Abstract
In the present study, acute onset of severe lupus nephritis was successfully treated in mice using a new, benzamide-linked, small molecule that targets immune modulation and the NLRP3 inflammasome. Specifically, 6-(2,4-difluorophenyl)-3-(3-(trifluoromethyl)phenyl)-2H-benzo[e][1,3]oxazine-2,4(3H)-dione (Cf-02) (a) reduced serum levels of IgG anti-dsDNA, IL-β, IL-6, and TNF-α, (b) inhibited activation of dendritic cells and differentially regulated T cell functions, and (c) suppressed the NF-κB/NLRP3 inflammasome axis, targeting priming and activating signals of the inflammasome. Moreover, treatment with Cf-02 significantly inhibited secretion of IL-1β in lipopolysaccharide-stimulated macrophages, but this effect was abolished by autophagy induction. These results recommend Cf-02 as a promising drug candidate for the serious renal conditions associated with systemic lupus erythematosus. Future investigations should examine whether Cf-02 may also be therapeutic in other types of chronic kidney disease involving NLRP3 inflammasome-driven signaling.
Keywords: severe lupus nephritis, autophagy, benzamide-linked small molecule, NF-κB/NLRP3 inflammasome axis
1 |. INTRODUCTION
Clinically, recurrence and/or transformation of renal pathology in lupus nephritis (LN), a major complication of systemic lupus erythematosus (SLE) from low grade to high grade (severe, diffuse proliferative glomerulonephritis) is not uncommon,1,2 the latter often featuring diffuse intrinsic cell proliferation in glomeruli, associated with neutrophil influx, cellular crescents, fibrinoid necrosis, and interstitial leukocyte infiltration.3 Growing evidence from multiple investigations suggests that increased systemic exposure to bacterial infection is implicated in SLE.4–6 Increased serum levels of antimicrobial response factors and of soluble CD14, a lipopolysaccharide (LPS) receptor, are observed in SLE patients.6–10 Moreover, neutrophil extracellular traps, a primary defense mechanism against microorganisms, contribute to the evolution of LN.11–13 Recently, we demonstrated that excessive production of IL-1β and activated T cells occur in an accelerated, severe LN (ASLN) model in NZB/W F1 mice.14–17 Furthermore, we found that NEK7, a mitotic serine/threonine kinase, can directly bind NLRP3, facilitating ASC, and leading to NLRP3 inflammasome activation in activated macrophages.18 On the other hand, autophagy inhibits IL-1β secretion in pathogen-associated, ligand-treated macrophages,19,20 and it may reduce IL-1β production by degrading pro-IL-1β and suppressing NLRP3 inflammasome activation.16,19–21
Treatment of LN includes immunosuppressive therapy involving either glucocorticoids and/or cytotoxic agents; however, the potentially severe contraindications of long-term use of these drugs remain a major concern.22–26 Therefore, it is worth developing new therapeutic agents that target specific pathogenic pathways for the renal condition, with tolerable side effects. In this regard, drug candidates for autoimmune diseases should have immunomodulatory effects with little or no toxicity.14,15,27 For this purpose, we succeeded in making a series of new 5-(2′,4′-difluorophenyl)-salicylanilide derivatives and their ring-fused analogs, such as 2-hydroxy-N-[3-(trifluoromethyl)phenyl]benzamide (HS-Cf),28 N-(4-chloro-2-fluorophenyl)-2-hydroxybenzamide (HS-Cm),29 and N-(3-chloro-4-fluorophenyl)-2-hydroxybenzamide (HS-Ck).30 These new, small molecules inhibited expression and/or activities of receptor activator of nuclear factor kappa-B ligand (RANKL) and RANKL-related effector genes, including NF-κB, nuclear factor of activated T cells 1, c-fos, triiodothyronine receptor auxiliary protein, and cathepsin K in RANKL-induced osteoclastogenesis.31,32 Interestingly, during the course of making potent derivatives from synthetic analogs of HS-Cf, a novel compound, 6-(2,4-difluorophenyl)-3-(3-(trifluoromethyl)phenyl)-2H-benzo[e][1,3]oxazine-2,4(3H)-dione (Cf-02) was identified. It shares some structural similarities with quercetin, a natural product in various fruits and vegetables, with potent immunomodulatory properties.33
In the present study, the accelerated, severe renal condition in ASLN mice simulated acute onset of high-grade pathological categories of LN and benefited from treatment with Cf-02 by differentially regulating the NLRP3 inflammasome, autophagy, and T cell functions. Our results suggest that this new compound may be efficacious for other types of chronic kidney disease involving excessive NLRP3 inflammasome activation.
2 |. MATERIALS AND METHODS
2.1 |. Synthesis of Cf-02
Cf-02, a novel benzamide-iinked small molecule, 6-(2,4-difluorophenyl)-3-phenyl-2H-benzo[e][1,3]oxazine-2,4(3H)-dione derivative, was synthesized as described previously.33
2.2 |. Clinical samples
Serum samples were collected from 65 patients with SLE, 24 with Sjögren syndrome and 30 healthy controls, while whole blood samples were collected from 14 SLE patients for the preparation of peripheral blood mononuclear cells (PBMCs) in Ficoll-Hypaque (Amersham Pharmacia Biotech, NJ, USA). All participants gave written informed consent and the research plan was approved by the Ethics Committee of Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan (approval number: 2-106-05-001).
2.3 |. ASLN mouse model and experimental protocol
To establish ASLN model mice, 8-week-old female NZB/WF1 mice (prior to autoantibody production) were given LPS (Sigma, MO, USA) (0.8 mg/kg body weight) twice a week by intraperitoneal (ip) injections.15 One week after the first injection of LPS, seven mice each received either Cf-02 (10 mg/kg body weight) or vehicle (PEG 400) (Sigma) daily via an ip route. Another group of such NZB/WF1 mice were treated with MCC950, a NLRP3 inhibitor (TargetMol, MA, USA), at 10 mg/kg body weight or saline every 2 days via ip injections. All mice were sacrificed 5 weeks after induction of the disease. Another group of age-matched female NZB/WF1 mice were injected with saline and served as normal controls. All animal experiments were performed in compliance with the NIH Guidelines for the Care and Use of Laboratory Animals with approval of the Institutional Animal Care and Use Committee of The National Defense Medical Center, Taipei, Taiwan.
2.4 |. Renal function, albuminuria, pathology
Urine samples were collected for albuminuria assessment using the ratio of urine albumin/urine Cr34 Serum BUN and Cr levels were determined, as described previously.14 Renal tissues were fixed in formalin buffer and paraffin-embedded and stained with hematoxylin and eosin (H&E). Scoring of renal pathology was evaluated as described previously, and glomerulonephritis activity was scored, as described previously.35 Methyl Carnoy solution-fixed and paraffin-embedded renal tissues were stained with antibodies against F4/80+ (Serotec, NC, USA), CD3+ (pan T cell; Dako, Glostrup, Denmark) or collagen IV (Santa Cruz, TX, USA), and frozen sections were stained with CD11c+ antibody (BioLegend, CA, USA).34 For scoring the number of CD3+-, F4/80+- , or CD11c+-positive cells, and collagen IV expression, quantitative image analysis software (Pax-it; Paxcam, IL, USA) was applied.36
2.5 |. Cultured cells
Murine macrophage cell line, J774A.1, was purchased from the American Type Culture Collection (USA) and was maintained in RPMI with 10% FBS (Invitrogen, USA).18
2.6 |. OVA-specific T cell proliferation assay
Bone marrow-derived dendritic cells (BMDCs) were isolated from tibias and femurs of 8-week-old female C57/BL6 mice.37 Cells were cultured in the presence of granulocyte-macrophage colony stimulating factor (GM-CSF; Invitrogen, CA USA) (10 ng/mL) for 6 days, to induce differentiation of DCs.37 For the proliferation assay, freshly isolated CD4+ T cells from lymph node cells of OT-II transgenic mice (kindly provided by Dr C. Lowell, University of California, USA) were co-cultured with LPS (100 ng/mL)-stimulated DCs and 1 μg/mL with OVA323-339 peptide (Genomics, Taiwan) at 1:4 DCs/T cell ratio by measuring uptake of [3H]-thymidine (Amersham Pharmacia Biotech, New Jersey, USA) in TopCount (PerkinElmer Life Sciences, Palo Alto, CA, USA).38 T cell proliferation of splenocytes was measured by [H3]-thymidine as described above.
2.7 |. Western blot analysis and immunoprecipitation
Protein lysates from tissues and cultured cells were run on SDS-PAGE gels.18 Anti-NLRP3 (AdiopGen, CA, USA), IL-18, caspase-1, β-actin (Santa Cruz, TX, USA), IL-1β (R&D, MN, USA), p-IκB, p-p38MAPK, p-ERK1/2, p-JNK1/2, double-stranded RNA-dependent protein kinase (PKR), NEK7 (Cell Signaling, MA, USA), ASC (Sigma), LC3B (GeneTex, CA, USA), p62 (Abcam, Bristol, UK) antibodies were used. For immunoprecipitation analysis, cell lysates were mixed with anti-NLRP3 antibody, and followed by addition of protein G beads. After elution, the samples were subjected to immunoblotting analysis using anti-PKR, NEK7 or ASC antibodies.
2.8 |. ASC oligomerization assay
Murine J774A.1 macrophages were lysed with 0.5% Triton X-100, protease inhibitors and phosphatase inhibitors, and followed by centrifugation. The ASC oligomerization were then analyzed by Western blotting as described previously.21
2.9 |. Real-time PCR assay
Total RNA was extracted from renal tissues with REzol (Protech Technology, Taipei, Taiwan) and cDNA was prepared as described previously.22 Real-time PCR reactions were conducted using SYBR Green RT-PCR Reagents Kit (Applied Biosystems, MA, USA). The primer pairs included: mouse iNOS forward: 5′-GGGCTGTCACGGAGATCA-3′; mouse iNOS reverse: 5′- CCATGATGGTCACATTCTGC-3′; and mouse Fizz-1 forward: 5′-ACCTTTCCTGAGATTCTGCCCC-3′; mouse Fizz-1 reverse: 5′-CAGTGGTCCAGTCAACGAGTAAGC-3′; mouse GAPDH forward: 5′-TCCGCCCCTTCTGCCGATG-3′; mouse GAPDH reverse: 5′-CACGGAAGGCCATGCCAGTGA-3′.
2.10 |. Flow cytometry
Mouse splenocytes were isolated and stained with allophycocyanin-conjugated anti-mouse CD3+ or CD4+, FITC-conjugated CD44+ and phycoerythrin-conjugated anti-mouse CD62L+ antibodies (all from BD Biosciences, CA, USA). For Treg cells, splenocytes were stained using a Mouse Regulatory T cell Staining Kit (eBioscience), according to the manufacturer’s instructions. Maturation of BMDCs was evaluated by CD11c+CD80+ and CD11c+CD86+ cells.37 A flow cytometer (FACSCalibur, BD Biosciences) was used.
2.11 |. ELISA and enzyme activity assay
Levels of LPS binding protein (LBP; MyBiosouce, Vancouver, Canada), IL-1β (R&D Systems, MN, USA), and anti-dsDNA antibody (Alpha Diagnostic, USA) were measured using commercial ELISA kits. Nuclear NF-κB p65 activity (Active Motif, Shinjuku-Ku, Tokyo, Japan) and cytoplasmic caspase-1 activity (R&D systems) were measured, according to the manufacturer’s instructions.
2.12 |. Renal levels of ROS
ROS levels in renal tissues were measured by a lucigeninenhanced chemiluminescence assay.35
2.13 |. Neutrophil influx in mice with MSU crystals-induced peritonitis
Eight-week-old female C57BL/6J were administered with MSU crystals ip for 0, 24, and 48 hours as described previously.39,40 Cf-02 (daily dose of 10 mg/kg body weight) dissolved in PEG 400 (Sigma) was given via an ip route (Cf-02 + MSU); another group of C57BL/6J was administered with vehicle only as the disease control group (Vehicle + MSU). Mice were sacrificed at 53 hours and the peritoneal cavity were lavaged with PBS. The absolute number of cells harvested was counted in a hemocytometer before subsequent analysis with flow cytometry as described previously.39,40
2.14 |. Statistical analysis
All data were presented as means ± SEM. Data from in vitro and in vivo experiments were analyzed using t-tests (two-tailed) for two groups or ANOVA (with Dunnett’s multiple comparisons test) for three or more groups. A P-value <.05 was considered significant.
3 |. RESULTS
3.1 |. Increased serum LBP levels in SLE patients and reduced IL-1β secretion in PBMCs by Cf-02 treatment
Ample evidence shows that patients with SLE and LN have experienced increased systemic exposure to bacteria, resulting in increased serum levels of LBP and its receptor CD14.6 Significantly, in patients with SLE, serum LBP levels were increased compared with those of Sjogren’s syndrome and normal subjects (Figure 1A). Furthermore, treatment with Cf-02, a small molecule that shows potent immunomodulatory effects, resulted in significantly reduced levels of IL-1β secretion by LPS-primed PBMCs collected from SLE patients, compared with those of saline controls (Figure 1B). These findings suggest that episodes of bacterial infection and may contribute to deterioration of the patients tested and Cf-02 may have therapeutic effects in SLE and LN.
FIGURE 1.
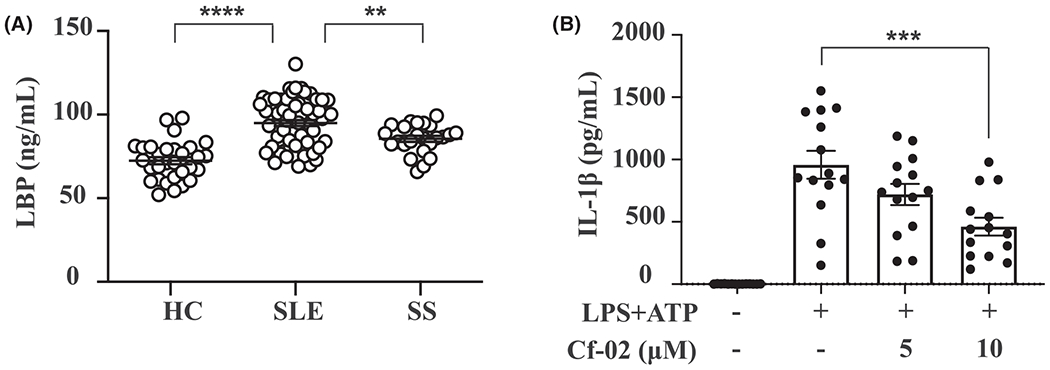
LPS-binding protein and IL-1β levels in SLE patients. A, Serum levels of LPS-binding protein. B, IL-1β secretion from PBMCs treated with LPS and ATP, followed by treatment with Cf-02. Data are presented as means ± SEM. Cf-02, a novel benzamide-linked small molecule; HC, healthy control; PBMCs, peripheral blood mononuclear cells; SLE, systemic lupus erythematosus; SS, Sjögren syndrome. **P < .01, ***P < .005, ****P < .001
3.2 |. Cf-02 treatment improves renal function, albuminuria and renal pathology
Next, we validated potential therapeutic effects of Cf-02 in an ASLN model in NZB/W F1 mice, which mimics acute onset of severe LN in human.17 LN was induced in these mice by intermittent LPS injections to simulate SLE patients following bacterial infections believed to act as environmental triggers. Cf-02 was administered daily after the onset of the renal condition in ASLN mice (ASLN + Cf-02 mice), while ASLN mice that received vehicle only (ASLN + Vehicle mice) were used as disease control mice. Elevated serum levels of BUN and Cr were seen in ASLN + Vehicle mice, but these effects were significantly inhibited in ASLN + Cf-02 mice (Figure 2A,B). In parallel, ASLN + Cf-02 mice exhibited greatly reduced albuminuria, compared with ASLN + Vehicle mice, which manifested overt albuminuria in comparison with saline control mice (Figure 2C). While ASLN + Vehicle mice developed serious pathological changes, including intrinsic cell proliferation, crescent formation, fibrinoid necrosis, neutrophil infiltration in the glomeruli, and periglomerular interstitial inflammation, these effects were significantly inhibited in ASLN + Cf-02 mice (Figure 2D–I ). ASLN + Cf-02 mice had significantly lower glomerulonephritis activity scores than ASLN + Vehicle mice (Figure 2J). In addition, significantly reduced renal collagen IV expression was observed in the ASLN + Cf-02 mice, compared with ASLN + Vehicle mice (Figure 2K,L). To elucidate the mechanism of action of the Cf-02 in its inhibiting neutrophil infiltration in the kidney, we performed an MSU crystals-induced peritonitis in mice.39,40 Although neutrophil recruitment was observed in peritoneal lavage fluid of the mice treated with MSU crystal, Cf-02 failed to reduce the severity of the peritonitis in the animals at the dose of 10 mg/kg of the compound (Figure 2M). Furthermore, we conducted an experiment to evaluate the effectiveness of MCC950, an NLRP3-specific inhibitor41 in the ASLN mice. The results show that reduced albuminuria and improved renal function were observed in the ASLN mice treated with MCC950, compared with those treated with saline alone (urine albumin/creatinine ratio, ASLN + MCC950, 0.35 ± 0.11 vs ASLN + Vehicle, 1.04 ± 0.30, P < .01; serum BUN, ASLN + MCC950, 65.91 ± 20.21 vs ASLN + Vehicle, 92.33 ± 15.66, P < .05; serum Cr, ASLN + MCC950, 0.42 ± 0.10 vs ASLN + Vehicle, 0.64 ± 0.15, P < .01).
FIGURE 2.
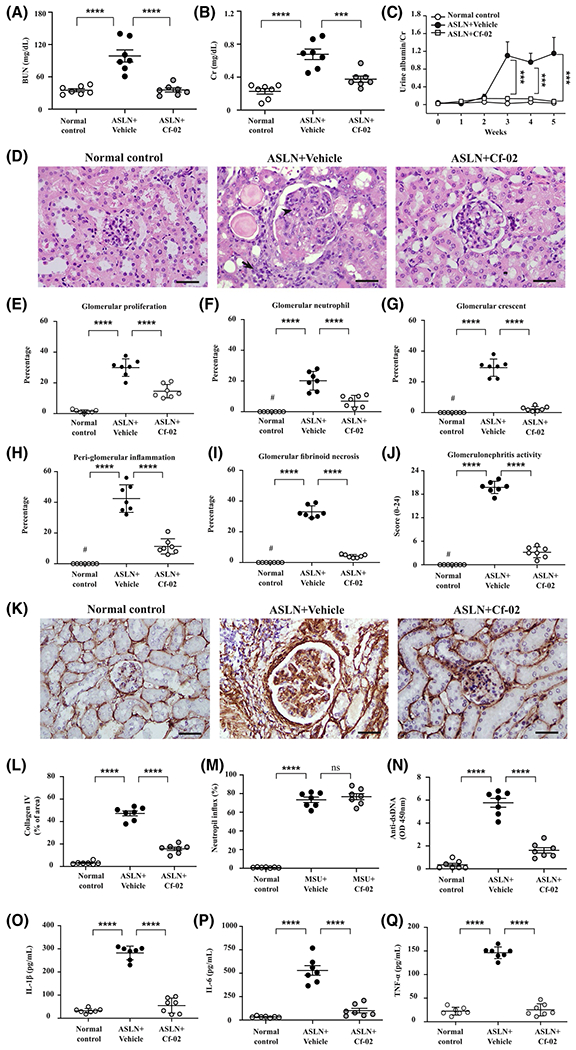
Renal function, albuminuria, pathology, neutrophil influx, IgG anti-dsDNA, and cytokines in ASLN mice. Serum levels of (A) BUN and (B) Cr; (C) Urine albumin levels; (D) Renal pathology, H&E staining. The arrows indicate mononuclear leukocyte infiltration. The arrowheads indicate neutrophil infiltration. E-I, Scoring for renal pathology; (J) Scoring for glomerulonephritis activity. K, Collagen IV by IHC staining. L, Scoring for IHC. M, Neutrophil influx was quantified in MSU crystals-induced peritonitis in mice by flow cytometry. N, Serum levels of IgG anti-dsDNA autoantibody; Serum levels of (O) IL-1β, (P) IL-6 and (Q) TNF-α. Scale bars = 50 μm. Original magnification 400×. Data are presented as means ± SEM for seven mice per group. ASLN, accelerated, severe lupus nephritis; BUN, blood urea nitrogen; Cf-02, a novel benzamide-linked small molecule; Cr, creatinine. ***P < .005, ****P < .001, #Not detectable. ns, no significant difference
3.3 |. Cf-02 treatment reduces levels of anti-dsDNA and cytokines in sera, inhibits renal immune cell infiltration, and regulates systemic T cell functions
Greatly reduced serum anti-dsDNA levels were observed in ASLN + Cf-02 mice, compared with increased serum anti-dsDNA levels in ASLN + Vehicle mice in relation to saline control mice (Figure 2N). In parallel, ASLN + Cf-02 mice showed significantly reduced serum levels of IL-1β, IL-6, and TNF-α compared with ASLN + Vehicle mice (Figure 2O–Q). In addition, treatment with Cf-02 resulted in greatly decreased levels of mononuclear leukocyte infiltration by F4/80+ macrophages, CD3+ T cells, and CD11c+ dendritic cells (DCs) in renal tissues of ASLN + Cf-02 mice, compared with those of ASLN + Vehicle mice, as demonstrated immunohistochemically (Figure 3AβF). Next, we evaluated the effect of Cf-02 on the polarization of M1 and M2 macrophages in renal tissues of the mice using real-time PCR assay. The results show that reduced renal mRNA levels of M1 (iNOS) and M2 (Fizz-1) was seen in ASLN + Cf-02 mice, compared to those of ASLN + Vehicle mice (Figure 3G,H), respectively, suggesting that treatment with Cf-02 affected macrophage polarization, which might be partly involved in the mechanism of action of the compound. Moreover, Cf-02 treatment inhibited CD4+ and CD8+ T cells (Figure 3I,J), but enhanced T reg cell differentiation (Figure 3K) and T cell proliferation (Figure 3L) in splenocytes from ASLN + Cf-02 mice. These results suggest that differential regulation of T cell activation and Treg differentiation may be involved in the therapeutic effect of this small molecule in this ASLN mouse model. Excessive activation of DCs and resultant dysregulation of T cell functions contribute to the progression of SLE and LN.42 Next, we examined whether Cf-02 can inhibit DCs by regulating T cell activation in a cell model. While ATP-activated, LPS-stimulated BMDCs showed increased percentages of CD11c+CD80+ and CD11c+CD86+ immune cells, treatment with Cf-02 abolished this effect of BMDCs maturation (Figure 3M,N). Furthermore, in an OVA-specific T cell proliferation assay, treatment with Cf-02 significantly reduced proliferation of CD4+ T cells that were co-incubated with activated DCs, compared with controls treated only with vehicle (DMSO) (Figure 3O).
FIGURE 3.
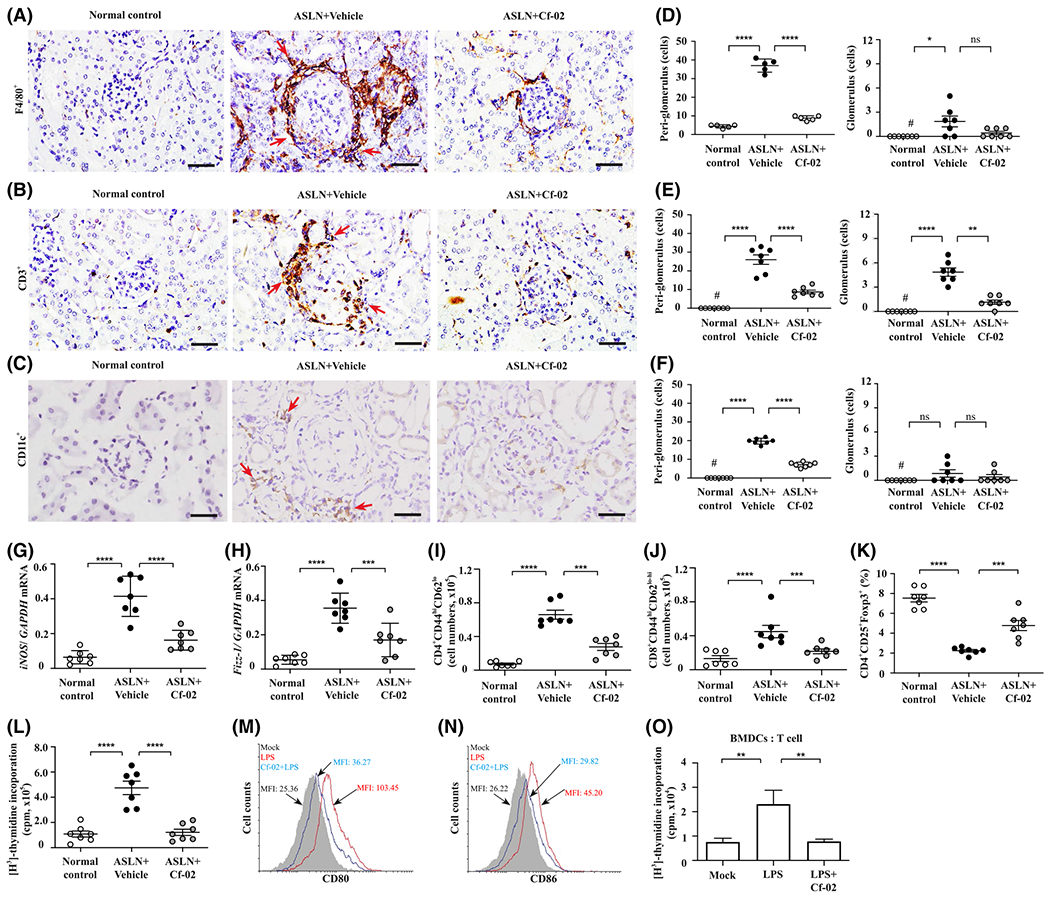
Immunohistochemistry, real-time PCR assay, flow cytometry in ASLN mice and OT-II antigen-specific T cell proliferation analysis. A, F4/80+ macrophages infiltrating the kidney; (B) CD3+ T cells infiltrating the kidney; (C) CD11c+ dendritic cells infiltrating the kidney. IHC, Original magnification, 400×. The arrows indicate mononuclear leukocyte infiltration; (D-F) Scoring for IHC. Renal mRNA levels of (G) M1 and (H) M2 by real-time PCR assay. I, CD4+CD44hiCD62Llo T memory cells; (J) CD8+CD44hiCD62Llo-hi T memory cells; (K) CD4+CD25+Foxp3+ Treg cells. Flow cytometry using splenocytes. L, T cell proliferation analysis using splenocytes by [H3]-thymidine incorporation assay. Data are presented as means ± SEM with seven mice per group. M, CD11c+CD80+ and (N) CD11c+CD86+ in BMDCs determined by flow cytometry. O, OT-II antigen-specific T cell proliferation analysis using [H3]-thymidine incorporation assay. A 1:4 ratio of BMDCs to CD4+ T cells was used. ASLN, accelerated, severe lupus nephritis; BMDCs, bone marrow derived dendritic cells; Cf-02, a novel benzamide-linked small molecule; MFI, mean fluorescent intensity. *P < .05, **P < .01, ***P < .005 and ****P < .001. #Not detectable. ns, no significant difference
3.4 |. Cf-02 treatment reduces renal ROS and inhibits NLRP3 inflammasome activation
Excessive production of ROS is implicated in activation of the NLRP3 inflammasome and subsequent involvement in development and progression of ASLN.15–17 ASLN + Cf-02 mice exhibited significantly reduced renal levels of ROS, compared with ASLN + Vehicle mice (Figure 4A). Additionally, significantly decreased renal NF-κB p65 activity was detected in ASLN + Cf-02 mice compared with ASLN + Vehicle mice (Figure 4B). Treatment with Cf-02 significantly reduced mRNA levels of NLRP3, IL-1β, and caspase-1 in renal tissues of ASLN + Cf-02 mice compared with ASLN + Vehicle mice (Figure 4C). In parallel, decreased protein levels of renal NLRP3, caspase-1 and IL-1β were observed in ASLN + Cf-02 mice, compared with those of ASLN + Vehicle mice (Figure 4D–G). Also, ASLN + Cf-02 mice showed significantly inhibited renal caspase-1 activity compared with ASLN + Vehicle mice (Figure 4H).
FIGURE 4.
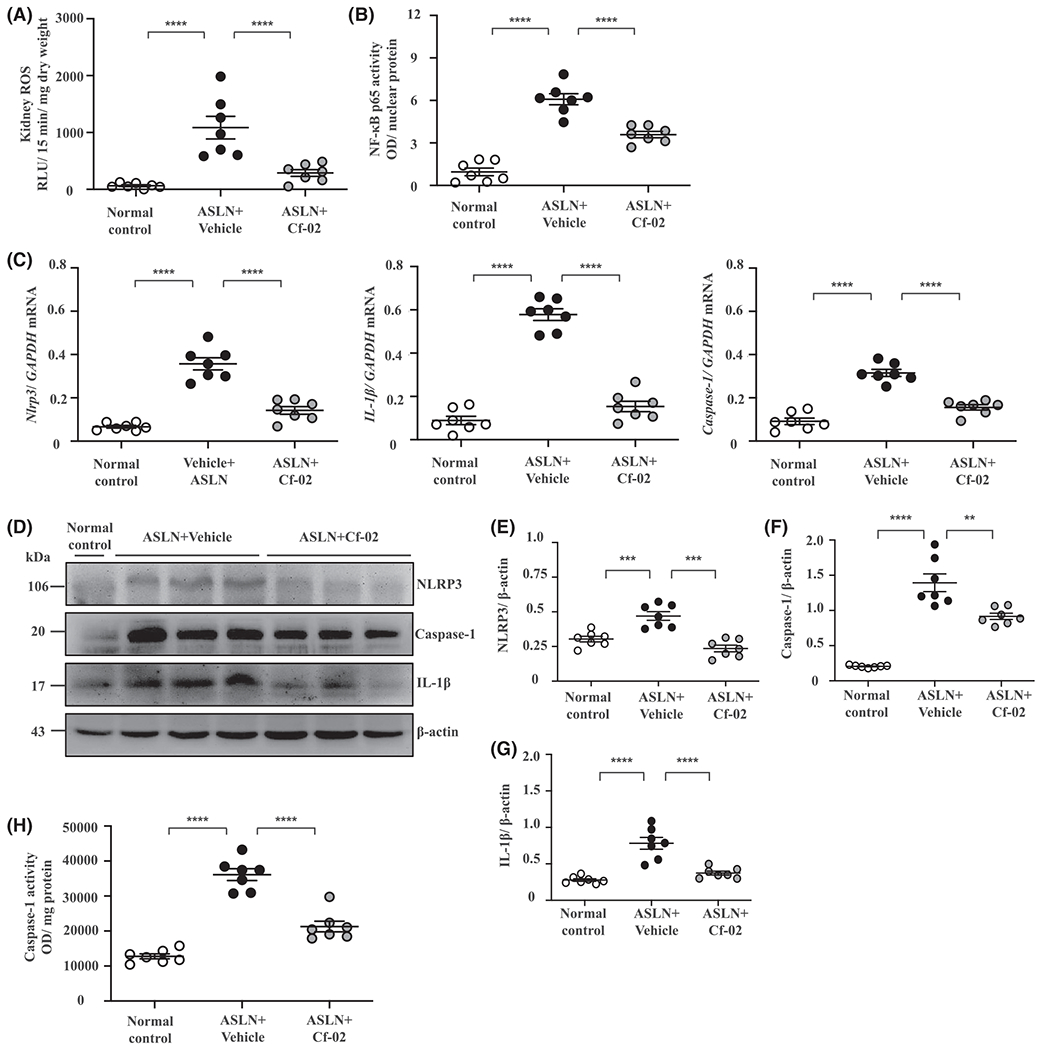
Renal ROS production and NLRP3 inflammasome activation in ASLN mice. A, ROS. B, NF-κB activity. C, mRNA levels of NLRP3, caspase-1, IL-1β. D, Protein levels NLRP3, caspase-1 and IL-1β; (E-H) Semiquantitative analysis. I, Caspase-1 activity. Data are presented as means ± SEM with seven mice per group. ASLN, accelerated, severe lupus nephritis; Cf-02, a novel benzamide-linked small molecule; ROS, reactive oxygen species. **P < .01, ***P < .005 and ****P < .001. #Not detectable. ns, no significant difference
3.5 |. Cf-02 treatment negatively regulates the NLRP3 inflammasome in vitro
3.5.1 |. Cf-02 inhibits priming and secondary signals during activation of the NLRP3 inflammasome
First, LPS-stimulated, ATP-activated macrophages secreted significantly more IL-1β compared with saline controls, as demonstrated by ELISA (Figure 5A). However, treatment with Cf-02 abolished this effect in a dose-dependent manner. Consistent with this, using Western blot analysis, it was clear that treatment with Cf-02 reduced production of IL-1β, IL- 18 (Figure 5B), caspase-1 (Figure 5C), NLRP3, and ASC (Figure 5D).
FIGURE 5.
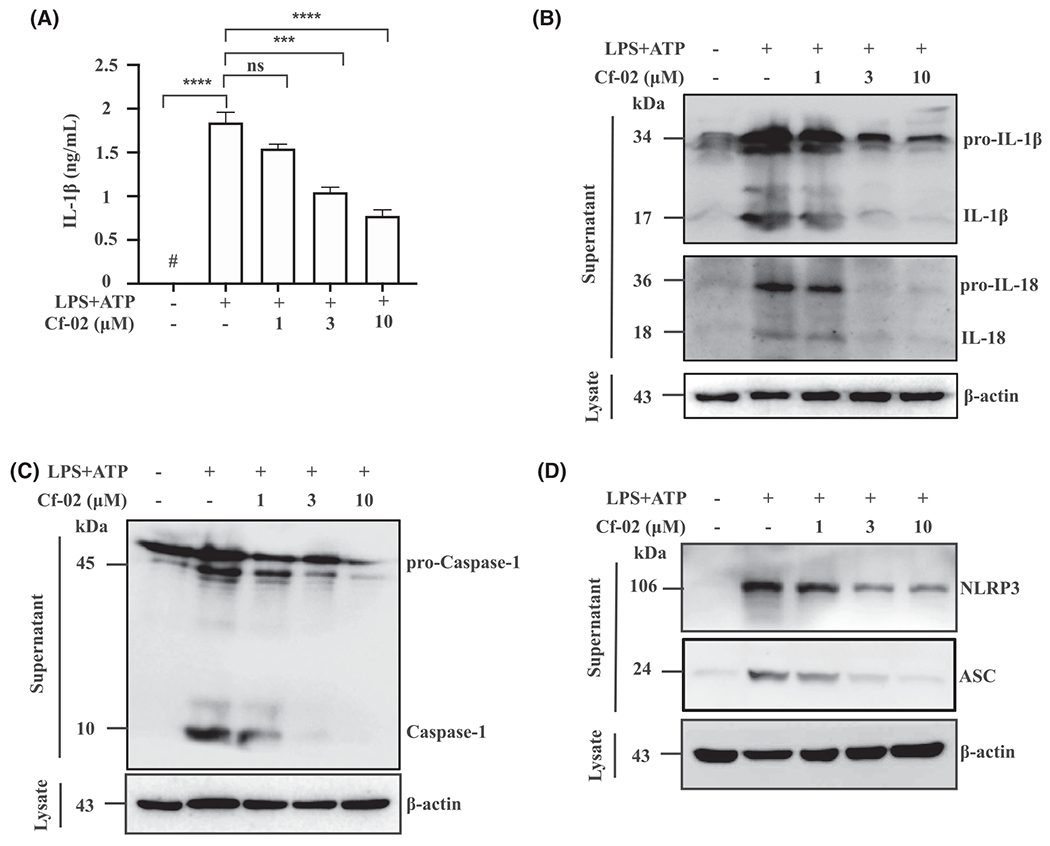
NLRP3 inflammasome activation in macrophages. A, IL-1β by ELISA. B, IL-1β, IL-18, (C) Caspase-1, (D) NLRP3 and ASC by Western blot analysis. Cells were incubated with 1 μg/mL of LPS for 5.5 hours, and then with Cf-02 at concentrations indicated for 30 minutes, followed by 5 mM ATP for 30 minutes. Data are presented as means ± SEM for three separate experiments, and each experiment was performed in triplicate. Casp-1, caspase-1; Cf-02, a novel benzamide-linked small molecule. ***P < .005 and ****P < .001. ns, no significant difference
Full activation of the NLRP3 inflammasome requires priming and secondary signals, in which the former determines expression of NLRP3 and pro-IL-1β, while the latter controls activation of caspase-1.43 Next, we investigated whether Cf-02 inhibited primary signal pathways of the NLRP3 inflammasome. Cf-02 significantly reduced levels of NLRP3 and pro-IL-1β in LPS-primed macrophage lysates in a dose-dependent manner (Figure 6A). In addition, MAPKs and NF-κB signal pathways are implicated in the priming stage of inflammasome activation. LPS-stimulated macrophages produced increased levels of p-ERK, p-JNK, and p-p38MAPK, but this effect was inhibited by treatment with Cf-02, except for p-p38MAPK (Figure 6B). In parallel, Cf-02 reduced p-I-κB levels in LPS-primed macrophages in a dose-dependent manner, compared with those of LPS-primed cells treated with vehicle (DMSO) only (Figure 6C). Together, these results suggest that Cf-02 inhibited activation of the NLRP3 inflammasome, by inhibiting part of MAPKs- or NF-κB-mediated inflammasome priming signal in macrophages.
FIGURE 6.
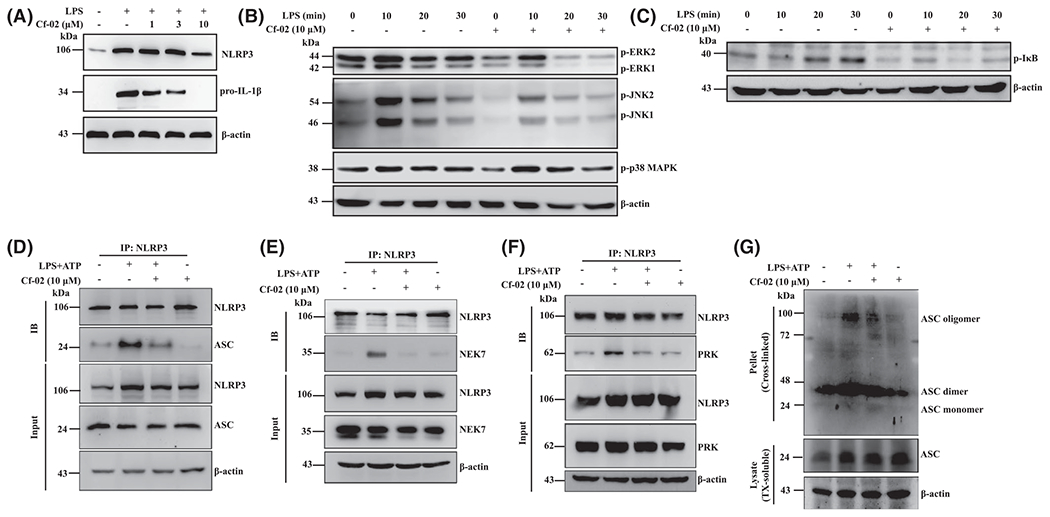
Phosphorylation of ERK1/2, JNK1/2, p38MAPK, I-κB, NLRP3 assembly and ASC oligomerization in macrophages. A, Protein levels of NLRP3, proIL-1β; (B) p-ERK1/2, p-JNK1/2, p-p38MAPK and (C) p-I-κB by Western blot analysis. Cells were incubated with Cf-02 at indicated concentrations for 30 minutes, followed by incubation with 1 μg/mL of LPS. NLRP3 assembly of (D) ASC-NLRP3; (E) NEK7-NLRP3; (F) PKR-NLRP3 by immunoprecipitation. G, ASC oligomerization by Western blot analysis. Cells were incubated with 1 μg/mL of LPS for 5.5 hours, and then with Cf-02 at concentrations indicated for 30 minutes, followed by 5 mM ATP for 30 minutes. Data are presented as means ± SEM with three separate experiments, and each experiment was performed in triplicate. Cf-02, a novel benzamide-linked small molecule; IB, immunoblotting; IP, immunoprecipitation
NLRP3 inflammasome assembly and ASC oligomerization are required to activate the NLRP3 inflammasome.44 NLRP3-NIMA-related kinase 7 (NEK7) interaction is involved in activation of the NLRP3 inflammasome, featuring ASC oligomerization and ASC speck formation.18,45 Amplification of PKR activates the NLRP3 inflammasome by physically interacting with NLRP3.18,46 Whereas the NLRP3-ASC complex formation was greatly enhanced in ATP-activated, LPS-stimulated macrophages, this effect was abrogated by Cf-02 (Figure 6D). Moreover, Cf-02 interrupted the interaction of NEK7 and NLRP3 (Figure 6E) or PKR and NLRP3 (Figure 6F) in ATP-activated, LPS-stimulated macrophages. Similarly, levels of ASC oligomerization were decreased by Cf-02 in ATP-activated, LPS-stimulated macrophages, compared with those treated with vehicle (DMSO) only (Figure 6G).
3.5.2 |. Cf-02 promotes autophagy-mediated inhibition of the NLRP3 inflammasome
Growing evidence confirms that autophagy negatively regulates activation of NLRP3 inflammation.16,19–21 First, treatment with Cf-02 enhanced LC3B-II and p62 expression in cultured macrophages at resting stage (without being activated by a particular stimulant) in a time-dependent manner (Figure 7A), and this effect was reversed by 3-MA, an autophagy inhibitor (Figure 7B). We then examined whether Cf-02 was able to activate autophagy, which further inhibited the NLRP3 inflammasome in activated macrophages. Cf-02 inhibited IL-1β secretion (Figure 7C) and production (Figure 7D) in ATP-activated, LPS-stimulated macrophages, an effect reversed by 3-MA.
FIGURE 7.
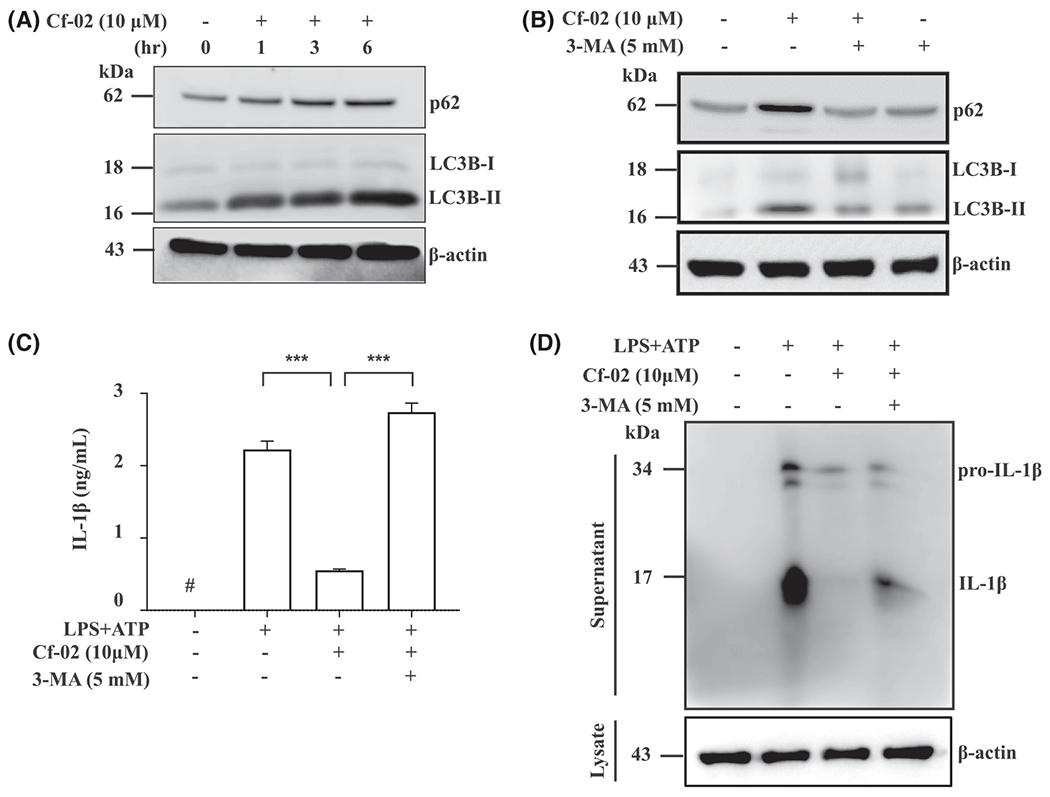
Activated autophagy-mediated inhibition of the NLRP3 inflammasome in macrophages. A, Protein levels of LC3B-I/II and p62 at a resting stage by Western blot analysis. Cells were incubated with Cf-02 at concentrations indicated in a time-dependent manner. B, Protein levels of LC3B-I/II and p62 by Western blot analysis. Cells were incubated with Cf-02 for 6 hours, and with or without 3-MA for 30 minutes (5 mM; an autophagy inhibitor). Protein levels of IL-1β by (C) ELISA and (D) Western blot analysis. Cells were incubated with 1 μg/mL of LPS for 5.5 hours, with or without 3-MA (5 mM) for 30 minutes, and Cf-02 for 30 minutes, followed by 5 mM ATP for 30 minutes. Data are presented as means ± SEM for three separate experiments, and each experiment was performed in triplicate. Cf-02, a benzamide-linked small molecule; 3-MA, 3-Methyladenine. ***P < .005. #Not detectable
4 |. DISCUSSION
LN remains a therapeutic enigma. The present study highlights potent therapeutic effects of Cf-02, a novel, ring-fused small molecule, in ASLN NZB/W F1 mice, a model of acute onset of progressive LN, and its mechanisms of action, which include differential regulation of the NF-κB/NLRP3 inflammasome axis, autophagy, and T cell functions. First, we demonstrated that treatment with Cf-02 significantly modulated activation of the NLRP3 inflammasome in ASLN mice. Treatment with this compound reduced infiltration of mononuclear leukocytes, including macrophages, DCs, and T cells into the kidneys of ASLN mice. Furthermore, treatment with Cf-02 suppressed T cell activation, but increased the proportion of Treg cells in ASLN mice. In specific cell models, we found that Cf-02 administration significantly decreased DCs maturation and inhibited proliferation in antigen-specific CD4+ T cells induced by activated DCs, as demonstrated in an in vitro OVA-specific T cell proliferation assay. These findings suggest that Cf-02 negatively regulates DCs function, thereby inhibiting the adaptive immune response in T cells. These combined actions may contribute to the mechanism of action by which Cf-02 exerts its therapeutic effects in this murine ASLN model.
Recently, we demonstrated that blockade of MAPK signaling pathways, p-ERK1/2, p-JNK1/2, and p-p38MAPK, resulted in inhibitory IL-1β mRNA and pro-IL-1β protein expression in LPS-activated macrophages.16,47 In the current study, blockade of p-ERK1/2, p-JNK1/2, and p-I-κB by Cf-02 inhibited NLRP3 expression in LPS-stimulated macrophages, suggesting that blocking of MAPK- and NF-κB-mediated priming of NLRP3 inflammasome activation may contribute to the mechanism by which Cf-02 exerts its therapeutic effects on ASLN in mice. Of note, PKR positively regulates the NLRP3 inflammasome by binding to NLRP3 and NEK7 serves as an essential mediator of NLRP3 inflammasome activation.18,45,46 Recently, we showed that PKR and NEK7 bind to NLRP3 upon ATP stimulation in LPS-activated macrophages.18 Consistent with these findings, Cf-02 treatment inhibited binding of NEK7 and PKR to NLRP3 in cultured macrophages. Cf-02 treatment abolished formation of a complex of ASC with NLRP3 and oligomerization of ASC upon ATP stimulation in LPS-stimulated macrophages. Therefore, negative regulation of NLRP3 inflammasome assembly and ASC oligomerization may be involved in the therapeutic effect of Cf-02 on ASLN in NZB/W F1 mice. On the other hand, whether there might be other molecular targets involved in the mechanism of action for the compound. In this regard, we found that (a) the potency of Cf-02 in suppressing the activation of NF-κB, STAT-3, and IRF-133 and (b) excessive activation of the NF-κB/NLRP3 inflammasome axis is involved in ASLN pathogenesis.16,17
Growing evidence confirms that autophagy negatively regulates activation of NLRP3 inflammation activation and controls production of IL-1β by degrading pro-IL-1β.16,19–21 Notably, autophagy participates in pathogenesis of SLE and LN and is implicated in transforming mild LN into severe LN.48,49 The current results show that Cf-02 confers its therapeutic effects in ASLN mice by inhibiting NLRP3 inflammasome activation, partially via autophagy-mediated blocking of the inflammasome. However, further investigation is required about whether other molecular targets that might be involved in the mechanism of action for the Cf-02 in the mouse model of ALSN.
To understand more about the mechanism of action of the Cf-02, we also think of the possibility whether it might involve another molecular pathway associated with IL-18, which is also produced by NLRP3 inflammasome activation.50 In the past, we found that IL-18 expression by mesangial cells is observed in the ASLN model, which is accompanied by neutrophil infiltration, intrinsic cell proliferation, and apoptosis in renal tissues.51,52 Moreover, IL-18 prolongs neutrophil survival in burns or other traumatic injuries,53 and this cytokine is involved in TNFα-mediated priming of neutrophil oxidase.54
In the present study, Cf-02 is a new drug candidate, the pharmacokinetic and dynamic (PK/PD) values of the compound deserve further investigation. Recently, we characterized the pharmacokinetic properties NSC765689, a derivative of the Cf-02. We presumed that its medicinal chemical properties and bioavailability of the Cf-02 could be extrapolated from the published data.55,56 However, PK/PD values of the Cf-02 and its dosing regimen as well as biochemical characteristics of the small compound should be further investigated to support this new tool compound as a credible drug candidate.
In summary, acute onset of severe LN was successfully treated in mice using a new, benzamide-linked, small molecule that targets immune modulation and the NLRP3 inflammasome (Figure 8). These results recommend Cf-02 as a promising drug candidate for the serious renal conditions associated with SLE. Future investigations should examine whether Cf-02 may also be therapeutic in other types of chronic kidney disease involving NLRP3 inflammasome-driven signaling.
FIGURE 8.
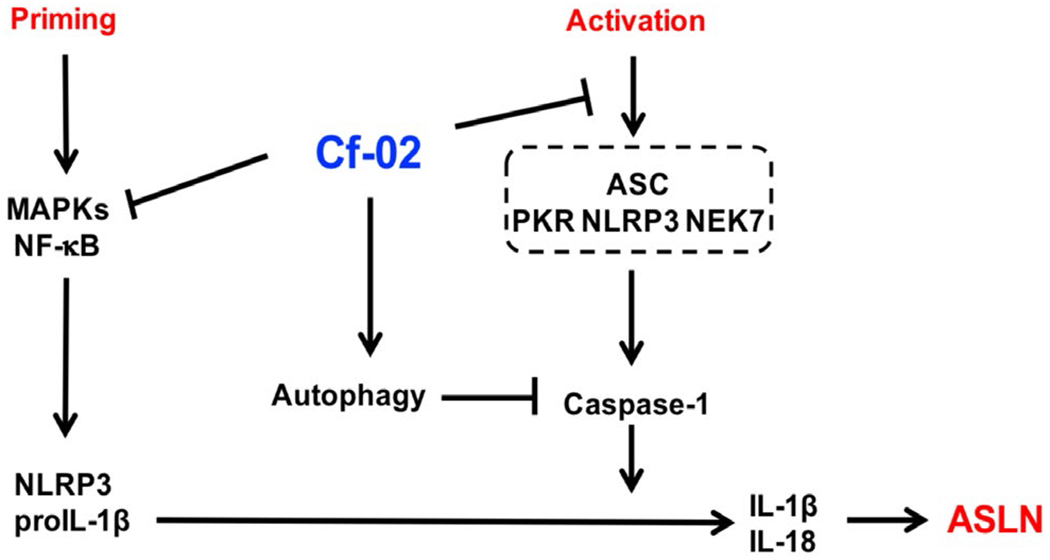
Schematic representation of the plausible mechanism of action for therapeutic effects of Cf-02 on ASLN. ASLN, accelerated, severe lupus nephritis; Cf-02, a novel benzamide-linked small molecule
ACKNOWLEDGMENTS
This work was supported by grants MOST 109-2320-B-016-008, MOST 109-2314-B-016-034-MY3 from the Ministry of Science and Technology, MAB-109-028 from the National Defense Medical Center, and TSGH-D-109090 from the Tri-Service General Hospital, Taipei, Taiwan.
Abbreviations:
- ASLN
accelerated, severe lupus nephritis
- BMDCs
bone marrow-derived dendritic cells
- BUN
blood urea nitrogen
- Cf-02
6-(2,4-difluorophenyl)-3-(3-(trifluoromethyl)phenyl)-2H-benzo[e][1,3]oxazine-2,4(3H)-dione
- Cr
creatinine
- H&E
hematoxylin and eosin
- HS-Cf
2-hydroxy-N-[3-(trifluoromethyl)phenyl]benzamide
- HS-Ck
N-(3-chloro-4-fluorophenyl)-2-hydroxybenzamide
- HS-Cm
N-(4-chloro-2-fluorophenyl)-2-hydroxybenzamide
- LBP
LPS binding protein
- LN
lupus nephritis
- LPS
lipopolysaccharide
- NEK7
NLRP3-NIMA-related kinase 7
- PBMCs
peripheral blood mononuclear cells
- PKR
double-stranded RNA-dependent protein kinase
- RANKL
receptor activator of nuclear factor kappa-B ligand
- ROS
reactive oxygen species
- SLE
systemic lupus erythematosus
Footnotes
CONFLICT OF INTEREST
Each author of this work declares no conflicts of interest.
REFERENCES
- 1.Tam LS, Li EK, Lai FM, Chan YK, Szeto CC. Mesangial lupus nephritis in Chinese is associated with a high rate of transformation to higher grade nephritis. Lupus. 2003;12:665–671. [DOI] [PubMed] [Google Scholar]
- 2.Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clin J Am Soc Nephrol. 2017;12:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–530. [DOI] [PubMed] [Google Scholar]
- 4.Kronbichler A, Kerschbaum J, Mayer G. The influence and role of microbial factors in autoimmune kidney diseases: a systematic review. J Immunol Res. 2015;2015:858027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whittall-Garcia LP, Torres-Ruiz J, Zentella-Dehesa A, et al. Neutrophil extracellular traps are a source of extracellular HMGB1 in lupus nephritis: associations with clinical and histopathological features. Lupus. 2019;28:1549–1557. [DOI] [PubMed] [Google Scholar]
- 6.Ayyappan P, Harms RZ, Buckner JH, Sarvetnick NE. Coordinated induction of antimicrobial response factors in systemic lupus erythematosus. Front Immunol. 2019;10:658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanoni I, Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front Cell Infect Microbiol. 2013;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landmann R, Knopf HP, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64:1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nockher W, Wigand R, Schoeppe W, Scherberich J. Elevated levels of soluble CD 14 in serum of patients with systemic lupus erythematosus. Clin Exp Immunol. 1994;96:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liphaus B, Kiss M, Carrasco S, Goldenstein-Schainberg C. Reduced expressions of Fas and Bcl-2 proteins in CD14+ monocytes and normal CD14 soluble levels in juvenile systemic lupus erythematosus. Lupus. 2013;22:940–947. [DOI] [PubMed] [Google Scholar]
- 11.El-Ghoneimy DH, Hesham M, Hasan R, Tarif M, Gouda S. The behavior of neutrophil extracellular traps and NADPH oxidative activity in pediatric systemic lupus erythematosus: relation to disease activity and lupus nephritis. Clin Rheumatol. 2019;38:2585–2593. [DOI] [PubMed] [Google Scholar]
- 12.Bruschi M, Bonanni A, Petretto A, et al. Neutrophil extracellular traps profiles in patients with incident systemic lupus erythematosus and lupus nephritis. J Rheumatol. 2020;47:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rother N, Pieterse E, Lubbers J, Hilbrands L, van der Vlag J. Acetylated histones in apoptotic microparticles drive the formation of neutrophil extracellular traps in active lupus nephritis. Front Immunol. 2017;8:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ka S-M, Lin J-C, Lin T-J, et al. Citral alleviates an accelerated and severe lupus nephritis model by inhibiting the activation signal of NLRP3 inflammasome and enhancing Nrf2 activation. Arthritis Res Ther. 2015;17:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin T-J, Wu C-Y, Tsai P-Y, et al. Accelerated and severe lupus nephritis benefits from M1, an active metabolite of ginsenoside, by regulating NLRP3 inflammasome and T cell functions in mice. Front Immunol. 2019;10:1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu CY, Hua KF, Chu CL, et al. Tris DBA ameliorates accelerated and severe lupus nephritis in mice by activating regulatory T cells and autophagy and inhibiting the NLRP3 inflammasome. J Immunol. 2020;204:1448–1461. [DOI] [PubMed] [Google Scholar]
- 17.Yang S-R, Hua K-F, Chu LJ, et al. Xenon blunts NF-κB/NLRP3 inflammasome activation and improves acute onset of accelerated and severe lupus nephritis in mice. Kidney Int. 2020;98(2):378–390. [DOI] [PubMed] [Google Scholar]
- 18.Chiu H-W, Li L-H, Hsieh C-Y, et al. Glucosamine inhibits IL-1β expression by preserving mitochondrial integrity and disrupting assembly of the NLRP3 inflammasome. Sci Rep. 2019;9:5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris J, Hartman M, Roche C, et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J Biol Chem. 2011;286:9587–9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahama M, Akira S, Saitoh T. Autophagy limits activation of the inflammasomes. Immunol Rev. 2018;281:62–73. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh C-Y, Li L-H, Lam Y, et al. Synthetic 4-hydroxy auxarconjugatin B, a novel autophagy inducer, attenuates gouty inflammation by inhibiting the NLRP3 inflammasome. Cells. 2020;9(2):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flanc RS, Roberts MA, Strippoli GF, Chadban SJ, Kerr PG, Atkins RC. Treatment of diffuse proliferative lupus nephritis: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2004;43:197–208. [DOI] [PubMed] [Google Scholar]
- 23.Illei GG, Austin HA, Crane M, et al. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med. 2001;135:248–257. [DOI] [PubMed] [Google Scholar]
- 24.Molino C, Fabbian F, Longhini C. Clinical approach to lupus nephritis: recent advances. Eur J Intern Med. 2009;20:447–453. [DOI] [PubMed] [Google Scholar]
- 25.Parikh SV, Rovin BH. Current and emerging therapies for lupus nephritis. J Am Soc Nephrol. 2016;27:2929–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponticelli C. New therapies for lupus nephritis. Clin J Am Soc Nephrol. 2006;1:863–868. [DOI] [PubMed] [Google Scholar]
- 27.Fu R, Guo C, Wang S, et al. Podocyte activation of NLRP3 inflammasomes contributes to the development of proteinuria in lupus nephritis. Arthritis Rheumatol. 2017;69:1636–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu FC, Huang HS, Huang CY, et al. A benzamide-linked small molecule HS-Cf inhibits TNF-α-induced interferon regulatory factor-1 in porcine chondrocytes: a potential disease-modifying drug for osteoarthritis therapeutics. J Clin Immunol. 2011;31:1131–1142. [DOI] [PubMed] [Google Scholar]
- 29.Liou JT, Huang HS, Chiang ML, et al. A salicylate-based small molecule HS-Cm exhibits immunomodulatory effects and inhibits dipeptidyl peptidase-IV activity in human T cells. Eur J Pharmacol. 2014;726:124–132. [DOI] [PubMed] [Google Scholar]
- 30.Lee C-C, Lo Y, Ho L-J, et al. A new application of parallel synthesis strategy for discovery of amide-linked small molecules as potent chondroprotective agents in TNF-α-stimulated chondrocytes. PLoS ONE. 2016;11:e0149317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C-C, Liu F-L, Chen C-L, et al. Novel inhibitors of RANKL-induced osteoclastogenesis: Design, synthesis, and biological evaluation of 6-(2, 4-difluorophenyl)-3-phenyl-2H-benzo [e][1, 3] oxazine-2, 4 (3H)-diones. Bioorg Med Chem. 2015;23:4522–4532. [DOI] [PubMed] [Google Scholar]
- 32.Lee C-C, Liu F-L, Chen C-L, Chen T-C, Chang D-M, Huang H-S. Discovery of 5-(2′, 4′-difluorophenyl)-salicylanilides as new inhibitors of receptor activator of NF-κB ligand (RANKL)-induced osteoclastogenesis. Eur J Med Chem. 2015;98:115–126. [DOI] [PubMed] [Google Scholar]
- 33.Liu F-C, Lu J-W, Chien C-Y, et al. Arthroprotective effects of Cf-02 sharing structural similarity with quercetin. Int J Mol Sci. 2018;19:1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang SM, Ka SM, Hua KF, et al. Antroquinonol mitigates an accelerated and progressive IgA nephropathy model in mice by activating the Nrf2 pathway and inhibiting T cells and NLRP3 inflammasome. Free Radic Biol Med. 2013;61:285–297. [DOI] [PubMed] [Google Scholar]
- 35.Hsu WH, Hua KF, Tuan LH, et al. Compound K inhibits priming and mitochondria-associated activating signals of NLRP3 inflammasome in renal tubulointerstitial lesions. Nephrol Dial Transplant. 2020;35:74–85. [DOI] [PubMed] [Google Scholar]
- 36.Tsai YL, Hua KF, Chen A, et al. NLRP3 inflammasome: pathogenic role and potential therapeutic target for IgA nephropathy. Sci Rep. 2017;7:41123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chi HH, Hua KF, Lin YC, et al. IL-36 signaling facilitates activation of the NLRP3 inflammasome and IL-23/IL-17 axis in renal inflammation and fibrosis. J Am Soc Nephrol. 2017;28:2022–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bickert T, Wohlleben G, Brinkman M, et al. Murine polyomavirus-like particles induce maturation of bone marrow-derived dendritic cells and proliferation of T cells. Med Microbiol Immunol. 2007;196:31–39. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh CY, Li LH, Rao YK, et al. Mechanistic insight into the attenuation of gouty inflammation by Taiwanese green propolis via inhibition of the NLRP3 inflammasome. J Cell Physiol. 2019;234:4081–4094. [DOI] [PubMed] [Google Scholar]
- 40.Wong W-T, Li L-H, Rao YK, et al. Repositioning of the β-blocker carvedilol as a novel autophagy inducer that inhibits the NLRP3 inflammasome. Front Immunol. 2018;9:1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coll RC, Robertson AAB, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan S, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol. 2007;178:271. [DOI] [PubMed] [Google Scholar]
- 43.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. [DOI] [PubMed] [Google Scholar]
- 45.He Y, Zeng MY, Yang D, Motro B, Nunez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu B, Nakamura T, Inouye K, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hung Y-L, Fang S-H, Wang S-C, et al. Corylin protects LPS-induced sepsis and attenuates LPS-induced inflammatory response. Sci Rep. 2017;7:46299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alessandri C, Barbati C, Vacirca D, et al. T lymphocytes from patients with systemic lupus erythematosus are resistant to induction of autophagy. FASEB J. 2012;26:4722–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou X-J, Klionsky DJ, Zhang H. Podocytes and autophagy: a potential therapeutic target in lupus nephritis. Autophagy. 2019;15:908–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shui H-A, Ka S-M, Wu W-M, et al. LPS-evoked IL-18 expression in mesangial cells plays a role in accelerating lupus nephritis. Rheumatology. 2007;46:1277–1284. [DOI] [PubMed] [Google Scholar]
- 52.Ka S-M, Lin J-C, Lin T-J, et al. Citral alleviates an accelerated and severe lupus nephritis model by inhibiting the activation signal of NLRP3 inflammasome and enhancing Nrf2 activation. Arthritis Res Ther. 2015;17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akhtar S, Li X, Kovacs EJ, Gamelli RL, Choudhry MA. Interleukin-18 delays neutrophil apoptosis following alcohol intoxication and burn injury. Mol Med. 2011;17:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silliman CC, Kelher MR, Gamboni-Robertson F, et al. Tumor necrosis factor-α causes release of cytosolic interleukin-18 from human neutrophils. Am J Physiol Cell Physiol. 2010;298:C714–C724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mokgautsi N, Wen Y-T, Lawal B, et al. An integrated bioinformatics study of a novel niclosamide derivative, nsc765689, a potential gsk3β/β-catenin/stat3/cd44 suppressor with anti-glioblastoma properties. Int J Mol Sci. 2021;22:2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawal B, Liu Y-L, Mokgautsi N, et al. Pharmacoinformatics and preclinical studies of nsc765690 and nsc765599, potential stat3/cdk2/4/6 inhibitors with antitumor activities against nci60 human tumor cell lines. Biomedicines. 2021;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]


