Abstract
Background
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) considers blood eosinophil counts < 100 cells/μL (BEC≤100) in people with COPD to predict poor inhaled corticosteroid (ICS) responsiveness. However, the BEC≤100 phenotype is inadequately characterized, especially in advanced COPD.
Research Question
Are there differences between GOLD group D patients with high BEC and those with low BEC regarding baseline characteristics and longitudinal outcomes?
Study Design and Methods
We used multivariable mixed models and logistic regression to contrast clinical characteristics and outcomes of BEC≤100 vs BEC > 100 (BEC100+) in all subjects with COPD (n = 1,414) and GOLD group D subjects (n = 185) not receiving ICS.
Results
We identified n = 485 with BEC≤100 (n = 61 GOLD group D) and n = 929 people with BEC100+ (n = 124 GOLD group D). BEC≤100 status was stable at 6 weeks and approximately 52 weeks (intraclass correlations of 0.78 and 0.71, respectively). Compared with BEC100+, BEC≤100 comprised more women, with greater current smoking, and less frequent childhood asthma. Among all analyzed participants, the two BEC-defined subsets showed similar rates of lung function decline (mean slope, BEC≤100 vs BEC100+, –50 vs –39 mL/y; P = .140), exacerbations (0.40 vs 0.36/y; P = .098), subsequent ICS initiation (2.5% vs 4.4%; P = .071), and mortality (7.8% vs 8.4%; P = .715). However, in GOLD group D, people with BEC≤100 showed higher exacerbation rates within 365 days of enrollment (0.62 vs 0.33/y; P = .002) and total follow-up (1.16 vs 0.83/y; P = .014). They also had greater lung function decline (mean slope of –68 mL/y vs –23 mL/y; P = .036) and had greater emphysema at baseline (voxels < 950 Hounsfield units at total lung capacity of 7.46% vs 4.61%; P = .029).
Interpretation
In non-ICS-treated GOLD group D COPD, people with BEC≤100 had more baseline emphysema, prospective exacerbations, and lung function decline. Our analysis has identified a particularly vulnerable subpopulation of people with COPD, suggesting the need for studies focused specifically on their therapeutic treatment.
Clinical Trial Registration
ClinicalTrials.gov; No.: NCT01969344; URL: www.clinicaltrials.gov
Key Words: COPD, eosinophil, GOLD group D, inhaled corticosteroid
FOR EDITORIAL COMMENT, SEE PAGE 467
Take-home Points.
StudyQuestion: Do patients with group D COPD and low blood eosinophil counts (BECs) differ from those with high BEC regarding baseline characteristics and longitudinal outcomes?
Results: We found that group D COPD patients with low BEC had greater emphysema at baseline, had higher exacerbation rates in the first year and throughout the course of the study, and had greater lung function decline during the course of the study compared with their peers with high BEC.
Interpretation: Group D COPD patients with low BEC represent a distinct cohort characterized by greater emphysema, more exacerbations, and greater lung function decline as compared with those with high BEC.
The Global Initiative for Obstructive Lung Disease (GOLD) identifies patients with COPD in GOLD group D (GOLD D) as symptomatic people with at least one severe or two moderate acute exacerbations in the year before assessment. Current guidelines propose considering avoiding use of inhaled corticosteroids (ICS) in GOLD group D patients with blood eosinophil counts (BECs) < 100/μL (BEC≤100), with limited evidence to support this recommendation.1 However, more recent results suggest that low BEC is associated with severity of emphysema2, 3, 4, 5 and is associated with worse survival and longer hospital stays in hospitalized patients with COPD exacerbations.6,7
To provide evidence to support management decisions in BEC≤100, we performed a longitudinal analysis of people with COPD not receiving ICS, contrasting BEC≤100 with normal or high blood eosinophil counts (BEC100+). We hypothesized that differences that occurred in smaller, more defined subgroups, such as GOLD group D, might be hidden in larger cohorts of subjects with a broader range of COPD severity. Therefore, we analyzed data for both groups in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS), a large, observational cohort of people with or at risk of developing COPD.
Study Design and Methods
Study Design and Participants
SPIROMICS is an ongoing multicenter, observational study that between 2010 and 2015 enrolled 2,983 participants aged 40 to 80 years.8 Participants included those who had either never (≤ 1 pack-year) or had currently or previously smoked cigarettes (≥ 20 pack-years); the latter two groups were with or without COPD as classified by GOLD guidelines.9 All investigations were conducted according to the principles of the Declaration of Helsinki; institutional review boards at each participating site reviewed and approved protocols; and all participants provided written informed consent. SPIROMICS did not mandate uniform therapies.
Data Collection
We evaluated participants at baseline and annual in-person visits, with additional quarterly telephone surveys. Each patient was monitored for 3 years. Pulmonary function testing (based on 2005 American Thoracic Society/European Respiratory Society guidelines10) was done, and 6-min walk distance (6MWD) was determined, at enrollment and annual visits. Chest CT scans, at full inspiration (total lung capacity) and expiration (residual volume), were acquired at enrollment and 1-year follow-up. Using those scans, we performed parametric response mapping to assess functional small-airway disease quantitatively.11 We assessed respiratory symptoms and disease-specific health status by self-report, using the modified Medical Research Council dyspnea score, COPD Assessment Test (CAT), and St. George’s Respiratory Questionnaire (SGRQ). We elicited acute COPD exacerbation (AECOPD) data through quarterly telephone calls and yearly follow-up visits, defining AECOPD as symptom worsening requiring antibiotics and/or systemic corticosteroids (moderate) or treatment in ED or hospital settings (severe). BECs were obtained by differential blood counts from the clinical laboratories at visits 1, 2, and 4, which we dichotomized as ≤ 100/μL for BEC≤100 and > 100/μL for BEC100+ according to the baseline values.
The value of 100 was included in the low BEC cohort because many laboratories report blood eosinophils in intervals of 100.
Statistical Analyses
Demographic, comorbidity, and baseline clinical characteristics of participants were evaluated by χ2 or Kruskal-Wallis test for categorical or continuous variables, respectively, and were stratified by BEC≤100 vs BEC100+. Using one-way analysis of variance to calculate the between-subject variation and within-subject variation, we performed repeatability analysis, using intraclass correlation coefficients (ICCs) between measures at enrollment and at 6-week and 1-year follow-up. ICC interpretations were as follows: < 0.50, poor; 0.50 to 0.75, moderate; 0.75 to 0.90, good; > 0.90, excellent.12 To evaluate the relationship of some baseline characteristics with BEC among those not receiving ICS or oral steroids at baseline, we built multivariate regression models adjusted for age, race, sex, BMI, history of asthma, chronic bronchitis, smoking status, and pack-years. These characteristics were lung function, 6MWD, imaging variables, and symptoms measured by CAT or SGRQ. Because of the skewed distribution of some data including complement C3, C-reactive protein, fibrinogen, and various quantitative CT scan characteristics, those values were log-transformed and summarized as geometric means to facilitate interpretation.
We used mixed-effects linear regression models adjusted for age, race, sex, BMI, history of asthma, chronic bronchitis, smoking status, and pack-years to evaluate the relationship between baseline BEC groups and longitudinal changes in clinical measures (FEV1, CAT, 6MWD, and SGRQ). Annual exacerbation rates were determined by dividing the number of reported exacerbations by the number of years in the study and were further analyzed using zero-inflated negative binomial regression models, adjusted as described above with the addition of exacerbations in the year before enrollment; in this analysis, we included only participants with complete 3-year follow-up data. P < .05 was considered statistically significant. All analyses were conducted with SAS 9.4 (SAS Institute).
Results
Study Cohort
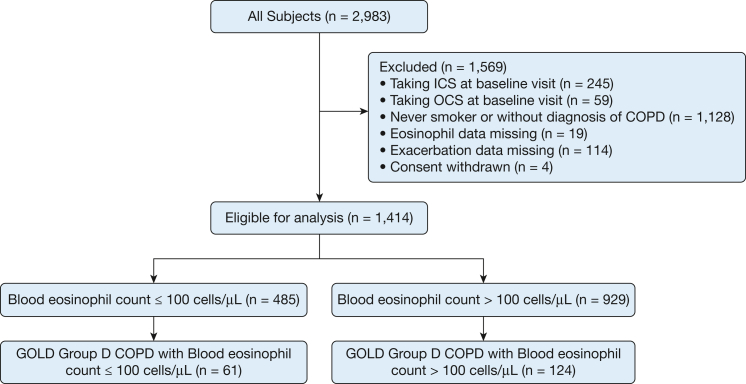
From the entire SPIROMICS cohort, we identified n = 1,414 participants diagnosed with COPD who were taking neither ICS nor oral corticosteroids at enrollment, of whom n = 185 were classified as GOLD D. At the baseline visit of these participants with COPD who were not taking ICS/oral corticosteroids, BEC≤100 was noted in a substantial fraction of both all (n = 485; 34.3%) and GOLD D participants (n = 61; 33.0%) (Fig 1).
Figure 1.
Enrollment and outcomes. GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled corticosteroids; OCS = oral corticosteroids.
Stability of BEC≤100 Classification
To evaluate the stability of BEC classification on repeated testing, we analyzed data from the SPIROMICS repeatability substudy, in which a subset of participants underwent repeated assessment 6 weeks after enrollment.13 Among 53 subjects who underwent repeated BEC analysis, BEC classifications were moderately repeatable (ICC, 0.78). To analyze longer-term stability, we compared the BEC values of all participants (n = 1,136) who underwent multiple analyses during the study and found similar repeatability (ICC, 0.71) of BEC classification.
Baseline Characteristics
BEC≤100 participants with COPD were more frequently women and currently smoking, but without higher smoking burden by pack-years than BEC100+ participants (Table 1). BEC≤100 participants had lower BMI and fibrinogen values, less frequently had a history of childhood asthma, and were less often prescribed a long-acting muscarinic antagonist or diagnosed with COPD before enrollment. However, there was no significant difference in baseline FEV1 (BEC≤100, 1.81 vs 1.83 L; P = .673) or different distribution of GOLD A-D groups between the two eosinophil subsets. In adjusted multivariable analysis, BEC≤100 participants had lower C-reactive protein than did BEC100+ participants (Table 2).
Table 1.
Baseline Univariate Analysis for Eligible Participants
| Variable | All Subjects With COPD |
GOLD Group D Subjects |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 ≤ Eosinophils ≤ 100 (n = 485) |
Eosinophils > 100 (n = 929) |
P Value | 0 ≤ Eosinophils ≤ 100 (n = 61) |
Eosinophils > 100 (n = 124) |
P Value | |||||
| No. | Value | No. | Value | No. | Value | No. | Value | |||
| Age, mean (SD), y | 485 | 65.18 (8.32) | 929 | 65.40 (7.79) | .6258 | 61 | 60.97 (8.74) | 124 | 62.90 (8.32) | .145 |
| Male, % | 267 | 55 | 573 | 62 | .0167 | 32 | 52 | 62 | 50 | .7574 |
| White, % | 405 | 84 | 759 | 82 | .3731 | 40 | 67 | 98 | 80 | .0678 |
| Hispanic, % | 15 | 3 | 41 | 4 | .2528 | 2 | 3 | 6 | 5 | .7224 |
| Current individuals who smoke, % | 188 | 39 | 300 | 33 | .0152 | 28 | 47 | 37 | 31 | .0478 |
| History of childhood asthma, % | 29 | 6 | 91 | 10 | .0118 | 5 | 9 | 13 | 12 | .6063 |
| BMI, mean (SD) | 485 | 26.64 (5.05) | 929 | 27.71 (5.23) | < .001 | 61 | 26.60 (5.65) | 124 | 27.33 (5.45) | .4004 |
| Smoking pack-years, mean (SD) | 485 | 54.32 (29.83) | 928 | 52.60 (25.06) | .2782 | 61 | 49.87 (21.03) | 124 | 51.66 (22.43) | .6041 |
| CRP, mean (SD), mg/dL | 282 | 5.39 (11.00) | 566 | 6.49 (9.69) | .1563 | 29 | 11.76 (22.78) | 64 | 7.30 (8.75) | .3159 |
| Fibrinogen, mean (SD), mg/dL | 282 | 5.12 (1.29) | 566 | 5.42 (1.50) | .0024 | 29 | 5.27 (1.62) | 64 | 5.77 (1.70) | .1868 |
| Ferritin, mean (SD), μg/L | 282 | 165.18 (178.82) | 566 | 164.55 (150.32) | .9594 | 29 | 192.72 (169.26) | 64 | 128.66 (120.38) | .0732 |
| IL-17, mean (SD), pg/mL | 282 | 1.58 (0.44) | 566 | 1.68 (0.83) | .0249 | 29 | 1.47 (0.04) | 64 | 1.61 (0.73) | .1195 |
| IL-6, mean (SD), pg/mL | 282 | 9.84 (86.36) | 566 | 4.39 (11.22) | .292 | 29 | 3.68 (1.87) | 64 | 3.96 (5.46) | .719 |
| TNF, mean (SD), pg/mL | 282 | 13.15 (6.34) | 566 | 14.61 (33.16) | .3128 | 29 | 13.47 (7.15) | 64 | 13.30 (5.98) | .906 |
| Post-FEV1, mean (SD), L | 485 | 1.81 (0.77) | 928 | 1.83 (0.77) | .6729 | 61 | 1.42 (0.59) | 124 | 1.40 (0.66) | .8578 |
| Post-FVC, mean (SD), L | 485 | 3.39 (1.05) | 928 | 3.43 (1.04) | .5271 | 61 | 3.11 (0.99) | 124 | 2.98 (0.92) | .3945 |
| PRMfSAD, mean (SD), % | 424 | 25.89 (13.42) | 832 | 25.99 (14.17) | .9019 | 50 | 29.74 (15.06) | 107 | 29.97 (14.16) | .9258 |
| Airway wall thickness, mean (SD), mm | 477 | 1.24 (0.19) | 907 | 1.26 (0.20) | .1805 | 59 | 1.24 (0.20) | 120 | 1.23 (0.20) | .8194 |
| Voxels < 865 HU at RV, mean (SD), % | 479 | 32.68 (20.65) | 921 | 31.88 (20.48) | .4881 | 58 | 40.08 (21.53) | 123 | 39.25 (20.97) | .8056 |
| Voxels < 950 HU at RV, mean (SD), % | 479 | 5.80 (8.93) | 921 | 5.40 (8.10) | .4197 | 58 | 8.90 (8.88) | 123 | 7.96 (9.33) | .5182 |
| 6-Min walk distance, mean (SD), m | 468 | 394.35 (112.04) | 883 | 386.61 (112.76) | .2291 | 55 | 351.55 (133.68) | 110 | 337.34 (109.80) | .468 |
| Voxels < 865 HU at TLC, mean (SD), % | 481 | 66.11 (14.48) | 924 | 65.29 (14.37) | .3091 | 59 | 63.51 (17.79) | 123 | 65.87 (16.08) | .3711 |
| Voxels < 865 HU at RV, mean (SD), % | 481 | 33.54 (17.36) | 924 | 32.51 (16.71) | .2821 | 59 | 36.74 (18.81) | 123 | 35.96 (17.75) | .7867 |
| Voxels < 950 HU at TLC in LLL, mean (SD), % | 481 | 8.38 (10.37) | 924 | 7.88 (9.80) | .3712 | 59 | 12.40 (12.74) | 123 | 9.75 (10.21) | .1657 |
| Voxels < 950 HU at TLC in LUL, mean (SD), % | 481 | 12.27 (13.64) | 924 | 11.14 (12.14) | .1269 | 59 | 16.32 (13.69) | 123 | 15.43 (14.68) | .6964 |
| Voxels < 950 HU at TLC in RUL, mean (SD), % | 481 | 13.46 (15.83) | 924 | 12.45 (14.61) | .2436 | 59 | 18.26 (16.44) | 123 | 17.22 (17.29) | .6981 |
| % Emphysema at TLC, mean (SD) | 481 | 11.18 (11.63) | 924 | 10.39 (10.78) | .191 | 59 | 15.36 (12.46) | 123 | 13.84 (12.20) | .435 |
| SGRQ, score (SD) | 437 | 35.15 (20.40) | 833 | 36.40 (19.21) | .2818 | 55 | 54.80 (15.12) | 117 | 51.47 (13.83) | .1557 |
| CAT, score (SD) | 469 | 14.75 (8.01) | 887 | 14.73 (7.90) | .9706 | 61 | 21.00 (6.67) | 124 | 20.38 (6.33) | .5384 |
| FEV1 bronchodilator response, % | 196 | 40 | 350 | 38 | .357 | 18 | 30 | 36 | 29 | 1.000 |
| Eosinophils (% of WBC count), mean (SD) | 168 | 0.62 (1.59) | 329 | 1.56 (5.01) | .0018 | 12 | 0.36 (0.58) | 21 | 6.31 (17.01) | .1245 |
| Eosinophils, mean (SD), cells/μL | 485 | 82.80 (32.70) | 929 | 275.76 (206.43) | < .001 | 61 | 76.36 (36.38) | 124 | 269.51 (120.17) | < .001 |
| History of exacerbations, % | ||||||||||
| 0 | 353 | 72.78 | 648 | 69.75 | … | 21 | 34.43 | 35 | 28.23 | … |
| 1 | 81 | 16.7 | 149 | 16.04 | … | 21 | 34.43 | 52 | 41.94 | … |
| 2 | 26 | 5.36 | 71 | 7.64 | … | 19 | 31.15 | 37 | 29.84 | … |
| 3 | 20 | 4.12 | 49 | 5.27 | … | 0 | 0 | 0 | 0 | … |
| Missing | 5 | 1.03 | 12 | 1.29 | .411 | 0 | 0 | 0 | 0 | .5693 |
| GOLD stage, % | ||||||||||
| 1 | 125 | 25.77 | 214 | 23.04 | … | 4 | 6.56 | 12 | 9.68 | … |
| 2 | 217 | 44.74 | 437 | 47.04 | … | 23 | 37.7 | 45 | 36.29 | … |
| 3 | 107 | 22.06 | 203 | 21.85 | … | 26 | 42.62 | 43 | 34.68 | … |
| 4 | 36 | 7.42 | 75 | 8.07 | … | 8 | 13.11 | 24 | 19.35 | … |
| Missing | 0 | 0 | 0 | 0 | .6735 | 0 | 0 | 0 | 0 | .5529 |
| mMRC, % | ||||||||||
| 0 | 125 | 25.77 | 230 | 24.76 | … | 0 | 0.0 | 2 | 1.61 | … |
| 1 | 219 | 45.15 | 422 | 45.43 | … | 3 | 4.92 | 10 | 8.06 | … |
| 2 | 85 | 17.53 | 166 | 17.87 | … | 23 | 37.70 | 49 | 39.52 | … |
| 3 | 42 | 8.66 | 80 | 8.61 | … | 23 | 37.70 | 32 | 25.81 | … |
| 4 | 12 | 2.47 | 26 | 2.8 | … | 8 | 13.11 | 24 | 19.35 | … |
| Missing | 2 | 0.41 | 5 | 0.54 | .9957 | 4 | 6.56 | 7 | 5.65 | .4773 |
| GOLD group based on mMRC, % | ||||||||||
| A | 316 | 65.15 | 558 | 60.06 | … | … | … | … | … | … |
| B | 90 | 18.56 | 184 | 19.81 | … | … | … | … | … | … |
| C | 32 | 6.6 | 84 | 9.04 | … | … | … | … | … | … |
| D | 35 | 7.22 | 68 | 7.32 | … | … | … | … | … | … |
| Missing | 12 | 2.47 | 35 | 3.77 | .2379 | … | … | … | … | … |
| GOLD group based on CAT, % | ||||||||||
| A | 136 | 28.04 | 232 | 24.97 | … | … | … | … | … | … |
| B | 254 | 52.37 | 479 | 51.56 | … | … | … | … | … | … |
| C | 6 | 1.24 | 20 | 2.15 | … | … | … | … | … | … |
| D | 61 | 12.58 | 124 | 13.35 | … | … | … | … | … | … |
| Missing | 28 | 5.77 | 74 | 7.97 | .2963 | … | … | … | … | … |
Data are presented as mean (SD) or No. (%). CAT = COPD Assessment Test; CRP = C-reactive protein; GOLD = Global Initiative for Chronic Obstructive Lung Disease; HU = Hounsfield unit; LLL = left lower lobe; LUL = left upper lobe; mMRC = modified Medical Research Council; PRMfSAD = parametric response mapping functional small-airway disease; RUL = right upper lobe; RV = residual volume; SGRQ = St. George’s Respiratory Questionnaire; TLC = total lung capacity; TNF = tumor necrosis factor.
Table 2.
Multivariate Analysis of Both All Analyzed Participants and Those With Group D COPD
| Variable | COPD (n = 1,414) |
Group D COPD (n = 185) |
||||
|---|---|---|---|---|---|---|
| BEC≤100 (n = 485) | BEC100+ (n = 929) | P Valuea | BEC≤100 (n = 61) | BEC100+ (n = 124) | P Valuea | |
| ICS initiation, No. (%) | 12 (2.5) | 41 (4.4) | .071 | 10 (16.4) | 16 (12.9) | .917 |
| Survival, No. (%) | 447 (92.2) | 851 (91.6) | .715 | 51 (83.6) | 108 (87.0) | .521 |
| 6MWD, mean (95% CI), m | 391.8 (381.7-401.9) | 390.98 (383.4-398.6) | .901 | 343.90 (309.8-378.0) | 346.2 (321.7-370.7) | .914 |
| SGRQ score, mean (95% CI) | 35.9 (34.3-37.3) | 35.1 (33.9-36.3) | .419 | 54.6 (50.8-58.5) | 51.14 (48.5-53.8) | .15 |
| CAT score, mean (95% CI) | 14.80 (14.2-15.5) | 14.4 (13.9-14.9) | .36 | 20.52 (18.8-22.3) | 20.4 (19.1-21.6) | .897 |
| Voxels < 865 HU at RV, mean (95% CI), % | 33.4 (31.8-35.0) | 31.1 (29.9-32.3) | .026 | 43.5 (38-2-48.8) | 38.8 (35.1-42.4) | .154 |
| Voxels < 950 HU at TLC, mean (95% CI), % | 3.9 (3.5-4.4) | 3.6 (3.3-3.9) | .232 | 7.5 (5.3-10.6) | 4.6 (3.6-5.9) | .029 |
| Airway wall thickness (Pi10), mean (95% CI), mm | 3.86 (3.8-3.9) | 3.86 (3.85-3.87) | .438 | 3.91 (3.87-3.96) | 3.85 (3.92-3.88) | .033 |
| PRMfSAD, mean (95% CI), % | 26.27 (25.1-27.5) | 25.54 (24.65-26.44) | .35 | 29.79 (25.66-33.92) | 29.74 (26.89-32.58) | .983 |
| CRP, mean (95% CI), mg/dL | 2.56 (2.2-3.0) | 3.12 (2.81-3.46) | .032 | 3.46 (1.97-6.1) | 5.25 (3.55-7.76) | .238 |
| Fibrinogen, mean (95% CI), g/L | 5.02 (4.9-5.2) | 5.21 (5.09-5.32) | .075 | 5.11 (4.54-5.75) | 5.71 (5.26-6.2) | .132 |
| Ferritin, mean (95% CI), μg/L | 107.3 (95.4-120.7) | 110.9 (101.83-120.78) | .657 | 142.22 (93.215.85) | 96.95 (72.63-129.42) | .141 |
| AEs, 1-y rate (95% CI) | 0.16 (0.1-0.2) | 0.13 (0.09-0.19) | .138 | 0.62 (0.35-1.11) | 0.33 (0.18-0.59) | .002 |
| AEs, total rate (95% CI) | 0.40 (0.3-0.5) | 0.36 (0.3-0.44) | .098 | 1.16 (0.72-1.86) | 0.83 (0.51-1.34) | .014 |
| Severe AEs, total rate (95% CI) | 0.23 (0.16-0.37) | 0.19 (0.13-0.27) | .097 | 0.48 (0.26-0.89) | 0.39 (0.2-0.76) | .258 |
| AEs requiring antibiotics, total rate (95% CI) | 0.34 (0.27-0.43) | 0.30 (0.24-0.37) | .184 | 0.71 (0.39-1.3) | 0.50 (0.28-0.92) | .020 |
| AEs requiring steroids, total rate (95% CI) | 0.35 (0.27-0.45) | 0.32 (0.25-0.41) | .365 | 0.93 (0.55-1.55) | 0.71 (0.42-1.20) | .102 |
Multivariate regression analysis was adjusted for age, race, sex, BMI, history of asthma, chronic bronchitis, smoking status, and pack-years. 6MWD = 6-min walk distance (m); AE = acute exacerbation; BEC = blood eosinophil count; CAT = COPD Assessment Test; CRP = C-reactive protein; HU = Hounsfield unit; ICS = inhaled corticosteroids; Pi10 = average wall thickness for an airway of 10-mm lumen perimeter on CT scan imaging; PRMfSAD = parametric response mapping functional small-airway disease; RV = residual volume; SGRQ = St. George’s Respiratory Questionnaire; TLC = total lung capacity.
Data are presented as means (95% CI), No. (%), or rate (95% CI) as appropriate.
Boldface entries indicate significance.
Among GOLD D participants, BEC≤100 participants more often reported current smoking, without other differences between groups (Table 1).
Radiographic Characteristics
In univariable analysis of the overall group, we found no difference in airway wall thickness (Pi10; the average wall thickness of a hypothetical airway of 10-mm lumen perimeter on CT scan imaging) or percent emphysema (% voxels < 950 Hounsfield units [HU] at total lung capacity), but in multivariable analysis, BEC≤100 participants had more air trapping defined by % voxels < 856 HU at expiration (BEC≤100, 33.4% vs 31.1%; P = .026).
Among GOLD D participants not using ICS, multivariable analysis detected increased airway wall thickness (P = .033), and more emphysema (P = .029) in BEC≤100 participants, without differences in air trapping (Table 2).
Exacerbations
In multivariable analysis of COPD participants not using ICS, there was no difference between BEC-defined subsets in AECOPD rate during follow-up (events per year, BEC≤100 vs BEC100+, 0.40 vs 0.36; P = .098), severe AECOPD (0.23 vs 0.19; P = .097), AECOPD over the first year (0.16 vs 0.13; P = .138), AECOPD requiring steroids (0.35 vs 0.32; P = .365), or AECOPD requiring antibiotics (0.34 vs 0.30; P = .184) (Table 2).
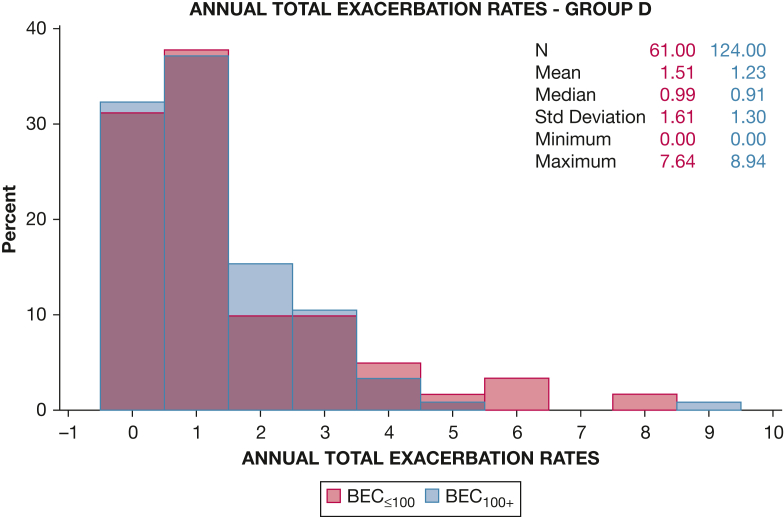
In contrast, in GOLD D participants multivariable analysis of prospective AECOPD revealed greater AECOPD rates in BEC≤100 participants during both total follow-up (BEC≤100 vs BEC100+, 1.16 vs 0.83; P = .014) (Fig 2) and over the first year (0.62 vs 0.33; P = .002), as well as greater rates of AECOPD requiring antibiotics (0.71 vs 0.50; P = .020). However, the two BEC-defined subsets did not differ in rates of severe AECOPD (BEC≤100 vs BEC100+, 0.48 vs 0.39; P = .258) or AECOPD requiring steroids (0.92 vs 0.71; P = .102) (Table 2).
Figure 2.
Annual exacerbation rates during the study in patients with group D COPD. Multivariate analysis of prospective acute exacerbation of the respiratory symptoms of COPD (AECOPD) in the GOLD D group revealed significant differences between BEC-defined subsets in AECOPD rates during the study follow-up period (1.16 vs. 0.83; P = .014). BEC = blood eosinophil count.
Initiation of ICS During the Follow-Up
To evaluate potential change in ICS use after baseline, we analyzed reported ICS use at the follow-up visits. Initiation of ICS was reported at some rate in both BEC-defined subsets without significant difference (BEC≤100 vs BEC100+, 12 [2.4%] vs 41 [4.4%]; P = .07).
Longitudinal Changes in Functional Status and Quality of Life
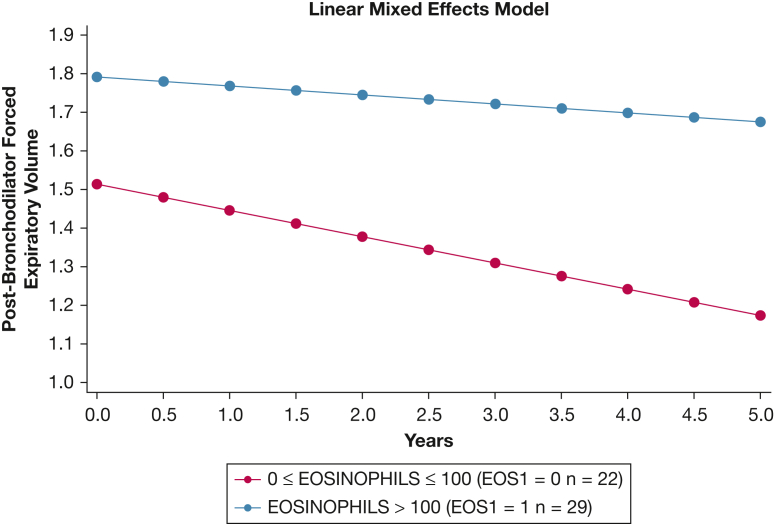
Excluding data from subjects with fewer than three outcome determinations, we identified n = 742 participants with COPD and n = 66 participants with GOLD group D COPD whose data we used for longitudinal analysis of functional performance, symptoms, and disease-specific health status. Using mixed-effects linear regression models to evaluate the changes in FEV1, we found no difference in lung function decline between the two BEC-defined subsets among all COPD participants not using ICS. In contrast, BEC≤100 GOLD D participants had greater decline of postbronchodilator FEV1 than did BEC100+ GOLD D participants (mean slope, BEC≤100 vs BEC100+, –68 vs –23 mL/y; P = .036) (Fig 3, Table 3). However, there were no differences between BEC-defined subsets among all analyzed participants or those with group D COPD for changes in 6MWD, CAT score, or SGRQ score (Table 3). We performed this same analysis while taking into account changes in ICS use throughout the study, and the results were not significantly changed.
Figure 3.
Longitudinal FEV1 decline (in L) in group D COPD participants not using inhaled corticosteroids over the 3 years of the study, separated by baseline blood eosinophil count counts.
Table 3.
Longitudinal Changes in Lung Function, Functional Status, and Quality of Life
| Variable | COPD (n = 742) |
Group D COPD (n = 66) |
||||||
|---|---|---|---|---|---|---|---|---|
| BEC≤100 | BEC100+ | Difference (SE) | P Value | BEC≤100 | BEC100+ | Difference (SE) | P Valuea | |
| Postbronchodilator FEV1, mL/y: mean slope (SE) [No.] | –50 (5.4) [248] | –39 (4.4) [404] | –10 (0.7) | .140 | –68 (13.4) [22] | –23 (16.2) [29] | –45 (21) | .036 |
| 6MWD, m/y: mean slope (SE) [No.] | –12.681 (2.1) [238] | –13.002 (1.8) [368] | 0.321 (2.71) | .906 | –13.979 (5.6) [19] | –13.328 (4.9) [25] | –0.651 (7.404) | .930 |
| CAT score, No./y: mean slope (SE) [No.] | 0.091 (0.2) [227] | 0.071 (0.1) [392] | 0.020 (0.185) | .910 | –0.558 (0.5) [21] | –0.468 (0.5) [28] | –0.091 (0.694) | .896 |
| SGRQ score, No./y: mean slope (SE) [No.] | 0.394 (0.3) [218] | 0.124 (0.2) [362] | 0.271 (0.352) | .442 | –1.237 (1.1) [20] | –1.360 (0.9) [28] | 0.122 (1.43) | .932 |
Mixed-effects linear regression models. Data are presented as the difference between mean slopes (SE) with [No.] where applicable. 6MWD = 6-min walk distance; BEC = blood eosinophil count; CAT = COPD Assessment Test; SGRQ = St. George’s Respiratory Questionnaire.
Boldface entry indicates significance.
Predictors of Prospective Exacerbations
To identify independent predictors of AECOPD in BEC≤100 participants, we used logistic regression to compare the two BEC-defined subsets among all participants with COPD not receiving ICS at enrollment. We limited this evaluation to the first 365 days from entry to avoid selection bias due to loss during follow-up. In both subsets, history of previous exacerbations was significantly associated with greater prospective exacerbations (Table 4), whereas postbronchodilator (BD) FEV1 was significantly associated with fewer prospective exacerbations. Also in both subsets, White race was associated with increased AECOPD risk (BEC≤100: OR, 2.34; 95% CI, 1.05-5.2; P = .038; BEC100+: OR, 1.68; 95% CI, 1.02-2.79; P = .043). History of asthma was associated with exacerbations only in the BEC100+ subset (OR, 1.79; 95% CI, 1.1-2.91; P = .02). In group D participants, there were no significant associations of BEC status with race or exacerbations within 1 year.
Table 4.
Predictors of Prospective AECOPD Among All COPD Participants Not Receiving ICS at Enrollment
| Predictor | COPD (n = 1,414) |
|||
|---|---|---|---|---|
| BEC≤100 (n = 485) | P Valuea | BEC100+ (n = 929) | P Valuea | |
| Sex, male | 0.7 (0.41-1.17) | .175 | 0.78 (0.53-1.13) | .186 |
| Race, White | 2.34 (1.05-5.2) | .038 | 1.68 (1.02-2.79) | .043 |
| History of asthma | 1.19 (0.57-2.47) | .643 | 1.79 (1.1-2.91) | .020 |
| History of childhood asthma | 1.11 (0.37-3.4) | .851 | 1.3 (0.66-2.55) | .455 |
| Current individuals who smoke | 0.55 (0.3-1.01) | .054 | 1.04 (0.68-1.6) | .845 |
| BMI | 0.98 (0.93-1.04) | .517 | 1 (0.96-1.03) | .773 |
| Postbronchodilator FEV1 | 0.98 (0.97-0.99) | .002 | 0.98 (0.97-0.99) | < .001 |
| Exacerbations, year before enrollment | 2.03 (1.47-2.8) | < .001 | 1.48 (1.21-1.81) | < .001 |
| Age | 0.97 (0.94-1.00) | .065 | 1 (0.97-1.02) | .717 |
| Pack-years smoked | 1.00 (0.99-1.01) | .652 | 1 (1-1.01) | .509 |
Analysis performed by logistic regression, limited to the first 365 days of follow-up. Data are presented as the OR of exacerbations (95% CI). AECOPD = acute exacerbation of the respiratory symptoms of COPD; BEC = blood eosinophil count; ICS = inhaled corticosteroid.
Boldface entries indicate significance.
Comparison With Those Using ICS at Enrollment
To investigate the effect of ICS on longitudinal outcomes in BEC≤100 participants with GOLD group D COPD, we compared those participants in this analysis (n = 61) with BEC≤100 GOLD D participants taking corticosteroids (oral or inhaled, n = 27) at baseline. As 81.5% of the 27 people taking corticosteroids at baseline did not report ICS continuation at all subsequent visits, we limited analysis to the first year of follow-up. We found no significant association between ICS use at baseline and AECOPD rate over the first year (ICS use vs no ICS use, 0.42 vs 0.41 exacerbation; P = .914) or initial lung function (1.15 vs 1.36 L; P = .110).
Discussion
Our analysis of a subset of the SPIROMICS cohort reveals several novel, clinically relevant findings about peripheral BEC in COPD. First, BEC≤100 is prevalent in COPD participants not taking corticosteroids, being present in more than one-third of these participants with a similar percentage of low vs normal/high BEC across all GOLD A through D groups. Second, BEC≤100 is stable over both short (6-week) and much longer intervals. Third, in GOLD D COPD, BEC≤100 participants had more emphysema, greater airway wall thickness, more rapid FEV1 decline, and more prospective AECOPD in the first year of follow-up. To our knowledge, this is the first study to investigate clinical characteristics and longitudinal outcomes for symptomatic COPD exacerbators with low BEC who are not using corticosteroids. Collectively, these findings suggest that a categorical definition of BEC identifies a distinct and particularly vulnerable population with advanced COPD.
These results advance our understanding of BEC to guide ICS management in COPD patients with frequent exacerbations.14 BECs are undoubtedly a useful biomarker in airway disease management. However, results of several analyses15, 16, 17 raised concern that, rather than using explicit thresholds, BECs may be more appropriately used as a continuous value in the context of other clinical features. In addition, those analyses introduced controversy about the role of ICS in pauci-eosinophilic COPD. However, although BEC≤100 has been associated with a less favorable response to ICS,18 few published reports describe clinical outcomes in people with BEC≤100 and with COPD not treated with ICS, a knowledge gap addressed by this analysis.
Nevertheless, BEC < 100 cells/μL has become a recommended criterion for avoiding ICS therapy in GOLD group D.19 The IMPACT (Informing the Pathway of COPD Treatment) study observed benefit from adding ICS to long-acting β-agonist/long-acting muscarinic antagonist in terms of exacerbation reduction regardless of BEC level, although there was greater reduction in exacerbation rates among those with BEC ≥ 150/μL.7 Subjects enrolled in the IMPACT trial all had a history of previous exacerbations as seen in group D.7 In an analysis of the CHAIN (COPD History Assessment in Spain) and BODE (BMI, degree of airflow obstruction, functional dyspnea, and exercise capacity) cohorts, BEC varied significantly over 2 years, whereas clinical characteristics, including exacerbation rates, did not differ between COPD patients with BEC ≥ 300/μL and those with BEC < 300/μL.20 Observed negative effects of ICS in BEC≤100 COPD patients mostly relate to increased pneumonia incidence,21 but studies suggest that this patient group is at increased risk of pneumonia even if untreated with ICS.22,23 Our analysis indicates that the group excluded by this recommendation may experience worse outcomes when compared with their peers with higher BEC.
Our current report complements a published SPIROMICS analysis24 that evaluated the relationship of blood and sputum eosinophilia, showing that BEC > 300/μL was associated with lower lung function and higher SGRQ score, but not increased history of exacerbations. Here, our current finding of worse outcomes in GOLD D participants with BEC≤100 vs those with similar clinical characteristics but with a higher BEC suggests that future therapeutic guidelines may need to offer more data and a better-defined approach to those with low BEC.
Our demonstration of temporal BEC stability significantly extends previous evaluations.20,25 One of the largest analyses26 found less stable BEC in those with vs without COPD, and that COPD participants with BEC < 340/μL demonstrated greater stability than those with higher BEC. Both findings were likely confounded by pharmacotherapy. By excluding those taking corticosteroids, we confirmed that BEC≤100 is a sustainable trait both in short- and longer-term assessment, and hence a COPD phenotype.27
Among our entire BEC≤100 COPD group, we confirm and extend several features that differ from those with higher BEC,28 including predominance among women and those currently smoking.18,29 Although a trend of increasing BEC has been recognized during smoking cessation,30 its mechanism is undetermined. Because smoking cessation is associated with reduced risk of COPD exacerbations31 and of hospital admission,32 both smoking status and low BEC could affect the exacerbation rates among participants with COPD not receiving ICS therapy. Our finding of a less frequent history of childhood asthma in BEC≤100 COPD is unsurprising. Eosinophilia characterizes subtypes of both asthma and COPD,33 and BECs tend to be higher in those with an asthma diagnosis.34
Our findings in adjusted multivariate analysis of multiple adverse outcomes in BEC≤100 GOLD D participants address an area of controversy. Previous data suggested an increased rate of emphysema progression in people with COPD and BEC < 2% but without observed differences in the extent of emphysema at baseline,29,35 and greater airway wall thickness in people with COPD, at a cutoff of BEC > 150/μL.36 With respect to emphysema, these data contrast with results from large cohort analyses,4 which, similar to our findings, showed a negative correlation of emphysema extent with BEC, although the differing BEC thresholds preclude direct comparison. Two reports, although differing in BEC cut points from our study, appear to support our findings: first, a UK study of 26,675 people with COPD37 found that the rate of change in FEV1 did not differ when stratified by eosinophil level, similar to our findings of no significant difference in longitudinal change for post-BD FEV1 in all SPIROMICS subjects; and second, the KOLD (Korean Obstructive Lung Disease) cohort study, which found that subjects with persistently high BEC (> 300/μL) had better survival than those with persistently low BEC (< 300/μL) over 6 years of follow-up,38 which is consistent with the observation of greater decline in post-BD FEV1 and more exacerbations in the low-BEC GOLD D group here. Collectively, the literature can be reconciled by recognizing that both higher-than-normal and lower-than-normal BEC may be associated with worsened disease progression if other conditions are present. Our data are compatible with data showing an association of decreasing eosinophil counts with higher mortality,39 an outcome that we did not examine. As well, the finding that group D COPD patients with low eosinophils more often required antibiotics for exacerbations could potentially indicate greater infectious causes of exacerbations among those with low eosinophils.
Few BEC≤100 COPD participants were started on ICS therapy during follow-up, and in participants in GOLD group D, the percentage did not differ from those with BEC100+. Because much of our study period preceded the GOLD recommendation to consider withholding ICS in BEC≤100 group D COPD,9 we speculate that BEC numbers may not have contributed significantly to therapeutic decisions. Our finding that use of ICS in BEC≤100 GOLD D COPD participants was not associated with greater total exacerbations in the first year, relative to BEC≤100 GOLD D COPD participants not receiving ICS, should be interpreted with caution. Because of the small numbers of participants, inadequate capture of pneumonias, and the observational nature of the SPIROMICS cohort, these findings cannot argue for or against ICS use in this patient population. Studies have demonstrated a relationship between increasing BEC and the benefits of ICS in symptomatic COPD exacerbators; however, these studies have also shown benefits even in those with low BEC.7,40 Our data instead argue for specifically investigating the role of ICS in group D BEC≤100 COPD, and in particular those with greater emphysema. Our results are concordant with a prior analysis by Nishimura et al3 that identified a group of “rapid decliners” characterized by greater radiographic emphysema as compared with lung function sustainers with higher levels of circulating eosinophils.
The analysis of risk of prospective AECOPD in BEC-defined subsets in GOLD group D made the novel observation that history of asthma was predictive only in BEC100+, whereas current smoking was predictive only in BEC≤100. The latter finding is compatible with the hypothesized effect of smoking to decrease both BEC numbers and AECOPD risk.31 It suggests that as a biomarker, BEC may be significantly affected by behavioral (smoking) or therapeutic (corticosteroids) interventions, limiting its applicability without consideration of additional variables.
Strengths include use of data from a large cohort of participants with significant smoking histories, at centers throughout the United States, with extensive baseline and longitudinal characterization, allowing adequate assessment of multiple clinical outcomes, particularly in smaller subgroups. Our design avoided treatment bias of BEC numbers by excluding those using any corticosteroid at enrollment. To interpret longitudinal changes adequately, we used mixed-effects models and assessed multiple testing points (minimum, three) over a period longer than 2 years.
Our study has several limitations. SPIROMICS was not designed specifically to examine the clinical implications of a low BEC. There are also clear limitations on our ability to evaluate the effect of therapeutic choices. This limitation was underscored by Harries et al,41 who found differences in the usefulness of BEC to predict the ability of ICS to decrease exacerbations between subjects in observational vs randomized studies. In addition, the number of group D participants is relatively small, so that conclusions drawn from it warrant caution. Multiple biases cannot be excluded entirely. Selection bias in this non-population-based cohort may limit applicability of findings to the general population; recall bias and potential misreporting of ICS usage are acknowledged. Decisions to withhold or start ICS were made without the study team’s involvement. Although limiting our analysis to those not receiving ICS at enrollment precluded evaluating the impact of that agent in treating BEC≤100 patients with COPD, it allowed us to monitor the natural course of people with BEC≤100 and to compare their outcomes with their BEC100+ counterparts.
Interpretation
Our data help to explain some of the discrepancies of prior analyses in examining the relationship between blood eosinophil level and patient outcomes in COPD. We demonstrate that both concomitant use of ICS and disease stage may modify these relationships. We found that GOLD D COPD participants with BEC≤100 in the absence of ICS therapy are a distinct phenotype in our cohort, with higher prevalence of current smoking, greater emphysema, and worse prospective outcomes (higher rate of lung function decline and more prospective exacerbations) than their BEC100+ counterparts. However, such differences in outcomes between BEC-defined subsets were not observed outside of GOLD group D. Our analysis sheds light on a particularly vulnerable COPD phenotype, suggesting the need for further studies focused on their therapeutic treatment.
Funding/Support
SPIROMICS was supported by contracts from the National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, and HHSN268200900020C) and grants from the NIH/NHLBI (U01 HL137880 and U24 HL141762), and was supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals; Chiesi Farmaceutici; Forest Research Institute; GlaxoSmithKline; Grifols Therapeutics; Ikaria; Novartis Pharmaceuticals; Nycomed; ProterixBio; Regeneron Pharmaceuticals; Sanofi; Sunovion; Takeda Pharmaceutical Company; and Theravance Biopharma and Mylan.
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: C. C. has received consulting fees from Nuvaira, MGC Diagnostics, and GlaxoSmithKline. J. L. C. has received grants from the NHLBI, the Department of Veterans Affairs, and the Department of Defense, and has received consultancy fees from AstraZeneca, CLS Behring, and Novartis. P. G. W. has received grants from the NIH and the COPD Foundation, and has received consulting fees from Sanofi, Regeneron, Glenmark Pharmaceuticals, the University of Wisconsin, NGM Pharma, GlaxoSmithKline, Theravance, and AstraZeneca. C. M. D. is funded by the NIH. D. P. T. has received honoraria from AstraZeneca, Sunovion, and Mylan. E. R. B. has undertaken clinical trials for AstraZeneca, MedImmune, Boehringer Ingelheim, Genentech, Johnson & Johnson (Janssen), Novartis, Regeneron, and Sanofi Genzyme and has also served as a paid consultant for AstraZeneca, MedImmune, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Regeneron, and Sanofi Genzyme. F. J. M. has received grants from AstraZeneca, GlaxoSmithKline, and Sanofi/Regeneron; consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Gala, GlaxoSmithKline, Novartis, Polarean, PulmonX, Sanofi/Regeneron, Sunovion, Teva, Theravance/Viatris, and Verona; and has received honoraria from UpToDate. I. B. has received grants from Theravance/Viatris and Amgen, and consulting fees from AstraZeneca, GlaxoSmithKline, Theravance/Viatris, Aerogen, Verona Pharma, Inhibrx, and Grifols. R. P. has received grants from the Department of Veterans Affairs and Partner Therapeutics, and consulting fees from Partner Therapeutics. V. E. O. has received grants from the NHLBI. M. T. D. has received consulting fees from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, Quark Pharmaceuticals, and Mereo. M.L. K. H. has received grants from the NHLBI, Sanofi, Novartis, and Nuvaira; consulting fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, PulmonX, Teva, Verona, Novartis, Merck, Sanofi, DevPro, Aerogen, and United Therapeutics; and honoraria from Cipla, Chiesi, AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline. R. B.P. has received grants from the NIH and consulting fees from GNS Health Care, Theravance, and GlaxoSmithKline. R. J. K. has received grants from Boehringer Ingelheim and honoraria from UpToDate, the France Foundation, Boehringer Ingelheim, and Genentech, and has stock in Air Cycle Systems. V. K. has received consulting fees from Gala Therapeutics and the American Board of Internal Medicine. W. H. A. has received grants from the NHLBI and COPD Foundation.
Acknowledgments
Author contributions: W. B. LeM., A. T. H., and I. B. act as guarantors for the content of this manuscript. W. B. LeM., D. C., P. M. Q., A. T. H., and I. B. contributed to the conception and design of the study. P. M. Q. and D. C. undertook data analysis. W. B. LeM., D. P. T., C. C., J. L. C., A. T. H., and I. B. drafted the manuscript. All authors contributed to data acquisition and/or interpretation and critically reviewed the manuscript before submission.
Other contributions: The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data is available at www.spiromics.org. The authors acknowledge the University of North Carolina at Chapel Hill BioSpecimen Processing Facility for sample processing, storage, and sample disbursements (https://bsp.web.unc.edu/). The authors acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E Alexis, MD; Wayne H. Anderson, PhD; Mehrdad Arjomandi, MD; Igor Barjaktarevic, MD, PhD; R. Graham Barr, MD, DrPH; Patricia Basta, PhD; Lori A. Bateman, MSc; Surya P. Bhatt, MD; Eugene R. Bleecker, MD; Richard C. Boucher, MD; Russell P. Bowler, MD, PhD; Stephanie A. Christenson, MD; Alejandro P. Comellas, MD; Christopher B. Cooper, MD, PhD; David J. Couper, PhD; Gerard J. Criner, MD; Ronald G. Crystal, MD; Jeffrey L. Curtis, MD; Claire M. Doerschuk, MD; Mark T. Dransfield, MD; Brad Drummond, MD; Christine M. Freeman, PhD; Craig Galban, PhD; MeiLan K. Han, MD, MS; Nadia N. Hansel, MD, MPH; Annette T. Hastie, PhD; Eric A. Hoffman, PhD; Yvonne Huang, MD; Robert J. Kaner, MD; Richard E. Kanner, MD; Eric C. Kleerup, MD; Jerry A. Krishnan, MD, PhD; Lisa M. LaVange, PhD; Stephen C. Lazarus, MD; Fernando J. Martinez, MD, MS; Deborah A. Meyers, PhD; Wendy C. Moore, MD; John D. Newell Jr, MD; Robert Paine III, MD; Laura Paulin, MD, MHS; Stephen P. Peters, MD, PhD; Cheryl Pirozzi, MD; Nirupama Putcha, MD, MHS; Elizabeth C. Oelsner, MD, MPH; Wanda K. O’Neal, PhD; Victor E. Ortega, MD, PhD; Sanjeev Raman, MBBS, MD; Stephen I. Rennard, MD; Donald P. Tashkin, MD; J. Michael Wells, MD; Robert A. Wise, MD; and Prescott G. Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD, and Lisa Viviano, BSN.
References
- 1.Bafadhel M., McKenna S., Terry S., et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(1):48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papaioannou A.I., Kostikas K., Papaporfyriou A., et al. Emphysematous phenotype is characterized by low blood eosinophils: a cross-sectional study. COPD. 2017;14(6):635–640. doi: 10.1080/15412555.2017.1386644. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura M., Makita H., Nagai K., et al. Hokkaido COPD Cohort Study Investigators. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(1):44–52. doi: 10.1164/rccm.201106-0992OC. [DOI] [PubMed] [Google Scholar]
- 4.Oh Y.-M., Lee K.S., Hong Y., et al. Blood eosinophil count as a prognostic biomarker in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3589–3596. doi: 10.2147/COPD.S179734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Garcia M.A., Faner R., Oscullo G., et al. Inhaled steroids, circulating eosinophils, chronic airway infection, and pneumonia risk in chronic obstructive pulmonary disease: a network analysis. Am J Respir Crit Care Med. 2020;201(9):1078–1085. doi: 10.1164/rccm.201908-1550OC. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald M.I., Osadnik C.R., Bulfin L., et al. Low and high blood eosinophil counts as biomarkers in hospitalized acute exacerbations of COPD. Chest. 2019;156(1):92–100. doi: 10.1016/j.chest.2019.02.406. [DOI] [PubMed] [Google Scholar]
- 7.Lipson D.A., Barnhart F., Brealey N., et al. IMPACT Investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi: 10.1056/NEJMoa1713901. [DOI] [PubMed] [Google Scholar]
- 8.Couper D., LaVange L.M., Han M., et al. SPIROMICS Research Group Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69(5):492–495. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogelmeier C.F., Criner G.J., Martinez F.J., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 10.Miller M.R., Hankinson J., Brusasco V., et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 11.Galbán C.J., Han M.K., Boes J.L., et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18(11):1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koo T.K., Li M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson W.H., Ha J.W., Couper D.J., et al. SPIROMICS Research Group Variability in objective and subjective measures affects baseline values in studies of patients with COPD. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald C.F. Eosinophils in chronic obstructive pulmonary disease: are they just another biomarker? Curr Opin Pulm Med. 2020;26(2):169–174. doi: 10.1097/MCP.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 15.Pascoe S., Barnes N., Brusselle G., et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med. 2019;7(9):745–756. doi: 10.1016/S2213-2600(19)30190-0. [DOI] [PubMed] [Google Scholar]
- 16.Pascoe S., Pavord I., Hinds D., Locantore N., Barnes N. The association between blood eosinophils and risk and treatment outcome in COPD is not dichotomised. Lancet Respir Med. 2018;6(5):e18. doi: 10.1016/S2213-2600(18)30137-1. [DOI] [PubMed] [Google Scholar]
- 17.Bafadhel M., Peterson S., Blas M.A.D., et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med. 2018;6(2):117–126. doi: 10.1016/S2213-2600(18)30006-7. [DOI] [PubMed] [Google Scholar]
- 18.Pascoe S., Locantore N., Dransfield M.T., Barnes N.C., Pavord I.D. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 19.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2021 GOLD Report. https://staging.goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf
- 20.Casanova C., Celli B.R., de-Torres J.P., et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J. 2017;50(5) doi: 10.1183/13993003.01162-2017. [DOI] [PubMed] [Google Scholar]
- 21.Calverley P.M.A., Anderson J.A., Celli B., et al. TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 22.Pavord I.D., Lettis S., Anzueto A., Barnes N. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysis. Lancet Respir Med. 2016;4(9):731–741. doi: 10.1016/S2213-2600(16)30148-5. [DOI] [PubMed] [Google Scholar]
- 23.Cheng S.-L. Blood eosinophils and inhaled corticosteroids in patients with COPD: systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:2775–2784. doi: 10.2147/COPD.S175017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hastie A.T., Martinez F.J., Curtis J.L., et al. SPIROMICS Investigators Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956–967. doi: 10.1016/S2213-2600(17)30432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Southworth T., Beech G., Foden P., Kolsum U., Singh D. The reproducibility of COPD blood eosinophil counts. Eur Respir J. 2018;52(1) doi: 10.1183/13993003.00427-2018. [DOI] [PubMed] [Google Scholar]
- 26.Oshagbemi O.A., Burden A.M., Braeken D.C.W., et al. Stability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline counts. Am J Respir Crit Care Med. 2017;195(10):1402–1404. doi: 10.1164/rccm.201701-0009LE. [DOI] [PubMed] [Google Scholar]
- 27.Han M.K., Agusti A., Calverley P.M., et al. Chronic obstructive pulmonary disease phenotypes. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landis S., Suruki R., Maskell J., Bonar K., Hilton E., Compton C. Demographic and clinical characteristics of COPD patients at different blood eosinophil levels in the UK Clinical Practice Research Datalink. COPD. 2018;15(2):177–184. doi: 10.1080/15412555.2018.1441275. [DOI] [PubMed] [Google Scholar]
- 29.Singh D., Kolsum U., Brightling C.E., Locantore N., Agusti A., Tal-Singer R. ECLIPSE Investigators. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–1700. doi: 10.1183/09031936.00162414. [DOI] [PubMed] [Google Scholar]
- 30.Jensen E.J., Pedersen B., Narvestadt E., Dahl R. Blood eosinophil and monocyte counts are related to smoking and lung function. Respir Med. 1998;92(1):63–69. doi: 10.1016/s0954-6111(98)90034-8. [DOI] [PubMed] [Google Scholar]
- 31.Au D.H., Bryson C.L., Chien J.W., et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24(4):457–463. doi: 10.1007/s11606-009-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godtfredsen N.S., Vestbo J., Osler M., Prescott E. Risk of hospital admission for COPD following smoking cessation and reduction: a Danish population study. Thorax. 2002;57(11):967–972. doi: 10.1136/thorax.57.11.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fahy J.V. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hancox R.J., Pavord I.D., Sears M.R. Associations between blood eosinophils and decline in lung function among adults with and without asthma. Eur Respir J. 2018;51(4) doi: 10.1183/13993003.02536-2017. [DOI] [PubMed] [Google Scholar]
- 35.Ostridge K., Williams N.P., Kim V., et al. AERIS Study Group Relationship of CT-quantified emphysema, small airways disease and bronchial wall dimensions with physiological, inflammatory and infective measures in COPD. Respir Res. 2018;19(1):31. doi: 10.1186/s12931-018-0734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan W.C., Bourbeau J., Nadeau G., et al. CanCOLD Collaborative Research Group High eosinophil counts predict decline in FEV1: results from the CanCOLD study. Eur Respir J. 2021;57(5) doi: 10.1183/13993003.00838-2020. [DOI] [PubMed] [Google Scholar]
- 37.Whittaker H.R., Müllerova H., Jarvis D., et al. Inhaled corticosteroids, blood eosinophils, and FEV1 decline in patients with COPD in a large UK primary health care setting. Int J Chron Obstruct Pulmon Dis. 2019;14:1063–1073. doi: 10.2147/COPD.S200919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin S.H., Park H.Y., Kang D., et al. KOLD Study Group Serial blood eosinophils and clinical outcome in patients with chronic obstructive pulmonary disease. Respir Res. 2018;19(1):134. doi: 10.1186/s12931-018-0840-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendy A., Forno E., Niyonsenga T., Gasana J. Blood biomarkers as predictors of long-term mortality in COPD. Clin Respir J. 2018;12(5):1891–1899. doi: 10.1111/crj.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabe K.F., Martinez F.J., Ferguson G.T., et al. ETHOS Investigators. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi: 10.1056/NEJMoa1916046. [DOI] [PubMed] [Google Scholar]
- 41.Harries T.H., Rowland V., Corrigan C.J., et al. Blood eosinophil count, a marker of inhaled corticosteroid effectiveness in preventing COPD exacerbations in post-hoc RCT and observational studies: systematic review and meta-analysis. Respir Res. 2020;21(1):3. doi: 10.1186/s12931-019-1268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]