TO THE EDITOR: The 5-hydroxytryptamine 4 (5-HT4) receptor in the enteric nervous system is involved in enhanced neurotransmission, leading to increased gastrointestinal motility, and its agonists, such as prucalopride and mosapride, are currently used in clinical practice as prokinetic drugs. Although both drugs are 5-HT4 agonists, their indications vary as chronic constipation and functional dyspepsia, respectively. Compared to prucalopride, mosapride exhibited a relatively weak effect on colonic motility, but the reason is not apparent. After absorption in the small intestine, mosapride is metabolized to M1 by the first-pass mechanism, which has a 5-HT3 antagonistic effect. We speculated that metabolite M1 could offset the prokinetic effect of mosapride. Therefore, it was hypothesized that direct colonic administration of mosapride could increase motility by acting directly on the colon before conversion to M1 compared with oral administration.
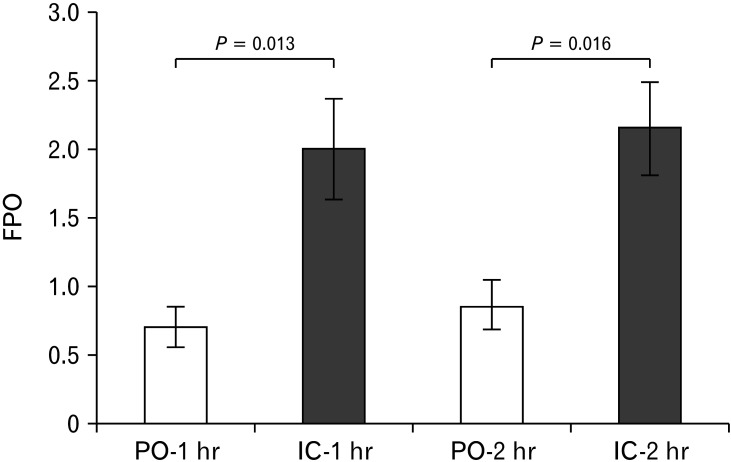
We performed a proof-of-concept experiment using a rat model that established an intracolonic administration route. After anesthesia, the right lower abdomen was incised, and the cecum was exteriorized. A PE 90 (BD company, Franklin Lakes, NJ, USA) tube was inserted into cecum and fixed by suture. After abdomen closure, the opposite end of the tube was exteriorized at the back of the neck via a subcutaneous tunnel from the right lower abdomen. The rats were fed freely during the experimental period and 20 mg/kg of mosapride or vehicle in 0.5 mL volume was administered by oral or intracolonic route on different days. A total of 7 animals were tested 2 times in a cross-over manner with a 3-day washout period. After either oral or intracolonic administration of mosapride, the number of fecal pellets that were expelled within 1 hour and 2 hours was counted. Accordingly, we could confirm that the number of expelled fecal pellets was significantly higher after colonic administration than oral administration, both at 1 hour and 2 hours (Figure). This result indicated that the intracolonic application of mosapride is more effective in increasing colon movement than oral intake. We proposed that the colon-specific drug delivery formulation of mosapride could be used as an effective and safe treatment for constipation.
Figure.
Fecal pellet output after mosapride administration. Data expressed as mean ± SE. To compare fecal pellet output (FPO) between intracolonic (IC) and peroral (PO) both at 1 hour and 2 hours, Wilcoxon rank sum test was performed.
Funding Statement
Financial support: This work is supported by Wonkwang University 2021 (M.Y.L.).
Footnotes
Conflicts of interest: None.
Author contributions: Moon Young Lee and Yong Sung Kim contributed the concept of design, data analysis, and manuscript writing and revision; and Min Seob Kim contributed the data acquisition and data analysis.