1. Introduction
The substance use crisis in the United States is dynamic and currently characterized as a “Third Wave” of increasing polysubstance use involving both opioids and stimulants.1,2 Approximately 85% of overdose deaths involve illicitly manufactured fentanyl, heroin, cocaine, or methamphetamine with a combination of two or more involved in a majority of these deaths.3 In a national sample of people with opioid use disorder (OUD) from 2015 to 2017, past-month methamphetamine use increased significantly from 6.2% to 19.1% for those using heroin and 3.8% to 7.9% for those using prescription opioids.4 In a study of people seeking OUD treatment over approximately the same period, the number of days of cocaine and amphetamine use doubled.5 Among people with past-year OUD, stimulant use disorders are frequently co-diagnosed. Specifically, 12.5% and 10.6% of people with OUD had co-occurring cocaine and methamphetamine use disorder, respectively.6 In a study of veterans, the prevalence of OUD in combination with methamphetamine use disorder increased at a faster rate between 2005 to 2019 compared to other SUD combinations.7
Thus, it is critical to elucidate shared and unique risk factors among people with co-occurring opioid and stimulant use disorders. The social ecological model8, which frames such factors into individual, interpersonal, community and societal levels of intersecting influence, has proven useful in understanding the etiologic underpinnings of the opioid epidemic.9,10 For drug use research, understanding the contribution of potentially modifiable components of the model are critically important given estimates that the risk of substance abuse is 50% genetic which are (presumably) not modifiable.11 These other etiologic components include the social determinants of health (SDOH) which influence health through multiple pathways such as socioeconomic status (SES)12, income13, employment, gender discrimination14, race/ethnicity disparity15, access to health care, educational attainment16,17, neighborhood context18,19, and housing stability.20
The impact of housing stability on people who use opioids and stimulants has emerged as an important topic in the polysubstance use and SDOH literature. Barocas et al. found that people experiencing a fatal overdose involving stimulants were more likely to be homeless compared to overdose from opioids alone.21 A national study found that respondents who used only methamphetamine were more likely to have unstable housing compared to those only using opioids.21,22 A larger proportion of patients with OUD were found to have unstable housing if they also tested positive for amphetamines.23 Likewise, around 30% of people with OUD experiencing homelessness reported stimulant abuse compared to 9% among those with stable housing.24 Emergency department patients with OUD or OUD with amphetamine use were more likely to have unstable housing.25
The social ecological model is amenable to data often only available in electronic medical records (EMRs) from hospital systems. EMRs can provide insights into SDOH and concurrent healthcare utilization at individual and neighborhood resolution.26,27 Obtaining neighborhood-level information, often referred to as area-based measures, for patients can be achieved through linking patient residential locations to US Census Bureau administrative units such as census tracts (e.g., neighborhoods). This linkage enables researchers to append patient data with quantitative neighborhood typologies that capture SDOH context through available single (e.g., poverty status) and combination measures (e.g., the CDC’s Social Vulnerability Index)28–30. Taken together, these measures can constitute a more informative social ecological model to help guide prevention and mitigation of SUDs.31
Thus, the objectives of this study are to examine variability in select domains of the social ecological model across cohorts of patients with diagnosed stimulant-only, opioid-only, and co-diagnosed SUDs. We focus attention on identifying variability between the cohorts on the SDOH dimensions of housing instability and neighborhood social context in Kentucky, a rural state with high rates of drug-related mortality and poverty.32
2. Materials and Methods
2.1. Substance use disorder cohorts
Patients were classified into three mutually exclusive cohorts on the basis of their earliest healthcare inpatient or outpatient encounter during the study period using EMRs: those with 1) only stimulant-related diagnosis codes, 2) only opioid-related diagnosis codes, and 3) both stimulant- and opioid-related diagnosis codes (“co-diagnosis”). We obtained EMRs from the University of Kentucky HealthCare (UKHC) system. UKHC is a large healthcare enterprise system that serves central Kentucky with two hospitals, two emergency departments, multiple outpatient clinics and centers, and regional satellite clinics. We included Kentucky resident patients aged ≥18 years old who had opioid or stimulant-related diagnosis codes based on International Classification of Disease Clinical Modification codes version 10 (ICD-10-CM)33 between January 1, 2017 and December 31, 2019. Stimulant-related diagnosis codes included: cocaine use disorders (F14.*), other stimulant use disorders (F15.*), and poisoning by cocaine (T40.5*) and poisoning by psychostimulants (T43.6*). Opioid use disorder diagnosis codes included: opioid-related disorders (F11.*) and opioid-related poisoning codes (T40.0*, T40.1*, T40.2*, T40.3*, T40.4*, T40.6*). For each patient’s first encounter, we obtained institutional EMRs, including demographics, diagnoses, procedures, labs, medications, and notes. This study was approved by the University of Kentucky IRB (#74501).
2.2. Domains of the Social Ecological Model
The individual subdomain demographic variables are conceptualized into demographic and current substance use subdomains. Demographic variables included age (categorized, continuous), race (Black, White, Other) and biological sex (male, female). The vast majority of UKHC data identifying sexual orientation was missing. Likewise, >95% of our data identifying Hispanic/Latino ethnicity was either non-Hispanic/Latino, not divulged or missing. Thus, we did not include these variables in the analysis. Insurance status was categorized as Medicaid, Medicare, other, and self-pay. Educational attainment is a patient-level indicator of SES commonly collected in EMRs.26 In the past decade, lack of educational attainment has been a more pronounced risk factor in mortality because it is associated with drug overdoses which have led to unprecedented declines in life expectancy in the United States.16,34 We categorized education as college/some college, career/technical training, high school, less than high school, unable to assess due to cognitive impairment, and unknown/other/missing.
2.2.1. Individual health variables
We examined mental and physical health disorders/conditions associated with high-risk drug use in the literature35: mental health disorders included alcohol use, attention deficit hyperactivity, major depressive, anxiety, bipolar, and schizophrenia and physical health conditions included HIV, Hepatitis C and A, endocarditis (B37.6, I33.*, I38, I39, A32.82), heart disease (I2-I5 and 41 and 42), and dental disorders (K00-K14). Hepatitis C and A and HIV were identified according to standards from Kentucky’s Injury Prevention Research Center.36 The Charlson Comorbidity index is a numeric measure of co-occurring health conditions frequently used as an indicator of overall health status in populations.37 Non-fatal suicide attempts and intentional self-harm were identified using standards from the National Center for Health Statistics.38
2.2.2. Substance use and toxicology
Laboratory drug tests are ordered for targeted drugs (tetrahydrocannabinol (THC), barbiturates, methadone, cannabinoids, breath alcohol, ethanol, cocaine metabolite, amphetamines, opiates, buprenorphine) and/or for drug abuse related urine/serum/plasma/blood panels. A positive drug test was defined for one of these targeted drugs having a laboratory value greater than zero or a drug name in the result list accompanying a confirmatory test. We did not include test results indicated as “presumptive positives” but considered these records as evidence that a drug screen was conducted. We examined the prevalence of select single and two-way drug combinations based on prevalent drugs in Kentucky’s drug related mortality reports.39 Thus, drugs presented here are not exhaustive. Smoking status was identified from a method using natural language processing on clinical notes developed in a prior study using UKHC EMR data.40
2.3. Geocoding
Patient location data was recorded in the EMRs. Location is pivotal to understanding environmental influences on health; patient-level, geospatial analyses required both access to sensitive data and access to a HIPAA-complaint computing environment. We geocoded the patient’s last-known address to obtain US state of residence, urban/rural status, Appalachian status, geospatial coordinates and census-designated regions. We calculated these using geocoding functions from an “in-house” HIPAA-compliant geodatabase, PostGIS41. PostGIS is an open-source extension adding geospatial objects and analytic functionality to the PostgreSQL42 database; we used wrappers for PostGIS that we developed for testing, geocoding, and mapping to census-designated regions.43,44 The US Census Bureau publishes geographic boundaries for census-designated regions which include, from smaller to larger: blocks, block groups, and tracts. Census tracts are smaller than counties and typically contain between 1,200 to 8,000 people with a reported optimum population of 4,000.45 Census tracts are commonly used to capture neighborhood-level variability in SDOH research.30
We removed spurious details from residential addresses such as suite or apartment numbers, and organizational names. We used address lines with the best road-like details. After computing the coordinates of the cleaned addresses, the geometries published by the US Census Bureau were used in coordinate intersection calculations to identify census-designated regions. 1.5% of addresses were not geocodable due to incomplete data or the presence of PO Box numbers. Our geocoded records covered all 82 census tracts possible in Fayette County, UKHC’s primary catchment area. UKHC’s secondary catchment area consists of 16 neighboring counties with 107 census tracts total; our records covered 106 of these counties. UKHC’s tertiary catchment area consists of 46 counties in central and eastern Kentucky; we also had patient representation from all 99 tracts within these counties. Overall, our geocoded data set has patients from 81% of all census tracts in Kentucky.
In PostGIS, a rating is given to describe the quality of the geocoding results; 31.7% had a perfect score, meaning the computed coordinates and the street names perfectly matched the PostGIS reference data. Another 46% had ratings indicating very strong matching. 9% had ratings indicating poor matching (~8% of the records had non-specific addresses without street names; for example, some addresses contained only a zip code or only city and state name combinations such as “Lexington, KY”). 4.7% had addresses implying homelessness, housing instability, or transitional living (see below). We classified census tracts as urban (codes 1–3) or rural (codes 4–9) using 2013 Rural Urban Classification Codes.46 Appalachian county-level status was defined by the Appalachian Regional Commission.47
2.4. Housing Instability
Different facets of the EMR data provided evidence that a patient experienced homelessness, housing instability, and/or a transitional living environment. 9.8% of our patient records had ICD-10-CM codes for problems related to housing and/or low-income economic circumstances (Z59*)24. We also leveraged the patient’s address data, which either explicitly stated some variation of the phrase “homeless” or implied housing issues by listing names/addresses for known homeless shelters or places for transitional living.48 Manual review of addresses occurring more than once (5.6% of all addresses) served as a first pass in identification of housing issues; distance calculations from known homeless shelters and housing organizations confirmed the accuracy of patient mappings. The majority of these distances were less than 100 meters. Using address data only, 6.8% of our population experienced some type of housing instability. In total, 14.1% of patients experienced housing instability by combining our ICD-10-CM results and our address data results which overlapped in some instances. Only 10% of patients with housing-related diagnosis codes had addresses implying housing instability; conversely, 18.7% of patients with addresses implying housing instability had matching diagnosis codes.
2.5. Census Tract SDOH data
We examined four core components of SDOH developed by Kolak et. al for census tract level research using 2014 Census data: socioeconomic advantage, limited mobility, urban core opportunity, and mixed immigrant cohesion and accessibility.29 General interpretation of these numeric scores for residents in each census tract are as follows: socioeconomic advantage (lower values have more disadvantage), limited mobility (lower values have less mobility), urban core opportunity (lower values have less opportunity), and mixed immigrant cohesion and accessibility (lower values have more multilingual families, traditional family structures, and/or accessibility stressors).29 These data were downloaded from the U.S. Social Determinants of Health Atlas49. Additionally, we examined the four themes of CDC’s 2014 Social Vulnerability Index (SVI) which has been used in injection drug use focused research: socioeconomic, household composition, minority status and language, housing type and transportation.50,51 We used 2014 scores as a direct comparison to Kolak’s timeframe, but more recent SVI years are available. For the SVI, a percentile value closer to 1 indicates higher vulnerability and tracts below the 90th percentile are set to zero.
For descriptive analyses, we used Chi-square tests for categorical variables and t-tests for quantitative variables in pairwise testing. We used an Upset plot to examine and demonstrate the high dimensional nature of polysubstance use in our co-diagnosis cohort.52,53 We used an adjusted, multinomial logistic regression to produce adjusted odds ratios (aORs) and 95% confidence intervals (CIs) to examine unstable housing and unique/shared factors associated with cohort membership using the opioid-only cohort as the reference group. To examine the association between the proportional distribution of the cohorts within census tracts and the eight SDOH indices, we conducted unadjusted linear regressions for census tracts in UKHC’s primary and secondary catchment areas (198 census tracts). All analyses were conducted in SAS software v9.4 for Windows (SAS Institute Inc, Cary, NC).
3. Results
Table 1. There were 14,032 patients included in the study. The stimulant-only, opioid-only, and co-diagnosis cohorts had 4,794, 7,385, and 1,853 patients, respectively. Table 1 shows the demographic characteristics of each cohort. Patients in the co-diagnosis cohort tended to be younger (36.3 y.o.) with the highest proportion of Medicaid recipients (78.4%). Black patients were more prevalently represented in the stimulant-only cohort (19.2%), as were residences in urban classified census tracts (62.3%) and non-Appalachian counties (67.8%). The race composition of the stimulant-only cohort was similar to Census Bureau data from UKHC’s primary catchment area (16% Black, 72% White). Patients in the opioid-only cohort tended to be older (42.7 y.o.) with a higher percentage of encounters covered by Medicare insurance (21.6%). The highest prevalence of smoking was observed in the co-diagnosis cohort (72.2%). Housing was unstable for 26.4% of the co-diagnosed cohort followed by the stimulant-only (17.6%) and the opioid-only (8.8%) cohorts. The co-diagnosed cohort had the largest percentages of high school completion (55.6%) and college/some college (10.5%) graduates.
Table 1.
Demographic and other individual domain characteristics by drug use cohorts in the University of Kentucky Healthcare System, 2017 to 2019.
| All | % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| STIMULANT-ONLY | OPIOID-ONLY | CO-DX | |||||||||
| N | % | p | N | % | N | % | P | p | N | ||
| AGE GROUP | |||||||||||
| 18–24 | 546 | 11.4 | *** | 548 | 7.4 | 200 | 10.8 | *** | *** | 1,294 | 9.2 |
| 25–34 | 1,159 | 24.2 | 2,219 | 30.0 | 700 | 37.8 | 4,078 | 29.1 | |||
| 35–44 | 1,275 | 26.6 | 1,673 | 22.7 | 585 | 31.6 | 3,533 | 25.2 | |||
| 45–54 | 1,054 | 22.0 | 1,216 | 16.5 | 261 | 14.1 | 2,531 | 18.0 | |||
| 55–64 | 619 | 12.9 | 1,004 | 13.6 | 97 | 5.2 | 1,720 | 12.3 | |||
| 65+ | 141 | 2.9 | 725 | 9.8 | 10 | 0.5 | 876 | 6.2 | |||
| Age (mean, SD) | 40.5 | (12.7) | *** | 42.7 | (15.1) | 36.3 | (10.1) | *** | 41.1 | ||
| RACE | |||||||||||
| BLACK | 922 | 19.2 | *** | 341 | 4.6 | 89 | 4.8 | 0.003 | *** | 1,352 | 9.6 |
| WHITE | 3,801 | 79.3 | 6,877 | 93.1 | 1,749 | 94.4 | 12,427 | 88.6 | |||
| OTHER | 71 | 1.5 | 167 | 2.3 | 15 | 0.8 | 253 | 1.8 | |||
| FEMALE | 2,020 | 42.1 | *** | 4,035 | 54.6 | 904 | 48.8 | *** | *** | 6,959 | 49.6 |
| EDUCATION | |||||||||||
| COLLEGE/SOME COLLEGE | 358 | 7.5 | *** | 719 | 9.7 | 194 | 10.5 | *** | *** | 1,271 | 9.1 |
| CAREER/TECHNICAL | 69 | 1.4 | 114 | 1.5 | 47 | 2.5 | 230 | 1.6 | |||
| HIGHSCHOOL | 2,132 | 44.5 | 2,744 | 37.2 | 1,030 | 55.6 | 5,906 | 42.1 | |||
| LESS THAN HIGHSCHOOL | 291 | 6.1 | 349 | 4.7 | 162 | 8.7 | 802 | 5.7 | |||
| COGNITIVE IMPAIRMENT | 241 | 5.0 | 260 | 3.5 | 112 | 6.0 | 613 | 4.4 | |||
| UNKNOWN/OTHER/MISSING | 1,703 | 35.5 | 3,199 | 43.3 | 308 | 16.6 | 5,210 | 37.1 | |||
| PAYER | |||||||||||
| MEDICAID | 2,901 | 60.5 | *** | 4,331 | 58.6 | 1,453 | 78.4 | *** | *** | 8,685 | 61.9 |
| MEDICARE | 630 | 13.1 | 1,594 | 21.6 | 164 | 8.9 | 2,388 | 17.0 | |||
| OTHER | 832 | 17.4 | 1,093 | 14.8 | 128 | 6.9 | 2,053 | 14.6 | |||
| SELF-PAY | 431 | 9.0 | 367 | 5.0 | 108 | 5.8 | 906 | 6.5 | |||
| URBAN/RURAL STATUS * | |||||||||||
| RURAL | 1,803 | 37.6 | *** | 3,578 | 48.4 | 908 | 49.0 | 0.856 | *** | 6,289 | 44.8 |
| URBAN | 2,987 | 62.3 | 3,801 | 51.5 | 943 | 50.9 | 7,731 | 55.1 | |||
| APPALACHIAN COUNTY | 1,542 | 32.2 | *** | 3,197 | 43.3 | 839 | 45.3 | 0.123 | 5,578 | 39.8 | |
| SMOKING STATUS | |||||||||||
| CURRENT/FORMER | 2,409 | 50.3 | *** | 2,708 | 36.7 | 1,338 | 72.2 | *** | *** | 6,455 | 46.0 |
| UNKNOWN | 1,810 | 37.8 | 3,494 | 47.3 | 340 | 18.3 | 5,644 | 40.2 | |||
| NO | 575 | 12.0 | 1,183 | 16.0 | 175 | 9.4 | 1,933 | 13.8 | |||
| UNSTABLE HOUSING | 842 | 17.6 | *** | 647 | 8.8 | 490 | 26.4 | *** | *** | 1,979 | 14.1 |
| All | 4,794 | 7,385 | 1,853 | 14,032 | |||||||
p-values in in cohort columns represent pairwise comparisons between independent variables (bolded) distributed in the cohort to the distribution in the opioid-only cohort. The last p-value is a test across all three cohorts.. *12 patients with missing urban/rural status.
p<0.05
p<0.001
p<0.0001
Table 2. The opioid-only cohort had lower prevalence of co-occurring alcohol use disorder (7.2%) with an overall prevalence of 11.4%. Approximately, one-third of the stimulant-only and the co-diagnosis cohorts had a diagnosed cocaine use disorder and ICD-code documented cocaine and amphetamine poisoning was highest in the co-diagnosis group (1.3% v 2.3% and 4.6% v 2.9% v 4.6%, respectively). Heroin poisoning was higher in the opioid-only group compared to the co-diagnosis group (7.3% v 4.5%, p<0.0001). Opioid poisoning incidents recorded at the first healthcare encounter were approximately 5 times higher in the opioid-only group compared to the co-diagnosis cohort (18.7% v 3.6%, p<0.0001, respectively).
Table 2.
Select co-occurring substance use disorders, mental and physical health conditions, and drug poisoning events by drug use cohort in University of Kentucky’s Healthcare System, 2017 to 2019.
| ALL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| STIMULANT-ONLY | OPIOID-ONLY | CO-DX | |||||||||
| N | % | p | N | % | N | % | p | p | N | % | |
| MENTAL HEALTH: SUBSTANCE USE DISORDERS | |||||||||||
| ALCOHOL USE DISORDER | 797 | 16.6 | *** | 535 | 7.2 | 266 | 14.4 | *** | *** | 1,598 | 11.4 |
| COCAINE USE DISORDER | 1,807 | 37.7 | n/a | n/a | 565 | 30.5 | n/a | *** | 2,372 | 16.9 | |
| OTHER STIMULANT USE DISORDER | 3,120 | 65.1 | n/a | n/a | 1,450 | 78.3 | n/a | *** | 4,570 | 32.6 | |
| OPIOID USE DISORDER | n/a | n/a | 5,672 | 76.8 | 1,763 | 95.1 | *** | *** | 7,435 | 53.0 | |
| OTHER DISORDERS/INDICATORS | |||||||||||
| ATTENTION DEFICIT HYPERACTIVITY DISORDER | 128 | 2.7 | *** | 61 | 0.8 | 42 | 2.3 | *** | *** | 231 | 1.6 |
| MAJOR DEPRESSIVE DISORDER | 779 | 16.2 | * | 1,347 | 18.2 | 445 | 24.0 | *** | *** | 2,571 | 18.3 |
| ANXIETY DISORDER | 777 | 16.2 | *** | 1,484 | 20.1 | 498 | 26.9 | *** | *** | 2,759 | 19.7 |
| BIPOLAR DISORDER | 332 | 6.9 | *** | 250 | 3.4 | 177 | 9.6 | *** | *** | 759 | 5.4 |
| SCHIZOPHRENIA | 162 | 3.4 | *** | 62 | 0.8 | 55 | 3.0 | *** | *** | 279 | 2.0 |
| SUICIDE ATTEMPT/SELF HARM | 206 | 4.3 | *** | 176 | 2.4 | 111 | 6.0 | *** | *** | 493 | 3.5 |
| PHYSICAL HEALTH: | |||||||||||
| CHARLSON COMORBIDITY INDEX | |||||||||||
| 0 | 2,314 | 48.3 | *** | 3,257 | 44.1 | 885 | 47.8 | *** | *** | 6,456 | 46.0 |
| 1 | 911 | 19.0 | 1,177 | 15.9 | 424 | 22.9 | 2,512 | 17.9 | |||
| 2 | 478 | 10.0 | 631 | 8.5 | 179 | 9.7 | 1,288 | 9.2 | |||
| >=3 | 1,091 | 22.8 | 2,320 | 31.4 | 365 | 19.7 | 3,776 | 26.9 | |||
| HIV | 89 | 1.9 | *** | 35 | 0.5 | 12 | 0.6 | 0.3475 | *** | 136 | 1.0 |
| HEPATITIS C | 622 | 13.0 | *** | 1,326 | 18.0 | 839 | 45.3 | *** | *** | 2,787 | 19.9 |
| HEPATITIS A | 60 | 1.3 | 0.0676 | 67 | 0.9 | 72 | 3.9 | *** | *** | 199 | 1.4 |
| ENDOCARDITIS | 62 | 1.3 | *** | 224 | 3.0 | 283 | 15.3 | *** | *** | 569 | 4.1 |
| HEART DISEASE | 1,198 | 25.0 | *** | 2,092 | 28.3 | 777 | 41.9 | *** | *** | 4,067 | 29.0 |
| DENTAL DISORDERS | 119 | 2.5 | * | 249 | 3.4 | 193 | 10.4 | *** | *** | 561 | 4.0 |
| DRUG POISONING | |||||||||||
| AMPHETAMINE | 139 | 2.9 | n/a | n/a | 86 | 4.6 | n/a | *** | 225 | 1.6 | |
| COCAINE | 64 | 1.3 | n/a | n/a | 43 | 2.3 | n/a | *** | 107 | 0.8 | |
| HEROIN | n/a | n/a | 542 | 7.3 | 83 | 4.5 | *** | *** | 625 | 4.5 | |
| OPIOID | n/a | n/a | 1,379 | 18.7 | 66 | 3.6 | *** | ** | 1,445 | 10.3 | |
| All | 4,794 | 7,385 | 1,853 | 14,032 | |||||||
DX: diagnosed. p-values in in cohort columns represent pairwise comparisons between independent variables (bolded) distributed in the cohort to the distribution in the opioid-only cohort.
The last p-value is a test across all three cohorts.
p<0.05
p<0.001
p<0.0001
The co-diagnosis cohort showed higher prevalence of nearly all of the physical and mental health diagnoses we examined. In particular, Hepatitis C was more than 2.5 times higher in the co-diagnosis group than in the opioid- or stimulant- only cohorts. Overall, our sample showed that 1% of the co-diagnosis cohort had an HIV diagnosis with the stimulant-only cohort at 1.9%. The Charlson Comorbidity Index indicated that the number of comorbidities were higher in the opioid-only cohort with approximately one-third of those patients diagnosed with three or more health conditions (31.4%). Endocarditis (i.e., bacterial infection in the heart) and heart disease were highest in the co-diagnosis cohort at 15.3% and 41.9%, respectively.
Table 3. The most prevalent drugs detected from laboratory samples were cannabis (12.7%) and methamphetamine (12.6%) followed by morphine (11.1%). Morphine is a metabolite of both medical (e.g., codeine) and non-medical (e.g., heroin) opioids and we could not distinguish between them on the basis of morphine positivity alone.54 The co-diagnosis cohort showed 37.6% positivity for methamphetamine, 25.5% for cannabis, 28% for buprenorphine (frequently used for the treatment of OUD), and 26.2% for fentanyl. The fentanyl prevalence in the co-diagnosis cohort was more than 3 times higher than the other cohorts (approximately 26% compared to 8% in the other cohorts). The co-diagnosis cohort also showed the highest positivity for all the two-drug combinations we examined. Out of 1,853 patients in the co-diagnosis cohort, we found 482 (26%) contained both methamphetamine and amphetamine regardless of the number of additional drugs. For patients with that combination, we found that 191 (39%) experienced some sort of housing instability compared with 26% of the patients overall in this cohort (data not shown).
Table 3.
Select co-occurring drugs present as determined by toxicology testing (*with the exception of the self-reported illicit drug use in the past year) conducted during a healthcare encounter at the University of Kentucky Healthcare System, 2017 to 2019.
| All | % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| STIMULANT-ONLY | OPIOID-ONLY | CO-DX | |||||||||
| N | % | p | N | % | N | % | p | p | N | ||
| OPIOIDS | |||||||||||
| Morphine | 382 | 8.0 | 0.0774 | 656 | 8.9 | 517 | 27.9 | *** | *** | 1,555 | 11.1 |
| Oxycodone | 247 | 5.2 | *** | 563 | 7.6 | 313 | 16.9 | *** | *** | 1,123 | 8.0 |
| Hydrocodone | 144 | 3.0 | * | 289 | 3.9 | 133 | 7.2 | *** | *** | 566 | 4.0 |
| Buprenorphine | 209 | 4.4 | *** | 793 | 10.7 | 518 | 28.0 | *** | *** | 1,520 | 10.8 |
| Methadone | 20 | 0.4 | *** | 143 | 1.9 | 72 | 3.9 | *** | *** | 235 | 1.7 |
| Fentanyl | 391 | 8.2 | 0.6780 | 618 | 8.4 | 485 | 26.2 | *** | *** | 1,494 | 10.6 |
| STIMULANTS | |||||||||||
| Amphetamine | 626 | 13.1 | *** | 202 | 2.7 | 531 | 28.7 | *** | *** | 1,359 | 9.7 |
| BENZODIAZEPINES | |||||||||||
| Alprazolam | 158 | 3.3 | 0.9871 | 243 | 3.3 | 138 | 7.4 | *** | *** | 539 | 3.8 |
| OTHER | |||||||||||
| Gabapentin | 86 | 1.8 | 0.0562 | 170 | 2.3 | 130 | 7.0 | *** | *** | 386 | 2.8 |
| Cannabis | 777 | 16.2 | *** | 532 | 7.2 | 472 | 25.5 | *** | *** | 1,781 | 12.7 |
| ILLICIT | |||||||||||
| Methamphetamine | 784 | 16.4 | *** | 289 | 3.9 | 696 | 37.6 | *** | *** | 1,769 | 12.6 |
| Heroin | 16 | 0.3 | *** | 140 | 1.9 | 108 | 5.8 | *** | *** | 264 | 1.9 |
| Cocaine | 560 | 11.7 | *** | 110 | 1.5 | 229 | 12.4 | *** | *** | 899 | 6.4 |
| Self-reported illicit drug use (past year) * | |||||||||||
| Yes | 1,895 | 39.5 | *** | 1,760 | 23.8 | 1,166 | 62.9 | *** | *** | 4,821 | 34.4 |
| Unknown | 1,840 | 38.4 | 3,238 | 43.8 | 416 | 22.5 | 5,494 | 39.2 | |||
| COMBINATIONS (2-WAY) | |||||||||||
| Cocaine & Methamphetamine | 86 | 1.8 | *** | 32 | 0.4 | 85 | 4.6 | *** | *** | 203 | 1.4 |
| Cocaine & Fentanyl | 83 | 1.7 | *** | 66 | 0.9 | 113 | 6.1 | *** | *** | 262 | 1.9 |
| Cocaine & Heroin | 8 | 0.2 | 0.2419 | 20 | 0.3 | 32 | 1.7 | *** | *** | 60 | 0.4 |
| Methamphetamine & Cannabis | 299 | 6.2 | *** | 89 | 1.2 | 255 | 13.8 | *** | *** | 643 | 4.6 |
| Methamphetamine & Fentanyl | 179 | 3.7 | *** | 143 | 1.9 | 283 | 15.3 | *** | *** | 605 | 4.3 |
| Methamphetamine & Amphetamine | 512 | 10.7 | *** | 173 | 2.3 | 482 | 26.0 | *** | *** | 1,167 | 8.3 |
| Fentanyl & Heroin | 12 | 0.3 | *** | 89 | 1.2 | 70 | 3.8 | *** | *** | 171 | 1.2 |
| Fentanyl & Amphetamine | 143 | 3.0 | *** | 102 | 1.4 | 207 | 11.2 | *** | *** | 452 | 3.2 |
| All | 4,794 | 7,385 | 1,853 | 14,032 | |||||||
p-values in in cohort columns represent pairwise comparisons between independent variables (bolded) distributed in the cohort to the distribution in the opioid-only cohort. The last p-value is a test across all three cohorts..
p<0.05
p<0.001
p<0.0001
Figure 1 shows the aORs and 95% CIs for the adjusted regression model examining social ecological model factors associated with cohort membership where variable distributions in the opioid-only cohort were used as the reference group (OR=1.0). The adjusted odds of having unstable housing were 2.75 times higher (95% CI: 2.39–3.12) in the co-diagnosed cohort and 1.86 times higher (95% CI: 1.66–2.09) in the stimulant-only cohort relative to the opioid-only group. Non-overlapping CIs suggest that the likelihood of unstable housing is significantly different between all three cohorts (lowest to highest: opioid-only>stimulant-only>co-diagnosis). Other demographics that are unique (i.e., non-overlapping CIs) across the three cohorts, include Black race, female sex, Medicaid insurance, a specified smoking status, and urban/rural residence. None of the educational levels were entirely unique but the adjusted odds of completing high school (aOR=0.74 95% CI: (0.60–0.91) were significantly lower for the co-diagnosis cohort compared to the opioid-only cohort and the opioid-only cohort was more likely to have a college degree. The odds of having a cognitive impairment that prevented a complete assessment of a patient’s education level was significantly higher when stimulants were involved. We conducted a sensitivity analysis and restricted our population to those with a diagnosed opioid or stimulant use disorder (excluding those with poisoning events only) and our findings were consistent. However, Medicare as a payer emerged as a unique factor distinguishing the cohorts.
Figure 1.
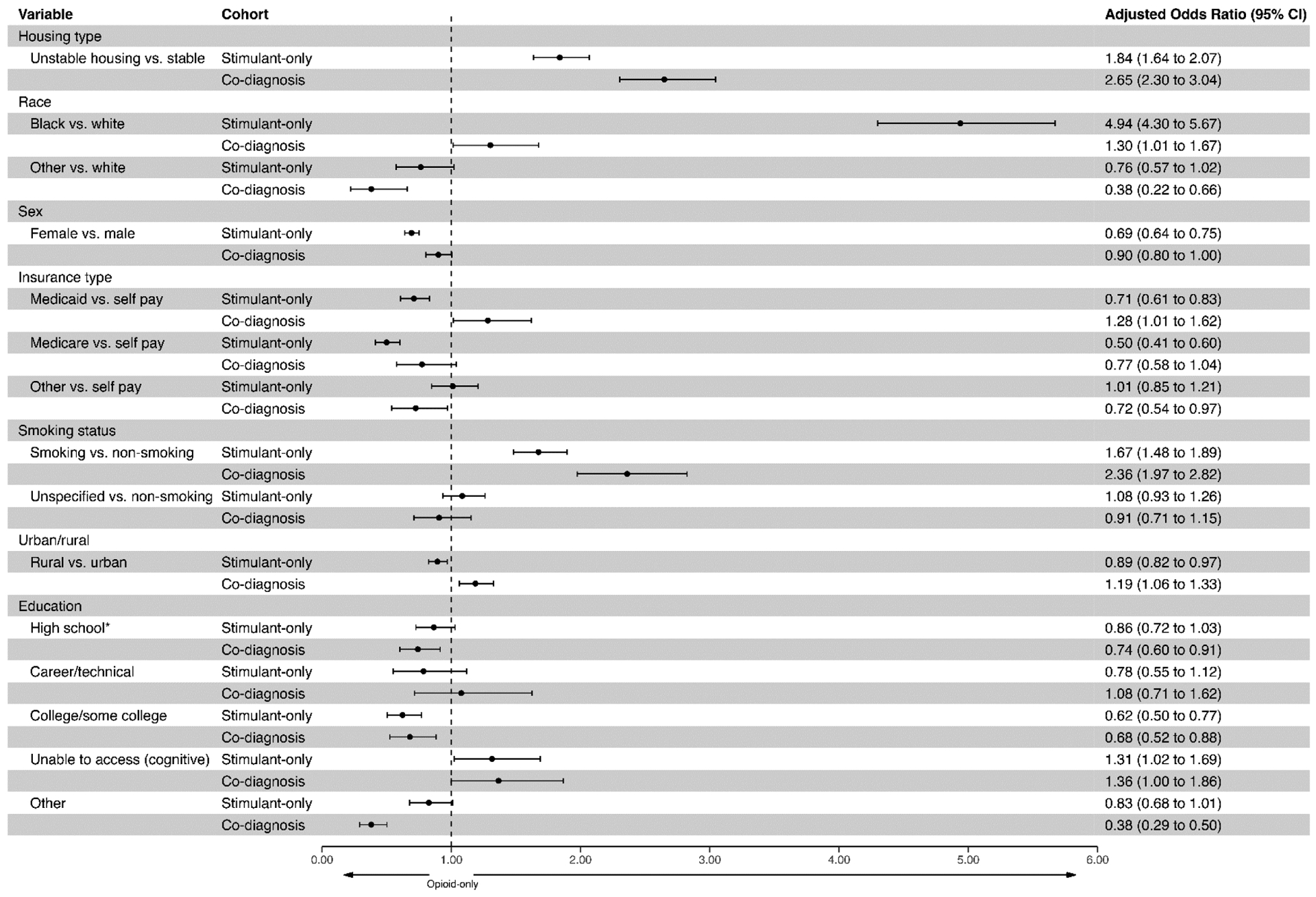
Adjusted odds ratios and 95% confidence intervals (CI) from multinomial regression analyses to identify unique (non-overlapping CIs) and shared factors associated with substance use cohort membership. Age (significant but not shown) is included in the adjusted model. Appalachian county status was removed since urban/rural status was included. *Reference group for educational status is “less than highschool”. The aOR=1.0 reference line represents the variables as distributed in the opioid-only cohort (comparison cohort).
Figure 2 shows the intersectionality of the top 10 drug combinations in the co-diagnosis cohort. The most common drug intersection among those selected for analysis was methamphetamine and amphetamine only (n=51), followed by the intersection with buprenorphine added (n=46). The methamphetamine/amphetamine pairing occurs in 6 of the top 10 intersections. Interpretation of this combination is limited since amphetamine can indicate both medical (e.g., ADHD treatment) and non-medical (i.e., methamphetamine metabolism) use. Thus, we cannot distinguish between exposures without a more extensive examination of data available in the EMR including written prescriptions for stimulant medications.
Figure 2.
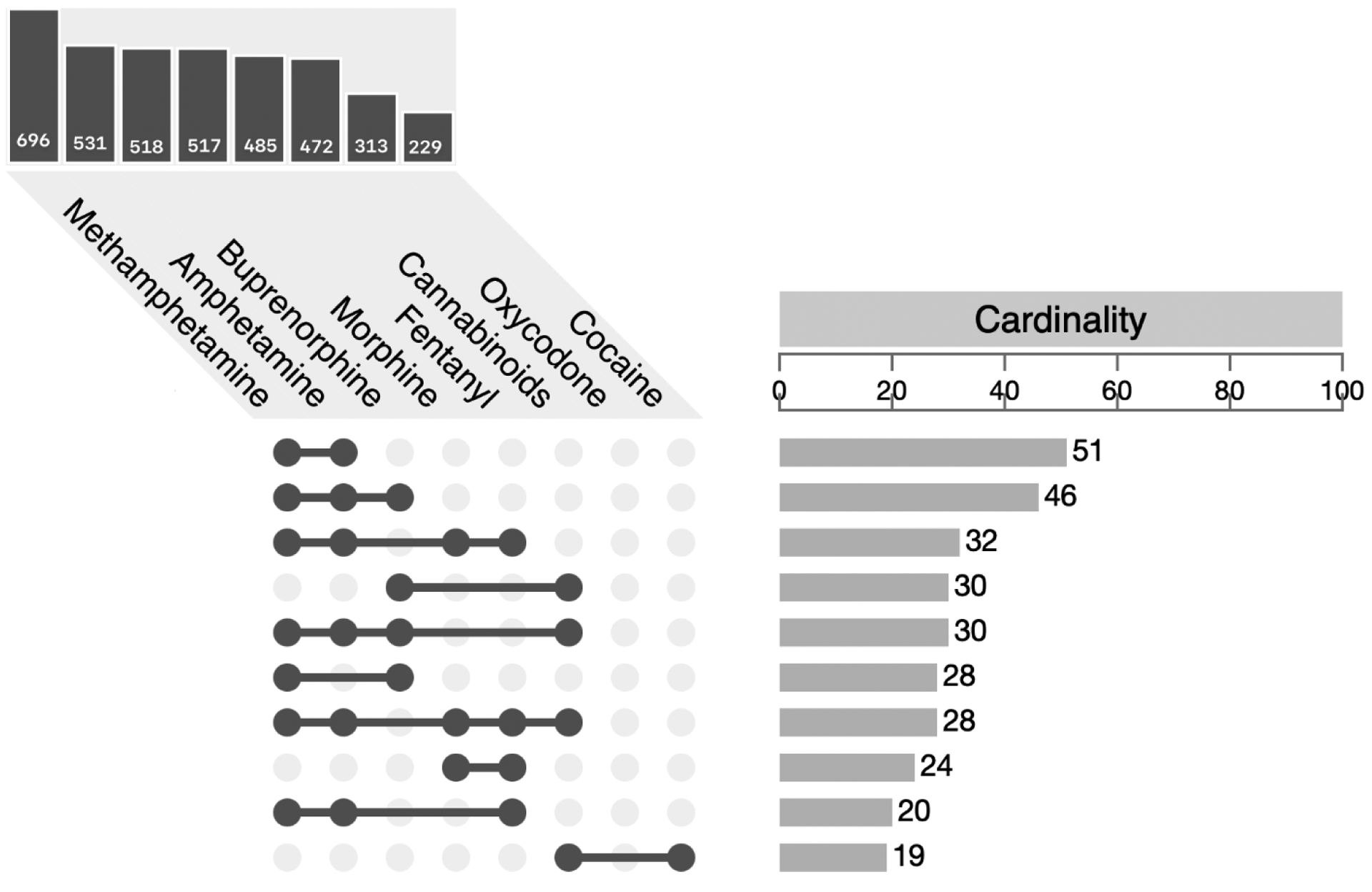
Top 10 co-occurring drug combinations in the co-diagnosed cohort (having both opioid and stimulant use disorders). The cardinality bar chart (right) shows the counts for specific combinations of drugs (only top 10 shown) and the bar chart (top left) counts single occurrences of select drugs.
3.1. SDOH and housing instability stratified results
There were 9,040 patients residing in UKHC’s primary and secondary catchment areas used for this part of the analysis. The Kolak and SVI mean, patient-level vulnerability scores showed variability across the SDOH themes and cohorts (see Figure 3). Examining Kolak’s socioeconomic theme descriptively, the stimulant-only cohort had lower mean scores (−0.36) followed by the co-diagnosis cohort (−0.25) and the opioid-only cohort (−0.07). The significantly lower, negative regression coefficients for the proportion of the stimulant and co-diagnosis cohort by census tract compared to the opioid-only proportion gives the same, quantified perspective (see Figure 4, Table 4). The interpretation is that for each additional 10% increase in stimulant-only cohort living in a census tract, we estimate that the SES score decreases by 0.42 [95% CI: −0.62, −0.23] points compared to the opioid-only cohort for two tracts with the same percentage of individuals in the co-diagnosis cohort.
Figure 3.
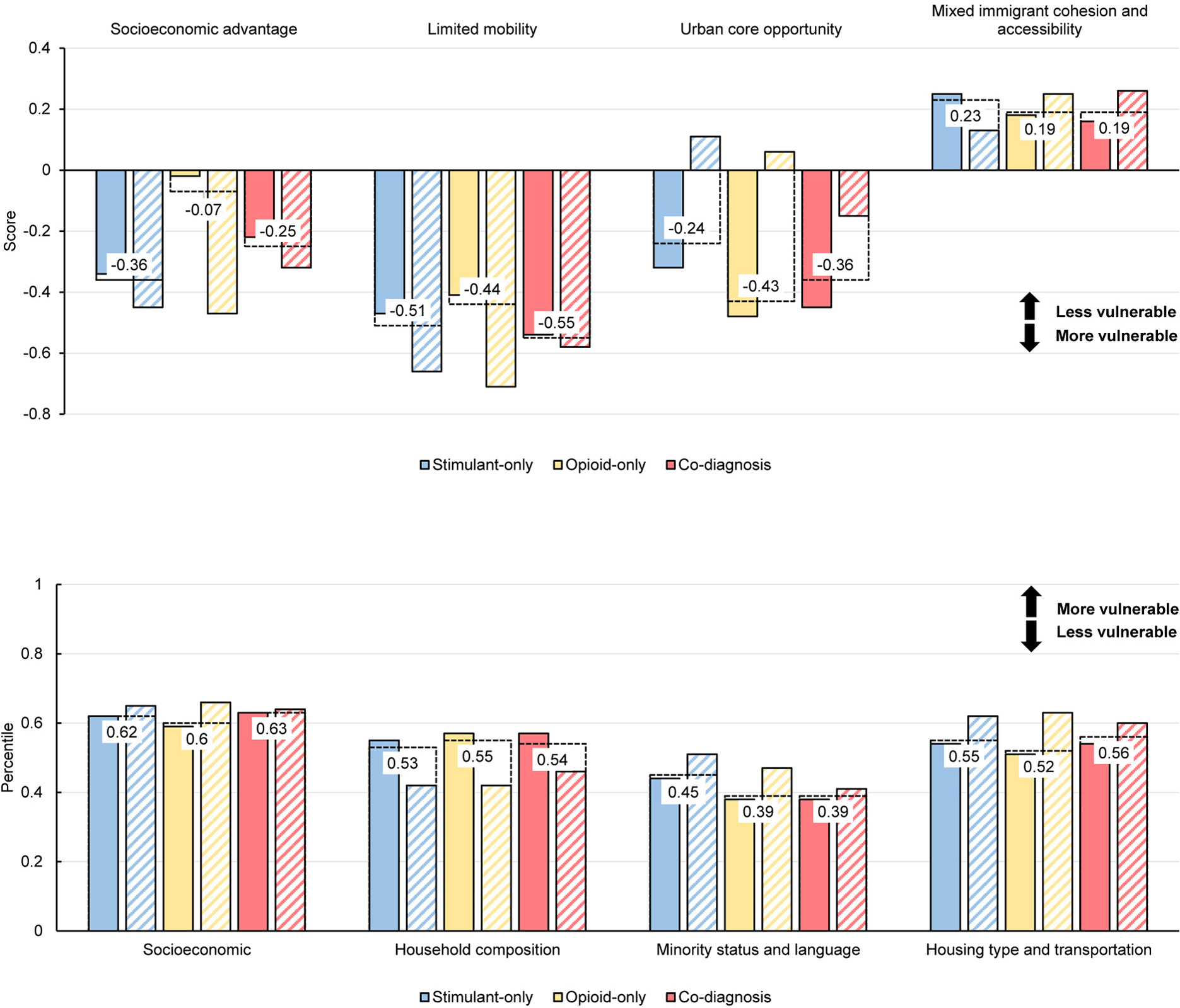
Top. Kolak’s social determinants of health (SDOH) components of US census tracts for patients residing in UKHC primary and secondary catchment areas (n=9,040). Lower scores indicate more vulnerability. Bottom. CDC’s Social Vulnerability Index Components. Higher percentiles indicate higher vulnerability. Hashed bars show values for those with unstable housing and dashed horizontal lines and labels show group mean values from SDOH scores assigned to each patient.
Figure 4.
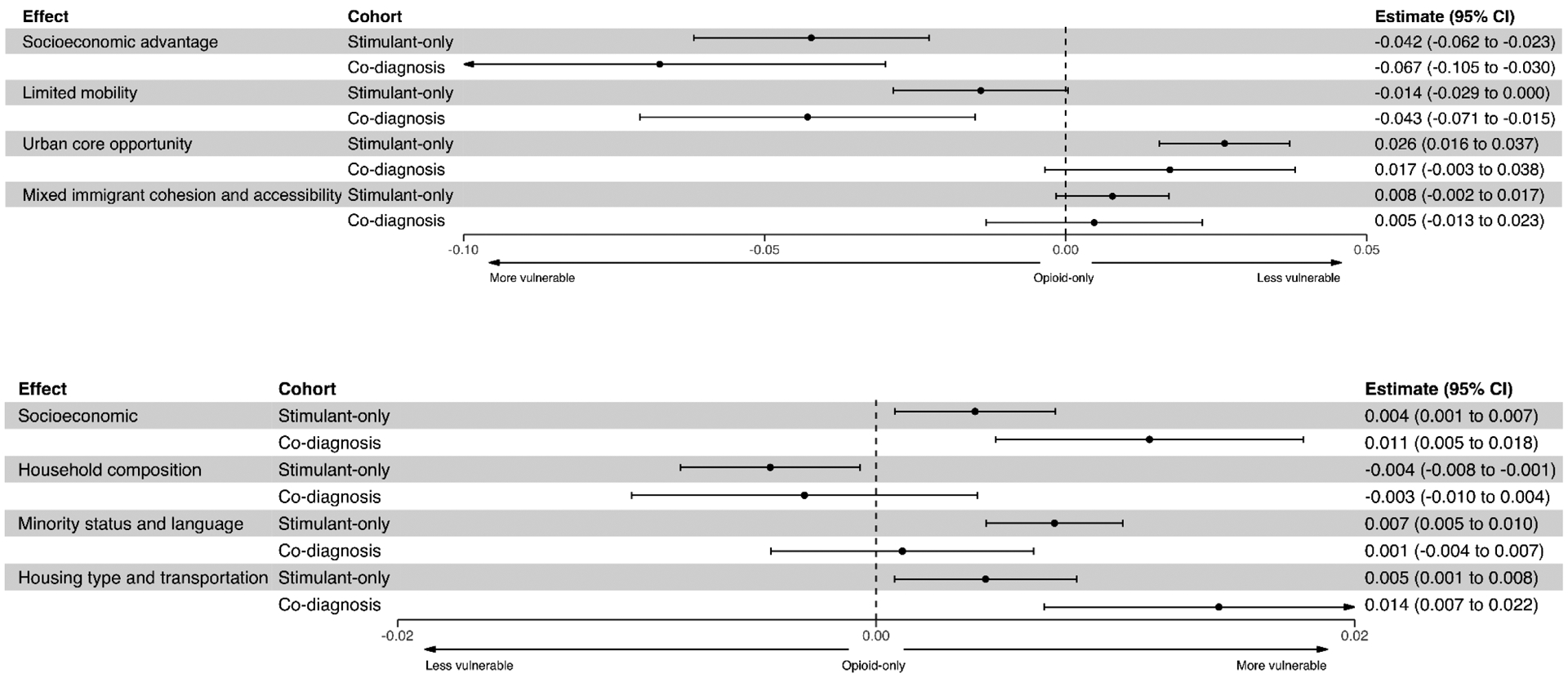
Linear regression estimates of proportion of each drug use cohort living in a census tract and the tracts vulnerability score. The estimate=0 reference line represents the opioid-only cohort (comparison group). The top graph are components of Kolak’s measures. The bottom graph are components of the US Centers for Disease Control and Prevention’s Social Vulnerability Index.
Table 4.
Regression coefficients for proportion of population in the specified cohort and social determinant of health score in the UKHC primary/secondary catchment areas. For, Kolak a negative sign means more vulnerable. For CDC’s SVI, a positive sign means less vulnerable.
| STIMULANT-ONLY | OPIOID-ONLY | CO-DX | UNSTABLE HOUSING | |
|---|---|---|---|---|
| Social determinant of health measure | Kolak | |||
| Socioeconomic Advantage | −0.0207* | 0.0097*** | −0.0425* | ns |
| Limited Mobility | ns | 0.0209** | −0.0345* | ns |
| Urban core opportunity | 0.0286*** | −0.0199** | ns | 0.0236*** |
| Mixed immigrant cohesion & accessibility | 0.0093* | Ns | ns | ns |
| CDC’s Social Vulnerability Index (SVI) | ||||
| Socioeconomic | ns | −0.0054** | 0.0089** | ns |
| Household composition | −0.0046** | Ns | Ns | −0.0071** |
| Minority status & language | 0.0052*** | −0.0057*** | Ns | 0.0031* |
| Housing type & transportation | ns | −0.0048** | 0.0116** | ns |
p<0.05
p<0.001
p<0.0001.
ns=not significant at the 0.05 level.
The co-diagnosis cohort was more vulnerable (−0.55) on the limited mobility theme than either the opioid-only (−0.44) or stimulant-only (−0.51) cohorts. On the urban core theme, the opioid-only cohort was more vulnerable (−0.43) than the stimulant-only cohort. The MICA measure was not associated with cohort composition. The Kolak socioeconomic measure appears to be the most sensitive to differences in cohort membership and housing stability. For a 10% increase in the percentage of the stimulant-only cohort living in a census tract, the socioeconomic score declines (more vulnerable) by 0.2 points. Furthermore, the association between percentage in the stimulant only cohort and SES score is significantly different than the association between percentage in the opioid only cohort and SES score (p <0.0001).
CDC’s SVI showed similar associations. For CDC’s SVI themes, despite limited variability on the visualized vulnerability score, the regression analysis indicated more vulnerability in census tracts with a larger proportion of stimulant-only patients. The interpretation is that for each additional 10% increase in stimulant-only cohort living in a census tract, we estimate that the SES score increases by 0.04 [95% CI: 0.01, 0.07] points compared to the opioid-only cohort for two tracts with the same percentage of individuals in the co-diagnosis cohort. The household composition measure was the only one to indicate more census tract vulnerability for the opioid-only cohort.
There was a positive association of proportion of patients with unstable housing and census tract vulnerability (0.0236, p<0.0001) for the urban core measure but was otherwise not statistically significant. The direction and magnitude of significant regression coefficients for unstable housing for Kolak and the SVI were similar to results found for the proportion of the stimulant-only cohort (Table 4). As a sensitivity analysis, we ran the adjusted logistic regression models for shared/unique risk factors for the catchment restricted sample and the results remained the same.
4. Discussion
In three cohorts of patients defined by opioid-only, stimulant-only, and co-diagnosis of opioid and stimulant use disorders from Kentucky’s largest primary and safety net healthcare system, we found substantial variability within multiple domains of the social ecological model. We draw several important conclusions from our focus on the social determinants of health, namely unstable housing and neighborhood vulnerability, which persisted after adjustment for multiple individual and other exposure factors in this study.
First, unstable housing was relatively prevalent and much higher among co-diagnosed patients (approximately one quarter of this cohort). Our findings represent a 3-year cross-section and we intentionally excluded CY2020 to avoid the high variability in healthcare utilization due to the COVID-19 pandemic.55 Even so, in follow up analyses we found that unstable housing for the co-diagnosed cohort increased from 21% (2017) to 35% in (2020). These findings, while derived from a single health care system, are consistent with a national survey that demonstrated increasing prevalence of housing instability associated with drug class involvement: opioids-only (7.6%), methamphetamine-only (11.7%) and both types (15%).22 Furthermore, homelessness in rural locations represents a growing and substantial share of the national total.56,57 Thus, it is not surprising that our observed unstable housing is higher than national estimates for multiple reasons including, but not limited to, Kentucky’s high rate of drug-related mortality58, large proportion of the population on Medicaid insurance and safety-net patients59, the underestimation associated with household-based survey sampling of populations that use drugs60, and our use of EMR data enhanced with keyword and patient address searching (i.e., increased sensitivity). To further apply advanced data science we will examine written clinical documents that may further assist in identifying patients experiencing some type of housing issue (<1% of clinical documents contained obvious key phrases such as “homeless”).61 The US Department of Health and Human Services recently emphasized national opportunities to data mine EMRs to advance health equity for SUDs.62
Second, those with unstable housing within each cohort tended to live in more economically-deprived neighborhoods according to the socioeconomic component of a novel and readily-available SDOH index. The stimulant-only group lived in more economically vulnerable neighborhoods in the UKHC catchment area. It is not surprising that other SDOH components showed variability within or between indices as each are designed to capture different aspects of social context. For example, it seems counterintuitive that the urban core opportunity component showed that patients with more stable housing in this population appeared to live in areas with less access to a “vibrant” urban core (i.e., more vulnerable due to less urban opportunity). However, this measure may be capturing different underlying aspects of neighborhood context in a rural, Appalachian state like Kentucky with few urban centers (the primary catchment area, Fayette County, is one of two counties in the state with population > 250,000). The Kolak index was originally validated against all-cause mortality in Chicago and this is the first time it has been applied to drug use research.
Several other findings related to co-morbid mental and physical health conditions and drug-related events point to the epidemiologic consistency between our healthcare system’s EMR-based data and other studies. By and large, the co-diagnosed cohort was at higher risk across multiple domains and factors with few exceptions. The prevalence of Hepatitis C, which is associated with non-sterile injection drug use, was particularly pronounced. The high rates of Hepatitis C and co-occurring OUD is consistent with multiple reports of high Hepatitis C transmission in Kentucky associated with opioid use.63,64 In the case of HIV, also associated with injection drug use65, the stimulant-only cohort had the highest proportions of people living with HIV (despite lower screening for HIV in UKHC, data not shown). The stimulant-only cohort also had the highest proportions of Black patients (somewhat higher than Census estimates for the area) and the HIV diagnosis rate for Black residents is consistently around 5 times higher than White residents in Kentucky.66 The opioid-only cohort was older, consistent with having the largest proportion of Medicare enrollees, and had higher comorbidities and rates of opioid poisoning. For the Medicare population, there could be a dimension of opioid poisoning related to medication adherence, dosing stabilization, and concurrent prescribing with other drugs (e.g., benzodiazepines67) that differs from the opioid poisoning associated with accidental overdose from non-medical use (e.g., heroin, illicitly manufactured fentanyl). Finally, in our overall patient population, 3.5% had a documented suicide attempt or self-harm. In a NSDUH sample, among people with varying stages of nonmedical prescription opioid use, 5% reported suicidal ideation.68 Suicide prevention is an urgent priority area for the CDC and additional research is planned.69
The ability to examine use and exposure to multiple drugs simultaneously is critical for research in the era of the “Third Wave”. Our toxicology findings are consistent with a national study of urine drug tests results submitted as a part of routine health care.70 Methamphetamine, cocaine, fentanyl and heroin showed prevalence of 8.4%, 4.9%, 4.7% and 1.9%, respectively. Our findings showed 12.6%, 6.4%, 10.6% and 1.9% positivity, respectively, with higher positivity likely explained by our focused identification of populations with confirmed SUDs. When we restricted to “routine” discharges, the percentages declined accordingly to 11.8%, 6.3%, 9.9%, and 1.6%, respectively. Even though we classified patients by diagnosed stimulant and opioid use disorders to isolate unique characteristics within these populations, the fact that multiple drugs were detected in these populations reflects the true dynamic and polysubstance nature of drug use among these patients. One hypothesis for the presence of buprenorphine in our stimulant-only cohort is that it may signal the emergence/undetected opioid addiction in this population but further work is needed.
4.1. Limitations
Though our data are consistent with multiple external studies, they represent a single healthcare system which, we estimate, covers 21% of the inpatient and 16% of outpatient population using stimulants in Kentucky. The total number of census tracts used for regression analyses was relatively small. We did not validate the diagnostic accuracy of the unstable housing ICD-10-CM code and approximately 45% of the Z59* series captures low income and extreme poverty status that is not necessarily associated with homelessness. We can only account for the patient’s last known residential address and use of area-based measures of socioeconomic status at the neighborhood-level is subject to ecological fallacy. We did not evaluate drug concentrations only whether lab values were positive and cannot identify when drug use occurred prior to the encounter. Furthermore, laboratory results for prescription medications do not indicate whether the drugs were use as prescribed. Drug and health condition screening rates varied substantially between the cohorts which could lead to bias. Cohorts may be misclassified if patients have undiagnosed and/or untreated SUDs which has been associated with unstable housing.25 We did not restrict cohorts to incident SUDs at the time of the first encounter and did not determine if the patient had prior encounters at UKHC.
5. Conclusions
Using electronic medicals records, we identified substantial variability within multiple domains of the social ecological model for individuals and communities experiencing the negative health outcomes associated with polysubstance use characterizing the “third wave”. These unique factors can be used to tailor interventions at multiple “touch points”71 for patients with substance use disorders.
Acknowledgements
Jungjun Bae for data visualization.
Funding
This project is fully supported by the Centers for Disease Control and Prevention of the U.S. Department of Health and Human Services (HHS) as part of grant 1R01CE003360–01-00. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by CDC/HHS, or the U.S. Government. Data for the project described was supported by the NIH National Center for Advancing Translational Sciences through grant number UL1TR001998. The authors declare that there is no conflict of interest.
References
- 1.Centers for Disease Control and Prevention. Other Drugs. Centers for Disease Control and Prevention. Published November 18, 2021. Accessed May 17, 2022. https://www.cdc.gov/drugoverdose/deaths/other-drugs.html
- 2.Centers for Disease Control and Prevention. Understanding the Epidemic. Published March 19, 2020. Accessed April 27, 2020. https://www.cdc.gov/drugoverdose/epidemic/index.html
- 3.Mattson CL, O’Donnell J, Kariisa M, Seth P, Scholl L, Gladden RM. Opportunities to Prevent Overdose Deaths Involving Prescription and Illicit Opioids, 11 States, July 2016–June 2017. MMWR Morbidity and Mortality Weekly Report. 2018;67(34):945–951. doi: 10.15585/mmwr.mm6734a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strickland JC, Havens JR, Stoops WW. A nationally representative analysis of “twin epidemics”: Rising rates of methamphetamine use among persons who use opioids. Drug Alcohol Depend. 2019;204:107592. doi: 10.1016/j.drugalcdep.2019.107592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder SM, Morse SA, Bride BE. A comparison of 2013 and 2017 baseline characteristics among treatment seeking patients who used opioids with co-occurring disorders. Journal of Substance Abuse Treatment. 2019;99:134–138. doi: 10.1016/j.jsat.2019.01.023 [DOI] [PubMed] [Google Scholar]
- 6.Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug and Alcohol Dependence. 2019;197:78–82. doi: 10.1016/j.drugalcdep.2018.12.030 [DOI] [PubMed] [Google Scholar]
- 7.Warfield SC, Bharat C, Bossarte RM, et al. Trends in comorbid opioid and stimulant use disorders among Veterans receiving care from the Veterans Health Administration, 2005–2019. Drug and Alcohol Dependence. 2022;232:109310. doi: 10.1016/j.drugalcdep.2022.109310 [DOI] [PubMed] [Google Scholar]
- 8.Bronfenbrenner U The Ecology of Human Development: Experiments by Nature and Design. Harvard University Press; 1996. [Google Scholar]
- 9.Jalali MS, Botticelli M, Hwang RC, Koh HK, McHugh RK. The opioid crisis: a contextual, social-ecological framework. Health Res Policy Sys. 2020;18(1):87. doi: 10.1186/s12961-020-00596-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Social-Ecological Model: A Framework for Prevention |Violence Prevention|Injury Center|CDC. Published January 29, 2021. Accessed March 13, 2021. https://www.cdc.gov/violenceprevention/about/social-ecologicalmodel.html
- 11.Volkow ND, Blanco C. The changing opioid crisis: development, challenges and opportunities. Mol Psychiatry. 2021;26(1):218–233. doi: 10.1038/s41380-020-0661-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Social Determinants of Health | CDC. Published September 30, 2021. Accessed April 5, 2022. https://www.cdc.gov/socialdeterminants/index.htm
- 13.Faller RW, Erausquin JT, McCoy TP. Misuse of Prescription and Illicit Drugs in Middle Adulthood in the Context of the Opioid Epidemic. Substance Use & Misuse. 2021;56(2):333–337. doi: 10.1080/10826084.2020.1858107 [DOI] [PubMed] [Google Scholar]
- 14.Carliner H, Sarvet AL, Gordon AR, Hasin DS. Gender discrimination, educational attainment, and illicit drug use among U.S. women. Soc Psychiatry Psychiatr Epidemiol. 2017;52(3):279–289. doi: 10.1007/s00127-016-1329-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen R, Kessler RC, Sadikova E, et al. Racial and ethnic differences in individual-level and area-based socioeconomic status and 12-month DSM-IV mental disorders. Journal of Psychiatric Research. 2019;119:48–59. doi: 10.1016/j.jpsychires.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hummer RA, Hernandez EM. The Effect of Educational Attainment on Adult Mortality in the United States. Popul Bull. 2013;68(1):1–16. [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis MS, Kasper ZA, Cicero TJ. Polysubstance use trends and variability among individuals with opioid use disorder in rural versus urban settings. Preventive Medicine. 2021;152:106729. doi: 10.1016/j.ypmed.2021.106729 [DOI] [PubMed] [Google Scholar]
- 18.Chichester K, Drawve G, Sisson M, McCleskey B, Dye DW, Cropsey K. Examining the neighborhood-level socioeconomic characteristics associated with fatal overdose by type of drug involved and overdose setting. Addict Behav. 2020;111:106555. doi: 10.1016/j.addbeh.2020.106555 [DOI] [PubMed] [Google Scholar]
- 19.Yedinak JL, Li Y, Krieger MS, et al. Machine learning takes a village: Assessing neighbourhood-level vulnerability for an overdose and infectious disease outbreak. Int J Drug Policy. 2021;96:103395. doi: 10.1016/j.drugpo.2021.103395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delcher C, Harris DR, Anthony N, Mir M. Opioid overdoses increase at home during the COVID-19 stay-at-home order period in Cook County, Illinois. AJPM Focus. Published online June 2022:100007. doi: 10.1016/j.focus.2022.100007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barocas JA, Wang J, Marshall BDL, et al. Sociodemographic factors and social determinants associated with toxicology confirmed polysubstance opioid-related deaths. Drug and Alcohol Dependence. 2019;200:59–63. doi: 10.1016/j.drugalcdep.2019.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shearer RD, Howell BA, Bart G, Winkelman TNA. Substance use patterns and health profiles among US adults who use opioids, methamphetamine, or both, 2015–2018. Drug and Alcohol Dependence. 2020;214:108162. doi: 10.1016/j.drugalcdep.2020.108162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chawarski MC, Hawk K, Edelman EJ, et al. Use of Amphetamine-Type Stimulants Among Emergency Department Patients With Untreated Opioid Use Disorder. Annals of Emergency Medicine. 2020;76(6):782–787. doi: 10.1016/j.annemergmed.2020.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali MM, Sutherland H, Rosenoff E. Comorbid Health Conditions and Treatment Utilization among Individuals with Opioid Use Disorder Experiencing Homelessness. Substance Use & Misuse. 2021;56(4):571–574. doi: 10.1080/10826084.2021.1884723 [DOI] [PubMed] [Google Scholar]
- 25.Chawarski MC, Hawk K, Edelman EJ, et al. Use of Amphetamine-Type Stimulants Among Emergency Department Patients With Untreated Opioid Use Disorder. Annals of Emergency Medicine. 2020;76(6):782–787. doi: 10.1016/j.annemergmed.2020.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golembiewski E, Allen KS, Blackmon AM, Hinrichs RJ, Vest JR. Combining Nonclinical Determinants of Health and Clinical Data for Research and Evaluation: Rapid Review. JMIR Public Health Surveill. 2019;5(4):e12846. doi: 10.2196/12846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berkowitz SA, Traore CY, Singer DE, Atlas SJ. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50(2):398–417. doi: 10.1111/1475-6773.12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC). Social Vulnerability Index (SVI). CDC Social Vulnerability Index (SVI). Published October 15, 2020. Accessed December 19, 2020. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- 29.Kolak M, Bhatt J, Park YH, Padrón NA, Molefe A. Quantification of Neighborhood-Level Social Determinants of Health in the Continental United States. JAMA Netw Open. 2020;3(1):e1919928. doi: 10.1001/jamanetworkopen.2019.19928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dolan C, Delcher C. Monitoring Health Inequities and Planning in Virginia: Poverty, Human Immunodeficiency Virus, and Sexually Transmitted Infections. Sexually Transmitted Diseases. 2008;35:981–984 10.1097/OLQ.0b013e318182a571. [DOI] [PubMed] [Google Scholar]
- 31.Nagasako EM, Reidhead M, Waterman B, Claiborne Dunagan W. Adding Socioeconomic Data To Hospital Readmissions Calculations May Produce More Useful Results. Health Affairs. 2014;33(5):786–791. doi: 10.1377/hlthaff.2013.1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark MW, Ziliak JP, Sheather S. Kentucky Annual Economic Report 2021. Center for Business and Economic Research, University of Kentucky; 2021. doi: 10.13023/CBER.KAER.2021 [DOI] [Google Scholar]
- 33.ICD- 10 - CM International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Published April 6, 2022. Accessed May 19, 2022. https://www.cdc.gov/nchs/icd/icd-10-cm.htm
- 34.Life Expectancy in the U.S. Declined a Year and Half in 2020. Published July 20, 2021. Accessed April 2, 2022. https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2021/202107.htm
- 35.Karriker-Jaffe KJ, Witbrodt J, Mericle AA, Polcin DL, Kaskutas LA. Testing a Socioecological Model of Relapse and Recovery from Alcohol Problems. Subst�Abuse. 2020;14:117822182093363. doi: 10.1177/1178221820933631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drug Overdose and Related Comorbidity County Profiles | KIPRC. Accessed May 23, 2022. https://kiprc.uky.edu/programs/overdose-data-action/county-profiles
- 37.NCI Comorbidity Index Overview. Accessed May 25, 2022. https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity.html
- 38.Hedegaard H, Schoenbaum M, Claassen C, Crosby A, Holland K, Proescholdbell S. Issues in Developing a Surveillance Case Definition for Nonfatal Suicide Attempt and Intentional Self-harm Using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) Coded Data. Natl Health Stat Report. 2018;(108):1–19. [PubMed] [Google Scholar]
- 39.Hargrove SL, Ward PJ, Mitchell LG, Bunn TL. Drug Overdose Fatality Surveillance System (DOFSS) 2017 Annual Technical Report. Published online 2017:85. [Google Scholar]
- 40.Harris DR, Henderson DW, Corbeau A. Improving the Utility of Tobacco-Related Problem List Entries Using Natural Language Processing. AMIA Annu Symp Proc. 2020;2020:534–543. [PMC free article] [PubMed] [Google Scholar]
- 41.About PostGIS | PostGIS. Accessed June 14, 2022. https://postgis.net/
- 42.PostgreSQL: The world’s most advanced open source database. Accessed June 14, 2022. https://www.postgresql.org/
- 43.Harris DR, Delcher C. bench4gis: Benchmarking Privacy-aware Geocoding with Open Big Data. Proc IEEE Int Conf Big Data. 2019;2019:4067–4070. doi: 10.1109/bigdata47090.2019.9006234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris DR. Leveraging Differential Privacy in Geospatial Analyses of Standardized Healthcare Data. Proc IEEE Int Conf Big Data. 2020;2020:3119–3122. doi: 10.1109/bigdata50022.2020.9378390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bureau UC. Glossary. Census.gov. Accessed May 19, 2022. https://www.census.gov/programs-surveys/geography/about/glossary.html
- 46.NCHS Urban-Rural Classification Scheme for Counties. Centers for Disease Control and Prevention. http://www.cdc.gov/nchs/data_access/urban_rural.htm
- 47.Appalachian Regional Commission. The Appalachian Region. Accessed May 8, 2019. https://www.arc.gov/appalachian_region/theappalachianregion.asp
- 48.Kentucky Housing Corporation Community Resource Guide. https://www.kyhousing.org/Pages/default.aspx
- 49.The SDOH Atlas. Accessed June 14, 2022. https://sdohatlas.github.io/
- 50.CDC/ATSDR’s Social Vulnerability Index (SVI). Published April 28, 2021. Accessed December 9, 2021. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- 51.Sharareh N, Hess R, White S, Dunn A, Singer PM, Cochran J. A vulnerability assessment for the HCV infections associated with injection drug use. Prev Med. 2020;134:106040. doi: 10.1016/j.ypmed.2020.106040 [DOI] [PubMed] [Google Scholar]
- 52.Lex A, Gehlenborg N. Sets and intersections. Nat Methods. 2014;11(8):779–779. doi: 10.1038/nmeth.3033 [DOI] [Google Scholar]
- 53.Lex A, Gehlenborg N, Strobelt H, Vuillemot R, Pfister H. UpSet: Visualization of Intersecting Sets. IEEE Trans Visual Comput Graphics. 2014;20(12):1983–1992. doi: 10.1109/TVCG.2014.2346248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84(7):613–624. doi: 10.1016/S0025-6196(11)60750-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reid LD, Fang Z. Changes in Hospitalizations and In-Hospital Deaths in the Initial Period of the COVID-19 Pandemic (April–September 2020), 13 States: Statistical Brief #282. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Accessed June 2, 2022. http://www.ncbi.nlm.nih.gov/books/NBK574671/ [Google Scholar]
- 56.Jackson A, Shannon L. Examining social support in a rural homeless population. Journal of Rural Social Sciences. 2014;29(1):3. [Google Scholar]
- 57.Henry M, De Sousa T, Roddey C, Gayen S, Bednar T. The 2020 Annual Homeless Assessment Report (AHAR) to Congress. Published online 2020.
- 58.Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and Geographic Patterns in Drug and Synthetic Opioid Overdose Deaths — United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202–207. doi: 10.15585/mmwr.mm7006a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oct 17 P, 2019. Medicaid State Fact Sheets. KFF. Published October 17, 2019. Accessed May 22, 2022. https://www.kff.org/interactive/medicaid-state-fact-sheets/
- 60.Barocas JA, White LF, Wang J, et al. Estimated Prevalence of Opioid Use Disorder in Massachusetts, 2011–2015: A Capture–Recapture Analysis. Am J Public Health. 2018;108(12):1675–1681. doi: 10.2105/AJPH.2018.304673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chapman AB, Jones A, Kelley AT, et al. ReHouSED: A novel measurement of Veteran housing stability using natural language processing. Journal of Biomedical Informatics. 2021;122:103903. doi: 10.1016/j.jbi.2021.103903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blanco C, Kato EU, Aklin WM, et al. Research to Move Policy — Using Evidence to Advance Health Equity for Substance Use Disorders. N Engl J Med. 2022;386(24):2253–2255. doi: 10.1056/NEJMp2202740 [DOI] [PubMed] [Google Scholar]
- 63.Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–458. [PMC free article] [PubMed] [Google Scholar]
- 64.Zibbell JE, Asher AK, Patel RC, et al. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am J Public Health. 2018;108(2):175–181. doi: 10.2105/AJPH.2017.304132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Conrad C, Bradley HM, Broz D, et al. Community Outbreak of HIV Infection Linked to Injection Drug Use of Oxymorphone--Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(16):443–444. [PMC free article] [PubMed] [Google Scholar]
- 66.Kentucky Cabinet for Health and Family Services, Department for Public Health, HIV/AIDS Section. HIV/AIDS Surveillance Report 2020. Published June 2021. Accessed August 3, 2021. https://chfs.ky.gov/agencies/dph/dehp/hab/Documents/AnnualReport2020.pdf
- 67.Lo‐Ciganic W, Hincapie‐Castillo J, Wang T, et al. Dosing profiles of concurrent opioid and benzodiazepine use associated with overdose risk among US Medicare beneficiaries: Group‐based multi‐trajectory models. Addiction. Published online February 28, 2022:add.15857. doi: 10.1111/add.15857 [DOI] [PubMed] [Google Scholar]
- 68.Kuramoto SJ, Chilcoat HD, Ko J, Martins SS. Suicidal Ideation and Suicide Attempt Across Stages of Nonmedical Prescription Opioid Use and Presence of Prescription Opioid Disorders Among US Adults. JOURNAL OF STUDIES ON ALCOHOL AND DRUGS. 2012;73(2):178–184. doi: 10.15288/jsad.2012.73.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Priority Areas | CDC. Published June 2, 2022. Accessed June 9, 2022. https://www.cdc.gov/injury/priority/index.html
- 70.Twillman RK, Dawson E, LaRue L, Guevara MG, Whitley P, Huskey A. Evaluation of Trends of Near-Real-Time Urine Drug Test Results for Methamphetamine, Cocaine, Heroin, and Fentanyl. JAMA Netw Open. 2020;3(1):e1918514. doi: 10.1001/jamanetworkopen.2019.18514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larochelle MR, Bernstein R, Bernson D, et al. Touchpoints – Opportunities to predict and prevent opioid overdose: A cohort study. Drug and Alcohol Dependence. 2019;204:107537. doi: 10.1016/j.drugalcdep.2019.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]


