Abstract
Introduction: While a wound caused by a minor cutaneous incision routinely heals in a short time, wounds from major surgical operations might need numerous days to heal and may leave an obvious cicatrix. The use of blue light therapy (BLT) to destroy infectious microorganisms and disrupt biofilm formation could be an efficient method for healing ulcers. This systematic review focused on the effects of BLT in different preclinical in vivo studies and clinical models of skin wound healing. Furthermore, this study attempted to determine what main light parameters should be tested in preclinical and clinical studies.
Methods: The online databases PubMed.gov, Google Scholar, Scopus, Web of Science, and Cochrane were searched using the keywords "blue light" and "wound healing" according to PRISMA guidelines. No publication time limit was enforced.
Results: A total of 858 articles were identified, and 17 articles in three distinct categories were included for review. They comprised two articles on humans, fourteen articles on healthy animals, and one article on diabetic animals.
Conclusion: Some studies have shown that the application of BLT on preclinical and clinical models of wound healing in vivo is able to significantly accelerate the healing process. Few studies, however, have explored the bactericidal effect of BLT on skin injury repair in burn patients. Further preclinical investigations designed to provide a better understanding of the bactericidal effect of BLT using standardized protocols, different BLT wavelengths, and different stages of the wound healing process of infected wounds and ulcers in healthy and diabetic animals should be carried out before clinical trials can be considered. BLT could eventually be a good option for treating infected chronic wounds, including those in diabetic patients.
Keywords: Photobiomodulation, Phototherapy, Wound healing, Ulcer, Review
Introduction
While a minor cutaneous incision routinely heals in a short time, wounds resulting from major surgical operations might need many days to fully heal and often deposit an obvious cicatrix. In the most severe cases, chronic wounds are a major problem all over western countries.1 Wound healing includes the spatial and chronological harmonization of many kinds of cells with different properties involved in the inflammatory, proliferative, and maturation stages of wound healing.2
For a skin defect to cure well, all four stages of wound healing should happen in good order and at appropriate intervals.3 Many issues can interfere with wound healing by disturbing one or more stages of the healing process, contributing to the overall delay of healing. Skin injuries which display delayed healing can be hindered acute injuries or chronic ulcers and often stall in their advancement over the usual course of healing.4 Most ulcers are characterized by prolonged or extreme inflammation, continuous microbial contamination, and the inability of skin cells to respond to restorative stimuli.5
Almost 2.5% of the entire USA population will experience ulcers during their lifetime,6 and medical expenses for the management of acute skin injuries and ulcers have been estimated to be about $62.5 billion.7 More importantly, ulcers normally shelter dangerous microbial biofilms, which are resistant to systemic and local antimicrobial therapy. Various schemes have been widely developed to propose recommended antibiotic applications and to control the development and increase of bacterial resistance; however, novel antibacterial methods currently require a further appraisal.8
A growing number of patients are seeking conservative approaches to improve clinical and cosmetic skin conditions, including wound healing.9 Many patients welcome new advances in the use of photobiomodulation (PBM) and light-emitting diodes (LEDs) for numerous medical, clinical, and beauty applications. Depending upon the desired chromophore, different wavelengths of light can be employed.10
Several studies have confirmed the beneficial properties of light of diverse wavelengths on skin injury repair.11 Blue light wavelengths range from about 380 nm to 460 nm. Blue light therapy (BLT) has been extensively applied for managing cutaneous diseases and for accelerating skin injury repair.12
Researchers have found that the application of BLT to the skin has bactericidal,13 anti-contamination,14 and anti-inflammatory15 effects. Consequently, antimicrobial BLT (aBLT) has shown an extensive variety of anti-bacterial effects against gram-positive and gram-negative bacteria. The details of its mechanism of action are not yet entirely known, but it is assumed that it relies on photodynamic effects following the light excitement of endogenic microbial chromophores.16,17 The preliminary favorable results of BLT applications have directed researchers toward the expansion of phototherapy as well as PBM therapy in wound healing.12
Several recent review articles have stated the importance of using blue light to accelerate wound healing.10,18,19
The effects of BLT on skin wound healing have received much notice during the previous two decades. Despite favorable results in the majority of these investigations, there is still no established protocol for BLT. To the extent that we have studied, our work is the first systematic review of the influence of BLT on preclinical models and clinical studies of wound healing.
Regarding too much studies which were performed on both in vitro, and in vivo (preclinical) models, in this systematic review investigation, it was focused on the effect of BLT on different preclinical and clinical models of skin wound healing to cover all in vivo studies which were done. Furthermore, we tried to discover the healing ability of light protocol of BLT on the wound healing evaluating methods, and attempted to grasp main light factors that are necessary to be deliberated in preclinical and clinical studies. According to our findings, we could select the most effective light factors of BLT for accelerating wound healing and likewise inspire upcoming investigations to use these required light factors of BLT.
Materials and Methods
Procedure
This systematic review followed the guidelines of PRISMA.
Acceptability and Inclusion Criteria
All original articles that investigated the influence of BLT on skin wound healing in preclinical and clinical studies in vivo were eligible for inclusion.
Exclusion Criteria
Review articles including systematic and narrative reviews, meta-analyses, news, editorials, case reports, letters to the editor, and conference papers were excluded. Studies in which BLT was used to treat clinical conditions other than skin injuries or ulcers, studies that lacked data on blue light parameters such as wavelength, duration, energy density, wound site and depth, studies for which the complete report was not provided and languages other than English were used, and studies performed on cell culture systems were all excluded.
Search Stratagem
The online databases of PubMed.gov, Google Scholar, Scopus, Web of Science, and Cochrane were searched using the keywords “blue light” and “wound healing;” no publication time limitation was imposed.
Study Selection Strategy
For article selection, these three steps were performed:
Articles whose titles met the inclusion criteria were included; duplicates were eliminated.
The abstracts of the selected articles were studied.
Any article that did not satisfy the inclusion criteria based on the content of the full text was excluded. In this step, two reviewers assessed the full text of each article separately. Any disagreement between the two reviewers was resolved by the third author.
Data Collection
Critical data were extracted from the selected studies, including (1) first author surname and release date of the paper; (2) studied animal and its species; (3) main BLT parameters such as wavelength (nm), duration and number of irradiations of light delivery method, and energy density; (4) site and depth of skin injury; (5) main results and/or conclusions; and (6) organizational score of each study.
Organizational Score of the Study
The quality of data reporting was evaluated for the existence of the following in the articles: randomization, blinding, conflict of interest statement, and ethical approval. While each case was observed, a 25% score was added to the organizational score of the study.20
Results
This review identified 244 articles on PubMed, 100 on Google Scholar, 319 on Scopus, and 195 on Web of Science databases for a total of 858 articles, 760 of which remained after duplicate studies were eliminated (98 cases). Subsequently, abstracts and full texts were screened, and 17 articles were included in this systematic review (Figure 1).
Figure 1.
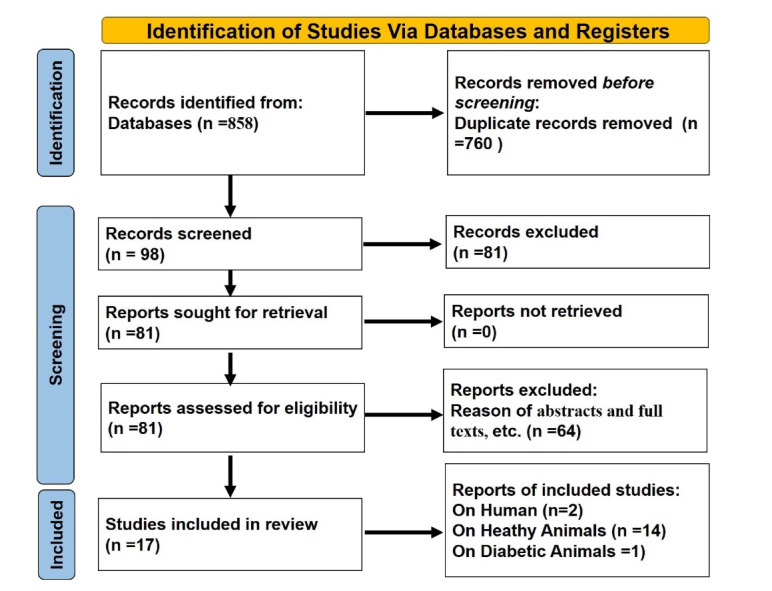
PRISMA Flow Chart of Article Gathering Course.
Description of Studies
To evaluate more deeply the impact of BLT in preclinical and clinical studies, we separated the accepted articles into three distinct categories, comprising two articles on humans (Table 1), fourteen articles on healthy animals (Table 2), and one article on diabetic animals (Table 3).
Table 1. Studies on the Effect of Blue Light Therapy on Wound Healing in Humans .
| First Author, Publication Year | Probable Disease; Type and Location of Wound | BLT Parameters | Main Results and/or Conclusions | Methodological Quality of Study |
| Fraccalvieri, 202221 | 90 Patients with vascular or non-healing surgical dehiscence ulcers | BLT, range of wavelength between 410-430 nm, 60 seconds, 120 mW/cm2; 7.2 J/cm2, weekly treatment. | BLT plus standard care of ulcers accelerated new epidermal cell formation in ulcers of lower limbs by 2.5 months. | 50%; randomization and blinding were not mentioned. |
| Dini, 202122 | 20 Patients with ulcers (12 patients with venous foot ulcers, 6 patients with skin vasculitis, 2 patients with traumatic ulcers) resistant to regular therapy. | BLT, range of wavelength between 400 - 430 nm, 120 mW/cm2. | BLT encouraged wound healing, improved WBS, and decreased soreness in patients. | 50%; randomization and blinding were not mentioned. |
All light was radiated from LED devices.
Abbreviations: WBS, wound bed score; BLT, blue light therapy.
Table 2. Studies on the Effect of Blue Light Therapy on Wound Healing in Healthy Animals .
| First Author, Publication Year | Probable Disease; Type and Location of Wound | BLT Parameters | Main Results and/or Conclusions | Methodological Quality of Study |
| Lv, 202224 | Adult male rats; wounds were infected with various drug-resistant bacteria. | BLT (465 nm) and RLT (625 nm, 75 mW/cm2). |
RLT and BLT both showed effects against drug-resistant bacteria. | 25%; conflicts of interest and medical ethics were not mentioned. |
| Lu, 202223 | Adult male rats; there were 4 full-thickness round excisional wounds. | photocurable chitosan bio-ink hydrogels were irradiated with BLT (405-nm, 10–30 s). | Adhesive quick gelling hydrogels showed hemostasis, bio-adaptability, and bactericidal properties under BLT for wound healing. | 50%; medical ethics and conflicts of interest were not mentioned. |
| Li, 201625 | Rabbits; 3 round full-thickness skin wounds on the rabbit’s back were generated. | RLT (630 nm, 50 mW/cm2) and BLT (460 nm, 50 mW/cm2). | The results of BLT on wound healing were inferior to that of RLT. | 50%; blinding and conflicts of interest were not mentioned. |
| Cicchi, 201626 | Adult rats; four abrasions made on the skin on the backs of rats were generated. | BLT (410 nm - 435 nm, 1.27 W/cm2, 25 s). | The irradiated injuries displayed a decreased inflammation and a greater amount of collagen. | 50%; randomization and conflicts of interest were not mentioned. |
| Nour, 201627 | One female adult (13-year-old) horse with an infected back ulcer. | BLT (460 nm, 250 mW, 20 minutes) plus AgNPs at 16 mg/g was formulated in a cellulose gel. | Combination therapy was effective in the management of an infected ulcer. | 50%; randomization and blinding were not mentioned. |
| Figurová, 201628 | Ten, 12-month-old female Minipigs; one incision, 10 cm in length, was created on the back of each minipig. |
RLT (685 nm; 4 sources, 0.05 W per source, diameter of aperture 0.15 cm) BLT (470 nm, 13 sources, 0.016 W source, 0.008 W/cm2) Both LEDs continuous mode; 420 s; energy density per session: 3.36 J/cm2. |
Combined RLT and BLT augmented increased new epidermal formation and intermolecular connections between collagen fibrils compared to sham irradiated control wounds. | 100% |
| Dungel, 201529 | Male rats; skin flap in the inferior epigastric neurovascular bundle was created. |
BLT (470 nm, 1W); RLT (629 nm, 1 W), 50 mW/cm2. | LED therapy of ischemic tissue enhanced the initial steps of skin injury repair by increasing new blood vessel formation regardless of the wavelength. | 75%; blinding was not mentioned. |
| Zhang, 201430 | BALB/c mice; a full-thickness scald was created utilizing a preheated brass block (95°C, 7 s) and infected with drug-resistant bacteria (107 CFU of Acinetobacter. baumannii). |
BLT (415 nm, 14.6 mW/cm2, 55.8 J/cm2). | BLT significantly decreased the microbial burden in scald wounds. | 50%; randomization and blinding were not mentioned. |
| Cheon, 201331 | Adult male rats; two round wounds (1 in diameter) were made in each animal. | BLT (470 nm, 3.55 mW/cm2, 60 minutes/day for 9 days, continuous); GLT (525 nm, 4.02 mW/cm2), RLT (633 nm, 6.78 mW/cm2). | RLT, GLT, and BLT all had a positive effect on skin injury repair and could substitute for low-level laser therapy. | 0%; All items were missed. |
| Dai, 201332 | Adult BALB/c mice; a full-thickness degree scald was created and infected with drug-resistant bacteria (3 × 106 CFU of P. aeruginosa). | BLT (55.8 J/cm2, 14.6 mW/cm2, 62 min). | BLT could be an effective and safe alternative to pharmacologic therapy for Pseudomonas aeruginosa scald infections. |
0%; All items were missed. |
| Fushimi, 201233 | Adult mice; a round full thickness excisional wound (8 mm diameter) was created on the back of mice. | BLT (456 nm, 0~2.31 W, 0~0.3 mW/cm2); GLT (518 nm, 0~2.28 W, 0~0.25 mW/cm2, 0.3 J/cm2); RLT (638 nm, 0~2.52 W, 0~0.65 mW/cm2). | GLT stimulated skin injury repair by promoting migration and growth mediators. | 50%; blinding and conflicts of interest were not mentioned. |
| Adamskaya, 201134 | Adult male Rats; two circular excisional wounds (full thickness) were made on the back of each rat. |
BLT (470 nm, 1 W, 10 min); RLT (629 nm, 1 W, 10 min). | BLT could have a significant effect on normal skin injury repair by affecting keratin expression. | 25%; only medical ethics considered. |
| Soyer, 201135 | Neonatal rat; one incision was made on the back area. | The experimental group was subjected to BLT (30-40 μW/cm2 per nm, the distance of the target from the source: 45 cm). |
VEGF was decreased in neonatal rat skin under the influence of BLT. | 50%; blinding and conflicts of interest were not mentioned. |
| de Sousa, 201036 | Adult male rats; one 1 × 1 cm skin wound was created. | RLT (700 nm, 15 mW, spot diameter 16 mm); GLT (530 nm, 8 mW, spot diameter 16 mm); BLT (460 nm, 22 mW, spot diameter 16 mm). |
RLT and GLT displayed a significant increase in fibroblast numbers compared to the control group. | 100% |
All light was from LED devices.
Abbreviations: RLT, red light therapy; AgNPs, silver nanoparticles; CFU, colony-forming unit; GLT, green light therapy; BLT, blue light therapy; VEGF, vascular endothelial growth factor; LED, light-emitting diode.
Table 3. Studies on the effects of Blue Light Therapy on Wound Healing in Non-healthy Animals .
| First Author, Publication Year | Probable Disease; Type and Location of Wound | BLT Parameters | Main Results and/or Conclusions | Methodological Quality of Study |
| Cai, 202237 | Adult diabetic rats; A 2-cm-diameter skin defect was made. Four groups: control, RLT, BLT, and RLT + BLT | A 5.0 cm2 array of 99 LED sources; each diode could deliver BLT (460 nm, 0.004 W/cm2) or RLT (630 nm, 0.004 W/cm2). |
Photobiomodulation with a mixture of BLT + RLT had a synergistic effect on skin injury repair in diabetic rats and could be a more effective approach to skin ulcer repair in diabetic patients | 75%; blinding was not mentioned |
All light was radiated from light emitting diode (LED) devices.
Abbreviations: RLT, red light therapy; BLT, blue light therapy; LED, light-emitting diode.
Effect of BLT on Wound Healing in Humans
Fraccalvieri et al at 2022 determine impact of BLT plus regular care of ulcers in relation to regular care alone in encouraging new epidermal cell formation of ulcers of lower limb in 8.5 months. Fraccalvieri et al concluded that BLT plus regular care hurries wound healing course of ulcers, particularly venous leg ulcers and surges the probabilities of entire wound healing in 8.5 months.21 In 2021, Dini et al tried to stimulate the healing course in 20 non-healing ulcers using a moveable LED array device that radiated blue light. They concluded that LED BLT favorably encouraged skin injury repair, improved the wound bed score (WBS), and decreased patient reports of soreness22(Table 1).
Effects of BLT on Wound Healing in Healthy Animals
In 2021, Lu et al designed special sticky hydrogels for the instant management of bleeding from non-compressible wounds. They concluded that the novel properties of the adhesive hydrogels, such as rapid gelling and bactericidal activity under the influence of BLT, suggested beneficial applications for skin injury repair.23 In 2022, Lv et al assessed and compared the healing effects of red light therapy (RLT) with those of BLT from LED devices on numerous drug-resistant bacteria in vitro and in infected wounds in rats. PBM therapy showed potentially significant improvement in healing infected wounds, suggesting it could be a cheap, available, painless, and safe intervention for drug-resistant infected wounds.24 In 2016, Li et al investigated the histological foundation of the healing effect of PBM (RLT, BLT) and the correlation between the duration of irradiation and the PBM effect of different LED wavelengths in an albino rabbit ear skin wound model. They reported that the effect of BLT on skin wound healing was inferior to that of RLT. This investigation offered a justification for the probable benefits of LED therapy in medical settings.25 In 2016, Cicchi et al investigated the effects of a blue-LED hemostatic device on mechanical abrasions on the backs of rats. The irradiated area showed decreased inflammation and increased connective tissue fibers. The blue LED produced a photothermal effect on cutaneous injuries.26 Also, in 2016, Nour et al examined the potential use of silver nanoparticles (AgNPs) in combination with BLT against numerous drug-resistant bacteria (Pseudomonas aeruginosa) in culture systems and in an animal model. They stated that the combined treatment had not previously been examined. They reported that their combination treatment was effective in handling contamination with numerous drug-resistant bacteria and could be applied as an alternative to standard pharmacologic treatment.27 In 2016, Figurová et al histopathologically evaluated the effect of PBM (laser therapy plus LED) on pig cutaneous wound healing. Their combination therapy increased new epidermal cell formation and the creation of intermolecular connections between collagen fibrils compared to sham-treated wounds.28 Dungel et al evaluated and compared the effects of LED BLT and LED RLT on skin wound healing in an ischemic flap model in rodents. They concluded that LED treatment of ischemic tissue increased the early healing of flaps by stimulating new blood vessel formation regardless of the wavelength. They suggested that this could be a conservative approach and an economical modality for the treatment of complex wounds.29 In 2014, Zhang et al investigated the use of bactericidal BLT against contamination with drug-resistant bacteria (Acinetobacter baumannii) in a scald wound mouse model. BLT (55.8 J/cm2) significantly decreased the microbial burden, and no noteworthy apoptosis was observed in the skin of mice using a TUNEL assay after BLT (195 J/cm2).30 In 2013, Cheon et al investigated the therapeutic effects of RLT, green LT (GLT), and BLT from separate LED devices on an excisional wound model in rats. They concluded that RLT, GLT, and BLT all had positive effects on skin injury repair and could possibly substitute for laser therapy.31 Dai et al reported the efficacy of BLT (415 nm) for treating fresh, possibly fatal scald contaminations in mice. Survival studies revealed that BLT increased the viability rate of contaminated mice from 18% to 100%. Dai et al suggested that BLT could be an operational and harmless substitute for routine pharmacological treatment for P. aeruginosa in contaminated scald wounds.32 In 2012, Fushimi et al explored the effects of RLT (638 nm), BLT (456 nm), and GLT (518 nm) from LEDs on skin injury repair. They reported that LED GLT encouraged skin injury repair by enhancing migration and cell signaling and suggested that not only LED RLT but also LED GLT could be a beneficial, novel approach for skin injury repair.33 In 2011, Adamskaya et al explored the effects of BLT and RLT from LEDs on the healing of an excisional wound model in rats. They reported that BLT significantly stimulated skin injury repair. They proposed that phototherapy could significantly improve the function of normal wound healing through keratin formation and could provide a simple, valid, harmless, and economical approach for managing superficial wounds.34 In 2011, Soyer et al evaluated the effect of light therapy on cytokine expression levels in the wound healing of the skin in newborn rats. They reported that vascular endothelial growth factor (VEGF), a mediator of new blood vessel formation, was reduced in the skin of newborn rats after phototherapy. They suggested that the lower values of VEGF after light therapy might alter new blood vessel formation and unfavorably disturb the healing of skin in infants.35 In 2010, de Sousa et al histopathologically evaluated the fibroblast proliferation in back skin wounds in rats irradiated with LEDs of three different wavelengths and without irradiation. They reported that LED RLT and LED GLT increased fibroblast proliferation when compared with the control group36 (Table 2).
Effect of BLT on Wound Healing in Diabetic Animals
In 2022, Cai et al designed a complete skin injury model in diabetic rats and a high glucose endothelial cell culture system to explore the effects of PBM with a mixture of RLT and BLT on wound healing in diabetic subjects. They concluded that PBM with RLT plus BLT had a synergistic effect on skin injury repair and could be a more effective approach to skin ulcer repair in diabetic patients37(Table 3).
Discussion
We have surveyed published literature without any publication date limitations pertaining to impact of blue light on wound healing and have focused on preclinical and clinical models. Some important points that could hold back the development of this field were highlighted. Firstly, only in two original studies did researchers examine the effects of BLT on skin injury repair in patients, and only one study evaluated the effect of BLT on a diabetic model in a preclinical animal model.
Secondly, inspection of the light parameters in the included studies revealed that some parameters were poorly characterized and ill-defined. In other words, inconsistent use of units, such as mW/cm2, minute, J, and μW/cm2, was observed. In addition, the ranges of power density and energy density used for stimulating skin cells were very wide: 50 mW/cm2 up to 1.27 W/cm2; 3.36 J/cm2 up to 55.8 J/cm2.
Inconsistencies were also found in the literature regarding the effect of LED BLT on wound healing. While Abe et al and Li et al (first group) showed that RLT had a superior effect on skin injury repair than that of BLT25 and increased recovery of the epidermal barrier,38 Dungel et al, Zhang et al, Figurová et al, and Cheon et al (second group) had different conclusions and reported that isolated LED irradiation with both wavelengths of red and blue light significantly increased angiogenesis and tissue perfusion up to 168 hours after ischemic damage29 and improved wound healing,28,31 and they also showed that BLT was more effective than RLT.26
More details on the above-mentioned inconsistencies are given here. In the first study of the first group, Abe et al reported that PBM (40 mW/cm2) stimulated retrieval of the epidermal barrier of the skin in a porcine skin sample. The researchers observed an accelerated recovery of the epidermal barrier by radiation of RLT; however, the radiations of BLT were ineffective in accelerating barrier recovery.38 Similarly in the second study of this group, Li et al induced three skin wounds in each rabbit, of which two wounds were radiated by RLT (630 nm, 50 mW/cm2) and BLT (460 nm, 50 mW/cm2) respectively, and the third one was the control. Li et al found that the results of RLT in terms of wound closure ratio, collagen fiber, skin thickness, and expression of growth factors were considerably better than those in other groups. Li et al concluded that BLT achieved poorer wound healing than RLT, and RLT seemed to hasten wound healing.25
On the other hand, in the first study of the second group, Dungel et al found that LED radiation (BLT, and RLT) meaningfully augmented new blood vessel formation in the papillary part of dermis and intramuscularly (skin muscle) which was linked with meaningfully better tissue perfusion seven days after the ischemic damage. Therefore, tissue death was meaningfully decreased and contraction meaningfully minus marked in the LED-radiated groups of both lights.29
Concurrently, Zhang et al investigated the utility of antimicrobial BLT (415 nm, 14.6 mW/cm2, 55.8 J/cm2, one shot) for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model. They found that the multidrug-resistant A. baumannii strain was considerably more vulnerable than keratinocytes to BLT inactivation. Zhang et al also found BLT-induced ultrastructural damage in A. baumannii cells.30
In the third study of this group, Figurová et al evaluated the effects of PBM with a combination of PBM plus LED on pig dermal injury repair from a histological point of view. They found that PBM (RLT and BLT) increased new epidermal cell formation and the creation of more intermolecular connections between collagen fibrils compared to sham-treated wounds.
In the fourth study of this group, Cheon et al used male rats in one control group and three LED therapy groups, i.e. RLT, GLT, and BLT for accelerating the wound healing process. They observed that using BLT (P < 0.05) was more effective than using RLT (P > 0.05). The RLT–GLT–BLT-irradiated groups had more collagen and also had a beneficial effect on skin injury repair compared to the control group and could possibly be a substitute for PBM.
The red LED (wavelength range between 620 nm—770 nm) has the maximum penetration (up to 0.6 cm below the skin surface) among all the LEDs available in the market (yellow, green, and blue).25 Moreover, the capability to directly affect dermal fibroblast proliferation39,40 made RLT useful for the management of cutaneous injury repair. The results suggested a possible direct effect of RLT on the growth of dermal fibroblasts41 as well as new blood vessel formation.42 This action occurs chiefly through PBM, in which RLT affects the mitochondria and increases the activity of intracellular enzymes, encourages cell proliferation and metabolism, and therefore accelerates skin injury repair.43 On the other hand, we should note that both the absorption and the scattering of light in tissue are much higher in blue light than in red light.44
aBLT is a light-based approach that exerts inherent bactericidal effects without the participation of any exogenous photosensitizers.45 Our systematic review of the literature revealed that while there was no study on the effect of BLT on infected skin wounds in healthy and diabetic patients or animals, in only two available studies did researchers report beneficial effects of BLT on burns infected with multidrug-resistant A. baumannii30 or P. aeruginosa.32 In the second study,32 BLT also prevented the occurrence of fatal bacteremia in the mice. In this situation, Fink et al emphasized that the P. aeruginosa strain could be fatal to mice.46 Lacking a cure, the bacteria could invade deep into the mouse tissue and reach the blood circulation within hours, leading to bacteremia and death in the mice. Dai et al also examined the BLT inactivation of P. aeruginosa. Transmission electron microscopy images showed that BLT destroyed P. aeruginosa cells by causing vacuoles inside the cytoplasm, suggesting that the bacterial death was caused by intracellular chromophores activated by BLT.47
To identify and measure intracellular porphyrins, Dai et al recommended that future investigations should be performed utilizing high-performance liquid chromatography or capillary electrophoresis coupled with authentic porphyrin standards.48
Because of the increasing worldwide problem of antibiotic resistance, there is a serious necessity to discover alternative therapies for infectious diseases.49 Moreover, the use of BLT for infections is of crucial significance for both military and civilian medicine. Currently, antimicrobial resistance could cause wound infections which cannot be cured with current antibiotics. Recently, a dangerous new enzyme (New Delhi metallo-β-lactamase 1), which creates some bacterial resistance to carbapenems (antibiotics administered as a last option when common antibiotics are unsuccessful) was observed in patients in the USA.50 Many clinicians are concerned about rapidly developing microbial wound infections which might not be curable, as was common in the days before the antibiotic era began.50 Consequently, there is a great demand for progress in alternative approaches for various drug-resistant bacteria, to which microbes will not be capable of developing resistance (e.g., antimicrobial BLT). A new non-antibiotic treatment, i.e. aBLT, is attracting increasing attention.51
In their 2022 review article, Leanse et al stated that BLT has the advantage of being more damaging to bacteria than to host cells, which could be in accord with Ehrlich’s principles of the “magic bullet.” Its ability to be combined with other modern or outdated antimicrobial approaches further confirms its probable utility as an antimicrobial treatment. It is obvious that while aBTL is safe and effective in curing contamination and certainly has a bright future, the beneficial window needs more definition as well as confirmation in human studies.52 Wang and Dai stated in their review article that it is generally assumed that the bactericidal property of aBLT can be attributed to the existence of endogenous photosensitizing chromophores inside bacterial cells that create cytotoxic reactive oxygen species after light irradiation. A large number of common bacterial species have been observed to be vulnerable to aBLT inactivation. In vivo investigations have also revealed that infected sites occur where microorganisms are inactivated by aBLT, while host cells are preserved unharmed. The combination of aBLT with additional modalities or medicines could synergistically increase bactericidal efficiency. Future studies should concentrate on assessing aBLT efficiency, clarifying the details of its mechanism, determining the optimum parameters, and translating this method into clinical practice.53
Conclusion
Some studies have shown that the application of BLT on preclinical and clinical models of wound healing in vivo significantly accelerated the healing process; however, few studies have explored the bactericidal effect of BLT on skin injury repair in burn patients. Further preclinical and clinical studies are suggested to aim at attaining a better understanding of the bactericidal effect of BLT using standardized protocols, including different wavelengths of BLT, during the healing course of infected wounds and ulcers in healthy and diabetic animals as well as humans. BLT could be a good option for treating infected chronic wounds, including hard-to-heal diabetic wounds.
Author Contributions
MB and RA introduced the idea. MB, RA, MRH, and SC performed the research. MB wrote the article; RA, MRH, and SC added their comments. MB submitted the article to the journal.
Conflicts of Interests
MRHdeclares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc., Cleveland, OH; Hologenix Inc., Santa Monica, CA; Vielight, Toronto, Canada; JOOVV Inc., Minneapolis-St. Paul, MN; Sunlighten, Kansas City, MO. Consulting: USHIO Corp., Japan; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholding: Niraxx Light Therapeutics, Inc., Irvine CA; JelikaLite Corp., New York, NY. The other authors declare they have no conflicts of interest.
Ethical Considerations
Not applicable.
Funding
RA provided partial support for accessing full text articles for this work. MRH was supported by US NIH Grants R01AI050875 and R21AI121700.
Please cite this article as follows: Bayat M, Albright R, Hamblin MR, Chien S. Impact of blue light therapy on wound healing in preclinical and clinical subjects: A systematic review. J Lasers Med Sci. 2022;13:e69. doi:10.34172/jlms.2022.69.
References
- 1.Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370–8. doi: 10.1111/bjd.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev. 2019;99(1):665–706. doi: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Merchan EC. Surgical wound healing in bleeding disorders. Haemophilia. 2012;18(4):487–90. doi: 10.1111/j.1365-2516.2012.02760.x. [DOI] [PubMed] [Google Scholar]
- 4.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care. 2012;25(7):304–14. doi: 10.1097/01.ASW.0000416006.55218.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–71. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen CK. Human wound and its burden: updated 2020 compendium of estimates. Adv Wound Care (New Rochelle) 2021;10(5):281–92. doi: 10.1089/wound.2021.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowler PG. Antibiotic resistance and biofilm tolerance: a combined threat in the treatment of chronic infections. J Wound Care. 2018;27(5):273–7. doi: 10.12968/jowc.2018.27.5.273. [DOI] [PubMed] [Google Scholar]
- 9.Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32(1):41–52. [PMC free article] [PubMed] [Google Scholar]
- 10.Ablon G. Phototherapy with light emitting diodes: treating a broad range of medical and aesthetic conditions in dermatology. J Clin Aesthet Dermatol. 2018;11(2):21–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Denzinger M, Held M, Krauss S, Knorr C, Memmel C, Daigeler A, et al. Does phototherapy promote wound healing? Limitations of blue light irradiation. Wounds. 2021;33(4):91–8. [PubMed] [Google Scholar]
- 12.Bonnans M, Fouque L, Pelletier M, Chabert R, Pinacolo S, Restellini L, et al. Blue light: friend or foe? J Photochem Photobiol B. 2020;212:112026. doi: 10.1016/j.jphotobiol.2020.112026. [DOI] [PubMed] [Google Scholar]
- 13.Halstead FD, Thwaite JE, Burt R, Laws TR, Raguse M, Moeller R, et al. Antibacterial activity of blue light against nosocomial wound pathogens growing planktonically and as mature biofilms. Appl Environ Microbiol. 2016;82(13):4006–16. doi: 10.1128/aem.00756-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Wang Y, Wang Y, Murray CK, Hamblin MR, Hooper DC, et al. Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resist Updat. 2017;33-35:1–22. doi: 10.1016/j.drup.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shnitkind E, Yaping E, Geen S, Shalita AR, Lee WL. Anti-inflammatory properties of narrow-band blue light. J Drugs Dermatol. 2006;5(7):605–10. [PubMed] [Google Scholar]
- 16.Plavskii VY, Mikulich AV, Tretyakova AI, Leusenka IA, Plavskaya LG, Kazyuchits OA, et al. Porphyrins and flavins as endogenous acceptors of optical radiation of blue spectral region determining photoinactivation of microbial cells. J Photochem Photobiol B. 2018;183:172–83. doi: 10.1016/j.jphotobiol.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Cieplik F, Späth A, Leibl C, Gollmer A, Regensburger J, Tabenski L, et al. Blue light kills Aggregatibacteractinomycetemcomitans due to its endogenous photosensitizers. Clin Oral Investig. 2014;18(7):1763–9. doi: 10.1007/s00784-013-1151-8. [DOI] [PubMed] [Google Scholar]
- 18.Mofazzal Jahromi MA, Sahandi Zangabad P, Moosavi Basri SM, Sahandi Zangabad K, Ghamarypour A, Aref AR, et al. Nanomedicine and advanced technologies for burns: preventing infection and facilitating wound healing. Adv Drug Deliv Rev. 2018;123:33–64. doi: 10.1016/j.addr.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs C, Negri LB, Pham L, Tam J. Light-based devices for wound healing. Curr Dermatol Rep. 2020;9(4):261–76. doi: 10.1007/s13671-020-00309-y. [DOI] [Google Scholar]
- 20.Rahmannia M, Amini A, Chien S, Bayat M. Impact of photobiomodulation on macrophages and their polarization during diabetic wound healing: a systematic review. Lasers Med Sci. 2022;37(7):2805–15. doi: 10.1007/s10103-022-03581-5. [DOI] [PubMed] [Google Scholar]
- 21.Fraccalvieri M, Amadeo G, Bortolotti P, Ciliberti M, Garrubba A, Mosti G, et al. Effectiveness of blue light photobiomodulation therapy in the treatment of chronic wounds. Results of the Blue Light for Ulcer Reduction (B.L.U.R.) Study. Ital J Dermatol Venerol. 2022;157(2):187–94. doi: 10.23736/s2784-8671.21.07067-5. [DOI] [PubMed] [Google Scholar]
- 22.Dini V, Romanelli M, Oranges T, Davini G, Janowska A. Blue light emission in the management of hard-to-heal wounds. Ital J Dermatol Venerol. 2021;156(6):709–13. doi: 10.23736/s2784-8671.20.06691-2. [DOI] [PubMed] [Google Scholar]
- 23.Lu S, Zhang X, Tang Z, Xiao H, Zhang M, Liu K, et al. Mussel-inspired blue-light-activated cellulose-based adhesive hydrogel with fast gelation, rapid haemostasis and antibacterial property for wound healing. Chem Eng J. 2021;417:129329. doi: 10.1016/j.cej.2021.129329. [DOI] [Google Scholar]
- 24.Lv Y, Chen Z, Yang Z, Yang W, Chu W, Tu Y, et al. Evaluation of the red & blue LED effects on cutaneous refractory wound healing in male Sprague-Dawley rat using 3 different multi-drug resistant bacteria. Lasers Surg Med. 2022;54(5):725–36. doi: 10.1002/lsm.23515. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Zhang J, Xu Y, Han Y, Jiang B, Huang L, et al. The histopathological investigation of red and blue light emitting diode on treating skin wounds in Japanese big-ear white rabbit. PLoS One. 2016;11(6):e0157898. doi: 10.1371/journal.pone.0157898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cicchi R, Rossi F, Alfieri D, Bacci S, Tatini F, De Siena G, et al. Observation of an improved healing process in superficial skin wounds after irradiation with a blue-LED haemostatic device. J Biophotonics. 2016;9(6):645–55. doi: 10.1002/jbio.201500191. [DOI] [PubMed] [Google Scholar]
- 27.Nour El Din S, El-Tayeb TA, Abou-Aisha K, El-Azizi M. In vitro and in vivo antimicrobial activity of combined therapy of silver nanoparticles and visible blue light against Pseudomonas aeruginosa. Int J Nanomedicine. 2016;11:1749–58. doi: 10.2147/ijn.s102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Figurová M, Ledecký V, Karasová M, Hluchý M, Trbolová A, Capík I, et al. Histological assessment of a combined low-level laser/light-emitting diode therapy (685 nm/470 nm) for sutured skin incisions in a porcine model: a short report. Photomed Laser Surg. 2016;34(2):53–5. doi: 10.1089/pho.2015.4013. [DOI] [PubMed] [Google Scholar]
- 29.Dungel P, Hartinger J, Chaudary S, Slezak P, Hofmann A, Hausner T, et al. Low level light therapy by LED of different wavelength induces angiogenesis and improves ischemic wound healing. Lasers Surg Med. 2014;46(10):773–80. doi: 10.1002/lsm.22299. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Zhu Y, Gupta A, Huang Y, Murray CK, Vrahas MS, et al. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections. J Infect Dis. 2014;209(12):1963–71. doi: 10.1093/infdis/jit842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheon MW, Kim TG, Lee YS, Kim SH. Low level light therapy by Red–Green–Blue LEDs improves healing in an excision model of Sprague–Dawley rats. Pers Ubiquitous Comput. 2013;17(7):1421–8. doi: 10.1007/s00779-012-0577-3. [DOI] [Google Scholar]
- 32.Dai T, Gupta A, Huang YY, Yin R, Murray CK, Vrahas MS, et al. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob Agents Chemother. 2013;57(3):1238–45. doi: 10.1128/aac.01652-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fushimi T, Inui S, Nakajima T, Ogasawara M, Hosokawa K, Itami S. Green light emitting diodes accelerate wound healing: characterization of the effect and its molecular basis in vitro and in vivo. Wound Repair Regen. 2012;20(2):226–35. doi: 10.1111/j.1524-475X.2012.00771.x. [DOI] [PubMed] [Google Scholar]
- 34.Adamskaya N, Dungel P, Mittermayr R, Hartinger J, Feichtinger G, Wassermann K, et al. Light therapy by blue LED improves wound healing in an excision model in rats. Injury. 2011;42(9):917–21. doi: 10.1016/j.injury.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 35.Soyer T, Ayva S, Aliefendioğlu D, Aktuna Z, Aslan MK, Senyücel MF, et al. Effect of phototherapy on growth factor levels in neonatal rat skin. J Pediatr Surg. 2011;46(11):2128–31. doi: 10.1016/j.jpedsurg.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 36.de Sousa AP, Santos JN, Dos Reis JA Jr, Ramos TA, de Souza J, Cangussú MC, et al. Effect of LED phototherapy of three distinct wavelengths on fibroblasts on wound healing: a histological study in a rodent model. Photomed Laser Surg. 2010;28(4):547–52. doi: 10.1089/pho.2009.2605. [DOI] [PubMed] [Google Scholar]
- 37.Cai W, Hamushan M, Zhang Y, Xu Z, Ren Z, Du J, et al. Synergistic effects of photobiomodulation therapy with combined wavelength on diabetic wound healing in vitro and in vivo. Photobiomodul Photomed Laser Surg. 2022;40(1):13–24. doi: 10.1089/photob.2021.0068. [DOI] [PubMed] [Google Scholar]
- 38.Abe Y, Konno H, Yoshida S, Yamauchi T, Yamasaki K, Denda M, et al. Red light-promoted skin barrier recovery: spatiotemporal evaluation by transepidermal potential. PLoS One. 2019;14(7):e0219198. doi: 10.1371/journal.pone.0219198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleinpenning MM, Smits T, Frunt MH, van Erp PE, van de Kerkhof PC, Gerritsen RM. Clinical and histological effects of blue light on normal skin. Photodermatol Photoimmunol Photomed. 2010;26(1):16–21. doi: 10.1111/j.1600-0781.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- 40.Simpson CR, Kohl M, Essenpreis M, Cope M. Near-infrared optical properties of ex vivo human skin and subcutaneous tissues measured using the Monte Carlo inversion technique. Phys Med Biol. 1998;43(9):2465–78. doi: 10.1088/0031-9155/43/9/003. [DOI] [PubMed] [Google Scholar]
- 41.Dai T, Gupta A, Murray CK, Vrahas MS, Tegos GP, Hamblin MR. Blue light for infectious diseases: propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist Updat. 2012;15(4):223–36. doi: 10.1016/j.drup.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colombo F, de Aguiar Pires Valença Neto A, de Sousa AP, Marchionni AM, Pinheiro AL, de Almeida Reis SR. Effect of low-level laser therapy (λ660 nm) on angiogenesis in wound healing: a immunohistochemical study in a rodent model. Braz Dent J. 2013;24(4):308–12. doi: 10.1590/0103-6440201301867. [DOI] [PubMed] [Google Scholar]
- 43.Rei W, Cheng HY, Sun HQ. Effect of LED red-light radiation on wound healing of combined radiation-trauma injury in a mouse model. J Third Mil Med Univ. 2013;35(10):981–4. [Google Scholar]
- 44.Hamblin MR, Demidova TN. Mechanisms of low level light therapy. Proc SPIE Int Soc Opt Eng. 2006;6140:1–12. doi: 10.1117/12.646294. [DOI] [Google Scholar]
- 45.Ferrer-Espada R, Wang Y, Goh XS, Dai T. Antimicrobial blue light inactivation of microbial isolates in biofilms. Lasers Surg Med. 2020;52(5):472–8. doi: 10.1002/lsm.23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fink D, Romanowski K, Valuckaite V, Babrowski T, Kim M, Matthews JB, et al. Pseudomonas aeruginosa potentiates the lethal effect of intestinal ischemia-reperfusion injury: the role of in vivo virulence activation. J Trauma. 2011;71(6):1575–82. doi: 10.1097/TA.0b013e31821cb7e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strauss WS, Sailer R, Schneckenburger H, Akgün N, Gottfried V, Chetwer L, et al. Photodynamic efficacy of naturally occurring porphyrins in endothelial cells in vitro and microvasculature in vivo. J Photochem Photobiol B. 1997;39(2):176–84. doi: 10.1016/s1011-1344(97)00002-x. [DOI] [PubMed] [Google Scholar]
- 48.Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob Agents Chemother. 2005;49(7):2822–7. doi: 10.1128/aac.49.7.2822-2827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Y, Dai T. Antimicrobial Blue Light to Treat Infections in Mouse Wounds. SPIE; 2015.
- 50. Centers for Disease Control and Prevention (CDC). Detection of Enterobacteriaceae isolates carrying metallo-betalactamase - United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59(24):750. [PubMed]
- 51.Ferrer-Espada R, Liu X, Goh XS, Dai T. Antimicrobial blue light inactivation of polymicrobial biofilms. Front Microbiol. 2019;10:721. doi: 10.3389/fmicb.2019.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leanse LG, Dos Anjos C, Mushtaq S, Dai T. Antimicrobial blue light: a ‘Magic Bullet’ for the 21st century and beyond? Adv Drug Deliv Rev. 2022;180:114057. doi: 10.1016/j.addr.2021.114057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang Y, Dai T. Antimicrobial blue light: a drug-free approach for inactivating pathogenic microbes. In: Light-Based Diagnosis and Treatment of Infectious Diseases. Vol 10479. SPIE; 2018. p. 28-33. 10.1117/12.2283019. [DOI]


