Abstract
Patient: Male, 31-year-old
Final Diagnosis: Lupus nephritis
Symptoms: Edema
Clinical Procedure: —
Specialty: Nephrology
Objective:
Unusual clinical course
Background:
Lupus nephritis (LN) is the most common and serious complication of systemic lupus erythematosus (SLE). Minimal change disease (MCD) and primary membranous nephropathy (PMN) are the 2 most common causes of primary nephrotic syndrome. Our purpose in publishing this case report is to introduce an unusual clinical course and initial renal biopsy revealed MCD and then PMN in second renal biopsy. Subsequently, a third renal biopsy resulted in a final diagnosis of LN. To the best of our knowledge, this is the first such report.
Case Report:
The 31-year-old male patient was initially diagnosed with MCD after the first renal biopsy in 2004. He improved with initial management and had a complete remission for 9 years. After 9 years, the patient again presented with heavy proteinuria without systemic lupus erythematous finding and he was diagnosed with MN following the second renal biopsy. Seven years later, he again developed proteinuria alone with concurrent systemic symptoms of systemic lupus erythematosus, and a third biopsy was performed, leading to final diagnosis as LN. He was well managed with the methylprednisolone and cyclophosphamide (CTX) regimen, which improved renal function and spared the patient from continuous hemodialysis.
Conclusions:
In rare case, MCD may represent an early phase of lupus nephritis, which may subsequently develop into severe lupus nephritis.
Keywords: Lupus Nephritis; Glomerulonephritis, Membranous; Lupus Erythematosus, Systemic; Nephrosis, Lipoid
Background
Lupus nephritis (LN) is one of the most common and serious complications of systemic lupus erythematosus (SLE) [1]. Minimal change disease (MCD) and primary membranous nephropathy (PMN) are the 2 most common causes of primary nephrotic syndrome. MCD followed by LN has rarely been reported, and MCD followed by PMN leading to LN is even rarer.
This report describes a case of a 31-year-old man who was initially diagnosed with MCD in 2004 and then PMN in 2013. Subsequently, a third renal biopsy resulted in a final diagnosis of LN.
Case Report
In 2004, 31-year-old man presented to our hospital with an initial presentation of facial edema for 7 days, with no history of diabetes, hypertension, or hepatitis B. Renal biopsy revealed MCD, with an almost normal appearance under light microscope, and absence of other immune deposits under immunofluorescence (Table 1), and other clinical and laboratory parameters are shown in Table 2. At that time, the patient was treated with oral prednisolone 50 mg/d for 12 months. After sustaining complete remission for 1 year, the predniso-lone was stopped with tapering dosage.
Table 1.
Pathological characteristics of the 3 renal biopsies.
| Parameters | First renal biopsy | Second renal biopsy | Third renal biopsy |
|---|---|---|---|
| Pathological diagnosis | Minimal change glomerulopathy | Membranous nephropathy (II period) | Membranous lupus nephritis |
| IgA in renal biopsy | Negative | negative | 2+, GCW and mesangium |
| IgG in renal biopsy | Negative | 3+, GCW | 3+, GCW and mesangium |
| IgM in renal biopsy | Negative | negative | 2+, GCW and mesangium |
| C3 in renal biopsy | Negative | 2+, GCW | 3+, GCW and mesangium |
| C1q in renal biopsy | Negative | 1–2+, GCW | 3+, GCW and mesangium |
| IgG1 in renal biopsy | ∼ | 2+, GCW | 3+, GCW and mesangium |
| IgG2 in renal biopsy | ∼ | ∼ | 2+, GCW and mesangium |
| IgG3 in renal biopsy | ∼ | ∼ | 2+, GCW and mesangium |
| IgG4 in renal biopsy | ∼ | 2+, GCW | – |
| κ in renal biopsy | ∼ | +− | +, GCW and mesangium |
| λ in renal biopsy | ∼ | + | +, GCW and mesangium |
| PLA2R in renal biopsy | ∼ | ∼ | – |
GCW – glomerular capillary walls’; “∼” – specific laboratory test not done; “−” – negative
Table 2.
Clinical and Laboratory findings during the 3 instances of renal biopsies.
| Parameters | First renal biopsy | Second renal biopsy | Third renal biopsy | Normal range |
|---|---|---|---|---|
| Blood pressure (mmHg) | 126/81 | 155/113 | 126/81 | – |
| Serum creatinine (mg/dL) | 1.53 | 0.92 | 7.51 | 0.65–1.26 |
| Serum urea (mmol/L) | 8.5 | 3.4 | 22.64 | 3.1–8 |
| Serum albumin (g/L) | 10.8 | 19.6 | 14.2 | 35–55 |
| Hb (g/L) | 152 | 85 | 71 | 120–180 |
| 24h urine protein (g) | 3.8 | 8.1 | 1.5 | <0.15 |
| Hematuresis (/µl) | 10 | 564.3 | 1498.4 | 0–12 |
| Serum a-PLA2R | ∼ | ∼ | 1:64 | >1: 20 |
| ANA | Negative | Negative | 1+ | Negative |
| Anti-dsDNA (IU/ml) | Negative | Negative | >200.0 | 0–10 |
| ENA | Negative | Negative | – | Negative |
| c-ANCA | – | Negative | Negative | Negative |
| p-ANCA | – | Negative | 1+ | Negative |
| MPO (RU/mL) | 0.0–19.9 | – | <2.0 | 0.0–19.9 |
| PR3 (RU/mL) | 0.0–19.9 | – | 36.9 | 0.0–19.9 |
| GBM (U/mL) | ∼ | ∼ | 3.6 | 0.0–19.9 |
| C3 in serum (mg/L) | Within normal limits | Within normal limits | 489 | 900–1800 |
| C4 in serum (mg/L) | Within normal limits | Within normal limits | 74 | 100–400 |
ANA – antinuclear antibody; anti-dsDNA – anti-double-stranded DNA; ENA – extractable nuclear antigen; ANCA – perinuclear antineutrophil cytoplasmic antibody; MPO – myeloperoxidase; PR3 – proteinase 3; GBM – glomerular basement membrane. “∼” – specific laboratory test not done; “–” – negative.
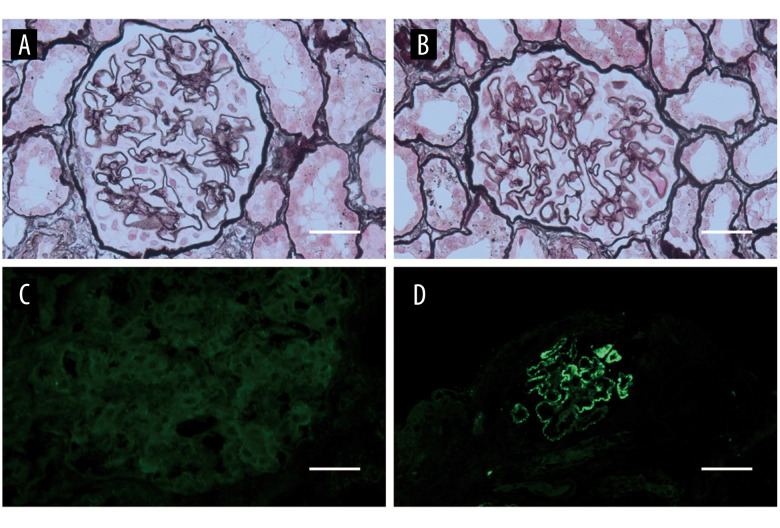
The patient remained apparently well without any clinical manifestation or relapse for about 9 years. In 2013, he was hospitalized with a history of recurrent edema. A second renal biopsy was performed and resulted in a diagnosis of PMN (Tables 1, 2 and Figure 1). His condition was controlled well with oral prednisolone 25 mg/d and oral cyclophosphamide (CTX) 100 mg/d (9 g in total). After 6 months of continuous treatment, the patient achieved complete remission.
Figure 1.
The second renal biopsy and histopathology of the patient in 2013. (A, B) Thickened glomerular basement membrane, normal mesangial cellularity (PASM ×400) (C, D) Immunofluorescence microscopy revealing negative findings for IgA and (++) findings for IgG1 (immunofluorescence ×400 and ×100). PASM – periodic Schiff-Methenamine.
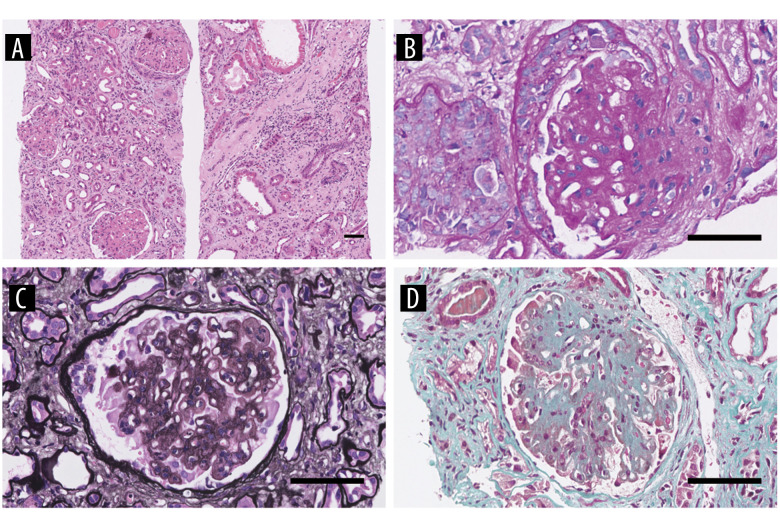
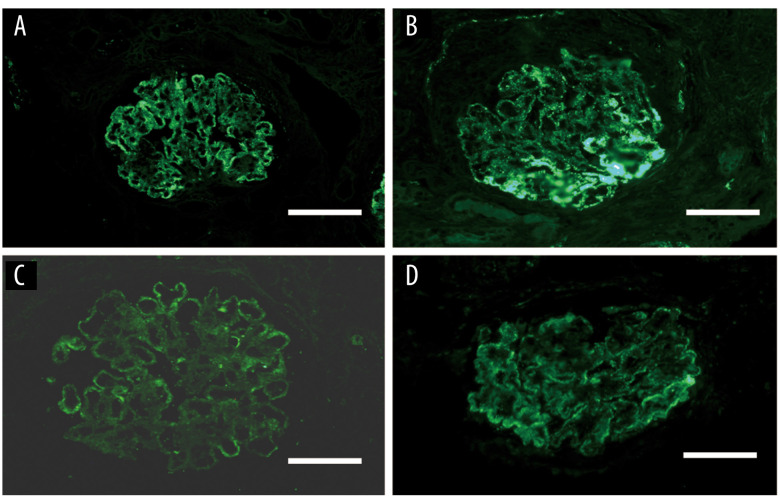
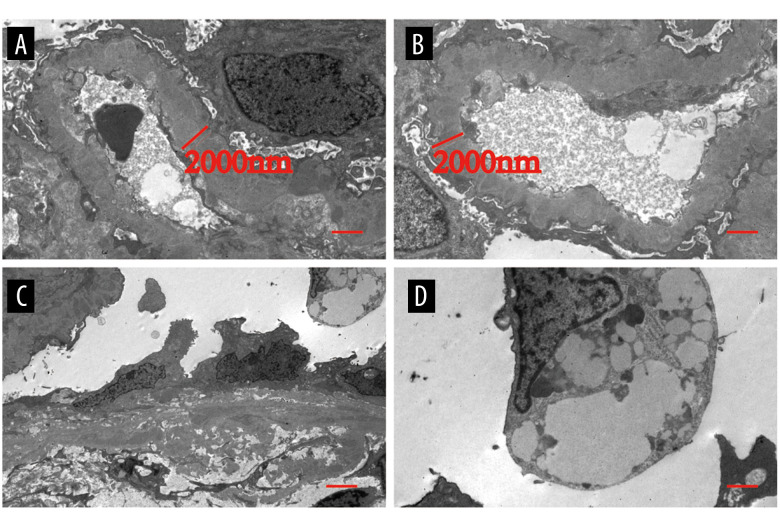
In May 2020, he was evaluated at the outpatient department for recurrence of severe edema and he was suggested for renal biopsy. The third renal biopsy showed diffuse nodular and sclerosing LN with membranous LN (Class IV-G (A/C) + V). The glomerular basement membranes were irregularly thickened, and the foot processes of the podocytes were diffusely coalescing (Tables 1–3, and Figures 2–4). The patient was administered oral methylprednisolone 50 mg/d and oral CTX 100mg/d(6 g in total) and received hemodialysis. After 2 months, hemodialysis was terminated and his serum creatinine was 3.13 mg/dL. The patient was maintained on follow-up at the outpatient department.
Table 3.
Treatment protocols following the 3 renal biopsies.
| Parameters | First renal biopsy | Second renal biopsy | Third renal biopsy |
|---|---|---|---|
| Therapy | Prednisone, 50 mg qd, 6 months | Prednisone, 25 mg qd + CTX, 100 mg qd (9 g in total) | Hemodialysis, prednisone, 50 mg + CTX, 100 mg qd (6 g in total) |
| Prognosis | Urine proteins turn negative | Repeated recurrence | ∼ |
“∼” – no specification.
Figure 2.
The third renal biopsy of the patient in 2020. (A–D) A light micrograph shows diffuse mesangial and endothelial proliferation, capillary occlusion, basement membrane thickening, nail-like structures, segmented mesangial insertion and double rail formation. (A) HE ×100 (B) PAS ×400 (C) PASM ×400 (D) Masson ×400.
Figure 3.
The third renal biopsy of the patient in 2020. (A–D) Membranous ++/+++ positivity for (A) IgG, (B) IgG1, (C) IgA, (D) C1q (immunofluorescence ×400).
Figure 4.
The third renal biopsy of the patient in 2020. (A–D) The basement membrane is thickened, epithelial cells are swollen, and vacuolar degeneration is observed. Epithelial cells are swollen, and vacuolar degeneration is observed. Foot processes are fused. HE – hematoxylin and eosin; PAS – periodic acid Schiff.
Discussion
The diagnosis of this case evolved from MCD to PMN, and then to SLE nephritis. Upon initial renal biopsy, MCD was diagnosed by histopathology. Many studies have reported that MCD is closely related to SLE [2,3]. Moreover, SLE can develop simultaneously with MCD or following MCD. Conversely, MCD can develop during the course of SLE. The relationship between MCD and SLE may be related to T-cell dysfunction [2]. The pathological mechanisms in lupus nephritis are mainly based on defective clearance of apoptotic cells and impaired neutrophil extracellular trap (NET) degradation [4]. Apoptosis fragments exist in the extracellular matrix and circulates in SLE patients. The abnormal process of apoptosis and insufficient clearance of apoptotic cells may lead to activation of the innate immune system and adaptive immune system. In addition, the abnormal presentation of peptide by antigen-presenting cells, the disorder of the lymphocyte selection process, and the imbalance of lymphocyte response may also add to the pathogenesis of SLE [5]. Membranous nephropathy (MN) is related to apoptosis via the PI3K/AKT/mTOR pathway [6]. In addition, Smad7 is up-regulated in podocytes of MCD, MN, and LN [7]. Therefore, the main pathogeneses for MCD, MN, and LN are all closely related to apoptosis and/or abnormal inflammation process or are disorder related.
Another interesting finding was that there were (1–2 +) C1q deposits in mesangial and capillary walls in the second renal biopsy and (3 +) C1q deposits in the third renal biopsy. This is also consistent with previous studies. Zhang et al [8] found that 22.9% of PMN patients had weak (1+) C1q deposits along the glomerular capillary walls. C1q is associated with secondary membranous nephropathy, especially SLE [9,10]. Song et al reported that most cases of LN exhibit strongly positive immunofluorescence findings for deposition for IgG1, IgG2, and IgG3 but negative for IgG4. Most PMN, on the other hand, were positive for IgG1 and IgG4 [11]. This is consistent with the diagnostic findings for separate instances of LN and PMN in this case. A previous study reported that a 25-year-old woman diagnosed with PMN was subsequently diagnosed with LN 4 years later [12]. Therefore, attention must be given to auto-antibody levels even when diagnosis of SLE among patients is precluded by current SLE diagnostic criteria.
Our patient was positive for serum circulating a-PLA2R(ELESA detection) and negative for PLA2R in the podocytes, based on the third renal biopsy. Laurence [13] first reported that circulating serum a-PLA2R was present among patients with PMN while serum samples from 6 MLN patients were negative for the marker. However, circulating a-PLA2R was detected in a separate study in 10 (5.3%) of 190 SLE patients [14]. That study and our case both suggest that circulating a-PLA2R is not specific for PMN and that LN may also be associated with this laboratory finding.
Conclusions
In summary, we described a very rare case of LN in a man previously diagnosed with PLA2R-associated membranous LN after 3 separate occasions of undergoing renal biopsy. Prior diagnosis of MCD and MN does not exclude the probability of subsequently developing SLE. Renal biopsy is crucial for the diagnosis of renal diseases and should be repeated as necessary.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References:
- 1.Borchers AT, Leibushor N, Naguwa SM, et al. Lupus nephritis: A critical review. Autoimmun Rev. 2012;12(2):174–94. doi: 10.1016/j.autrev.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Wei X, Wang Y, Tu L, et al. Acute kidney injury associated with minimal change disease in systemic lupus erythematosus: A case report. J Med Case Rep. 2014;8:422. doi: 10.1186/1752-1947-8-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang YT, Chou HH, Chen FF, et al. A case of minimal-change nephrotic syndrome in pediatric lupus erythematosus: Just a coincidence? Lupus. 2006;15(4):244–47. doi: 10.1191/0961203306lu2285cr. [DOI] [PubMed] [Google Scholar]
- 4.Chauhan SK, Rai R, Singh VV, et al. Differential clearance mechanisms, neutrophil extracellular trap degradation and phagocytosis, are operative in systemic lupus erythematosus patients with distinct autoantibody specificities. Immunol Lett. 2015;168(2):254–59. doi: 10.1016/j.imlet.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Rekvig OP, Van der Vlag J. The pathogenesis and diagnosis of systemic lupus erythematosus: Still not resolved. Semin Immunopathol. 2014;36(3):301–11. doi: 10.1007/s00281-014-0428-6. [DOI] [PubMed] [Google Scholar]
- 6.Chiou TT, Chau YY, Chen JB, et al. Rapamycin attenuates PLA2R activation-mediated podocyte apoptosis via the PI3K/AKT/mTOR pathway. Biomed Pharmacother. 2021;144:112349. doi: 10.1016/j.biopha.2021.112349. [DOI] [PubMed] [Google Scholar]
- 7.Schiffer M, Schiffer LE, Gupta A, et al. Inhibitory smads and tgf-Beta signaling in glomerular cells. J Am Soc Nephrol. 2002;13(11):2657–66. doi: 10.1097/01.asn.0000033276.06451.50. [DOI] [PubMed] [Google Scholar]
- 8.Zhang MF, Cui Z, Zhang YM, et al. Clinical and prognostic significance of glomerular C1q deposits in primary MN. Clin Chim Acta. 2018;485:152–57. doi: 10.1016/j.cca.2018.06.050. [DOI] [PubMed] [Google Scholar]
- 9.Walport MJ, Davies KA, Botto M. C1q and systemic lupus erythematosus. Immunobiology. 1998;199(2):265–85. doi: 10.1016/S0171-2985(98)80032-6. [DOI] [PubMed] [Google Scholar]
- 10.Robson MG, Cook HT, Botto M, et al. Accelerated nephrotoxic nephritis is exacerbated in C1q-deficient mice. J Immunol. 2001;166(11):6820–28. doi: 10.4049/jimmunol.166.11.6820. [DOI] [PubMed] [Google Scholar]
- 11.Song YS, Min KW, Kim JH, et al. Differential diagnosis of lupus and primary membranous nephropathies by IgG subclass analysis. Clin J Am Soc Nephrol. 2012;7(12):1947–55. doi: 10.2215/CJN.04800511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shearn MA, Hopper J, Jr, Biava CG. Membranous lupus nephropathy initially seen as idiopathic membranous nephropathy. Possible diagnostic value of tubular reticular structures. Arch Intern Med. 1980;140(11):1521–23. [PubMed] [Google Scholar]
- 13.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Vives E, Solé C, Moliné T, et al. Antibodies to M-type phospholipase A2 receptor (PLA2R) in membranous lupus nephritis. Lupus. 2019;28(3):396–405. doi: 10.1177/0961203319828521. [DOI] [PubMed] [Google Scholar]