Abstract
Introduction: Bone healing depends on inflammation control and tissue repair time. Low-level laser therapy (LLLT) has been investigated to accelerate this process. Methylene blue (MB), together with LLLT, has been investigated for its antioxidant and anti-inflammatory potential; however, the effects of photosensitizers (photodynamic therapy, PDT) are controversial. This study aimed to verify whether the combination of MB and LLLT changes the course of the consolidation of experimental bone defects.
Methods: Sixteen Wistar rats underwent femoral bone defects. In the control group (n=4), LLLT simulations were performed without MB. The MB group (n=4) received MB and simulation of LLLT. The LLLT group (n=4) was exposed to LLLT. The PDT+LLLT group (n=4) received MB and LLLT. At the end of 7 or 14 days, the animals were euthanized, and samples were collected.
Results: PDT and LLLT induced osteogenic formation with cellularity (after seven days) and union of bony edges (14 days). On the seventh day, LLLT combined with PDT induced an increase (P<0.05) of 484% in the area of bone neoformation compared to the control. On the fourteenth day, LLLT combined with PDT or alone increased (P<0.05) the area of bone neoformation by 214% and 240% respectively, compared to the control group. The PDT/LLLT combination was associated with increased radiopacity (P<0.038).
Conclusion: The combined use of MB with LLLT initiated during the transoperative phase may stimulate the bone repair process in rats.
Keywords: Bone, Laser, Methylene blue, Ossification
Introduction
Orthopedic surgery has been a common procedure in humans and animals to repair the bone discontinuities that occur as a result of fractures caused by high energy impacts, metabolic disorders, gaps resulting from osteotomies, neoplasms, infections, and “union delayed” and “non-union” resulting from complications in the bone healing process.1 In humans, trauma costs in the United States have been estimated at about $56 billion per year, of which 37% are related to the treatment of 7.9 million fractures. Of these, 5%–10% experience delayed healing, and 1% (~100 000) progressed to “non-union”.2 In animals, it is estimated that the largest number of vet visits is related to orthopedic surgery.3
The success of the bone healing process for fractures depends, among other aspects, on the correct reduction and stabilization of bone fragments, control of the inflammatory response, and tissue repair time. To accelerate this process, local filling with biological and/or synthetic materials, such as calcium aluminate cement, hydroxyapatite, bioactive glass autogenous bone grafts, platelet-rich plasma, autologous stem cells, and methylene blue (MB) deposits,4 has been used.5 Additionally, transcutaneous stimulation with low-intensity pulsed ultrasound,6,7 extracorporeal therapy with shock waves,8 and low-level laser therapy (LLLT)9 have been widely investigated.
In vivo experimental models have shown that LLLT stimulates angiogenesis, increases the number of osteoblasts,10 induces better organization of the bone matrix,11 increases bone density,12 and promotes greater expression of collagen,13 greater proliferation of fibroblasts, and activation of osteogenic factors such as TGF-β, FGF2, OPG/RANK, and osteocalcin.14
However, the use of laser therapy associated with a low-toxicity photosensitizer, called photodynamic therapy (PDT), has been controversial. On the one hand, PDT has reported effects including antimicrobials,15 antineoplastics,16 and regulation of bone loss and bone metabolism.17 On the other hand, there is evidence that PDT causes stress and oxidative damage18 and inflammation.19,20
MB (3,7-bis (dimethylamino)-phenothiazin-5-ium chloride), a cyclic aromatic chemical component (C16H18N3SCl1), which is a diaminophenothiazine with low oxide reduction potential, has frequently been used in biological applications.21,22 It has low toxicity and is highly permeable, as it is soluble in water and organic solvents, reaching compartments such as mitochondria, lysosomes, and nuclei.22 MB is an antioxidant23 and anti-inflammatory, and it has been used in several clinical fields for the treatment of acute and chronic methemoglobinemia, carbon monoxide poisoning, urinary tract infection, septic shock, cardiopulmonary bypass,24 and Alzheimer’s disease.25 We hypothesize that MB may be used as a photosensitizer associated with LLLT because it has the property of absorbing light in the wavelength range of 620–660 nm, and at the same time it is able to penetrate deeply into the tissues.26
The objective of this study was to verify whether the association between methylene blue and LLLT modifies the course of the process of consolidation of experimental bone defects in the femur of Wistar rats.
Materials and Methods
Animals and Experimental Groups
Sixteen (N = 16) male Wistar rats (Rattus norvegicus), aged 90 days and weighing between 250 and 300 g, were used. They were kept individually in mini insulators lined with shavings, arranged on ventilated shelves at a controlled temperature of 22 ± 2°C, under a 12 h controlled light/dark cycle, in the Vivarium of the Reproduction Biology Center (CBR) of the Federal University of Juiz de Fora (UFJF), with feed (pelleted in trough) and water (baby bottle) ad libitum. Two bone defects were created in the right femur of each animal, one proximal and the other distal, which were used for the application of the protocols, totaling 32 bone defects.
In the first part of the study (Figure 1A), eight animals underwent surgery to expose the femurs, followed by the creation of 16 proximal (n = 8) and distal (n = 8) bone defects. In four proximal bone defects (n = 4), only a simulation of laser exposure was performed, without methylene blue (control group, GI). MB was added to four distal bone defects (n = 4), and a simulation of laser exposure in the postoperative period (methylene blue group - GII) was performed. Postoperative laser exposure was performed in four proximal bone defects (Group LLLT-GIII) and the remaining four distal bone defects (n = 4) were treated with methylene blue, transoperative laser exposure at the edges of the bone defects (PDT), and laser therapy in the postoperative period (PDT + LLLT group). At the end of seven days, the animals were euthanized, and the samples were collected for analysis. In the second part of the study (Figure 1B), the same procedures described above were performed on the other eight animals; however, euthanasia was performed 14 days after the procedures.
Figure 1.
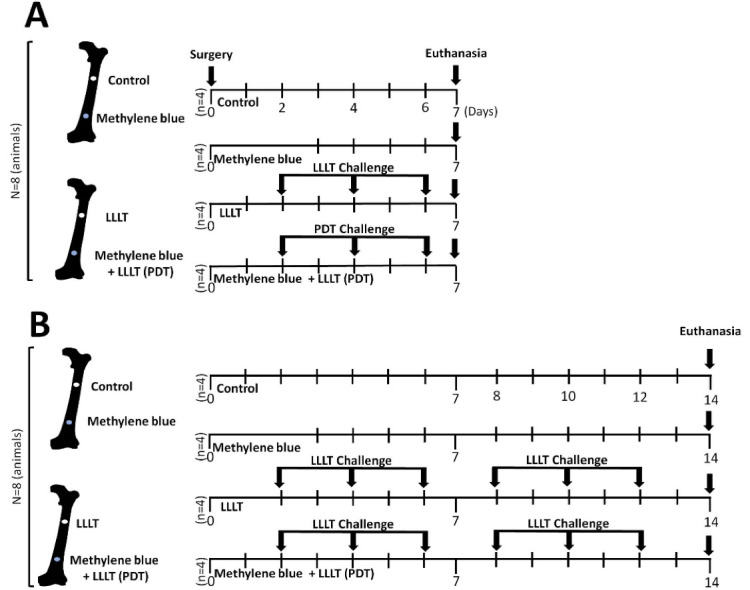
Experimental Timeline of Bone Defects Challenged With Laser and Methylene Blue on Wistar Rat. Control (GI) - Proximal bone defects (n = 4) exposed to only a simulation of laser exposure, without methylene blue. Methylene blue (GII) - Distal bone defects (n = 4) challenged with methylene blue (10 µL to 0.01%) and simulation of laser exposure in the postoperative period. LLLT (GIII) - Proximal bone defects exposed to the laser in the postoperative period. PDT + LLLT (GIV) - Distal bone defects (n = 4) challenged with methylene blue (10 µL to 0.01%), with transoperative laser exposure (PDT), and exposed to postoperative laser therapy. PDT + LLLT (GIV) - Distal bone defects (n = 4) challenged with methylene blue (10 µL to 0.01%), with transoperative laser exposure (PDT), and exposed to postoperative laser therapy. PDT = emission of red (arsenic and gallium) laser, only one point (90 s), pulsatile, λ = 660 nm, 40 mW and total dose of 2.3 J/cm2. LLLT = emission of red (arsenic and gallium) laser, five points, pulsatile, 90 s/point, λ = 660 nm, 40 mW
Surgical Procedure, Bone Defects and Deposition of Methylene Blue
The animals were anesthetized by intraperitoneal injection of ketamine (100 mg/kg), 2% xylazine (10 mg/kg), and fentanyl (0.03 mg/kg). The skin was shaved and disinfected with alcohol 70, and femoral exposure took place from a side view through a longitudinal incision and folding of the femoral quadriceps. Two circular femoral bone defects (200 000 µm2 each) were made on the side of the right femur, one proximal (at 20 mm from the distal femoral end) and the other distal (at 10 mm from the distal femoral end), with a micromotor (Giramatic micro grinder, 3.6 V, 1200 W, 10 000 RPM - Conthey Comércio e Indústria Ltda São Paulo - S, model D1-13233) and a 2-mm dental drill (Figure 2A). The samples that received MB were soaked with 10 µL of a 0.01% methylene blue solution (CHIMIOLUX® Methylene Blue, DMC Import and Export of Equipment LTDA, São Carlos - SP), followed by single transoperative irradiation (Figure 2B). The PDT was carried out with point red light emission using gallium arsenide semiconductors through an optical fiber, at five points (20 mm2) along the femur, at a distance of 0.5 mm, for 90 seconds/point (4 mm2), at an angle of 90° in relation to the femur, at a 660-nm wavelength, with a peak power of 40 mW, and a total dose of 2.3 J/cm2 (MM OPTICS LTDA of São Carlos - SP / TWIN LASER®). The femoral quadriceps muscles and subcutaneous tissue were then sutured in a simple continuous pattern with an absorbable multifilament thread (Polyglactin 910 4-0), and the skin was sutured in a separate simple pattern with a non-absorbable monofilament thread (Nylon 4-0). After surgery, all animals received two daily doses (every 12 hours subcutaneously) of tramadol hydrochloride (5 mg/kg) for four consecutive days. The surgical wound was cleaned daily with saline solution to prevent secondary infection. All surgical procedures were performed by a single professional who had technical training and who was qualified under Brazilian law.
Figure 2.
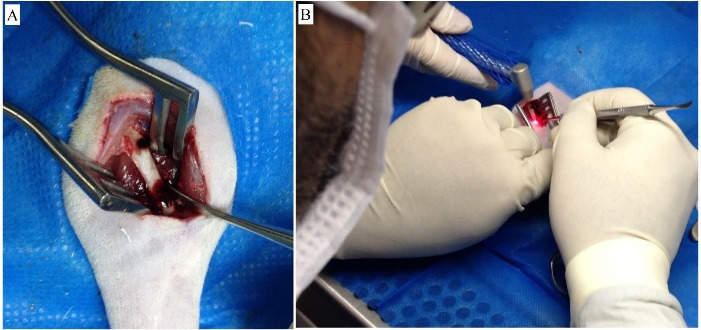
(A) Proximal and distal bone defects induced by a micromotor in femurs of rats (side view). (B) Photodynamic Therapy (PDT) on bone lesions soaked with methylene blue (10 µL to 0.01%) during the operation. PDT = emission of red (arsenic and gallium) laser, only one point (90 s), pulsatile, λ = 660 nm, 40 mW and total dose of 2.3 J/cm2
LLLT Protocol
After the surgery, LLLT was performed transcutaneously on alternate days after 48 hours of the surgery (Figure 1), using the same treatment parameters as for the transoperative irradiation.
Radiographic Evaluation
Femoral radiographs (X-ray SPECTRO 70X Eletronic Class I - Type B - Common, with intermittent operation/DABI ATLANTE Indústrias Médico Odontológicas Ltda, São Paulo, SP, Brazil) were performed in craniocaudal and mid-lateral views on the same day as the surgical procedure after making the bone defects and on the seventh or 14th postoperative day prior to the moment of euthanasia under the effect of anesthetic. The radiographs were obtained using occlusal dental films (DENTUS E-Speed Intra-Oral X-Ray Film 3x4 × Size 2/150 1 AGFA) and were manually developed with an EXSIL MX fixative (Silpachem Indústria e Comércio de Produtos Químicos Ltda.). An aluminum bar containing eight steps (each 0.5 mm high by 0.6 mm wide, following Nascimento et al27 and Ribeiro et al28) was used as a graduation parameter. The parameters of radiodensity were defined from the first four steps of the bar, numbered as follows: (1) absent; (2) mild/discrete; (3) moderate, and (4) advanced. The focal distance was standardized at 40 cm and the incidence of the x-ray beam was 70 kVp, 8 mA and 1 second. The images were analyzed, using a negatoscope with the aid of a magnifying glass.
Euthanasia and Sample Preparations
The animals were euthanized on the seventh (n = 8) or 14th day (n = 8) after surgery through anesthetic overdose with ketamine hydrochloride 10% (200 mg/kg) and xylazine hydrochloride 2% (20 mg/kg), followed by exsanguination and diaphragmatic rupture. The right femurs were disinserted, disarticulated, collected, fixed in formalin (10%), diaphanized, and embedded in paraffin. The tissues were sectioned at 5 µm and stained with hematoxylin and eosin (H&E).
Histomorphometric and Histological Analysis
Histological tissue analysis was performed using optical microscopy (AxioStar Zeiss AxioCam CHF5, Hallbergmoos, Germany) coupled to a camera (AxioCam Vision). A descriptive histological analysis was carried out to identify the following: the presence of inflammation; presence, maturation, and organization of the granulation tissue; presence, maturation, and cellularity of the osteogenic membrane; osteoid deposition activity; presence of trabecular bone; presence of bone with the onset of lamellar formation; presence of mature bone with Haversian systems. Morphometric analyses were performed in 14 consecutive histological fields on the edges of the bone defects. Histological sections were scanned at 400x magnification for semiautomatic quantification of areas of bone neoformation, using the software Zen 2.3 Blue Edition (Zeiss, Germany, 2012). To determine the areas of bone neoformation, each histological slide was manually delimited, and the areas in µm2 were measured from 10 photomicrographs/groups.
Statistical Analysis
Qualitative analysis of the radiodensity of radiographic images was performed, considering the values that showed progressive radiodensity (parameters categorized as 2, 3, and 4) as ‘radiopaque’ and those that did not (categorized parameter 1) as ‘radiolucent’. The Chi-square test was used to verify a possible association between the frequency distribution of the presence or absence of radiopacity or radiolucency and the presence or absence of methylene blue associated with LLLT. The normality of the parametric data was verified using the D’Agostino & Pearson omnibus test. Data are expressed as the simple arithmetic mean ± standard deviation from the mean. The arithmetic means were compared using the Mann-Whitney U test (GraphPad Prism version 5.0, GraphPad Software, San Diego, CA, USA). To interpret and discuss the data and refute or support the hypothesis of the study, a 95% compatibility interval was considered, and the probability value was accepted when P < 0.05.
Results
Histopathological analysis of all groups revealed the presence of new bone formation with varying degrees of maturity and quality (Figure 3). The samples from the control group showed an inflamed lesion area seven days after surgery, with a mixed inflammatory exudate. 25% of the samples exhibited a discrete area of osteogenic membrane on only one of the edges with the beginning of formation of ossification centers. On the 14th day, the presence of inflammatory infiltrate interspersed with granulation tissue was also observed.
Figure 3.
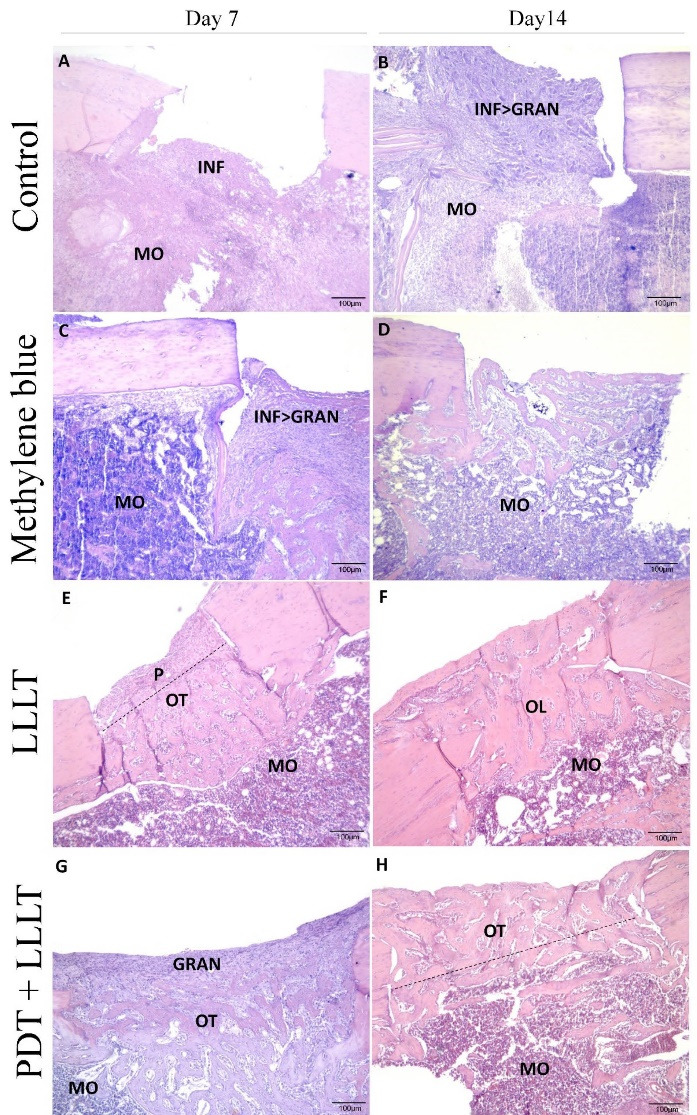
Bone Defects Stained in Hematoxylin and Eosin (5 µm Thick) From Femur Challenged With Laser and Methylene Blue. A: bone defects in the control group at seven days; B: bone defects in the control group at fourteen days; C: bone defects of the methylene blue group at seven days; D: bone defects of the methylene blue group at fourteen days; E: bone defects in the LLLT group at seven days; F: bone defects in the LLLT group at fourteen days; G: bone defects in the LLLT + PDT group at seven days; H: bone defects in the LLLT + PDT group at fourteen days. INF, inflammatory infiltrate; MO, bone marrow; GRAN, granulation tissue; P, periosteum; OT, trabecular bone; OL, lamellar bone
In the histological sections of the groups of the samples that received only MB, regions of bone formation were observed between the edges of the lesion, both seven and 14 days after surgery. However, on the seventh day, the presence of inflammatory infiltrate and granulation tissue was observed in different proportions to those observed on the seventh day of the control group. In 14 days, the bone was organized in lamellae and a reorganization of the bone marrow underlying the bone defect was observed.
Irradiation with the laser not combined with methylene blue induced a filling by immature bone tissue in depth on the seventh day and an external surface covered by mature periosteum. On the 14th day, LLLT induced complete closure of the defect by lamellar bone.
The combination of PDT and LLLT induced the formation of an osteogenic membrane with intense cellularity with already mature areas of trabecular bone, especially on the lateral edges of the lesion on the seventh day. On the 14th day, the wound was closed with a complete union of the edges but still with immature, trabecular bone.
Morphometric analyses showed that on the seventh day after surgery, LLLT combined with PDT induced an increase (P < 0.05) of 484% in the area of bone neoformation (GIV: 20.30 ± 6.574 µm2 × 10-3) when compared to the control group (GI: 4.192 ± 4.366 µm2 × 10-3). The LLLT (GIII: 11.74 ± 4.088 µm2 × 10-3) or methylene blue (GII: 8.024 ± 9.580 µm2 × 10 -3) alone induced increases of 280% and 191% respectively, although these results go beyond the range of compatibility admitted in the present study. On the fourteenth day after surgery, combined LLLT and PDT (GIV: 33.71 ± 5.665 µm2 × 10-3) or LLLT alone (GIII: 37.83 ± 9.448 µm2 × 10-3) increased (p < 0.05) the area of bone neoformation by 214% and 240% respectively when compared to the control group (IG: 15.74 ± 7.782 µm2 × 10 -3). Mb (GII: 18.93 ± 10.51 µm2 × 10-3) alone induced an increase of 120%, although these results go beyond the range of compatibility admitted in the present study (Figure 4).
Figure 4.
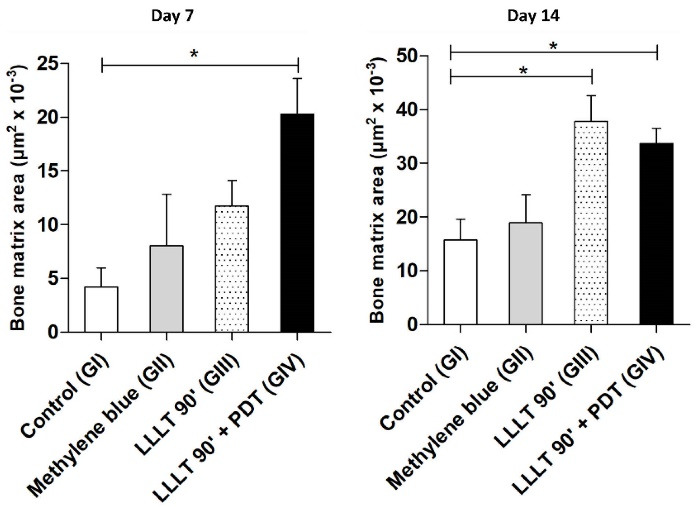
Effects of Laser and Photodynamic Therapy on Bone Neoformation Microareas in Bone Defects in Rat. Control (GI) - Proximal bone defects (n = 4) exposed to only a simulation of laser exposure, without methylene blue. Methylene blue (GII) - Distal bone defects (n = 4) challenged with methylene blue (10 µL to 0.01%) and simulation of laser exposure in the postoperative period. LLLT (GIII) - Proximal bone defects exposed to the laser in the postoperative period. PDT + LLLT (GIV) - Distal bone defects (n = 4) challenged with methylene blue (10 µL to 0.01%), with transoperative laser exposure (PDT), and exposed to postoperative laser therapy. PDT = emission of red (Arsenic and Gallium) laser, only one point (90s), pulsatile, λ = 660nm, 40mW and total dose of 2.3 J/cm2. LLLT = emission of red (arsenic and gallium) laser, 5 points, pulsatile, 90 s/point, λ = 660 nm, 40 mW. Was used a Kruskal-Wallis followed by the Dunn’s post hoc test. In all instances, significance levels were set at 5%. *P < 0.05 compared to the control group (n = 8 per group). PDT, photodynamic therapy
From the radiographic point of view, none of the bone defects had been completely consolidated (Figure 5), with all showing some aspect of translucency. The frequency distribution of the number of bone defects that were exposed to LLLT, combined with PDT or not, according to the radiographic aspect is shown in Table 1. We show that the PDT/LLLT combination was associated with increased radiopacity (P < 0.038).
Figure 5.
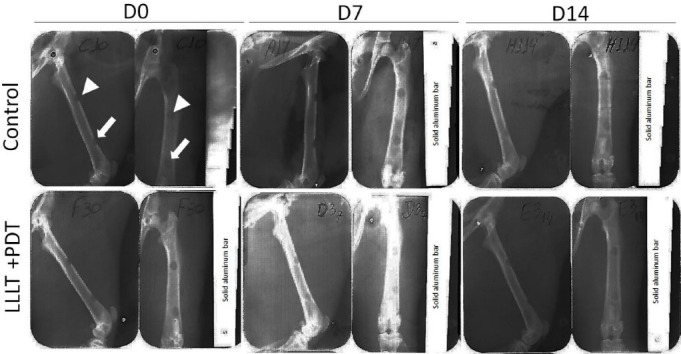
Effects of Low-Level Laser and Photodynamic Therapy on Bone Defects Aspect Radiographic in Wistar Rat. White arrowhead - proximal bone defects, without methylene blue; White arrow - bone defects with methylene blue. ‘D0’ - day when bone defects were performed in the right femurs, methylene blue was administered (10 µL to 0.01%) and LLLT treatment was performed during the operation (PDT - Photodynamic Therapy). ‘D7’ and ‘D14’ - indicate, respectively, the seventh and 14th days after surgery (euthanasia). Radiographic images were taken at a distance (focus/film) = 40cm, mid-lateral and cranio-caudal view, at 70 kV, at 8 mA, for 1.0 s (Spectro 70X Electronic Class I, Type B. Dentus E-Speed). PDT = emission of red (arsenic and gallium) laser, only one point (90 s), pulsatile, λ = 660 nm, 40 mW and total dose of 2.3 J/cm2. LLLT = emission of red (arsenic and gallium) laser, five points, pulsatile, 90 s/point, λ = 660 nm, 40 mW.
Table 1. Frequency Distribution of the Sample Number (Bone Defects) vs. Exposure to LLLT or Photodynamic Therapy Per Aspect Radiographics .
| Models of Therapy | Hyperlucency | Radio-opaque | P |
| LLLT | 5 | 3 | 0.038 |
| PDT + LLLT | 1 | 7 |
PDT, Photodynamic Therapy;LLLT, low level light therapy
Note: PDT, Photodynamic Therapy; LLLT, low level light therapy (emission of red (Arsenic and Gallium) laser, only one point (90s), pulsatile, λ = 660nm, 40mW and total dose of 2.3 J/cm2. Proximal bone defects were exposed to the laser in the postoperative period. Distal bone defects were challenged (PDT + LLLT) with methylene blue (10 μL to 0.01%), with transoperative laser exposure (PDT), and exposed to postoperative laser therapy. For statistical analysis was used Chi Square test (χ2); CI - confidence interval equal 95%; Degrees of freedom (df) = n-1.
For statistical analysis was used chi-square test.
Discussion
This study demonstrated that the LLLT associated with PDT, still in the transoperative phase, resulted in a greater presence of bone neoformation micro-areas after seven days of bone defect induction, while still in the presence of inflammatory cells. After 14 days, both LLLT/PDT and LLLT alone were associated with proportional elevations of the newly formed bone micro-areas.
The biological effects induced by laser photomodulatory stimuli have been investigated since the 1960s.29 It has been shown that the laser promotes upregulation of transcription factors linked to cell proliferation cycles, such as MAPK11, second messengers with Ca + +, endothelial and vascular growth factor, apoptosis, respiratory chain, collagen synthesis, and increase in the number and size of mitochondria.30 Anti-inflammation,31 immunomodulation,32 and action on oxide-reduction balance have also been reported.33
Yamada demonstrated that LLLT was able to induce proliferation and differentiation of osteoblastic cells in vitro.34 The consideration of this type of evidence and the recurrent investigation of the effects of lasers on experimental bone healing11,35 led us to test the hypothesis that LLLT could have an effect enhanced by the biomodulatory properties of methylene blue.
In the present study, we did not test the potential antioxidant effects of MB. However, it has already been demonstrated that MB is capable of suppressing the production of superoxide anion acting as an alternative receptor of electrons from xanthine oxidase and NADPH-oxidase32 and that MB is able to facilitate the transport of electrons in the mitochondria, reducing the production of mitochondrial superoxide.24 There is evidence that the control of oxidative stress by MB results in an increase in collagen.33 The limitation of the experimental design of the present study did not allow us to determine whether the antioxidant effect of MB influenced the results. If this occurred, we believe that combined with LLLT, MB may have favored an early osteogenic response (especially coinciding with the seventh experimental day). Bone lesions would therefore be under less attack by free radicals and oxidative stress. New studies may help to clarify these speculations.
The MB/LLLT combination on the seventh postoperative day revealed the mature areas of osteogenic membrane, especially at the edges of the lesion, at the same time that there was still preservation of cellularity with a mixed pattern in the foci of inflammation. There is therefore some evidence that MB has anti-inflammatory properties, with a potential effect on reducing caspase-1 activity.24 On the other hand, the presence of granulation tissue at this stage was also observed in the bone defects of the tibia of Wistar rats treated with low-power laser.36
We were unable to explain these findings with the resources used in the present study. Interestingly, a recent study showed, in an experimental model, that the high presence of cellularity on the seventh day after injury to the marrow of rats may not necessarily be associated with a negative effect. In this case, the low-power laser facilitated the activation of microgliocytes/macrophage polarization still present in the inflammatory process, accompanied by an increase in IL4 and IL13, an increase in the number of M2 cells, a reduction in the number of M1 cells, and a reduction in the expression of induced nitric oxide synthase, resulting in late functional recovery.32
The lack of parameters to reinforce the effectiveness of the laser seems to be supported by the diversity of the experimental models. The problem begins with the choice of the wavelength to be tested (from 248 to 10 600 nm), as can be seen from the diversity of experimental designs for the realization of bone defects5,9,29,35,37,38
The bone mineralization status of the femur of Wistar rats can differ according to the method used for radiodensity analysis. Nascimento et al,27 Ribeiro et al,28 and Briteño-Vázquez et al35 digitally assessed radiodensity, whereas Barbosa et al39 evaluated the femurs after removal from the animal’s body without interference from soft tissues. In the present study, the negatoscope analyses were carried out by an observer with clinical training in the area, similar to what occurs in clinical practice.
Considering the diversity of techniques used in radiographic investigations, we sought to study the association between the presence or absence of radiopacity and the combined use of LLLT. We showed that the use of LLLT was associated with radiopacity. These findings suggest that, if meticulously evaluated by a specialized professional, radiographs can serve as an important tool even on the seventh day of bone injury, although this is a controversial issue. Hoerth et al12 found that it is only possible to observe a high degree of mineralization in the bone callus formed twenty-one days after bone repair.
However, LLLT has been mentioned as a technique capable of promoting the beginning of tissue repair and improvement in its organization,11,12,10,13 as well as stimulates osteoblast cells for improved bone formation.40 Our samples showed a histopathological aspect with anticipation of bone repair, we sought to confirm this precisely41 and corroborate the literature, mainly supported by histomorphometric techniques.36
Conclusion
The results suggest that the combined use of PDT with the laser in the transoperative phase, particularly the MB and LLLT combined, can serve as a stimulus to begin the bone repair process, especially stimulating osteogenesis. Further studies may establish whether this strategy is promising as a prophylactic measure for surgically treated bone injuries.
Acknowledgments
The authors thank the Center for Reproductive Biology—CRB, Federal University of Juiz de Fora, Juiz de Fora, Minas Gerais, Brazil, for technical support in the care and handling of animals in the biotery.
Authors’ Contribution
Conceptualization: Fabiano Luiz Dulce de Oliveira; Akinori Cardozo Nagato; Fernando Monteiro Aarestrup; Beatriz Julião Vieira Aarestrup.
Methodology: Fabiano Luiz Dulce de Oliveira; Beatriz Julião Vieira Aarestrup
Validation: Fabiano Luiz Dulce de Oliveira; Akinori Cardozo Nagato; Fernando Monteiro Aarestrup; Beatriz Julião Vieira Aarestrup.
Formal Analysis: Fabiano Luiz Dulce de Oliveira; Akinori Cardozo Nagato; Fernando Monteiro Aarestrup; Beatriz Julião Vieira Aarestrup.
Investigation: Fabiano Luiz Dulce de Oliveira; Akinori Cardozo Nagato; Fernando Monteiro Aarestrup; Beatriz Julião Vieira Aarestrup.
Resources: Fabiano Luiz Dulce de Oliveira; Akinori Cardozo Nagato; Fernando Monteiro Aarestrup; Beatriz Julião Vieira Aarestrup.
Data Curation: Fabiano Luiz Dulce de Oliveira; Akinori Cardozo Nagato; Beatriz Julião Vieira Aarestrup.
Writing—Original Draft Preparation: Fabiano Luiz Dulce de Oliveira; Akinori Cardozo Nagato; Beatriz Julião Vieira Aarestrup.
Writing—Review and Editing: Fabiano Luiz Dulce de Oliveira; Akinori Cardozo Nagato; Fernando Monteiro Aarestrup; Beatriz Julião Vieira Aarestrup.
Visualization: Fabiano Luiz Dulce de Oliveira; Akinori Cardozo Nagato; Fernando Monteiro Aarestrup; Beatriz Julião Vieira Aarestrup.
Supervision: Fabiano Luiz Dulce de Oliveira; Fernando Monteiro Aarestrup; Beatriz Julião Vieira Aarestrup.
Project Administration: Fabiano Luiz Dulce de Oliveira; Beatriz Julião Vieira Aarestrup.
Funding Acquisition: Fabiano Luiz Dulce de Oliveira; Akinori Cardozo Nagato; Fernando Monteiro Aarestrup; Beatriz Julião Vieira Aarestrup.
Availability of Data and Materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Conflicts of Interests
The authors declare that they have no competing interests.
Ethics Considerations
All authors declare that all procedures comply with the ethical standards of the national guides on the care and use of laboratory animals of CONCEA (Conselho Nacional de Controle de Experimentação Animal) of Brazil. All experimental methods were approved by the Ethics Committee on the Use of Animals of the Federal University ofJuiz de Fora and registered under number 033/2016.
Funding
This work was supported by grants from Redes Mineiras de Bioterismo e Toxicologia – TOXIFAR from the Fundação de Amparo à Pesquisa de Minas Gerais–FAPEMIG.
Please cite this article as follows: de Oliveira FLD, Nagato AC, Monteiro Aarestrup F, Vieira Aarestrup BJ. Bone neoformation induced by low-level laser and methylene blue suggests early ossification in rats. J Lasers Med Sci. 2022;13:e48. doi:10.34172/jlms.2022.48.
References
- 1.Ramadanov N, Toma I, Herkner H, Klein R, Behringer W, Matthes G. Factors that influence the complications and outcomes of femoral neck fractures treated by cannulated screw fixation. Sci Rep. 2020;10(1):758. doi: 10.1038/s41598-020-57696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buza JA 3rd, Einhorn T. Bone healing in 2016. Clin Cases Miner Bone Metab. 2016;13(2):101–5. doi: 10.11138/ccmbm/2016.13.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitehair JG, Vasseur PB. Fractures of the femur. Vet Clin North Am Small Anim Pract. 1992;22(1):149–59. doi: 10.1016/s0195-5616(92)50010-9. [DOI] [PubMed] [Google Scholar]
- 4.Souza EQM, Costa Klaus AE, Espósito Santos BF, Carvalho da Costa M, Ervolino E, Coelho de Lima D, et al. Evaluations of hydroxyapatite and bioactive glass in the repair of critical size bone defects in rat calvaria. J Oral Biol Craniofac Res. 2020;10(4):422–9. doi: 10.1016/j.jobcr.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis G. Effect of methylene blue on the fracture toughness of acrylic bone cement. Biomaterials. 1994;15(12):1024–8. doi: 10.1016/0142-9612(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 6.Farkash U, Bain O, Gam A, Nyska M, Sagiv P. Low-intensity pulsed ultrasound for treating delayed union scaphoid fractures: case series. J Orthop Surg Res. 2015;10:72. doi: 10.1186/s13018-015-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leighton R, Watson JT, Giannoudis P, Papakostidis C, Harrison A, Steen RG. Healing of fracture nonunions treated with low-intensity pulsed ultrasound (LIPUS): a systematic review and meta-analysis. Injury. 2017;48(7):1339–47. doi: 10.1016/j.injury.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Kieves NR, MacKay CS, Adducci K, Rao S, Goh C, Palmer RH, et al. High energy focused shock wave therapy accelerates bone healing A blinded, prospective, randomized canine clinical trial. Vet Comp Orthop Traumatol. 2015;28(6):425–32. doi: 10.3415/vcot-15-05-0084. [DOI] [PubMed] [Google Scholar]
- 9.Aurégan JC, Coyle RM, Danoff JR, Burky RE, Akelina Y, Rosenwasser MP. The rat model of femur fracture for bone and mineral research: an improved description of expected comminution, quantity of soft callus and incidence of complications. Bone Joint Res. 2013;2(8):149–54. doi: 10.1302/2046-3758.28.2000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mostafavinia A, Masteri Farahani R, Abbasian M, Vasheghani Farahani M, Fridoni M, Zandpazandi S, et al. Effect of pulsed wave low-level laser therapy on tibial complete osteotomy model of fracture healing with an intramedullary fixation. Iran Red Crescent Med J. 2015;17(12):e32076. doi: 10.5812/ircmj.32076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bossini PS, Rennó AC, Ribeiro DA, Fangel R, Ribeiro AC, de Assis Lahoz M, et al. Low level laser therapy (830nm) improves bone repair in osteoporotic rats: similar outcomes at two different dosages. Exp Gerontol. 2012;47(2):136–42. doi: 10.1016/j.exger.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Hoerth RM, Kerschnitzki M, Aido M, Schmidt I, Burghammer M, Duda GN, et al. Correlations between nanostructure and micromechanical properties of healing bone. J Mech Behav Biomed Mater. 2018;77:258–66. doi: 10.1016/j.jmbbm.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Tim CR, Bossini PS, Kido HW, Malavazi I, von Zeska Kress MR, Carazzolle MF, et al. Effects of low-level laser therapy on the expression of osteogenic genes during the initial stages of bone healing in rats: a microarray analysis. Lasers Med Sci. 2015;30(9):2325–33. doi: 10.1007/s10103-015-1807-5. [DOI] [PubMed] [Google Scholar]
- 14.de Oliveira LSS, de Araújo AA, de Araújo Júnior RF, Barboza CAG, Borges BCD, da Silva JSP. Low-level laser therapy (780 nm) combined with collagen sponge scaffold promotes repair of rat cranial critical-size defects and increases TGF-β, FGF-2, OPG/RANK and osteocalcin expression. Int J Exp Pathol. 2017;98(2):75–85. doi: 10.1111/iep.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosa LP, da Silva FC, Nader SA, Meira GA, Viana MS. Antimicrobial photodynamic inactivation of Staphylococcus aureus biofilms in bone specimens using methylene blue, toluidine blue ortho and malachite green: an in vitro study. Arch Oral Biol. 2015;60(5):675–80. doi: 10.1016/j.archoralbio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Rosa LP, da Silva FC, Vieira RL, Tanajura BR, da Silva Gusmão AG, de Oliveira JM, et al. Application of photodynamic therapy, laser therapy, and a cellulose membrane for calcaneal pressure ulcer treatment in a diabetic patient: a case report. Photodiagnosis Photodyn Ther. 2017;19:235–8. doi: 10.1016/j.pdpdt.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Theodoro LH, Longo M, Novaes VCN, Miessi DMJ, Ferro-Alves ML, Ervolino E, et al. Low-level laser and antimicrobial photodynamic therapy on experimental periodontitis in rats submitted to chemotherapy by 5-fluorouracil. Support Care Cancer. 2017;25(10):3261–71. doi: 10.1007/s00520-017-3738-0. [DOI] [PubMed] [Google Scholar]
- 18.Dos Santos AF, Terra LF, Wailemann RA, Oliveira TC, de Morais Gomes V, Mineiro MF, et al. Methylene blue photodynamic therapy induces selective and massive cell death in human breast cancer cells. BMC Cancer. 2017;17(1):194. doi: 10.1186/s12885-017-3179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barathan M, Mariappan V, Shankar EM, Abdullah BJ, Goh KL, Vadivelu J. Hypericin-photodynamic therapy leads to interleukin-6 secretion by HepG2 cells and their apoptosis via recruitment of BH3 interacting-domain death agonist and caspases. Cell Death Dis. 2013;4(6):e697. doi: 10.1038/cddis.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo VC, Akens MK, Wise-Milestone L, Yee AJ, Wilson BC, Whyne CM. The benefits of photodynamic therapy on vertebral bone are maintained and enhanced by combination treatment with bisphosphonates and radiation therapy. J Orthop Res. 2013;31(9):1398–405. doi: 10.1002/jor.22373. [DOI] [PubMed] [Google Scholar]
- 21. Schirmer RH, Adler H, Pickhardt M, Mandelkow E. “Lest we forget you--methylene blue...”. Neurobiol Aging 2011;32(12):2325.e7-16. 10.1016/j.neurobiolaging.2010.12.012. [DOI] [PubMed]
- 22.Xiong ZM, O’Donovan M, Sun L, Choi JY, Ren M, Cao K. Anti-aging potentials of methylene blue for human skin longevity. Sci Rep. 2017;7(1):2475. doi: 10.1038/s41598-017-02419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bozkurt B, Dumlu EG, Tokac M, Ozkardes AB, Ergin M, Orhun S, et al. Methylene blue as an antioxidant agent in experimentally-induced injury in rat liver. Bratisl Lek Listy. 2015;116(3):157–61. doi: 10.4149/bll_2015_032. [DOI] [PubMed] [Google Scholar]
- 24.Ahn H, Kang SG, Yoon SI, Ko HJ, Kim PH, Hong EJ, et al. Methylene blue inhibits NLRP3, NLRC4, AIM2, and non-canonical inflammasome activation. Sci Rep. 2017;7(1):12409. doi: 10.1038/s41598-017-12635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atamna H, Kumar R. Protective role of methylene blue in Alzheimer’s disease via mitochondria and cytochrome c oxidase. J Alzheimers Dis. 2010;20 Suppl 2:S439–52. doi: 10.3233/jad-2010-100414. [DOI] [PubMed] [Google Scholar]
- 26.Veeranarayanan S, Mohamed MS, Poulose AC, Rinya M, Sakamoto Y, Maekawa T, et al. Photodynamic therapy at ultra-low NIR laser power and X-Ray imaging using Cu3BiS3 nanocrystals. Theranostics. 2018;8(19):5231–45. doi: 10.7150/thno.25286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nascimento SB, Cardoso CA, Ribeiro TP, Almeida JD, Albertini R, Munin E, et al. Effect of low-level laser therapy and calcitonin on bone repair in castrated rats: a densitometric study. Photomed Laser Surg. 2010;28(1):45–9. doi: 10.1089/pho.2008.2396. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro DA, Paiotti AP, Medalha CC. Dual role of cyclooxygenase-2 during tissue repair induced by low level laser therapy: an intriguing issue. J Cosmet Laser Ther. 2012;14(4):184–8. doi: 10.3109/14764172.2012.685479. [DOI] [PubMed] [Google Scholar]
- 29.Moskvin SV. Low-level laser therapy in Russia: history, science and practice. J Lasers Med Sci. 2017;8(2):56–65. doi: 10.15171/jlms.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Lasers Med Sci. 2014;5(2):58–62. [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JH, Chiang MH, Chen PH, Ho ML, Lee HE, Wang YH. Anti-inflammatory effects of low-level laser therapy on human periodontal ligament cells: in vitro study. Lasers Med Sci. 2018;33(3):469–77. doi: 10.1007/s10103-017-2376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song JW, Li K, Liang ZW, Dai C, Shen XF, Gong YZ, et al. Low-level laser facilitates alternatively activated macrophage/microglia polarization and promotes functional recovery after crush spinal cord injury in rats. Sci Rep. 2017;7(1):620. doi: 10.1038/s41598-017-00553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tatmatsu-Rocha JC, Ferraresi C, Hamblin MR, Damasceno Maia F, do Nascimento NR, Driusso P, et al. Low-level laser therapy (904nm) can increase collagen and reduce oxidative and nitrosative stress in diabetic wounded mouse skin. J Photochem Photobiol B. 2016;164:96–102. doi: 10.1016/j.jphotobiol.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada K. Biological effects of low power laser irradiation on clonal osteoblastic cells (MC3T3-E1) Nihon Seikeigeka Gakkai Zasshi. 1991;65(9):787–99. [PubMed] [Google Scholar]
- 35.Briteño-Vázquez M, Santillán-Díaz G, González-Pérez M, Gallego-Izquierdo T, Pecos-Martín D, Plaza-Manzano G, et al. Low power laser stimulation of the bone consolidation in tibial fractures of rats: a radiologic and histopathological analysis. Lasers Med Sci. 2015;30(1):333–8. doi: 10.1007/s10103-014-1673-6. [DOI] [PubMed] [Google Scholar]
- 36.Tim CR, Bossini PS, Kido HW, Malavazi I, von Zeska Kress MR, Carazzolle MF, et al. Low-level laser therapy induces an upregulation of collagen gene expression during the initial process of bone healing: a microarray analysis. J Biomed Opt. 2016;21(8):88001. doi: 10.1117/1.jbo.21.8.088001. [DOI] [PubMed] [Google Scholar]
- 37.Alves AMM, de Miranda Fortaleza LM, Filho A, Ferreira DCL, da Costa CLS, Viana VGF, et al. Evaluation of bone repair after application of a norbixin membrane scaffold with and without laser photobiomodulation (λ 780 nm) Lasers Med Sci. 2018;33(7):1493–504. doi: 10.1007/s10103-018-2506-9. [DOI] [PubMed] [Google Scholar]
- 38.Jiang C, Yang W, Wang C, Qin W, Ming J, Zhang M, et al. Methylene blue-mediated photodynamic therapy induces macrophage apoptosis via ROS and reduces bone resorption in periodontitis. Oxid Med Cell Longev. 2019;2019:1529520. doi: 10.1155/2019/1529520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbosa D, de Souza RA, Xavier M, da Silva FF, Arisawa EA, Villaverde AG. Effects of low-level laser therapy (LLLT) on bone repair in rats: optical densitometry analysis. Lasers Med Sci. 2013;28(2):651–656. doi: 10.1007/s10103-012-1125-0. [DOI] [PubMed] [Google Scholar]
- 40.Jawad MM, Husein A, Azlina A, Alam MK, Hassan R, Shaari R. Effect of 940 nm low-level laser therapy on osteogenesis in vitro. J Biomed Opt. 2013;18(12):128001. doi: 10.1117/1.jbo.18.12.128001. [DOI] [PubMed] [Google Scholar]
- 41.Sella VR, do Bomfim FR, Machado PC, da Silva Morsoleto MJ, Chohfi M, Plapler H. Effect of low-level laser therapy on bone repair: a randomized controlled experimental study. Lasers Med Sci. 2015;30(3):1061–8. doi: 10.1007/s10103-015-1710-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


