Abstract
Background
Coronaviruses, generally known to cause a mild degree of respiratory illness have in the recent past caused three serious disease outbreaks. The world is yet to be released from the grip of the most recent coronavirus disease 2019 (COVID-19) pandemic due to emerging mutant strains. Age, presence of comorbidities, clinical severity, and laboratory markers such as C-reactive protein and D-dimer are some of the factors being employed to prioritize patients for hospital care. It is known that comorbidities themselves are an outcome of inflammation and can induce a pro-inflammatory state. Our study aims to elucidate the influence of age and comorbidities on laboratory markers in patients with COVID-19.
Methodology
This is a single-center retrospective study of patients with a laboratory diagnosis of COVID-19 admitted to our hospital between September 21, 2020, and October 1, 2020. A total of 387 patients above the age of 18 years were included in the analysis and categorized based on the age-adjusted Charlson comorbidity index (ACCI) score into group A (score ≤4) and group B (score >4). Demographic, clinical, and laboratory factors as well as outcomes were compared.
Results
Group B exhibited higher intensive care unit admission and mortality, as well as statistically significant higher mean values of most laboratory markers. A correlation was also observed between the ACCI score and biomarker values. On comparison between the two groups regarding cut-offs predicting mortality for laboratory determinants, no consistent pattern was observed.
Conclusions
A correlation between age, the number of comorbidities, and laboratory markers was observed in our analysis of COVID-19-affected patients. Aging and comorbid conditions can produce a state of meta-inflammation and can thereby contribute to hyperinflammation in COVID-19. This can be an explanation for the higher risk of COVID-19-related mortality in older individuals and those with underlying comorbidities.
Keywords: meta-inflammation, covid-19, india, biomarkers, comorbidity, age-adjusted charlson comorbidity index
Introduction
Coronaviruses are a group of viruses that generally cause a mild degree of respiratory disease [1]. However, the last two decades saw three disease outbreaks by coronaviruses causing severe to fatal disease, namely, SARS-CoV causing severe acute respiratory syndrome (SARS), MERS-CoV causing Middle East respiratory syndrome (MERS), and SARS-CoV-2 leading to coronavirus disease 2019 (COVID-19) [2]. The last one has evolved into a pandemic that has gripped the world since the first outbreak in the Hubei province of China in December 2019. The novel coronavirus infection that was declared a pandemic by the World Health Organization on March 11, 2020, has infected both high and low-income countries alike. However, the brunt of it was borne by countries with limited health resources given the explosive nature of the pandemic [3]. The world is still grappling with the pandemic. Mutant strains have prevented the reining in of the outbreak [4].
Much of the research on the previous two coronavirus outbreaks has contributed immensely toward guiding research and management of the novel coronavirus infection [5]. It is imperative that we continue our quest for triaging as well as refining the management of patients with COVID-19, which will surely be an asset for similar challenges in the future.
During the current pandemic, it was evident that regions with limited resources needed to categorize and prioritize patients for optimal use of health resources and better delivery of healthcare. In this endeavor, several risk factors of COVID-19-related mortality were identified. This included demographic and clinical factors as well as laboratory parameters [6-8]. C-reactive protein (CRP) and D-dimer are some of the biomarkers that have been used as predictors of mortality in triaging patients globally [8]. Age and the presence of comorbidities are other known risk factors for COVID-19-related mortality [8]. However, the effect of the presence of comorbidities on these laboratory parameters has not yet been extensively unraveled.
Hyperinflammation is a key event in COVID-19-affected patients with adverse outcomes. However, comorbidities themselves can independently induce a pro-inflammatory state with the derangement of laboratory markers such as acute-phase reactants [9,10]. Additionally, research has shown inflammation to be an underlying risk factor for morbidities such as cardiovascular disease, diabetes, and cancer [10]. Moreover, studies have also demonstrated that increased levels of inflammatory markers can predict the development of these diseases. The National Survey of Mid-life in the United States showed that circulating levels of interleukin 6 (IL-6) and CRP increased with increasing numbers of chronic conditions [11]. Shaheer et al. reported elevated levels of serum high-sensitivity CRP in patients with type 2 diabetes mellitus and diabetic nephropathy in comparison with non-diabetic controls [12]. Similarly, age is also known to influence the levels of many of these laboratory predictors even in the absence of COVID-19. This is attributed to chronic low-grade inflammation associated with aging also known as inflammaging [13]. This calls for the need to take into account age and the presence of comorbidities while analyzing laboratory values in COVID-19 patients.
Some studies have combined factors related to comorbidity and laboratory predictors to derive prognostic scores such as the 4C mortality score and CoLACD (CoVID-19 lymphocyte ratio, age, Charlson comorbidity index (CCI) score, dyspnea) score to predict COVID-19 related mortality [14,15]. Eight important predictors of COVID-19-related mortality were taken up for computing the 4C mortality score. This included age, sex, number of comorbidities defined according to the modified CCI, respiratory rate, peripheral oxygen saturation, level of consciousness, urea level, and CRP. The parameters taken into account in the CoLACD score combine lymphocyte percentage, age, CCI, and the presence of dyspnea. A study conducted in Mysore, India explored the effect of the presence of multiple comorbidities on laboratory cut-offs of biomarkers [16]. For this, the study categorized patients according to CCI and compared both the biomarker values as well as the cut-offs between the different categories.
CCI that combines 19 parameters is a long-used validated score for assessment of the presence of multiple comorbidities in a patient to predict disease outcome [17]. During the current pandemic, it has been shown to be a useful predictor of mortality in COVID-19-affected patients [18]. Age-adjusted Charlson comorbidity index (ACCI) score, a modified score with age as an additional index, has also been shown to be a similar predictor of adverse disease outcomes in patients with COVID-19 [19]. In our current analysis, we attempted to explore the correlation between comorbidities and laboratory parameters in COVID-19 by employing ACCI.
Materials and methods
This is a single-center, record-based, cross-sectional study conducted in Government Medical College Hospital, Thiruvananthapuram, which is a public sector tertiary care center and teaching hospital. The study was conducted after obtaining ethical clearance from the Institutional Ethics Committee (04/87/2020/MCT). The study population was laboratory-confirmed consecutive cases of COVID-19 admitted to our hospital between September 21, 2020, and October 1, 2020. We had determined the laboratory predictors of COVID-19-related mortality in our earlier analysis, the detailed methodology and findings of which have been published [20]. For the current analysis, we further categorized patients according to the ACCI score, as shown in Table 1.
Table 1. Age-adjusted Charlson comorbidity index score.
| Score | Comorbidity |
| 1 | For each decade over 40 years add 1 point up to a maximum of 4 points for the age group 71 or older |
| 1 | Myocardial infarction, congestive heart failure, peripheral vascular disease, cerebral vascular disease, dementia, chronic obstructive pulmonary disease, connective tissue disease, peptic ulcer disease, mild liver disease, diabetes without end-organ damage |
| 2 | Hemiplegia, moderate/severe renal disease, diabetes with end-organ damage, any solid tumor without metastasis, leukemia, lymphoma |
| 3 | Moderate/severe liver disease |
| 6 | Metastatic solid tumor, acquired immunodeficiency syndrome |
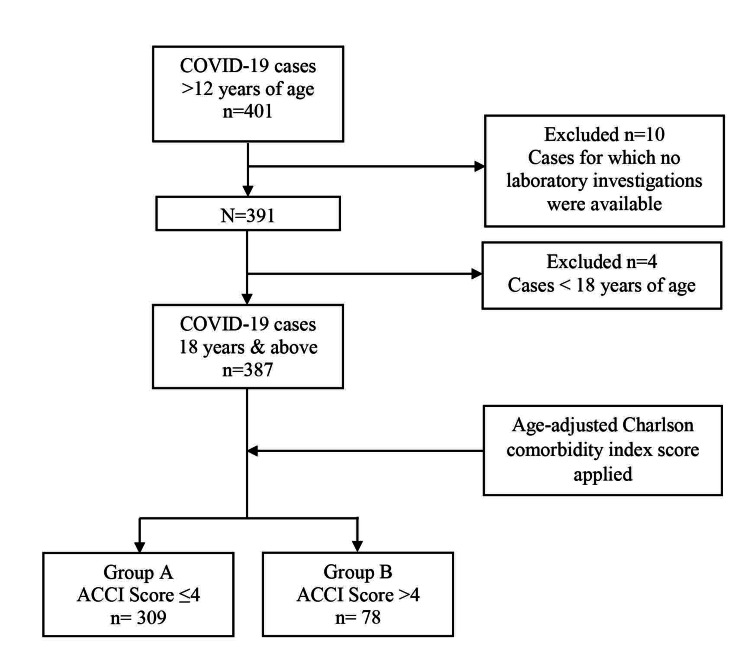
A total of 387 patients aged 18 years and above were included in the present analysis. The ACCI score was calculated by assigning scores for age and 19 medical comorbidities as per the data obtained from case records. Further, the score obtained was used to classify patients into two groups, namely, group A (score ≤4) and group B (score >4) [19]. See the Strobe flowchart in Figure 1.
Figure 1. Strobe flowchart.
ACCI: age-adjusted Charlson comorbidity index
Subsequently, we tabulated the demographic features and laboratory findings at the time of admission as well as outcomes in the two groups. For the present analysis, we additionally calculated the derived neutrophil-to-lymphocyte ratio (dNLR), platelet-to-lymphocyte ratio (PLR), and blood urea nitrogen-to-serum albumin ratio (BAR) from the prior laboratory results.
Statistical analysis
Statistical analysis was performed using SPSS for Windows version 25.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean and standard deviation and categorical variables as proportions. For univariate analyses, an unpaired t-test was performed for continuous variables and the chi-square test for categorical variables. Spearman’s correlation was performed to explore the relationship between biomarker values and the ACCI score.
Potential cut-off values were calculated in both groups for laboratory parameters that were found to be mortality predictors in our earlier analysis. For each group, we performed receiver operating characteristic analysis (ROC) to explore the ability of each biomarker to classify survivors and non-survivors using a cut-off value. We generated ROC curves, the area under the curve (AUC), p-values, and coordinate points of the curve. Youden’s J was computed for each coordinate point and the point with the highest J value was listed as the optimal cut-off predicting mortality. The sensitivity and specificity of respective cut-off points were also reported.
Results
There were a total of 309 patients in the lower ACCI group (group A) and 78 in the higher ACCI group (group B).
Table 2 shows the demographic characteristics, severity, and mortality in each of the study groups. Group B had a significantly higher mean age and larger proportion of males when compared to group A. Similarly, higher intensive care unit (ICU) admission and mortality were seen in group B.
Table 2. Comparison of demographic features and outcomes between group A and group B.
ICU: intensive care unit
| Group A (n = 309 ) | Group B (n = 78) | P-value | ||
| Age in years | Mean (SD) | 49.3 (15.2) | 70.4 (10.5) | <0.001 |
| Sex | Male | 156 (50.5%) | 49 (62.8%) | 0.051 |
| Female | 153 (49.5%) | 29 (37.2%) | ||
| ICU admission | Yes | 47 (15.2%) | 30 (38.5%) | <0.001 |
| No | 262 (84.8%) | 48 (61.5%) | ||
| Mortality | Died | 27 (8.7%) | 33 (42.3%) | <0.001 |
| Survived | 282 (91.3%) | 45 (57.7%) |
Table 3 presents the distribution (mean and standard deviation) of the biomarkers in group A in comparison to group B. Most biomarkers showed a statistically significant difference in mean between the two groups except for platelet count. Of these biomarkers, mean values of total Leukocyte count (TLC), absolute neutrophil count (ANC), neutrophil-to-lymphocyte ratio (NLR), dNLR, PLR, CRP, ferritin, lactate dehydrogenase (LDH), D-dimer, blood urea nitrogen (BUN), serum creatinine, BAR, and prothrombin international normalized ratio (PT-INR) were higher in group B in comparison to group A while the mean values of absolute lymphocyte count (ALC) and serum albumin were lower in group B.
Table 3. Comparison of laboratory parameters between group A and group B.
TLC: total leukocyte count; ANC: absolute neutrophil count; ALC: absolute lymphocyte count; NLR: neutrophil-to-lymphocyte ratio; dNLR: derived neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; CRP: C-reactive protein; LDH: lactate dehydrogenase; BUN: blood urea nitrogen; BAR: blood urea nitrogen-to-serum albumin ratio; PT-INR: prothrombin time international normalized ratio
| Group A (n = 309) | Group B (n = 78) | P-value | |
| TLC (×109/L) | 7.68 (4.55) | 10.14 (6.80) | <0.001 |
| ANC (×109/L) | 5.54 (4.39) | 8.61 (6.81) | <0.001 |
| ALC (×109/L) | 1.99 (0.96) | 1.46 (0.84) | <0.001 |
| NLR | 3.7 (3.8) | 8.8 (12.7) | <0.001 |
| dNLR | 3.5 (3.7) | 8.6 (12.7) | <0.001 |
| Platelet count (×109/L) | 220 (90) | 210 (100) | 0.405 |
| PLR | 140.0 (96.5) | 209.3 (182.2) | <0.001 |
| CRP (mg/L) | 60 (94) | 115 (128) | <0.001 |
| Ferritin (ng/mL) | 381.3 (433.6) | 514.3 (493.3) | 0.033 |
| LDH (U/L) | 537.2 (427.1) | 687.9 (474.3) | 0.041 |
| D-dimer (μg/mL) | 1.3 (1.9) | 3.1 (3.9) | <0.001 |
| PT-INR | 1.0 (0.2) | 1.2 (0.4) | <0.001 |
| BUN (mg/dL) | 32.9 (31.7) | 91.6 (83.6) | <0.001 |
| Creatinine (mg/dL) | 1.1 (1.8) | 3.0 (3.5) | <0.001 |
| Albumin (g/dL) | 3.8 (2.1) | 3.1 (0.7) | 0.010 |
| BAR (mg/g) | 9.5 (9.6) | 33.2 (34.9) | <0.001 |
Table 4 shows the correlation of the biomarkers with the ACCI score of the study population. There was a significant correlation between the biomarkers and the ACCI score. All biomarkers except ALC and serum albumin showed a positive correlation with the ACCI score. Both ALC and serum albumin showed a downward trend in values with increasing ACCI scores.
Table 4. Correlation between age-adjusted Charlson comorbidity index scores and laboratory parameters.
TLC: total leukocyte count; ANC: absolute neutrophil count; ALC: absolute lymphocyte count; NLR: neutrophil-to-lymphocyte ratio; dNLR: derived neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; CRP: C-reactive protein; LDH: lactate dehydrogenase; BUN: blood urea nitrogen; BAR: blood urea nitrogen-to-serum albumin ratio; PT-INR:prothrombin time international normalized ratio
| Spearman’s correlation with ACCI score | P-value | |
| TLC | 0.207 | <0.001 |
| ANC | 0.288 | <0.001 |
| ALC | -0.274 | <0.001 |
| NLR | 0.385 | <0.001 |
| dNLR | 0.384 | <0.001 |
| PLR | 0.190 | <0.001 |
| CRP | 0.373 | <0.001 |
| Ferritin | 0.230 | <0.001 |
| LDH | 0.232 | <0.001 |
| D-dimer | 0.408 | <0.001 |
| PT-INR | 0.160 | 0.022 |
| BUN | 0.523 | <0.001 |
| Creatinine | 0.342 | <0.001 |
| Albumin | -0.468 | <0.001 |
| BAR | 0.556 | <0.001 |
We classified and compared the optimal biomarker cut-off predicting mortality between the two groups. The cut-off values for each laboratory test for group A and group B are presented in Table 5 together with the AUC, sensitivity, and specificity of each cut-off value.
Table 5. Comparison of the optimal biomarker cut-off predicting mortality between group A and group B.
ACCI: age-adjusted Charlson comorbidity index; ANC: absolute neutrophil count; CRP: C-reactive protein; BUN: blood urea nitrogen; AUC: area under the curve
| ACCI category | AUC | P-value | Cut-off | Sensitivity | Specificity | |
| ANC | Group A | 0.752 | <0.001 | 8.01 (×109/L) | 55.6 | 87.1 |
| Group B | 0.695 | 0.005 | 7.39 (×109/L) | 63.3 | 74.4 | |
| CRP | Group A | 0.840 | <0.001 | 58.6 (mg/L) | 82.6 | 80.0 |
| Group B | 0.796 | <0.001 | 87.5 (mg/L) | 68.0 | 83.3 | |
| D-dimer | Group A | 0.811 | <0.001 | 1.05 (μg/mL) | 82.6 | 74.4 |
| Group B | 0.786 | <0.001 | 1.01 (μg/mL) | 91.3 | 60.5 | |
| BUN | Group A | 0.806 | <0.001 | 31.5 (mg/dL) | 77.8 | 74.1 |
| Group B | 0.789 | <0.001 | 52.5 (mg/dL) | 82.1 | 68.2 |
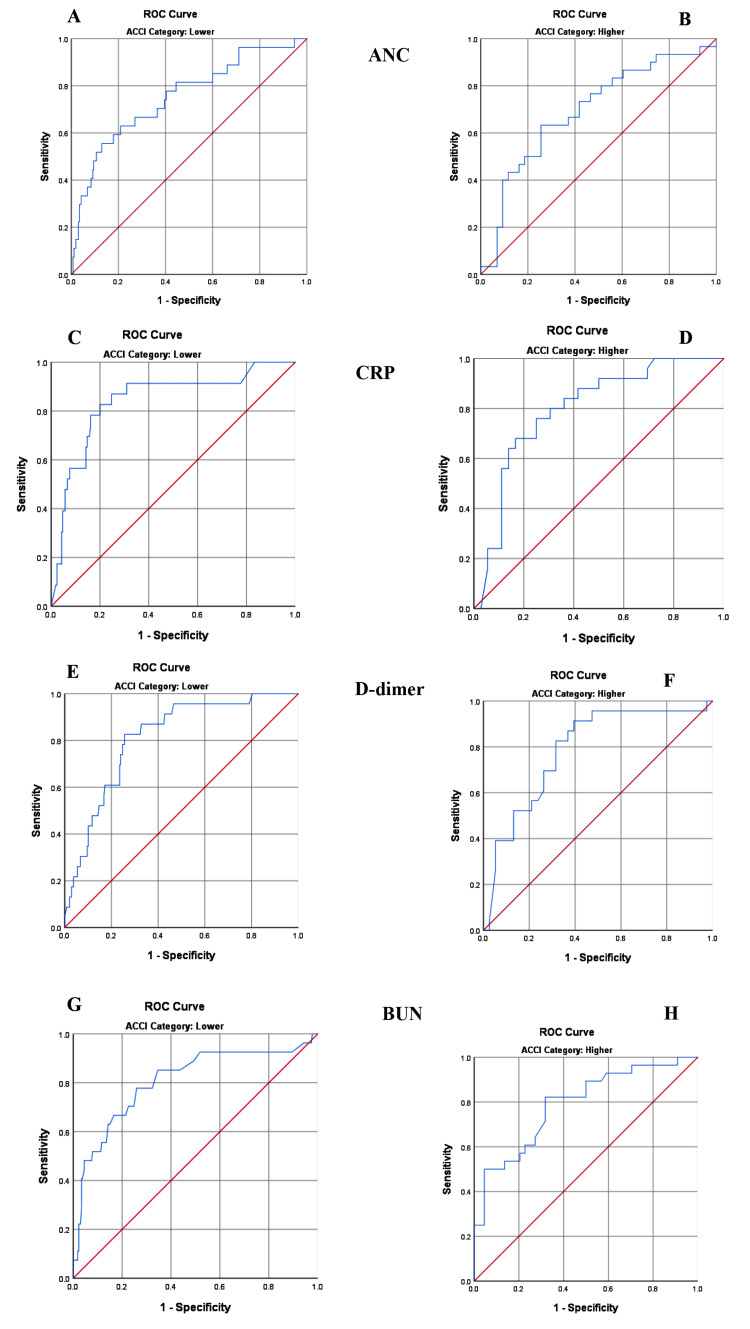
In our earlier analysis, ANC, CRP, D-dimer, and BUN were shown to be independent predictors of COVID-19-related mortality [20]. Cut-off values predicting mortality were derived for the same biomarkers in the present analysis in both group A and group B. ANC and D dimer showed lower cut-off values in group B when compared to group A while CRP and BUN demonstrated a higher cut-off in group B. Figure 2 illustrates the ROC analysis for selected biomarkers.
Figure 2. Receiver operating characteristic curve analysis for selected biomarkers.
A and B illustrate ANC (group A and group B), C and D illustrate CRP (group A and group B), E and F illustrate D-dimer (group A and group B), and G and H illustrate BUN (group A and group B).
ANC: absolute neutrophil count; CRP: C-reactive protein; BUN: blood urea nitrogen; AUC: area under the curve
Discussion
Of the total 387 patients included in the analysis, 309 had an ACCI score of ≤4 (group A) and 78 had a score of >4 (group B). Group B showed a higher mean age and proportion of males. This group also exhibited a higher proportion of ICU admission as well as mortality indicating increased disease severity. This demonstrates that the number of comorbidities and age has a significant influence on disease severity and progression. This is in line with earlier studies that have shown both ACCI as well as CCI to be predictors of adverse disease outcomes in COVID-19 patients [18,19].
The mean values of laboratory markers such as TLC, ANC, NLR, dNLR, PLR, CRP, ferritin, LDH, D-dimer, BUN, serum creatinine, BAR, and PT-INR showed a significant difference between the two groups with higher values in group B. Mean values of ALC and albumin were significantly lower in group B. There was also a significant correlation between the laboratory values and the ACCI score. These findings indicate that the number of comorbidities and age significantly influence the serum levels of biomarkers in patients with COVID-19. This is consistent with the findings of Parthasarathy et al. who showed a higher impairment of laboratory determinants in the group with more number of comorbidities [16]. They also reported a similar positive correlation between the laboratory parameters and the CCI score. Elevated neutrophil count, NLR, and dNLR point toward a rapid innate immunity with subsequent hyperactivation of neutrophils and monocytes leading to cytokine storm while lymphopenia indicates a dysregulated T-cell response and suppressed adaptive immunity [21,22]. Similarly, elevated CRP, LDH, and ferritin are all markers of the inflammatory process in COVID-19. It has been shown that multimorbidities themselves are a consequence of inflammation with the elevation of inflammatory markers. The statistically significant increase in serum levels of inflammatory markers in group B supports the hypothesis that pre-existing chronic low-grade inflammation due to comorbid diseases might have a cascading effect in COVID-19 and consequent adverse outcomes.
On exploring the cut-offs between group A and group B for the biomarkers that were shown to predict mortality in the study population, ANC and D-dimer showed a lower cut-off in the group with higher ACCI score (group B) in comparison to the group with lower ACCI score (group A) while CRP and BUN showed a higher cut-off. Parthasarathy et al. in their study had shown that TLC, ANC, NLR, dNLR, and CRP had a consistently lower cut-off in the group with more comorbidities. They employed the CCI score for patient categorization. However, our analysis did not demonstrate such consistent results between groups across all the selected biomarkers. The reasons for this discrepancy are manifold. Because ours is a referral tertiary care center, moderately or severely ill constituted a large proportion of the inpatients, and laboratory derangements were seen in patients belonging to both ACCI categories. Hypertension and obesity, both of which can induce a state of meta-inflammation, are not part of the ACCI score [23,24]. This is one of the limitations of our study. In fact, hypertension was the second highest comorbidity in our study population [20]. Other limitations that could have affected the results are the smaller sample size and the retrospective nature of our study.
Conclusions
COVID-19, otherwise a mild infection, can be catastrophic in some individuals, with comorbidity and age being significant risk factors. Chronic low-grade inflammation seems to be a contributory factor in this scenario. As the evaluation of laboratory markers holds a key to this, we recommend exploring the cut-off for biomarkers by classifying patients using a validated comorbidity score in a multicentric study with a larger sample size. We believe that hypertension and obesity need to be incorporated in scoring for comorbidity in COVID-19 patients as both can produce a state of meta-inflammation.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Human Ethics Committee, Government Medical College, Thiruvananthapuram issued approval 04/87/2020/MCT
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Coronaviruses and SARS-CoV-2: a brief overview. Ludwig S, Zarbock A. Anesth Analg. 2020;131:93–96. doi: 10.1213/ANE.0000000000004845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. Yang Y, Peng F, Wang R, et al. J Autoimmun. 2020;109:102434. doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19. 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 4.New coronavirus variants are creating more challenges to global healthcare system: a brief report on the current knowledge. Islam S, Islam T, Islam MR. Clin Pathol. 2022;15:2632010. doi: 10.1177/2632010X221075584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.From SARS to MERS: evidence and speculation. Gao H, Yao H, Yang S, Li L. Front Med. 2016;10:377–382. doi: 10.1007/s11684-016-0466-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. Pijls BG, Jolani S, Atherley A, et al. BMJ Open. 2021;11:0. doi: 10.1136/bmjopen-2020-044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical risk factors for mortality of hospitalized patients with COVID-19: systematic review and meta-analysis. Xiang G, Xie L, Chen Z, et al. Ann Palliat Med. 2021;10:2723–2735. doi: 10.21037/apm-20-1278. [DOI] [PubMed] [Google Scholar]
- 8.Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. Dessie ZG, Zewotir T. https://doi.org/10.1186/s12879-021-06536-3. BMC Infect Dis. 2021;21:855. doi: 10.1186/s12879-021-06536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Effects of age and comorbidities on serum levels of inflammatory markers in community-acquired pneumonia. Viasus D, Simonetti AF, Estupiñan-Bohórquez AF, Carratalà J. Eur J Clin Invest. 2021;51:0. doi: 10.1111/eci.13480. [DOI] [PubMed] [Google Scholar]
- 10.Inflammatory biomarkers, multi-morbidity, and biologic aging. St Sauver J, Rocca W, LeBrasseur N, et al. J Int Med Res. 2022;50:3000605221109393. doi: 10.1177/03000605221109393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Living well with medical comorbidities: a biopsychosocial perspective. Friedman EM, Ryff CD. J Gerontol B Psychol Sci Soc Sci. 2012;67:535–544. doi: 10.1093/geronb/gbr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.A comparative study of high sensitivity C-reactive protein and metabolic variables in type 2 diabetes mellitus with and without nephropathy. Shaheer AK, Tharayil JK, Krishna PW. J Clin Diagn Res. 2017;11:0–4. doi: 10.7860/JCDR/2017/30272.10528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. Franceschi C, Campisi J. J Gerontol A Biol Sci Med Sci. 2014;69 Suppl 1:0–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 14.Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. Knight SR, Ho A, Pius R, et al. BMJ. 2020;370:0. doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The impact of Charlson comorbidity index on mortality from SARS-CoV-2 virus infection and a novel COVID-19 mortality index: CoLACD. Varol Y, Hakoglu B, Kadri Cirak A, et al. Int J Clin Pract. 2021;75:0. doi: 10.1111/ijcp.13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comorbidities influence the predictive power of hematological markers for mortality in hospitalized COVID-19 patients. Parthasarathi A, Basavaraja CK, Arunachala S, Chandran S, Venkataraman H, Satheesh A, Mahesh PA. Adv Respir Med. 2022;90:49–59. doi: 10.5603/ARM.a2022.0017. [DOI] [PubMed] [Google Scholar]
- 17.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Charlson ME, Pompei P, Ales KL, MacKenzie CR. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Does the Charlson comorbidity index help predict the risk of death in COVID-19 patients? Comoglu S, Kant A. North Clin Istanb. 2022;9:117–121. doi: 10.14744/nci.2022.33349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Utility of age-adjusted Charlson comorbidity index as a predictor of need for invasive mechanical ventilation, length of hospital stay, and survival in COVID-19 patients. Shanbhag V, Arjun NR, Chaudhuri S, Pandey AK. Indian J Crit Care Med. 2021;25:987–991. doi: 10.5005/jp-journals-10071-23946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical profile and determinants of mortality in patients with COVID-19: a retrospective analytical cross-sectional study in a tertiary care center in South India. Kurien SS, David RS, Chellappan AK, Varma RP, Pillai PR, Yadev I. Cureus. 2022;14:0. doi: 10.7759/cureus.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The roles of neutrophils in cytokine storms. Chan L, Karimi N, Morovati S, et al. Viruses. 2021;13:2318. doi: 10.3390/v13112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Qin C, Zhou L, Hu Z, et al. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The role of inflammation in hypertension: novel concepts. Patrick DM, Van Beusecum JP, Kirabo A. Curr Opin Physiol. 2021;19:92–98. doi: 10.1016/j.cophys.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meta-inflammation and metabolic reprogramming of macrophages in diabetes and obesity: the importance of metabolites. Russo S, Kwiatkowski M, Govorukhina N, Bischoff R, Melgert BN. Front Immunol. 2021;12:746151. doi: 10.3389/fimmu.2021.746151. [DOI] [PMC free article] [PubMed] [Google Scholar]