Abstract
Natural regeneration of degraded reefs relies on the recruitment of larvae to restore populations. Intervention strategies are being developed to enhance this process through aquaculture production of coral larvae and their deployment as spat. Larval settlement relies on cues associated with crustose coralline algae (CCA) that are known to induce attachment and metamorphosis. To understand processes underpinning recruitment, we tested larval settlement responses of 15 coral species, to 15 species of CCA from the Great Barrier Reef (GBR). CCA in the family Lithophyllaceae were overall the best inducer across most coral species, with Titanoderma cf. tessellatum being the most effective species that induced at least 50% settlement in 14 of the coral species (mean 81%). Taxonomic level associations were found, with species of Porolithon inducing high settlement in the genus Acropora; while a previously understudied CCA, Sporolithon sp., was a strong inducer for the Lobophyllidae. Habitat-specific associations were detected, with CCA collected from similar light environment as the coral inducing higher levels of settlement. This study revealed the intimate relationships between coral larvae and CCA and provides optimal coral-algal species pairings that could be utilized to increase the success of larval settlement to generate healthy spat for reef restoration.
Subject terms: Behavioural ecology, Restoration ecology, Marine biology
Introduction
For sessile marine invertebrates, the generation of motile planktonic reproductive propagules, such as larvae, is critical for range expansion and the genetic mixing and maintenance of populations1–3. Larvae may remain in the water column for periods ranging from minutes4 to months5, with longer durations aiding dispersal from natal habitats. Upon reaching competency for metamorphosis, larvae migrate to the benthos, where they explore and sense the substate in search of a suitable habitat6. The process of habitat selection can be complex, with several factors including substrate orientation, microtopography, and biochemical cues (reviewed in7) reported to induce larvae to attach and metamorphose, a process commonly termed ‘settlement’. The habitat where larvae choose to settle can have significant repercussions on post-settlement survival and growth8,9.
The larvae of scleractinian corals may encounter a variety of suitable habitats for settlement on tropical reefs. An optimal habitat for settlement would promote the survival of spat (early single polyp recruits) through consolidation (e.g. reef matrix that won’t easily be dislodged10,11), protection from grazing, predation and sediment smothering12,13, and adequate irradiance, requirements which could differ amongst coral species14. Importantly, substrata that have developed a microbial biofilm community can often induce metamorphosis15. In addition to microbial biofilms, colonisers such as coralline algae (phylum Rhodophyta, subclass Corallinophycidae) have been demonstrated as important biochemical inducers for coral larval settlement16,17. The potential for coralline algae to facilitate the recovery of coral populations, highlights their importance for the resilience of coral reefs, which are in a state of global decline.
Coralline red algae are a group of calcifying macroalgae that deposit primarily high-magnesium calcite in their cell walls. Coralline algae include two major functional groups: the non-geniculate crustose coralline algae (CCA), and the geniculate, articulated coralline algae, both of which are comprised of > 750 described species18. CCA are distributed worldwide from the tropics to polar regions, and from the intertidal to depths of > 260 m. On tropical coral reefs, in addition to their role in reef resilience, they are important for reef cementation and accretion. However, our understanding of the role of CCA in inducing settlement in coral larvae is limited to a handful of species, across both the algal and coral counterparts. Research in the Caribbean and Pacific on well-studied coral species in the families Agariicidae and Acroporidae has shown that larvae exhibit selectivity and hierarchical settlement responses to different species of CCA, with Titanoderma spp. (family Lithophylloideae) typically settlement across a range of coral species from both regions8,19–22. Other coralline algal genera that have been investigated previously for their inductive potential include Hydrolithon, Porolithon, Amphiroa, Lithophorella, and Neogoniolithon, with varying degrees of success. For example, acroporid larvae have been shown to avoid settlement to Neogoniolithon fosliei, possibly as a result of settlement inhibition strategies deployed by the CCA (e.g. epithallus shedding, overgrowth and chemical deterrents8). Clearly, these intimate CCA-coral associations could have direct implications for the survival of early-life stages and, consequently, species distributions. However, whether the inductive or inhibitory properties of these CCA are relevant beyond the Agariicidae and Acroporidae, and if larval settlement responses across CCA species are consistent within, and between, coral families is currently unknown.
To better understand the role of CCA in coral larval settlement more broadly, we tested settlement responses of larvae across 15 Great Barrier Reef (GBR) coral species from 5 taxonomic families that included the Acroporidae, Merulinidae, Lobophyllidae, Poritidae and Fungiidae from two consecutive spawning periods in October and November 2021 (Table 1), to 14 species of CCA from 7 taxonomic subfamilies/families that included the Lithophylloideae, Hydrolithoideae, Metagoniolithoideae, Mesophyllumaceae, Hapalidiaceae and Sporolithaceae, and a non-coralline species of crustose red calcifying alga, Ramicrusta sp. (family Peyssonneliaceae) (Fig. 1); collectively termed CCA hereafter for ease of communication. Here, we aimed to elucidate species-specific, intra-generic, and familial patterns of coral settlement preferences to common CCA species. Additionally, we assessed whether the origin and habitat from which CCA species were collected had an influence on coral larval settlement. Using a broad range of coral and coralline algal taxonomic groups, our study provides a comprehensive platform for further investigations into the intimate interaction between coral larval settlement and coralline algae and provides fundamental information on coral-algal species pairings useful to optimise larval settlement in aquaculture for reef restoration.
Table 1.
Collection, modes of reproduction, spawning details, and the age of larvae at the start of the experiment for the 15 coral species used in the study.
| Family | Coral species | Mode of reproduction | Gamete release type | Collection location | Number of colonies | Spawning date | Spawning time | Larval age (days) |
|---|---|---|---|---|---|---|---|---|
| Acroporidae | Acropora tenuis (October) | Hermaphroditic | Egg and sperm bundles; gentle | Palm and Magnetic Islands | 13 | 24th October 2021 | 18:00–19:05 h | 6 |
| Acropora tenuis (November) | Hermaphroditic | Egg and sperm bundles; gentle | Palm Islands | 6 | 24th November 2021 | 18:35 h | 7 | |
| Acropora anthocercis | Hermaphroditic | Egg and sperm bundles; gentle | Magnetic Island | 7 | 20th October 2021 | 21:16–22:30 h | 6 | |
| Acropora hyacinthus | Hermaphroditic | Egg and sperm bundles; gentle | Davies Reef | 14 | 29th November 2021 | 21:45–23:37 h | 7 | |
| Montipora aequituberculata | Hermaphroditic | Egg and sperm bundles; gentle | Palm Islands | 4 | 25th October 2021 | 19:38–20:50 h | 6 | |
| Merulinidae | Coeloastrea aspera | Hermaphroditic | Egg and sperm bundles; gentle | Magnetic Island | 14 | 24th October 2021 | 21:20–21:34 h | 5, 8, 18 |
| Caulastrea furcata | Hermaphroditic | Egg and sperm bundles; gentle | Palm Islands | 3 | 23rd November 2021 | 19:50–20:39 h | 6 | |
| Dipsastrea favus | Hermaphroditic | Egg and sperm bundles; vigorous | Magnetic Island | 4 | 23rd October 2021 | 19:45–20:07 h | 4 | |
| Goniastrea favulus | Hermaphroditic | Eggs and sperm separately; passive | Magnetic Island | 6 | 24th October 2021 | 19:20 h | 8 | |
| Mycedium elephantotus | Hermaphroditic | Egg and sperm bundles; gentle | Davies Reef | 5 | 24th November 2021 | 20:54 h | 8 | |
| Platygyra sinensis | Hermaphroditic | Egg and sperm bundles; gentle | Magnetic Island | 2 | 24th October 2021 | 19:34–20:04 h | 4 | |
| Platygyra daedalea | Hermaphroditic | Egg and sperm bundles; gentle | Palm Islands | 6 | 23rd November 2021 | 18:50 h | 6 | |
| Lobophyllidae | Echinophyllia aspera | Hermaphroditic | Egg and sperm bundles; gentle | Magnetic Island | 4 | 28th October 2021 | 20:15–20:30 h | 5 |
| Lobophyllia corymbosa | Hermaphroditic | Eggs and sperm separately; gentle | Palm Islands | 6 | 26th November 2021 | 19:27 h | 8 | |
| Poritidae | Porites lobata | Gonochoric | Eggs and sperm separately; vigorous | Palm Islands | 1 male and 4 females | 25th November 2021 | 21:15–2131 h | 6 |
| Fungiidae | Fungia fungites | Gonochoric | Eggs and sperm separately; vigorous | SeaSim captive | 4 males and 2 females | 25th November 2021 | 18:37–19:26 h | 8 |
The full moon was on the 21st of October (00:56 h) and 19th of November 2021 (18:57 h) during the respective spawning periods.
Figure 1.
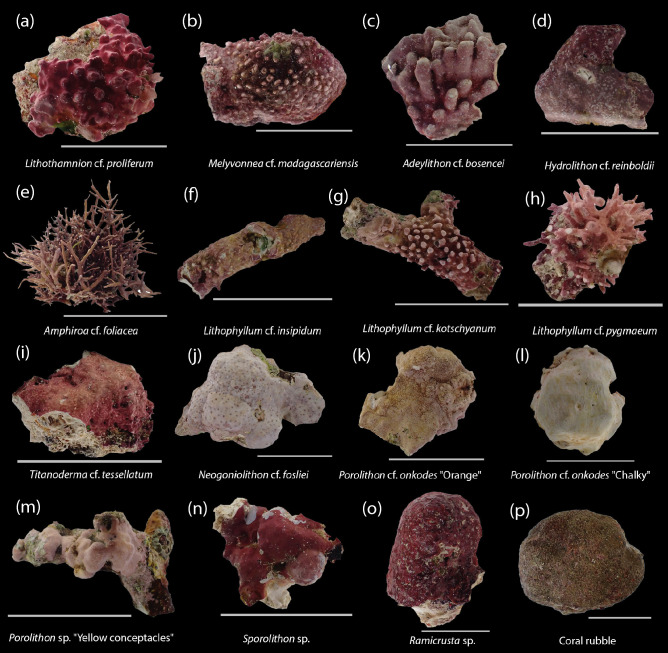
(a‒n) Sample fragments of coralline red algae (including crustose coralline algae and articulated, geniculate coralline algae), (o) Ramicrusta sp. (Peyssonneliaceae) and (p) coral rubble collected from the Great Barrier Reef and tested in the study. Scale bar = 5 cm.
This study aimed to test the hypotheses that (1) there are taxonomic associations between CCA and coral species for larval settlement, and (2) CCA species that occur in similar habitats to their coral counterpart would induce higher levels of settlement.
Results
Taxonomic identification and species delineation of CCA
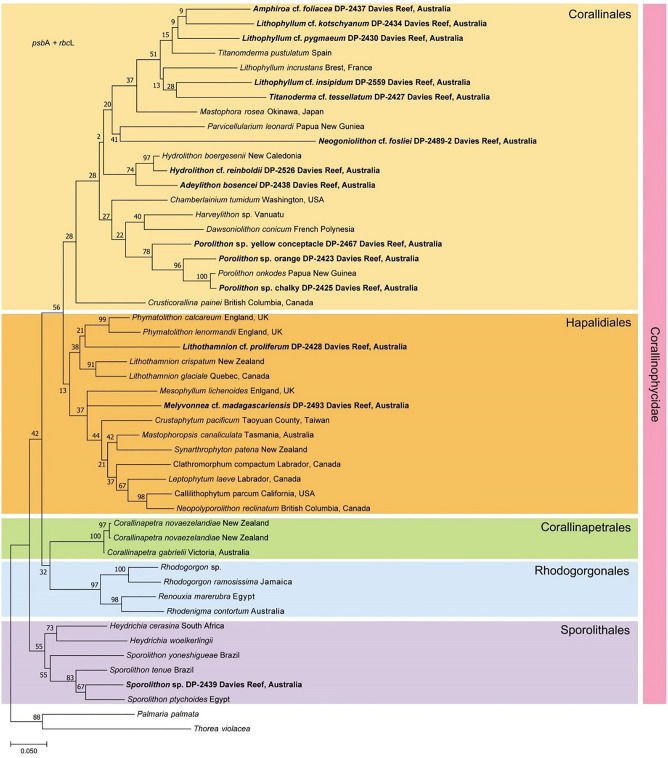
A total of 30 DNA sequences were generated from representative specimens of each of the species used in the experiment (recently published in23), including 15 for psbA (368‒972 bp) and 15 for rbcL (349‒758 bp). The concatenated dataset comprised of 51 taxa with sequences ranging from 654 to 1173 nucleotides (Table 2, Fig. 2). Phylogenetic trees inferred from the psbA and rbcL sequences showed similar topologies in maximum-likelihood analyses to one another and to the concatenated phylogeny (Fig. 2, Supplementary Material 1). In total, eleven sequences were identified in the order Corallinales, two in the order Hapalidiales, and one in the order Sporolithales (Fig. 2). Identifications of majority of the studied genera were supported by psbA and rbcL sequences from type specimens (from recognised herbaria) including for Lithophyllum, Adeylithon, Porolithon and Sporolithon (Supplementary Material 1). At the species level, we were unable to match our collections to any sequences of type specimens as these were either different to the type sequence, or unavailable; we therefore referred to specimens from our collection with the suffix “cf.”, which refers to the species that they most closely resembled based on original species descriptions. The sequence of Ramicrusta sp., which is not included in the concatenated tree, had 96.2% match to the sequence of Ramicrusta textilis (MT215161.1) in psbA and 97.9% match to the sequence of Ramicrusta arenea (JX969780.1) in rbcL through a blast search on GenBank. The phylogenetic analyses support the delineation of species that was performed using morphological assessment for the sorting of the CCA collection used in this study.
Table 2.
Taxonomic, morphological characters and ecological distributions (habitat types, irradiance and relative abundance across the Great Barrier Reef) of CCA species tested in this study.
| Species | Family/Sub-family | Collection site | Key morpho-anatomical characteristics | Distribution | Relative abundance (GBR shelf) | Herbarium numbers | Genbank | Reference (Distribution and GBR abundance) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Habitat | Irradiance level | Inner | Mid | Outer | psbA | rbcL | ||||||
| Lithothamnion cf. proliferum | Hapalidiaceae | Davies Reef | Branching. Lobed branches; very smooth surface; multiporate conceptacles; cell fusions | Crevices, caves | Low | Rare | Rare | Rare | DP-2428 | OP830448 | OP830463 | Dean et al.15 |
| Melyvonnea cf. madagascariensis | Mesophyllumaceae | Davies Reef | Branching. Surface with protuberances; multiporate conceptacles; coaxial hypothallus and cell fusions | Shallow–deep reef | Low–mid | Rare | Common | Rare | DP-2493; DP-2436 | OP830452 | OP830467 | Dean et al.15, G.D.-P. pers. obs |
| Adeylithon cf. bosencei | Hydrolithoideae | Davies Reef | Branching. Cylindrical branches; strongly tessellate surface; trichocytes in loosely defined fields; cell fusions | Shallow–deep reef | Low–high | Rare | Rare | Common | DP-2438 | OP830454 | OP830469 | Peña et al.49, G.D.-P. pers. obs |
| Hydrolithon cf. reinboldii | Hydrolithoideae | Palm Island Group | Encrusting. Knobby protuberances; strongly tessellate surface; dimerous hypothallus; cell fusions | Shallow–deep reef | Mid | Common | Moderate | Moderate | DP-2526 | OP830457 | OP830472 | Dean et al.15, G.D.-P. pers. obs |
| Amphiroa cf. foliacea | Lithophylloideae | Davies Reef | Branching. Articulated (geniculate); cylindrical to flattened branches; secondary pit connections | Shallow–mid reef | Mid–high | Rare | Moderate | Moderate | DP-2437 | OP830453 | OP830468 | G.D.-P. pers. obs |
| Lithophyllum cf. insipidum | Lithophylloideae | Palm Island Group | Encrusting. Dimerous thallus; strongly tessellate surface; secondary pit connections present | Crest, shallow reef | Mid–high | Rare | Common | Common | DP-2559 | OP830456 | OP830471 | Dean et al.15 |
| Lithophyllum cf. kotschyanum | Lithophylloideae | Davies Reef | Branching. Thick and robust branches; slightly tessellate surface; secondary pit connections | Reef crest | Mid—high | Moderate | Moderate | Common | DP-2434 | OP830451 | OP830466 | Dean et al.15 |
| Lithophyllum cf. pygmaeum | Lithophylloideae | Davies Reef | Branching. Pointy to round branches; slightly tessellate surface; secondary pit connections | Crest, shallow reef | Mid–high | Rare | Moderate | Moderate | DP-2430 | OP830449 | OP830464 | Dean et al.15 |
| Titanoderma cf. tessellatum | Lithophylloideae | Davies Reef | Encrusting. Concentric whorls; large green–brown conceptacles; secondary pit connections | Shallow–deep reef | Low–mid | Rare | Rare | Rare | DP-2427 | OP830447 | OP830462 | Dean et al.15 |
| Neogoniolithon cf. fosliei | Neogoniolithoideae | Davies Reef | Encrusting. Very large conceptacles; skin chicken like surface; individual trichocytes; cell fusions | Reef crest | High | Rare | Common | Common | DP-2489-2 | OP830450 | OP830465 | Dean et al.15 |
| Porolithon cf. onkodes "Orange" | Metagoniolithoideae | Davies Reef | Encrusting. ‘Orange’ species in Porolithon cf. onkodes complex; trichocytes in well-defined fields; cell fusions | Reef crest | High | Moderate | Common | Common | DP-2423 | OP830444 | OP830460 | Dean et al.15, G.D.-P. pers. obs |
| Porolithon cf. onkodes "Chalky" | Metagoniolithoideae | Davies Reef | Encrusting. ‘Chalky’ species in Porolithon cf. onkodes complex; trichocytes in well-defined fields; cell fusions | Reef crest | High | Moderate | Common | Common | DP-2425 | OP830445 | OP830460 | Dean et al.15, G.D.-P. pers. obs |
| Porolithon cf. onkodes "Yellow conceptacles" | Metagoniolithoideae | Palm Island Group | Encrusting. Conspicuous yellow-green conceptacles; trichocytes in well-defined fields; cell fusions | Reef crest | High | Common | Rare | Rare | DP-2467 | OP830446 | OP830461 | G.D.-P. pers. obs |
| Sporolithon sp. | Sporolithaceae | Davies Reef | Encrusting. Thick thallus; smooth surface; presence of sori | Crevices, caves | Low | Moderate | Rare | Rare | DP-2439 | OP830455 | OP830470 | Dean et al.15, G.D.-P. pers. obs |
| Ramicrusta sp. | Peyssonneliaceae | Davies Reef | Encrusting. Thick thallus only partially calcified (surface soft) | Crevices, caves | Low | Rare | Moderate | Moderate | DP-2435 | OP830458 | OP830473 | G.D.-P. pers. obs |
Herbarium numbers refer to the collection of Diaz-Pulido at Griffith University, Brisbane, Australia. Representative sequences of each CCA species for the psbA and rbcL gene regions were submitted to GeneBank. G.D.-P. refers to Guillermo Diaz-Pulido.
Figure 2.
Phylogenetic tree based on Maximum Likelihood (ML) analysis of concatenated psbA and rbcL alignment. Values above branches denote maximum likelihood bootstrap values (BS) in %. Sequences obtained from this study are in bold.
CCA inductive properties across coral species
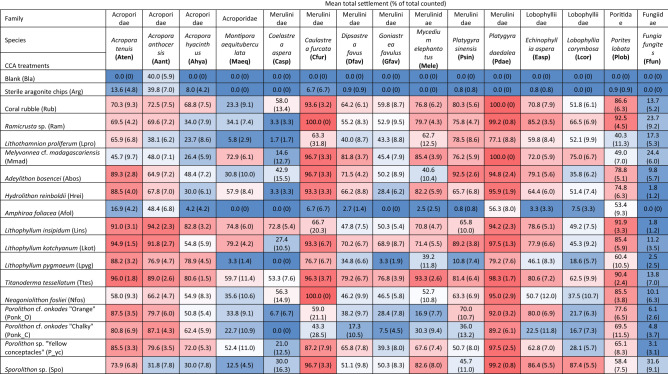
A total of 15 coral species across 5 taxonomic families were tested, and there was a significant effect of CCA treatment on settlement across all coral species (Kruskal–Wallis: H = 876.062, df = 17, p < 0.001). Averaging across all coral species (nspecies = 15, nassay = 165 per CCA species), members of the CCA family Lithophyllaceae induced the highest settlement (~ 68–77%) and was comparable to settlement in the coral rubble treatment (64.8 ± 2.5%; see Supplementary Material 2 for coral species level results). Dunn’s pairwise tests showed that settlement in the P. cf. onkodes “Orange”, L. cf. proliferum, L. cf. pygmaeum, P. cf. onkodes “Chalky” and A. cf. foliacea were significantly lower than the coral rubble treatment (p < 0.05; Supplementary Material 2) while settlement in response to A. cf. foliacea was similar to that found in the sterile aragonite treatment (p > 0.05).
Titanoderma cf. tessellatum induced settlement of > 75% in 11 out of the 15 coral species, with moderate settlement in C. aspera (53.3 ± 7.6%), M. aequituberculata (59.7 ± 11.4%), L. corymbosa (62.5%) and F. fungites (13.8%; Table 3). Overall, T. cf. tessellatum, L. cf. insipidum and L. cf. kotschyanum were the most effective inducers based on mean settlement (see Supplementary Material 2 for pairwise comparisons). However, settlement in non-acroporid species (C. furcata, M. elephantotus, P. daedalea, L. corymbosa, and F. fungites) was consistently higher in the coral rubble and Ramicrusta sp. treatments than in the aforementioned CCA species (Table 3). Amphiroa cf. foliacea was the weakest inducer for settlement when all coral species were considered (Supplementary Material 2). While L. cf. pygmaneum and P. cf. onkodes “Chalky” performed well in most Acropora spp. assays and attained settlement of up to > 75%, they do not perform as well across non-Acropora taxa (Table 3).
Table 3.
Mean total settlement (%; SE in parentheses) of coral larvae from 15 species across 5 taxonomic families when presented with 15 common encrusting red algal species across 8 taxonomic families from the Great Barrier Reef.
Coral and CCA abbreviations are provided in parentheses. Cells with warmer colours represent higher settlement values. Refer to Table 1 for details on coral larval species.
Larval settlement to the living surface of CCA was highly variable across all coral and CCA species tested, with live-surface settlement ranging from 0 to 56.3% across coral species, for between 0 and 13 CCA treatments (Supplementary Material 2). Species in the Acroporidae and Poritidae had the highest settlement onto the living surface of CCA and are detailed in family-level descriptions in Supplementary Material 2.
Settlement trends of coral species and families to common CCA and their ecological traits
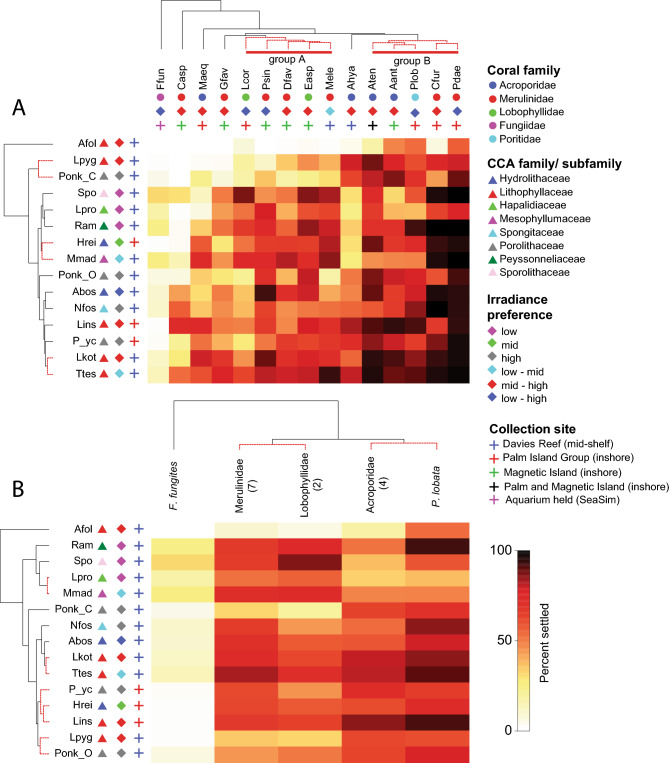
Multivariate analyses using settlement data averaged at both the coral species and family levels, were performed to elucidate any ecologically relevant settlement trends associated with taxonomic grouping, irradiance preference, and CCA collection site. At the coral species level, two statistically distinct groups were found: group A was comprised of members of the Lobophyllidae and Merulinidae (L. corymbosa, E. aspera, P. sinensis, D. favus, and M. elephantotus), while group B comprised members of the Acroporidae, Merulinae and Poritidae (A. tenuis, A. anthocercis, C. furcata, P. daedalea and P. lobata; Fig. 3A, see Supplementary Material 3 for habitat and depth distribution of studied coral species). SIMPER analyses showed that both groups had high within-group similarity of > 90%, with a between-group comparison (dissimilarity = 12.4%) showing higher average settlement of group B corals to CCAs adapted to living under high irradiance (A. cf. foliacea, P. cf. onkodes “Chalky”, L. cf. pygmaeum, P. cf. onkodes “Orange”, N. cf. fosliei, Porolithon sp. “Yellow conceptacles” and A. cf. boscensei, contributing 65% to the between group dissimilarity). This trend corresponded to the structure in the CCA species dendrogram (Fig. 3A). Of note, two large clusters were recovered, one of which comprised CCA that prefer low-mid irradiance (Sporolithon sp., L. cf. proliferum, Ramicrusta sp., H. cf. reinboldii and M. cf. madagascariensis), and another that comprised mid-high irradiance adapted CCAs (P. cf. onkodes “Orange”, A. cf. bosencei, N. cf. fosliei, L. cf. insipidum, Porolithon sp. “Yellow conceptacles”, L. cf. kotschyanum), and an outlier T. cf. tessellatum that prefers low-mid irradiance. Titanoderma cf. tessellatum and L. cf. kotschyanum formed a distinct SIMPROF cluster, reflecting their broadly inductive properties across the 15 coral species tested (Fig. 3A). Lithophyllum cf. pygmaeum and P. cf. onkodes “Chalky”, which also formed a distinct cluster induced higher settlement in group B corals (Fig. 3A). Amphiroa cf. foliacea was an outlier that induced settlement in only some members of group A corals. There was no clear trend in the CCA species dendrogram when the CCA family and collection site were considered (Fig. 3A).
Figure 3.
Shade plots ordered by cluster analyses dendrogram of coral and CCA taxa at the (A) coral species level and (B) coral family level (number of species represented per family indicated in parentheses). Cluster analyses were performed using the Bray–Curtis resemblance matrix for coral taxa and index of association matrix for CCA taxa, on the total settlement data averaged at the coral species and family level. Red dashed lines on the coral and CCA dendrograms reflect distinct groups as determined by SIMPROF analyses. Symbols corresponding to CCA and coral taxonomic families, irradiance preferences and collection sites (see legend) are included after the species abbreviations (See Table 3 for full species names).
At the coral family level, the Merulinidae and Lobophyllidae formed a cluster distinct from the Acroporidae and Poritidae (Fig. 3B). SIMPER analysis showed that both clusters had high within group similarity of > 94%, with between group dissimilarity of 9.56%. Similar to the species level analyses, the separation of these clusters could be explained by the irradiance preference of CCAs, with Merulinidae and Lobophyllidae species settling in higher numbers to low-light adapted CCA species (Sporolithon sp., M. cf. madagascariensis and L. cf. proliferum), while Acroporidae and Poritidae species settled in response to light-adapted CCA species (P. cf. onkodes “Chalky”, A. cf. foliacea, L. cf. pygmaeum, P. cf. onkodes “Orange” and L. cf. insipidum); the aforementioned 8 CCA species contributed to 72% of the between-group dissimilarity. The CCA species dendrogram recovered 2 major clusters and identified A. cf. foliacea as an outlier. Similar to the species-level analyses, the first cluster comprised of CCA species that are adapted to low irradiance (Ramicrusta sp., Sporolithon sp., L. cf. proliferum and M. cf. madagascariensis), while the other cluster comprised species that are adapted to medium to high light (Fig. 3B).
Discussion
Knowledge of the processes that underpin larval settlement behavior is critical for understanding how coral populations are maintained and recover following mortality events24. Our study tested the settlement of 15 common coral species against 15 species of coralline red algae from the GBR, and represents one of the most comprehensive studies undertaken to investigate the intimate relationships between coral larvae and crustose coralline algae (CCA) on coral reefs. We found that coral larval settlement to CCA species varied considerably amongst species, even within the same coral family and genus. This variability notwithstanding, our experiment confirmed the alga Titanoderma cf. tessellatum as a strong inducer of larvae settlement across Acroporidae and Merulinidae8. We also identified previously unknown species of CCA that are important inducers of larval settlement in the coral family Lobophyllidae, and in Fungia fungites and Porites lobata. We demonstrate that coral larval behaviour in response to CCA is highly dependent on the habitat of origin, with coral species from low-irradiance habitats settling in higher proportion on CCA adapted to similar light environments. Although intuitive, this has not been consistently demonstrated across coral and algal species (but see25). Our study provides important foundational information and identifies optimal coral-CCA associations that can be used to produce coral spat for reef restoration across a broad range of taxonomic families.
Larval settlement to CCA species varied considerably among and within the coral families examined. Within the Acroporidae, the plating coral M. aequituberculata, which is common around the turbid inshore reefs of the Great Barrier Reef (GBR;26,27), displayed settlement responses that were distinct from three Acropora spp. tested, which settled well (> 75%) in response to CCA species that are adapted to living in high light and high wave-energy environments (Porolithon spp. and Lithophyllum cf. pygmaeum). Interestingly, out of the three Porolithon species tested, M. aequituberculata settled in higher numbers to P. cf. onkodes “Yellow conceptacles” (~ 52%) when compared to the other two Porolithon spp. (< 35%). Both the coral and CCA species were collected from inshore reefs, which may suggest that some of the variability in CCA-larval interactions may be related to the type of environment that the species pair share.
Intra-generic differences in larval settlement behaviour were also found within the Acropora and Platygyra. For example, A. hyacinthus, which is typically found on reef crest environments, settled up to 50% less on moderate‒low light adapted CCAs compared to A. tenuis and A. anthocercis, both of which occur in deeper and inshore habitats, indicating a potential innate ability of A. hyacinthus larvae to discriminate between CCA adapted to differing irradiance environments. This behaviour could significantly influence habitat selection by A. hyacinthus and may, consequently, influence post-settlement survival, growth, and ultimately species distributions on the reef. Within the genus Platygyra, P. daedalea is a generalist that settled in response to all the CCA species tested except A. cf. foliacea, while P. sinensis was more selective. As P. daedalea and P. sinensis co-occur in similar habitats across the GBR, this variability in settlement response may be driven by species-specific associations, as seen in other sympatric congeneric coral species16.
Larval settlement also varied widely depending on the taxonomic subfamilies of coralline algae considered. For example, while CCA species within the Metagoniolithoideae (which includes Porolithon spp.) were moderate to good (> 63% settlement) inducers for the Acroporidae, they did not perform as well for corals from other taxonomic families. For instance, in the Lobophyllidae, while none of the Porolithon species induced settlement of > 50% (and recording as low as 20% in P. cf. onkodes “Chalky”), Sporolithon sp., Ramicrusta sp., and M. cf. madagascariensis induced settlement of up to 87%. A similar trend was found for the Merulinidae and Fungia fungites, again highlighting the poor performance of Porolithon spp. in inducing larval settlement in these groups. The reverse is however true for the Acroporidae, whereby the latter 3 CCA species did not induce settlement over 60%. Interestingly, Sporolithon sp., Ramicrusta sp., and M. cf. madagascariensis are all adapted to living in habitats having low irradiances. It seems therefore that the coral taxa that occur in deeper or more turbid waters (e.g. inshore compared to mid-shelf reefs), seem to prefer CCA from low-light environments. At the broader level, these observed larval behaviours, and thus potential habitat selectivity, suggests that habitat selection and subsequent ecological partitioning of species on reefal habitats could occur as early as the larval settlement phase (see28).
The subfamily Lithophylloideae is overall the best inducer of coral settlement across the range of coral species and families tested, in particular for the Acroporidae. This finding corroborates a recent study in Guam using Acropora surculosa, which found that larvae settled 9 × more on an undescribed Lithophylloideae sp.1, compared to 26 other CCA species presented together on conditioned substrate29. Interestingly, CCA within the genus Lithophyllum, and the subfamily Lithophylloideae more broadly, include species that are morphologically diverse and that illicit a broad range of settlement responses in corals. Within the genus Lithophyllum, L. cf. kotschyanum (with short protuberances) and L. cf. insipidum (smooth encrusting), consistently out-performed L. cf. pygmaeum (longer branches) across most of the coral species tested, except for Acropora and some generalist Merulinidae (e.g. C. furcata and P. daedalea). The geniculate A. cf. foliacea was the poorest performing Lithophylloideae in which very low to no settlement was found across most coral species. Despite the variability in coral settlement capabilities across the Lithophylloideae, a species that gave relatively consistent settlement results across the 5 coral taxonomic families was Titanoderma cf. tessellatum. Why this species of Titanoderma is such an effective inducer across several coral taxa is little known, although recent studies indicate that the unique metabolites and microbial community associated with this CCA may be responsible for the settlement of some species of Acropora22,30,31.
Individual CCA species were presented in isolation and therefore may not fully elucidate the preferential behaviour of larvae as compared with a choice experiment. For example, Neogoniolithon fosliei has been shown to induce 15 × less settlement compared to Titanoderma prototypum in species of Acropora if they were presented together8. In our study, fairly high settlement to N. cf. fosliei (60%) was observed, although it remained lower than the congeneric T. cf. tessellatum (82%). Whether the use of freshly cut CCA chips, which may release cell-bound settlement cues, could have an effect on settlement is unknown, however species of CCA are known to heal injuries rapidly (e.g. < 2 weeks32); nevertheless, our method allowed for the isolation of CCA-specific morphogens, which would otherwise complicate the interpretation of inductive cues when larvae are tested in choice experiments (i.e. larvae in CCA choice experiments may recognize water-borne or surface cues from one CCA species but attach elsewhere, masking the origin of the cue). Importantly, our study confirms that coralline algae are, overall, strong inducers of larval settlement across all families of corals investigated, and the strength of the inductive potential not only varies depending on the families of corals and algae considered, but also the type of habitat from which both pairs originate.
The causes of the hierarchy in inductive potential of CCA species are not well understood. External algal morphology doesn’t seem to be a good predictor of coral larval settlement because species with very similar morphology elicit very different settlement responses. For example, both M. cf. madagascariensis and L. kotschyanum have a branching morphology with the presence of protuberances (see Fig. 1b and g) yet they had contrasting effects on A. anthocercis settlement. Similarly, smooth species with nearly identical morphological and anatomical features, such as the 3 taxonomically cryptic species within the Porolithon led to large differences in settlement responses (i.e. 22 vs. 87% settlement) in some coral species. A greater focus will therefore need to be placed on the accurate identification of CCA, most likely from using a combination of morphological and molecular approaches as performed in this study, for future studies on coral-CCA interactions.
Biological characteristics of some CCA species have been shown to affect coral larval settlement. For example, epithallial shedding (process by which the uppermost, surficial tissue, the epithallus, sloughs off) has been suggested as an important mechanism by which CCA deter coral larval settlement8. However, N. cf. fosliei, a CCA with strong epithallial shedding induced variable settlement across species. Another possible factor influencing variable settlement induction by Lithophylloideae could be the absence (or scarcity) of trichocytes on the thallus surface, as trichocytes may easily come off the CCA surface dislodging settled larvae. CCA from the other subfamilies in the Corallinaceae such as Hydrolithoideae (Hydrolithon, Adeylithon), Metagoniolithoideae (Porolithon) and Neogoniolithoideae (Neogoniolithon) have abundant trichocytes; however, this hypothesis needs further examination. Algal derived metabolites have also been suggested to drive the settlement behavior of coral larvae and could vary amongst CCA species, resulting in hierarchical induction30,33. Alternatively, microbially derived cues have also been identified as important inducers of coral larval settlement and these vary amongst CCA taxa15,31. It is also possible that cues from both the algal and microbial components interact to modulate coral settlement, as shown for Acropora on the GBR30, and it is also plausible that the nature of these cues is species-specific, given the large variability in surficial microbiome composition across CCA taxa22,31,34,35 and chemical constituents of the alga thallus36. Detailed microbiome and metabolomic studies across a range of coral and algal taxa (e.g.22) would be useful for a better understanding of the nature of larval settlement behavior and their preferences for CCA.
This study summarises the settlement responses of coral larvae to CCA across a broad range of taxa, both for the corals and algae, and will serve as an important platform from which further detailed investigations into the intimate mechanistic pathways for coral larval settlement could be progressed. Our findings here demonstrate the important role of an understudied CCA, Sporolithon sp., on the settlement of corals in the Lobophyllidae and Fungia fungites, and further investigations may uncover novel pathways for habitat selection and larval settlement in non-acroporid coral species. While some studies investigating larval settlement behaviours and competencies have previously used Porolithon onkodes as a routine biological morphogenic inducer37,38, we show here that variable settlement results could inevitably surface due to the cryptic species that are present and currently unresolved in this species complex (e.g.39). The same may be true for other coralline algae groups studied here. For reliable and consistent settlement across coral taxa, an alternative option would be to use Titanoderma cf. tessellatum, which is simpler to identify in the field due to its distinctive morphological characters (i.e. presence of whorls), although detailed taxonomic work is also needed to resolve the taxonomy and test for the presence of cryptic species within this group. Finally, with the world’s coral reefs currently under increasing stress, the optimal coral-algal species combinations reported in this study could be utilized to increase the success of larval settlement to generate healthy spat across a wide range of coral families to supply corals for reef restoration.
Materials and methods
To assess the settlement preferences of coral larvae to CCA, we collected gravid coral colonies and CCA from several localities on the central GBR and transferred them to the National Sea Simulator aquarium facility at the Australian Institute of Marine Science, Townsville (AIMS SeaSim). Settlement assays were performed over a period of ~ 48 h under controlled environmental conditions to test the efficacy of each CCA species in inducing coral larval settlement.
Coral collection, spawning and larval culture
Coral spawning was performed in October and November 2021 (refer to Table 1 for the list of species, reproductive modes and spawning details) to capture inshore and offshore GBR spawning, respectively. Reproductively mature corals were collected from depths of 1–9 m from reefal habitats around Magnetic Island (19°07′45.78″S 146°52′40.14″E), the Palm Island Group (18°45′56.4″S 146°32′2.58″E) and Davies Reef (18°49′13.5″S 147°38′40.32″E), central GBR between the 9th to 20th of October and 14th to 21st of November 2021 (GBRMPA Permit G21/45348.1). Habitats around Magnetic Island and the Palm Island group are representative of fringing reefs around inshore islands of the GBR, and Davies Reef is representative of a low-turbidity mid-shelf reef.
Approximately 1 week before the full moon, mature coral colonies were determined by visual inspection of pigmented eggs within the coral tissue in situ, and whole colonies, or fragments, collected using a hammer and chisel on SCUBA. As eggs were small (~ 200 μm) and unpigmented in the gonochoric coral Porites lobata, small fragments (n = 4–5; ~ 5 × 5 mm) containing live tissue were stained in 10 mL of Neutral Red for 20 min (following40) to highlight the mesenteries, ovaries and testes, and observed under a stereo microscope to confirm the sex and maturity of the colonies prior to collection. Coral colonies and fragments were left on the reef until the morning of transfer to the research facility, where they were transported in 70L bins (1‒4 colonies per bin) receiving constant flow-through seawater on board the vessel over a period of 4‒6 h. Upon reaching the AIMS SeaSim, corals were held in outdoor semi-recirculating aquaria that received new input of 1 µm filtered seawater (FSW) at a rate of ~ 3 turnovers per day and that profiled the ambient temperature experienced at mid-shelf reefs, based on the historic daily mean reef temperature at Davies Reef measured at 4 m water depth between 1991 and 2012 (~ 27.2 °C; see Supplementary Material 2, Fig. S4, for temperature profile).
Depending on species and colony sizes, between 10 and 20 individual colonies were held in each aquarium (deep aquaria: 100 × 280 × 50 cm, 2000 L; shallow aquaria: 80 × 140 × 28 cm, 280L). All aquaria received natural sunlight (midday max intensity ~ 200 μmol quanta m−2 s−1) and photoperiod (12‒13 h daylight) and were fitted with 2 gyres (Maxspect 350 series) at each end of the aquarium to provide consistent water circulation across aquaria.
Corals were monitored throughout the predicted evenings of spawning and individual colonies were isolated in 60 L aquaria when setting of gametes was observed. For coral species that released egg-sperm bundles, gametes were collected within 1 h of release by skimming from the water surface using a clean plastic cup and plastic pipettes. The bundles were gently agitated and filtered through a 106 μm mesh screen to separate eggs and sperm. Eggs were washed with FSW. For each coral species, gametes from all parent colonies were pooled for cross fertilization in a 60 L aquarium at approximately 1 × 106 sperm mL−1. After 1 h, embryos were gently rinsed in FSW to remove excess sperm and transferred to either 500 L or 70 L flow-through culture tanks at a stocking density of approximately 0.3 larvae mL−1. For the gonochoric species (P. lobata and Fungia fungites), and hermaphroditic species that release eggs and sperm separately (Lobophyllia corymbosa and Goniastrea favulus), fertilization was performed in 60 L aquaria by transferring water containing sperm across aquaria and embryos transferred to culture tanks when first signs of cleavage were detected (~ after 30–45 min). Gentle aeration was provided around the culture tank standpipes to prevent embryos from sticking to the outlet filters (106–212 μm mesh depending on species embryo sizes) and aeration was gradually increased after 24 h (beyond the gastrula stage) to allow for in-water circulation. Larvae were maintained in the culture tanks until used in the settlement experiment.
Algal collections and identifications
Thirteen common species of non-geniculate crustose coralline algae, a geniculate articulated coralline algal species, and an encrusting calcifying red algal species from the family Peyssonneliaceae, were collected from reefal habitats at Davies Reef and Palm Island Group where coral species co-occurred at depths of 1–10 m, between the 9th and 20th of October 2021 (Fig. 1). Collections were performed on SCUBA using a hammer and chisel. CCA were held in 70 L flow-through aquaria and were subsequently identified and sorted onboard the research vessel. CCA samples were identified based on morphological and anatomical characters, including thallus surficial texture, presence/absence of trichocytes and trichocyte fields, types of reproductive structures (conceptacles), and hypothallus arrangement, using a dissecting microscope and cell connections (fusions and secondary pits) under a compound microscope (e.g.41,42) and, where possible, species-level identification assigned. Representative individuals of the different species used in the experiment were preserved in silica gel for molecular identifications and voucher specimens deposited in the Coral Reef Algae Laboratory at Griffith University, Brisbane. Genomic DNA extraction and amplification followed43. Briefly, genomic DNA was extracted using a NucleoSpin Plant II Kit (Macherey–Nagel, Düren, Germany) following the manufacturer’s instructions. Two plastid-encoded markers (psbA; psbAF1 and psbAR2 primers and rbcL; F57/R1150 and F993/RrbcStart primers) were amplified to infer phylogenetic relationships of the experimental CCA species44,45. Each PCR reaction comprised a 30 μl mixture of 4–12 μl genomic DNA, 1 μl of 10 pmol forward + 1 μl of reverse primers, 0–8 μl distilled water, and 16 μl HelixAmp Ready-2x-Go Series (NanoHelix, Daejeon, Korea).Cycle sequencing was performed by Macrogen (Seoul, South Korea). Detailed description of sequence alignments and phylogenetic analyses are provided in Supplementary Material 1, with the concatenated psbA and rbcL tree presented here. Newly generated sequences were deposited in GenBank (Table 2).
All CCA species were collected from Davies Reef except for Hydrolithon cf. reinboldii, Lithophyllum cf. insipidum and Porolithon cf. onkodes “Yellow conceptacles”, which were collected from the Palm Island Group (Table 2). For Davies Reef CCA, species adapted to low-light conditions were collected from crevices and under overhangs and included Ramicrusta sp., Lithothamnion cf. proliferum and Sporolithon sp.; other species occurred commonly on shallow reef crests exposed to high light and high flow, and included species of Porolithon spp., Neogoniolithon cf. fosliei, Adeylithon cf. bosencei and Lithophyllum cf. pygmaeum. Lithophyllum cf. kotschyanum was collected from a field of branching acroporid coral rubble at ~ 3 m. Titanoderma cf. tessellatum and Melyvonnea cf. madagascariensis occurred in habitats with moderate illumination; T. cf. tessellatum was found on vertical walls or at the intersection of light–dark habitats (i.e. at the edge of overhangs) while M. cf. madagascariensis was found growing on deeper substrates (~ 8–9 m). The articulated coralline alga Amphiroa cf. foliacea was collected from the front of Davies Reef at 5‒6 m depth. Collectively, these CCA species were chosen because they are among the most common taxa on the GBR and they encompass the major orders of the subclass Corallinophycidae (Rhodophyta). ‘Coral rubble’ was also included as a treatment as this presents a common reefal substrate, comprising diverse community of potential alternative inducers for coral larvae46. Coral rubble chips were prepared from recently dead rubble of massive Porites sp. covered with thin microbial and algal biofilms (cyanobacteria, diatoms), brown algae Sphacelaria sp., green algae Cladophora sp., and red algae Polysiphonia spp., Ceramium spp., and ~ 40% cover of mixed CCA (characterized from n = 8 fragments).
CCA were transported in flow-through 70L bins, similar to the method used for corals, to AIMS SeaSim where they were held in indoor semi-recirculating aquaria (80 × 140 × 28 cm, 280 L) with water exchange flow rates and circulation as described above. Aquaria were outfitted with 2 LED panel lights, with a lighting profile comprising a linear ramp-up period of 6.5 h from darkness (05:30 h) to a maximum of ~ 120 μmol quanta m−2 s−1 (12:00 h) and a ramp-down over 6.5 h to darkness (18:30 h). Light intensities for low-light adapted (max midday 12.7‒15 μmol quanta m−2 s−1; LI-COR LI-250A) and moderate-light adapted (max midday 56‒58 μmol quanta m−2 s−1) CCA species were controlled using one to several 50% shade cloths in the holding tank.
CCA maintenance and settlement assays
To assess settlement responses of coral larval species to the suite of CCA species, assays using live CCA chips were performed during the October and November 2021 spawning periods. CCA fragments were cut into 10 × 10 mm pieces using a wet diamond band saw (Gryphon), glued onto a poly-vinyl-chloride (PVC) rack, and allowed to recover for at least 2-weeks in their respective holding tanks. Immediately prior (< 1 h) to setting up settlement assays, CCA fragments were resized to 5 × 5 mm pieces to minimize any water quality issues (e.g. excessive organic loading and anoxic conditions) during the assays that could result from high biomass. Experimental treatments included the 15 algal species, the coral rubble treatment, a negative control that did not contain any substrate apart from the plastic surfaces of wells (blanks), and a procedural control that had a 5 × 5 mm fragment of sterile aragonite (autoclaved at 120 °C for 20 min).
Settlement assays were performed in wells (Costar® 6-well plates) filled with 10 ml of 0.1 μm FSW. Ten active and normal swimming larvae and one treatment chip (placed in the centre of the well with the live tissue facing up) were added to each well. The experiment was conducted in a temperature-controlled room (27.2 °C). The position of CCA treatments across wells was randomized to minimize any well or plate positioning bias. LED panel lights were positioned above the plates to maintain the healthy condition of live CCA in the assays with a linear ramp-up period of 6.5 h from darkness (05:30 h) to a maximum of ~ 50 μmol quanta m−2 s−1 (12:00 h) and a ramp-down over 6.5 h to darkness (18:30 h). Maximum illumination intensity of ~ 50 μmol quanta m−2 s−1 was selected to emulate light conditions on benthic substrates onto which coral larvae are likely to settle47.
Settlement assays were performed over 46‒55 h. Total larval settlement and the position on which larvae had successfully settled (i.e. on live CCA tissue or on the side or underside of CCA, in the matrix of CCA, and on the plastic surface of wells) were recorded (see Supplementary Material 2, Fig. S42, for images of larval settlement endpoints). Settlement was defined as the permanent attachment and metamorphosis of a larva into a primary polyp that is flattened on the oral-aboral axis to form a disc-shaped structure with obvious radial septal mesenteries17. Fluorescence was used to assist in the detection of smaller sized larvae, using a stereo microscope fluorescence adaptor (https://nightsea.com/; SFA RB – excitation 440–460 nm, emission filter 500 nm longpass) that excites the larval green fluorescent proteins.
As the same CCA specimens collected in October were used for assays following both spawning periods, any temporal changes in their bioactivity (e.g. from aging or being held in aquaria) were assessed by performing a duplicate assay in November using the coral species Acropora tenuis. Similar conditions were applied across the two assays, with the only difference being the testing of 6-day old larvae in October compared with 7-day old larvae in November (Table 1). In addition, assays were repeated for the coral species Coeloastrea aspera across three larval ages (5, 8 and 18 d) in October, because 5-day old larvae had low settlement across treatments and thus may not have been competent. Twelve treatment replicates were used in all coral settlement assays, except for 8- and 18-day old C. aspera (n = 6) and Caulastrea furcata (n = 3; Table 1), and all larvae tested were between 4 and 8 days old unless otherwise stated (Table 1).
Statistical analyses
To assess broad settlement trends, settlement data for each CCA species were pooled to coral family (Acroporidae nassays = 48; Merulinidae nassays = 69; Lobophyllidae nassays = 24; Poritidae nassays = 12; and Fungiidae nassays = 12). The assay for A. tenuis from November was omitted from family level analyses as this species exhibited similar settlement responses across the two test periods (see Supplementary Material 2 for temporal comparisons). Assay data from 5-day old C. aspera (Merulinidae) were omitted from the analysis as settlement competency was not reached for this species until day 8. Similarly, assay data from 18-day old C. aspera assay were omitted because results were similar to those obtained from 8-day old larvae (Supplementary Material 2) and to keep age consistent with other coral species tested. Additionally, data were pooled across all coral species to identify overall patterns in response to the CCA species. Family-specific settlement data did not meet model assumptions for parametric tests; therefore Kruskal–Wallis and Dunn’s post-hoc pairwise tests with Bonferroni adjustments were performed using the function kwPlot in the package ‘GMAMisc’, using the aragonite treatment as an experimental control. Figures were generated using the package ‘ggplot2’. Similarly, coral species level analyses were performed using Kruskal–Wallis and Dunn’s pairwise tests, as assumptions for parametric tests were not met. Only total settlement, regardless of settlement position in the assays, was assessed. General trends of larval settlement onto the living surface of CCA are briefly reported. Univariate analyses were performed in R Studio, using R version 4.0.4.
To assess whether groups of coral species or families has specific settlement preferences for CCA species, a multivariate dataset was generated by averaging total settlement in response to each CCA species at (1) the coral species level and (2) the coral family level. Cluster analyses (9999 permutations) were performed separately for coral species- and family-level data, on the Bray–Curtis similarity resemblance matrix (samples), and on the Index of Association similarity resemblance matrix for the CCA species (variables). Similarity profile tests (SIMPROF) were concurrently performed to identify statistically distinct clusters from the analyses. To visualize any trends in settlement by group, shade plots of average percent settlement, ordered by coral and CCA dendrograms, were plotted to include CCA family, irradiance preference and collection sites for each CCA species. CCA habitat and irradiance preferences were determined from48,49 and personal observations by Diaz-Pulido, where high irradiance refers to shallow reef crest environments, low irradiance to crevices and cave habitats, and moderate irradiance to reef slope and wall habitats (Table 2). Similarity percentage (SIMPER) analysis on the resulting SIMPROF groups identified CCA species that contributed to ~ 70% of the between-group dissimilarity. Multivariate analyses were performed in PRIMER v7.
Supplementary Information
Acknowledgements
This research was supported by the Reef Restoration and Adaptation Program, which aims to develop effective interventions to help the Reef resist, adapt and recover from the impacts of climate change, and which is funded by the partnership between the Australian Governments Reef Trust and the Great Barrier Reef Foundation. We thank the Australian Biological Resource Study (ABRS; grant no. RG19-35) for support to GDP and SJ. We would also like to acknowledge and pay our respects to the Bindal and Wulgurukaba people, the Traditional Custodians of the land and sea country on which this research was performed. We would like to thank the editor and 2 anonymous Reviewers for providing constructive feedback that improved the manuscript.
Author contributions
M.A.A.W. and G.D.-P. conceptualized the study. M.A.A.W., S.F., V.K.S. and G.M. performed the larval settlement assays. S.J. performed molecular analyses of CCA. M.A.A.W. performed the analyses, prepared figures/ tables and drafted the manuscript. All authors revised the manuscript and approved the final draft.
Data availability
All summary data generated in the study are provided as tables in the main article and the Electronic Supplementary Materials (ECM). Raw data is available from the Australian Institute of Marine Science Data Centre repository (https://apps.aims.gov.au/metadata/search). Molecular data for CCA species identifications used during the current study were uploaded to GenBank under accession numbers OP830444 to OP830473. Data from GenBank can be accessed by following instructions in the article https://academic.oup.com/nar/article/45/D1/D37/2605704.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-32676-4.
References
- 1.Vance RR. On reproductive strategies in marine benthic invertebrates. Am. Nat. 1973;107:339–352. doi: 10.1086/282838. [DOI] [Google Scholar]
- 2.Pineda J, Reyns NB, Starczak VR. Complexity and simplification in understanding recruitment in benthic populations. Popul. Ecol. 2009;51:17–32. doi: 10.1007/s10144-008-0118-0. [DOI] [Google Scholar]
- 3.Aguirre JD, Marshall DJ. Genetic diversity increases population productivity in a sessile marine invertebrate. Ecology. 2012;93:1134–1142. doi: 10.1890/11-1448.1. [DOI] [PubMed] [Google Scholar]
- 4.Abdul Wahab MA, de Nys R, Webster N, Whalan S. Larval behaviours and their contribution to the distribution of the intertidal coral reef sponge Carteriospongia foliascens. PLoS ONE. 2014;9:e98181. doi: 10.1371/journal.pone.0098181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham E, Baird A, Connolly S. Survival dynamics of scleractinian coral larvae and implications for dispersal. Coral Reefs. 2008;27:529–539. doi: 10.1007/s00338-008-0361-z. [DOI] [Google Scholar]
- 6.Harrison PL, Wallace C. Reproduction, dispersal and recruitment of scleractinian corals. Ecosyst. World. 1990;25:133–207. [Google Scholar]
- 7.Randall CJ, Giuliano C, Heyward AJ, Negri AP. Enhancing coral survival on deployment devices with microrefugia. Front. Mar. Sci. 2021;8:662263. doi: 10.3389/fmars.2021.662263. [DOI] [Google Scholar]
- 8.Harrington L, Fabricius K, De’Ath G, Negri A. Recognition and selection of settlement substrata determine post-settlement survival in corals. Ecology. 2004;85:3428–3437. doi: 10.1890/04-0298. [DOI] [Google Scholar]
- 9.Abdul Wahab MA, Maldonado M, Luter HM, Jones R, Ricardo G. Effects of sediment resuspension on the larval stage of the model sponge Carteriospongia foliascens. Sci. Total Environ. 2019;695:133837. doi: 10.1016/j.scitotenv.2019.133837. [DOI] [PubMed] [Google Scholar]
- 10.Yadav S, Rathod P, Alcoverro T, Arthur R. “Choice” and destiny: The substrate composition and mechanical stability of settlement structures can mediate coral recruit fate in post-bleached reefs. Coral Reefs. 2016;35:211–222. doi: 10.1007/s00338-015-1358-z. [DOI] [Google Scholar]
- 11.Johns KA, et al. Macroalgal feedbacks and substrate properties maintain a coral reef regime shift. Ecosphere. 2018;9:e02349. doi: 10.1002/ecs2.2349. [DOI] [Google Scholar]
- 12.Trapon M, Pratchett M, Hoey A, Baird A. Influence of fish grazing and sedimentation on the early post-settlement survival of the tabular coral Acropora cytherea. Coral Reefs. 2013;32:1051–1059. doi: 10.1007/s00338-013-1059-4. [DOI] [Google Scholar]
- 13.Doropoulos C, et al. Characterizing the ecological trade-offs throughout the early ontogeny of coral recruitment. Ecol. Monogr. 2016;86:20–44. doi: 10.1890/15-0668.1. [DOI] [Google Scholar]
- 14.Anthony K, Connolly SR. Environmental limits to growth: Physiological niche boundaries of corals along turbidity–light gradients. Oecologia. 2004;141:373–384. doi: 10.1007/s00442-004-1647-7. [DOI] [PubMed] [Google Scholar]
- 15.Webster NS, et al. Metamorphosis of a scleractinian coral in response to microbial biofilms. Appl. Environ. Microbiol. 2004;70:1213–1221. doi: 10.1128/AEM.70.2.1213-1221.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morse DE, Hooker N, Morse AN, Jensen RA. Control of larval metamorphosis and recruitment in sympatric agariciid corals. J. Exp. Mar. Biol. Ecol. 1988;116:193–217. doi: 10.1016/0022-0981(88)90027-5. [DOI] [Google Scholar]
- 17.Heyward A, Negri A. Natural inducers for coral larval metamorphosis. Coral Reefs. 1999;18:273–279. doi: 10.1007/s003380050193. [DOI] [Google Scholar]
- 18.Peña V, et al. Radiation of the coralline red algae (Corallinophycidae, Rhodophyta) crown group as inferred from a multilocus time-calibrated phylogeny. Mol. Phylogenet. Evol. 2020;150:106845. doi: 10.1016/j.ympev.2020.106845. [DOI] [PubMed] [Google Scholar]
- 19.Price N. Habitat selection, facilitation, and biotic settlement cues affect distribution and performance of coral recruits in French Polynesia. Oecologia. 2010;163:747–758. doi: 10.1007/s00442-010-1578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritson-Williams R, Arnold S, Paul VJ, Steneck R. Larval settlement preferences of Acropora palmata and Montastraea faveolata in response to diverse red algae. Coral Reefs. 2014;33:59–66. doi: 10.1007/s00338-013-1113-2. [DOI] [Google Scholar]
- 21.Ritson-Williams R, Arnold SN, Paul VJ. Patterns of larval settlement preferences and post-settlement survival for seven Caribbean corals. Mar. Ecol. Prog. Ser. 2016;548:127–138. doi: 10.3354/meps11688. [DOI] [Google Scholar]
- 22.Jorissen H, et al. Coral larval settlement preferences linked to crustose coralline algae with distinct chemical and microbial signatures. Sci. Rep. 2021;11:1–11. doi: 10.1038/s41598-021-94096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doll PC, et al. Settlement cue selectivity by larvae of the destructive crown-of-thorns starfish. Biol. Lett. 2023;19(1):20220399. doi: 10.1098/rsbl.2022.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De’Ath G, Fabricius KE, Sweatman H, Puotinen M. The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17995–17999. doi: 10.1073/pnas.1208909109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorissen H, Baumgartner C, Steneck RS, Nugues MM. Contrasting effects of crustose coralline algae from exposed and subcryptic habitats on coral recruits. Coral Reefs. 2020;39:1767–1778. doi: 10.1007/s00338-020-02002-9. [DOI] [Google Scholar]
- 26.DeVantier L, De’Ath G, Done T, Turak E. Ecological assessment of a complex natural system: a case study from the Great Barrier Reef. Ecol. Appl. 1998;8:480–496. doi: 10.1890/1051-0761(1998)008[0480:EAOACN]2.0.CO;2. [DOI] [Google Scholar]
- 27.Cacciapaglia C, van Woesik R. Climate-change refugia: Shading reef corals by turbidity. Glob. Change Biol. 2016;22:1145–1154. doi: 10.1111/gcb.13166. [DOI] [PubMed] [Google Scholar]
- 28.Davies S, Meyer E, Guermond SM, Matz MV. A cross-ocean comparison of responses to settlement cures in reef building corals. PeerJ. 2014;2:e333. doi: 10.7717/peerj.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deinhart M, Mills MS, Schils T. Community assessment of crustose calcifying red algae as coral recruitment substrates. PLoS ONE. 2022;17:e0271438. doi: 10.1371/journal.pone.0271438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gómez-Lemos LA, Doropoulos C, Bayraktarov E, Diaz-Pulido G. Coralline algal metabolites induce settlement and mediate the inductive effect of epiphytic microbes on coral larvae. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-35206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siboni N, et al. Crustose coralline algae that promote coral larval settlement harbor distinct surface bacterial communities. Coral Reefs. 2020;39:1703–1713. doi: 10.1007/s00338-020-01997-5. [DOI] [Google Scholar]
- 32.Reyes Nivia, M. C. Effect of future climate scenarios on reef bioerosion processes. PhD Thesis, The University of Queensland, St Lucia, Australia (2013)
- 33.Tebben J. Chemical mediation of coral larval settlement by crustose coralline algae. Sci. Rep. 2015;5:10803. doi: 10.1038/srep10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sneed JM, Sharp KH, Ritchie KB, Paul VJ. The chemical cue tetrabromopyrrole from a biofilm bacterium induces settlement of multiple Caribbean corals. Proc. R. Soc. B. 2014;281:20133086. doi: 10.1098/rspb.2013.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinlan ZA, Ritson-Williams R, Carroll BJ, Carlson CA, Nelson CE. Species-specific differences in the microbiomes and organic exudates of crustose coralline algae influence bacterioplankton communities. Front. Microbiol. 2019;10:2397. doi: 10.3389/fmicb.2019.02397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergstrom E, et al. Cell wall organic matrix composition and biomineralization across reef-building coralline algae under global change. J. Phycol. 2022;59:111–125. doi: 10.1111/jpy.13290. [DOI] [PubMed] [Google Scholar]
- 37.Diaz-Pulido G, Harii S, McCook L, Hoegh-Guldberg O. The impact of benthic algae on the settlement of a reef-building coral. Coral Reefs. 2010;29:203–208. doi: 10.1007/s00338-009-0573-x. [DOI] [Google Scholar]
- 38.Whitman TN, Negri AP, Bourne DG, Randall CJ. Settlement of larvae from four families of corals in response to a crustose coralline alga and its biochemical morphogens. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-73103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrielson PW, Hughey JR, Diaz-Pulido G. Genomics reveals abundant speciation in the coral reef building alga Porolithon onkodes (Corallinales, Rhodophyta) J. Phycol. 2018;54:429–434. doi: 10.1111/jpy.12761. [DOI] [PubMed] [Google Scholar]
- 40.Elliott DT, Tang KW. Simple staining method for differentiating live and dead marine zooplankton in field samples. Limnol. Oceanogr. Methods. 2009;7:585–594. doi: 10.4319/lom.2009.7.585. [DOI] [Google Scholar]
- 41.Adey, W. H., Townsend, R. & Boykins, W. The crustose coralline algae Rhodophyta: (Corallinaceae) of the Hawaiian Islands. Smithson. Contribu. Mar. Sci. 1–74 (1982)
- 42.Ringeltaube P, Harvey A. Non-geniculate coralline algae (Corallinales, Rhodophyta) on Heron Reef, Great Barrier Reef (Australia) Bot. Mar. 2000;43:431–454. doi: 10.1515/BOT.2000.045. [DOI] [Google Scholar]
- 43.Jeong S, Won B, Cho TO. Two new encrusting species from the genus Phymatolithon (Hapalidiales, Corallinophycidae, Rhodophyta) from Korea. Phycologia. 2019;58:592–604. doi: 10.1080/00318884.2019.1625608. [DOI] [Google Scholar]
- 44.Freshwater DW, Rueness J. Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis. Phycologia. 1994;33:187–194. doi: 10.2216/i0031-8884-33-3-187.1. [DOI] [Google Scholar]
- 45.Yoon HS, Hackett JD, Bhattacharya D. A single origin of the peridinin- and fucoxanthin-containing plastids in dinoflagellates through tertiary endosymbiosis. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11724–11729. doi: 10.1073/pnas.172234799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfe K, Kenyon TM, Mumby PJ. The biology and ecology of coral rubble and implications for the future of coral reefs. Coral Reefs. 2021;40:1769–1806. doi: 10.1007/s00338-021-02185-9. [DOI] [Google Scholar]
- 47.Ricardo GF, et al. Impacts of water quality on Acropora coral settlement: The relative importance of substrate quality and light. Sci. Total Environ. 2021;777:146079. doi: 10.1016/j.scitotenv.2021.146079. [DOI] [PubMed] [Google Scholar]
- 48.Dean AJ, Steneck RS, Tager D, Pandolfi JM. Distribution, abundance and diversity of crustose coralline algae on the Great Barrier Reef. Coral Reefs. 2015;34:581–594. doi: 10.1007/s00338-015-1263-5. [DOI] [Google Scholar]
- 49.Peña V, Le Gall L, Rösler A, Payri CE, Braga JC. Adeylithon bosencei gen. et sp. Nov. (Corallinales, Rhodophyta): A new reef-building genus with anatomical affinities with the fossil Aethesolithon. J. Phycol. 2019;55(1):134–145. doi: 10.1111/jpy.12799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All summary data generated in the study are provided as tables in the main article and the Electronic Supplementary Materials (ECM). Raw data is available from the Australian Institute of Marine Science Data Centre repository (https://apps.aims.gov.au/metadata/search). Molecular data for CCA species identifications used during the current study were uploaded to GenBank under accession numbers OP830444 to OP830473. Data from GenBank can be accessed by following instructions in the article https://academic.oup.com/nar/article/45/D1/D37/2605704.