). Irradiated animals in the short-term and long-term groups exhibited a statistically significant decrease (P = 0.0322 and P = 0.0162) in the percentage of freezing time in the conditioning fear memory test based on the student’s t test between the control group and its exposure group. A two-way ANOVA analysis indicated no significant interaction of exposure time and groups (F(1,28) = 0.179; P = 0.6752), a significant main impact of groups (F(1,28) = 13.0; P = 0.0012), and a significant impact of exposure time (days) (F(1,28) = 8.09; P = 0.0082). During the experiment, freezing was observed to see if the behavioral strategies affected fear in a particular environment. Based on the data shown in Figure 2, it can be inferred that exposure to EMF in the 2.45 GHz range reduced the rate of immobility and the loss of conditioned memory, in terms of spatial and fear.
Figure 2.
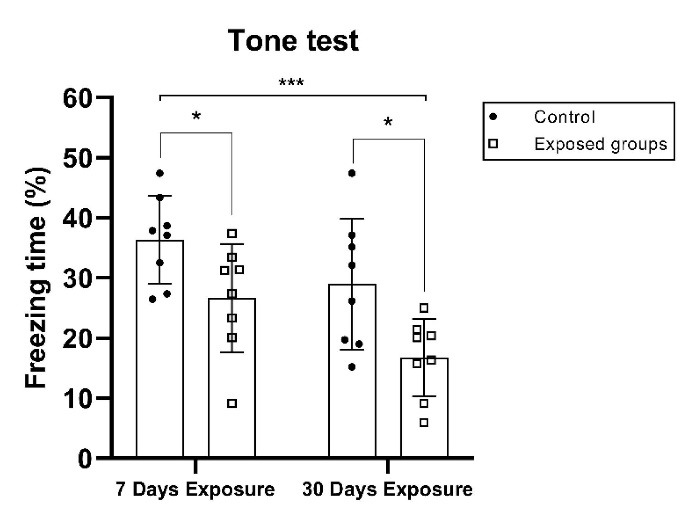
Mean Percentage of Freezing to Context and Tone Data Following a 24-Hour Contextual Fear Conditioning Test (tone: 4000 Hz, 90 dB, 30 s; foot shock: 0.5 mA, 2 s). The freezing time of the control and EMF exposure groups was monitored, recorded, and evaluated by the software application. All data are presented as mean ± SD and were analyzed using one-way and two-way ANOVA (comparing between all groups) or Student’s t test to compare the short-term group and its control group, and the long-term group and its control group; ns = not significant; *P < 0.05 and ***P < 0.001.
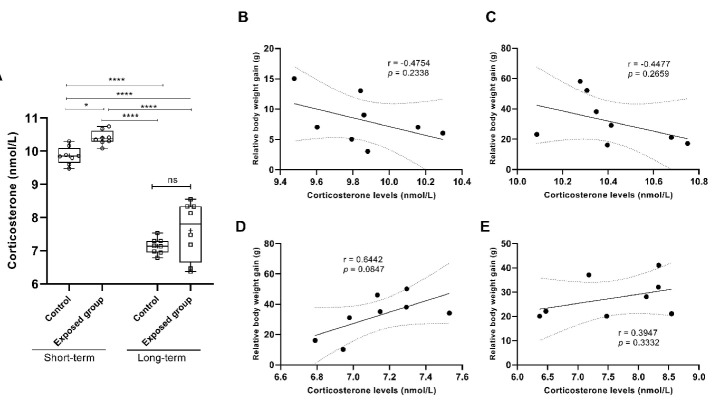
Next, we investigated whether CORT levels changed following the fear memory test and the context tone test time exposure. Figure 3 depicts the CORT levels in rats following the conditioning fear memory test. After fear conditioning, significantly, the student’s t test revealed a tendency toward a higher increase in CORT levels in the short-term exposure group compared to the control group (P = 0.0295, Figure 3A). The long-term exposed group showed no significant difference compared to its control group at P = 0.0668 (Figure 3A).
Figure 3.
The Correlations of Physiological Factors Such as Body Weight and Corticosterone Levels Together. (A) Plasmacorticosterone (nmol/L) measured in rats in response to behavioral tests immediately after the fear memory test. (B) corticosterone levels in the control short-term group, negatively correlated with body weight gain after seven days of the experiment. In the exposed short-term group, relative body weight was (C) negatively correlated with corticosterone levels. (D) Control long-term, and (E) exposed long-term groups positively correlated with corticosterone levels after one month. The data are expressed as the mean ± SD. One-way ANOVA using the Tukey test was used to compare all groups. r = Pearson’s correlation, P = significant value. ns indicated no significant,*P < 0.05, ***P < 0.001 and ****P < 0.0001. Student’s t test was used to compare the short-term and its control group, and the long-term group and its control group.
The indices were examined by a two-way ANOVA using various groups (tested and control groups) and the length of exposure time. The two-way ANOVA using the Bonferroni post hoc test showed a significant difference in groups (control and exposed) effects (F(1,6) = 9.874, P = 0.0200), no significant difference in groups × length of exposure time interaction (F(1,6) = 1.134, P = 0.3279), and a significant difference in the length of exposure time (F(1,6) = 117.4, P < 0.0001). The effects on irradiated and non-irradiated (control) animals were unaffected by group or exposure length. A Pearson’s correlation revealed a significant difference between plasma CORT levels and groups’ weight gain (g) (Figure 3B-3E). Based on the findings, CORT levels were only in long-term groups (control and exposed) positively correlated with body weight gain following 30 days of the experiment (r = 0.6442, 95% CI: -0.1107-0.9277, P = 0.0847, Figure 3D; r = 0.3947, 95% CI: -0.4294-0.8601, P = 0.3332, Figure 3E).
Discussion
Different EMFs are thought to have both beneficial and detrimental biological effects.27 In this study, the effect of EMF at a frequency of 2.45 GHz on the fear memory of male rats was investigated. The study groups were divided into four groups of eight rats for the radiation period experiment. These groups included two groups of short-term (control and its exposed group) for one week and long-term (control and its exposed group) for thirty consecutive days. According to existing guidelines, the mean power density limit for occupational RF exposure was 10 W/m2.28,29 However, we used a lower power density than this limit (6 mW/cm2 = 0.6 W/m2). Particularly, the widespread usage of 2.45 GHz radiation is on the rise, raising concerns about potential health risks, especially to the neurological system.30 In the present study, we used the fear conditioning test to assess the effects of EMF exposure on fear learning and memory. CFC may be a fundamental key learning experiment.31
Fear is an anxious and exciting reaction to an express threat related to defense system activation. Fear conditioning is predicated on a Pavlovian condition and the induction of fear to respond to unconditional stimuli. Both mechanisms are complicated and depend on the release of CORT.32 Our study showed that both short-term and long-term exposure groups were biologically different from their control groups. However, we observed a significant increase in body weight in the short-term exposure group. After exposure, the body weight of the short-term groups of rats exhibited a significant difference compared to the control group (P < 0.05) on the last day. This discovery could imply that the mechanism(s) that controls body fuel is sensitive to 2.45 GHz during this period. Virtually identical research conducted by Shahabi et al2 found that chronic exposure for two months to mobile radiofrequency at 900 MHz had no impact on animal body weight, while acute exposure (only for one month) showed a significant difference. However, a systematic review by Sienkiewicz et al in 2019 showed that many papers, using weight as biological evidence, have indicated a detrimental effect on memory and spatial learning from exposure to EMF.6
In the current study, we carefully monitored CORT plasma levels throughout the experiment to determine how variations in CORT plasma levels may be related to fear memory generalization. Regarding CORT levels, de Kloet et al33 mentioned that dysregulation in HPA axis performance and alterations in CORT levels have been linked to the development of anxiety disorders. However, in our study, the time course of EMF did not show any significant difference in the long-term exposure group. Some other studies also confirmed that their results regarding EMF exposure did not affect CORT levels.34 As we discussed earlier, compared with the control groups, there was a significant difference in the CORT levels in only the short-term exposed groups. Also, we found a significant difference between these two groups (short-term exposure and long-term exposure groups). However, a study by Bouji et al showed that RF-EMF exposure improved Alzheimer’s disease memory impairments in animal models.35 In the animal model of Alzheimer’s disease, they found that RF-EMF exposure increased hippocampal HO1 staining while decreasing CORT levels.35
In contrast but with similar results to our findings, Sandi and Loscertales discovered that their findings might be attributed to plastic alterations in the hypothalamus that explain the consequences of chronic exogenous glucocorticoid increases in the negative responses of the HPA axis.36 Another study by Roozendaal investigated whether glucocorticoids decrease memory function. Memory recall activities are often affected by elevated circulating levels of glucocorticoids or following hippocampal infusions of glucocorticoid receptor agonists.37
On the basis of the body weight and CORT levels depicted in Figures 1B, 1C, 1D, and 3, it can be concluded that long-term radiation may increase stress, which is associated with significant weight loss in rats. As CORT levels did not change in the animals exposed, this data may show that exposure to 2.45 GHz long-term does not depend on adrenal gland activity. However, our findings indicated that EMF exposure in the long-term study affected both hippocampus-dependent and hippocampus-independent learning and memory represent hippocampus-dependent and hippocampus-independent learning and memory, respectively.38 EMF at a frequency of 2.45 GHz caused damage to the fear memory in our rats during the long-term study. However, Szemerszky et al mentioned that prolonged and continuous exposure to a reasonably high-intensity EMF may constitute a moderate stress scenario and may have a role in the development of a depressed mood or metabolic abnormalities,27 which we observed in our long-term exposed animal group in weight gain data. As a result, further research is needed to understand the impact of ELF-EMF on behavior and sex and to investigate memory-related genes.
Conclusion
Based on the results obtained from the fear memory extinction between the two different groups of short-term and long-term exposure to 2.45 GHz radiation and their significant differences, it can be concluded that both short- and long-term radiation has a significantly more destructive effect on fear memory in rats. Also, the results of weighing rats showed that the group under short-term radiation compared to the control group had a significant weight reduction. During both short-term and long-term exposure to EMF, we observed an increase in CORT levels in rats, which was related to increased stress compared to their corresponding control groups. However, there was a negligible and non-significant difference in the long-term exposure. On the other hand, increasing stress showed a significant decrease in the weight of rats. Further research under controlled settings is needed to fully understand and corroborate these occurrences and findings.
Conflicts of Interests
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Ethical Considerations
All tests were performed following guidelines established by the Malek-Ashtar University Medical Ethics Committee and Shahid Beheshti Medical Ethics Committee (approval ethics No. IR.SBMU.RETECH.REC.1395.671), the UK Animals (Scientific Procedures) Act, 1986 and associated guidelines, and the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
Please cite this article as follows: Dehghani Z, Mahdavi SM, Modarresi Chahardehi A, Mansouri V, Jahani Sherafat S. The effect of 2.45 Ghz electromagnetic fields on fear memory extinction in male rats. J Lasers Med Sci. 2022;13:e58. doi:10.34172/jlms.2022.58.
References
- 1.Singh S, Kapoor N. Health implications of electromagnetic fields, mechanisms of action, and research needs. Adv Biol. 2014;2014:198609. doi: 10.1155/2014/198609. [DOI] [Google Scholar]
- 2.Shahabi S, Hassanzadeh Taji I, Hoseinnezhaddarzi M, Mousavi F, Shirchi S, Nazari A, et al. Exposure to cell phone radiofrequency changes corticotrophin hormone levels and histology of the brain and adrenal glands in male Wistar rat. Iran J Basic Med Sci. 2018;21(12):1269–74. doi: 10.22038/ijbms.2018.29567.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahdavi SM. Whole body 12 Hz electromagnetic field exposure and stress-related hormones changes. Int J Basic Sci Appl Res. 2017;6(3):201–8. [Google Scholar]
- 4.PaK Aeen M, Mahdavi SM, Maghami P, Modarresi Chahardehi A. The effect of non-ionizing electromagnetic fields in the range of 2.4 GHz on memory, thermal sensitivity and serum protein in male rats. Act Nerv Super Rediviva. 2022;64(2-3):77–85. [Google Scholar]
- 5.Prochnow N, Gebing T, Ladage K, Krause-Finkeldey D, El Ouardi A, Bitz A, et al. Electromagnetic field effect or simply stress? Effects of UMTS exposure on hippocampal longterm plasticity in the context of procedure related hormone release. PLoS One. 2011;6(5):e19437. doi: 10.1371/journal.pone.0019437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sienkiewicz Z, van Rongen E. Can low-level exposure to radiofrequency fields effect cognitive behaviour in laboratory animals? A systematic review of the literature related to spatial learning and place memory. Int J Environ Res Public Health. 2019;16(9):1607. doi: 10.3390/ijerph16091607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim TH, Huang TQ, Jang JJ, Kim MH, Kim HJ, Lee JS, et al. Local exposure of 849 MHz and 1763 MHz radiofrequency radiation to mouse heads does not induce cell death or cell proliferation in brain. Exp Mol Med. 2008;40(3):294–303. doi: 10.3858/emm.2008.40.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbar Shemirani S, Nasiri Khalili MA, Mahdavi SM, Modarresi Chahardehi A. The effect of mirtazapine on reducing chronic stress in male rats. Int Clin Neurosci J. 2022;9:e21. doi: 10.34172/icnj.2022.21. [DOI] [Google Scholar]
- 9.Singh KV, Gautam R, Meena R, Nirala JP, Jha SK, Rajamani P. Effect of mobile phone radiation on oxidative stress, inflammatory response, and contextual fear memory in Wistar rat. Environ Sci Pollut Res Int. 2020;27(16):19340–51. doi: 10.1007/s11356-020-07916-z. [DOI] [PubMed] [Google Scholar]
- 10.Kolodziejczyk MH, Fendt M. Corticosterone treatment and incubation time after contextual fear conditioning synergistically induce fear memory generalization in neuropeptide S receptor-deficient mice. Front Neurosci. 2020;14:128. doi: 10.3389/fnins.2020.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesuis SL, Catsburg LAE, Lucassen PJ, Krugers HJ. Effects of corticosterone on mild auditory fear conditioning and extinction; role of sex and training paradigm. Learn Mem. 2018;25(10):544–9. doi: 10.1101/lm.047811.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beylin AV, Shors TJ. Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Horm Behav. 2003;43(1):124–31. doi: 10.1016/s0018-506x(02)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pai CS, Sharma PK, Huang HT, Loganathan S, Lin H, Hsu YL, et al. The activating transcription factor 3 (Atf3) homozygous knockout mice exhibit enhanced conditioned fear and down regulation of hippocampal GELSOLIN. Front Mol Neurosci. 2018;11:37. doi: 10.3389/fnmol.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izquierdo I, Furini CR, Myskiw JC. Fear memory. Physiol Rev. 2016;96(2):695–750. doi: 10.1152/physrev.00018.2015. [DOI] [PubMed] [Google Scholar]
- 15.Taherian F, Vafaei AA, Vaezi GH, Eskandarian S, Kashef A, Rashidy-Pour A. Propranolol-induced impairment of contextual fear memory reconsolidation in rats: a similar effect on weak and strong recent and remote memories. Basic Clin Neurosci. 2014;5(3):231–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132(5):1033–44. doi: 10.1016/j.jaci.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duma D, Collins JB, Chou JW, Cidlowski JA. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci Signal. 2010;3(143):ra74. doi: 10.1126/scisignal.2001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35(3):565–72. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chester JA, Kirchhoff AM, Barrenha GD. Relation between corticosterone and fear-related behavior in mice selectively bred for high or low alcohol preference. Addict Biol. 2014;19(4):663–75. doi: 10.1111/adb.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaaya N, Jacques A, Belmer A, Beecher K, Ali SA, Chehrehasa F, et al. Contextual fear conditioning alter microglia number and morphology in the rat dorsal hippocampus. Front Cell Neurosci. 2019;13:214. doi: 10.3389/fncel.2019.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albrecht A, Çalışkan G, Oitzl MS, Heinemann U, Stork O. Long-lasting increase of corticosterone after fear memory reactivation: anxiolytic effects and network activity modulation in the ventral hippocampus. Neuropsychopharmacology. 2013;38(3):386–94. doi: 10.1038/npp.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi M, Ahn S, Yang EJ, Kim H, Chong YH, Kim HS. Hippocampus-based contextual memory alters the morphological characteristics of astrocytes in the dentate gyrus. Mol Brain. 2016;9(1):72. doi: 10.1186/s13041-016-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim WB, Cho JH. Encoding of contextual fear memory in hippocampal-amygdala circuit. Nat Commun. 2020;11(1):1382. doi: 10.1038/s41467-020-15121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sweatt JD. Rodent behavioral learning and memory models. In: Sweatt JD, ed. Mechanisms of Memory. 2nd ed. London: Academic Press; 2010. p. 76-103. 10.1016/b978-0-12-374951-2.00004-4. [DOI]
- 25. Hoffman KL. What can animal models tell us about depressive disorders? In: Hoffman KL, ed. Modeling Neuropsychiatric Disorders in Laboratory Animals. Cambridge, UK: Woodhead Publishing; 2016. p. 35-86. 10.1016/b978-0-08-100099-1.00002-9. [DOI]
- 26. Curzon P, Rustay NR, Browman KE. Cued and contextual fear conditioning for rodents. In: Buccafusco JJ, ed. Methods of Behavior Analysis in Neuroscience. 2nd ed. Boca Raton, FL: CRC Press, Taylor & Francis; 2009. [PubMed]
- 27.Szemerszky R, Zelena D, Barna I, Bárdos G. Stress-related endocrinological and psychopathological effects of short- and long-term 50Hz electromagnetic field exposure in rats. Brain Res Bull. 2010;81(1):92–9. doi: 10.1016/j.brainresbull.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 28.International Commission on Non-Ionizing Radiation Protection. Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz) Health Phys. 1998;74(4):494–522. [PubMed] [Google Scholar]
- 29. IEEE International Committee on Electromagnetic Safety (SCC39), “IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz,” IEEE Std C95.1-2005 (Revision of IEEE Std C95.1-1991), pp. 1–238 (2006). 10.1109/IEEESTD.2006.99501.
- 30.Othman H, López-Furelos A, Leiro-Vidal JM, Ammari M, Sakly M, Abdelmelek H, et al. Exposure to 2.45 GHz radiation triggers changes in HSP-70, glucocorticoid receptors and GFAP biomarkers in rat brain. Int J Mol Sci. 2021;22(10):5103. doi: 10.3390/ijms22105103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee JLC. Mechanisms and functions of hippocampal memory reconsolidation. In: Alberini CM, ed. Memory Reconsolidation. San Diego: Academic Press; 2013. p. 43-68. 10.1016/b978-0-12-386892-3.00003-2. [DOI]
- 32.Bouji M, Lecomte A, Hode Y, de Seze R, Villégier AS. Effects of 900 MHz radiofrequency on corticosterone, emotional memory and neuroinflammation in middle-aged rats. Exp Gerontol. 2012;47(6):444–51. doi: 10.1016/j.exger.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 33.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 34.Kim HS, Choi HD, Pack JK, Kim N, Ahn YH. Biological effects of exposure to a radiofrequency electromagnetic field on the placental barrier in pregnant rats. Bioelectromagnetics. 2021;42(3):191–9. doi: 10.1002/bem.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouji M, Lecomte A, Gamez C, Blazy K, Villégier AS. Impact of cerebral radiofrequency exposures on oxidative stress and corticosterone in a rat model of Alzheimer’s disease. J Alzheimers Dis. 2020;73(2):467–76. doi: 10.3233/jad-190593. [DOI] [PubMed] [Google Scholar]
- 36.Sandi C, Loscertales M. Opposite effects on NCAM expression in the rat frontal cortex induced by acute vs. chronic corticosterone treatments. Brain Res. 1999;828(1-2):127–34. doi: 10.1016/s0006-8993(99)01346-3. [DOI] [PubMed] [Google Scholar]
- 37.Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 2002;78(3):578–95. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- 38.Lai J, Zhang Y, Liu X, Zhang J, Ruan G, Chaugai S, et al. Effects of extremely low frequency electromagnetic fields (100μT) on behaviors in rats. Neurotoxicology. 2016;52:104–13. doi: 10.1016/j.neuro.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]